Abstract
Background
Cardiac arrhythmias, mainly atrial fibrillation (AF), is frequently reported in COVID-19 patients, more often in Intensive Care Unit (ICU) patients, yet causality has not been virtually explored. Moreover, non-Covid ICU patients frequently present AF, sepsis being the major trigger. We aimed to examine whether sepsis or other factors-apart from Covid-19 myocardial involvement-contribute to elicit New Onset AF (NOAF) in intubated ICU patients.
Methods
Consecutive intubated, Covid-19ARDS patients, were prospectively studied for factors triggering NOAF. Demographics, data on Covid-19 infection duration, laboratory findings (troponin as well), severity of illness and ARDS were compared between NOAF and control group (no AF) on admission. In NOAF patients, echocardiographic findings, laboratory and secondary infection data on the AF day were compared to the preceding days and/or ICU admission data.
Results
Among 105 patients screened, 79 were eligible; nineteen presented NOAF (24%). Baseline characteristics did not differ between the NOAF and control groups. Troponin levels were mildly elevated upon ICU admission in both groups. Left ventricular global longitudinal strain was impaired (<16.5%) in 63% vs 78% in the two groups, respectively. The right ventricle was mildly dilated, and pericardial effusion was present in 52 vs 43%, respectively. NOAF occurred on the 18 ± 4.8 days from Covid-19 symptoms’ onset, and the 8.5 ± 2.1 ICUday. A septic secondary infection episode occurred in 89.5% of the patients in the NOAF group ( vs 41.6% in the control group (p < 0.001). In fact, NOAF occurred concurrently with a secondary septic episode in 84.2% of the patients. Sepsis presence was the only factor associated to NOAF occurrence (OR 16.63, p = 0.002). Noradrenaline, lactate and inflammation biomarkers gradually increased in the days before AF (all p < 0.05). Echocardiographic findings did not change on NOAF occurrence.
Conclusion
Secondary infections seem to be major contributors for NOAF occurrence in Covid-19 patients, probably playing the role of the “second hit” in an affected myocardium from Covid-19.
Keywords: Covid-19 ARDS, New Onset Atrial Fibrillation, Sepsis, Septic shock, ICU
Background
The main manifestation of coronavirus disease 2019 (Covid-19) is pneumonia leading to Acute Respiratory Distress Syndrome (ARDS) in 6% of cases [1], [2], [3]. Additionally, cardiovascular complications are frequently reported in Covid-19 patients [4]. Yet, cardiac involvement is mainly based on mildly increased troponin levels [5], [6], [7], [8]; more detailed examinations are still rather scarce [9], [10].
Cardiac arrhythmias, mainly atrial fibrillation (AF), have been reported in Covid-19 patients, but with a wide range in the incidence between 3% in patients hospitalized in the general ward and 41% in ICU patients [11], [12], [13]. Therefore, AF is more frequent in ICU Covid-19 patients and seems to occur late in the course of the disease, after respiratory worsening leading to ICU admission [1], [14], [15]. However, the actual time of new onset AF (NOAF) is not clearly reported [11], [12], [14], [15], [16]. Covid-19 per se could be the cause, but in ICU patients, multiple triggers may be implicated in AF occurrence.
In the non-Covid-19 era, NOAF is a frequently encountered arrhythmia in ICU patients and sepsis is one of the leading factors [17]. As sepsis has been increasingly reported in Covid-19 ARDS patients, partly attributed to the immunosuppressive drug protocols adopted, we hypothesized that sepsis or even other factors than SARS-CoV-2 infection may contribute to NOAF occurrence in ICU patients with Covid-19 ARDS. [18], [19]. Therefore, we aimed to evaluate in a cohort of intubated, ICU, Covid-19 ARDS patients (with no pre-existing factors known to trigger AF): 1). the incidence of NOAF and investigate possible factors leading to its occurrence, 2). the course of NOAF during ICU stay, and 3). the cardiac involvement using echocardiography and troponin levels upon ICU admission.
Methods
Study population
Consecutive patients admitted in the ICU (March 2020-February 2021) at the University Hospital of Larissa, Greece with laboratory confirmed (real-time PCR) SARS-CoV-2 infection and ARDS were included in this prospective study. The study was approved by the local ethics committee (55951/2020), with a waiver for informed consent. All patients were admitted after they had been intubated (intubation took in the general ward in rooms with negative pressure) and were transferred in the ICU. Patients having been intubated in the general ward for more than 24 h were excluded from the analysis. Evaluation included the period from the first ICU day until the 28th day (either still in the ICU, discharged or dead).
All the patients admitted in the ICU with Covid-19 ARDS were screened for inclusion in the study. The patients were included in the study if 1). they were positive for SARS-COV-2 and 2). presented ARDS upon ICU admission. Patients were excluded if they had 1). known history of cardiac disease [a). history of recent myocardial infarction or previous echocardiography presenting wall motion abnormalities indicating ischemic disease, b). recent admission for either coronary artery bypass graft, cardiac surgery or percutaneous transluminal coronary angioplasty (PTCA), c). severe aortic or mitral stenosis or regurgitation, d). history of heart failure from any cause or previous echocardiographic findings indicating Left Ventricular Ejection Fraction (LVEF) below 45%, e). known Right Ventricular (RV) dysfunction, f). cardiomyopathy of any type, g). presence of pacemaker, h). congenital heart disease, i). permanent AF, j) history of paroxysmal atrial fibrillation (PAF)]. 2) history of severe lung disease 3). brief AF episodes not meeting inclusion criteria, 4). history of NOAF occurring in the ward or presenting upon ICU admission. 5). Patients dying during the first 48 h of ICU admission were finally excluded from the analysis All eligible (according to inclusion/exclusion criteria) patients were divided in two groups: the NOAF group including patients with new onset AF during ICU stay without previous history of AF, and the control group, including all other patients not presenting AF. Patients were included in the NOAF group if they presented at least one AF episode lasting more than 10 min or suffered multiple AF episodes during a 24-hour period, or AF episodes needing direct electrical cardioversion due to hemodynamic instability.
Data collection
Baseline characteristics and disease severity (APACHE II, SOFA score) were recorded on admission. Demographics, medical history, and data concerning COVID-19 infection prior to hospital admission were collected from patients’ medical records and/or next-of-kin. Laboratory findings [inflammation markers (CRP, ferritin), coagulation, electrolytes] and the SOFA score were recorded daily. Troponin levels were recorded on ICU admission, the NOAF day and whenever indicated, according to attending physicians.
Blood, urinary and endotracheal aspirate (ETA) cultures were collected on admission, every 3 days (per local protocol) and whenever indicated, according to the attending physicians, but always on the day of AF occurrence and the day after. Heart rhythm was assessed continuously from the patients’ monitor (General Electric, Carescape B850); ECG tracings (12-lead) could be reviewed for the preceding 72-hours (GE monitor’s software), while 12-lead ECG was performed daily.
All patients received enhanced prophylactic anticoagulation according to current suggestions for Covid-19, except in patients with contraindication (coagulation abnormalities, thrombocytopenia, active bleeding).
Echocardiography was performed on admission and whenever indicated, but always on the AF day, according to AHA guidelines [20], by trained operators (VT, NK, VV), (General Electric, Vivid E95). We used a standard procedure to assess LV and RV sizes, function and filling measurements (2D imaging, color doppler, Tissue Doppler Imaging (Appendix) [21]. Left ventricular myocardial performance was assessed using the two-dimensional speckle-tracking method [21].
In patients needing cardioversion after 24 h due to AF persistence, a transesophageal echocardiography (TEE) preceded. If pulmonary embolism (PE) was suspected, Computed Tomography Pulmonary Angiography (CTPA) was performed.
All patients were assessed under satisfactory loading conditions (Appendix). Central Venous Oxygen Saturation (ScVO2) measurements were performed on admission, in episodes of hemodynamic instability, and whenever indicated according to attending physicians.
Definitions
Sepsis, septic shock, and types of infections were defined according to recently updated terms [22], [23]. Secondary infections included all hospital acquired Blood Stream Infections (BSI), Hospital/Ventilator-Associated Pneumonia (HAP/VAP) and Urinary Tract Infections (UTI) occurring after 48 h of hospital admission (Appendix).
Atrial fibrillation management protocol
All NOAF episodes received amiodarone (750 mg daily) after a loading dose of 150–300 mg administration ( ± b-blockers for rate control). Direct electrical cardioversion was performed only in patients with hemodynamic instability, defined as a significant increase in vasopressor dosage after AF appearance, according to the attending physicians’ assessment. However, attending physicians were encouraged to postpone electrical cardioversion until the patient had received the amiodarone loading dose.
Statistical analyses
Results are given as mean ( ± Standard Deviation, SD) in normally distributed parameters and as median (interquartile range) in not normally distributed values. Normally distributed continuous indices were compared with Student’s t-test (between two groups) and paired t-test was used to compare serial variables in the same group.; non-normally distributed indices were compared via the Mann-Whitney-U and Wilcoxon test. To test possible factors associated to the occurrence of NOAF, binary logistic regression analysis, using NOAF as the dependent was performed. Indeed, binary logistic regression models were built for NOAF, with odds ratio (OR) and the corresponding 95% confidence interval (CI) reported in relation to the model covariates. Variables that turned statistically significant in univariate logistic regression models on NOAF as the dependent variable, were entered in the final model. In univariate analysis, baseline variables (i.e demographics, Charlson comorbidity index, history of hypertension, diabetes mellitus, baseline laboratory findings, baseline echocardiographic data) were tested for significance. Finally, Chi-square was used when testing categorical data. Data were analyzed using SPSS (IBM SPSS statistics version 25).
Results
Among one hundred and five patients with Covid-19 ARDS admitted in the ICU, seventy-nine patients were eligible for the study ( Fig. 1). Nineteen patients presented NOAF (24%), constituting the NOAF group; the rest 60 patients comprised the control group. The two groups did not differ in baseline characteristics, Charlson comorbidity index, laboratory findings, disease severity upon admission and lung mechanics ( Table 1). Troponin levels were mildly elevated upon ICU admission in both groups (0.17 ± 0.4 vs 0.15 ± 0.55, p = 1) (Table 1). Corticosteroids received 73% in the NOAF and 84%in the control groups (p = 0.08) (Table 1). Tocilizumab had received 26% vs 32% (p = 0.66), while 21% vs 18 30% had received anti-IL-1 therapy (p = 0.64), respectively. Six patients, in whom severe pulmonary embolism was suspected, underwent CTPA. One in the control and none in the NOAF group was diagnosed with significant PE.
Fig. 1.
Study Flowchart, AF, atrial fibrillation; CAD, Coronary Artery Disease; Covid-19, Coronavirus disease-2019; ECHO, Echocardiography; EF, Ejection Fraction; HF, Heart Failure; ICU, Intensive Care Unit; MI, Myocardial Infarction; NOAF, New Onset Atrial Fibrillation.
Table 1.
Demographics and baseline characteristics in patients without AF (controls) and NOAF patients upon ICU admission.
| Control (n = 60) |
NOAF (n = 19) |
p |
|
|---|---|---|---|
| Demographic | |||
| Age | 68.2 ± 3.1 | 69.7 ± 3.1 | 0.64 |
| Males N (%) | 46 (76%) | 14 (74%) | 0.88 |
| Charlson comorbidity index | 3.2 ± 0.4 | 3.8 ± 0.88 | 0.11 |
| Hypertension | 46 (76.6%) | 10 (52.6%) | 0.05 |
| Corticosteroids (N) (%) | 54 (84%) | 14 (74%) | 0.075 |
| Clinical Data | |||
| APACHE II | 16.9 ± 1.8 | 14.4 ± 2.8 | 0.12 |
| SOFA | 8 ± 1.3 | 7.4 ± 1 | 0.63 |
| PαΟ2/FιΟ2, mmHg | 111.2 ± 45.7 | 124.6 ± 42.2 | 0.35 |
| CRS | 36.7 ± 2.5 | 37.1 ± 8.9 | 0.89 |
| Noradrenaline (μg/kg/min) | 0.39 ± 0.18 | 0.24 ± 0.13 | 0.27 |
| Laboratory Data | |||
| D-Dimers (ng/ml) (<300) | 819 ± 398.5 | 895 ± 353.6 | 0.52 |
| Ferritin, (ng/ml), (< 330) | 1205.7 ± 952.8 | 1380 ± 801.5 | 0.28 |
| WBC, 103/L (<10 ×103/L) | 9273.2 ± 6498 | 9543.8 ± 2108 | 0.08 |
| CRP (mg/dL), (<0.5) | 8.4 ± 0.4 | 10.9 ± 4.2 | 0.13 |
| Troponin, ng/ml (<0.04) | 0.15 ± 0.34 | 0.16 ± 0.31 | 1.0 |
APACHE II, Acute Physiology and Chronic Health Evaluation II; CRP, C-reactive protein; CRS, Respiratory System Compliance; NOAF, New Onset Atrial Fibrillation; PaO2/FiO2, Partial Oxygen Pressure/Fraction of Inspired Oxygen; SOFA, Sequential Organ Failure Assessment; WBC, white blood cells;
New Onset AF occurred late in the course of hospitalization, 18 ± 4.8 days from Covid-19 symptoms’ onset and on 8.5 ± 2.1 ICU day. Only one patient (70-years-old) presented a short AF episode lasting 3 min (2nd ICU day) and another two (65 and 72-years-old) presented with NOAF on ICU admission and were excluded. In all other patients, NOAF occurred after/on the 3rd ICU day (range 3–23 ICU day).
Laboratory and clinical data on AF day, compared to data on the third preceding the AF day are presented in Table 2.
Table 2.
NOAF group: Clinical and laboratory data on the day of AF compared to data three days before AF occurrence.
| Day − 3 of AF occurrence | Day of AF occurrence | p | |
|---|---|---|---|
| Clinical Data | |||
| PaO2/FiO2, mmHg | 162.3 ± 24.3 | 199.05 ± 35.5 | 0.056 |
| Heart rate | 65.9 ± 14.6 | 100 ± 6.8 | 0.002 |
| Noradrenaline (μg/kg/min) | 0.08 ± 0.06 | 0.44 ± 0.22 | 0.01 |
| Lactate, mmol/lt | 1.1 ± 0.4 | 2.3 ± 0.5 | < 0.001 |
| ScVO2, %a | 69.6 ± 3.6 | 75.8 ± 3 | < 0.001 |
| Laboratory Data | |||
| WBC, 103/L (<10 ×103/L) | 8680 ± 2679 | 10,627 ± 1972 | 0.71 |
| CRP (mg/dl) (<0.5) | 7.41 ± 4.3 | 12.33 ± 4.1 | 0.01 |
| Ferritin (ng/ml) (<330) | 1188 ± 453 | 999 ± 787 | 0.46 |
| Na (mmol/lt) | 143.6 ± 4.7 | 145.6 ± 3.8 | 0.57 |
| K (mmol/lt) | 4.3 ± 0.3 | 4.4 ± 0.3 | 0.62 |
| Troponin, ng/ml Ia(<0.02) | 0.16 ± 0.31 | 0.64 ± 1.04 | 0.017 |
| Culture results | |||
| Positive blood culture, n(%)b | 0 | 13 (81%) | – |
| Positive ETA, N(%) | 0 | 5 (26%) | – |
CRP, C-reactive protein; ETA, Endotracheal Aspirate sample; NOAF, New Onset Atrial Fibrillation; PaO2/FiO2, Partial Oxygen Pressure/Fraction of Inspired Oxygen; ScVO2, Central Venous Oxygen Saturation; WBC, white blood cells;
For troponin and ScVO2, the value in the first column refers on admission data
Eleven patients presented primary bacteremia on the day NOAF occurred. Two more patients diagnosed with VAP on NOAF day, presented positive blood cultures, with the same isolate as the one responsible for VAP (bacteremic VAP)
In order to discriminate factors associated with the occurrence of NOAF, multivariate regression analysis was performed, with NOAF serving as the dependent variable. The univariate logistic regression on NOAF as the dependent variable did not show statistical significance with the following variables: i) age ii) sex, iii) diabetes mellitus, iv) APACHE II score, v) SOFA score, vi) CRP (admission), vii) WBC (admission), viii) Levophed (admission), ix) echocardiographic variables of the left and right ventricle. On the contrary, i) day since SARS-COV-2 infection onset, ii) diagnosis of sepsis iii) Ferritin levels upon admission, iv) history of hypertension, v) receipt of corticosteroids for Covid-19, all showed statistical significance (p < 0.001) and were entered in the multivariate model.
Binary logistic regression analysis revealed that only for sepsis presence the statistical significance was maintained. The respective adjusted OR was 16.63 (p = 0.002) ( Table 3).
Table 3.
Logistic regression model for predicting New Onset Atrial Fibrillation. Odds ratios (OR) with 95% confidence intervals (95% CI) and the covariate p-value are reported.
| Variables | OR | 95% CI | p-value |
|---|---|---|---|
| Sepsis yes (vs no) | 16.04 | 2.87 – 96.37 | 0.002 |
| Day since infection onset | 0.92 | 0.82 – 1.05 | 0.926 |
| Ferritin levels (admission) | 1.00 | 1.00 – 1.00 | 0.502 |
| Hypertension yes (vs no) | 0.183 | 0.03 –1.26 | 0.084 |
| Corticosteroids yes (vs no) | 3.40 | 0.19 – 60.79 | 0.406 |
Odds ratios (OR) with 95% confidence intervals (95% CI) and the covariate p-value are reported.
Septic secondary infection episodes
A secondary (at least one) infection episode occurred in 41.6% patients in the control group (upon ICU admission in two) and 89.5% in the NOAF group during their ICU stay (p < 0.001) (Fig. 1). Secondary infections occurred after the sixth ICU day in 71.4% patients. Fig. 2.
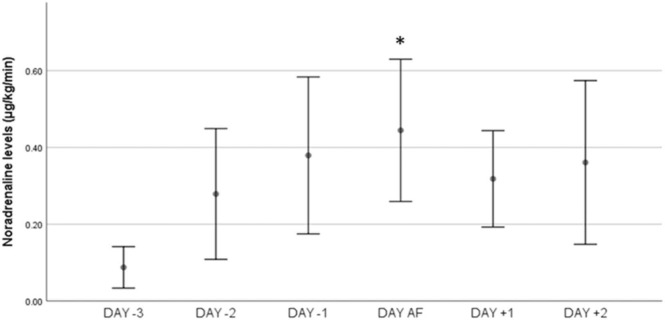
Fig. 2.
: Time course of vasopressor dose in the period around NOAF occurrence. Noradrenaline dose was increased from 0.08 ± 0.06 μg/kg/min three days before NOAF to 0.44 ± 0.22 μg/kg/min exactly before AF appearance (p = 0.01), reaching the highest value (0.52 ± 0.06 μg/kg/min) after NOAF occurrence (p = 0.033, compared to NOAF occurrence), Comparisons have been performed with the Day-3 (reference value) and, also, between each day, * : p = 0.01 refers to the difference between the noradrenaline dose on the day AF occurred (just before AF appearance) and the dose that the patients were receiving on the Day − 3 (reference day).
NOAF group. In 84.2% of the patients a septic secondary infection occurred concurrently with NOAF, in81.3% presenting with septic shock. Primary bacteremia occurred in57.9% and 26.3% suffered ventilator-associated pneumonia (VAP)-two accompanied with bacteremia. All NOAF patients had negative blood, urine and ETA cultures on admission. In 87.5% NOAF presented during the first septic episode. Notably, antibiotics were added or modified in 94.7% of the patients, in the two preceding or the NOAF day, for suspected septic secondary infections, according to the attending physicians.
Noradrenaline dose was increased from 0.08 ± 0.06 μg/kg/min three days before NOAF to 0.44 ± 0.22 μg/kg/min exactly before AF appearance (p = 0.01), reaching the highest value (0.52 ± 0.06 μg/kg/min) after NOAF (p = 0.033 compared to the value on NOAF occurrence). Lactate increased (1.1 ± 0.3 vs 2.3 ± 0.5, p < 0.001); in 13 patients lactate increased> 2 mmol/lt. No modification in sedation had been done that could have provoked hypotension. A positive fluid balance was noted in patients with sepsis in the preceding 3 days (6948.13 ± 2829 ml), while ScVO2 presented a significant rise on the day of AF compared to admission (75.8 ± 3 vs 69.6 ± 3.6%, p < 0.001)(Table 2).
Laboratory findings
CRP values showed a gradual increase during the days preceding AF (Table 2) and a subsequent decrease thereafter. Troponin levels significantly increased on the AF day compared to admission (0.64 ± 1.04 vs 0.16 ± 0.31 ng/dl, p = 0.017).
Echocardiographic findings
Left Ventricular function did not differ between groups, although RV function was mildly impaired in the control group. ( Table 4). Left Ventricular Global Longitudinal Strain (GLSLV) was − 12 ± 4% in patients without AF and − 14 ± 6% in NOAF patients. Noteworthy, GLSLV was abnormal (<16.6%) in 78% of the patients in the control and 63% in the NOAF group [22]. Covid-19 patients presented moderate RV dilation (Right Ventricular End Diastolic Area/Left Ventricular End Diastolic Area>0.6 in both groups). Mild pericardial effusion was present in43% vs 52% in the control and NOAF groups respectively, presented (p = 0.48). Echocardiographic measurements did not significantly change on NOAF occurrence (Table 4).
Table 4.
Comparison of echocardiographic variables between the control and NOAF group on admission, and between admission and the NOAF day, in the NOAF group.
| Control Group (n = 60) | NOAF group (n = 19) |
||
|---|---|---|---|
| ICU Admission | ICU admission | AF Day | |
| Left atrial area, cm2 | 19.7 ± 3.1 | 21.2 ± 3.6 | 22.2 ± 4.7 |
| Left Ventricular End Diastolic Diameter, cm | 4.5 ± 0.7 | 4.6 ± 0.4 | 4.6 ± 0.5 |
| Left Ventricular EF (Simpson method) (%) | 59.9 ± 14.4 | 55.1 ± 19.5 | 56.6 ± 15 |
| GLSLV, % | -12.3 ± 4.2 | -14.7 ± 5.5 | -11.9 ± 3.1 |
| GLSLV < 16.6%, n (%) | 47 (78%) | 12 (63%) | 0.075 |
| VTILVOT, cm | 22.2 ± 5.4 | 21.6 ± 7.1 | 22.8 ± 5.3 |
| E, cm/s | 63 ± 21 | 74 ± 8 | 76 ± 16 |
| A, cm/sec | 67 ± 8 | 72 ± 15 | |
| E’, cm/s | 8 ± 2 | 7 ± 1 | 8 ± 2 |
| E/E’ | 7.4 ± 5.1 | 9.9 ± 2.3 | 10.1 ± 2.3 |
| S’, cm/s | 10 ± 3 | 8 ± 1 | 8 ± 2 |
| RVEDA/LVEDA | 0.7 ± 0.2 | 0.7 ± 0.4 | 0.7 ± 0.3 |
| RV FAC, % | 38.7 ± 13.3 * | 51 ± 21.4 | 36.3 ± 10 |
| TAPSE, mm | 22.1 ± 5.1 | 25.4 ± 5.9 | 21.1 ± 5 |
| RV s’, cm/sec | 15 ± 4 * | 19 ± 3# | 13 ± 5 |
| Pericardial effusion | 26/60 (43%) | 10/19 (52%) | 11/19 (58%) |
Α: late diastolic ventricular filling velocity with atrial contraction; AF, Atrial Fibrillation; EF: Ejection Fraction, E, left ventricular early diastolic peak velocity, E’, early diastolic tissue Doppler velocity; ICU, Intensive Care Unit; GLSLV, global longitudinal strain of the left ventricle; RVEDA/LVEDA, Right Ventricular End Diastolic Area to Left Ventricular End Diastolic Area; RVFAC, Right Ventricular Fractional Area Change; S’, systolic tissue Doppler velocity; RV s’, Tissue doppler peak systolic velocity at the tricuspid annulus, TAPSE: Tricuspid Annular Plane Systolic Excursion; VTILVOT, Left Ventricular Outflow Tract Velocity Time Integral;
Data are expressed as mean± standard deviation.
* : p < 0.05 for comparisons between the control and NOAF group upon ICU admission
#: p < 0.05 for comparisons between echocardiographic data on admission and the NOAF day, in the NOAF group
Outcome
Arrhythmia In all patients, NOAF lasted more than one hour. Sinus rhythm (SR) was restored in 84% of the patients, in 68.4% during the first 24 h and the rest during 48 h, although short recurrent AF episodes (after cardioversion), lasting less than 30 min were recorded in 4 patients. Only one patient, presenting severe hemodynamic instability, was electrically cardioverted, one hour after unsuccessful amiodarone infusion; in this patient, AF recurred, returning to sinus rhythm after 24 h. Nine TEE were performed in five patients; in all, cardiac chambers, including the appendage were free from thrombi.
In six patients (including three in whom SR was not restored) signs of sepsis (due to XDR species) were not resolving until death. In three, AF recurred after 4–9 days on SR, coinciding with a new septic episode, returning to SR with sepsis resolution. Amiodarone infusion was continued until ICU discharge or death.
Mortality
In NOAF group, 28th day mortality was 47% vs 41.7% in the control group (p = 0.57). Three patients died without converting to SR. All ten survivors were discharged on SR, under amiodarone. Among them, we were able to contact three patients (aged 45, 56, 76 years) discharged home; patients are on SR (2–7 months later); amiodarone has been stopped.
Discussion
Our study demonstrates that myocardial dysfunction is present in intubated ICU patients with severe Covid-19 ARDS and no pre-existing history of cardiac disease, as depicted by the echocardiographic findings of impaired left and right ventricular function, mild pericardial effusion, and mild elevation of troponin levels. However, New Onset Atrial Fibrillation was independently associated only with a secondary infection that led to severe sepsis. Importantly, 84.2% of NOAF occurred in the early phase (onset) of a septic episode, usually with septic shock. History of hypertension, days since Covid-19 onset, ferritin levels, corticosteroid treatment (revealed significant in univariate analysis) did not affect NOAF occurrence. Demographics, Charlson comorbidity index, ARDS severity, respiratory system mechanics, mechanical ventilatory modes and electrolytes did not differ between groups, while hypoxemia degree was quite improving in NOAF patients on the day AF occurred. We suggest that sepsis triggered NOAF occurrence, in the setting of an affected, from Covid-19, myocardium; sepsis resolution was crucial to maintain SR (under amiodarone infusion).
Our understanding on the cardiovascular effects of Covid-19 is still limited [6]. In our cohort, Left Ventricular EF was rather normal, although impaired global longitudinal strain indicated occult myocardial injury in the majority (74%) of Covid-19 ARDS patients upon ICU admission; abnormal GLSLV (<16.6%) has been reported in 42% of Covid-19 patients admitted in the ward, while data on ICU patients are scarce [24], [25], [26]. In addition, a moderate RV enlargement was observed, which is in accordance with various Covid-19 reports [27], [28]. However, multiple factors may explain this finding apart from Covid-19; RV dilation is exacerbated by mechanical ventilator settings (PEEP), especially when lung compliance is preserved [29], [30]. Interestingly, 45.6% of the patients had a mild pericardial effusion. Pericardial effusion incidence has not been thoroughly evaluated in Covid-19 [31]. In addition, troponin levels were elevated on ICU admission, a finding that has been linked to myocardial involvement in Covid-19 [5], [6], [7], [8]. The above data support the notion that a degree of myocardial injury is present in severe Covid-19 patients, admitted in the ICU. However, there was no difference between the NOAF and control group, in any parameter concerning the cardiac involvement. Moreover, patients with a known cardiac history were initially excluded, so that the results of the study would be more straightforward.
Among atrial arrhythmias, AF is the most frequent in Covid-19 patients; NOAF prevalence varies between 3% and 10% in non-ICU patients [12], [13]. In our study, NOAF incidence was 24%, which is in accordance with the higher incidence reported in ICU patients [11], [12], [13], [14], [32], [33]. Colon et al., reported NOAF in 16.5% ICU patients [15]. However, no reference is made on possible secondary conditions and the timing of arrhythmia occurrence. Increased inflammatory markers and vasopressor need were reported during AF appearance, without specifying whether AF occurrence was coincidental to a secondary infection episode [4], [11]. Other studies confirm the increased NOAF incidence in ICU Covid-19 patients, varying between 16.5% and 40%, yet, without specifying whether the virus or other factors, frequently present in critically-ill patients, are associated to its occurrence [11], [12], [13], [14], [15], [16], [32], [33]. Similarly, existing data lack information about the exact time of NOAF appearance in the course of Covid-19 [12], [13], [14], [15], [32], [33]. An early, in the course of the infection, virally driven hyperinflammation-cytokine storm has been proposed as a possible mechanism, partially explaining NOAF occurrence in patients hospitalized in the wards [4], [11]. In our study, NOAF appeared late in the course of the disease, approximately during the 18th post symptom onset day (8th ICU-day), when COVID-19 symptoms usually subside [32]. Although myocarditis has also been suggested as a possible mechanism for arrythmias in Covid-19, histological findings indicate macrophage infiltration, with no clear association to myocardial injury, and, although troponin is high, true myocarditis is established in only 4.5% of the severely ill, Covid-19 patients with heart failure, undergoing endomyocardial biopsies; thus, the virus does not seem to directly invade the cardiac cells in order to initiate AF [34]. Our findings support that non cardiac causes, such as systemic infection, may contribute to NOAF.
New-onset AF is a common arrhythmia in non-Covid-19 ICU patients, occurring in 19–35% of patients, sepsis being the main triggering factor [17], [35], [36]. Walkey et. al, reported an increased incidence (35%) of NOAF among septic patients, further increasing with disease severity [35]. In our study, 84.2% of the patients presented sepsis and 68.4% had septic shock on NOAF episodes. Inflammation markers, vasopressor need, and lactate levels presented a gradual increase in the preceding the AF days. The positive fluid balance during the last three days, and the rise in ScVO2 values, further supported NOAF’s association to sepsis-induced vasodilation [37].
An increased incidence of secondary infections has been observed in our cohort; 42% in the control group and 89% in the NOAF group, consistent with recent reports, indicating an increased incidence after the 7th ICU day [18], [19]. Corticosteroids, Tocilizumab and Anakinra, used in COVID-19 ARDS, may be partly responsible [18], [19]. In our study, NOAF occurred on the 8.5 ± 2.1 ICU day, mostly coinciding with the first BSI/VAP septic episode. We suggest that sepsis, with adrenergic overstimulation, due to endogenous elevated catecholamine levels and exogenous catecholamine administration (as in septic shock), constitutes the second “hit”, to trigger AF in a diseased/affected, from SARS-CoV-2, myocardium [4], [17]. Interestingly, patients in both groups were of comparable age and did not present factors indicating apparent cardiovascular disease, known to increase AF occurrence risk [13].
Troponin levels were significantly raised on the AF day compared to admission, further supporting the association of NOAF to secondary sepsis. Troponin elevation has been repeatedly reported in bacterial sepsis, reflecting altered cardiomyocyte permeability or some degree of necrosis, frequently associated with cardiac dysfunction (sepsis cardiomyopathy) [38], [39]. Sepsis induced myocardial dysfunction is very frequent, attributed to increased circulating catecholamine and cytokine levels, found in severe sepsis and septic shock [40], [41]. However, decreased systemic vascular resistance may mask the altered myocardial performance. We believe that sepsis-induced vasoplegia is responsible for the apparently preserved LVEF in our patients when NOAF appeared.
Rhythm control has been found more beneficial than rate control in ICU patients [36]. Most patients in our study returned to sinus rhythm with pharmacologic cardioversion (amiodarone); AF could not be restored in patients with non-resolving sepsis or re-occurred in those with recurrent septic episodes.
Our study has limitations. It was conducted in a single center serving an urban population; thus the number of patients is limited. However, although our findings may not be generalizable across the world, they may have particular importance in South Europe and other countries with an increased incidence of nosocomial infections from PDR/XDR, as in our study [42]. In addition, we consider an advantage that the study population was rather homogenous: we prospectively enrolled consecutive, intubated patients with severe Covid-19, with no obvious preexisting cardiovascular disease in order to eliminate known factors triggering AF. Cardiac Magnetic Resonance tomography was not performed, but its utility in ICU is limited by the out-of-hour availability and the requirement for some breath-holding, while no patients underwent endomyocardial biopsy (caring inherent risks), as it is not suggested due to the low incidence of myocarditis in Covid-19 [34]. Instead, in all patients, troponin levels and a full echocardiographic examination were performed, which seem appropriate to reveal cardiac involvement in Covid-19.
Conclusion
In conclusion, myocardial function is altered in Covid-19 patients, probably lowering the threshold for arrhythmogenicity. Secondary infections seem to be major contributors for NOAF occurrence in ICU Covid-19 ARDS patients, probably playing the role of the “second hit” in an affected myocardium. Sepsis should be suspected in case of late NOAF occurrence in these patients. Furthermore, AF did not resolve or re-occurred if sepsis persisted. Further research on the arrhythmogenicity of COVID-19 in severe ICU Covid-19 ARDS patients is needed.
Ethics approval and consent to participate
This study was approved by the Institutional Ethics Committees of the University Hospital of Larissa (55951/2020), with a waiver for informed consent.
Sponsorship
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
None.
CRediT authorship contribution statement
GEZ contributed to the concept, design, and data collection; conducted the analyses; and drafted the manuscript. VT contributed to concept and design, data collection, and critical revisions of the manuscript. DM contributed to concept and design, data collection, and critical revisions of the manuscript. NK, GD, VV and KM, contributed to concept and design and critical revisions of the manuscript. GV contributed to statistical analysis and critical revisions of the manuscript.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgements
None.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jiph.2022.06.006.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Phua J., Weng L., Ling L., Egi M., Lim C.M., Divatia J.V., et al. Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. COVID-19 Lombardy ICU Network Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet (Lond, Engl) 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gawałko M., Kapłon-Cieślicka A., Hohl M., Dobrev D., Linz D. COVID-19 associated atrial fibrillation: Incidence, putative mechanisms and potential clinical implications. Int J Cardiol Heart Vasc. 2020;30 doi: 10.1016/j.ijcha.2020.100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Shu H., Liu H., Li X., Zhou X., Zou X., et al. The peak levels of highly sensitive troponin I predicts in-hospital mortality in COVID-19 patients with cardiac injury: a retrospective study. Eur Heart J Acute Cardiovasc Care. 2020:zuaa019. doi: 10.1093/ehjacc/zuaa019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsolaki V., Zakynthinos G.E. Are patients with COVID-19 dying of or with cardiac injury. Am J Respir Crit Care Med. 2020;202(2):300–301. doi: 10.1164/rccm.202004-1083LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siripanthong B., Nazarian S., Muser D., Deo R., Santangeli P., Khanji M.Y., et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imazio M., Klingel K., Kindermann I., Brucato A., De Rosa F.G., Adler Y., et al. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis. Heart. 2020;106(15):1127–1131. doi: 10.1136/heartjnl-2020-317186. [DOI] [PubMed] [Google Scholar]
- 9.D’Alto M., Marra A.M., Severino S., Salzano A., Romeo E., De Rosa R., et al. Right ventricular-arterial uncoupling independently predicts survival in COVID-19 ARDS. Crit Care. 2020;24(1):670. doi: 10.1186/s13054-020-03385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagate F., Masi P., d’Humières T., Al-Assaad L., Chakra L.A., Razazi K., et al. Advanced echocardiographic phenotyping of critically ill patients with coronavirus-19 sepsis: a prospective cohort study. J Intensive Care. 2021;9(1):12. doi: 10.1186/s40560-020-00516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yarmohammadi H., Morrow J.P., Dizon J., Biviano A., Ehlert F., Saluja D., et al. Frequency of atrial arrhythmia in hospitalized patients with COVID-19. Am J Cardiol. 2021;S0002–9149(21):00150–00158. doi: 10.1016/j.amjcard.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angeli F., Spanevello A., De Ponti R., Visca D., Marazzato J., Palmiotto G., et al. Electrocardiographic features of patients with COVID-19 pneumonia. Eur J Intern Med. 2020;78:101–106. doi: 10.1016/j.ejim.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatla A., Mayer M.M., Adusumalli S., Hyman M.C., Oh E., Tierney A., et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17(9):1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iacopino S., Placentino F., Colella J., Pesce F., Pardeo A., Filannino P., et al. New-onset cardiac arrhythmias during COVID-19 hospitalization. Circ Arrhythm Electro. 2020;13(11) doi: 10.1161/CIRCEP.120.009040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colon C.M., Barrios J.G., Chiles J.W., McElwee S.K., Russell D.W., Maddox W.R., et al. Atrial arrhythmias in COVID-19 patients. JACC Clin Electro. 2020;6(9):1189–1190. doi: 10.1016/j.jacep.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A., et al. Clinical characteristics of covid-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosch N.A., Cimini J., Walkey A.J. Atrial fibrillation in the ICU. Chest. 2018;154(6):1424–1434. doi: 10.1016/j.chest.2018.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buetti N., Ruckly S., de Montmollin E., Reignier J., Terzi N., Cohen Y., et al. COVID-19 increased the risk of ICU-acquired bloodstream infections: a case-cohort study from the multicentric OUTCOMEREA network. Intensive Care Med. 2021;47(2):180–187. doi: 10.1007/s00134-021-06346-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouzé A., Martin-Loeches I., Povoa P., Makris D., Artigas A., Bouchereau M., et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med. 2021;47(2):188–198. doi: 10.1007/s00134-020-06323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheitlin M.D., Alpert J.S., Armstrong W.F., Aurigemma G.P., Beller G.A., Bierman F.Z., et al. ACC/AHA Guidelines for the Clinical Application of Echocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Application of Echocardiography). Developed in collaboration with the American Society of Echocardiography. Circulation. 1997;95(6):1686–1744. doi: 10.1161/01.cir.95.6.1686. [DOI] [PubMed] [Google Scholar]
- 21.Lang R.M., Bierig M., Devereux R.B., Flachskampf F.A., Foster E., Pellikka P.A., et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horan T.C., Andrus M., Dudeck M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Rothschild E., Baruch G., Szekely Y., Lichter Y., Kaplan A., Taieb P., et al. The predictive role of left and right ventricular speckle-tracking echocardiography in COVID-19. JACC Cardiovasc Imaging. 2020;13(11):2471–2474. doi: 10.1016/j.jcmg.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bursi F., Santangelo G., Sansalone D., Valli F., Vella A.M., Toriello F., et al. Prognostic utility of quantitative offline 2D-echocardiography in hospitalized patients with COVID-19 disease. Echocardiography. 2020;37(12)):2029–2039. doi: 10.1111/echo.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baycan O.F., Barman H.A., Atici A., Tatlisu A., Bolen F., Ergen P., et al. Evaluation of biventricular function in patients with COVID-19 using speckle tracking echocardiography. Int J Cardiovasc Imaging. 2021;37(1):135–144. doi: 10.1007/s10554-020-01968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bleakley C., Singh S., Garfield B., Morosin M., Surkova E., Mandalia M.S., et al. Right ventricular dysfunction in critically ill COVID-19 ARDS. Int J Cardiol. 2021;327:251–258. doi: 10.1016/j.ijcard.2020.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szekely Y., Lichter Y., Taieb P., Banai A., Hochstadt A., Merdler I., et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142(4):342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsolaki V., Zakynthinos G.E. RV dysfunction in Covid-19 ARDS: Is there a difference in the impact of mechanical ventilation and ECMO? Int J Cardiol. 2021;330:273. doi: 10.1016/j.ijcard.2021.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsolaki V., Siempos I., Magira E., Kokkoris S., Zakynthinos G.E., Zakynthinos S. PEEP levels in COVID-19 pneumonia. Crit Care. 2020;24(1):303. doi: 10.1186/s13054-020-03049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimopoulou D., Spyridis N., Dasoula F., Krepis P., Eleftheriou E., Liaska M., et al. Pericarditis as the main clinical manifestation of COVID-19 in adolescents. Pedia Infect Dis J. 2021;40(5):e197–e199. doi: 10.1097/INF.0000000000003096. [DOI] [PubMed] [Google Scholar]
- 32.Babapoor-Farrokhran S., Rasekhi R.T., Gill D., Babapoor S., Amanullah A. Arrhythmia in COVID-19. SN Compr Clin Med. 2020:1–6. doi: 10.1007/s42399-020-00454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vahey G.M., Marshall K.E., McDonald E., Martin S.W., Tate J.E., Midgley C.M., et al. Colorado Investigation Team2. Symptom profiles and progression in hospitalized and nonhospitalized patients with coronavirus disease, Colorado, USA, 2020. Emerg Infect Dis. 2021;27(2):385–395. doi: 10.3201/eid2702.203729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawakami R., Sakamoto A., Kawai K., Gianatti A., Pellegrini D., Nasr A., et al. Pathological evidence for SARS-CoV-2 as a cause of myocarditis: JACC review topic of the week. J Am Coll Cardiol. 2021;77(3):314–325. doi: 10.1016/j.jacc.2020.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walkey A.J., Benjamin E.J., Lubitz S.A. New-onset atrial fibrillation during hospitalization. J Am Coll Cardiol. 2014;64(22):2432–2433. doi: 10.1016/j.jacc.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arrigo M., Ishihara S., Feliot E., Rudiger A., Deye N., Cariou A., et al. New-onset atrial fibrillation in critically ill patients and its association with mortality: a report from the FROG-ICU study. Int J Cardiol. 2018;266:95–99. doi: 10.1016/j.ijcard.2018.03.051. [DOI] [PubMed] [Google Scholar]
- 37.Textoris J., Fouché L., Wiramus S., Antonini F., Tho S., Martin C., Leone M. High central venous oxygen saturation in the latter stages of septic shock is associated with increased mortality. Crit Care. 2011;15(4):R176. doi: 10.1186/cc10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J.S., Kim M., Kim Y.J., Ryoo S.M., Sohn C.H., Ahn S., et al. Troponin testing for assessing sepsis-induced myocardial dysfunction in patients with septic shock. J Clin Med. 2019;8(2):239. doi: 10.3390/jcm8020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu A.H. Increased troponin in patients with sepsis and septic shock: myocardial necrosis or reversible myocardial depression? Intensive Care Med. 2001;27(6):959–961. doi: 10.1007/s001340100970. [DOI] [PubMed] [Google Scholar]
- 40.Jeong H.S., Lee T.H., Bang C.H., Kim J.H., Hong S.J. Risk factors and outcomes of sepsis-induced myocardial dysfunction and stress-induced cardiomyopathy in sepsis or septic shock: a comparative retrospective study. Med (Baltim) 2018;97(13) doi: 10.1097/MD.0000000000010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vieillard-Baron A., Caille V., Charron C., Belliard G., Page B., Jardin F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med. 2008;36(6):1701–1706. doi: 10.1097/CCM.0b013e318174db05. PMID: 18496368. [DOI] [PubMed] [Google Scholar]
- 42.Tsolaki V., Mantzarlis K., Mpakalis A., Malli E., Tsimpoukas F., Tsirogianni A., et al. Ceftazidime-avibactam to treat life-threatening infections by carbapenem-resistant pathogens in critically ill mechanically ventilated patients. Antimicrob Agents Chemother. 2020;64(3) doi: 10.1128/AAC.02320-19. https://doi:10.1128/AAC.02320-19 PMID: 31818820; PMCID: PMC7038311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material