Abstract
Current methods for total RNA extraction are time-consuming and require several hands-on steps and specialized equipment. Microfluidic devices can offer the opportunity to reduce the number of hands-on steps, decrease the volumes of reagents required for purification, and make extraction high throughput. Here, we investigated the translation of a high volume magnetic bead-based total RNA extraction method (from human whole blood) onto a low input volume microfluidic device. Our results first show that RNA integrity is maintained when the reagent volumes are scaled down by a factor of 22 and the wash buffers are combined 1:1. With our microfluidic method, the number of wash steps can be reduced from four to one. Thus, the time to complete RNA extraction can be reduced from 2 h to 40 min. These manipulations to the conventional protocol yielded RNA amplifiable within 40 cycles of reverse transcription quantitative PCR (RT-qPCR) when using the microfluidic device to simplify the wash steps. To improve the purification of the RNA during the bead transport through the microchannel, we also investigated the effect of a synergetic application of the electrokinetic flow. Our results show that DNase I and other contaminants surrounding the beads get washed away more effectively via electrophoretic transport. Most notably, RNA adsorption on the beads is strong enough to counter electrophoretically-driven desorption. In all, our work opens new ways to extract high-quality total RNA rapidly and simply from a small quantity of blood, making the process of RNA extraction more accessible.
I. INTRODUCTION
DNA and RNA can be isolated from viruses, circulating tumor cells, and more to assess a patient for various pathologies. The isolation and purification of nucleic acids from a biofluid such as blood or saliva requires several steps and complex equipment. When analyzing multiple peoples' biofluids for research studies or clinical diagnoses, the large number of preparation steps, the need for cold storage, and the lack of low-cost automation1 limit the performance of nucleic acid preparations to well-resourced laboratories. Automated equipment, large numbers of personnel, sufficient quantities of reagents, equipment for cold storage, and more are required for high-quality nucleic acid extraction from multiple samples within a reasonable timeframe. RNA isolation and purification, though, has further challenges as RNA molecules are less stable than DNA, and are degraded by ribonucleases (RNases) that are present on surfaces and in the air.2 Here, we focus on total RNA extraction from white blood cells (WBCs), which requires isolation of white blood cells from whole blood, lysis of white blood cells, DNase treatment to degrade the DNA, and finally, purification of the RNA. To reduce the volume of solutions used, increase throughput, and decrease the amount of time to extract total RNA from cells, this paper shows the significance of using a microfluidic device in combination with magnetic bead-based solid phase extraction for the miniaturization and simplification of RNA purification.
Microfluidic devices allow for miniaturization of processes, which often reduces the volume of reagents and samples required. Additionally, they are portable, can utilize fluid dynamic phenomena for actions like mixing and separation and are automation-friendly.3 All these characteristics make microfluidic devices excellent candidates for simplifying nucleic acid extraction for low-resource settings. However, due to the ubiquitous presence of RNases in the environment, the creation of microfluidic chips for RNA extraction is difficult, which likely has limited their development.4 In addition, whole blood is a complex biofluid that contains inhibitors of downstream nucleic acid analysis techniques, like PCR.5 Thus, there are a few studies that have developed microfluidic workflows starting from whole blood to simplify RNA extraction.
Of the devices that have extracted total RNA from human whole blood on a microfluidic chip, most use a capture matrix.3–11 These methods involve drying of the membrane with a vacuum and/or a pump for constant fluid flow, which increases the volume of reagents and samples necessary for extraction. Magnetic beads are more automation-friendly and require only a magnet to manipulate them (compared to a centrifuge for non-microfluidic capture matrices). One of the few studies that use magnetic beads to extract total RNA from whole blood lysates using microfluidics was done by Jebrail et al., who designed a digital microfluidic device.12 Digital microfluidic devices are automation-friendly and can integrate further analyses methods like reverse transcription and PCR, which helps reduce the need for cold storage of the eluted RNA. With this device, researchers were able to complete the RNA extraction process in one device, but their protocol, which does not include DNase digestion, was tested by our group and the RNA eluate contained significant DNA contamination, lowering the quality of RNA eluted (data not shown). Additionally, digital microfluidics are complex in their design and their method required multiple external pieces of equipment.
Du et al. used magnetic beads for purification in a microfluidic device developed to isolate and fluorescently identify Ebola virus RNA from a spiked whole blood sample.13 This device is great for point of care (POC), but since they focused on hybridizing the RNA of interest to the beads for fluorescence detection, their method did not have to consider the washing away of contaminants in blood lysate to yield RT-PCR-amplifiable RNA. Wash steps will follow the lysing of the cells or viruses, to wash away contaminants from the cells and viruses as well as wash away other reagents used in the purification process. Lysis buffers often contain guanidium salts, wash buffers contain ethanol or isopropanol, and DNase solutions contain DNases, which all inhibit PCR.14,15 Therefore, insufficient removal of buffers used in nucleic acid extraction can affect the integrity of diagnoses and sequencing.
To maximize impact for low-resourced laboratories, minimal laboratory equipment is essential, but many devices, such as those developed for point of care (POC), still use methods that require specialized equipment.16 Here, a method to simplify the washing of RNA extracted from whole blood was developed, without the need for equipment beyond the microfluidic device, pipets, a magnet, and the reagents involved. We also investigated the use of electrophoretic transport for improved purification. Previous work in our group has used electroosmosis and magnetic beads on microfluidic chips for DNA purification.17,18 In the study by Schneider et al., adapter-dimer DNA fragments were being purified out of a solution for downstream sequencing using magnetic beads.17 Deraney et al. purified PCR inhibitors for DNA extraction from plasma.18 Their work showed strong evidence of the utility of electroosmosis in those applications for getting rid of the contaminants of interest, which is why its applicability was investigated in this work. Here, the contaminants differ in terms of electrical charge, type, and amount. Overall, this novel work developed a simple microfluidic-based protocol for RNA purification and used the combination of electrokinetics and magnetic bead-based purification to increase the integrity of RNA following RNA extraction from whole blood.
II. MATERIALS AND METHODS
A. Human whole blood
Human male whole blood was purchased from Golden West Biosolutions (Temecula, California, USA). The blood samples contained either sodium heparin or disodium EDTA anticoagulants. The samples were tested for common infectious diseases by Gold West Biosolutions. All samples were stored at 4 °C.
B. Conventional RNA extraction reagents
Reagents from the Chemagic™ RNA Blood Kit Special H96 (PerkinElmer, Waltham, Massachusetts, USA) were used for RNA extraction from whole blood β-mercaptoethanol (Millipore Sigma, Burlington, Massachusetts, USA) was used to stabilize the RNA, as recommended by the ChemagicTM (tm) RNA Blood Kit Special H96. Millipore Sigma's Red Blood Cell Lysis Buffer (RLB) (Burlington, Massachusetts, USA) was used for cell lysis and isolation for the white blood cells. Instead of a 2:1 ratio of RLB to whole blood, a 3:1 ratio of RLB to whole blood was used for the first red blood cell (RBC) lysis step to lyse the red blood cells more efficiently. Adjusting the ratio of RLB to whole blood was suggested by the manufacturer's protocol if one did not see sufficient lysis, i.e., the resulting solution was not semi-transparent. We found that with a 3:1 ratio, the lysis was more efficient.
C. Determination of RNA yield and integrity
RNA yield and integrity were found using the Agilent Bioanalyzer 2100 and either the Agilent RNA 6000 Pico Kit or the Agilent RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, California, USA). RNA integrity numbers (RINs) were calculated by the Agilent Technologies 2100 Bioanalyzer 2100 Expert software, version B.02.11.SI811.
D. Microfluidic chip fabrication
The microfluidic chip was fabricated using polydimethylsiloxane (PDMS) soft lithography. The microfluidic channels were designed using Fusion360 (version 2.0.11415, Salt Lake City, Utah, USA) and the SU-8 mold was made by FlowJem (Toronto, Ontario, Canada). Following the PDMS curing directions for Sylgard 184, a 10:1 mixture of Sylgard 184 elastomer base and a curing agent from Krayden, Inc. (Denver, Colorado, USA) was made and placed into a vacuum chamber for 30 min to remove air bubbles.
The channel wells of the microfluidic chip were formed by pouring the PDMS into a sandwich mold composed of an SU-8 master mold to form the microfluidic features (150 μm in depth) and a machined aluminum mold to form the wells of the device.15 The two molds are aligned and held together with spring clamps. Uncured PDMS is then poured into the top opening into the gap between both molds, which is 13.4 mm in width. Once cured at 70 °C, the film of PDMS formed in the gap is released from both molds. The wells have been formed by the aluminum mold, but a ∼3 mm length of PDMS occludes them, which is hole-punched out using 3.5 or 1.5 mm diameter hole punchers, according to the respective well diameter. The PDMS film is then bonded to a 75 × 50 mm glass slide. This method allows for more precise well formation when compared to conventional PDMS hole-punching methods. After plasma bonding, the microfluidic chip stayed at 70 °C overnight and then was brought to room temperature for at least 30 min. Depending on the ionic strength of the solutions used in a microfluidic chip, the temperature may play a more significant role in the zeta potential uniformity.19
E. Magnet movement
To remove variability in the magnet's movement when performing experiments, an in-house device was used to standardize magnet motion. The device consists of an x-stage for left–right motion. The stage is a linear screw-drive stage (igus® plastics for longer life®, Providence, Rhode Island, USA) with a stepper motor used to control the stage motion (Applied Motion Products, Inc., Watsonville, California, USA) based on an in-house script. The magnet is a 2 x 1/2 × 1/4 in.3 thick neodymium bar magnet (Grade N42, Item No. BY084), with a surface field of 3424 G, purchased from K & J Magnetics, Inc. (Pipersville, Pennsylvania, USA). To move any magnetic beads that did not move with the (non-optimized) in-house device movement, a bar magnet was used manually.
F. Primers for reverse transcription qPCR (RT-qPCR)
Reverse transcription was performed with the High Capacity cDNA Reverse Transcription Kit from Thermo Fisher Scientific Inc. (Waltham, Massachusetts, USA) and was performed on the day of RNA extraction, and the resulting cDNA was stored at −20 °C. Real-time PCR was performed on the CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, California, USA). TaqMan™ Gene Expression Assay ID# Hs00168719_m1, a primer for the peptidylpropyl isomerase B (PPIB) gene was used, which is a good reference gene for human peripheral blood (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA).20 The TaqMan™ Gene Expression Master Mix (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA) was used for the assay.
G. Human Peripheral Blood Leukocytes Total RNA —RT-qPCR standard curve
Human Peripheral Blood Leukocytes Total RNA (ThermoFisher Scientific Inc., Waltham, Massachusetts, USA) was used to make a standard curve RT-qPCR. The standard curve was not used for quantification, but to ensure integrity of the RT-qPCR reaction.
H. Autodesk® Fusion360™ and COMSOL Multiphysics®—Theoretical modeling
Theoretical modeling was performed using COMSOL Multiphysics® Software (Stockholm, Sweden), version 5.6. Two-dimensional computer-aided design (CAD) models were designed in Fusion360 (Draper, Utah, USA) and imported into COMSOL as the geometry for the simulations.
I. Statistical analyses
Statistical analyses were performed in GraphPad Prism 8 (San Diego, California, USA) or Microsoft Excel (Redmond, Washington, USA) with alpha = 0.05. Statistical analyses were performed in GraphPad Prism 8 (San Diego, CA, USA). *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001. Further information regarding statistical comparisons can be found in the supplementary material.
III. RESULTS AND DISCUSSION
A. Conventional protocol
The extraction of RNA from whole blood requires several biochemical steps, including lysis of cells, separation of DNA and RNA, isolation of RNA, and buffer exchanges. Since the protocols are lengthy and often complex to understand, we designed a visual way to describe the protocol used here in Fig. 1, where all steps and conditions are described in three parts, I, II, and III. In Part I, the red blood cells are lysed, and the white blood cells are isolated following the protocol for Millipore Sigma's Red Blood Cell Lysis Buffer (RLB). About 1.1 × 107 white blood cells (∼5500 per μl) were theoretically expected in this step.21 Once the white blood cells were isolated, reagents from the RNA extraction kit are used to lyse the white blood cells, and β-mercaptoethanol is used to stabilize the RNA. At this point the white blood cell lysate can be stored at −20 °C or used immediately for extraction. In Part II, the RNA and DNA in the white blood cell lysate are bound to magnetic beads using a binding buffer, and the solution is constantly mixed for 5 min via pipetting up and down. The supernatant is removed, and similar mix steps are done for the wash steps. The solution is then degraded by a DNase I solution for 20 min at room temperature. This step removes any DNA contamination, which is a prerequisite for any RNA-based expression profiling. From there, in Part III, the RNA is re-bound to the magnetic beads using a binding buffer, and the RNA–magnetic bead complex is washed. The RNA is eluted using an elution buffer. About 3.2–16 μg of RNA was expected from 2 ml of blood,22 which is a concentration of 16–80 ng/μl in 200 μl of elution buffer. This protocol takes approximately 3 h to complete for one sample.
FIG. 1.
RNA extraction from whole blood protocol. The protocol for red blood cell (RBC) lysis and white blood cell (WBC) isolation used Millipore Sigma's Red Blood Cell Lysis Buffer (RLB) and RNA extraction used the Chemagic™ RNA Blood Kit Special H96. All steps were performed at room temperature.
Following the method in Fig. 1, three human whole blood samples were tested, using disodium EDTA or sodium heparin as anticoagulants. These samples were stored for a variety of times after the blood was drawn: 2, 5, and 8 days, contributing to the standard deviation of the yield measurements. Disodium EDTA and sodium heparin anticoagulated blood yielded average RNA concentrations of 25 and 20 ng/μl, respectively (Table I). The average RNA integrity numbers (RINs) and average Cq values were 4.3 and 30.06, respectively, for disodium EDTA anticoagulated blood. The average RNA integrity numbers (RINs) and average Cq values were 6.7 and 29.01, respectively, for sodium heparin anticoagulated blood. There was no statistically significant difference between the values measured for disodium EDTA vs sodium heparin. In comparison to other methods, RNA integrity for the conventional protocol is similar; the concentration of the samples tested here is 2–3× fold higher than other methods.23
TABLE I.
The conventional protocol was performed on three disodium EDTA and sodium heparin anticoagulated human whole blood. Using a t-test assuming unequal variance, the results of average concentration, average RIN, and average Cq value for three samples were compared between disodium EDTA and sodium heparin anticoagulants. There was no significant difference (p > 0.05) between any of the aforementioned values between the two anticoagulants. The volume of the reagents in the conventional protocol was scaled down by a factor of 22, and a separate experiment mixed the two wash buffers 1:1 to reduce the number of wash steps. Using the conventional protocol as a reference group, all measured values of the adjusted protocols were compared to that of the reference group and were not statistically significant. Note: For the “1/22 scale down” concentration values in the elution buffer—This is not the actual yield of the 1/22 scale down protocol, but for comparison purposes, the measured value was multiplied by 22.
| Anticoagulant | Protocol | Average RNA yield (ng/μl elution buffer) ± standard deviation | Average actual yield (ng/μl blood) | Expected yield (ng/μl blood) | Average RIN ± standard deviation | Average Cq value ± standard deviation |
|---|---|---|---|---|---|---|
| Disodium EDTA | Conventional protocol | 25 ± 7.21 | 2.5 | 4.3 ± 3.0 | 30.06 ± 4.43 | |
| 1/22 scale down | 54.05 ± 24.42 | 0.54 | 3.4 ± 0.5 | 29.60 ± 0.85 | ||
| 1:1 Wash buffer mix | 26.22 ± 4.00 | 2.6 | 1.6–8 | 4.7 ± 1.9 | 26.49 ± 1.16 | |
| Sodium heparin | Conventional protocol | 20 ± 5.67 | 2 | 6.7 ± 1.6 | 29.01 ± 2.97 | |
| 1/22 scale down | 36.66 ± 15.02 | 0.37 | 3.6 ± 0.7 | 30.25 ± 1.54 | ||
| 1:1 Wash buffer mix | 31.83 ± 14.90 | 3.2 | 4.6 ± 0.6 | 26.78 ± 0.95 |
B. Scale down of Part I of the conventional protocol
Reducing the starting volume of the blood or the white blood cell lysate and scaling down the subsequent reagents linearly was essential for future microfluidic protocol development. Part I of the conventional protocol (Fig. 1) involves centrifuging 2 ml of blood and lysing the red blood cells to isolate the white blood cells. Thus, two methods of reducing the volume of the reagents in these steps were investigated. The first was reducing the starting volume of blood (from 2 ml to 500, 250, 100, or 50 μl) and the second was reducing the starting volume of the isolated white blood cells, lysis buffer, and β-mercaptoethanol (from 625 to 156, 78, 39, or 28.4 μl). The rest of the reagents were scaled down linearly with the starting sample. At the two highest volume reductions, reducing the starting volume of blood resulted in a lower concentration and RIN (measured using the Agilent Bioanalyzer 2100) than by reducing the volume of the white blood cell mixture. However, at the two lowest volume reductions, the concentration and RIN were similar between the two methods (data not shown).
We decided to move forward with the second method to sample from the white blood cell lysate from Part I of the protocolto reduce the sample preparation time for future experiments. Using this method, the starting volume of blood is always 2 ml and multiple small aliquots (28.4 μl) of the white blood cell lysate can be used for multiple experiments and analyses. When using the first method we investigated, many individual replicates of the white blood cell lysate would have needed to be prepared, while the second requires storing one large solution of white blood cell lysate that can be sampled from multiple times. Either method would be sufficient for reducing the volume of the conventional protocol. However, when isolating RNA from samples with lower white blood cell counts, the first method using lower volumes of blood may not have sufficient yield of less concentrated RNA.
C. Scale down of Parts II and III of the conventional protocol and combining wash buffers
The lowest volume tested used 1/22 of the 625 μl white blood cell lysate mixture, which is a starting volume of 28.4 μl after performing Part I of the conventional protocol. Therefore, the volumes of the reagents in Parts II and III of the conventional protocol were scaled down by a factor of 22. The scaled-down elution volume of 9 μl was multiplied by 2.2 for a final 20 μl elution volume to ensure sufficient volume for pipetting and downstream analyses. These experiments were done with five-day old blood. Additionally, in a separate experiment, to investigate the possibility of reducing the number of wash steps, wash buffers No. 3 and No. 4 were mixed 1:1. These experiments were done with two-day old blood, with two common anticoagulants: disodium EDTA and sodium heparin. We used two types of anticoagulated blood for investigational purposes, not for comparison. The results for average concentration (in elution buffer), average RIN, and average Cq are shown in Table I. The results for the altered protocols were compared to the conventional protocol, and no statistically significant difference was found between them. The actual yield per μl of blood for the scale downs is lower than the expected yield, which could be due to the age of the blood and lower volumes being more sensitive to measurements since the scaled-down concentration was found using the RNA Pico Kit. However, since the Cq and RIN values were comparable to the conventional protocol, we concluded that reducing the volume of reagents and combining the wash buffers into one wash buffer is acceptable to translate onto the microfluidic chip.
D. The microfluidic separator
Previous work in our group led to the development of a microfluidic device that integrates magnetic separation and electrokinetic purification (MSEP) of DNA.15–17 These microfluidic devices were developed to simplify the washing and purification steps of the DNA extraction process and are made of a PDMS mold bonded to a glass slide. The device operates by first placing a wash solution into the primary wash buffer loading well, which flows throughout the wash channel to the secondary wash buffer loading well [green, Figs. 2(a) and 2(b)]. Then, the sample, which contains nucleic acids, is pipetted into the sample well simultaneously with the elution well solution (a wash buffer or an elution buffer) [yellow, Fig. 2(c)]. Our prior work used a purified DNA sample17 or diluted whole blood as the starting sample.15 A magnet is then placed beneath the separator to transport the magnetic beads through the wash solution in the Wash Channel, washing the beads. This movement is repeated until all beads have been transported to the elution well, and the solution from the elution well is removed from the microfluidic separator simulatenous with the sample well's contents, which are discarded [Figs. 2(d) and 2(e)]. The elution well's contents are then used for downstream processing. The addition and removal of the sample well and elution wells' contents must be done simultaneously such that they do not flow into the elution well. Multiple separators are designed to be on one microfluidic chip, with six in parallel and in two rows (for a total of 12 separators), with each separator being 9 mm apart such that multiple purifications can be performed simultaneously with a multi-channel pipettor and a long magnet.
FIG. 2.
Solution loading and dimensions of the microfluidic separator. (a) and (b) A wash buffer loaded into the primary wash buffer loading well, which flows throughout the wash channel into the secondary wash buffer loading well. This takes approximately 20 s. (c) A solution with nucleic acids is pipetted into the sample well (top, yellow) at the same time as either a wash buffer or elution buffer into the Elution Well (bottom, blue). (d) and (e) Then, using a magnet placed beneath the microfluidic separator, the magnetic beads are transported from the sample well to the elution well, through the wash buffer in the wash channel. (d) and (e) are repeated until all magnetic beads have moved to the elution well, which takes 2–3 min. (f) The dimensions of a microfluidic separator.
For RNA purification, which is investigated here, several changes were made to the microfluidic separator design used by Lee et al. First, the number of beads used in the RNA extraction protocol compared to the DNA extraction protocol used by Lee et al. is 4.25× higher, so the width of the narrowest section of the wash channel and the width of the entrance point of the sample and elution wells were increased to be 4.25× larger. This helped reduce the possibility of the magnetic beads getting stuck and unable to move through the separator and to keep the time to move the magnetic beads through the entire separator similar to that of the DNA extraction protocol. Next, the width of the entrance to the elution well was made to be the same as that of the sample well (instead of smaller) to ensure the magnetic beads could efficiently enter the elution well. The secondary wash buffer loading channel was made to be twice the width of the primary one because with a previous iteration of this RNA microfluidic separator, the wash solution would not flow from the primary wash buffer loading well to the secondary wash buffer loading well but would stop once the wash channel was filled. To reduce the hydrodynamic resistance in the secondary wash buffer loading well, the width of the channel was doubled, which did cause the wash channel and wells to be filled as desired. Finally, the height of the sample and elution wells (and subsequently the wash buffer loading wells) was increased via increasing the depth of the aluminum mold used to make the microfluidic chip, such that 100 μl of solution could fit into the Sample and Elution Wells.
E. Investigation of a reduced number of wash steps and hands-on time from the conventional protocol
Parts II and III of the conventional protocol have ∼25 min of hands-on washing steps each. The microfluidic separator was designed to simplify washing of magnetic beads; therefore, we investigated how the number of steps could be reduced for microfluidic purification. The blood samples here were from two different 2 ml aliquots from the same donor and were two days old. The controls (following the 1/22 scale down protocol) had an average Cq value of 26.88 for disodium EDTA anticoagulated blood and 28.08 for sodium heparin anticoagulated blood. There was no statistically significant difference between the two average Cq values of each anticoagulant.
To first test a reduction of the number of wash steps, all the wash steps were removed from the 1/22-scaled-down protocol (termed “No washes”), and the resulting products' Cq values were measured. Without the wash steps, the average Cq value for disodium EDTA anticoagulated blood was 34.05, and that of sodium heparin anticoagulated blood was 34.90 (Fig. 3), which were both higher than their respective control protocols. Additionally, out of six disodium EDTA replicates, only three amplified; for sodium heparin, two out of the six amplified. The inconsistency in amplification via RT-qPCR indicates that removing all the wash steps is not repeatable and that some of the unwashed species inhibit PCR.
FIG. 3.
Cq values of trials with the removal of wash steps at different stages in the 1/22-scaled-down protocol using disodium EDTA and sodium heparin anticoagulated blood. Four samples were tested for the control protocol and six samples were tested for the experimental protocols. The values in parentheses are the number of samples that amplified within 40 cyles of RT-qPCR (numerator) over the total number of samples analyzed (denominator). All samples were generated following the 1/22-scaled-down volumes. “Control” refers to trials following the 1/22-scaled-down protocol. “No washes” refers to trials in which none of the wash steps were performed. “No wash 2” followed the 1/22-scaled-down protocol and also used a 1:1 mixture of the wash buffers for the wash steps in Part II, and only skips the wash steps in Part III, after DNase I treatment. Finally, “No mixing” refers to all mixing steps being replaced with 5-min incubations. To compare the mean Cq value of the trials to the positive controls, one-way ANOVA and Dunnett's multiple comparisons test were performed. (a) Disodium EDTA anticoagulated blood. (b) Sodium heparin anticoagulated blood.
We hypothesized that DNase I was essential to being washed off the magnetic beads for successful qPCR. To test this, the 1/22-scaled-down protocol was performed but without the wash steps after the DNase digestion (termed “No wash 2”). This trial was performed with the 1:1 mixture of wash buffers in place of two separate wash steps. The disodium EDTA samples had an average Cq value of 33.86, and the sodium heparin anticoagulated samples had an average Cq value of 37.61, which were statistically significantly higher than their respective controls. Four of the six disodium EDTA samples amplified, and one of the six sodium heparin samples amplified, leading to a similar conclusion to that of the “No washes” samples, where skipping the wash steps after the DNase I digestion are not repeatable.
Finally, to further reduce the number of hands-on steps for the microfluidic protocol, the need for the 5-min constant mixing after every reagent addition was investigated. For this experiment, the 1/22-scaled-down protocol was performed without any mixing where the conventional protocol indicates to do so. Instead, the sample was incubated for 5 min at room temperature during the indicated mixing times. For the disodium EDTA anticoagulated blood, the average Cq value was 33.14, and that of sodium heparin was 29.54. There was no statistically significant difference between no mixing with sodium heparin anticoagulated blood and the positive control; there was a statistically significant difference between no mixing with disodium EDTA anticoagulated blood and the positive control. Sodium heparin and disodium EDTA have both been shown to affect PCR.24 Disodium EDTA chelates Mg2+ ions, while sodium heparin is thought to directly interact with nucleic acids.25 This could explain the more significant difference between the sodium heparin protocol variations and their corresponding control since RNA amplification may be more affected by the presence of heparin in the absence of washes.
To summarize, all of the conventional protocol variations tested showed that (1) the scaled-down volume of 1/22 and combining the wash steps into one 1:1 mixture had comparable Cq values and RINs to the conventional protocol, (2) washing of the RNA–magnetic bead complex after the DNase digestion was an essential step for repeatable results, and (3) the manual mixing steps could be replaced with room temperature incubations. DNase I is a PCR inhibitor since it degrades DNA, and this shows that DNase I must be sufficiently washed away for the resulting RNA sample to be RT-qPCR amplifiable. Taken together, these results allowed us to reduce the starting volume of the white blood cell lysate, reduce the volumes of other reagents used, combine the wash buffers, and reduce the amount of hands-on time needed by the researcher to perform RNA extraction. These are significant steps toward simplifying the RNA extraction process, and reducing the volume of reagents implies a reduction in cost to do RNA extraction. A detailed analysis into conventional protocol reduction has rarely been described in the literature, which is important for not only microfluidic translation but also in understanding essential steps in RNA extraction and purification.
F. The microfluidic separator—Solution loading procedure for RNA purification
We first tested the microfluidic separator to replace the wash steps in Part II. The starting sample at this stage contained white blood cell lysate, DNA–RNA-bound magnetic beads and binding buffer, and the 1:1 mix of the wash buffers was in the wash channel and elution well. We found that due to the high nucleic acid content of the sample, the magnetic beads were too difficult to transport from the sample well to the elution well. This is because both DNA and RNA are on the beads, and they have strong bonds between them, which cannot be broken up by the magnetic pull on the magnetic beads.15 Thus, our focus shifted to using the microfluidic separator for the wash steps in Part III, after the DNAse I digestion and re-binding of the RNA to the magnetic beads.
Using the insight from the conventional protocol's variations, the finalized microfluidic protocol is shown in Fig. 4. Part I remains the same between the conventional protocol and the microfluidic protocol. 28.4 μl of the solution from Part I was mixed with 3.4 μl of magnetic beads and 41 μl binding buffer No. 2 and incubated for 5 min at room temperature. The supernatant was removed and 27.4 μl of DNase I solution was added to the magnetic beads. The wash steps in Part II of the conventional protocol were not performed. Then, 41 μl of binding buffer No. 2 was added to the DNase I/beads solution and incubated for 5 min at room temperature. ∼50 μl of a 1:1 mixture of wash buffers 3 and 4 were added to the wash channel. Then, the DNase I/beads/binding buffer No. 2 solution (72 μl) was added to the sample well of a microfluidic separator simultaneously with a 1:1 mixture of the two wash buffers added to the elution well. The microfluidic separator was then used to clean the RNA–magnetic bead complex. The magnetic beads were moved from the sample well to the elution well until all magnetic beads are transported to the elution well, which was verified by visual inspection. Then, the solution from the elution well was placed into a tube, and the supernatant was removed. 20 μl of the elution buffer was added to the RNA–magnetic bead complex and incubated for 5 min. The supernatant contains the isolated total RNA. The 1:1 wash buffer mix was prepared fresh daily due to the uncertainty around the stability of that solution long-term. The volume of 85 μl was removed from the sample well (a larger volume than was input) to ensure all the contents of that well were removed from the separator such that none flows into the elution well. If the sample well contents flow into the elution well, the RNA did not amplify in RT-qPCR, due to the presence of DNase I (data not shown).
FIG. 4.
Procedure for solution loading for microfluidic washing of total RNA after DNase I digestion. Part I remains the same as the conventional protocol. In Part II, the wash steps are skipped compared to the conventional protocol, and the solutions are incubated where indicated instead of mixed. In Part III, the microfluidic chip is used to perform the wash steps, and the elution is performed off-chip.
G. Can the microfluidic separator replace the post-DNase I treatment wash steps?
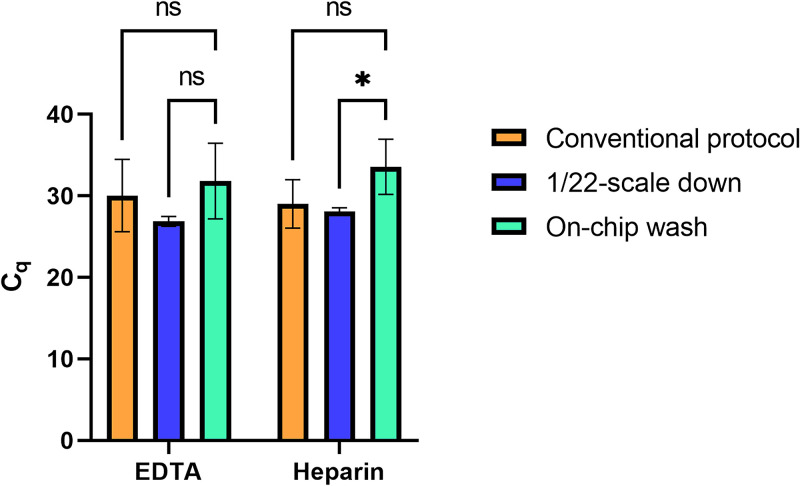
Following the steps in Fig. 4, we tested if the microfluidic separator was sufficient to perform the wash after the DNase I digestion and re-binding of the RNA to the beads (Fig. 5). The average Cq value for disodium EDTA anticoagulated samples was 31.82 and that of sodium heparin anticoagulated samples was 33.57. In this experiment, all six samples tested amplified, indicating repeatability and that the microfluidic separator was sufficient to wash away any PCR inhibitors, including DNase I. Additionally, there was no significant difference between using the microfluidic separator for washing compared to the conventional protocol or the 1/22-scaled-down protocol. “On-chip wash” does have more variability in Cq value than the control, which is likely due to the slight differences between separators and their formation. “On-chip wash” was performed on an earlier iteration of the microfluidic chip in which the elution well was a smaller diameter than the sample well (instead of being the same diameter), which made removing solutions from it via pipetting slightly challenging. This issue was solved by making the elution well's diameter larger and the variability between microfluidic separators was reduced as seen in subsequent results.
FIG. 5.
On-chip washing vs the conventional protocol and the 1/22-scale down. “On-chip wash” uses the volumes of the 1/22-scale down, skips the wash step in Part II, and the washes for Part III are combined and performed on the microfluidic chip (Fig. 4). To compare the mean Cq value of the trials to that of the 1/22-scale down and the conventional protocol, two-way ANOVA was performed, with Tukey's multiple comparisons test.
H. Electrophoretic applications to the microfluidic separator for increased purification
Building upon previous work in our group, we sought to investigate the use of electrokinetics to improve purification of the RNA samples. Here, when referring to electrokinetics, we are referring to both electroosmotic flow and electrophoretic transport. The total velocity of the solute would be the sum of the electroosmotic and the electrophoretic velocities,
Electroosmotic flow is the bulk flow of fluid in the presence of an electric field. For this study, the glass slide to which the PDMS microfluidic chip is bonded is negatively charged, causing the positive charges of the fluid to be attracted to the glass slide creating an electric double layer.26 Thus, along the surface of the glass slide, in the presence of an electric field, the bulk flow of the fluid is toward the negative electrode due to the positive charges of the fluid. Electrophoresis is the movement of a solute in response to an electric field, depending upon the charge of the solute. Cations will move toward a negative electrode, and anions will move toward a positive electrode.27 Schneider et al. and Deraney et al.17,18 found that electroosmotic flow improved the purity of the magnetic bead-bound DNA samples they were purifying because contaminants in the bulk solution would flow out of the wash channel into the wash buffer loading well that contained the negative electrode.
Here, there are multiple contaminants in the solution that surrounds the beads, which are positively and negatively charged and will reduce RNA purity. The solutes are likely the DNase I enzyme, binding buffer salts, and fragmented genomic DNA. DNase I also requires positive ions such as Mg2+ and Ca2+ to degrade DNA.28 The magnetic beads were dragged through the microchannel and the fluid drag on beads separate any unbound DNase, salts, or fragmented genomic DNA. Fluid viscosity, bead aggregate radius, and bead velocity are denoted by η, R, and , respectively. However, the no-slip condition on the bead aggregate is less likely to separate the unbound species close to bead surfaces. Hence, electric field driven electrokinetic flows were investigated to effectively separate unbound species next to beads. More specifically, we were interested in exploiting electrophoretic transport as this would theoretically remove both positively and negatively charged contaminants from the wash channel.
I. Experimental investigation of electrophoresis for improving RNA purification on the microfluidic separator
Similar to our prior work, we applied an electric field onto the microfluidic separator via placing electrodes into the primary and secondary wash buffer loading wells to induce electrophoretic and electroosmotic velocities. To obtain uniform zeta potential between different microfluidic chips, it was essential for the microfluidic chip to not be used until it sat overnight following plasma bonding between the PDMS and glass slide. A negative electrode was placed into the primary wash buffer loading well, and a positive electrode was placed into the secondary one, causing the electroosmotic velocity to be in the opposite direction as magnetic bead motion and electrophoretic transport to occur according to the respective electrode (i.e., negatively charged contaminants flow toward the positive electrode and vice versa) [Fig. 6(a)]. Electroosmotic flow in the opposite direction of the bead motion would mean that the bulk flow of the fluid and contaminants would be away from the elution well to aid in purifying the sample. Electrophoretic transport toward both electrodes would mean that both positively and negatively charged contaminants are flowing out of the wash channel to the wash buffer loading wells.
FIG. 6.
Cq values, concentrations, and RINs of eluted RNA when voltages were applied in the microfluidic separator in the positive configuration using sodium heparin anticoagulated blood. (a) The positive configuration of the electric potential, shown with 300 V. (b) Six replicates were tested for the off-chip. Four replicates were tested for 0, 50, and 300 V. Five replicates were tested for 150 V. To compare the mean Cq value of the trials to that of the off-chip protocol, one-way ANOVA was performed, with Dunnett's multiple comparisons test. (c) To compare the mean concentration of off-chip to the mean concentrations of 0, 50, 150, and 300 V one-way ANOVA was performed, with Dunnett's multiple comparisons test. One replicate did not yield a value for RIN for both 150 and 300 V; therefore, the concentration values for those samples were excluded.
We compared the use of the electric field to an off-chip sample that followed the microfluidic protocol (Fig. 4), but instead of using the microfluidic chip in Part III, the wash was performed in a tube. We tested off-chip (average Cq = 27.28) and compared them to 0 V (average Cq = 27.12), 50 V (average Cq = 26.31), 150 V (average Cq = 27.35), and 300 V (average Cq = 25.62). There was no significant difference between the average Cq values of any of the tested conditions vs off-chip [Fig. 6(b)].
Cq values are a function of both RNA concentration and RNA sample quality, i.e., the presence of inhibitors or degradation of RNA. Therefore, to further characterize the RNA extracted, capillary gel electrophoresis was performed using the Agilent Bioanalyzer [Fig. 6(c)]. In this analysis, each sample's replicate was measured three times with the Bioanalyzer, and those values were averaged. Then each replicate's measurements were averaged. The Bioanalyzer measures concentration and an RNA integrity number (RIN). The concentration values were highly variable with the Bioanalyzer measurements, but the yields of 0, 50, 150, and 300 V were not statistically significantly different than that of the off-chip. The lack of statistical difference is likely due to the high variability in the off-chip concentration measurements. The off-chip concentration measurements ranged from 871 pg/μl (RIN of 4) to 14 001 pg/μl (RIN of 2.1), and the median was 1,351 pg/μl (Table ST1 in the supplementary material). With RIN, there was a statistically significant difference between off-chip and 150 V, and off-chip and 300 V, with the RIN of 150 and 300 V being higher than that of off-chip.
Since Cq values depend on a combination of purity and concentration, the combination of the RNA concentration and purity values in Fig. 6 explains why Cq does not show differences in purity. The average off-chip concentration was higher than that of any of the on-chip samples, and the average RIN for the on-chip samples were higher than those of the off-chip samples. Thus, this results in similar Cq values for all samples. A study by White et al.29 showed some correlation between RIN and Cq value, with Cq values being lower with higher RIN. However, it has been shown that amplification of transcripts can happen despite RNA degradation.30
We tested a reversal of the electrodes, termed the negative configuration, to ensure electrophoretic transport was dominating over electroosmotic flow. If electroosmotic velocity was dominating, we would see a reduction of purity of the RNA samples because the contaminants would flow in the direction of the elution well,18 which we did not see (Fig. S1 in the supplementary material). We also tested disodium EDTA anticoagulated blood in the positive configuration and had similar results for the Cq values comparisons (Fig. S2 in the supplementary material). For the Bioanalyzer results, however, the RIN values could not be measured reliably, likely due to the concentration of EDTA being higher than the recommended limits for the RNA Pico 6000 Kit. These results are in Table ST2 in the supplementary material. The electropherogram traces for disodium EDTA samples were similar to that of the sodium heparin ones; thus, the threshold for the RIN measurements could be adjusted to estimate RIN values, but with less confidence.
Overall, these results show that electrophoretic transport increased purity of the RNA extracted using the microfluidic separator. The higher the voltage the better the purity, i.e. the RIN of the sample increases. Even in the absence of electrokinetics, 0 V appeared to improve purity of the RNA (though not statistically significant) indicating that the microfluidic separator alone may clean the sample better than the off-chip protocol. This could be due to the differing geometries between an Eppendorf tube and the microfluidic wash channel, where the channel is more spread out allowing for the magnetic beads to experience advection more strongly. On the other hand, all samples tested on the microfluidic separator had a lower concentration than when using a tube off-chip. This demonstrates the importance of treating the microfluidic device with a solution to eliminate RNases (which was not done here), since the concentration of RNA purified on the microfluidic device was lower than that of the off-chip. The lower concentration could also be due to the adsorption of RNA to the walls of the PDMS or glass of the microfluidic chip.31 Finally, since the concentration was not significantly reduced between the voltages tested, this shows that the RNA remained bound to the magnetic beads despite the effects of electrophoresis present in the microfluidic separator.
J. Mechanistic understanding of the flow of unbound species in the microfluidic channel
To validate our experimental results, we mathematically modeled the electric field on the microfluidic separator using COMSOL Multiphysics to observe the effect of electrokinetic velocities. For the wash channel, the solution chosen for modeling was ethanol, which is likely a primary component of the proprietary wash buffer used experimentally. The solute was chosen to be DNA fragments of 150 bp in size with a large negative charge, though experimentally the solutes are much more complex than that. Three modules were used in COMSOL: electric currents, laminar flow, and transport of diluted species, to solve for the electric field, electroosmotic flow, and the convection, diffusion, and electrophoresis of charged species, respectively. For further details regarding the model, see the supplementary material. Figure 7 shows the concentration of the solute at 3 min of the simulation time for 150 and 300 V with zi = −9000, where zi is the charge number of the ionic species (dimensionless). The electroosmotic velocity, electrophoretic flux, and electric field for 150 and 300 V are shown in Figs. S3 and S4 in the supplementary material. This shows that electrophoresis is what dominates the velocity of the solutes since the solutions (in red) are moving toward the positive electrode instead of the negative one. If the solution has a positive charge of the same magnitude (zi = 9000), the same profile is seen but toward the negative electrode (Fig. S5 in the supplementary material). Electroosmotic velocity is also occurring (Fig. S3 in the supplementary material), though it is not greater than electrophoretic velocity as evidenced by the concentration profile showing electrophretic flow despite increased electroosmotic velocity with increasing voltage.
FIG. 7.
Movement of negatively charged solutes' concentration boundary when 150 and 300 V are applied for 3 min and a depiction of electroosmotic and electrophoretic velocities. Top: the red color indicates the solute concentration (set as 0.001 mol/m3) and the boundary can be seen moving toward the secondary wash buffer loading well due to electrophoresis. Bottom: Electroosmotic velocity and electrophoretic velocity in this microfluidic separator are in opposition for negatively charged molecules but not for positively charged molecules. The magnitudes of the velocities are depicted by the arrow thickness and length, based on the experimental results. (a) 150 V is shown. (b) 300 V is shown.
Modeling in COMSOL aided in understanding the movement of the unbound species in the wash channel of the microfluidic separator. This showed that in the positive configuration, negatively charged ions move toward the bottom of the chip due to electrophoresis, while the positive ions travel upward due to electroosmosis and electrophoresis.
IV. CONCLUSIONS
High-quality total RNA extraction is essential for various downstream applications like transcriptomic or viral load analyses. Our aim was to investigate ways to shorten the total RNA extraction process while yielding high-quality RNA. Compared to the conventional protocol, the microfluidic protocol reduced the starting blood volume from 2 ml to 91 μl, reduced the number of wash steps from 4 to 1, reduced the total volume of reagents from ∼5500 to ∼400 μl, and eliminated the manual mixing steps. This reduction allows for a lower volume of both reagents and blood sample, which is beneficial to both the patient and researchers. A lower volume of reagents lowers the cost and reduces the physical space needed for the storage of these reagents. Additionally, all the reagents are stored at room temperature except for DNase I (which can be lyophilized and rehydrated), expanding access to performing RNA extraction in a variety of environments. The use of a microfluidic design and magnetic beads for RNA extraction allows for automation of this process and for multiple RNA extractions to be performed in parallel. For example, performing the extractions in parallel was possible with this microfluidic design and was done in the 0 V experiments, which made the extraction process significantly faster. Compared to other magnetic bead-based microfluidic devices developed for RNA extraction and purification, our method uses minimal equipment and does not require the use of electricity or fluid pumping, which greatly simplifies this process.
COMSOL modeling showed that electrophoresis dominates in the microfluidic wash channel, which means that this microfluidic design introduces a novel way to minimize electroosmotic velocity in the absence of additives like coatings. Electrophoretic velocity further purified positively and negatively charged contaminants while maintaining the eluted RNA concentration at higher voltages. Both negatively charged genomic DNA fragments and positively charged salts needed to be washed away to maximize the purity of eluted RNA, and this microfluidic separator was able to wash away both types of charged contaminants, with and without an applied voltage. Thus, we showed that a microfluidic device was essential for the success of lower volume protocols, since the modifications made to the conventional protocol were best performed on the microfluidic chip.
A limitation of this study is that the gene used for qPCR amplification was one that was abundant in white blood cells. Future studies should investigate the purification of a less abundant gene, perhaps with a virus such as human immunodeficiency virus (HIV) RNA in plasma. We did not alter the concentrations of buffers relative to each other, which would be a good study to investigate more ways to increase RNA yield. Future work should incorporate RNA amplification and detection platforms to the microfluidic protocol for the POC to reduce the need for RNA storage. To improve the precision and accuracy of the PDMS's formation, alterations to the SU-8 can be made to align the aluminum mold more precisely with it, improving the accuracy of the intended location of the well formations. The parameters of the microfluidic design as well as surface modifications can be investigated to further explore the role of electrokinetic forces on the purification of RNA. Overall, this study provided an in-depth insight and understanding of the essential steps of RNA purification protocols. The RNA purification process was greatly simplified, which will aid in expanding access to RNA purification to lower resourced laboratories and eventually to the point of care.
SUPPLEMENTARY MATERIAL
The supplementary material as referenced throughout the manuscript is found here. This includes further information about the statistical analyses, Cq values and Bioanalyzer results for disodium EDTA anticoagulated blood in the positive configuration, results for sodium heparin anticoagulated blood in the negative configuration, COMSOL model development, and additional COMSOL model results.
ACKNOWLEDGMENTS
K.L. would like to acknowledge the National Science Foundation Graduate Research Fellowship and the GEM Fellowship for graduate student support. K.L. also received the First-Year Graduate School Fellowship and Brown's Initiative to Maximize Student Development (IMSD) Fellowship (NIGMS Grant No. R25GM083270). This work was in part supported by Perkin Elmer's research grant to Brown University. K.L. and A.T. would also like to thank Tim Weissbach for his insight in the early stages of this project.
AUTHOR DECLARATIONS
Conflict of Interest
A.T. is a paid scientific advisor/consultant and lecturer for PerkinElmer. K.L. has no conflicts to disclose.
Author Contributions
Kiara Lee: Conceptualization (equal); Data curation (lead); Formal analysis (equal); Funding acquisition (supporting); Investigation (equal); Methodology (equal); Validation (lead); Visualization (lead); Writing – original draft (lead); Writing – review & editing (lead). Anubhav Tripathi: Conceptualization (equal); Funding acquisition (lead); Project administration (lead); Supervision (lead); Writing – review & editing (supporting).
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.32
REFERENCES
- 1.Thatcher S. A., “DNA/RNA preparation for molecular detection,” Clin. Chem. 61(1), 89–99 (2015). 10.1373/clinchem.2014.221374 [DOI] [PubMed] [Google Scholar]
- 2.Pasloske B. L., “Ribonuclease inhibitors,” in Nuclease Methods and Protocols, 160th ed., edited by Schein C. H. (Totowa Humana Press Inc., 2001), pp. 105–111. [Google Scholar]
- 3.Fan A., Byrnes S., and Klapperich C., “Purification of DNA/RNA in a microfluidic device,” Methods Mol. Biol. 949, 403–411 (2013). 10.1007/978-1-62703-134-9_25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Root B. E., Agarwal A. K., Kelso D. M., and Barron A. E., “Purification of HIV RNA from serum using a polymer capture matrix in a microfluidic device,” Anal. Chem. 83(3), 982–988 (2011). 10.1021/ac102736g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akane A., Matsubara K., Nakamura H., Takahashi S., and Kimura K., “Identification of the heme compound copurified with deoxyribonucleic acid (DNA) from bloodstains, a major inhibitor of polymerase chain reaction (PCR) amplification,” J. Forensic Sci. 39(2), 362–372 (1994). 10.1520/JFS13607J [DOI] [PubMed] [Google Scholar]
- 6.Kolluri N. et al. , “SNAPflex: A paper-and-plastic device for instrument-free RNA and DNA extraction from whole blood,” Lab Chip 20, 3386 (2020). 10.1039/D0LC00277A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kokoris M. et al. , “Rare cancer cell analyzer for whole blood applications: Automated nucleic acid purification in a microfluidic disposable card,” Methods 37(1), 114–119 (2005). 10.1016/j.ymeth.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 8.Mauk M. G., Liu C., Sadik M., and Bau H. H., “Microfluidic devices for nucleic acid (NA) isolation, isothermal NA amplification, and real-time detection,” Methods Mol. Biol. 1256, 15–40 (2015). 10.1007/978-1-4939-2172-0_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price C. W., Leslie D. C., and Landers J. P., “Nucleic acid extraction techniques and application to the microchip,” Lab Chip 9(17), 2484 (2009). 10.1039/b907652m [DOI] [PubMed] [Google Scholar]
- 10.Wen J., Legendre L. A., Bienvenue J. M., and Landers J. P., “Purification of nucleic acids in microfluidic devices,” Anal. Chem. 80(17), 6472–6479 (2008). 10.1021/ac8014998 [DOI] [PubMed] [Google Scholar]
- 11.Witek M. A., Hupert M. L., Park D. S. W., Fears K., Murphy M. C., and Soper S. A., “96-well polycarbonate-based microfluidic titer plate for high-throughput purification of DNA and RNA,” Anal. Chem. 80(9), 3483–3491 (2008). 10.1021/ac8002352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jebrail M. J. et al. , “World-to-digital-microfluidic interface enabling extraction and purification of RNA from human whole blood,” Anal. Chem. 86(8), 3856–3862 (2014). 10.1021/ac404085p [DOI] [PubMed] [Google Scholar]
- 13.Du K. et al. , “Microfluidic system for detection of viral RNA in blood using a barcode fluorescence reporter and a photocleavable capture probe,” Anal. Chem. 89(22), 12433 (2017). 10.1021/acs.analchem.7b03527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sur K. et al. , “Immiscible phase nucleic acid purification eliminates PCR inhibitors with a single pass of paramagnetic particles through a hydrophobic liquid,” J. Mol. Diagn. 12(5), 620 (2010). 10.2353/jmoldx.2010.090190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee K. and Tripathi A., “Parallel DNA extraction from whole blood for rapid sample generation in genetic epidemiological studies,” Front. Genet. 11, 374 (2020). 10.3389/fgene.2020.00374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang P. and Kricka L. J., “Current and emerging trends in point-of-care technology and strategies for clinical validation and implementation,” Clin. Chem. 64(10), 1439–1452 (2018). 10.1373/clinchem.2018.287052 [DOI] [PubMed] [Google Scholar]
- 17.Schneider L., Cui F., and Tripathi A., “Isolation of target DNA using synergistic magnetic bead transport and electrokinetic flow,” Biomicrofluidics 15(2), 024104 (2021). 10.1063/5.0045307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deraney R. N., Schneider L., and Tripathi A., “Synergistic use of electroosmotic flow and magnetic forces for nucleic acid extraction,” Analyst 145(6), 2412–2419 (2020). 10.1039/C9AN02191D [DOI] [PubMed] [Google Scholar]
- 19.Al Mahrouqi D., Vinogradov J., and Jackson M. D., “Temperature dependence of the zeta potential in intact natural carbonates,” Geophys. Res. Lett. 43(22), 11578, 10.1002/2016GL071151 (2016). 10.1002/2016GL071151 [DOI] [Google Scholar]
- 20.Pachot A., Blond J. L., Mougin B., and Miossec P., “Peptidylpropyl isomerase B (PPIB): A suitable reference gene for mRNA quantification in peripheral whole blood,” J. Biotechnol. 114(1–2), 121–124 (2004). 10.1016/j.jbiotec.2004.07.001 [DOI] [PubMed] [Google Scholar]
- 21.Blumenreich M. S., The White Blood Cell and Differential Count (Butterworths, 1990). [PubMed] [Google Scholar]
- 22.See https://www.qiagen.com/us/resources/faq?id=06a192c2-e72d-42e8-9b40-3171e1eb4cb8&lang=en for “How much RNA does a typical mammalian cell contain?—QIAGEN.”
- 23.Schwochow D., Serieys L. E. K., Wayne R. K., and Thalmann O., “Efficient recovery of whole blood RNA—A comparison of commercial RNA extraction protocols for high-throughput applications in wildlife species,” BMC Biotechnol. 12, 33 (2012). 10.1186/1472-6750-12-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huggett J. F. et al. , “Differential susceptibility of PCR reactions to inhibitors: An important and unrecognised phenomenon,” BMC Res. Notes 1, 70 (2008). 10.1186/1756-0500-1-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrader C., Schielke A., Ellerbroek L., and Johne R., “PCR inhibitors—Occurrence, properties and removal,” J. Appl. Microbiol. 113(5), 1014–1026 (2012). 10.1111/j.1365-2672.2012.05384.x [DOI] [PubMed] [Google Scholar]
- 26.Bhagat A. A. S., Dasgupta S., Banerjee R. K., and Papautsky I., “Effects of microchannel cross-section and applied electric field on electroosmotic mobility,” in TRANSDUCERS 2007—2007 International Solid-State Sensors, Actuators and Microsystems Conference (IEEE, 2007), pp. 1853–1856. [Google Scholar]
- 27.Harvey D., see https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Analytical_Chemistry_2.1_(Harvey)/12%3A_Chromatographic_and_Electrophoretic_Methods/12.07%3A_Electrophoresis for “12.7: Electrophoresis - Chemistry LibreTexts,” Chemistry LibreTexts, 2021.
- 28.Guéroult M., Picot D., Abi-Ghanem J., Hartmann B., and Baaden M., “How cations can assist DNase I in DNA binding and hydrolysis,” PLoS Comput. Biol. 6(11), e1001000 (2010). 10.1371/journal.pcbi.1001000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White K., Yang P., Li L., Farshori A., Medina A. E., and Zielke H. R., “Effect of postmortem interval and years in storage on RNA quality of tissue at a repository of the NIH NeuroBioBank,” Biopreserv. Biobank. 16(2), 148–157 (2018). 10.1089/bio.2017.0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sidova M., Tomankova S., Abaffy P., Kubista M., and Sindelka R., “Effects of post-mortem and physical degradation on RNA integrity and quality,” Biomol. Detect. Quantif. 5, 3–9 (2015). 10.1016/j.bdq.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon J.-Y. and Garrell R. L., “Biomolecular adsorption in microfluidics,” in Encyclopedia of Microfluidics and Nanofluidics, edited by Li D. (Springer, Boston, MA, 2008), pp. 68–76. [Google Scholar]
- 32.See 10.7910/DVN/DFBFEVW for the data that supports the findings of this paper. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary material as referenced throughout the manuscript is found here. This includes further information about the statistical analyses, Cq values and Bioanalyzer results for disodium EDTA anticoagulated blood in the positive configuration, results for sodium heparin anticoagulated blood in the negative configuration, COMSOL model development, and additional COMSOL model results.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.32