Abstract
The early detection of biomarker proteins in clinical samples is of great significance for the diagnosis of diseases. However, it is still a challenge to detect low-concentration protein. Herein, a label-free aptamer-based amplification assay, termed the ATC-TA system, that allows fluorescence detection of very low numbers of protein without time-consuming washing steps and pre-treatment was developed. The target induces a conformational change in the allosteric aptasensor, triggers the target cycling and transcription amplification, and ultimately converts the input of the target protein into the output of the light-up aptamer (R-Pepper). It exhibits ultrahigh sensitivity with a detection limit of 5.62 fM at 37 ℃ and the accuracy is comparable to conventional ELISA. ATC-TA has potential application for the detection of endogenous PDGF-BB in serum samples to distinguish tumor mice from healthy mice at an early stage. It also successfully detects exogenous SARS-CoV-2 spike proteins in human serum. Therefore, this high-sensitive, universality, easy-to-operate and cost-effective biosensing platform holds great clinical application potential in early clinical diagnosis.
Keywords: Allosteric aptasensor, Target cycling signal amplification, Transcription amplification, Protein detection, Light-up RNA aptamer
1. Introduction
Proteins play a critical role in multifarious biological processes and are deemed to be important biomarkers for clinical diagnosis and prognosis. Early detection of serum biomarker proteins is of great significance for the early diagnosis and treatment of diseases, such as cancer and viral infection [1], [2], [3]. Protein can be measured by different methods including enzyme-linked immunosorbent assays (ELISA) [4], mass spectrometry-based proteomics [5], [6], surface plasmon resonance (SPR) [7], electrochemiluminescence [8] and the lateral flow assay-based method [9], [10]. Among them, ELISA has been the classical method for the quantitative assessment of water-soluble proteins. It is performed in the form of a commercial kit which has the advantage of convenience. Nevertheless, due to the low sensitivity, generally ranging from ng/ml to μg/ml [11], it can't meet the growing demand for early diagnosis of diseases based on low-abundance biomarkers. To improve the sensitivity of ELISA, various signal amplification techniques, such as redox-cycling-based colorimetric ELISA [12], digital ELISA [13] and plasmonic ELISA [14], have been developed. However, the time-consuming washing steps in such assays not only make them labor-intensive but also prevent analysis in real-time, limiting further application. Although the lateral flow assay-based method is simple and suitable for rapid detection [15], [16], it can only be used for semi-quantitative analysis and isn't sensitive enough, which is not suitable for low-concentration protein quantification or early diagnosis [9], [10].
Alternatively, aptamer-based amplification assays have been developed with great effort. Aptamers are short single-stranded DNA or RNA that can interact with corresponding targets with high affinity and specificity [17], [18]. Based on the structure of nucleic acid, aptamers can be used for allosteric regulation to develop aptamer-based amplification assays [19], such as recombinase polymerase amplification (RPA) [20], rolling circle amplification (RCA) [21], [22], hybridization chain reaction (HCR) [23], [24], CRISPR-Cas13a/12a amplification reaction [25], [26]. However, the sensitivity for most methods is at the picomolar level [27]. Therefore, it is still necessary to develop a highly sensitive technique for the detection of low concentrations of proteins.
In recent years, the light-up RNA aptamers, such as Spinach, Broccoli and Pepper, which can bind fluorogens (DFHBI/DFHBI-1 T, HBC, et) and enhance their fluorescence by several orders of magnitude upon binding, have been applied as a promising label-free reporter for in biological detection [28]. Compared with light-up RNA aptamers, the non-specific binding of sequences, such as SYBR Gold and SYBR Green I, inevitably, leads to high background or nonspecific signals[29]. Although covalently labeled probes would allow specific labeling, however, compromised with complicated design and high cost [30]. Hence, the advantages of light-up RNA aptamers could be exploited as an ideal reporter for target-specific detection.
Herein, we designed a label-free allosteric aptasensor (Aa) to develop an aptamer-based cascade amplification assay for protein detection, termed the “allosteric aptasensor-initiated target cycling and transcription amplification” (ATC-TA) system, to overcome the limitation of sensitivity and cumbersome procedures in protein detection. As a proof of concept, platelet-derived growth factor-BB (PDGF-BB) was chosen as the model analyte, which is an important biomarker for tumor diagnosis [31], [32]. The accuracy of this system is comparable to ELISA in complex samples, but exhibits ultrahigh sensitivity. This system not only realizes fluorescent detection but also can detect protein in real-time and visual detection. Additionally, using the aptamer of SARS-CoV-2 spike protein previously reported by our group [33], an ATC-TA system for detecting spike protein was established, which showed that this system has universality, making it more applicable for protein detection.
2. Materials and methods
2.1. Materials
The PDGF-BB aptamer (ssDNA, 5′- CAGGCTACGGCACGTAGAGCATCACCATGATCCTG-3′), the aptamer of SARS-CoV-2 spike protein (ssDNA, 5′- GGGGAGGGCGGGTGGATTGGATGCCGA −3′) and all other oligonucleotides (Table S1) were purchased from Sangon Biotech (Shanghai, China) and purified by HPLC.
2.2. Binding assays of an allosteric aptasensor to PDGF-BB
PDGF-BB proteins (100 ng per well) were coated in the 96-well ELISA microplates with 100 μL coating buffer at 4 °C overnight. The PDGF-BB -protein-coated plates were washed twice with washing buffer before blocking with 200 μL of the solution (2% BSA in PBST) at RT for 1 h. Then, 200 nM final concentration biotin-labeled Aa at the 5′-end were added into the wells and incubated in binding buffer at RT for 30 min with gentle shaking. After washing two times, streptavidin-horseradish peroxidase (HRP) and its substrates were sequentially added to the reactions. Color development was carried out using TMB substrate and measured using a microplate reader at 450 nm absorbance.
2.3. ATC-TA for protein detection
The amplification assay was carried out in a 10 μL mixture containing 5 nM Aa, 5 nM Tp, 1 × Bst buffer (20 mM Tris-HCl (pH 8.8), 10 mM KCl, 10 mM (NH4)2SO4, 2 mM MgSO4 and 0.1% Triton X-100), 10 mM NaCl, 4 mM MgSO4, 0.5 mM dNTPs, 0.12 units/μL of Bst DNA polymerase and protein at 37 °C for 1 h. After this extended reaction, the transcription was carried out. ATC products were transcribed in a 20 μL reaction containing 10 μL of ATC product, 0.75 mM of NTP, 1 × T7 RNA polymerase buffer (40 mM Tris-HCl, pH 7.9, 10 mM NaCl, 10 mM DTT, 6 mM MgCl2, 2 mM spermidine), and 0.75 units/μL of T7 RNA polymerase at 37 °C for 2 h. Afterward, fluorescence was measured at respective wavelengths.
2.4. Spectroscopic measurements
A solution of the ATC-TA products and HBC (10 µM) were incubated with the target in a buffer containing 40 mM HEPES (pH=7.4), 100 mM KCl and 10 mM MgCl2 at 25 ℃ for 10 min. Fluorescence spectra were measured using Bio-Tek Synergy H1 equipped with 25 ℃. The emission wavelength window, 495–600 nm; slit widths, 5 nm. The excitation wavelength was 460 nm; and the emission wavelength was 514 nm.
3. Results and discussion
3.1. The principle of the ATC-TA system
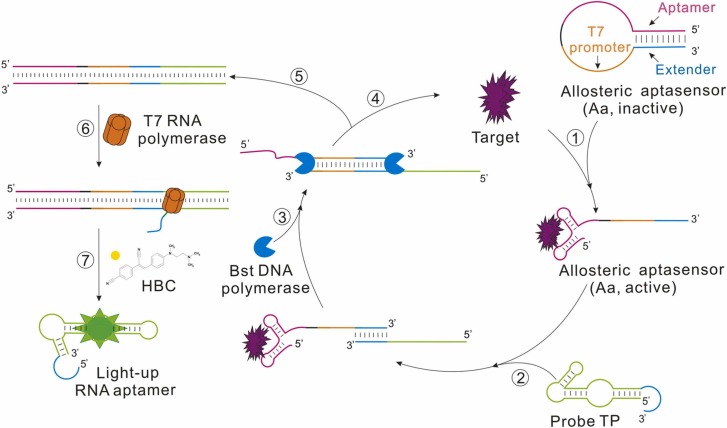
The principle of the designed strategy of ATC-TA is illustrated in Fig. 1. Two label-free DNA probes, namely Aa and Tp are ingeniously designed. Allosteric aptasensor (Aa) without sticky end contained four functional modules: (1) aptamer modules (carmine) for specific recognition of target protein (purple); (2) T7 promoter modules (orange); (3) 6 successive adenine bases (black) between the aptamer and T7 promoter to reduce any possible steric hindrance effect, which has no interaction with other DNA sequences; (4) extend modules (blue) which is complementary with part of Tp on the 3′ terminals to initiate the downstream reaction.
Fig. 1.
Schematic illustration of the allosteric aptasensor-initiated target cycling and transcription amplification of RNA aptamer for protein detection.
In the absence of a target, the Aa is in an inactive conformation (stem-loop conformation). The extended modules form a stem with the 5′-end of aptamer, blocking the downstream reaction. Once the protein binds to the aptamer domain, the protein would mediate the conformational switch of the aptamer module, restoring the active structure and releasing the extended modules to hybridize to the probe Tp, which consisted of two parts from the 3′- to 5′-end: the antisense sequence of the extend modules (blue) and the antisense sequence of light-up aptamer (green). Once the extended modules were captured by the probe Tp, DNA polymerase would extend on the Aa-Tp duplex to yield double-stranded DNA, followed by the displacement of the target, which will continue to bind with another Aa, initiating the target cycle. Meanwhile, T7 RNA polymerase recognized the T7 promoter sequence in double-stranded DNA and conducted RNA transcription to generate a large amount of RNA product, the light-up RNA aptamer with a tail on the 5′-end. Finally, those multiple light-up RNA aptamers bind to HBC to generate fluorescence signals as a reporter. The target cycle and transcription amplification are repeated continuously and enrichment of multiple light-up RNA aptamers, resulting in a cascade of reactions and amplified fluorescence signals.
3.2. Feasibility studies of ATC-TA
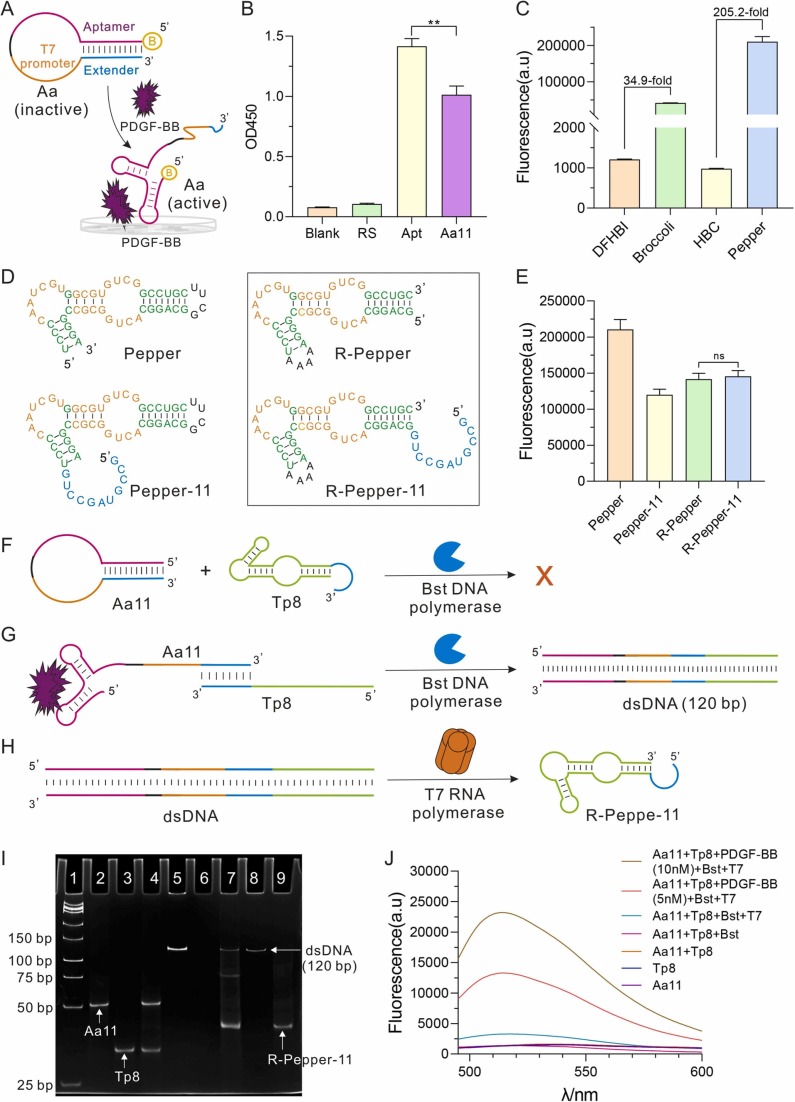
To analyze the feasibility of this strategy, Aa with 11 bp stem (Aa11) and Tp with 8 bases (Tp8) complementary to Aa were designed. Firstly, we analyze whether the PDGF-BB aptamer (Kd = 0.1 nM) domain of Aa11 could bind to PDGF-BB protein by ELISA. The result revealed that Aa11 can successfully bind with PDGF-BB ( Fig. 2A-B). Secondly, to select the light-up RNA aptamers, we compare the fluorescence of Peppers, which have superior properties, with that of Broccoli, which is one of the widely used light-up RNA aptamers [28], [34], [35]. Pepper exhibits a higher signal-to-noise ratio (S/N) (205.2-fold, Fig. 2C) and is used as the fluorescent reporter in this work.
Fig. 2.
The feasibility analysis of the ATC-TA system. A. Schematic illustrates the ELISA principle of PDGF-BB binding to the biotin-labeled Aa. B. ELISA reveals the ability of Aa to bind human PDGF-BB. Apt: aptamer of PDGF-BB. RS: random sequence. Blank: without DNA. All the P values were determined using paired t-test. * * P ≤ 0.01. C. Fluorescence intensities of Broccoli (1 μM) binding to DFHBI (10 µM) and Pepper (1 μM) binding to HBC (10 µM). The excitation and emission wavelengths are 460 nm and 500 nm for DFHBI. D. Comparison of the structure and sequence of Pepper, R-Pepper, Pepper-11, R-Pepper-11. The sequence binding to HBC is labelled in orange. R-Pepper and R-Pepper-11 are in the black box. E. The fluorescence peak density of those light-up aptamers in the presence of HBC, respectively. F. Aa11 and Tp8 do not extend without PDGF-BB. G. Bst DNA polymerase extends on the Aa11-Tp8 duplex to yield double-stranded DNA with PDGF-BB. H. T7 RNA polymerase recognizes the T7 promoter sequence and transcribes R-pepper-11. I. 16% native PAGE analysis of experimental feasibility. Lane 1: DNA ladder; Lane 2: Aa11; Lane 3: Tp8; Lane 4: Aa11 + Tp8 + Bst; Lane 5: Aa11 + PDGF-BB+ Tp8 + Bst; Lane 6: Aa11 + Tp8 + Bst+ T7; Lane 7: PDGF-BB+ Aa11 + Tp8 + Bst+ T7; The synthetic dsDNA (120 bp, Lane 8) and the transcribed R-Pepper-11 (Lane 9) were also used as the marker. J. Fluorescence spectra of each step of the ATC-TA system. Error bars were shown as means ± S.D., n = 3, triplicate.
Because the light-up RNA corresponding to Aa11 carries an 11-base tail at the 5 'end (Pepper-11), we asked whether the tail would interfere with the fluorescence signals. The result showed that the tail did interfere with Pepper 's fluorescence (Fig. 2D-E). To reduce this interference, we tried to reverse the Pepper sequence and obtained the R-Pepper (Reversed Pepper). Although the R-Pepper's fluorescence is weaker than that of Pepper, the fluorescence of R-Pepper-11 (with the tail at R-Pepper) is stronger than that of Pepper-11 and is consistent with that of R-Pepper (Fig. 2D-E), indicating that R-Pepper can tolerate more unrelated sequences than Pepper, which broadened the application range of the light-up aptamer. Therefore, R-Pepper was chosen for the subsequent experiments.
Subsequently, to verify the proposed scheme step by step, native polyacrylamide gel (PAGE) was performed. Firstly, it is necessary to investigate whether the Aa11 can be opened by Tp8 and then extended by Bst DNA polymerase. The result shows that the product band was not observed (Fig. 2F, Lane 4; Fig. 2I). When PDGF-BB was added to the reaction, the Aa11 is allosteric, hybridizing with Tp8, and an extended product with predicted length was observed in PAGE analysis (Fig. 2G, Lane 5; Fig. 2I). Afterward, the T7 RNA polymerase is bound to the T7 promoter sequence in double-stranded DNA and transcript RNA (Fig. 2H, Lane 7; Fig. 2I). The bands in Lane 7 and Lane 9 (in vitro transcribed R-Pepper-11, as the marker) were consistent with each other, suggesting that in the presence of PDGF-BB, those two probes can work well and result in amplified fluorescence signals. It is worth noting that there is one extra band in the middle of lane 7. It was proved to be the hybridization product of R-Pepper-11 and Tp8(Fig. S1A-B). Secondly, the possible false-positive results were also analyzed using native PAGE (Fig. S1C), indicating that the reaction could only be triggered by PDGF-BB. Moreover, to further confirm those results, we also measured the fluorescence intensity of each step (Fig. 2J) and the results were consistent with the native PAGE. All those results indicated that the strong fluorescence signal could be detected only in the presence of the PDGF-BB.
3.3. Optimization of reaction parameters
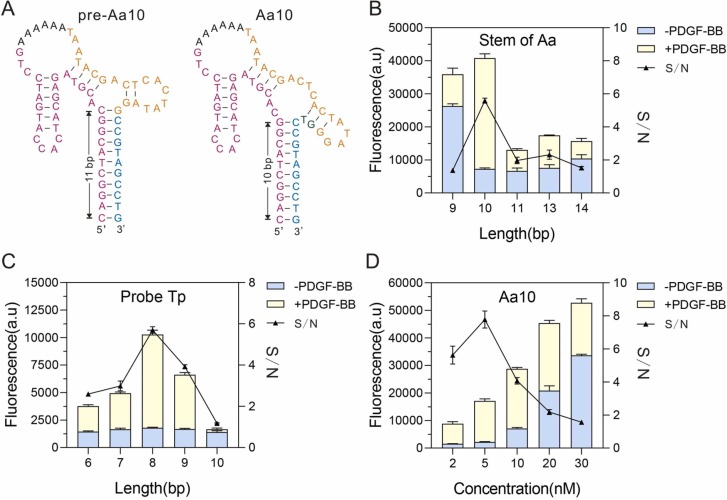
To acquire optimal performance for PDGF-BB detection, several important aspects were systematically investigated. The stem region of Aa was crucial for the binding efficiency of PDGF-BB. We designed different Aa variants for screening. Among them, when we designed the Aa10, aptamer and T7 promoter are just complementary, resulting in a stem with 11 bp (pre-Aa10; Fig. 3A). To form the stem with 10 bp, we added the GT (dark green) bases to pre-Aa10, yielding Aa10 which exhibits the optimum S/N (Fig. 3B). The ability of Aa10 to bind proteins is comparable to that of individual aptamer (Fig. S2A). Thus, Aa10 was selected as the optimum allosteric aptasensor to conduct the subsequent experiments.
Fig. 3.
Optimization of reaction parameters. A. The structure and sequence of pre-Aa10 and Aa10 were predicted using UNAFold. Carmine: aptamer; Orange: T7 promoter; Blue: extender. Dark green: GT bases. B. Optimizing the stem length of Aa. C. Comparison of different Tp with varied lengths which are complementary with Aa. D. The concentration of Aa10 tested in ATC-TA. Error bars were shown as means ± S.D., n = 3, triplicate.
Likewise, the length of complementary bases between Aa and Tp affects their binding efficiency and 8 nt was found to have the optimal activity (Fig. 3C). Furthermore, we also evaluated the fluorescence intensity of R-Pepper-GU-10 corresponding to Aa10 (Fig. S2B-C), further confirming that R-Pepper can tolerate more unrelated sequences than Pepper. Additionally, the optimum concentration of Aa10 and Tp8 was found to be 5 nM in a reaction volume of 10 μL with 4 mM NaCl, 10 mM MgCl2, 0.12 U/μL Bst DNA polymerase, 1.5 U/μL T7 RNA polymerase and 0.75 mM NTP (Fig. 3D, Fig. S3).
3.4. Analytical performance of the ATC-TA system
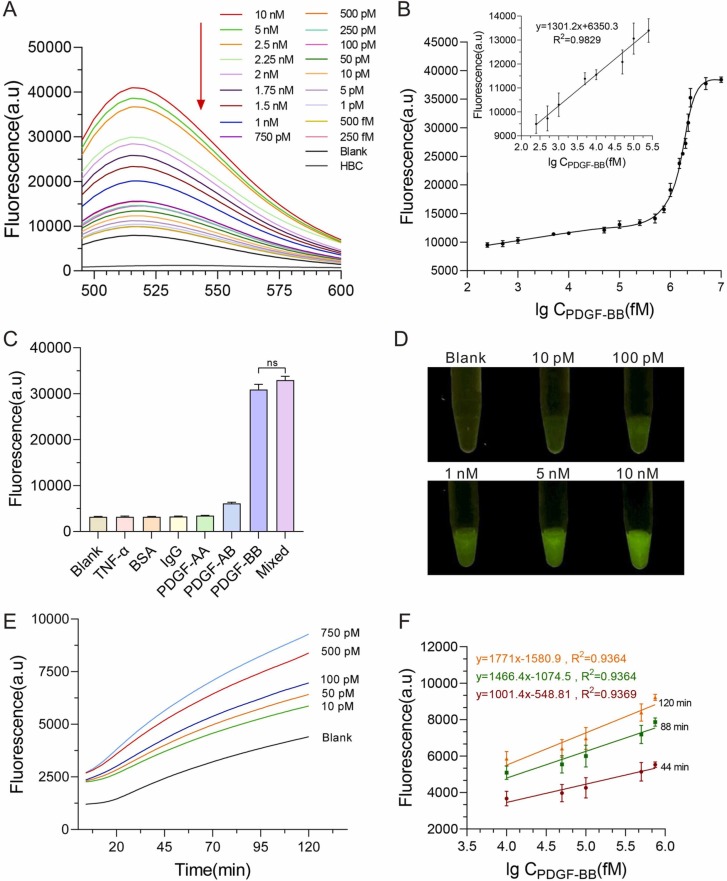
To investigate the sensitivity of ATC-TA, we investigated various concentrations of PDGF-BB under optimal conditions. The fluorescence intensity increased with the elevated PDGF-BB concentration, showing a positive correlation ( Fig. 4A). At low concentrations, especially in the range of 250 fM–250 pM, an equation is obtained by linear regression analysis of the calibration curve (Fig. 4B). The limit of detection (LOD) was calculated as our previously reported[36]. The LOD is defined as 3σ/S (in which S is the slope of the calibration curve in the range of 250 fM–750 pM, and σ is the standard deviation of the blank, n = 3) and the calculated LOD is 5.62 fM. Additionally, the detection limit and linear range of previously reported methods were compared in Table S2. Those results demonstrat that this ATC-TA system has ultrahigh sensitivity.
Fig. 4.
Performance of ATC-TA system. A. Fluorescence spectra of ATC-TA in response to different concentrations of PDGF-BB. Blank: without PDGF-BB. B. Calibrated curve of the average fluorescence intensity at 514 nm. Inset shows the linear responses at low PDGF-BB concentrations. C. Selectivity evaluation of ATC-TA system. Mixed: the mix of PDGF-BB, PDGF-AB, PDGF-AA, TNF-α IgG and BSA, each at a concentration of 5 nM. D. Colorimetric detection of PDGF-BB with different concentrations. E. Real-time detection of PDGF-BB at different concentrations by ATC-TA. F. Linear analysis of real-time detection of PDGF-BB at different times. Error bars were shown as means ± S.D., n = 3, triplicate.
Furthermore, to avoid interference from other proteins, several kinds of proteins were investigated to assess the selectivity of ATC-TA. The result showed that the fluorescence was significantly increased only with the addition of PDGF-BB. Conversely, the addition of the other protein, such as IgG, TNF-a, BSA and the two isomers (PDGF-AA and PDGF-AB) presented a weak fluorescence signal (Fig. 4C), demonstrating that ATC-TA is highly specific. The reaction tube was illuminated with a blue gel imager (excitation with 488 nm), and PDGF-BB could even be visualized in high concentrations with the naked eye (Fig. 4 D), which provides a variety of optional analysis methods for rapid on-site detection.
Because of the laborious washing steps required in ELISA, it is unusable for real-time analysis. Nevertheless, to achieve a more accurate quantitative detection of targets, it is better to monitor the progress of amplification reaction on a real-time basis. To detect PDGF-BB in real-time, real-time ATC-TA was carried out and performed at 37 °C. The result exhibits the typical fluorescence intensity curves in the presence of varying PDGF-BB concentrations (Fig. 4E-F). Those results indicated that the ATC-TA system was convenient and effectively detected PDGF-BB in real-time.
3.5. Detection of endogenous PDGF-BB protein in complex biological samples
To evaluate the applicability of ATC-TA system in real samples, the fluorescence of PDGF-BB was measured in human serum solutions diluted in different proportions, from 10-fold to 10000-fold dilutions ( Fig. 5A). Their concentrations were then calculated from the calibration curve. We also compared the ATC-TA with the commercialized ELISA (Fig. S4A-B) and the accuracy was consistent with the ELISA (Table S3), demonstrating that this ATC-TA system could accurately detect the target in complex biological samples without interference from the matrix. Notably, as expected, when the serum is diluted to 10000-fold, the concentration is undetectable by ELISA. Nevertheless, the ATC-TA still successfully detected PDGF-BB, indicating that ATC-TA can be used as a sensitive platform for complex biological samples.
Fig. 5.
Detection of the PDGF-BB in serum. A. Fluorescence peak density of PDGF-BB in different dilutions of human serum solution. B. ELISA reveals the ability of Aa to bind mouse PDGF-BB. The P values were determined using paired t-test. * P ≤ 0.05. C. Schematic illustration for the construction of mouse MC38 colon tumor models and blood collection at the indicated time. 59 mm3 and 97 mm3 represent the average tumor volume at the indicated time, respectively. D. Mouse PDGF-BB expression curves versus time of six infected mice (n = 6) and six healthy mice ((n = 6, dash-dot lines), with the average trace (red line, black line) shown. E. Histogram shows the difference in mouse PDGF-BB content between model mice and the healthy group at the indicated time. Day 5 for the healthy Group. All the P values were determined using paired t-test. * ** P ≤ 0.001; * ** * P ≤ 0.0001. All experiments were repeated three times. Error bars were shown as means ± S.D., n = 3, triplicate.
To ask whether the ATC-TA system can be used for the early diagnosis and prognosis of the tumor, we first demonstrated that Aa10 was also capable of binding to mouse PDGF-BB (Fig. 5B). Subsequently, we established the mouse MC38 colon tumor model to evaluate its ability for early diagnosis of tumor (Fig. 5C). The mouse PDGF-BB abundance in serum showed an upward trend with tumor growth. Compared with healthy mice, ATC-TA could effectively distinguish tumor mice from healthy mice in the early stage (3-day after subcutaneous inoculation of MC38 cells to 9-day) (Fig. 5D-E), demonstrating that the ATC-TA system has great application potential in complex substrate samples, especially in the early clinical diagnosis of a biomarker.
3.6. Detection of the exogenous SARS-CoV-2 spike protein in human serum
Having demonstrated the ability of ATC-TA to detect endogenous proteins in serum, we wondered whether it could detect exogenous proteins in serum for the detection of viral infection. We designed an allosteric aptasensor (S-Aa) based on the aptamer ST-6–2 (27 nt, Kd = 80 nM)[33] targeting the SARS-CoV-2 spike protein and analyzed its feasibility (Fig. S5A -B). The optimized stem length of S-Aa was 12 bp and the concentration was 10 nM (Fig. S5C-E). The fluorescence exhibited significantly increased in the presence of spike protein ( Fig. 6A) and there was a good linear relationship (Fig. 6B). It also has ideal selectivity to distinguish SARS-CoV-2 from other spike proteins of viruses, reducing the risk of contamination from other coronaviruses (Fig. 6C). Additionally, this system exerts ideal universality for SARS-CoV-2 variants, including the Delta, Lambda and Omicron, whose fluorescence is essentially identical to that of the wild type (Fig. 6D). Those demonstrat that this system has the potential to detect SARS-CoV-2 and its variants.
Fig. 6.
Detection of the SARS-CoV-2 spike protein in human serum. A. Fluorescence spectra of ATC-TA in response to different concentrations of the SARS-CoV-2 spike protein. Blank: without spike protein. B. The calibrated curve was made according to the fluorescence spectra. C. Selectivity study of ATC-TA system against the spike protein of coronaviruses. D. Fluorescence peak intensity to assess the performance of the ATC-TA system for the spike protein of wild-type SARS-CoV-2 to detect the spike protein from SARS-CoV-2 variants. E. The general workflow to detect contrived samples using ATC-TA. F. Fluorescence spectra were made by adding spike protein of SARS-CoV-2 to human serum. All the P values were determined using paired t-test. * P<0.05. Error bars were shown as means ± S.D., n = 3, triplicate.
Afterward, a series of contrived samples were prepared by adding known concentrations of spike protein to human serum and the final concentrations were 0.25, 0.5, 1 and 1.5 nM, respectively. Then, we use the ATC-TA system to measure the fluorescence signal of these samples (Fig. 6E-F) and calculate those concentrations. The recoveries ranged from 91% to 117% (Table S4) and the matrix did not affect the detection of exogenous proteins (Fig. S5F), indicating that the ATC-TA system can be used as an alternative for the screening of virus infections, having potential utility in clinical settings.
4. Conclusion
In summary, a highly sensitive and specific ATC-TA system for fluorescence detection of protein was developed. It exhibits several advantages: first, compared with the current common method, it has high sensitivity, providing a simple method for low-concentration protein quantification or early diagnosis of biomarker proteins. Second, it does not require time-consuming washing steps, pre-treatment and other cumbersome operations, making the process simple and easy to practical application. Third, all probes used in this system require no modification, which is important for retaining its high target-binding activity and making the system more convenient and cost-effective. Fourth, it not only realizes fluorescent detection but also can detect protein in real-time and visual detection, providing a variety of optional analysis methods. Additionally, this system is technically variable, offering great potential for simple, low-cost detection of many other targets by designing corresponding allosteric aptasensor, including proteins, metabolic molecules and pathogenic microorganisms. Taken together, this ATC-TA system provides a simple and sensitive biosensing platform with practical utility in the clinical setting, particularly in the early stages of disease diagnosis.
CRediT authorship contribution statement
Danxia Song: Methodology, Formal analysis. Deyu Yuan: Methodology, Formal analysis. Xuemei Tan: Formal analysis, Validation. Ling Li: Formal analysis. Huan He: Software. Liang Zhao: Investigation. Gang Yang: Investigation. Sirui Pan: Resources. Hongyuan Dai: Resources. Xu Song: Supervision, Methodology, Reviewing. Yongyun Zhao: Conceptualization, Methodology, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the National Key Research and Development Project (2017YFA0504300), Chengdu Science and Technology Program (2021-YF05-01681-SN).
Biographies
Danxia Song: She is currently studying for an M.D. in biology. Her research interest is in biological detection methods.
Deyu Yuan: He is currently studying for a Ph.D. in biology. His research interest is in biological detection methods.
Xuemei Tan: She is currently working as a researcher in biology. Her research interest is in RNA.
Ling Li: He is currently working as a researcher in biology. His research interest is in lncRNA.
Huan He: He is currently studying for an M.D. in biology. His research interest is in biological detection methods.
Liang Zhao: He is currently studying for an M.D. in biology. His research interest is in the screening of functional nucleic acid.
Gang Yang: He is currently studying for a Ph.D. in biology. His research interest is in the screening of functional nucleic acid.
Sirui Pan: She is currently studying for an M.D. in biology. Her research interest is bioimaging.
Hongyuan Dai: He is currently studying for an M.D. in biology. His research interest is in bioimaging.
Xu Song: He is currently working as a professor in biology. His research interests include lncRNA and biological detection methods.
Yongyun Zhao: She is currently working as a researcher in biology. Her research interests include the screening of functional nucleic acid, biological detection methods and bioimaging.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.snb.2022.132526.
Appendix A. Supplementary material
Supplementary material
.
Data Availability
Data will be made available on request.
References
- 1.Zhang Z., Bast R.C., Yu Y.H., Li J.N., Sokoll L.J., Rai A.J., et al. Three biomarkers identified from serum proteomic analysis for the detection of early stage ovarian cancer. Cancer Res. 2004;64:5882–5890. doi: 10.1158/0008-5472.CAN-04-0746. [DOI] [PubMed] [Google Scholar]
- 2.Rusling J.F., Kumar C.V., Gutkind J.S., Patel V. Measurement of biomarker proteins for point-of-care early detection and monitoring of cancer. Analyst. 2010;135:2496–2511. doi: 10.1039/c0an00204f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin. Infect. Dis. 2020;71:2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gosling J.P. A decade of development in immunoassay methodology. Clin. Chem. 1990;36:1408–1427. [PubMed] [Google Scholar]
- 5.Domon B., Aebersold R. Review - Mass spectrometry and protein analysis. Science. 2006;312:212–217. doi: 10.1126/science.1124619. [DOI] [PubMed] [Google Scholar]
- 6.Lin T.T., Zhang T., Kitata R.B., Liu T., Smith R.D., Qian W.J., et al. Mass spectrometry-based targeted proteomics for analysis of protein mutations. Mass Spectrom. Rev. 2021 doi: 10.1002/mas.21741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masson J.F. Surface plasmon resonance clinical biosensors for medical diagnostics. ACS Sens. 2017;2:16–30. doi: 10.1021/acssensors.6b00763. [DOI] [PubMed] [Google Scholar]
- 8.Li P., Luo L., Cheng D., Sun Y., Zhang Y., Liu M., et al. Regulation of the structure of zirconium-based porphyrinic metal-organic framework as highly electrochemiluminescence sensing platform for thrombin. Anal. Chem. 2022;94:5707–5714. doi: 10.1021/acs.analchem.2c00737. [DOI] [PubMed] [Google Scholar]
- 9.Huang L., Tian S., Zhao W., Liu K., Ma X., Guo J. Aptamer-based lateral flow assay on-site biosensors. Biosens. Bioelectron. 2021;186 doi: 10.1016/j.bios.2021.113279. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y., Zhan L., Qin Z., Sackrison J., Bischof J.C. Ultrasensitive and highly specific lateral flow assays for point-of-care diagnosis. ACS Nano. 2021;15:3593–3611. doi: 10.1021/acsnano.0c10035. [DOI] [PubMed] [Google Scholar]
- 11.de la Rica R., Stevens M.M. Plasmonic ELISA for the detection of analytes at ultralow concentrations with the naked eye. Nat. Protoc. 2013;8:1759–1764. doi: 10.1038/nprot.2013.085. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z., Wang H., Zhang Z., Chen L. Chemical redox-cycling for improving the sensitivity of colorimetric enzyme-linked immunosorbent assay. Anal. Chem. 2019;91:1254–1259. doi: 10.1021/acs.analchem.8b05095. [DOI] [PubMed] [Google Scholar]
- 13.Rissin D.M., Kan C.W., Campbell T.G., Howes S.C., Fournier D.R., Song L., et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 2010;28:595–599. doi: 10.1038/nbt.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Rica R., Stevens M.M. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat. Nanotechnol. 2012;7:821–824. doi: 10.1038/nnano.2012.186. [DOI] [PubMed] [Google Scholar]
- 15.Liu D., Ju C., Han C., Shi R., Chen X., Duan D., et al. Nanozyme chemiluminescence paper test for rapid and sensitive detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021;173 doi: 10.1016/j.bios.2020.112817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sajid M., Kawde A.N., Daud M. Designs, formats and applications of lateral flow assay: a literature review. J. Saudi Chem. Soc. 2015;19:689–705. [Google Scholar]
- 17.Toh S.Y., Citartan M., Gopinath S.C.B., Tang T.H. Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2015;64:392–403. doi: 10.1016/j.bios.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 18.Zhou W., Huang P.J.J., Ding J., Liu J. Aptamer-based biosensors for biomedical diagnostics. Analyst. 2014;139:2627–2640. doi: 10.1039/c4an00132j. [DOI] [PubMed] [Google Scholar]
- 19.Vinkenborg J.L., Karnowski N., Famulok M. Aptamers for allosteric regulation. Nat. Chem. Biol. 2011;7:519–527. doi: 10.1038/nchembio.609. [DOI] [PubMed] [Google Scholar]
- 20.Kang J., Jang H., Yeom G., Kim M.G. Ultrasensitive detection platform of disease biomarkers based on recombinase polymerase amplification with H-sandwich aptamers. Anal. Chem. 2021;93:992–1000. doi: 10.1021/acs.analchem.0c03822. [DOI] [PubMed] [Google Scholar]
- 21.Chen Q., Tian R., Liu G., Wen Y., Bian X., Luan D., et al. Fishing unfunctionalized SERS tags with DNA hydrogel network generated by ligation-rolling circle amplification for simple and ultrasensitive detection of kanamycin. Biosens. Bioelectron. 2022;207 doi: 10.1016/j.bios.2022.114187. [DOI] [PubMed] [Google Scholar]
- 22.Xu X., Su Y., Zhang Y., Wang X., Tian H., Ma X., et al. Novel rolling circle amplification biosensors for food-borne microorganism detection. Trac Trends Anal. Chem. 2021;141 [Google Scholar]
- 23.Wang X., Jiang A., Hou T., Li H., Li F. Enzyme-free and label-free fluorescence aptasensing strategy for highly sensitive detection of protein based on target-triggered hybridization chain reaction amplification. Biosens. Bioelectron. 2015;70:324–329. doi: 10.1016/j.bios.2015.03.053. [DOI] [PubMed] [Google Scholar]
- 24.Ling P., Wang L., Cheng S., Gao X., Sun X., Gao F. Ultrasensitive electrochemical biosensor for protein detection based on target-triggering cascade enzyme-free signal amplification strategy. Anal. Chim. Acta. 2022;1202 doi: 10.1016/j.aca.2022.339675. [DOI] [PubMed] [Google Scholar]
- 25.Zhang D., Yan Y., Cheng X., Yang T., Li X., Ding S., et al. Controlling the trans-cleavage of CRISPR-Cas12a with nicked PAM: Universal platform for biosensing. Sens. Actuators B Chem. 2022;353 [Google Scholar]
- 26.Wang Y., Xue T., Wang M., Ledesma-Amaro R., Lu Y., Hu X., et al. CRISPR-Cas13a cascade-based viral RNA assay for detecting SARS-CoV-2 and its mutations in clinical samples. Sens. Actuators B Chem. 2022;362 doi: 10.1016/j.snb.2022.131765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razmi N., Baradaran B., Hejazi M., Hasanzadeh M., Mosafer J., Mokhtarzadeh A., et al. Recent advances on aptamer-based biosensors to detection of platelet-derived growth factor. Biosens. Bioelectron. 2018;113:58–71. doi: 10.1016/j.bios.2018.04.048. [DOI] [PubMed] [Google Scholar]
- 28.Swetha P., Fan Z., Wang F., Jiang J.H. Genetically encoded light-up RNA aptamers and their applications for imaging and biosensing. J. Mater. Chem. B. 2020;8:3382–3392. doi: 10.1039/c9tb02668a. [DOI] [PubMed] [Google Scholar]
- 29.Zheng G., Dai J., Wang H., Li L., Yuan D., Bai S., et al. A hairpin-mediated nicking enzymatic signal amplification for nucleic acids detection. Talanta. 2021;225 doi: 10.1016/j.talanta.2020.121991. [DOI] [PubMed] [Google Scholar]
- 30.Shen J., Zhou X., Shan Y., Yue H., Huang R., Hu J., et al. Sensitive detection of a bacterial pathogen using allosteric probe-initiated catalysis and CRISPR-Cas13a amplification reaction. Nat. Commun. 2020;11:267. doi: 10.1038/s41467-019-14135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura Y., Tanaka F., Yoshikawa Y., Mimori K., Inoue H., Yanaga K., et al. PDGF-BB is a novel prognostic factor in colorectal cancer. Ann. Surg. Oncol. 2008;15:2129–2136. doi: 10.1245/s10434-008-9943-9. [DOI] [PubMed] [Google Scholar]
- 32.Hosaka K., Yang Y., Seki T., Nakamura M., Andersson P., Rouhi P., et al. Tumour PDGF-BB expression levels determine dual effects of anti-PDGF drugs on vascular remodelling and metastasis. Nat. Commun. 2013;4:2129. doi: 10.1038/ncomms3129. [DOI] [PubMed] [Google Scholar]
- 33.Yang G., Zhang S., Wang Y., Li L., Li Y., Yuan D., et al. Aptamer blocking S-TLR4 interaction selectively inhibits SARS-CoV-2 induced inflammation. Signal Transduct. Tar. Ther. 2022;7:120. doi: 10.1038/s41392-022-00968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X., Zhang D., Su N., Bao B., Xie X., Zuo F., et al. Visualizing RNA dynamics in live cells with bright and stable fluorescent RNAs. Nat. Biotechnol. 2019;37:1287. doi: 10.1038/s41587-019-0249-1. [DOI] [PubMed] [Google Scholar]
- 35.Huang K., Chen X., Li C., Song Q., Li H., Zhu L., et al. Structure-based investigation of fluorogenic Pepper aptamer. Nat. Chem. Biol. 2021;17:1289–1295. doi: 10.1038/s41589-021-00884-6. [DOI] [PubMed] [Google Scholar]
- 36.Zheng G., Zhao L., Yuan D., Li J., Yang G., Song D., et al. A genetically encoded fluorescent biosensor for monitoring ATP in living cells with heterobifunctional aptamers. Biosens. Bioelectron. 2022;198 doi: 10.1016/j.bios.2021.113827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.