Abstract
Sympathetic activation has been long appreciated exclusively as a fundamental compensatory mechanism of the failing heart and, thus, welcome and to be supported. In the initial clinical phases of heart failure (HF), the sympathetic nervous system overdrive plays a compensatory function aimed at maintaining an adequate cardiac output despite the inotropic dysfunction affecting the myocardium. However, when the sympathetic reflex response is exaggerated it triggers a sequence of unfavourable remodelling processes causing a further contractile deterioration that unleashes major adverse cardiovascular consequences, favouring the HF progression and the occurrence of fatal events. Eventually, the sympathetic nervous system in HF was demonstrated to be a ‘lethality factor’ and thus became a prominent therapeutic target. The existence of an effective highly specialized intracardiac neuronal network immediately rules out the old concept that sympathetic activation in HF is merely the consequence of a drop in cardiac output. When a cardiac damage occurs, such as myocardial ischaemia or a primary myocardial disorder, the adaptive capability of the system may be overcame, leading to excessive sympatho-excitation coupled with attenuation till to abolishment of central parasympathetic drive. Myocardial infarction causes, within a very short time, both a functional and anatomical remodelling with a diffuse up-regulation of nerve growth factor (NGF). The subsequent nerve sprouting signal, facilitated by a rise in the levels of NGF in the left stellate ganglion and in the serum, triggers an increase in cardiac nerve density in both peri-infarct and non-infarcted areas. Finally, NFG production decreases over time, supposedly as an adaptative response to the prolonged exposure to sympathetic overactivity, leading in the end to a reduction in sympathetic nerve density. Accordingly, NGF levels were markedly reduced in patients with severe congestive heart failure. The kidney is the other key player of the sympathetic response to HF as it indeed reacts to under-perfusion and to loop diuretics to preserve filtration at the cost of many pathological consequences on its physiology. This vicious loop ultimately participates to the chronic and disruptive sympathetic overdrive.
In conclusion, sympathetic activation is the natural physiological consequence to life stressors but also to any condition that may harm our body. It is the first system of reaction to any potential life-threatening event. However, in any aspect of life over reaction is never effective but, in many instances, is, actually, life threatening. One for all is the case of ischaemia-related ventricular fibrillation which is, strongly facilitated by sympathetic hyperactivity. The take home message? When, in a condition of harm, everybody is yelling failure is just around the corner.
Keywords: Heart failure, Autonomic nervous system, Baroreflex activation
The premises
Sympathetic activation has been long appreciated exclusively as a fundamental compensatory mechanism of the failing heart and, thus, welcome and to be supported. Along this line, the hypothesis of modulating the reflex sympathetic response to the loss of cardiac function was conceived by few long vision investigators but strongly denied by the vast majority of the cardiologic and physiologic ‘intelligentia’. Heart failure with reduced ejection fraction (HFrEF) was perceived as a disease of the myocyte that needed support to retrieve strength. Myocyte strength recovery would have restored haemodynamic balance, relieved symptoms and reactivated the inhibitory action of baroreceptors on sympathetic outflow that, at this point, would not have been needed any more. Such a path of thinking resulted in trials (beta-agonist) early interrupted because of an excess of mortality in the treated.1
It was in the seventies that the first observational study2 provided clue on benefit of beta-blockers in congestive cardiomyopathy. Unfortunately, it took more than 10 years before the US Carvedilol trial3 pointed to the striking evidence that shielding the failing heart from exceeding sympathetic activation could dramatically and favourably change quality of life and, most of all, could provide impressive survival improvement. At the same time, the debated issue of vagal innervation of the ventricles was finally resolved by a bulk of experimental and clinical evidence proving that vagal nerve reaches the ventricles and modulates local sympathetic circuits.4
It is in this context that the understanding of pathophysiological autonomic responses to the drop in cardiac output was eventually revisited opening a completely new approach to heart failure detection and treatment. More so happened when the progressive increase in life span brought about the growing entity of the HF with preserved ejection fraction (HFpEF). Indeed, while the clinical phenotype of patients with HFpEF is not different from the HRrEF ones, the neural mechanism involved in origin and initial progression of the clinical entities is quite different and may contribute to explain the unequal prognosis.
The pivotal study, the ATRAMI,5 and other studies from the same scientific group addressed the time course of cardiac autonomic control remodelling after a first myocardial infarction (MI) and its influence on the later left ventricular remodelling leading to HFpEF. The key message emerging from those studies and from experimental studies conducted on an NIH sponsored model6–8 was that the intrinsic individual autonomic profile, involving both anatomical and functional aspects, was one of the driving elements determining survival and cardiac function preservation or deterioration after MI.9 In a very condensed sentence: too much sympathetic cardiac drive leads to HF and/or death, adequate vagal inhibitory control produces longer and better life. This is, in essence, the key message that generated a novel line of research aimed at restoring a balanced cardiac control as soon as possible after a cardiac perturbation, specifically if of ischaemic origin.
One concept which became prominent through the years is related to the evidence that in the initial clinical phases of HFrEF, the sympathetic nervous system (SNS) overdrive (and more in general the neuro-humoral activation) plays a compensatory function aimed at maintaining an adequate cardiac output despite the inotropic dysfunction affecting the myocardium. However, with time, it triggers the switch of adult myocardial cell phenotype to fetal phenotype10 causing a further contractile deterioration that unleashes major adverse cardiovascular consequences, favouring the HF progression and the occurrence of fatal events. Eventually, the SNS in HF was demonstrated to be a ‘lethality factor’, and thus became a prominent therapeutic target.
The cardiac neural network and its activation pathways
In the last 50 years, our understanding of the anatomical and functional organization of cardiac neuraxis has dramatically improved.11 Cardiac neuronal control is realized through a series of reflex control networks involving somata in the (i) intrinsic cardiac ganglia (heart), (ii) intrathoracic extracardiac ganglia (stellate, middle cervical), (iii) superior cervical ganglia, (iv) spinal cord, (v) brainstem, and (vi) higher centres. Each one of these processing centres harbours afferent, efferent, and local circuit neurons, which interact both locally and in an interdependent fashion with the other levels to orchestrate electrical and mechanical local cardiac indices on a beat-to-beat basis. The peripheral afferent branch of these complex cardiac reflexes is elicited by inputs from baro-, chemo- and mechanoreceptors disperse within the entire cardiovascular system. This neuronal control system portrays impressive homeostatic capabilities, being able to assure the maintenance of a proper cardiac output over a wide spectrum of physiological stressors such as standing and physical activity, thanks to its high plasticity and memory capacity. For instance, arterial baroreflex resetting due to a combination of feedback and feedforward mechanisms, with the last ones prevailing, is crucial for the physiological response to exercise. Yet, when a cardiac damage occurs, such as myocardial ischaemia or a primary myocardial disorder, the adaptive capability of the system may be overcame, leading to excessive sympatho-excitation coupled with attenuation till to abolishment of central parasympathetic drive.
In turn, autonomic dysregulation plays a central role in the progression of HF and in the development of life-threatening arrhythmias. As such, the implementation of successful neuromodulation therapies aimed to restore autonomic balance requires full understanding of the anatomical and physiological basis for cardiovascular neuronal control.
For decades, the leading theory used to explain cardiac neuronal control, better known as centrally determined cardiac neuronal command, had focus on the pivotal role of forebrain centres and their neuronal projections in controlling peripheral post-ganglionic sympathetic and parasympathetic motor neurons.12 The two major central stations included the following: (i) parasympathetic efferent pre-ganglionic neurons, mostly located in the nucleus ambiguus of the medulla oblongata13,14 and (ii) sympathetic efferent pre-ganglionic neurons located in the intermediolateral cell column of the spinal cord, at the caudal cervical (C8) and at the cranial thoracic level (T1–T4).15 Within this scenario, the peripheral afferent inputs were only provided by sensory neurons, whose somata was either located in nodose ganglia or in thoracic dorsal root ganglia (DRG),16 which, in turn, projected to medullary and spinal cord second neurons, respectively. Cardiac afferent fibres reaching the nodose ganglia travel along vagal nerve branches and are commonly referred to as cardiac parasympathetic afferent fibres. Cardiac afferent fibres reach the DRG travel across the paravertebral sympathetic ganglia (without having synapsis) and are commonly referred to as cardiac sympathetic afferent fibres. Afferent inputs also converge to other forebrain neurons and to cortical neurons, particularly those in the insular cortex17 leading to a tonic descendant influence on efferent stations (central command). According to this theory, the first integrative stations of cardiovascular inputs were in the medulla and at the spinal cord level, while the intrinsic cardiac ganglia located within the epicardial fat pads only contained parasympathetic post-ganglionic neuronal bodies.
It was only in the late ‘90-early 2000s that a new paradigm emerged, after the realization that intracardiac, as well as extracardiac peripheral stations below the spinal cord were also involved in afferent signalling processing thanks to the presence of a rich network of interconnected neurons.18 The landmark discovery driving this new conception of a multiple layers, highly integrated, cardiovascular autonomic control was the realization that neuronal bodies located in the intrinsic cardiac ganglia contain all the essential elements for a functionally independent neuronal network, such as efferent neurons (both sympathetic and parasympathetic), afferent neurons and interneurons.19 This impressive intracardiac neuronal network started to be referred to as the intrinsic cardiac nervous system (ICNS), or, emphasizing its central role in the short-loop dynamic reflex control of regional cardiac function,20 as the little brain in the heart.21 The precise anatomical delineation of each one of the ganglionated plexuses located within the human ICNS and of their connectivity is hampered by the continuum nature of the epicardial fat pads. Yet at least 10 major groups of ganglia have been identified, mostly located on the posterior surfaces of the atria and on the superior aspect of the ventricles. Overall, the ICNS in humans is estimated to contain more than 14,000 neurons. The ICNS behaves as a distributive centre, meaning that post-ganglionic cholinergic neurons within each major intrinsic cardiac ganglion exert a widespread control over different cardiac regions, rather than selectively controlling discrete cardiac sites and/or indices, although broad areas of preferential influence have been identified.22,23 For instance, the right atrial ganglionated plexus, whose structure and function has been recently revisited,24 not only plays a pivotal role in controlling cardiac chronotropic function, together with the posterior atrial, and the dorsal atrial intrinsic cardiac ganglionated plexi, but it also exerts dromotropic and chronotropic effects.24 Accordingly, pre-clinical data demonstrated that discrete ablation of one element of the ICNS is followed by adaptative responses of the entire network, finally leading to restoration of cardiac functional control.25 These data are very important because at present they discourage the over simplistic approach concept that chronic ablation/stimulation of selected neuronal populations of the ICNS can be used to selectively modulate a specific aspect of cardiac function with a chronic, stable effect and without any other consequence for cardiac autonomic control.
Once the cardiac neural network is described, the understanding of the primary mechanisms involved in the autonomic derangement of the failing heart comes as a logical consequence.
The existence of an effective highly specialized intracardiac neuronal network immediately rules out the old concept that sympathetic activation in HF is merely the consequence of a drop in cardiac output. If this was the case, it would be impossible to explain on one side, why patients with the same mechanical dysfunction may have completely different autonomic profile, several with a preserved autonomic balance, and, on the other side, why autonomic imbalance may be detected very early in same patients, largely before the cardiac output is affected. As a matter of fact, sympathetic-parasympathetic imbalance e is a primary cause of HF development and NOT a pure consequence of it. Experimental evidence of cardio-cardiac autonomic reflexes triggered by intracardiac receptors is as old as 50 years, although at the beginning, as previously stated, integration was believed to only occur at the spinal cord level. This line of research documented that the primary vagal inhibition mechanism comes from the activation of cardiac sympathetic afferent fibres.26 These receptors linked to sympathetic afferent fibres are sensitive to very local chemo and mechanical stretches. Thus, any alteration in local PH, O2 concentration, and wall stress triggers autonomic responses in fraction of a second. Such responses become over time chronic as the enhanced sympathetic drive sustain all the unfavourable mechanisms perpetuating LV dysfunction progression and leading to overt HF occurrence. The crucial concept that the vagal withdrawal that may follow any cardiac damage that leads to an abnormal afferent signalling has a functional rather than an anatomical base as initially suggested by Zipes,27 and therefore, may be reverted, was recently reinforced by an elegant porcine study. Vaseghi et al.28 demonstrated that cardiac acetylcholine levels are preserved 6–8 weeks after a coronary occlusion in the border zones and in the viable myocardium of infarcted hearts, but their resting firing frequency is abnormal, as well as their response to stressor. Although the conclusive explanation for the largely preserved anatomical integrity of the cardiac parasympathetic network even after a cardiac damage such as MI has not being provided yet, an intrinsic consistent regenerative potential provided by the near stations of the ICNS has been suggested. Regardless of the mechanisms, this anatomical integrity is crucial to understand the rationale for neuromodulation strategies aimed to increase cardiac vagal output. Once HF becomes a chronic condition, then other mechanisms become the drive, specifically in advanced NYHA classes. As far as the baroreflex control, if the only signal was persistently lowered arterial pressure, adaptation would occur over days, with resetting, so that the lower pressure would be perceived as the norm, and no longer an abnormal condition. Instead, baroreflex dysfunction is sustained by the abnormal afferent signalling coming from several cardiovascular receptors including the cardiopulmonary ones. Accordingly, the increased cardiac sympathetic output in congestive HF is directly proportional to the increase in pulmonary artery pressure and in pulmonary wedge pressure and the reduction of these two parameters with the infusion of a vasodilator such as nitroprusside reduces cardiac sympathetic outflow, despite the reduction in arterial pressure. This evidence contributed to the current use of pulmonary arterial pressure monitoring as very early sensitive marker of later acute HF occurrence.
Anatomical and functional remodelling after MI and in HF: the role of nerve growth factor
The nerve growth factor (NGF) is the most representative member of a neurotrophin family that plays a pivotal role in regulating the neuronal healing and sprouting processes occurring after cardiac damage.29 Indeed, NGF activates an important developmental and regulatory pathway shared by two neuronal types, namely the cardiac sensory neurons and the cardiac efferent sympathetic neurons, that are also united by a common embryological origin from the trunk neuronal crest cells.30,31 Accordingly, the development of cardiac nociceptive sensory nerves, of DRG, and of the intermediolateral column of the dorsal horns was found to be markedly disrupted in NGF-deficient mice, whereas cardiac-specific NGF overexpression rescued these deficits.32 Nerve growth factor mostly behaves as a paracrine factor, synthesized, and secreted by sympathetic and sensory target organs and acting on membrane receptors of neuronal terminals. At the cardiac level, NFG expression influences the density of sympathetic post-ganglionic innervation, the sympathetic nerve survival and the synaptic transmission between neurons and cardiac myocytes.33,34 Back in 1979, it was demonstrated that NGF exposure was able to enhance cardiac sympathetic reinnervation of surgically denervated canine hearts.35 Subsequently, experimental data in conscious dogs confirmed that NGF infusion in the left stellate ganglion (LSG) induces a remarkable cardiac sympathetic nerve sprouting as assessed by the analysis of the tyrosine-hydroxylase (TH) and the growth-associated protein 43 (GAP43) expression, leading to sympathetic hyperinnervation and increased risk of ventricular fibrillation and sudden cardiac death.36,37 Notably, although GAP-43 is specific marker for sympathetic nerve sprouting, it must be considered that sprouting fibres may regress if they do not establish synaptic contacts; therefore, TH represents a better indicator for stable cardiac sympathetic innervation.38
Later on, a dog model of MI induced by coronary artery ligation was used to study NGF protein levels and mRNA levels in the infarct site, in the remote left ventricle free wall and in the LSG: a significant raise was observed in all three sites 39Specifically, the increase occurred earlier and was more pronounced in the border zone of the myocardial lesion, where NGF protein levels were significantly higher after 3.5 h, whereas NGF mRNA peaked within 3 days after coronary ligation. The raise in transcardiac NGF concentration (difference in NGF concentration between coronary sinus and aorta) occurred immediately after MI, before any change in NGF mRNA expression could be detected, therefore suggesting a rapid local release of NGF from intracardiac storages. The same was true for NGF and GAP43 protein levels in the LSG: the early increase detected after MI was not paired with an increased mRNA content, supporting the involvement of a retrograde axonal transport from the infarct site to the LSG, although an extra cardiac contribution cannot be completely excluded. From the LSG, the nerve sprouting signal induced a generalized enhancement in cardiac nerve density (hyperinnervation) throughout the heart (including both ventricles and atria) as assessed by GAP43 positive nerve fibres, especially at the non-infarcted left ventricular free-wall sites.
A similar study was performed in a murinae model of MI.40 In this case, NGF mRNA expression was significantly elevated at an even earlier time point (3 h) compared with the canine model. Acute MI also resulted in an increase of GAP-43 within 3 h that peaked at 1 week, then progressively declined. As opposed to GAP-43, TH-positive nerve fibre density at 1 month was more elevated in the peri-infarcted area (border zone) than in the remote sites.
Concerning NGF production over time after the acute MI, animal data 41showed a progressive decline months after the injury, that has been potentially related to the prolonged exposure to elevated concentrations of catecholamines, although the data are conflicting. Indeed, neonatal cardiomyocytes treated with NE show a consistent reduction of NGF mRNA that is largely abolished by the alfa-antagonist prazosin.42 On the other side, beta-adrenergic receptors 43might be implicated in the initial NGF rise after coronary ligation in the setting of strong sympathetic reflexes elicited by acute myocardial ischaemia.
Overall, pre-clinical models consistently showed that MI causes, within a very short time, a diffuse up-regulation of NGF that is more pronounced in the border zone. The subsequent nerve sprouting signal, facilitated by a rise in the levels of NGF in the LSG and in the serum, triggers an increase in cardiac nerve density in both peri-infarct and non-infarcted areas. Finally, NFG production decrease over time, supposedly as an adaptative response to the prolonged exposure to sympathetic overactivity, leading in the end to a reduction in sympathetic nerve density. Accordingly, NGF levels were markedly reduced in patients with severe congestive heart failure 41compared to controls, and a reduced overall cardiac sympathetic innervation and function as estimated semiquantitative and non-invasively by scintigraphy using an isotope analogue of norepinephrine, namely the 123I-metaiodobenzylguanidine (MIBG), was consistently associated with an increased risk of cardiac death and of cardiac event,44 including ventricular arrhythmias and SCD,45,46 in patients with HFrEF. Notably, in the AdreView Myocardial Imaging for Risk Evaluation in Heart Failure study, the heart-to-mediastinum ratio at 123I-MIBG cardiac imaging proved to be of additional independent prognostic value for risk stratification of both all cardiac events 47and arrhythmic events only 45in the setting of HFrEF, on top of all conventional predictors such as LVEF, B-type natriuretic peptide and blood pressure, with a consistent increase in the performance of the model, once again underlying the pivotal and independent pathophysiological and prognostic information provided by the assessment of cardiac autonomic asset.
The cardio–renal game
In the normal conditions, the cardiopulmonary reflex provides crucial control of sympathetic nerve activity. The elevation of heart filling pressure, indeed, engages the baroreceptors in the pulmonary venous-atrial junction resulting in reflex vagal activation. This leads to heart rate increase modulation occurring under sympathetic system domain and restrain of renal sympathetic activation, with consequent increase in urinary flow and sodium excretion via atrial natriuretic peptides release.48,49 The net effect of the inhibitory cardiopulmonary reflex is the minute per minute maintenance of the balanced circulatory volume and of ventricular preload.
When an injury impairs heart performance, cardiac output dips and the low blood flow is sensed by baroreceptor as a signal of circulation underfilling. The low flow signal ignites sympathetic activation in the renal vasculature blunting vagal response, while activating avid sodium and water reuptake from the glomerular filtrate. Those are compensatory mechanisms directed to counteract hypotension, due to the perception of the cardiac output drop as a drop of circulating volume.50
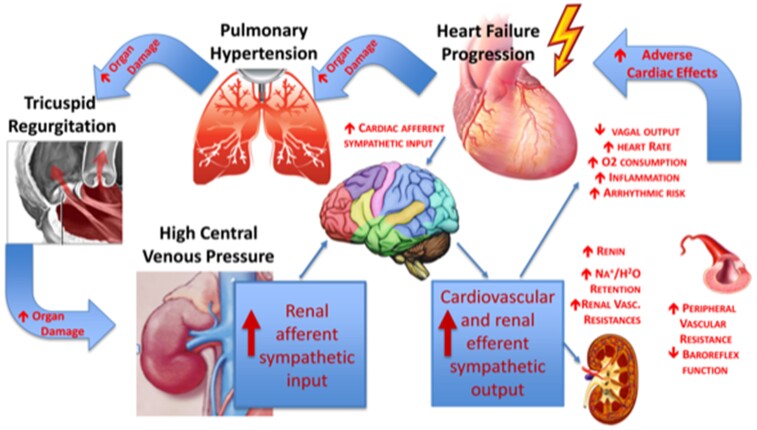
The abnormal reactive intensity of these responses not only drives inappropriate fluid retention but builds up, over time, the conditions for unfavourable cardiac remodelling. These mechanisms run the maladaptive process linked to HF progression that, in turn, enhances the SNS activation and the neurohormonal cascade, increasing vascular congestion and worsening renal function. The excess of intravascular load, coupled with the renal filtration decline, further negatively affects heart and kidney function, providing the pathway to CRS persistence and aggravation.51 In addition, afferent fibres originating in the kidney reach the midbrain where they activate neural cardiovascular control centres.52 Ischaemia and adenosine release, both generated by intense vasoconstriction, activate the response of these afferent nervous fibres. Also, renin release by the macula densa induces production of angiotensin II (ATII) through the renin–angiotensin–aldosterone system (RAAS), and consequent activation of SNA.53 In patients with end-stage renal disease (ESRD) who underwent removal of the native kidney or in renal transplant recipients, instead, the SNA declines.54 The abnormal persistent activation of the afferent-efferent ‘sympathorenal axis’ (Figure 1) provides path to a generalized, sustained, self-perpetuating SNA cycle, that can be modified by changes in some humoral substances, including natriuretic peptides (NP).56
Figure 1.
The cardio–renal engagement in heart failure progression. Heart failure leads to increased cardiac filling pressures that, in turn, drive backward pressure increase in lung circulation and in central venous system, leading to vascular congestion. The ensue of tricuspid regurgitation led by high pulmonary pressure, is the marker of right ventricular failure and addresses the loss of all cardiac compensatory mechanism. Congestion due to intravascular overload is the most potent driver of sympathetic nervous system activation that increases arterial vascular resistance and organs hypo-perfusion. Kidney reacts to the higher intra-parenchimal vascular resistance by increasing the sympathetic response and the neurohormonal activation, with major consequence in fluid retention. The increased sympathetic renal afferent signalling significantly contributes to the increased cardiovascular sympathetic output and the decreased cardiac vagal output in the setting of HFrEF. The overall effect worsens heart function and target organs damage contributing to maintain and to aggravate heart failure progression. Modified from Gonda et al.55
In normal physiology, ∼25% of the stroke volume is delivered to the renal perfusion. A decreased cardiac output causes a huge decrease in renal perfusion, through arterial vasculature underfilling that allows flow shunting to heart and brain circulation. This is how in congestive HF the kidney contributes to the muscle SNA enhancement and to the overall NE spillover, which is a documented marker of negative outcome.56,57 The total excess of sympathetic drive in HF patients depends indeed both on renal and cardiac NE spillover.58 To this regard, it has also been shown that renal NE spillover is highly predictive of outcomes, despite concomitant therapy with neurohormonal inhibitor drugs,59 completely confirming previous data.60 Efficacy of beta-blockers in HF with reduced ejection fraction patients, at any level of disease severity, is the most important evidence of the link between excessive SNA activation and poor prognosis in HF.59 Of note, however, the inhibition at central level of pre-synaptic NE release with moxonidine administration in HF subjects was associated with enhanced mortality, probably due to hypotension led by an inappropriate fall in plasma NE levels.61 As matter of fact, excessive central sympathetic effect inhibition by bucindolol was suggested as contributing to the negative outcome of the BEST trial.62
In addition, renal sympathetic denervation was associated with improved outcomes in an experimental MI model.63
The last but not least piece of the puzzle comes from the therapy: congestion is the obvious main cause of diuretics use, and kidney dysfunction often occurs during intensive treatment with loop diuretics. Large evidence documents the consequences of kidney dysfunction on SNA either directly or through activation of the RAAS.64 Grassi et al65studied mild-to-moderate CKD patients who were divided into four groups based on their eGFR, with highest quartile of eGFR (95.4 ± 1.6 mL/min/1.73 m2) in Group I and lowest quartile of eGFR (31.4 ± 1.8 mL/min/1.73 m2) in Group IV. There was a significant and progressive increase in resting MSNA from the highest quartile to the lowest quartile of eGFR.65
In summary, the kidney is a key player of the sympathetic response to HF as it indeed reacts to under-perfusion and to loop diuretics to preserve filtration at the cost of many pathological consequences on its physiology. This vicious loop ultimately participates to the chronic and disruptive sympathetic overdrive.
Conclusions
Sympathetic activation is the natural physiological consequence to life stressors but also to any condition that may harm our body. It is the first system of reaction to any potential life-threatening event. However, in any aspect of life over reaction is never effective but, in many instances, is actually, life threatening. One for all is the case of ischaemia-related ventricular fibrillation which is, strongly facilitated by sympathetic hyperactivity. The take home message? When, in a condition of harm, everybody is yelling failure is just around the corner.
Funding
This paper was published as part of a supplement financially supported by CVRx, Inc.
Contributor Information
E Gronda, U.O.C. Nefrologia, Dialisi e Trapianto Renale dell’Adulto, Programma Cardiorenale, Dipartimento di Medicina e Specialità Mediche, Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Milano, Italy; Area Cardiorenale Metabolica Associazione nazionale Medici Cardiologi Ospedalieri, Italia.
V Dusi, Cardiology Division, Department of Medical Sciences, University of Turin, Torino, Italy.
E D’Elia, Cardiovascular Department, Papa Giovanni XXIII Hospital, Bergamo, Italy.
M Iacoviello, Area Cardiorenale Metabolica Associazione nazionale Medici Cardiologi Ospedalieri, Italia; S.C. Cardiologia, AOU Policlinico Riuniti di Foggia, Dipartimento di Scienze Mediche e Chirurgiche, Università degli Studi, Foggia, Italy.
E Benvenuto, Area Cardiorenale Metabolica Associazione nazionale Medici Cardiologi Ospedalieri, Italia; U.O.C. di Cardiologia-UTIC-Emodinamica PO ‘G. Mazzini’ Teramo, Italy.
E Vanoli, Department of Molecular Medicine, University of Pavia, Pavia, Italy; Department of Medicine, Cardiology and Rehabilitation Sacra Famiglia Hospital, Erba, Italy.
References
- 1. Adamson PB, Suarez J, Ellis E, Kanaly T, Vanoli E. Ephedrine increases ventricular arrhythmias in conscious dogs after myocardial infarction. J Am Coll Cardiol 2004;44:1675–1678. [DOI] [PubMed] [Google Scholar]
- 2. Swedberg K, Hjalmarson A, Waagstein F, Wallentin I. Prolongation of survival in congestive cardiomyopathy during treatment with beta-receptor blockade. Lancet 1979;1:1374–1376. [DOI] [PubMed] [Google Scholar]
- 3. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 4. Ardell JL, Armour JA. Neurocardiology: structure-based function. Compr Physiol 2016;6:1635–1653. [DOI] [PubMed] [Google Scholar]
- 5. La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (autonomic tone and reflexes after myocardial infarction) investigators. Lancet 1998;351:478–484. [DOI] [PubMed] [Google Scholar]
- 6. Adamson PB, Huang MH, Vanoli E, Foreman RD, Schwartz PJ, Hull SS Jr. Unexpected interaction between beta-adrenergic blockade and heart rate variability before and after myocardial infarction. A longitudinal study in dogs at high and low risk for sudden death. Circulation 1994;90:976–982. [DOI] [PubMed] [Google Scholar]
- 7. Vanoli E, Adamson PB, Hull SS Jr, Foreman RD, Schwartz PJ. Prediction of unexpected sudden death among healthy dogs by a novel marker of autonomic neural activity. Heart Rhythm 2008;5:300–305. [DOI] [PubMed] [Google Scholar]
- 8. Adamson PB, Vanoli E. Early autonomic and repolarization abnormalities contribute to lethal arrhythmias in chronic ischemic heart failure: characteristics of a novel heart failure model in dogs with postmyocardial infarction left ventricular dysfunction. J Am Coll Cardiol 2001;37:1741–1748. [DOI] [PubMed] [Google Scholar]
- 9. La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, et al. Autonomic tone and reflexes after myocardial infarcton. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation 2001 Apr 24;103:2072–2077. [DOI] [PubMed] [Google Scholar]
- 10. Lowes BD, Gilbert EM, Abraham WT, Minobe WA, Larrabee P, Ferguson D, et al. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med 2002;346:1357–1365. [DOI] [PubMed] [Google Scholar]
- 11. Dusi V, Ardell JL. Brain-Heart afferent-efferent traffic. In: Brain and Heart Dynamics. Springer; 2020;3.– . [Google Scholar]
- 12. Williamson JW, Fadel PJ, Mitchell JH. New insights into central cardiovascular control during exercise in humans: a central command update. Exp Physiol 2006;91:51–58. [DOI] [PubMed] [Google Scholar]
- 13. Hopkins DA, Armour JA. Localization of sympathetic postganglionic and parasympathetic preganglionic neurons which innervate different regions of the dog heart. J Comp Neurol 1984;229:186–198. [DOI] [PubMed] [Google Scholar]
- 14. Gray AL, Johnson TA, Lauenstein JM, Newton SS, Ardell JL, Massari VJ. Parasympathetic control of the heart. III. Neuropeptide Y-immunoreactive nerve terminals synapse on three populations of negative chronotropic vagal preganglionic neurons. J Appl Physiol (1985) 2004;96:2279–2287. [DOI] [PubMed] [Google Scholar]
- 15. Levy M, Martin P. Neural control of the heart. In: Berne RM, ed. Handb. Physiol. Sect. 2 Cardiovasc. Syst. Vol. 1 Hear. Bethesda: The American Physiological Society; 1979:581–620. [Google Scholar]
- 16. Paintal AS. Vagal sensory receptors and their reflex effects. Physiol Rev 1973;53:159–227. [DOI] [PubMed] [Google Scholar]
- 17. Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology 1992;42:1727–1732. [DOI] [PubMed] [Google Scholar]
- 18. Armour JA. Cardiac neuronal hierarchy in health and disease. Am J Physiol Regul Integr Comp Physiol 2004;287:R262–R271. [DOI] [PubMed] [Google Scholar]
- 19. Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec 1997;247:289–298. [DOI] [PubMed] [Google Scholar]
- 20. Murphy DA, O'Blenes S, Hanna BD, Armour JA. Capacity of intrinsic cardiac neurons to modify the acutely autotransplanted mammalian heart. J Heart Lung Transplant 1994;13:847–856. [PubMed] [Google Scholar]
- 21. Armour JA. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol 2008;93:165–176. [DOI] [PubMed] [Google Scholar]
- 22. Ardell JL, Butler CK, Smith FM, Hopkins DA, Armour JA. Activity of in vivo atrial and ventricular neurons in chronically decentralized canine hearts. Am J Physiol 1991;260:H713–H721. [DOI] [PubMed] [Google Scholar]
- 23. Yuan BX, Ardell JL, Hopkins DA, Armour JA. Differential cardiac responses induced by nicotine sensitive canine atrial and ventricular neurones. Cardiovasc Res 1993 May;27:760–769. [DOI] [PubMed] [Google Scholar]
- 24. Hanna P, Dacey MJ, Brennan J, Moss A, Robbins S, Achanta S, et al. Innervation and neuronal control of the mammalian sinoatrial node a comprehensive atlas. Circ Res 2021;128:1279–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leiria TL, Glavinovic T, Armour JA, Cardinal R, de Lima GG, Kus T. Longterm effects of cardiac mediastinal nerve cryoablation on neural inducibility of atrial fibrillation in canines. Auton Neurosci 2011;161:68–74. [DOI] [PubMed] [Google Scholar]
- 26. Schwartz PJ, Pagani M, Lombardi F, Malliani A, Brown AM. A cardiocardiac sympathovagal reflex in the cat. Circ Res 1973;32:215–220. [DOI] [PubMed] [Google Scholar]
- 27. Chilson DA, Peigh P, Mahomed Y, Zipes DP. Encircling endocardial incision interrupts efferent vagal-induced prolongation of endocardial and epicardial refractoriness in the dog. J Am Coll Cardiol 1985;5:290–296. [DOI] [PubMed] [Google Scholar]
- 28. Vaseghi M, Salavatian S, Rajendran PS, Yagishita D, Woodward WR, Hamon D, et al. Parasympathetic dysfunction and antiarrhythmic effect of vagal nerve stimulation following myocardial infarction. JCI Insight 2017;2:e86715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Govoni S, Pascale A, Amadio M, Calvillo L, D'Elia E, Cereda C, et al. NGF And heart: is there a role in heart disease? Pharmacol Res 2011;63:266–277. [DOI] [PubMed] [Google Scholar]
- 30. Kimura K, Ieda M, Fukuda K. Development, maturation, and transdifferentiation of cardiac sympathetic nerves. Circ Res 2012;110: 325–336. [DOI] [PubMed] [Google Scholar]
- 31. Hasan W. Autonomic cardiac innervation: development and adult plasticity. Organogenesis 2013;9:176–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ieda M, Kanazawa H, Ieda Y, Kimura K, Matsumura K, Tomita Y, et al. Nerve growth factor is critical for cardiac sensory innervation and rescues neuropathy in diabetic hearts. Circulation 2006;114:2351–2363. [DOI] [PubMed] [Google Scholar]
- 33. Lockhart ST, Turrigiano GG, Birren SJ. Nerve growth factor modulates synaptic transmission between sympathetic neurons and cardiac myocytes. J Neurosci 1997;17:9573–9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lockhart ST, Mead JN, Pisano JM, Slonimsky JD, Birren SJ. Nerve growth factor collaborates with myocyte-derived factors to promote development of presynaptic sites in cultured sympathetic neurons. J Neurobiol 2000;42:460–476. [PubMed] [Google Scholar]
- 35. Kaye MP, Wells DJ, Tyce GM. Nerve growth factor-enhanced reinnervation of surgically denervated canine heart. Am J Physiol 1979;236:624–628. [DOI] [PubMed] [Google Scholar]
- 36. Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res 2001;50:409–416. [DOI] [PubMed] [Google Scholar]
- 37. Voroshilovsky O, Qu Z, Lee MH, Ohara T, Fishbein GA, Huang HLA, et al. Mechanisms of ventricular fibrillation induction by 60-Hz alternating current in isolated swine right ventricle. Circulation 2000;102:1569–1574. [DOI] [PubMed] [Google Scholar]
- 38. Chen LS, Zhou S, Fishbein MC, Chen PS. New perspectives on the role of autonomic nervous system in the genesis of arrhythmias. J Cardiovasc Electrophysiol 2007;18:123–127. [DOI] [PubMed] [Google Scholar]
- 39. Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, et al. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res 2004;95:76–83. [DOI] [PubMed] [Google Scholar]
- 40. Oh YS, Jong AY, Kim DT, Li H, Wang C, Zemljic-Harpf A, et al. Spatial distribution of nerve sprouting after myocardial infarction in mice. Heart Rhythm 2006;3:728–736. [DOI] [PubMed] [Google Scholar]
- 41. Kaye DM, Vaddadi G, Gruskin SL, Du XJ, Esler MD. Reduced myocardial nerve growth factor expression in human and experimental heart failure. Circ Res 2000;86:80–84. [DOI] [PubMed] [Google Scholar]
- 42. Qin F, Vulapalli RS, Stevens SY, Liang CS. Loss of cardiac sympathetic neurotransmitters in heart failure and NE infusion is associated with reduced NGF. Am J Physiol Heart Circ Physiol 2002;282:363–371. [DOI] [PubMed] [Google Scholar]
- 43. Riaz SS, Tomlinson DR. Pharmacological modulation of nerve growth factor synthesis: a mechanistic comparison of vitamin D receptor and adrenoceptor 2 agonists. Mol Brain Res 2000;85:179–188. [DOI] [PubMed] [Google Scholar]
- 44. Verberne HJ, Brewster LM, Somsen GA, van Eck-Smit BL. Prognostic value of myocardial 123I-metaiodobenzylguanidine (MIBG) parameters in patients with heart failure: a systematic review. Eur Heart J 2008;29:1147–1159. [DOI] [PubMed] [Google Scholar]
- 45. Tamaki S, Yamada T, Okuyama Y, Morita T, Sanada S, Tsukamoto Y, et al. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J Am Coll Cardiol 2009;53:426–435. [DOI] [PubMed] [Google Scholar]
- 46. Al Badarin FJ, Wimmer AP, Kennedy KF, Jacobson AF, Bateman TM. The utility of ADMIRE-HF risk score in predicting serious arrhythmic events in heart failure patients: incremental prognostic benefit of cardiac 123I-mIBG scintigraphy. J Nucl Cardiol 2014;21:756–762; quiz 753-55, 763-5. [DOI] [PubMed] [Google Scholar]
- 47. Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView myocardial imaging for risk evaluation in heart failure) study. J Am Coll Cardiol 2010;55:2212–2221. [DOI] [PubMed] [Google Scholar]
- 48. Robbe HW, Mulder LJ, Rüddel H, Langewitz WA, Veldman JB, Mulder G. Assessment of baroreceptor reflex sensitivity by means of spectral analysis. Hypertension 1987;10:538–543. [DOI] [PubMed] [Google Scholar]
- 49. Linden RJ. Atrial reflexes and renal function. Am J Cardiol 1979;44:879–883. [DOI] [PubMed] [Google Scholar]
- 50. Dibner-Dunlap ME, Thames MD. Control of sympathetic nerve activity by vagal mechanoreflexes is blunted in heart failure. Circulation. 1992;86:1929–1934. [DOI] [PubMed] [Google Scholar]
- 51. Shlipak MG, Massie BM. The clinical challenge of cardiorenal syndrome. Circulation. 2004;110:1514–1517. [DOI] [PubMed] [Google Scholar]
- 52. DiBona GF. Neural control of the kidney: past, present, and future. Hypertension. 2003;41:621–624. [DOI] [PubMed] [Google Scholar]
- 53. Campese VM, Kogosov E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension. 1995;25:878–882. [DOI] [PubMed] [Google Scholar]
- 54. Hausberg M, Kosch M, Harmelink P, Barenbrock M, Hohage H, Kisters K, et al. Sympathetic nerve activity in end-stage renal disease. Circulation 2002;106:1974–1979. [DOI] [PubMed] [Google Scholar]
- 55. Gronda E, Vanoli E. The cardiorenal cross talk: the autonomic pathway regulating the cardiocirculatory balance. In: Brain and Heart Dynamics. Springer; 2020;43–53. [Google Scholar]
- 56. Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 2009;54:1747–1762. [DOI] [PubMed] [Google Scholar]
- 57. Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984;311:819–823. [DOI] [PubMed] [Google Scholar]
- 58. Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation 1986;73:615–621. [DOI] [PubMed] [Google Scholar]
- 59. Goldsmith SR, Sobotka PA, Bart BA. The sympathorenal axis in hypertension and heart failure. J Card Fail 2010 May;16:369–373. [DOI] [PubMed] [Google Scholar]
- 60. Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure. Drugs 1990;39 Suppl 4:10–21; discussion 22-4. [DOI] [PubMed] [Google Scholar]
- 61. Cohn JN, Pfeffer MA, Rouleau J, Sharpe N, Swedberg K, Straub M, et al. Adverse mortality effect of central sympathetic inhibition with sustained-release moxonidine in patients with heart failure (MOXCON). Eur J Heart Fail 2003;5:659–667. [DOI] [PubMed] [Google Scholar]
- 62. Eichhorn EJ, Domanski MJ, Krause-Steinrauf H, Bristow MR, Lavori PW. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001 May 31;344:1659–1667. [DOI] [PubMed] [Google Scholar]
- 63. Nozawa T, Igawa A, Fujii N, Kato B, Yoshida N, Asanoi H, et al. Effects of long-term renal sympathetic denervation on heart failure after myocardial infarction in rats. Heart Vessels 2002;16:51–56. [DOI] [PubMed] [Google Scholar]
- 64. Francis GS, Siegel RM, Goldsmith SR, Olivari MT, Levine TB, Cohn JN. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohumoral axis. Ann Intern Med 1985;103:1–6. [DOI] [PubMed] [Google Scholar]
- 65. Grassi G, Quarti-Trevano F, Seravalle G, Arenare F, Volpe M, Furiani S, et al. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension 2011 Apr;57:846–851. [DOI] [PubMed] [Google Scholar]