Abstract
Platelets play an important role in the development and progression of respiratory distress. Functional platelets are known to seal inflammatory endothelial gaps and loss of platelet function has been shown to result in loss of integrity of pulmonary vessels. This leads to fluid accumulation in the pulmonary interstitium, eventually resulting in respiratory distress. Streptococcus pneumoniae is one of the major pathogens causing community-acquired pneumonia. Previously, we have shown that its major toxin pneumolysin forms pores in platelet membranes and renders them nonfunctional. In vitro, this process was inhibited by polyvalent intravenous immunoglobulins (IVIGs). In this study, we compared the efficacy of a standard IVIG preparation (IVIG, 98% immunoglobulin G [IgG]; Privigen, CSL Behring, United States) and an IgM/IgA-enriched immunoglobulin preparation (21% IgA, 23% IgM, 56% IgG; trimodulin, Biotest AG, Germany) to inhibit pneumolysin-induced platelet destruction. Platelet destruction and functionality were assessed by flow cytometry, intracellular calcium release, aggregometry, platelet viability, transwell, and flow chamber assays. Overall, both immunoglobulin preparations efficiently inhibited pneumolysin-induced platelet destruction. The capacity to antagonize pneumolysin mainly depended on the final IgG content. As both polyvalent immunoglobulin preparations efficiently prevent pneumolysin-induced platelet destruction and maintain platelet function in vitro, they represent promising candidates for clinical studies on supportive treatment of pneumococcal pneumonia to reduce progression of respiratory distress.
Keywords: immunoglobulins, platelets, pneumolysin, pneumonia, Streptococcus pneumoniae
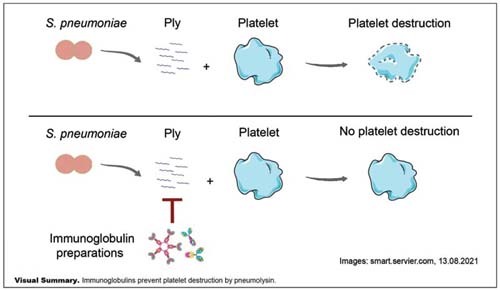
Introduction
Community-acquired pneumonia (CAP) is frequent and can cause acute respiratory distress syndrome (ARDS), 1 a medical condition with high mortality. 2 One of the major pathogens causing CAP is Streptococcus pneumoniae, and its toxin pneumolysin contributes to the development of CAP and ARDS. 3 4 5 6 Recently, we have shown that pneumolysin induces platelet destruction and renders them nonfunctional. 7 Platelets play a role in lung diseases 8 and impaired platelet function leads to loss of vascular integrity, 9 10 also in the pulmonary vessels. 11 12 This results in fluid accumulation in the pulmonary interstitium and alveoli, eventually resulting in respiratory distress. Already in the 1990s, it has been shown that washed platelets prevent lung edema. 13 14 Prevention of pneumolysin-induced platelet destruction may represent a supportive therapeutic strategy in patients with pneumococcal CAP. Among other approaches, 15 16 inhibition of pneumolysin can be achieved by polyvalent immunoglobulin preparations pooled from human plasma. We have shown that a human immunoglobulin G (IgG) preparation (intravenous immunoglobulin [IVIG]; 98% IgG) prevents pneumolysin-induced platelet destruction in vitro. 7 Furthermore, the IgM/IgA-enriched immunoglobulin preparation trimodulin (23% IgM, 21% IgA, 56% IgG) reduced mortality in severe CAP (sCAP) patients who had high C-reactive protein and/or low IgM levels. 17 More targeted analysis revealed that trimodulin also reduced mortality in a small patient subgroup with pneumococcal microbiological etiology compared to placebo, and was shown to prevent lysis of erythrocytes by pneumolysin. 7
In this study, we investigate the impact of trimodulin on pneumolysin-induced platelet destruction compared to an IVIG preparation in vitro.
Methods
Polyvalent Immunoglobulin Preparations
In all experiments, IVIG (98% IgG; Privigen, CSL Behring, United States) was used at a final concentration of 1 mg/mL. The IgM/IgA-enriched immunoglobulin preparation trimodulin (23% IgM, 21% IgA, 56% IgG; Biotest AG, Germany) was used in two final concentrations, 1 and 1.79 mg/mL. IVIG 1 mg/mL and trimodulin 1.79 mg/mL contain the same final concentration of IgG, whereas IVIG 1 mg/mL and trimodulin 1 mg/mL contain the same final overall concentration of human plasma immunoglobulin proteins.
Determination of Ply-Specific Antibodies Using the Luminex xMAP Technology
Human plasma samples to measure and compare Ply-specific antibody titers were either derived from the excellence network for Community Acquired Pneumonia (CAPNETZ) or the SHIP-TREND-0 cohort of the Study of Health in Pomerania (SHIP). 18 The observational CAPNETZ study includes more than 12,000 patients diagnosed with CAP from whom comprehensive data and biomaterials were collected ( https://clinicaltrials.gov/ct2/show/NCT02139163?term=NCT02139163&draw=2&rank=1 or https://www.capnetz.de/html/capnetz/project?set-language-to=en ). Samples from the SHIP-TREND-0 cohort were used as a healthy control group and comprised a stratified random sample of adults living in the region of Western Pomerania whose health status was assessed. 19 The study protocol of the CAPNETZ cohort was approved by the local ethics committee of the Medical School Hannover, Germany (registration number 301-2008). The study protocol of the SHIP-TREND-0 cohort was approved by the local ethics committee of the University of Greifswald (registration number BB39/08), with all participants providing written consent. A total of 138 human plasma samples were included from each study, which were matched for age and gender. Samples: n = 138 CAPNETZ and n = 138 SHIP-TREND-0. Mean age in years: 54.3 years; men 54.3%, women 45.7%.
The determination of Ply-specific antibody titers in IVIG and human plasma samples was performed via a Luminex-based serological assay described recently. 20 In brief, recombinant pneumolysin was immobilized on xMAP MagPlex beads (Luminex, United States), which were incubated with fourfold serially diluted IVIG (98% IgG; Privigen, CSL Behring, United States) or plasma samples (seven dilutions ranging from 1:50 to 1:204,800). Detection of primary antibody binding was enabled by incubation with an R-Phycoerythrin-labeled anti-human IgG (Jackson ImmunoResearch Europe Ltd, Ely, United Kingdom) followed by measurement in the FLEXMAP 3D (Luminex, United States). The xMAPr analysis tool 21 was used for curve fitting and calculation of Ply-specific IgG response values.
Detection of Ply-Specific Antibodies Using Western Blot
Different concentrations of recombinant pneumolysin (0.125 , 0.25 , 0.5, and 1 µg/mL) were applied on a 12% SDS gel and blotted on a nitrocellulose membrane. After blocking in 5% skimmed milk a rabbit anti-pneumolysin polyclonal Ab (1:500) generated as described, 7 serum of a defined donor (1:500), IVIG (1 mg/mL), and trimodulin (1.79 mg/mL) were used as primary antibodies. After washing, the blots were incubated with HRP-labeled anti-human or anti-rabbit IgG. Detection was done with a ChemoCam (Intas, Science Imaging) with 15 second exposure time for all immunoblots. The immunoblot image was brightness-adjusted using Photoshop CS5 64 bit.
Platelet Preparation
Platelets were purified from ACD-A whole blood obtained from healthy volunteers matching the criteria for blood donation in Germany. All donors gave written and informed consent. Ethics were approved by the Ethics Committee of the Universitätsmedizin Greifswald (BB 044/18). Platelets were prepared as described. 7 Briefly, platelet-rich plasma was washed twice with Tyrode's buffer (0.35% BSA, 0.1% glucose, 2.5 U/mL apyrase, 1 U/mL hirudin, pH 6.3) and the final platelet pellet was resuspended in a bicarbonate-based suspension buffer (0.137 M NaCl, 0.027 M KCl, 0.012 M NaHCO 3 , 0.42 mM NaH 2 PO 4 , 0.35% BSA, 0.1% glucose, 0.212 M MgCl 2 , 0.196 M CaCl2, pH 7.2) and adjusted to 300.000 platelets/µL.
Pneumolysin
Purification of Strep-tagged pneumolysin and cytolytic activity assessment were performed as described. 7 Pneumolysin stock solution (0.296 mg/mL) was diluted in PBS (w/o Ca 2+ /Mg 2+ , pH = 7.0) to different concentrations, depending on the assay. Prior to the platelet assays, pneumolysin in different concentrations was incubated with IVIG or trimodulin (30 minutes, at room temperature [RT]). Pneumolysin concentrations were tested over a wide range (final concentrations of mainly 300–900 ng/mL) to test for potential differences in the efficacy of immunoglobulins.
Flow Cytometry (CD62P Staining)
To avoid artifacts of platelet activation caused by FcγRIIa-dependent platelet activation (e.g., due to blood group antibodies anti-A or anti-B IgG in the immunoglobulin preparations), platelets were preincubated with the monoclonal antibody (mAb) IV.3 (supernatant of cell culture, 45 minutes, 37°C in a 1:15 dilution) to block platelet FcγRIIa. Pneumolysin of different concentrations (final concentrations: 900, 600, 300, 30 ng/mL) was preincubated with IVIG (final concentration 1 mg/mL), trimodulin (final concentrations: 1 and 1.79 mg/mL) or buffer for 20 seconds, 60 seconds, 5 minutes, or 30 minutes and then incubated with platelets for 10 minutes at RT. To test remaining platelet functionality, samples were split and 50% were incubated with thrombin receptor activator peptide (TRAP-6, Hart Biologicals, United Kingdom; final concentration 20 µM) for 10 minutes at RT, the other 50% with PBS. Then, platelets were labeled with anti-human CD62P PE-Cy5-labelled antibody (BD Pharmingen, United States, 10 minutes, RT, 1:10 dilution), fixed with paraformaldehyde 2% (Morphisto, Germany, 15 minutes, RT), washed with PBS (650 g, 7 minutes), and assessed by flow cytometry (Cytomics FC500; Beckmann, United States; CXP 2.2 software). A platelet gate was predefined using the forward–sideward scatter followed by CD61-positive cells. Results are presented as mean fluorescence intensity of the CD62P-positive population multiplied by the percentage of gated events. The experimental setup is shown in Supplementary Fig. S1 (available in the online version).
Comparison of Different IVIG and Trimodulin Batches
To test batch-to-batch variability, four different trimodulin batches (Lot 1, 2, 3, 4) and two different IVIG batches (Lot 1, 2) were assessed in the CD62P flow cytometry assay (performed as described above) and a hemolysis assay. For the hemolysis assay, ACD-A whole blood was washed with PBS four times (211 g, 10 minutes, brake 0), the pellet was resuspended and diluted in PBS (w/o Ca 2+ /Mg 2+ , pH = 7, 1:5). Samples were then incubated with pneumolysin or pneumolysin preincubated with IVIG or trimodulin (final concentrations: pneumolysin: 300, 600, 900 ng/mL; IVIG: 1 mg/mL; trimodulin: 1.79 mg/mL) in a 96-well plate for 10 minutes, 37°C. The plate was then centrifuged (10 minutes, 475 g, brake 0), and the supernatant was measured for absorption at 450 nm for free hemoglobin content (Tecan Infinite 200 PRO, Tecan Group, Switzerland). The experimental setup is shown in Supplementary Fig. S2 (available in the online version).
Measurement of Intracellular Calcium Release
The release of calcium from intracellular stores was measured using the fluorescent calcium indicator FLUO-4 AM (ThermoFisher, United States). To avoid artifacts, platelets were washed and resuspended in a calcium-free Tyrode's buffer as described. 7 Platelets were stained with FLUO-4 AM (ThermoFisher, United States; 5.4 ng/µL), and FcγRIIa was blocked by mAb IV.3 (1:15, 45 minutes, 37°C). Measurements were performed with Fluoroskan Ascent fluorometer (ThermoFisher, United States) applying Ascent software 2.6 as described. 7 In brief, pneumolysin (final concentrations: 900, 600, 300 ng/mL) preincubated with IVIG (final concentration 1 mg/mL), trimodulin (final concentrations: 1 and 1.79 mg/mL), or buffer was added after 15 seconds to the platelets and measurement continued for 225 seconds. Then, the remaining platelet functionality was tested by the addition of TRAP-6 (final concentration 20 µM). The amount of free intracellular calcium was normalized to the baseline measurement. The detailed experimental setup is shown in Supplementary Fig. S3 (available in the online version).
Light Transmission Aggregometry (Platelet Lysis and Aggregation)
FcγRIIa of washed platelets was blocked using mAb IV.3 (1:15, 45 minutes, 37°C) and fibrinogen (Invitrogen, United States; 2.25 mg/mL) was added to allow platelet aggregation. Platelets were assessed for aggregation or platelet lysis using an APACT4 aggregometer (500 rpm, 37°C, Haemochrom, Germany) applying APACT LPC-software as described. 7 In brief, pneumolysin (final concentrations: 900, 600, or 300 ng/mL) preincubated with IVIG (final concentration 1 mg/mL), trimodulin (final concentrations: 1 and 1.79 mg/mL), or buffer was added to the samples after 15 seconds and change in light transmission was followed for 225 seconds, then TRAP-6 (final concentration 20 µM) was added to test the remaining platelet function. The detailed experimental set up is shown in Supplementary Fig. S4 (available in the online version).
Platelet Viability
To determine platelet viability, RealTime-Glo Cell Viability Assay (Promega, United States) was used. RealTime-Glo reagents were prepared according to the manufacturer's protocol and mixed with platelets and pneumolysin (final concentrations: 900, 600, 300 ng/mL), which was preincubated with IVIG (final concentration 1 mg/mL) or trimodulin (final concentrations: 1 and 1.79 mg/mL) in a 96-well plate. Real-time luminescence measurement was performed for 30 minutes (FLUOstar Omega, BMG LABTECH, Germany) as described. 7 All samples were tested in duplicates. The mean of the relative luminescence units of duplicate measurements was subtracted by the blank and represents cell viability. The detailed experimental setup is shown in Supplementary Fig. S5 (available in the online version).
Transwell Assay (Pore Sealing Capacity)
To determine the pore sealing capacity of platelets, transwell assays with fibronectin-coated membranes (Corning 6.5 mm Transwell with 3.0 µm Pore Polycarbonate Membrane Insert, Corning, United States) were performed as described. 7 Briefly, platelets were incubated with pneumolysin (final concentrations: 600, 300 ng/mL ) preincubated with IVIG (final concentration 1 mg/mL), trimodulin (final concentration 1.79 mg/mL), or buffer for 45 minutes at 37°C in the inserts of the transwell plates. The supernatant of the inserts was discarded, and the inserts transferred into new wells containing PBS (w/o Ca 2+ /Mg 2+ , pH 7.0). FITC-labeled bovine serum albumin (BSA-FITC, ThermoFisher, United States; final concentration 0.25 mg/mL) was then added to the inserts (10 minutes, 37°C, darkness). The platelets' pore sealing capacity was determined by fluorometric measurement of BSA-FITC flow through in the liquid of the lower chamber (Fluoroskan Ascent FL, ThermoFisher, United States, Ascent Software 2.6). The detailed experimental setup is shown in Supplementary Fig. S6 (available in the online version).
Thrombus Formation under Shear Flow
Platelets in hirudinized whole blood were stained with FITC-labeled anti-human CD61 antibody (BD Pharmingen, United States; 1:100) and then incubated with pneumolysin (final concentration: 600 ng/mL), which was preincubated with IVIG (1 mg/mL) or trimodulin (1 and 1.79 mg/mL) for 10 minutes at RT. Blood samples were flown over collagen (horse tendon, Nycomed, Germany; 200 µg/mL HORM collagen type I) coated channels of ibidi µ-slides VI 0.1 (ibidi, Germany) at a wall shear rate of 1,000 s −1 . Every 10 seconds, time-lapse images were captured on a confocal laser scanning microscope (Leica SP5, Leica, Germany) as described. 7 Quantitative assessment of thrombus formation was performed by image segmentation in Bitplane Imaris version 7.65. (Oxford Instruments, Abingdon, United Kingdom) using the surface creation wizard algorithm as previously described. 7 All flow experiments were performed according to International Society on Thrombosis and Haemostasis Scientific and Standardization Committee subcommittee on Biorheology recommendations. 22 The detailed experimental setup is shown in Supplementary Fig. S7 (available in the online version).
Statistics
Statistical analysis was performed using GraphPad Prism 9. Data are shown as median, except where indicated. Nonnormally distributed data were analyzed using the Friedman test followed by the uncorrected Dunn's test for multiple comparisons. Normally distributed data were analyzed using one-way repeated measures ANOVA (analysis of variance), followed by the Bonferroni method for multiple comparisons. A p -value <0.05 was considered to be statistically significant.
Results
Trimodulin and IVIG Inhibit Pneumolysin-Induced Platelet Damage
Permeabilization of Platelets
We have shown earlier that pneumolysin treatment of platelets results in intracellular CD62P staining due to pneumolysin-induced pore formation in the platelet membrane. 7 This allows anti-CD62P antibodies to pass the membrane and stain intraplatelet CD62P. Therefore, CD62P staining after pneumolysin treatment is a marker for platelet destruction. 7 In the presence of IVIG or trimodulin, pneumolysin-induced increase in CD62P staining in platelets was reduced in a concentration-dependent manner ( Fig. 1A ). Both immunoglobulin preparations significantly reduced CD62P staining induced by pneumolysin concentrations up to 300 ng/mL. At higher concentrations of pneumolysin (600–900 ng/mL), the values of CD62P staining in platelets in the presence of trimodulin 1.79 mg/mL and IVIG 1 mg/mL were similar. In contrast, CD62P staining in platelets was higher when only 1 mg/mL trimodulin was used ( Fig. 1A ). Pneumolysin preincubations of >60 seconds with immunoglobulin preparations were sufficient to reduce CD62P values ( Supplementary Fig. S8 [available in the online version]). As control, neither IVIG nor trimodulin increased CD62P staining in the absence of pneumolysin ( Fig. 1A ).
Fig. 1.
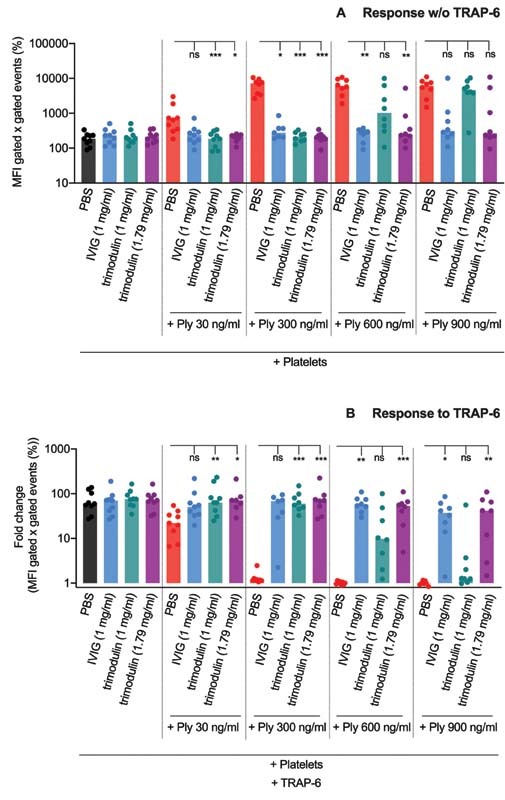
CD62P staining in platelets upon pneumolysin treatment. ( A ) Increased CD62P staining upon treatment with pneumolysin (Ply) or pneumolysin preincubated with IVIG or trimodulin. CD62P staining is presented as mean fluorescence intensity (MFI) of CD62P-positive gated events multiplied with the percent of CD62P-positive gated events. As a control, samples were incubated only with PBS. ( B ) CD62P staining upon TRAP-6 (20 µM) addition after preincubation of platelets with pneumolysin or pneumolysin preincubated with IVIG or trimodulin. Response to TRAP-6 is presented as a fold change of the MFI of CD62P-positive gated events multiplied with the percent of CD62P-positive events compared to the PBS control. As a control, samples were incubated with PBS only. Statistical analysis was performed using the Friedman test followed by uncorrected Dunn's test for multiple comparisons. A p -value <0.05 was considered to be significant ( * > 0.033, ** > 0.002 , *** > 0.001). IVIG, intravenous immunoglobulin; PBS, phosphate-buffered saline.
Remaining Platelet Function
A similar pattern was observed for the remaining platelet functionality when the response to TRAP-6 was tested ( Fig. 1B ). IVIG 1 mg/mL and trimodulin 1.79 mg/mL maintained TRAP-6 responsiveness up to 900 ng/mL pneumolysin, whereas trimodulin 1 mg/mL failed to do so when more than 300 ng/mL pneumolysin was used. As a control, neither IVIG nor trimodulin inhibited CD62P expression on platelets after activation with TRAP-6 in the absence of pneumolysin ( Fig. 1B and Supplementary Fig. S8 [available in the online version]).
Batch-to-Batch Variability
To test batch-to-batch variability in the capacity to antagonize pneumolysin, e.g., due to different antipneumolysin immunoglobulin concentrations in different batches, four batches of trimodulin and two batches of IVIG were tested in the CD62P ( Supplementary Fig. S9A [available in the online version]) and hemolysis assay ( Supplementary Fig. S9B [available in the online version]). Overall, no relevant batch-to-batch variability in the capacity to antagonize pneumolysin was observed ( Supplementary Fig. S9 [available in the online version]). Therefore, all other experiments were performed with only one batch per immunoglobulin preparation (IVIG lot 1 and trimodulin lot 1).
Calcium Release
Platelets physiologically mobilize calcium from intracellular stores upon activation over a short time, here demonstrated by TRAP-6 addition ( Fig. 2A1 – A3 ). In contrast, calcium release upon pneumolysin treatment is permanent ( Fig. 2A1 – A3 ). 7 Consistent with the flow cytometry results, both immunoglobulin preparations showed sufficient inhibition of calcium release and maintenance of TRAP-6 responsiveness up to 300 ng/mL pneumolysin ( Fig. 2A1 ). At 600 ng/mL pneumolysin, trimodulin 1.79 mg/mL and IVIG 1 mg/mL still showed sufficient inhibition of calcium release and maintenance of TRAP-6 responsiveness, whereas trimodulin 1 mg/mL did not ( Fig. 2A2 ). At 900 ng/mL pneumolysin, trimodulin 1.79 mg/mL showed a delay of pathological calcium release and only IVIG 1 mg/mL showed a reduction of calcium release and maintenance of TRAP-6 responsiveness similar to the control ( Fig. 2A3 ). In contrast, trimodulin 1 mg/mL was not sufficient to inhibit the 900 ng/mL pneumolysin effect ( Fig. 2A3 ). As a control, neither IVIG nor trimodulin inhibited calcium release of platelets after activation with TRAP-6 in the absence of pneumolysin ( Supplementary Fig. S10A [available in the online version]).
Fig. 2.
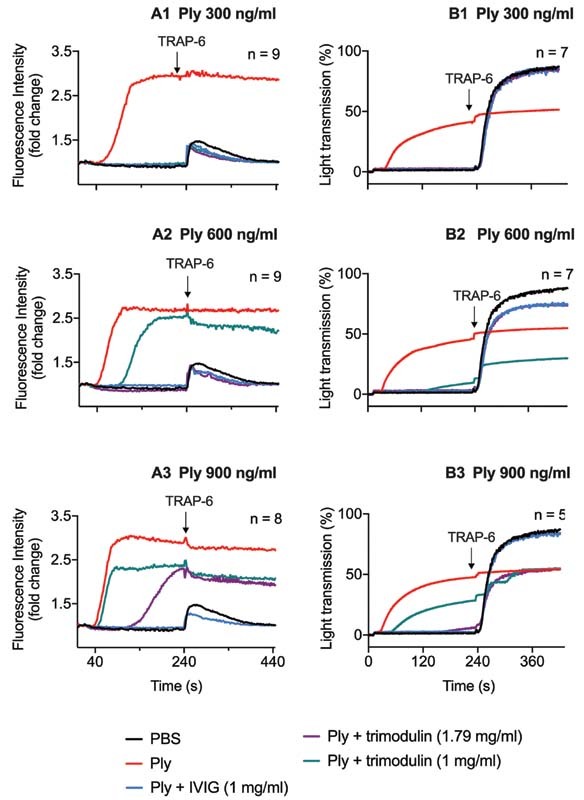
Kinetics of ( A ) intracellular calcium release and ( B ) platelet aggregation/lysis upon pneumolysin treatment. ( A ) Intracellular calcium release in platelets upon treatment with different pneumolysin (Ply) concentrations (A1–A3) or pneumolysin (Ply) preincubated with IVIG or trimodulin, respectively. Intracellular calcium was quantified by fluorescence measurement, resulting from the fluorescence intracellular calcium indicator FLUO-4-AM. After 240 seconds, platelet functionality was assessed by TRAP-6 (20 µM) addition. Curves represent the median of platelets of ≥8 donors. As a control, platelets were incubated with PBS. ( B ) Light transmission through platelet suspension upon treatment with different pneumolysin concentrations (B1–B3) or pneumolysin preincubated with IVIG or trimodulin in aggregometry, respectively. Increased light transmission upon pneumolysin treatment results from platelet lysis. After 240 seconds, platelet functionality was assessed by TRAP-6 (20 µM) addition. Increased light transmission upon TRAP-6 treatment results from platelet aggregation. Curves represent the median of at least five donors. As a control, platelets were incubated with PBS only. IVIG, intravenous immunoglobulin; PBS, phosphate-buffered saline.
Fig. 3.
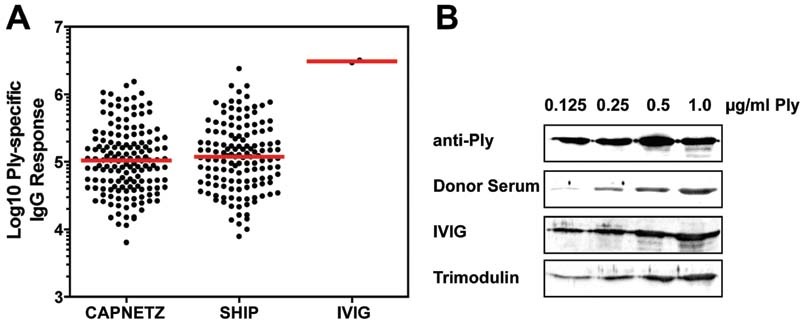
Pneumolysin IgG antibody levels in human sera and immunoglobulin preparations. ( A ) Anti-pneumolysin IgG antibody levels in a cohort of pneumonia patients ( n = 138), a healthy cohort ( n = 138), and IVIG. The mean age was 54.3 years with 54.3% men and 45.7% women. The pneumolysin IgG antibody titers were determined by the Luminex xMAP technology and the xMAPr analysis tool was used for curve fitting and calculation of Ply-specific IgG response values. ( B ) Detection of IgG specific for pneumolysin by immunoblot analysis. Different amounts of pneumolysin (0.125, 0.25, 0.5, and 1 µg/mL) were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and incubated with a rabbit polyclonal antipneumolysin antibody (1:500, Davids Biotechnologie GmbH, Regensburg, Germany), a donor serum (1:500), IVIG (1 mg/mL), and trimodulin (1.79 mg/mL). Detection of bound IgG was conducted with a secondary HRP-labeled anti-human or anti-rabbit IgG. Detection was done with a ChemoCam (Intas, Science Imaging) and the immunoblot image was brightness-adjusted using Photoshop CS5 64 bit. IgG, immunoglobulin G; IVIG, intravenous immunoglobulin; HRP, horseradish peroxidase.
Platelet Aggregation
In aggregometry with functional platelets, increased light transmission upon activation (here following TRAP-6 stimulation) is a measure for platelet aggregation and represents the physiological platelet reaction ( Fig. 2B1 – B3 ). In previous experiments we indicated that changes in light transmission upon pneumolysin treatment are not inhibited by the addition of RGDS, which blocks platelet aggregation. 7 In this case, increased light transmission upon pneumolysin treatment is not a measure for platelet aggregation but for platelet lysis. 7 Both immunoglobulin preparations inhibited platelet lysis and maintained TRAP-6 responsiveness up to 300 ng/mL pneumolysin ( Fig. 2B1 ). At 600 ng/mL pneumolysin, trimodulin 1 mg/mL showed an incomplete reduction of platelet lysis, and TRAP-6 responsiveness was lost, whereas trimodulin 1.79 mg/mL and IVIG 1 mg/mL showed a complete reduction of platelet lysis and maintenance of TRAP-6 responsiveness ( Fig. 2B2 ). At 900 ng/mL pneumolysin, trimodulin 1.79 mg/mL and IVIG 1 mg/mL inhibited platelet lysis, but only IVIG maintained TRAP-6 responsiveness completely ( Fig. 2B3 ). Neither IVIG nor trimodulin inhibited platelet aggregation after activation with TRAP-6 in the absence of pneumolysin ( Supplementary Fig. S10B [available in the online version]).
Antipneumolysin Antibody Levels in Immunoglobulin Preparations
To demonstrate that the rescue of platelet function by IVIG and trimodulin is due to neutralization of the toxin by specific antipneumolysin antibodies, the relative IgG antibody levels were determined in IVIG. These antibody levels were compared to antipneumolysin IgG levels of a healthy cohort (SHIP cohort) and a cohort of patients suffering on pneumonia (CAPNETZ cohort). The results showed high antipneumolysin IgG levels in IVIG ( Fig. 3A ). In addition, the direct interaction of antibodies present in IVIG, trimodulin, and a healthy donor serum was indicated by immunoblot analysis with pneumolysin. Consistent with the findings of our functional experiments, IVIG was more reactive than trimodulin ( Fig. 3B ).
Trimodulin and IVIG Prevent Loss of Platelet Viability, Pore Sealing Capacity, and Clot Formation upon Pneumolysin Treatment
After assessing the inhibition of pneumolysin-induced platelet damage and the maintenance of TRAP-6 responsiveness by trimodulin and IVIG, the maintenance of more complex platelet functions, which are likely relevant in the development of pulmonary vascular leakage and respiratory distress, was assessed.
Platelet Viability
Impaired platelet metabolism and enzymatic functionality have been shown to result in lung damage due to vascular leakage. 11 13 In platelet viability assays, metabolically active viable platelets converted the RealTime-Glo substrate to a luminescent product; metabolically nonactive dead platelets did not. Pneumolysin treatment impaired platelet viability and induced cell death ( Fig. 4A – C ). Both immunoglobulin preparations inhibited cell death of platelets up to 300 ng/mL pneumolysin ( Fig. 4A ). At 600 ng/mL pneumolysin, trimodulin 1.79 mg/mL and IVIG 1 mg/mL inhibited cell death ( Fig. 4B ). At 900 ng/mL pneumolysin, only IVIG 1 mg/mL significantly inhibited cell death, and trimodulin 1 mg/mL inhibited cell death the least ( Fig. 4C ). As a control, neither IVIG nor trimodulin inhibited platelet viability in the absence of pneumolysin ( Supplementary Fig. S10D [available in the online version]).
Fig. 4.
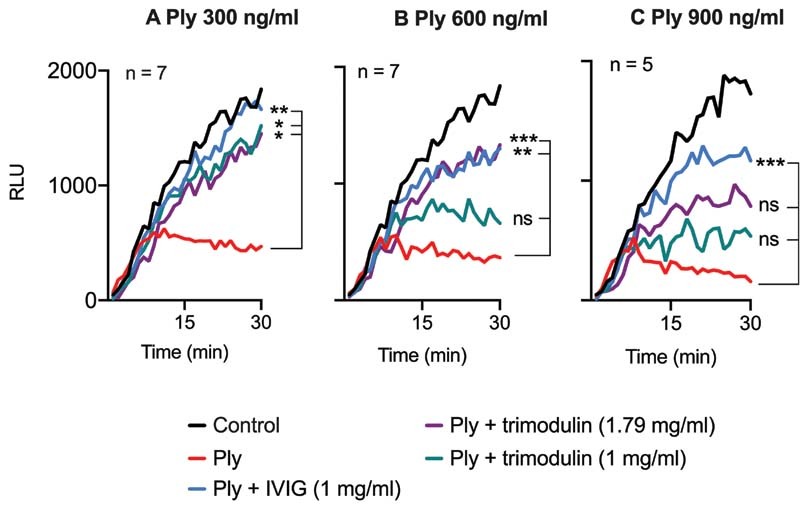
Platelet viability upon pneumolysin treatment. Platelet viability upon treatment with different pneumolysin (Ply) concentrations ( A–C ) or with pneumolysin (Ply) preincubated with IVIG or trimodulin, respectively. Viability was assessed by real-time measurement of relative luminescence units (RLU) resulting from the enzymatic conversion of a substrate by viable platelets. Curves represent the median of platelets of ≥5 donors. As a control, platelets were incubated with PBS only. For statistical analysis, the measurement endpoints (RLU of the final minute) were compared applying the Friedman test, followed by uncorrected Dunn's test for multiple comparisons. A p -value <0.05 was considered to be significant (* > 0.033, ** > 0.002 , *** > 0.001). IVIG, intravenous immunoglobulin; PBS, phosphate-buffered saline.
Pore Sealing Capacity of Platelets
In transwell assays, pneumolysin reduced the capacity of platelets to inhibit BSA-FITC diffusion through a perforated membrane. Such impaired pore sealing capacity of platelets could result in vascular leakage and fluid accumulation in the pulmonary interstitium during inflammation in vivo . In vitro, both immunoglobulin preparations maintained the platelet pore sealing capacity at 300 ng/mL pneumolysin ( Fig. 5A ). At 600 ng/mL pneumolysin, only trimodulin 1 mg/mL was less efficient ( Fig. 5B ). As control, neither IVIG nor trimodulin increased BSA-FITC flow through in the absence of pneumolysin to a relevant extent ( Supplementary Fig. S9C [available in the online version]).
Fig. 5.
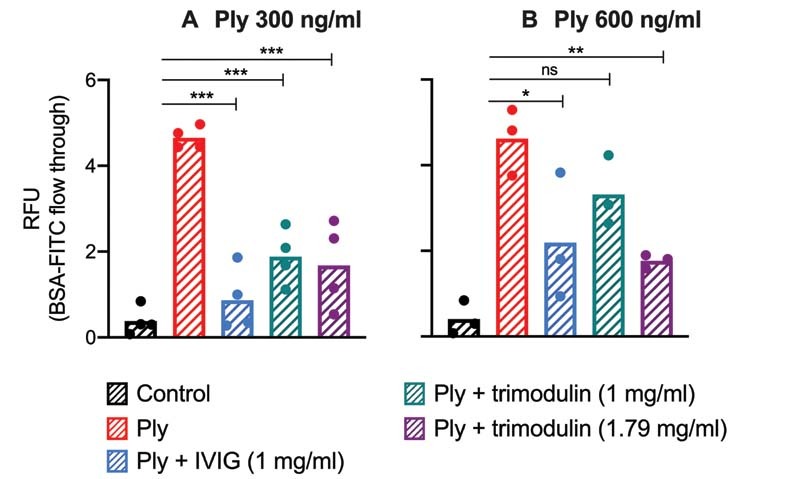
Platelet's pore sealing capacity upon pneumolysin treatment. The pore sealing capacity of platelets upon treatment with different pneumolysin concentrations ( A, B ) or with pneumolysin preincubated with IVIG or trimodulin, respectively, was assessed by their capability to inhibit BSA-FITC diffusion through a perforated fibronectin-coated membrane. BSA-FITC diffusion was quantified by the measurement of relative fluorescence units (RFU). Bars represent the mean of platelets of at least three donors. As a control, platelets were incubated with PBS only. Statistical analysis was performed using the Shapiro–Wilk normality test and repeated measures one-way ANOVA. A p -value <0.05 was considered to be significant ( * > 0.033, ** > 0.002 , *** > 0.001). ANOVA, analysis of variance.
Thrombus Formation
In whole blood, pneumolysin inhibited platelet adherence and subsequent thrombus formation ( Fig. 6A ). Reduced platelet adherence and insufficient thrombus formation were preserved by trimodulin and IVIG ( Fig. 6A ). The lower effect of trimodulin compared to IVIG is an artifact as the formulation buffer (without any immunoglobulins) of trimodulin reduced blood clot formation ( Fig. 6B ). In vivo, agents contained in the formulation buffer will be rapidly diluted and cleared from the blood and therefore will likely have no effect on platelet function.
Fig. 6.
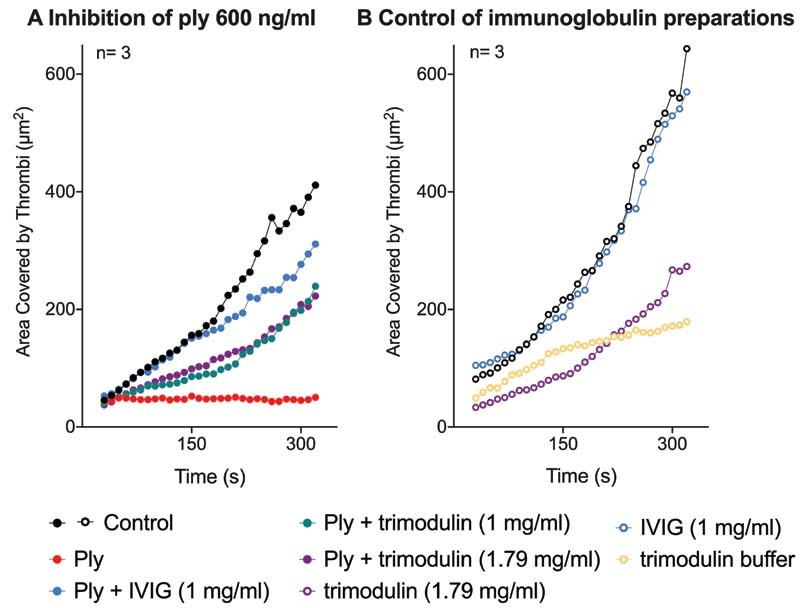
Thrombus formation in whole blood. ( A ) Thrombus formation upon treatment with 600 ng/mL pneumolysin (Ply) or pneumolysin preincubated with IVIG or trimodulin. A quantitative assessment of thrombus formation was performed by analyzing the area of the collagen-coated microchannel slide that was covered by thrombi. Curves represent the mean of platelets of three donors. As a control, whole blood was incubated with PBS only. ( B ) Thrombus formation upon treatment with immunoglobulin preparations (without pneumolysin) to investigate their effects alone on the thrombus formation. A quantitative assessment of thrombus formation was performed by analyzing the area covered. Curves represent the mean of platelets of three donors. As a control, whole blood was incubated with PBS only. IVIG, intravenous immunoglobulin; PBS, phosphate-buffered saline.
Discussion
This study aimed to provide further information about the impairment of platelets by pneumolysin and the impact of two immunoglobulin preparations, the IgM/IgA-enriched immunoglobulin preparation trimodulin and a standard IVIG, on pneumolysin-induced platelet destruction. We found that both pharmaceutical immunoglobulin preparations prevent the destruction of platelets by pneumolysin in vitro as indicated by flow cytometry, calcium release assay, and light transmission aggregometry. Further, the immunoglobulins maintain complex platelet functions as shown in in vitro viability and transwell assays and even in whole blood an effect can be observed as indicated by thrombus formation.
Importantly, the extent of prevention of pneumolysin-induced platelet damage depends mainly on the IgG content in the immunoglobulin preparation. When the total content of IgG was similar, IVIG and trimodulin showed similar in vitro effects. However, when the two preparations were adjusted to the same overall human plasma immunoglobulin concentration, trimodulin showed lower efficacy. This indicates that IgM and IgA antibodies do not contribute similarly to IgG to the inhibition of pneumolysin in the in vitro assays tested here. These data are strongly supported by the Luminex-based serological assay, which indicated high levels of antipneumolysin IgGs in IVIG. Although IVIG is diluted by patient blood after transfusion, the high levels of antipneumolysin will result in clinically meaningful concentrations of these inhibitory antibodies by IVIG and trimodulin. However, trimodulin reacted with a lower efficiency with pneumolysin compared to IVIG, which supports the observed differences on the in vitro neutralization of pneumolysin for IVIG and trimodulin.
All assays were done with IVIG at a concentration of 1 mg/mL and trimodulin at a concentration of 1 and 1.79 mg/mL. During treatment of patients with immunoglobulin preparations, immunoglobulin concentrations will be even higher. When trimodulin was given to patients with sCAP at a dose of 182.6 mg/kg body weight daily on 5 consecutive days, serum concentrations of up to 11.5 mg/mL IgG, 2.0 mg/mL IgM, and 4.1 mg/mL IgA were observed. 23 When IVIG was used in doses of 138.0 to 554.0 mg/kg body weight in patients with primary immunodeficiency, IgG concentrations of up to 16.6 mg/mL were reached 1 hour after infusion and 9 mg/mL IgG 2 weeks after infusion. 24 These pharmacokinetic properties indicate that the concentrations, which had been sufficient in our studies to prevent pneumolysin-induced platelet destruction in vitro, are even below those that can be reached in vivo using standard therapeutic regimens. The inhibitory effect of immunoglobulin preparations on pneumolysin is already measured after 60 seconds. Thus, treatment of patients suffering from pneumococcal pneumonia with immunoglobulin preparations will be clinically relevant, in particular in clinical settings where pneumolysin is released during therapeutic intervention.
Pneumolysin was used mainly at concentrations of 300 to 900 ng/mL in our experiments. In vivo data on the concentration of pneumolysin in pneumococci-induced pneumonia are difficult to obtain, because its local concentration in the lung is most likely much higher than the concentrations found in the venous blood taken from patients after it had circulated through the entire vessel system. The in vivo pneumolysin concentrations in humans, which probably come closest to the local concentrations of pneumolysin, are measured in cerebrospinal fluid from pneumococcal meningitis patients, where no dilution of pneumolysin occurs by the bloodstream. Reported concentrations range from 0.85 to 180 ng/mL, 25 with individual cases exceeding 600 ng/mL. 26 In a murine mouse model, pneumolysin concentrations in bronchoalveolar lavage were found up to 1 ng/mL. 4 Although there remains some uncertainty about in vivo pneumolysin concentrations at the local site of infection, the ratios of immunoglobulin preparations and pneumolysin used in our experiments are likely within a clinically relevant range.
Surprisingly, for the pneumolysin concentration of 900 ng/mL, differences between IVIG (1 mg/mL) and trimodulin with adjusted IgG content (1.79 mg/mL) were observed in aggregometry, calcium release ( Fig. 2A, B ), and viability assay ( Fig. 4 ), but not in flow cytometry ( Fig. 1 ). This might be either a matter of different sensitivity of the assays to platelet damage or donor-specific reasons.
In addition, trimodulin buffer showed an effect itself on thrombus formation in the flow chamber assay ( Fig. 6 ). As the blood samples tested in the assay function as a closed system without any clearance, this effect is likely to be an experimental artifact. In vivo, agents contained in the trimodulin buffer will likely be rapidly diluted and cleared from the circulation.
The negligible impact of IgM in preventing pneumolysin-induced platelet destruction by trimodulin can very likely be explained by its origin. Trimodulin is prepared from pooled human plasma obtained from healthy blood donors. Blood donors with symptoms of acute infection within the last 2 weeks are typically excluded from blood donation, but antipneumolysin IgM concentrations in human plasma are highest during pneumococcal infection. 27 In addition, in contrast to antipneumococcal capsular polysaccharide IgM, antipneumolysin IgM titers remain relatively low, even during acute infection. 27
An experimental limitation of our study is that pneumolysin was first preincubated with the immunoglobulin preparations for 30 minutes before platelets were added. In vivo, patients will already be infected with pneumococci, which release pneumolysin 28 29 before they are treated with immunoglobulins. However, pneumonia can progress to sCAP associated with sepsis and immunoglobulin treatment may prevent worsening of pulmonary leakage. Interestingly, in a clinical study in patients with sCAP due to confirmed S. pneumoniae infection, in those receiving trimodulin, platelet counts increased faster compared to placebo. 7 The underlying mechanisms remain unclear. Our experiments indicate that one potential explanation among others might be that neutralization of pneumolysin supports the faster increase of platelet counts. However, other studies indicate more pronounced immune modulation by trimodulin compared to IVIG. 30 Furthermore, trimodulin but not IVIG was found to interact with the complement system in an either activating or inhibiting way, depending on the concentration. 23 Which of these factors finally contributes to clinical efficacy in acute pneumonia is unresolved.
In conclusion, our results indicate that both IVIG and the IgM/IgA-enriched immunoglobulin preparation trimodulin represent promising candidates for supportive treatment of pneumococcal pneumonia. By preventing pneumolysin-induced platelet destruction and maintaining platelet functionality, immunoglobulin preparations could potentially prevent the worsening of respiratory distress. In pharmaceutical immunoglobulin preparations, IgG seems to be the determining component for the effective prevention of platelet destruction by pneumolysin in vitro.
Acknowledgement
The investigators of this scientific work acknowledge CAPNETZ STIFTUNG and the competence network CAPNETZ for project support with regard to using biomaterials and clinical data. CAPNETZ is a multidisciplinary approach to better understand and treat patients with CAP. The network has been made possible by the contributions of many investigators.
Funding Statement
Funding This study was funded by Deutsche Forschungsgemeinschaft, Grant/Award Number: Projektnummer 374031971-TRR240; Research support by Biotest. CAPNETZ is supported by the German Center for Lung Research (DZL).
Footnotes
Conflict of Interest A.G. reports grants and nonfinancial support from Aspen, Boehringer Ingelheim, MSD, Bristol Myers Squibb (BMS), Bayer Healthcare, Instrumentation Laboratory; personal fees from Aspen, MSD, Macopharma, BMS, Chromatec, Instrumentation Laboratory, nonfinancial support from Portola, Ergomed, Biokit outside the submitted work. S.W. and J.S. are employees of Biotest AG. All other authors declare no conflict of interest.
What is known about this topic?
Pneumolysin, the main toxin of Streptococcus pneumoniae, forms pores in platelets and renders them nonfunctional. Impaired platelet function plays a role in the development of respiratory distress, which occurs as a complication of severe community-acquired pneumonia (sCAP), often caused by S. pneumoniae .
In vitro, a human IgG immunoglobulin preparation was shown to prevent pneumolysin-induced platelet destruction.
In vivo, a human IgM/IgA-enriched immunoglobulin preparation was shown to reduce mortality in sCAP patients.
What does this paper add?
Both IgG and IgM/IgA-enriched immunoglobulin preparations sufficiently inhibit pneumolysin-induced platelet destruction in vitro .
Compared to IgG preparations, additional IgM and IgA do not increase the capacity to inhibit pneumolysin-induced platelet destruction in vitro. This indicates that IgG is the main component for an inhibition of pneumolysin-induced platelet destruction.
Supplementary Material
References
- 1.Cilloniz C, Ferrer M, Liapikou A. Acute respiratory distress syndrome in mechanically ventilated patients with community-acquired pneumonia. Eur Respir J. 2018;51(03):1.702215E6. doi: 10.1183/13993003.02215-2017. [DOI] [PubMed] [Google Scholar]
- 2.Thompson B T, Chambers R C, Liu K D. Acute respiratory distress syndrome. N Engl J Med. 2017;377(06):562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 3.Witzenrath M, Gutbier B, Hocke A C. Role of pneumolysin for the development of acute lung injury in pneumococcal pneumonia. Crit Care Med. 2006;34(07):1947–1954. doi: 10.1097/01.CCM.0000220496.48295.A9. [DOI] [PubMed] [Google Scholar]
- 4.García-Suárez M del M, Flórez N, Astudillo A. The role of pneumolysin in mediating lung damage in a lethal pneumococcal pneumonia murine model. Respir Res. 2007;8(01):3. doi: 10.1186/1465-9921-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson R, Nel J G, Feldman C. Multifaceted role of pneumolysin in the pathogenesis of myocardial injury in community-acquired pneumonia. Int J Mol Sci. 2018;19(04):E1147. doi: 10.3390/ijms19041147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubins J B, Duane P G, Clawson D, Charboneau D, Young J, Niewoehner D E. Toxicity of pneumolysin to pulmonary alveolar epithelial cells. Infect Immun. 1993;61(04):1352–1358. doi: 10.1128/iai.61.4.1352-1358.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jahn K, Handtke S, Palankar R. Pneumolysin induces platelet destruction, not platelet activation, which can be prevented by immunoglobulin preparations in vitro. Blood Adv. 2020;4(24):6315–6326. doi: 10.1182/bloodadvances.2020002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Middleton E A, Weyrich A S, Zimmerman G A. Platelets in pulmonary immune responses and inflammatory lung diseases. Physiol Rev. 2016;96(04):1211–1259. doi: 10.1152/physrev.00038.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues S F, Granger D N.Blood cells and endothelial barrier function Tissue Barriers 20153(1–2):e978720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho-Tin-Noé B, Demers M, Wagner D D. How platelets safeguard vascular integrity. J Thromb Haemost. 2011;9 01:56–65. doi: 10.1111/j.1538-7836.2011.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Porto A PNA, Claushuis T AM, van der Donk L EH. Platelet Btk is required for maintaining lung vascular integrity during murine pneumococcal pneumosepsis. Thromb Haemost. 2019;119(06):930–940. doi: 10.1055/s-0039-1681046. [DOI] [PubMed] [Google Scholar]
- 12.Lo S K, Burhop K E, Kaplan J E, Malik A B.Role of platelets in maintenance of pulmonary vascular permeability to protein Am J Physiol 1988254(4, Pt 2):H763–H771. [DOI] [PubMed] [Google Scholar]
- 13.Zamora C A, Baron D, Heffner J E. Washed human platelets prevent ischemia-reperfusion edema in isolated rabbit lungs. J Appl Physiol. 1991;70(03):1075–1084. doi: 10.1152/jappl.1991.70.3.1075. [DOI] [PubMed] [Google Scholar]
- 14.Heffner J E, Cook J A, Halushka P V. Human platelets modulate edema formation in isolated rabbit lungs. J Clin Invest. 1989;84(03):757–764. doi: 10.1172/JCI114233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimoto A T, Rosch J W, Tuomanen E I. Pneumolysin: pathogenesis and therapeutic target. Front Microbiol. 2020;11:1543. doi: 10.3389/fmicb.2020.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson R, Feldman C. Pneumolysin as a potential therapeutic target in severe pneumococcal disease. J Infect. 2017;74(06):527–544. doi: 10.1016/j.jinf.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Welte T, Dellinger R P, Ebelt H. Efficacy and safety of trimodulin, a novel polyclonal antibody preparation, in patients with severe community-acquired pneumonia: a randomized, placebo-controlled, double-blind, multicenter, phase II trial (CIGMA study) Intensive Care Med. 2018;44(04):438–448. doi: 10.1007/s00134-018-5143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Völzke H, Alte D, Schmidt C O. Cohort profile: the study of health in Pomerania. Int J Epidemiol. 2011;40(02):294–307. doi: 10.1093/ije/dyp394. [DOI] [PubMed] [Google Scholar]
- 19.Meyer T C, Michalik S, Holtfreter S. A comprehensive view on the human antibody repertoire against Staphylococcus aureus antigens in the general population . Front Immunol. 2021;12:651619. doi: 10.3389/fimmu.2021.651619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seinen J, Engelke R, Abdullah M R. Sputum proteome signatures of mechanically ventilated intensive care unit patients distinguish samples with or without anti-pneumococcal activity. mSystems. 2021;6(02):e00702–e00720. doi: 10.1128/mSystems.00702-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer T C, Schmidt F, Steinke J, Bröker B M, Völker U, Michalik S. Technical report: xMAPr - high-dynamic-range (HDR) quantification of antigen-specific antibody binding. J Proteomics. 2020;212:103577. doi: 10.1016/j.jprot.2019.103577. [DOI] [PubMed] [Google Scholar]
- 22.Subcommittee on Biorheology . Mangin P H, Gardiner E E, Nesbitt W S. In vitro flow based systems to study platelet function and thrombus formation: Recommendations for standardization: communication from the SSC on Biorheology of the ISTH. J Thromb Haemost. 2020;18(03):748–752. doi: 10.1111/jth.14717. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt C, Weißmüller S, Bohländer F. The dual role of a polyvalent IgM/IgA-enriched immunoglobulin preparation in activating and inhibiting the complement system. Biomedicines. 2021;9(07):817. doi: 10.3390/biomedicines9070817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morio T, Baheti G, Tortorici M A, Hofmann J, Rojavin M A. Pharmacokinetic properties of Privigen ® in Japanese patients with primary immunodeficiency . Immunol Med. 2019;42(04):162–168. doi: 10.1080/25785826.2019.1700085. [DOI] [PubMed] [Google Scholar]
- 25.Spreer A, Kerstan H, Böttcher T. Reduced release of pneumolysin by Streptococcus pneumoniae in vitro and in vivo after treatment with nonbacteriolytic antibiotics in comparison to ceftriaxone. Antimicrob Agents Chemother. 2003;47(08):2649–2654. doi: 10.1128/AAC.47.8.2649-2654.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wall E C, Gordon S B, Hussain S. Persistence of pneumolysin in the cerebrospinal fluid of patients with pneumococcal meningitis is associated with mortality. Clin Infect Dis. 2012;54(05):701–705. doi: 10.1093/cid/cir926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huo Z, Spencer O, Miles J.Antibody response to pneumolysin and to pneumococcal capsular polysaccharide in healthy individuals and Streptococcus pneumoniae infected patients Vaccine 200422(9–10):1157–1161. [DOI] [PubMed] [Google Scholar]
- 28.Loughran A J, Orihuela C J, Tuomanen E I. Streptococcus pneumoniae : invasion and inflammation . Microbiol Spectr. 2019;7(02):1–31. doi: 10.1128/microbiolspec.gpp3-0004-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balachandran P, Hollingshead S K, Paton J C, Briles D E. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J Bacteriol. 2001;183(10):3108–3116. doi: 10.1128/JB.183.10.3108-3116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bohländer F, Riehl D, Weißmüller S, Gutscher M, Schüttrumpf J, Faust S. Immunomodulation: immunoglobulin preparations suppress hyperinflammation in a COVID-19 model via FcγRIIA and FcαRI . Front Immunol. 2021;12:700429. doi: 10.3389/fimmu.2021.700429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


