Abstract
COVID-19 causes not only acute but also subacute medical conditions during the clinical course. COVID-19 causes severe inflammatory conditions; therefore, patients may develop long-term complications. Among patients with acute COVID-19, some patients can experience persistent symptoms, such as fatigue, joint pain, and smell and taste abnormalities, known as the long COVID-19 syndrome. The symptoms can be severe and require continuous medical care. Patients with severe clinical courses of COVID-19 may have critical symptoms again after the cure of the acute infections, especially among older patients. We encountered a case of neutropenia and myositis one month after contracting COVID-19. An 89-year-old man presented to our hospital with acute-onset systemic muscle pain and difficulty in movement and speaking. The patient had neutropenia and myositis with an extremely high level of immunoglobulin G caused by COVID-19. A granulocyte colony-stimulating factor could be effective for treating neutropenia. Besides, prednisolone was effective for treating myositis. In community hospitals, after developing COVID-19, appropriate history taking and physical examination should be performed in older patients with ambiguous symptoms, as they might have critical medical conditions such as neutropenia and myositis. The appropriate diagnosis and treatments of older patients with the complications of COVID-19 should be performed.
Keywords: community hospitals, japan, rural, autoantibody myositis, idiopathic neutropenia, covid-19
Introduction
Coronavirus disease 2019 (COVID-19) is a respiratory infection induced by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), affecting human lives since 2019. COVID-19 causes severe respiratory infections with an average mortality rate of 3%-4% [1]. There are no definitive treatments for the virus, and vaccination is the only measure to prevent the spread of the infection [2,3]. COVID-19 also causes various systemic symptoms by triggering inflammatory conditions with high concentrations of interleukin 6 and tumor necrosis factors in the blood [4]. Concrete symptoms include fatigue, muscle pain, headache, and joint pains [5]. Moreover, the infection can cause myositis and hematological abnormalities during the acute infection phase; therefore, intensive monitoring of the infection is required [5].
COVID-19 causes not only acute but also subacute medical conditions during the clinical course. COVID-19 also causes severe inflammatory conditions; therefore, patients may develop long-term complications [6]. Among patients with acute COVID-19, some can experience continuous symptoms such as fatigue, joint pain, and smell and taste abnormalities, known as long COVID-19 syndrome [6,7]. Their symptoms can be severe and require continuous medical care [6,7]. COVID-19 patients with severe acute symptoms may develop critical symptoms again after the cure, which could be fatal, especially among older patients [6,8].
This time, we encountered an older patient with post-COVID-19 infections with complaints of appetite loss and systemic muscle pain who were transferred to the emergency room of our rural hospital. The patient developed myositis and pancytopenia, following acute COVID-19 infection. Myositis and pancytopenia rarely coexist in patients as COVID-19 complications. Only a few case reports on patients with the same conditions, especially among older patients, have been published [9,10]. We describe this rare case and discuss how COVID-19 causes these complications and the appropriate treatment for these patients in rural hospitals.
Case presentation
An 89-year-old man presented to our hospital with acute-onset generalized muscle pain and difficulty in standing and speaking. His past medical histories were hypertension, angina pectoris, dementia, clavicle fracture, and lumbar vertebral compression fracture, and was admitted to our hospital after contracting COVID-19 one month before the present visit. He was under treatment with bisoprolol fumarate, tolvaptan, azosemide, aspirin, furosemide, and rupatadine fumarate.
Upon arrival, his vital signs were as follows: temperature, 37.7°C; pulse rate, 81 beats/min; respiration, 16 breaths/min; and blood pressure, 132/73 mmHg. Physical examination revealed tenderness of the proximal muscles of the extremities and bilateral pedal edema. He had stable breathing and an absence of chills, shivering, or cyanosis. The blood tests revealed pancytopenia (white blood cells: 900/µL, neutrophils: 1.2%, red blood cells: 282 × 104/µL, hemoglobin: 9.8 g/dL, and platelet count: 11.8 × 104/µL) and elevated creatinine kinase (2581 U/L) (Table 1).
Table 1. Patients’ initial laboratory data.
PT, prothrombin time; INR, international normalized ratio; APTT, activated partial thromboplastin time; eGFR, estimated glomerular filtration rate; CK, creatine kinase; CRP, C-reactive protein; TSH, thyroid-stimulating hormone; Ig, immunoglobulin; HCV, hepatitis C virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; HIV, human immunodeficiency virus; HBs, hepatitis B surface antigen; HBc, hepatitis B core antigen; C3, complement component3; C4, complement component4; KL-6, Krebs von den Lungen-6; MPO-ANCA, myeloperoxidase-antineutrophil cytoplasmic antibodies; anti-SSA/Ro autoantibodies, anti-Sjogren’s syndrome type A autoantibodies; anti-SSB/La autoantibodies, anti-Sjogren syndrome antigen type B autoantibodies; CCP antibodies, cyclic citrullinated peptide antibodies
| Marker | Level | Reference |
| White blood cells | 0.9 | 3.5–9.1 × 103/μL |
| Neutrophils | 1.2 | 44.0–72.0% |
| Lymphocytes | 86.9 | 18.0–59.0% |
| Monocytes | 6.0 | 0.0–12.0% |
| Eosinophils | 0.0 | 0.0–10.0% |
| Basophils | 0.0 | 0.0–3.0% |
| Red blood cells | 2.82 | 3.76–5.50 × 106/μL |
| Reticulocytes (%) | 14.3 | /μL (%) |
| Hemoglobin | 9.8 | 11.3–15.2 g/dL |
| Hematocrit | 29.0 | 33.4–44.9% |
| Mean corpuscular volume | 102.8 | 79.0–100.0 fl |
| Platelets | 11.8 | 13.0–36.9 × 104/μL |
| PT-INR | 0.98 | |
| APTT | 27.5 | 25–40 seconds |
| Total protein | 8.2 | 6.5–8.3 g/dL |
| Albumin | 3.6 | 3.8–5.3 g/dL |
| Total bilirubin | 1.6 | 0.2–1.2 mg/dL |
| Aspartate aminotransferase | 66 | 8–38 IU/L |
| Alanine aminotransferase | 18 | 4–43 IU/L |
| Alkaline phosphatase | 85 | 106–322 U/L |
| γ-Glutamyl transpeptidase | 19 | <48 IU/L |
| Lactate dehydrogenase | 232 | 121–245 U/L |
| Blood urea nitrogen | 32.2 | 8–20 mg/dL |
| Creatinine | 2.13 | 0.40–1.10 mg/dL |
| eGFR | 23.3 | > 60.0 mL/min/L |
| Serum Na | 140 | 135–150 mEq/L |
| Serum K | 4.1 | 3.5–5.3 mEq/L |
| Serum Cl | 102 | 98–110 mEq/L |
| Ferritin | 330.8 | 14.4–303.7 ng/mL |
| CK | 2581 | 56–244 U/L |
| CRP | 11.79 | <0.30 mg/dL |
| Procalcitonin | 0.48 | 0–0.05 ng/mL |
| TSH | 3.07 | 0.35–4.94 μIU/mL |
| Free T4 | 0.6 | 0.70–1.48 ng/dL |
| Vitamin B12 | 362 | 187–883 pg/mL |
| Folic acid | 9.8 | 3.1–20.5 ng/mL |
| IgG | 111 | 870–1,700 mg/dL |
| SARS-CoV-2 antigen | Negative | |
| SARS-CoV-2 IgG | >40,000.0 | <49.9 AU/mL |
| Antinuclear antibody | <40 | <40 |
| Homogeneous | 0 | <40 |
| Speckled | 0 | <40 |
| Nucleolar | 0 | <40 |
| Peripheral | 0 | <40 |
| Discrete | 0 | <40 |
| Cytoplasm | 0 | <40 |
| C3 | 126 | 86–160 mg/dL |
| C4 | 31 | 17–45 mg/mL |
| KL-6 | 332 | 105.3–401.2 U/mL |
| MPO-ANCA | <1.0 | <3.5 U/mL |
| Anti-SSA/Ro autoantibodies | <1.0 | <10 U/mL |
| Anti-SSB/La autoantibodies | <1.0 | <10 U/mL |
| CCP Antibodies | <0.6 | <5 U/mL |
| Cardiolipin antibodies | <4.0 | <12.3 U/mL |
| IgG4 | 111 | 11–121 |
| Urine test | ||
| Leukocyte | Negative | |
| Nitrite | Negative | |
| Protein | 2+ | |
| Glucose | Negative | |
| Urobilinogen | 1+ | |
| Bilirubin | Negative | |
| Ketone | Negative | |
| Blood | 3+ | |
| pH | 8.0 | |
| Specific gravity | 1.016 | |
| Bacteria | 2+ |
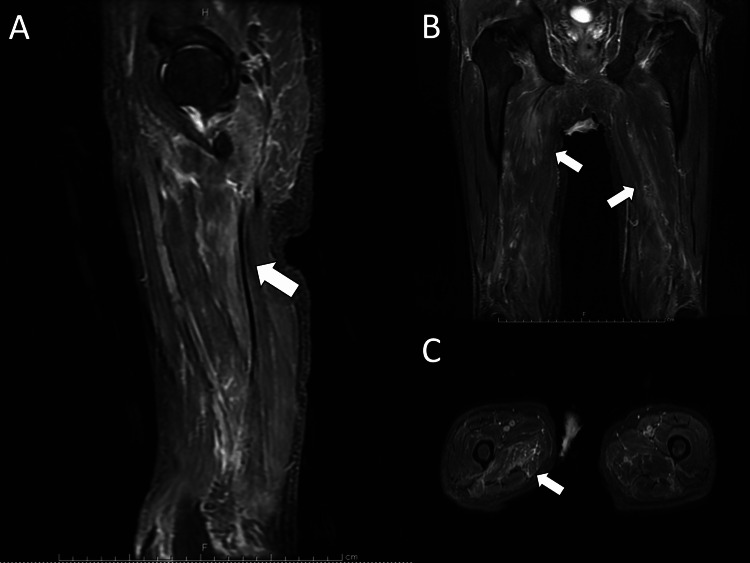
Short Tau inversion recovery (STIR) imaging of the thighs showed irregular high-intensity areas in both adductor muscle groups, suggesting necrotizing fasciitis (Figure 1).
Figure 1. Short TI inversion recovery images of thigh muscle groups (A: Sagittal, B: Coronal, C: Transverse) showing muscle inflammation (white arrow).
A chest computed tomography scan was performed and did not show obvious interstitial pneumonia or lymphadenopathy suspicious for lymphoid species. Antibody tests showed the absence of collagen-related antigen antibodies but demonstrated high levels of anti-coronavirus immunoglobulin G (40,000 U/mL) (Table 1). Based on these findings, febrile neutropenia due to COVID-19 and COVID-19 antibody-associated myositis was diagnosed.
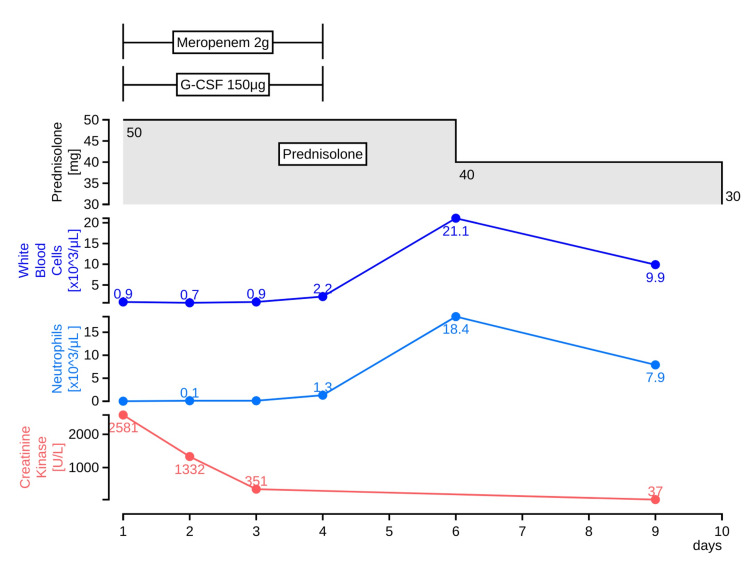
The patient did not exhibit significant elevation of ferritin levels (330.8 ng/mL), and the inflammation affecting the bone marrow was considered mild. On the day of admission, a granulocyte-colony stimulating factor (G-CSF) of 75 μg was initiated as a treatment for neutropenia (neutrophil count of <100/μL). Since febrile neutropenia was observed until the fourth day of admission, the dose was increased to 150 μg; the febrile neutropenia was suspected to be caused by multidrug-resistant Pseudomonas aeruginosa. For COVID-19 antibody-associated myositis, prednisolone 50 mg was administered orally for five days (Figure 2).
Figure 2. The clinical course of the case.
G-CSF: a granulocyte-colony stimulating factor
On the fourth day of hospitalization, P. aeruginosa was reported in the urine culture submitted at the visit; on the fifth day of hospitalization, the white blood cell count increased to 2,200/µL, while the neutrophil count increased to 1,300/µL. Therefore, G-CSF and meropenem were discontinued, and prednisolone for myositis was tapered off on the sixth day of admission. The patient was discharged on the 10th day of hospitalization and underwent outpatient follow-up.
Discussion
In this case, the patient presented with myositis and febrile neutropenia following acute COVID-19. This case suggests that neutropenia and myositis could be caused when the inflammation due to COVID-19 is severe and that G-CSF and prednisolone treatment may be effective against febrile neutropenia myositis following COVID-19 infection, although no clear literature evidence exists to support this finding.
Systemic inflammation and consequent autoantibody production after COVID-19 may affect the bone marrow and muscle, causing febrile neutropenia and autoantibody myositis, and should be considered in future cases of COVID-19. In our case report, the patient had no major symptoms at the time of COVID-19 but developed severe long-term complications. The complications of COVID-19 develop regardless of their severity, and the common symptoms include fatigue, dyspnea, anxiety, and depression [11,12]. Abnormalities in multiple organs, such as the lungs, renal tubules, heart, and liver, may also occur. In cases reported to date, neutropenia, and autoantibody myositis developed after contracting COVID-19 [13,14]. However, the pathophysiology of these cases remains unclear. According to previous studies, autoimmunity is temporarily activated by an abnormal increase in antibodies to COVID-19 and its cross-reactivity [13,14]. Most of the current case reports described neutropenia and myositis after developing COVID-19 [13-15]. However, none of the existing reports described the occurrence of only one of these conditions in COVID-19 patients. The present case suggests that the activation of autoimmunity by COVID-19 may spread to multiple organs rather than to a single organ, and further investigation is needed.
Further academic research regarding the use of G-CSF for febrile neutropenia after developing COVID-19 and prednisolone for antibody-associated myositis is warranted. In our patient, the continued use of G-CSF for febrile neutropenia and antimicrobial therapy enabled symptom control without abrupt changes. Previous literature suggests that G-CSF may be effective in the treatment of febrile neutropenia after contracting COVID-19; the post-COVID-19 hyperinflammatory state begins several weeks after onset, and there is no sufficient literature reporting the duration of this state [15,16]. There is a lack of evidence reporting the duration of the G-CSF administration. The mechanism is different from that of an immunocompromised host with febrile neutropenia after developing malignancy and undergoing chemotherapy, and the condition may be less likely to be severe. However, it is important to continue monitoring the neutrophil count and treating patients in the same manner as those with febrile neutropenia. With regard to COVID-19 antibody-associated myositis, a relationship between COVID-19 infection and autoimmune disease has been reported, although the mechanism of this relationship remains unclear [15,16]. In our case, the patient was suspected of having an autoimmune disease that could cause myositis and underwent blood tests, which showed the absence of antibodies except for an abnormal SARS-CoV-2 immunoglobulin G (IgG) level elevation. Based on the clinical course of this case, myositis was considered to have been caused by the release of autoantibodies during COVID-19. Although the appropriate treatment for this case was not clearly indicated in the previous literature, there were scattered reports of cases treated with prednisolone [15,16]. In the present case, the use of 50 mg prednisolone reduced the pain, which could be tapered off and discontinued from the 10th day of the disease. In conclusion, antibody-associated myositis is due to the transient elicitation of autoinflammation rather than the general spectrum of autoimmune diseases and may be in remission after short-term steroid use.
The current spread of COVID-19 may lead to an increase in similar cases. In community hospitals in Japan, many patients are older, and their symptoms are often vague [17,18]. Older individuals also have a variety of medical treatment behaviors and may not be able to appropriately self-manage their illnesses or use medical resources [17]. The spread and fear of COVID-19 may lead to an increase in the number of older patients with a variety of symptoms [19]. Clinicians working in the community should take a detailed history and physical examination; if there is a history of COVID-19, high inflammation caused by COVID-19 and inflammatory conditions caused by autoantibodies should be considered as differential diagnoses, and appropriate diagnosis and treatment should be provided [20].
Conclusions
This patient presented with myositis and febrile neutropenia induced following acute COVID-19. This case suggests that neutropenia and myositis could be caused by severe infection of COVID-19 and high titer of IgG against COVID-19. G-CSF and prednisolone therapy may be effective in treating febrile neutropenia and myositis that develops after COVID-19 infection. In community hospitals, it is necessary to take an appropriate history and physical examination of elderly patients with ambiguous symptoms after COVID-19 and to diagnose various complications by acute inflammation caused by COVID-19.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.COVID-19 presents high risk to older persons. Applegate WB, Ouslander JG. J Am Geriatr Soc. 2020;68:681. doi: 10.1111/jgs.16426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Effect of emergency declaration for the COVID −19 outbreak in Tokyo, Japan in the first two weeks: medRxiv. [ Apr; 2020 ];Kurita J, Sugawara T, Ohkusa Y. https://www.medrxiv.org/content/10.1101/2020.04.16.20067447v2.full.pdf 2020
- 3.Progression of COVID-19 from urban to rural areas in the United States: a spatiotemporal analysis of prevalence rates. Paul R, Arif AA, Adeyemi O, Ghosh S, Han D. J Rural Health. 2020;36:591–601. doi: 10.1111/jrh.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID- 19: a global challenge with old history, epidemiology and progress so far. Khan M, Adil SF, Alkhathlan HZ, Tahir MN, Saif S, Khan M, Khan ST. Molecules. 2020;26:39. doi: 10.3390/molecules26010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. Ayoubkhani D, Bermingham C, Pouwels KB, et al. BMJ. 2022;377:0. doi: 10.1136/bmj-2021-069676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Huang L, Yao Q, Gu X, et al. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Temporal trends in primary care-recorded self-harm during and beyond the first year of the COVID-19 pandemic: Time series analysis of electronic healthcare records for 2.8 million patients in the Greater Manchester Care Record. Steeg S, Bojanić L, Tilston G, et al. EClinicalMedicine. 2021;41:101175. doi: 10.1016/j.eclinm.2021.101175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epidemiology of Covid-19 in a long-term care facility in King county, Washington. McMichael TM, Currie DW, Clark S, et al. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prolonged self-resolving neutropenia following asymptomatic COVID-19 infection. Desai S, Quraishi J, Citrin D. Cureus. 2021;13:0. doi: 10.7759/cureus.16451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.New onset of autoimmune diseases following COVID-19 diagnosis. Gracia-Ramos AE, Martin-Nares E, Hernández-Molina G. Cells. 2021;10:3592. doi: 10.3390/cells10123592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covid-19: what new variants are emerging and how are they being investigated? Mahase E. BMJ. 2021;372:0. doi: 10.1136/bmj.n158. [DOI] [PubMed] [Google Scholar]
- 12.COVID-19 infection in the Veterans Health Administration: gender-specific racial and ethnic differences. Upchurch DM, Wong MS, Yuan AH, Haderlein TP, McClendon J, Christy A, Washington DL. Womens Health Issues. 2022;32:41–50. doi: 10.1016/j.whi.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Febrile neutropenia due to COVID-19 in an immunocompetent patient. Devi YM, Sehrawat A, Panda PK, Nath UK. BMJ Case Rep. 2021;14:0. doi: 10.1136/bcr-2021-242683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.COVID-19 disease and dermatomyositis: a mini-review. Qian J, Xu H. Front Immunol. 2021;12:747116. doi: 10.3389/fimmu.2021.747116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neutrophils in COVID-19. Reusch N, De Domenico E, Bonaguro L, Schulte-Schrepping J, Baßler K, Schultze JL, Aschenbrenner AC. Front Immunol. 2021;12:652470. doi: 10.3389/fimmu.2021.652470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Post-COVID-19 severe neutropenia. Bouslama B, Pierret C, Khelfaoui F, Bellanné-Chantelot C, Donadieu J, Héritier S. Pediatr Blood Cancer. 2021;68:0. doi: 10.1002/pbc.28866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.What resources do elderly people choose for managing their symptoms? Clarification of rural older people's choices of help-seeking behaviors in Japan. Ohta R, Sato M, Ryu Y, Kitayuguchi J, Maeno T, Sano C. BMC Health Serv Res. 2021;21:640. doi: 10.1186/s12913-021-06684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Challenges and solutions in the continuity of home care for rural older people: a thematic analysis. Ohta R, Ryu Y, Kitayuguchi J, Gomi T, Katsube T. Home Health Care Serv Q. 2020;39:126–139. doi: 10.1080/01621424.2020.1739185. [DOI] [PubMed] [Google Scholar]
- 19.Fears related to COVID-19 among rural older people in Japan. Ohta R, Ryu Y, Sano C. Healthcare (Basel) 2021;9:524. doi: 10.3390/healthcare9050524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Impact of COVID-19 on the analytical diagnosing ability of family medicine residents. Ohta R, Nishikura N, Sano C. J Gen Fam Med. 2021;22:109–110. doi: 10.1002/jgf2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]