Abstract
Background and Objective:
To perform a systematic review and meta-analysis to evaluate the efficacy of perioperative acetazolamide (ACTZ) administration with laparoscopy for reducing postoperative referred pain.
Methods:
The following databases were searched from inception to March 1, 2020: Cochrane, PubMed, PubMed Central, Ovid, and Embase. Electronic search used: Acetazolamide AND (laparoscopy OR laparoscopic OR Celioscopy OR Celioscopies OR Peritoneoscopy OR Peritoneoscopies). No limits or filters were used. We included only studies of patients who underwent abdominal laparoscopy (LSC), had a pain assessment at approximately 24 hours postoperatively, and included a treatment with ACTZ group and a no-treatment or minimal-treatment comparison group.
Results:
Five studies met inclusion criteria, with a combined total of 253 participants, 116 in the ACTZ group and 137 in the control group. A Bayesian hierarchical model was assumed for the study specific treatment effects. Posterior sampling was conducted via Markov Chain Monte Carlo methods, and posterior inference carried out on the hierarchical treatment effect. ACTZ significantly decreased average pain scores compared to control group by −0.726 points (95% confidence interval −1.175–0.264). The posterior probability that ACTZ decreases mean pain scores by ≥ 0.5 was 0.846.
Conclusion:
Current available evidence demonstrates that perioperative ACTZ may provide a modest improvement in postoperative referred pain following LSC.
Keywords: Acetazolamide, Laparoscopy, Perioperative Care, Postoperative Pain
INTRODUCTION
The number of abdominal laparoscopic cases continues to rise with the development of new technologies and innovations in procedure techniques. Laparoscopic (LSC) surgery has added benefits over laparotomy, including shorter hospital stays and potential decrease in postoperative pain leading to decreased opioid use. However, it does require insufflation of the abdomen for visualization, and this is most often achieved with carbon dioxide (CO2).
Despite improvement in postoperative pain compared to laparotomy, pain can still be an issue for patients undergoing LSC. Beyond the typical soreness expected after a procedure, these patients can have a unique type of pain as a result of the insufflation of CO2 which manifests as pain radiating to the shoulder. This specific pain, traditionally referred to as Kehr’s sign, is referred pain signaled by the phrenic nerve to the shoulder caused by irritation of the diaphragm. Several modalities are offered to reduce this pain, including use of local anesthetics, saline fluid instillation, nonsteroidal anti-inflammatory drugs (NSAIDs), intraperitoneal drains, and even filters which heat and hydrate laparoscopic gas.1–3 In addition, studies have also proposed a novel use of the medication acetazolamide (ACTZ).4–8
ACTZ is a medication that is currently approved for treatment of glaucoma acute altitude sickness, and epilepsy; however, given its mechanism of action, it has been utilized and studied as a means of reducing postoperative pain after laparoscopy. It works by inhibiting the carbonic anhydrase enzyme, which is responsible for the conversion of CO2 and water to carbonic acid.9 The CO2 gas that is used for insufflation is converted to carbonic acid by carbonic anhydrase, leading to intraperitoneal irritation due to acid buildup.10 Perioperative administration of ACTZ may decrease the amount of carbonic acid production and thereby decrease diaphragm irritation. Although there is no consensus regarding optimal administration timing, it can be administered pre-operatively, intra-operatively, or postoperatively via per os or intravenous route. ACTZ reaches maximum serum concertation in 2–4 hours and is rapidly cleared by the kidneys, with 90%–100% medication excretion in 24 hours.11 This represents a potential cost-effective strategy with few side effects or contraindications, making it an ideal option for improving postoperative pain. Current studies to date examining the efficacy of ACTZ for postoperative pain contain conflicting data, differing endpoints, and are limited in size, making it difficult to draw conclusions. The aim of this study is to perform a systematic review and meta-analysis to investigate the effect of ACTZ administration with LSC for reducing postoperative referred pain.
METHODS
This meta-analysis was conducted in accordance with the PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.12 This review was not registered.
Literature Search
The following databases were searched from inception to March 1, 2020: Cochrane, PubMed, PubMed Central, Ovid, and Embase. Electronic search used: Acetazolamide AND (laparoscopy OR laparoscopic OR Celioscopy OR Celioscopies OR Peritoneoscopy OR Peritoneoscopies). No limits or filters were used. The total reference list was reviewed for relevant articles and only papers available in English were included. We included prospective and retrospective studies of patients who underwent abdominal LSC, received treatment with ACTZ for pain management, had a pain assessment at approximately 24 hours postoperatively, and included a no-treatment or minimal-treatment comparison group. We excluded studies not available in English, manuscripts that were not full publications (i.e. conference abstracts), and reviews. All studies which resulted from the abovementioned search were considered. Two authors, NRC and EFS, reviewed and screened titles and abstracts independently. KM was consulted to resolve any discrepancy that arose following article screening. Two authors, NRC and EFS, independently extracted outcome data (pain scores 24 hours after surgery), population characteristics, and study design characteristics using a standardized data collection form.
Assessment of Risk of Bias
Quality of evidence and risk of bias was assessed using the Cochrane Risk of Bias in Randomized Trials (RoB 2.0) tool for randomized controlled trials and the Risk of Bias in Nonrandomized Studies (ROBINS-I) tool for observational studies by two authors independently, EFS and GS. NRC was consulted if consensus on assessment between the authors could not be reached.
Statistical Analysis
The primary outcome was postoperative pain score approximately 24 hours after laparoscopic surgery. Data were analyzed using R statistical software (R Core Team, 2019). A Bayesian hierarchical model was assumed for the study specific treatment effects, with a random overall hierarchical treatment effect, which was estimated through information from each of the five studies considered. Posterior sampling was conducted via Markov Chain Monte Carlo methods, and posterior inference was carried out on the hierarchical treatment effect to determine if ACTZ decreased pain scores. Detailed analytic methods and code used is included in Appendix.
RESULTS
Retrieved Papers
The search yielded 252 publications, and five studies met eligibility criteria (Figure 1). All publications were full-text publications, and publication dates ranged from October 1, 2003 to November 30, 2018.
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses flow (PRISMA) diagram.
Publication Bias and Evidence Quality
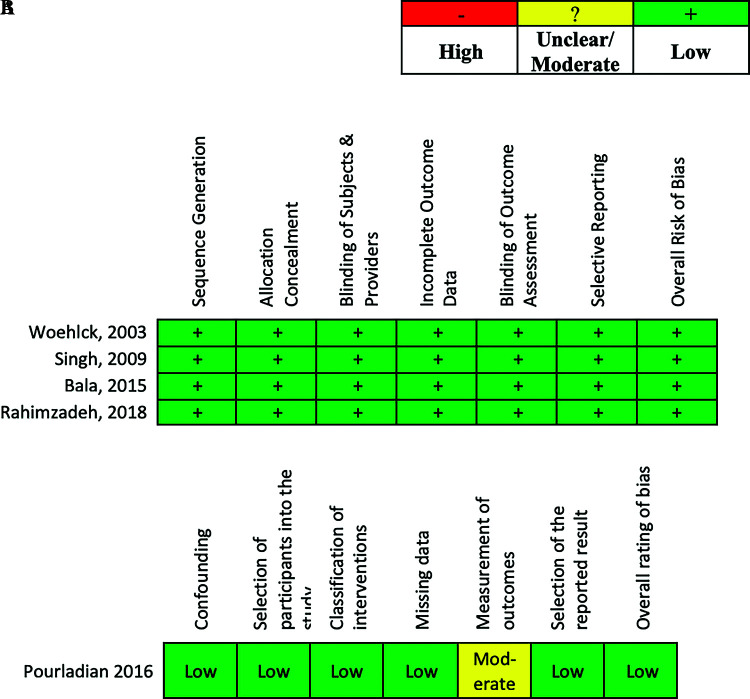
The Cochrane quality assessment method was used by two independent investigators to evaluate the five publications used in this study. The Risk of Bias (RoB 2.0) tool was used to establish low publication bias for the four randomized control trials4,6–8 (Figure 2A). The Risk of Bias in Nonrandomized Studies of Intervention (ROBIN-S) tool was used to assess a low level of bias overall and across all categories except measurement of outcome, where it ranked moderate, due to a lack of sufficient blindness5 (Figure 2B).
Figure 2.
Risk assessment. (A) Using the cochrane risk of bias in randomized trials (RoB 2.0) tool, all seven domains revealed a low risk of bias for the four articles within which randomized controlled trials were executed for data ascertainment. (B) Using the cochrane risk of bias in nonrandomized studies (ROBINS-I) tool, all domains were determined low risk of bias, except measurement of outcomes was considered moderate.
Key Characteristics and Findings of Studies Included
A summary of the study characteristics included in the meta-analysis is in Table 1. Two of the studies involved patients undergoing laparoscopic cholecystectomy.4,6 One study included patients undergoing LSC for donor nephrectomy.7 Another study included patients who were undergoing inguinal herniorrhaphy.5 The final study included patients receiving LSC for several different surgical procedures, including gynecologic, cholecystectomy, gastric bypass, herniorrhaphy, and lymph node dissection.8
Table 1.
Individual Study Information
| Study | Study Design | Intervention (n) | Outcome | Main Findings |
|---|---|---|---|---|
| Singh R et al. 20096 | RCT |
|
Postoperative pain intensity using VAS | Acetazolamide + bupivacaine group had reduced postoperative pain scores compared to bupivacaine only group |
| Pourladian et al. 20164 | Observational |
|
Postoperative pain intensity using VAS | Acetazolamide group had reduced postoperative pain scores compared to no acetazolamide group |
| Woehlck HJ et al. 20037 | RCT |
|
Postoperative pain intensity using VAS | Acetazolamide group had reduced postoperative pain scores compared to saline placebo group |
| Rahimzadeh R et al. 20185 | RCT |
|
Postoperative pain intensity using VAS | Acetazolamide group had reduced postoperative pain scores compared to saline placebo group |
| Bala I et al. 20153 | RCT |
|
Postoperative pain intensity using VAS | Acetazolamide group and saline group had similarly reduced postoperative pain scores compared to no acetazolamide group |
Abbreviations: RCT, randomized controlled trial; VAS, visual analog scale.
In regard to timing of administration of the ACTZ, three of the included studies had patients in the treatment groups receive the ACTZ intra-operatively.6–8 In the study by Singh et al., ACTZ was administered via orogastric tube following induction of anesthesia at a dose of 5 mg/kg diluted in 10 mL of normal saline. Both Woehick et al. and Rahimzadeh et al. administered ACTZ intravenously; Woehick et al. used a dose of 5 mg/kg whereas Rahimzadeh et al. used a dose of 00.5 mg/kg. The two other included studies both administered the ACTZ preoperatively.4,5 In the study by Pourladian et al., patients in the treatment group received 250 mg of ACTZ prior to surgery, though the route of administration was not specified. Patients in the treatment group in the study by Bala et al. were given 5 mg/kg oral ACTZ preoperatively. All of the included studies examined postoperative pain using the visual analog scale (VAS) (0–10) up to 24 hours after surgery. A combined total of 253 participants were included, with 116 in the ACTZ group and 137 in the control group.
Effect of Acetazolamide
The average and standard deviation of VAS scores for the ACTZ and control groups used for posterior sampling are shown in Table 2. The largest observed mean difference in 24-hour VAS scores between the control and ACTZ groups was 1.7, which was seen in Pourladian et al. Of interest, the standard deviation of VAS scores in the ACTZ group was smaller than the control group. The weighted average pain scores across all the studies was 2.45 for the ACTZ group and 3.35 for the control group, respectively. The posterior mean for the hierarchical treatment effect was –0.726 (95% credible interval = –1.175, –0.264) indicating that ACTZ decreases average pain scores compared to control. The posterior probability that ACTZ reduced pain scores (i.e. P[τ < 0|Data]) was 0.997. This is nearly definitive evidence that ACTZ can be used therapeutically. The posterior probability that ACTZ decreases mean pain scores by 00.5 or more was 0.846.
Table 2.
Effect of Acetazolamide on 24-Hour Pain Score
| Study | N Acetazolamide | Mean (SD) 24 h Pain score -Acetazolamide | N Control | Mean (SD) 24 h Pain score - Control |
|---|---|---|---|---|
| Singh et al. | 40 | 1.15 (1.44) | 39 | 1.67 (1.67) |
| Pourladian et al. | 22 | 2.30 (0.90) | 44 | 4.00 (2.10) |
| Woehlck et al. | 14 | 1.71 (1.98) | 14 | 1.96 (2.17) |
| Bala et al. | 20 | 6.00 (0.75) | 20 | 6.00 (1.00) |
| Rahimzadeh et al. | 20 | 2.22 (0.63) | 20 | 3.55 (1.12) |
Abbreviation: SD, standard deviation.
DISCUSSION
Minimally invasive surgery is improving at a remarkable pace and increasingly becoming the standard approach in procedures across surgical specialties. One of the benefits of LSC is reduced pain compared to laparotomy, though postoperative pain control remains a priority. Pain is a complex entity, and there are several etiologies of postoperative pain to consider including incisional pain, inflammation from healing, as well as referred pain from the stretching of the peritoneum from instillation of CO2 gas in LSC.
In our present study, ACTZ administration resulted in moderate, decreased pain the day after a LSC procedure. ACTZ is a well-known, inexpensive medication with few side effects and few contraindications.9 Some patients with sulfa allergies may have cross reactions as it is a sulfonamide derivative, and consideration should be given to patients with renal disease or glaucoma.9 Otherwise, this intervention is a reasonable adjunct to a minimally invasive procedure to potentially decrease pain, shorten hospital stays, improve patient satisfaction, and decrease the need for postoperative pain control.
The strengths of our study include rigorous statistical methods employed in this systematic review. The trials performed to date all had multiple endpoints, and thus, focusing on a single common measurement was critical to bolster the strength of the analysis. While the randomized controlled trials available for this meta-analysis showed benefit, each had their own separate endpoints, making it difficult to draw a singular conclusion. Herein, our investigation highlights a significant benefit that can be extrapolated for the justification of clinical use, or at the very least, future studies with more rigor and clinically relevant endpoints. The authors would charge that a well-defined, blinded, prospective randomized trial with a set dose of ACTZ compared with placebo would be most appropriate to further our understanding.
As with any meta-analysis, the limitations of the present study are related to the weaknesses of the original research used in the analysis. While the risk of bias for the studies were all low (four studies) or moderate (one study), different surgical procedures with different endpoints make it more challenging to confirm the efficacy of ACTZ for any one particular intervention. Furthermore, the studies all examined generalized postoperative pain and did not specify pain location. However, the included studies looked at the 24-hour mark, thus allowing this to be a reasonable endpoint to investigate in this meta-analysis. Further, given the diversity of the procedures, the external validity of our findings is enhanced, allowing consideration for this intervention to be applicable across the minimally invasive spectrum. Second, our study design does not allow for any conclusions to be drawn regarding adverse side effects of ACTZ, though the studies reporting on adverse events were overall reassuring.
CONCLUSION
The addition of perioperative ACTZ with abdominal LSC procedures may provide a modest, though clinically significant improvement in postoperative referred shoulder pain. This may be considered in appropriately selected patients, though future studies with rigorous scientific methodology are needed to confirm these results.
Appendix.
STATISTICAL ANALYSIS
We assume the following Bayesian hierarchical structure, where denotes the VAS score 24 hours after surgery for patient from the k-th study and denotes a treatment indicator for ACTZ. VAS score is discrete, but we will approximate the distribution of VAS scores assuming normality. This assumption was necessary to perform the meta-analysis, as each of the studies considered only reported sample averages and variances. Formally, we assume that which is the 24 hour VAS score for the patient who received treatment ( for Acetazolamide, for control). This has a population mean of and its own study specific variance . We assume the following hierarchical prior configuration shown in Appendix Figure 1.
This hierarchical structure is set to borrow strength across the study specific treatment effects (since there are five studies used in this meta-analysis) for each study to estimate the overall treatment effect , which is assumed to have a weakly information standard normal distribution. A negative value of indicates that Acetazolamide significantly decreases the VAS score at 24 hours compared to placebo. Since we are assuming conjugate normal priors in our hierarchical structure along with improper priors that lead to inverse γ posterior distributions, we only need sample sizes, average VAS, and standard deviation of VAS for the two treatment groups. This is appealing since the full datasets are not available for these studies. The parameters are not sampled, but are determined directly through posterior sampling for and .
Posterior sampling was performed via Gibbs sampling and Markov Chain Monte Carlo (MCMC). All parameters in Appendix Figure 1 have a conjugate conditional posterior distributions and can be sampled from directly using R statistical software. Ten thousand samples were drawn, with the first half discarded as burnin. Code used to sample from Appendix Figure 1 is included in the Appendix.
R Code for Implementing the Bayesian Hierarchical Sample Described in Appendix Figure 1
##Average 24 VAS for treatment and control groups
AVG_Treat = c(1.15, 20.3, 1.71, 6,2.22)
AVG_Control = c(1.67, 40.0,1.96, 6,3.55)
##Sd 24 hour VAS
SD_Treat=c(1.44, 0.9, 1.98, .75,.63)
SD_Control=c(1.67,20.1,2.17, 1,1.12)
##Sample size for treatment and control groups
N_Treat= c(40,22,14,20,20)
N_Control = c(39,44,14,20,20)
###Square Standard Deviations to get variance
SD_Treat=SD_Treat^2
SD_Control=SD_Control^2
##Set random seed to make results reproducibleset.
seed(1)
###Set up storage matrices
B = 10000 ##Number of iterations
TAU_STORE = matrix(nrow=B,ncol=length(AVG_Treat))
SIG_STORE = TAU_STORE
TREATEFF_STORE = rep(NA,B) ##overall treatment effect
SIGTAU_STORE = rep(NA,B)
###Starting Values for parameters
MU_Treat = AVG_Treat
MU_Control = AVG_Control
TAU = AVG_Treat
SD = SD_Treat
TreatEff = 0
SigTreat = 1for(b in 1:B){
###Sample muk, it's conjugate
for(j in 1:length(MU_Treat)){
MeanVal = TAU[j]+MU_Control[j]
##Posterior is normal
Mu1 = (N_Treat[j]*AVG_Treat[j]*SigTreat+MeanVal*SD[j])/(SigTreat*N_Treat[j]+SD[j])
Sd1 = SigTreat*SD[j]/(SigTreat*N_Treat[j]+SD[j])
Sd1 = sqrt(Sd1)
##Randomly draw study specific acetazolamide mean
MU_Treat[j]=rnorm(1,Mu1,Sd1)
}
##For control arm:
for(j in 1:length(MU_Treat)){
MeanVal = MU_Treat[j]-TAU[j]
##Posterior is normal
Mu1 = (N_Control[j]*AVG_Control[j]*SigTreat+MeanVal*SD[j])/(SigTreat*N_Control[j]+SD[j])
Sd1 = SigTreat*SD[j]/(SigTreat*N_Control[j]+SD[j])
Sd1 = sqrt(Sd1)
##Randomly draw study specific control mean
MU_Control[j]=rnorm(1,Mu1,Sd1)
}
##Sample σ k, inverse γ conditional posterior
for(j in 1:length(SD)){
a1= N_Treat[j]+N_Control[j]+2
b1 = SD_Treat[j]*(N_Treat[j]–1) + N_Treat[j]*(AVG_Treat[j]-MU_Treat[j])^2
b1 = b1+SD_Control[j]*(N_Control[j]–1) + N_Control[j]*(AVG_Control[j]-MU_Control[j])^2
b1 = b1/2
##Randomly draw study specific variance
SD[j]=1/rgamma(1,a1,rate=b1)
}
TAU = MU_Treat-MU_Control
###Sample overall treatment effect τ, which has a Normal conditional posterior
mu1 = sum(TAU)/(length(TAU)+SigTreat)
sd1 = SigTreat/(length(TAU)+SigTreat)
sd1 = sqrt(sd1)
TreatEff=rnorm(1,mu1,sd1)
##Sample σ_τ, which has a
##Inverse γ posterior distribution
a1 = length(N_Treat)+2
b1 = 0.5*sum((TAU-TreatEff)^2)
SigTreat = 1/rgamma(1,a1,b1)
##Storage for posterior samples
TREATEFF_STORE[b]=TreatEff
TAU_STORE[b,]=TAU
SIG_STORE[b,]=SD
SIGTAU_STORE[b]=SigTreat
}
###Traceplots
plot(1:B,SIG_STORE[,1],type=“l”)
plot(1:B,SIGTAU_STORE,type=“l”)
plot(1:B,TAU_STORE[,2],type=“l”)
plot(1:B,TREATEFF_STORE,type=“l”)
###Posterior density of treatment 2
plot(density(TAU_STORE[,2]))
TREATEFF_STORE=TREATEFF_STORE[(B/2):B]
###Try with metrop hastings…
plot(density(TREATEFF_STORE),main=“Posterior Distribution of Hierarchical Treatment Effect”,xlab=“Hierarchicial Treatment Effect”)
mean(TREATEFF_STORE)
mean(TREATEFF_STORE< (-0.5))
mean(TREATEFF_STORE< 0)
quantile(TREATEFF_STORE,c(.025, .975))
Appendix Figure 1.
Hierarchical prior configuration for Bayesian meta-analysis.
Footnotes
Acknowledgements: The authors would like to thank Ms. Louise McLaughlin, MSLS, MPS and the Health Sciences Library at Woman’s Hospital, Baton Rouge, Louisiana for their dedicated support to this study.
Conflict of interests: none.
Disclosure: none.
Funding sources: none.
Informed consent: Dr. Neil R. Chappell declares that written informed consent was obtained from the patient/s for publication of this study/report and any accompanying images.
Contributor Information
Kaitlin McGrail, Department of Obstetrics and Gynecology, Louisiana State University Health Sciences Center, Baton Rouge, Louisiana..
Andrew G. Chapple, Department of Biostatics, Louisiana State University Health Sciences Center, New Orleans, Louisiana..
Gabrielle Stone, Department of Obstetrics and Gynecology, Louisiana State University Health Sciences Center, Baton Rouge, Louisiana..
Elizabeth F. Sutton, Woman’s Hospital, Baton Rouge, Louisiana..
Neil R. Chappell, Fertility Answers, Woman’s Hospital, Baton Rouge, Louisiana..
References:
- 1.Kaloo P, Armstrong S, Kaloo C, Jordan V. Interventions to reduce shoulder pain following gynaecological laparoscopic procedures. Cochrane Database Syst Rev. 2019;1(1):CD011101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong M, Morris S, Wang K, Simpson K. Managing postoperative pain after minimally invasive gynecologic surgery in the era of the opioid epidemic. J Minim Invasive Gynecol. 2018;25(7):1165–1178. [DOI] [PubMed] [Google Scholar]
- 3.Ott DE, Reich H, Love B, et al. Reduction of laparoscopic-induced hypothermia, postoperative pain and recovery room stay by pre-conditioning gas with the Insuflox device: a prospective randomized controlled multi-center study. JSLS. 1998;22(4):321–329. [PMC free article] [PubMed] [Google Scholar]
- 4.Bala I, Bhatia N, Mishra P, Verma GR, Kaman L. Comparison of preoperative oral acetazolamide and intraperitoneal normal saline irrigation for reduction of postoperative pain after laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A. 2015;25(4):285–290. [DOI] [PubMed] [Google Scholar]
- 5.Pourladian I, Lois AW, Frelich MJ, et al. Acetazolamide reduces postoperative pain following laparoscopic inguinal heriorrhaphy. Surg Endosc. 2016;30(7):2685–2689. [DOI] [PubMed] [Google Scholar]
- 6.Rahimzadeh P, Faiz SHR, Hoseini M, Mousavie SH, Imani F, Negah AR. Comparison of intraperitoneal bupivacaine, acetazolamide, and placebo on pain relief after laparoscopic cholecystectomy surgery: a clinical trial. Med J. Islam Repub Iran. 2018;32:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh R, Sen I, Wig J, Minz M, Sharma A, Bala I. An acetazolamide based multimodal analgesic approach versus conventional pain management in patients undergoing laparoscopic living donor nephrectomy. Indian J Anaesth. 2009;53(4):434–441. [PMC free article] [PubMed] [Google Scholar]
- 8.Woehlck HJ, Otterson M, Yun H, Connolly LA, Eastwood D, Colpaert K. Acetazolamide reduces referred postoperative pain after laparoscopic surgery with carbon dioxide insufflation. Anesthesiology. 2003;99(4):924–928. [DOI] [PubMed] [Google Scholar]
- 9.Van Berkel MA, Elefritz JL. Evaluating off-label uses of acetazolamide. Am J Health Syst Pharm. 2018;75(8):524–531. [DOI] [PubMed] [Google Scholar]
- 10.Sao C-H, Chan-Tiopianco M, Chung K-C, et al. Pain after laparoscopic surgery: focus on shoulder-tip pain after gynecological laparoscopic surgery. J Chin Med Assoc. 2019;82(11):819–826. [DOI] [PubMed] [Google Scholar]
- 11.Movassaghi R, Peirovifar A, Aghamohammadi D, Mohammadipour Anvari H, Golzari SE, Kourehpaz Z. Premedication with single dose of acetazolamide for the control of referral shoulder pain after laparoscopic cholecystectomy. Anesth Pain Med. 2015;5(6):e29366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systemic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. [DOI] [PubMed] [Google Scholar]