Abstract
Objectives
To compare mortality of hospitalized COVID-19 patients under two low–molecular weight heparin (LMWH) thromboprophylaxis strategies: standard dose and variable dose (standard dose increased to intermediate dose in the presence of laboratory abnormalities indicating an increased thrombosis risk).
Study Design and Setting
Target trial emulation using observational data from 2,613 adults admitted with a COVID-19 diagnosis in Madrid, Spain between March 16 and April 15, 2020.
Results
A total of 1,284 patients were eligible. Among 503 patients without increased baseline thrombotic risk, 28-day mortality risk (95% confidence interval [CI]) was 9.0% (6.6, 11.7) under the standard dose strategy and 5.6% (3.3, 8.3) under the variable dose strategy; risk difference 3.4% (95% CI: −0.24, 6.9); mortality hazard ratio 1.61 (95% CI: 0.97, 2.89). Among 781 patients with increased baseline thrombotic risk, the 28-day mortality risk was 25.8% (22.7, 29.0) under the standard dose strategy and 18.1% (9.3, 28.9) under the intermediate dose strategy; risk difference 7.7% (95% CI: −3.5, 17.2); mortality hazard ratio 1.45 (95% CI: 0.81, 3.17). Major bleeding and LMWH-induced coagulopathy were rare under all strategies.
Conclusion
Escalating anticoagulation intensity after signs of thrombosis risk may increase the survival of hospitalized COVID-19 patients. However, effect estimates were imprecise and additional studies are warranted.
Keywords: Anticoagulants, COVID-19, COVID-19 drug treatment, Epidemiological methods, Low–molecular weight heparin, Target trial emulation
What is new?
Key findings
-
•
In this target trial emulation including 1,284 hospitalized COVID-19 patients, a flexible thromboprophylaxis strategy that increases to a higher nontherapeutic dose (intermediate dose) after increased thrombotic risk is suspected was superior to the standard low dose strategy among patients without increased thrombotic risk at baseline.
What this adds to what was known?
-
•
Therapeutic-dose heparin is known to reduce organ support-free days among hospitalized COVID-19 patients in comparison to standard low-dose heparin. This study expands existing evidence on thromboprophylaxis strategies for hospitalized COVID-19 patients by examining a widely-adopted and recommended dynamic thromboprophylaxis strategy where intermediate (non-therapeutic) doses of heparin are used only in presence of increased thrombotic risk.
What is the implication and what should change now?
-
•
Standard-dose heparin should be escalated to intermediate (non-therapeutic) dose if signs of thrombosis risk are detected among COVID-19 hospitalized patients.
1. Introduction
Coagulopathy and thrombotic complications are frequent and serious complications among hospitalized COVID-19 patients [[1], [2], [3], [4], [5]]. Therefore, most clinical guidelines recommend thromboprophylaxis with low–molecular weight heparin (LMWH), for example, 4,000 IU of enoxaparin subcutaneously daily for nonobese patients with normal renal function, for patients not previously on therapeutic anticoagulation and without thrombosis [[6], [7], [8], [9], [10], [11], [12]].
The optimal dose of LMWH, however, remains under debate [13,14] because evidence suggests some degree of heparin resistance especially in severe [15] and critical [16] COVID-19 patients. A recent randomized trial examined the role of therapeutic anticoagulation (e.g., 1 IU of enoxaparin subcutaneously twice per day or comparable dosage of dalteparin or tinzaparin) for thromboprophylaxis among hospitalized COVID-19 patients. Compared with the standard prophylactic dose, therapeutic anticoagulation increased organ support-free days by 27% among noncritically ill patients [17] but was futile in critically ill counterparts [18] and increased risk of major bleeding two-fold. Some expert panels [19] and clinical guidelines [20,21] recommend intermediate doses of LMWH that are higher than the standard prophylactic dose but lower than the therapeutic dose. These intermediate doses are especially recommended in the presence of laboratory abnormalities that suggest increased thrombosis risk (e.g., elevated D-dimer level) [22,23].
In hospitalized COVID-19 patients, some observational studies found a lower risk of thrombotic complications [23,24] and death [23,[25], [26], [27]] for intermediate dose compared with standard-dose LMWH prophylaxis. However, a randomized trial in COVID-19 patients admitted to the intensive care unit found a similar risk of a composite outcome (including thromboembolic complications, treatment with extracorporeal membrane oxygenation, and 30-day mortality) in the intermediate-dose and standard-dose groups. The risks of severe thrombocytopenia and bleeding were slightly higher in the intermediate-dose group than in the standard-dose group [28].
Here we used electronic health records to emulate a (hypothetical) target trial that compares two LMWH thromboprophylaxis strategies for hospitalized COVID-19 patients: a standard dose strategy and a variable dose strategy based on standard dose that is increased after laboratory abnormalities suggesting an increased risk of thrombosis. We first specify the protocol of the target trial that would have answered the causal question of interest and then define the observational analysis that explicitly emulates this target trial.
2. Methods
2.1. Target trial specification
We specified the protocol of a target trial to estimate the effect of standard dose vs. variable dose of LMWH on the mortality of hospitalized COVID-19 patients. Table 1 summarizes the key protocol components. Briefly, the eligibility criteria include age ≥ 18 years, admission for ≥ 24 hours to La Paz University Hospital (a large teaching hospital in Madrid, Spain) between March 16 and April 15, 2020 with an RT-PCR–confirmed COVID-19 diagnosis, no active bleeding, no severe thrombocytopenia (platelet count <50,000/mL), no long-term use of anticoagulation, no thrombosis, and no baseline analytical abnormalities indicating an increased thrombotic risk (C-reactive protein >150 mg/dL, D-dimer >1,500 ng/mL, ferritin >1,000 μg/L, or lymphocyte count ≤ 800/mL).
Table 1.
Outline of specification and emulation of a target trial of thromboprophylaxis in COVID-19 patients with no signs of increased thrombotic risk
| Component | Target trial specification | Target trial emulation |
|---|---|---|
| Eligibility |
|
Same. |
| Treatment strategies |
Individuals are excused from following the assigned strategy after thrombosis (when anticoagulation treatment must be started) and after active bleeding or platelet count <50,000/mL (when anticoagulation must be discontinued). |
Same. |
| Treatment assignment | Individuals are randomly assigned to one of the strategies. Individuals are aware of the strategy they are assigned to. | Individuals are assigned to the strategy their baseline data are compatible with. |
| Follow-up | For each individual, follow-up starts at the time of assignment to a strategy and ends at death, hospital discharge, or 28 days after hospitalization. | Same. |
| Primary end point | All-cause mortality at 28 days. We assume no deaths occurred after hospital discharge. | Same. |
| Causal contrast | Per-protocol effect. Intention-to-treat effect. |
Observational analog of the per-protocol effect. |
| Statistical analysis | Intention-to-treat analysis: 28-day survival curves and hazard ratios are estimated using pooled logistic regression models. Individuals who are discharged are assumed to remain alive through day 28. Per-protocol analysis: Same as intention-to-treat analyses, with two exceptions. First, individuals are censored when their data stop being compatible with the strategy they are assigned to. Second, each individual receives a time-varying inverse probability weight to adjust for the potential selection bias introduced by censoring. |
Same as per-protocol analysis except that, for individuals having treatment and laboratory data compatible with both strategies at baseline, we created two clones and assigned each clone to a different strategy. |
Eligible individuals would be randomly assigned to either standard-dose or variable-dose thromboprophylaxis strategies with LMWH. Under the standard strategy, individuals would receive LMWH at a standard prophylactic dose (e.g., 4,000 IU of enoxaparin subcutaneously every 24 hours for a nonobese individual). Under the variable strategy, the initial standard dose will be increased to an intermediate prophylactic dose (e.g., 6,000 IU of enoxaparin subcutaneously every 24 hours for a nonobese individual), which does not reach therapeutic dose (e.g., > 9,000 IU of enoxaparin subcutaneously every 24 hours) within 48 hours of the recording of laboratory abnormalities indicating increased thrombotic risk. The choice of drug is left to the physician's discretion. Individuals are excused from following the assigned strategy after thrombosis (when anticoagulation treatment must be started) and after active bleeding or platelet count <50,000/mL (when anticoagulation must be discontinued). The causal contrasts of interest would be the intention-to-treat and the per-protocol effect.
2.2. Statistical analysis of the target trial
The intention-to-treat analysis is based on the comparison of 28-day survival curves and of mortality hazard ratios. The survival curves can be estimated using a nonparametric Kaplan–Meier estimator or a pooled logistic regression model with an indicator for treatment group, a flexible time-varying intercept, and product terms between treatment group and time. The hazard ratios can be estimated using the same logistic model without the product terms [29]. Individuals who are discharged are assumed to remain alive through day 28.
The per-protocol analysis is the same as the intention-to-treat analysis with two exceptions. First, individuals are censored when they deviate from their assigned strategy. Individuals assigned to the standard-dose strategy are censored if/when they receive an intermediate dose. Individuals assigned to the variable dose strategy are censored if/when they receive an intermediate dose in the absence of analytic abnormalities or if they do not receive an intermediate dose within 48 hours of analytic abnormalities. Second, to adjust for the potential selection bias introduced by censoring, we would use nonstabilized inverse probability weights [30] (Supplement) to adjust for baseline and time-varying covariates. Baseline covariates include age, gender, migration status, disability, high blood pressure, diabetes mellitus, dyslipidemia, chronic heart disease, chronic kidney disease, active oncological disease, active smoking, Charlson Comorbidity Index, CURB-65 score for pneumonia severity, and heart rate at admission. Time-varying variables include C-reactive protein, D-dimer, and lymphocyte count. The weights can be truncated at the 99th percentile to avoid undue influence of outliers. The validity of the per-protocol effect estimates require no unmeasured confounding given the above covariates and no model misspecification.
2.3. Target trial emulation
We emulated this target trial using observational data from La Paz University Hospital. Data on individuals’ sociodemographic characteristics, previous medical history and regular medications, vital signs at admission and during hospitalization, comorbidity and pneumonia severity scales, treatments received during hospitalization, including start and stop dates, and clinical outcomes, including intensive care admission, mortality, hospital discharge, nonclinically significant coagulopathy (i.e., prothrombin time or international normalized ratio variations without clinical impact), clinically significant coagulopathy (i.e., prothrombin time or international normalized ratio variations and clinically compatible symptoms), and incident major bleeding (i.e., driving >2 g/dL drops in hemoglobin levels or requiring transfusion of blood or blood products) were retrieved from electronic health records by resident physicians or supervised last year medical students. For each individual, we linked these data with every laboratory determination performed during hospitalization. Ferritin was not monitored routinely in most individuals. We defined time zero as the hospitalization date.
To estimate the observational analog of the per-protocol effect, we conducted the statistical analysis described above with a difference: we created two copies (clones) of each eligible individual, one per strategy, and conducted the analysis in the expanded dataset [30]. We could not estimate an observational analog of the intention-to-treat effect because individuals could not be assigned to a single strategy at time zero.
We also emulated a second identical target trial except that it was restricted to individuals with baseline laboratory abnormalities indicating increased thrombotic risk (C-reactive protein >150 mg/dL, D-dimer >1,500 ng/mL, ferritin >1,000 μg/L, or lymphocyte count ≤ 800/mL). These individuals have a worse prognosis than individuals without baseline laboratory abnormalities and thus need to be considered separately. In this second trial, both strategies are constant given that either the standard dose or the intermediate dose is assigned at baseline and the emulation does not require cloning because the strategy followed by each individual is known at baseline.
2.4. Sensitivity analyses
We conducted two sensitivity analyses. First, to assess the magnitude of the measured confounding, we conducted only age-adjusted and gender-adjusted analyses. Second, we repeated our analyses in COVID-19 patients who, at admission, had both increased thrombotic risk and severe COVID-19 pneumonia (as defined by the World Health Organization's guidelines [31]).
Study procedures were approved by La Paz University Hospital's Institutional Review Board. Statistical analyses were programmed in R Studio. Study data are available on request from the corresponding author upon approval by the steering committee of the COVID@HULP group. Analyses were finalized on July 15, 2021. All analyses were conducted using R version 3.6.2. The R code used in this manuscript is publicly available in: https://www.hsph.harvard.edu/miguel-hernan/causal-inference-book/.
3. Results
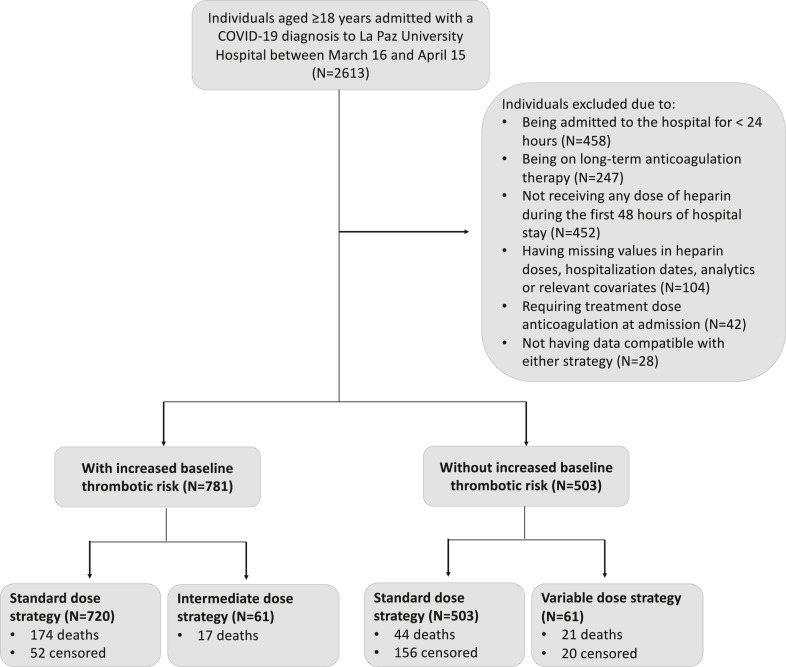
Of 2,613 individuals aged 18 years or more admitted with a COVID-19 diagnosis between March 16 and April 15, 1,284 (503 without increased baseline thrombotic risk and 781 with increased baseline thrombotic risk) were eligible (Fig. 1 ). Individuals with increased baseline thrombotic risk were on average 9 years older and more likely to be men, to be nonimmigrants, and to have comorbidities than those without increased thrombotic risk (Table 2 ).
Fig. 1.
Flowchart of eligible and included individuals for the emulation of a target trial of thromboprophylaxis in COVID-19 patients, Hospital Universitario La Paz, Madrid, Spain; March 16–April 15, 2020.
Table 2.
Baseline characteristics of individuals eligible for the emulation of a target trial of thromboprophylaxis in COVID-19 patients, Hospital Universitario La Paz, Madrid, Spain; March 16–April 15, 2020
| Variable | Increased baseline thrombotic riskb |
|
|---|---|---|
| Yes (N = 781) | No (N = 503) | |
| Age (yr), median (IQR) | 70 (59, 81) | 61.0 (51.0, 76.0) |
| Gender (% Male) | 60.2 | 47.1 |
| Immigrant (%) | 18.8 | 31.2 |
| Disability (%)a | 7.8 | 6.6 |
| Suspected hospital acquisition (%) | 15.1 | 16.7 |
| Chronic heart disease (%) | 16.3 | 14.11 |
| High blood pressure (%) | 52.1 | 42.1 |
| Diabetes mellitus (%) | 22.2 | 18.5 |
| Obesity (%) | 17.2 | 18.7 |
| Dyslipidemia (%) | 37.6 | 36.6 |
| Active smoking (%) | 7.4 | 4.4 |
| Chronic kidney disease (%) | 10.8 | 2.8 |
| Malignant neoplasms (%) | 11.1 | 5.9 |
| Hematological disease (%) | 52.5 | 6.2 |
| Charlson Comorbidity Index, median (IQR) | 3 (2, 5) | 2 (1, 4) |
| CURB-65 score, median (IQR) | 1 (0, 2) | 1 (0, 1) |
| Heart rate at admission, bpm, median (IQR) | 91 (80, 104) | 91 (78, 105) |
| Admission before/after April 5 (%) | 85.3 | 84.3 |
| D-dimer at baseline | 1,088.5 (609.5; 2,159.5) | 540.0 (368.0, 802.0) |
Abbreviations: IQR, interquartile range.
Disability as indicated by a Katz Basic Activities of Daily Living Score ≤ 2.
C-reactive protein >150 mg/dL, D-dimer >1,500 ng/mL, ferritin >1,000 μg/L, or lymphocyte count <800/mL.
3.1. Individuals without increased baseline thrombotic risk
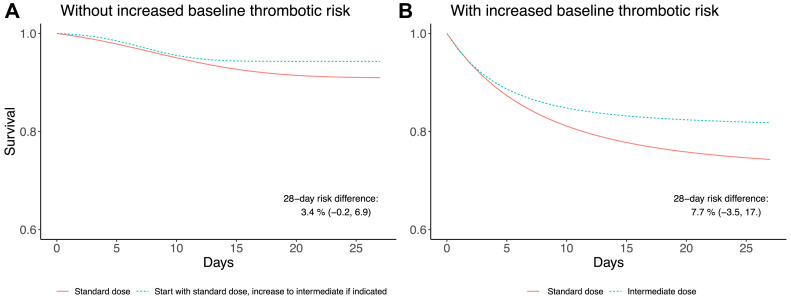
The 28-day mortality risk was 9.0% (95% confidence interval [CI]: 6.6, 11.7) under the standard dose strategy and 5.6% (95% CI: 3.3, 8.3) under the variable dose strategy; risk difference 3.4% (95% CI: −0.24, 6.9). Figure 2 shows the survival curves. The mortality hazard ratio (95% CI) for the standard vs. the variable dose strategy was 1.58 (0.93, 2.88) (Table 3 ).
Fig. 2.
Estimated survival curves under two thromboprophylaxis strategies for COVID-19 patients, Hospital Universitario La Paz, Madrid, Spain; March 16–April 15, 2020 for (A) individuals without increased baseline thrombotic risk and (B) individuals with increased baseline thrombotic risk.
Table 3.
Estimated 28-day mortality risks under two thromboprophylaxis strategies for COVID-19 patients, Hospital Universitario La Paz, Madrid, Spain; March 16–April 15, 2020
| 28-day mortality risk (No. deaths) | Risk differencea (95% CI) | Hazard ratioa,b (95% CI) | ||
|---|---|---|---|---|
| Always standard dose | Start with standard dose, increase to intermediate dose if indicated | |||
| No increased baseline thrombotic risk (N = 503) | 8.7% (44) | 4.2% (21) | 3.4 (−0.2, 6.9) | 1.58 (0.93, 2.88) |
| Always standard dose | Always intermediate dose | |||
| Increased baseline thrombotic risk (N = 781) | 24.2% (174) | 27.9% (17) | 7.7 (−3.5, 17.2) | 1.45 (0.81, 3.17) |
Increased baseline thrombotic risk is indicated by presence of the following laboratory abnormalities at baseline: C-reactive protein >150 mg/dL, D-dimer >1,500 ng/mL, ferritin >1,000 μg/L, or lymphocyte count ≤ 800/mL.
Adjusted for baseline confounders using inverse probability weighting: age, gender, migration status, disability, high blood pressure, diabetes mellitus, dyslipidemia, active smoking, Charlson Comorbidity Index, CURB-65 score for pneumonia severity, chronic heart disease, chronic kidney disease, malignant neoplasm, and heart rate at admission.
Additionally adjusted for time-varying indicators using inverse probability weighting: C-reactive protein >150 mg/dL, D-dimer >1,500 ng/mL, and lymphocyte count <800/mL (only for individuals without an increased baseline thrombotic risk).
Overall, there were one episode of incident major active bleeding, five episodes of nonclinically significant coagulopathy, and two episodes of clinically significant coagulopathy.
3.2. Individuals with increased baseline thrombotic risk
The 28-day mortality risk was 25.8% (95% CI: 22.7, 29.0) under the standard-dose strategy and 18.1% (95% CI: 9.3, 28.9) under the intermediate-dose strategy; risk difference 7.7% (95% CI: −3.5, 17.2). Figure 2 shows the survival curves. The mortality hazard ratio (95% CI) for the standard-dose vs. intermediate-dose strategy was 1.45 (0.81, 3.17) (Table 3).
Under the standard-dose strategy, there were three episodes of incident major active bleeding, 25 episodes of nonclinically significant coagulopathy, and 11 episodes of clinically significant coagulopathy. Under the intermediate-dose strategy, there were no episodes of incident major active bleeding, three episodes of nonclinically significant coagulopathy, and one episode of clinically significant coagulopathy.
3.3. Sensitivity analyses
When only adjusting the models for age and gender, the mortality hazard ratio (95% CI) was 1.70 (1.03, 3.0) for standard-dose vs. variable-dose strategies among individuals without increased baseline thrombotic risk and 0.91 (0.58, 1.58) for standard vs. intermediate dose among individuals with increased baseline thrombotic risk. The main confounders driving the difference between the hazard ratios for unadjusted and adjusted models for individuals with increased baseline thrombotic risk (0.91 unadjusted vs. 1.45 adjusted) were disability, diabetes mellitus, CURB-65 score for pneumonia severity, and heart rate at admission.
When restricting the analyses to patients with both increased thrombotic risk and severe COVID-19 pneumonia, the mortality hazard ratio (95% CI) was 1.25 (0.56, 3.07) for standard vs. intermediate dose.
4. Discussion
We used observational data from 1,283 hospitalized COVID-19 patients to emulate two target trials of thromboprophylaxis with LMWH. In individuals without signs of increased thrombotic risk at baseline, the estimated 28-day mortality risk was 3 percentage points higher under the standard prophylactic dose than under a strategy that increases to a higher nontherapeutic dose (intermediate dose) after increased thrombotic risk is suspected, with values between 0 and 7 percentage points being very compatible with our data. In individuals with signs of increased thrombotic risk, the difference was about 8 percentage points higher for standard dose compared with intermediate dose, with values between −4 and 17 percentage points being very compatible with our data. There were no differences between strategies in terms of coagulopathy and major bleeding, which were overall rare.
Our findings are consistent with several observational studies of noncritical COVID-19 patients, which found a lower mortality among hospitalized patients receiving intermediate-dose LMWH than in those receiving standard-dose [23,[25], [26], [27]]. In contrast, when comparing standard-dose vs. intermediate-dose thromboprophylaxis among critical patients, one observational study did not find 14-day mortality differences [24] and the Intermediate vs Standard-Dose Prophylactic Anticoagulation in Critically-ill Patients With COVID-19 randomized trial found no clinically significant differences in a composite outcome including thromboembolic complications, treatment with extracorporeal membrane oxygenation, and 30-day mortality [28]. Similarly, a large randomized trial found that, in comparison with standard-dose thromboprophylaxis, therapeutic doses reduce organ support-free days in noncritical patients but not in critical patients [17,18]. In our study, analyses restricted to severely ill patients with increased baseline thrombotic risk yielded inconclusive results with very wide 95% CIs.
By examining a dynamic strategy in which the intermediate dose is only used after analytic signs of increased thrombotic risk, as empirically recommended by expert panels [17] and widely adopted clinical guidelines [18,19], our study expands existing evidence and generates estimates more relevant for clinical practice. Moreover, the explicit emulation of a target trial overcomes some common limitations of observational analyses [32,33] and enhances the clinical interpretability of results.
This study has limitations. First, as with any observational data study with a causal goal, we cannot rule out the possibility of residual confounding. However, we adjusted for key baseline and time-varying prognostic factors and the adjustment did not have a substantial impact of the effect estimates for patients without elevated thrombotic risk, which suggests that large residual confounding is unlikely. Second, by only examining in-hospital mortality, we may have somewhat underestimated mortality risk due to thromboembolic events [34,35] and other causes [36] following hospital discharge. However, we do not have reasons to believe that outpatient outcomes differed across treatment strategy groups. Third, we did not study anticoagulant agents (i.e., direct oral anticoagulants) other than LMWH [37] because the latter is the most frequently recommended agent for prophylactic anticoagulation in hospitalized COVID-19 patients, mostly because of their wide availability and extensive experience of use in hospitalized patients [38,39]. In addition, LMWH might possess anti-inflammatory [40] and antiviral [41] properties.
In conclusion, our results suggest that escalating the intensity of anticoagulation after detection of laboratory markers of thrombosis risk increases survival among noncritical hospitalized COVID-19 patients. However, our estimates were imprecise and additional studies are warranted.
Acknowledgments
This work was supported by a “la Caixa” Foundation fellowship (ID 100010434, code “LCF/BQ/DR19/11740016”) and an ASISA-Harvard fellowship. Funders had no role in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
G Martínez-Alés and A Domingo-Relloso contributed equally.
Conflict of interest: All authors declare no competing interests related to this work.
Author Contributions: G. Martínez-Alés, A. Domingo-Relloso, M. Quintana-Díaz, and M. A. Hernán conceptualized the study and designed the analyses. G. Martínez-Alés and M. Quintana-Díaz acquired the data. G. Martínez-Alés and M. A. Hernán drafted the manuscript. A. Domingo-Relloso processed the data and implemented the analyses. All authors contributed to study design and interpretation of results and reviewed, revised, and approved the manuscript. The corresponding author accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jclinepi.2022.08.006.
Appendix A. Supplementary Data
References
- 1.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York city health system. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Samkari H., Karp Leaf R.S., Dzik W.H., Carlson J.C.T., Fogerty A.E., Waheed A., et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moores L.K., Tritschler T., Brosnahan S., Carrier M., Collen J.F., Doerschug K., et al. Diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158:1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McBane R.D., Torres Roldan V.D., Niven A.S., Pruthi R.K., Franco P.M., Linderbaum J.A., et al. Anticoagulation in COVID-19: a systematic Review, meta-analysis, and rapid guidance from mayo clinic. Mayo Clin Proc. 2020;95:2467–2486. doi: 10.1016/j.mayocp.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godino C., Scotti A., Maugeri N., Mancini N., Fominskiy E., Margonato A., et al. Antithrombotic therapy in patients with COVID-19? -Rationale and Evidence. Int J Cardiol. 2021;324:261–266. doi: 10.1016/j.ijcard.2020.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antithrombotic Therapy. COVID-19 Treatment Guidelines.
- 12.Clinical management of COVID-19.
- 13.Bikdeli B. Anticoagulation in COVID-19: randomized trials should set the balance between excitement and evidence. Thromb Res. 2020;196:638–640. doi: 10.1016/j.thromres.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cattaneo M., Bertinato E.M., Birocchi S., Brizio C., Malavolta D., Manzoni M., et al. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020;120:1230–1232. doi: 10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beun R., Kusadasi N., Sikma M., Westerink J., Huisman A. Thromboembolic events and apparent heparin resistance in patients infected with SARS-CoV-2. Int J Lab Hematol. 2020;42:19–20. doi: 10.1111/ijlh.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White D., MacDonald S., Bull T., Hayman M., de Monteverde-Robb R., Sapsford D., et al. Heparin resistance in COVID-19 patients in the intensive care unit. J Thromb Thrombolysis. 2020;50:287–291. doi: 10.1007/s11239-020-02145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ATTACC Investigators, ACTIV-4a Investigators, REMAP-CAP Investigators. Lawler P.R., Goligher E.C., Berger J.S., Neal M.D., McVerry B.J., Nicolau J.C., et al. Therapeutic anticoagulation with heparin in noncritically ill patients with covid-19. N Engl J Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.REMAP-CAP Investigators, ACTIV-4a Investigators, ATTACC Investigators. Goligher E.C., Bradbury C.A., McVerry B.J., Lawler P.R., Berger J.S., Gong M.N., et al. Therapeutic anticoagulation with heparin in critically ill patients with covid-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernández-Capitán C., Barba R., Díaz-Pedroche M.D.C., Sigüenza P., Demelo-Rodriguez P., Siniscalchi C., et al. Presenting Characteristics, Treatment Patterns, and Outcomes among Patients with Venous Thromboembolism during Hospitalization for COVID-19. Semin Thromb Hemost. 2020;47:351–361. doi: 10.1055/s-0040-1718402. [DOI] [PubMed] [Google Scholar]
- 20.Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T., et al. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vivas D., Roldán V., Esteve-Pastor M.A., Roldán I., Tello-Montoliu A., Ruiz-Nodar J.M., et al. Recommendations on antithrombotic treatment during the COVID-19 pandemic. Position statement of the Working Group on Cardiovascular Thrombosis of the Spanish Society of Cardiology. Rev Esp Cardiol (Engl Ed) 2020;73:749–757. doi: 10.1016/j.rec.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattioli M., Benfaremo D., Mancini M., Mucci L., Mainquà P., Polenta A., et al. Safety of intermediate dose of low molecular weight heparin in COVID-19 patients. J Thromb Thrombolysis. 2020;51:286–292. doi: 10.1007/s11239-020-02243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinelli I., Ciavarella A., Abbattista M., Aliberti S., De Zan V., Folli C., et al. Increasing dosages of low-molecular-weight heparin in hospitalized patients with Covid-19. Intern Emerg Med. 2021;16:1223–1229. doi: 10.1007/s11739-020-02585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tacquard C., Mansour A., Godon A., Godet J., Poissy J., Garrigue D., et al. IMpact of high dose prophylactic anticoagulation in critically ill patients with COVID-19 pneumonia. Chest. 2021;159:2417–2427. doi: 10.1016/j.chest.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meizlish M.L., Goshua G., Liu Y., Fine R., Amin K., Chang E., et al. Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: a propensity score-matched analysis. Am J Hematol. 2021;96:471–479. doi: 10.1002/ajh.26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ionescu F., Jaiyesimi I., Petrescu I., Lawler P.R., Castillo E., Munoz-Maldonado Y., et al. Association of anticoagulation dose and survival in hospitalized COVID-19 patients: a retrospective propensity score-weighted analysis. Eur J Haematol. 2021;106:165–174. doi: 10.1111/ejh.13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu A., Liu Y., Zayac A.S., Olszewski A.J., Reagan J.L. Intensity of anticoagulation and survival in patients hospitalized with COVID-19 pneumonia. Thromb Res. 2020;196:375–378. doi: 10.1016/j.thromres.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.INSPIRATION Investigators. Sadeghipour P., Talasaz A.H., Rashidi F., Sharif-Kashani B., Beigmohammadi M.T., Farrokhpour M., et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson W.A. On the treatment of grouped observations in life studies. Biometrics. 1977;33:463–470. [PubMed] [Google Scholar]
- 30.Cain L.E., Robins J.M., Lanoy E., Logan R., Costagliola D., Hernán M.A. When to start treatment? A systematic approach to the comparison of dynamic regimes using observational data. Int J Biostat. 2010;6 doi: 10.2202/1557-4679.1212. Article 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO/2019-nCoV/clinical/2020.5
- 32.Hernán M.A., Robins J.M. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183:758–764. doi: 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernán M.A., Sauer B.C., Hernández-Díaz S., Platt R., Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70–75. doi: 10.1016/j.jclinepi.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patell R., Bogue T., Koshy A., Bindal P., Merrill M., Aird W.C., et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136:1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giannis D., Allen S.L., Tsang J., Flint S., Pinhasov T., Williams S., et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. 2021;137:2838–2847. doi: 10.1182/blood.2020010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chopra V., Flanders S.A., O’Malley M., Malani A.N., Prescott H.C. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2020;174:576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schutgens R.E. DOAC in COVID-19: yes or No? HemaSphere. 2020;5:e526. doi: 10.1097/HS9.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samama M.M., Cohen A.T., Darmon J.Y., Desjardins L., Eldor A., Janbon C., et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341:793–800. doi: 10.1056/NEJM199909093411103. [DOI] [PubMed] [Google Scholar]
- 39.Leizorovicz A., Cohen A.T., Turpie A.G.G., Olsson C.-G., Vaitkus P.T., Goldhaber S.Z. PREVENT Medical Thromboprophylaxis Study Group. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110:874–879. doi: 10.1161/01.CIR.0000138928.83266.24. [DOI] [PubMed] [Google Scholar]
- 40.Mousavi S., Moradi M., Khorshidahmad T., Motamedi M. Anti-inflammatory effects of heparin and its derivatives: a systematic Review. Adv Pharmacol Sci. 2015;2015:507151. doi: 10.1155/2015/507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim S.Y., Jin W., Sood A., Montgomery D.W., Grant O.C., Fuster M.M., et al. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antiviral Res. 2020;181:104873. doi: 10.1016/j.antiviral.2020.104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.