Abstract
The airway epithelial glycocalyx plays an important role in preventing severe acute respiratory syndrome coronavirus 2 entry into the epithelial cells, while the endothelial glycocalyx contributes to vascular permeability and tone, as well as modulating immune, inflammatory, and coagulation responses. With ample evidence in the scientific literature that coronavirus disease 2019 (COVID-19) is related to epithelial and endothelial dysfunction, preserving the glycocalyx should be the main focus of any COVID-19 treatment protocol. The most studied functional unit of the glycocalyx is the glycosaminoglycan heparan sulfate, where the degree and position of the sulfate groups determine the biological activity. N-acetylcysteine (NAC) and other sulfur donors contribute to the inorganic sulfate pool, the rate-limiting molecule in sulfation. NAC is not only a precursor to glutathione but also converts to hydrogen sulfide, inorganic sulfate, taurine, Coenzyme A, and albumin. By optimising inorganic sulfate availability, and therefore sulfation, it is proposed that COVID-19 can be prevented or at least most of the symptoms attenuated. A comprehensive COVID-19 treatment protocol is needed to preserve the glycocalyx in both the prevention and treatment of COVID-19. The use of NAC at a dosage of 600 mg bid for the prevention of COVID-19 is proposed, but a higher dosage of NAC (1200 mg bid) should be administered upon the first onset of symptoms. In the severe to critically ill, it is advised that IV NAC should be administered immediately upon hospital admission, and in the late stage of the disease, IV sodium thiosulfate should be considered. Doxycycline as a protease inhibitor will prevent shedding and further degradation of the glycocalyx.
1. Introduction
The pandemic surrounding coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, has been associated with immense economic and social disruption around the globe, apart from high mortality rates. More than two years later, there is still a need to find effective therapeutic solutions to prevent and treat established disease. Because of their various physiological functions in viral infection and inflammation, the use of N-acetylcysteine (NAC) and sodium thiosulfate (STS) [1, 2] was recommended in the very early stages of the pandemic, by the authors and various other scientists, for both prevention and treatment of COVID-19. It is important to understand the science underlying these recommendations, which will be explained in this review.
We have recently reviewed the pathogenesis of COVID-19 [3] that provides, inter alia, a comprehensive overview of sulfation of the glycocalyx (GL), as well as highlighting the importance of an intact sulfated GL for natural protection against SARS-CoV-2 infection [3, 4]. We provide a short summary here of our undersulfation hypothesis, so that the remainder of the manuscript (treatment of COVID-19) may be understood better. GL will refer to both the epithelial glycocalyx (EpGL) and endothelial glycocalyx (EnGL), unless specified.
Since the GL surrounds all eukaryotic cells, the EpGL serves as the interface between our internal cells and the external world. It therefore serves as the first defence of the innate immune system, and if compromised, we are vulnerable to the onslaught of various pathogens, viruses, and environmental toxins [3]. The GL is a dense layer of proteins and carbohydrate chains that forms a mesh that extends into the extracellular matrix and is involved in many critical biological processes. Composed of membrane-bound glycoproteins, proteoglycans (PGs), and highly sulfated glycosaminoglycan (GAG) side-chains (Figure 1), the GL is particularly essential for barrier functions [5], as well as modulating immunity [3], inflammation [6, 7], and coagulation [8–11]. As a consequence of their interaction with multiple proteins, cell surface heparan sulfate (HS) PGs (HSPGs) are involved in developmental, regeneratory, infectious, and inflammatory processes [12]. It is important to note that the density and position of sulfate groups on the HS structure will mainly determine these HS-protein interactions [3, 13], and therefore the biological activity of sulfation.
Figure 1.
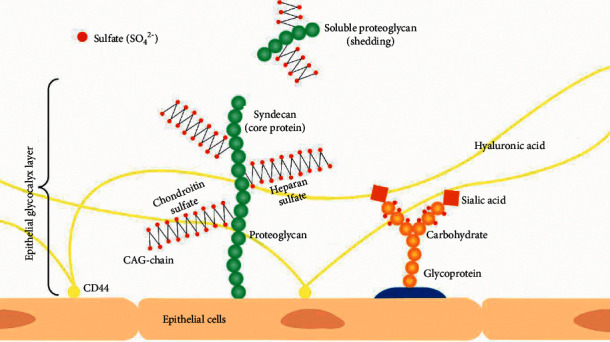
Schematic presentation of the epithelial glycocalyx (GL) in mammalian cells, forming a mesh-like structure that projects into the extracellular matrix (ECM). The proteoglycan presented is a syndecan with heparan sulfate and chondroitin sulfate glycosaminoglycan (GAG) side chains. The sulfate groups (SO42−) are indicated in red dots, attached to the GAG and glycoprotein side chains. Hyaluronic acid is unsulfated and anchored to the receptor CD44 on the cell membrane. The glycoprotein consists of a protein base with carbohydrate chains (orange circles) extending into the extracellular space with sialic acid (orange square) bound at the terminal position of the oligosaccharide chains. Soluble proteoglycans and free GAG chains can appear in the extracellular space.
Sulfation occurs in all tissues and involves the transfer of a sulfate group to various substrates, such as proteins, GAGs, lipids, hormones, various drugs, and toxins [14–18], [3, 19]. The availability of inorganic sulfate in the host was identified as the rate-limiting factor to sulfation [18, 20, 21]. The degree of sulfation and the trans-sulfuration pathway [22–24] is involved in many aspects of health, since it controls such a wide range of substances. The intact GL layer consists of many highly sulfated GAG chains, providing a negative charge density for the epithelial (Ep) and endothelial (En) surface layer [3, 25], and binding capacity for a variety of positively charged protein ligands [26]. The negative charge, mainly due to the presence of sulfate groups, is critical to biological and barrier function, especially in preventing the binding of negatively charged pathogens [3]. Changes in the charge density, and thus the degree of GAG sulfation, are often associated with various disorders [3, 27]. While different tissues and cell types vary in HS structure, the structure of HS can vary between individuals and with age. It seems evident that these differences in HS composition may contribute to tissue tropism and/or host susceptibility to infection by viruses and various other pathogens [3, 27–29].
Pre-COVID-19 vaccination, the elderly were most susceptible to SARS-CoV-2, where the typical profile of the critically ill patient was 65 years and older, presenting with comorbidities and ARDS [30, 31]. The main comorbidities related to COVID-19 were preexisting lung, heart and kidney disease, diabetes mellitus (DM) [32], obesity [33, 34], cancer, and cerebrovascular pathologies [35]. Dietary factors, such as low vitamin D and glutathione status, also increase susceptibility to COVID-19 [36, 37]. Today, more than two years later, immunosuppression in a younger population group seems to be a major predisposing factor for SARS-CoV-2 infection [38, 39]. Accumulating research relates dysfunction of the GL to ageing and these COVID-19 comorbid conditions [40, 41], which are also associated with chronic inflammation [3, 7, 34] and oxidative stress [42]. This highlights the probability that a disrupted GL is the main predisposing factor to COVID-19 susceptibility [3, 43]. As evident in COVID-19-related comorbid conditions, oxidative stress and chronic inflammation result in attenuation of the GL [7, 34, 42]. Moreover, En dysfunction increases systemic inflammation and oxidative stress, degradation of GL, and induces a procoagulant and antifibrinolytic state [35].
Since we are all exposed to SARS-CoV-2, the main question is why more than 80% of the world population did not become infected before the introduction of the COVID-19 vaccine, were asymptomatic, or showed only very mild symptoms [44–46], compared to the small percentage of people that became severely ill [22, 47]. The variable factor does not seem so much the expression of angiotensin converting enzyme 2 (ACE2) receptors, but rather the degree of GAG sulfation, which will affect the integrity of the GL, increasing susceptibility to SARS-CoV-2 infection and attenuating the ability to modulate inflammatory and coagulatory responses when the GL is undersulfated [3]. ACE2 expression is low in a well-preserved sulfated GL, which acts as a structural barrier [4]. Therefore, it is evident that the GL's structural and functional properties play a critical role in the pathogenesis of COVID-19. In this review, we will highlight the treatment strategies to best preserve the GL, with an emphasis on NAC and other sulfur donors as precursors to inorganic sulfate to support sulfation.
2. Glycocalyx
2.1. Structural Properties of the Glycocalyx (GL)
The extracellular matrix that surrounds all cells is a complex external layer of PGs, glycoproteins bound to sialic acid, GAGs, glycolipids, and proteins such as collagen [5, 48]. This glycoprotein polysaccharide covering is referred to as the GL. Plasma proteins such as antithrombin (AT) and albumin are also bound within the EnGL [49]. PGs are core proteins anchored to the apical membrane of Ep- and EnCs, with several GAG side-chains attached covalently to the PGs (Figure 1).
Glycosaminoglycan chains that bind to PGs are long linear, hydrophilic [50], negatively charged polysaccharides (PSs), namely, HS, chondroitin sulfate, dermatan sulfate, keratan sulfate, and nonsulfated hyaluronan [34, 48, 51, 52]. HS is the most studied and predominant GAG and acts as a receptor and reservoir for cytokines [53], enzymes, growth factor proteins, such as AT [12], and cell adhesion proteins, thereby modulating their distribution, concentration, and biological activity [54–56]. HSPG plays a critical role in maintaining and regulating various functions, including viral entry into target cells, vascular permeability [57], inflammatory and immune responses, and coagulation activity [3]. The GAG chain length and the number and location of sulfate groups in the various domains determine the binding affinity to the specific proteins [51, 58–60] and, therefore, biological function [12, 61, 62]. The degree of sulfation exerts the main influence on biological activity, therefore the GL's ability to modulate inflammation and coagulation, apart from barrier functions [3].
2.2. Sulfation
In mammalian cells, the sulfation process begins with the uptake of inorganic sulfate from the extracellular milieu [15], where the inorganic sulfate must be activated before reacting with the acceptor molecule. In mammals, this activated form of sulfate is sulfonucleotide 3-phosphoadenosine 5-phosphosulfate (PAPS), which is the universal sulfate donor for sulfotransferase (ST or SULT) reactions [11, 15]. STs transfer sulfate from PAPS to a specific position on the GAG structure [15], and therefore contribute significantly to the diversity of GAGs [3, 27].
2.3. Physiological Role of the Glycocalyx
Under normal healthy physiological conditions, HSPGs interact with and modulate the activity of several molecules [63], including protective enzymes such as superoxide dismutase (SOD) [64], cytokines [65], growth factors [25, 66], anticoagulant factors, albumin [57], Hedgehogs, Wingless, and protease inhibitors [67]. Therefore, apart from its essential role in barrier function and pathogen evasion [3], the GL will contribute to the regulation of coagulation, leukocyte adhesion [1, 65, 68], and protection against inflammation and oxidative stress [3, 49]. Any change in these functions is correlated with a wide range of pathophysiological consequences such as accelerated inflammation, capillary leak syndrome, consequent oedema formation [6], hypercoagulation, platelet hyperaggregation, and loss of vascular responsiveness [3, 25, 69]. The GL and HS do not only regulate physiological processes but are also implicated in many pathologies, including cancer, infectious and vascular diseases, and neurodegenerative disorders [1, 3, 5, 27, 56, 63, 70–73].
Of relevance to COVID-19, the primary role of the EpGL is the ability of a highly sulfated intact, negatively charged GL, to repel SARS-CoV-2 through electrostatic forces, thereby preventing cell invasion [3]. Most viral particles have a net negative charge at neutral pH, and SARS-CoV-2 is no exception. Various research reports confirmed the SARS-CoV-2 spike protein (SP) and net GRAVY score to be highly negative [74–77]. Nonetheless, if the GL is undersulfated and 3-O-sulfotransferase 3B (3OST-3B) is overexpressed, as is the case during chronic inflammatory conditions, the GL may facilitate SARS-CoV-2 entry [3]. Moreover, an undersulfated and degraded GL will not only increase susceptibility to SARS-CoV-2 infection, but will also hamper the ability to modulate the immune and inflammatory response and coagulation.
3. Sepsis Model, Degradation of the Glycocalyx
In COVID-19 patients that present with severe symptoms, sepsis has set in, which is a complicated condition that can be defined as severe organ dysfunction caused by an uncontrollable host response to infection [78]. After exposure to an infectious pathogen or inflammatory insult, alteration in the composition of the GL is one of the earliest features during sepsis [65, 68]. Following infection, acute injury, or inflammatory conditions, glucuronidases, such as heparanases (HPSEs) [58, 66, 79], reactive oxygen species (ROS) [65], and other proteases [5, 80, 81], cause shedding of HS and PGs [25, 57, 82]. Subsequently, adhesion molecules such as E-selectin and intercellular adhesion molecules are exposed on the denuded endothelium [6, 40, 80, 83], which result in accelerated inflammation [84], vascular permeability [58, 85], oedema, platelet aggregation, hypercoagulation, and a loss of vascular responsiveness [3, 40, 65, 68, 86, 87].
Pulmonary EnGL degradation increases the availability of En surface adhesion molecules to circulating microspheres. It contributes to neutrophil adhesion, leading to diffuse alveolar damage, interstitial lung oedema, and clot formation during acute lung injury in sepsis and ARDS [61, 68, 78]. There exists a clear association between alveolar epithelial and endothelial GL shedding and the establishment of ARDS [81, 82] and COVID-19 [4]. Moreover, circulating HS fragments are capable of influencing growth factors and other signaling pathways distant to the site of GL injury, which explains the systemic (i.e. para- or endocrine) consequences of GL degradation [51, 88], and supports the relationship between renal damage and the systemic proinflammatory state observed in sepsis [58] and COVID-19. Sepsis-associated induction of HPSE triggers degradation of vascular HS, leading to the collapse of the pulmonary and renal EnGL [51, 58]. While pulmonary EnGL loss contributes to lung injury via promotion of lung oedema and neutrophil adhesion, it was found that HPSE-mediated glomerular HS degradation could induce an early loss of glomerular filtration in the absence of kidney oedema or inflammation [51].
Apart from viral infections [82], multiple factors can cause degradation of the GL, including major surgery, ischemia/reperfusion, hypoxia/reoxygenation, mechanical ventilation [3, 48, 69], sodium overload [64, 80], ox-LDLs [5, 40, 86], sepsis, haemorrhagic shock, excessive shear stress, hypervolemia [48, 49], hyperglycaemia [68, 80], inflammatory cytokines such as tumour necrosis factor α (TNFα) [5, 85], interleukin (IL)-1β, IL-6, and IL-10 [48, 57, 58], matrix metalloproteinases (MMPs) [64, 65, 87], and reactive oxygen/nitrogen species [6].
Preserving the integrity of the GL is paramount both in the prevention and treatment of infectious diseases. Since most of the physiological properties of the GL are determined by the degree of GAG sulfation, ways to optimise inorganic sulfate, the rate-limiting substrate for sulfation, should be the primary focus of treatment. The intact Ep- and EnGL, with adequate sulfated GAGs, prevent viral infection and replication [3], determine vascular permeability, anticoagulant, anti-thrombotic, and anti-adhesive effects [11, 89], attenuate blood cell–vessel wall interactions [1], mediate shear stress sensing [40], enable balanced signaling, antioxidant properties [49], modulate inflammation [5], and fulfil a vasculoprotective role [25, 26, 48, 73]. In this review, we will expand on the role of NAC as a precursor to inorganic sulfate and its potential role as a preventive and therapeutic agent for combatting COVID-19.
4. NAC as a Physiological Precursor
Most of the literature to date focuses on NAC as a precursor to glutathione (GSH) [30, 42, 90–93]. However, it is important to realise that after free NAC enters a cell, it is rapidly hydrolysed to release cysteine (Cys), the rate-limiting substrate for intracellular GSH [94, 95], as well as hydrogen sulfide (H2S) [22, 30, 96, 97], inorganic sulfate [18], taurine [24, 98], Coenzyme A [99] and albumin synthesis [100] (Figure 2). NAC, therefore, has many physiological functions, apart from being an antioxidant precursor to GSH.
Figure 2.
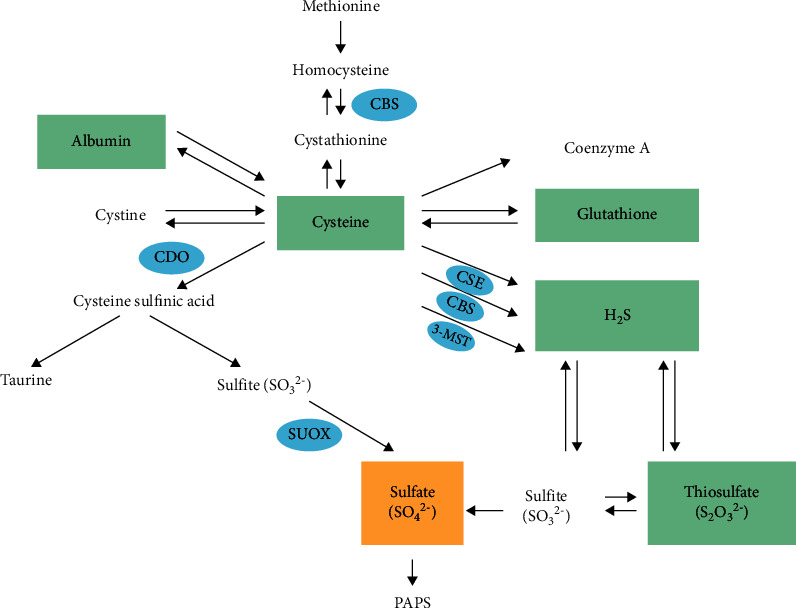
Sulfur metabolism diagram. The essential sulfur amino acid methionine converts to cysteine, a precursor to albumin, cystine, coenzyme A, glutathione, hydrogen sulfite (H2S), taurine, and sulfate (SO42−). H2S can convert to thiosulfate (S2O32−) and sulfite (SO32−), which are oxidised to sulfate. The enzyme cystathionine-β-synthase (CBS) converts homocysteine to cystathionine, while cysteine dioxygenase (CDO) is responsible for the conversion of cysteine to cysteine sulfinic acid. Sulfite oxidase (SUOX) oxidises sulfite to sulfate. H2S is generated from cysteine by three different pathways through either CBS, cystathionine-γ-lyase (CSE), or 3-mercaptopyruvate sulfurtransferase (3-MST). Adapted with permission by CC by 4.0 [3].
Since SARS-CoV-2 infection has been demonstrated to cause rapid depletion of sulfur amino acids (SAAs) due to oxidative stress or inflammation-induced proteolysis [22], amino acid replenishment becomes even more critical [18]. NAC treatment has been demonstrated to successfully replenish SAA levels, where a rapid increase in circulating Cys levels has been observed within hours following NAC supplementation [22]. During a rat study, the pharmacokinetics of NAC revealed that after oral administration, 77% NAC was maintained in the body and only 3% was excreted in faeces, while another human study indicated that after oral supplementation of NAC at 400 mg per single dose, the level of Cys increased to 4 mg/L in plasma [101].
4.1. Glutathione
Since Cys is the rate-limiting substrate in GSH and inorganic sulfate intracellular synthesis, low Cys levels will therefore result in lower GSH and inorganic sulfate levels. Through a series of redox reactions in the plasma, NAC directly affects the amino acid pool of extracellular cystine and intracellular Cys [18, 31]. NAC will favour GSH synthesis during oxidative stress. If there is a higher demand for GSH because of oxidative stress, such as during viral infection [102], extreme or endurance exercise [103], and hypoxic conditions [22, 94], it will have an inhibitory effect on inorganic sulfate synthesis. Lowered redox potential, common in high-risk COVID-19 patients, including older adults and those with uncontrolled DM, causes alterations in the TNFα receptor activity towards a proinflammatory state [22, 31, 95]. It is known that infection by RNA-viruses induces oxidative stress in host cells, and growing evidence indicates that viral replication is regulated by the redox state of the host cell [30, 94, 104, 105]. The aged patients with moderate and severe COVID-19 illness, and men had lower plasma levels of reduced GSH [30, 37], higher ROS levels, and greater redox status (ROS/GSH ratio) than COVID-19 patients with mild illness [37, 94, 106]. Erel et al. found that thiol levels decreased as the severity of the disease increased [107], while du Preez et al. hypothesised that low thiol levels/sulfur deficiency is an underlying cause of COVID-19 [3]. If these patients were low in GSH [108, 109], one can assume that Cys levels were low [102] and consequently inorganic sulfate, or it could be ascribed to a higher demand for Cys or inorganic sulfate during severe illness [106, 107, 110]. Bartonlini et al. demonstrated in vitro that SARS-CoV-2 infection impairs the metabolism of cellular GSH and its role in the redox homeostasis of cellular proteins, and results in changes in the composition of extracellular thiols [102, 111]. Even though it was found that COVID-19 patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency [112, 113] and those with the glutathione S-transferase (GST) theta 1 (GSTT1)−/− genotype or Ile 105Val glutathione S-transferase P1 (GSTP1) polymorphism, were more susceptible to infection and had higher mortality rates [114, 115], the availability of Cys will have a greater influence on redox status and outcome. The age-dependent decline of GSH and Cys in extracellular fluids has been hypothesised to not only be the actual causative factor [3], but also a molecular marker of increased risk of infection and development of serious COVID-19 [106, 107, 109, 116].
4.2. Hydrogen Sulfide (H2S)
Endogenous H2S supports basal, physiological, cellular bioenergetic functions, while the activity of this metabolic support decreases with physiological ageing [117]. Multiple biological regulatory roles for H2S as an endogenous gaseous transmitter have been established over the last decade [22, 97, 118]. H2S is produced, respectively, by cystathionine-β-synthase (CBS), CSE, and 3-mercaptopyruvate sulfurtransferase (3-MST) [22, 96, 118] (Figure 2). CBS and CSE serve as endogenous stimulators of cellular bioenergetics, while 3-MST serves as a regulator of cellular bioenergetics [118]. Moreover, during physiological oxygen reduction, H2S serves as a stimulator of electron transport in mammalian cells, by acting as a mitochondrial electron donor [117, 118]. It seems probable that stress and hypoxic states [97, 117] favour H2S as an “emergency” substrate that balances and complements the electron-donating effect of Krebs cycle-derived electron donors [119]. Of note is that the cell's free H2S concentration is likely to be constantly in dynamic equilibrium with a much larger pool of bound forms of sulfur, including thiol groups of proteins. Mitochondrial production of H2S starts with Cys [97, 106, 117], and the assumption can therefore be made that under hypoxic conditions, there would be an increased demand for H2S as electron donor [22], with a consequent inhibitory effect on inorganic sulfate and GSH synthesis, with Cys as the rate-limiting compound [119]. Even though one of the main H2S catabolic pathways in the mitochondria leads to the formation of thiosulfate, which is ultimately converted into sulfate [96], H2S would be favoured as an electron donor under hypoxic and stress conditions. This will affect the degree of HS sulfation [54]. Moreover, increases in intracellular H2S and ROS levels, mediated through hypoxic conditions [22], may synergistically induce membrane depolarisation [117]. This will result in increased levels of cytosolic calcium ions, which will lead to the activation of the endoplasmic reticulum (ER) stress response involved in the initiation of apoptosis [120]. Indeed, corona-derived viral protein deposits were found to result in ER stress and mitochondrial dysfunction in the affected EpCs [121].
Intrinsically synthesised H2S is extensively studied because of the role it plays as a proinflammatory mediator at high concentrations. Inhibition of endogenous H2S alleviates the diseased inflammatory condition, whereas exogenous H2S, released from natural sulfur compounds and SAAs, has shown protective effects in biological systems [117, 119]. H2S is stimulatory at lower concentrations, while it has an inhibitory effect on cellular bioenergetic functions at higher concentrations [118]. Inflammation, hypoxia, and calcium overload upregulate CSE gene expression and a simultaneous increase in H2S concentration [118, 119]. Conversely, H2S at low physiological concentrations [22, 97, 122] has been demonstrated to exhibit potent antiviral and anti-inflammatory properties [106, 123], mucolytic activity [96], as well as ameliorating various manifestations of inflammation, including ROS, nitric oxide, TNFα, and IL-6 [22, 122], and prevents endothelial dysfunction in cardiovascular-related pathologies [96]. Moreover, H2S attenuates pulmonary tissue injury by inducing ACE2 upregulation, while H2S may exhibit its antiviral activity against SARS-CoV-2 by interfering with both ACE2 and transmembrane protease serine 2 [22, 106]. COVID-19 patients with a more favourable outcome displayed higher circulating H2S levels than those found in patients with severe COVID-19 pneumonia [43, 96]. It seems evident that the H2S concentration must remain in a homeostatic balance to exert its protective effects. Even though Cys is the rate-limiting substrate for H2S production, pyridoxal 5′-phosphate (vitamin B6) is a very important cofactor, not only supporting CBS and CSE, but also the conversion of Cys to inorganic sulfate via CDO, along with iron. When supplementing with NAC to increase Cys levels, it is imperative to ensure a deficiency of the important cofactor nutrients does not impair the metabolic conversions in the sulfur metabolism pathway.
4.3. Inorganic Sulfate
Even though a small percentage of inorganic sulfate is found in foods and various sources of drinking water, the major source of inorganic sulfate for humans is from the biodegradation of dietary proteins. First, the essential sulfur amino acid methionine (Met) is converted to Cys [18]. Thereafter, the cytosolic enzyme, CDO, catalyses the conversion of Cys to cysteine sulfinic acid, the initial step in the transsulfuration pathway that leads to the formation of inorganic sulfate and taurine [124] (Figure 2). CDO is found primarily in the liver and brain, with some heart, kidney, and thyroid activity. It is known that the CDO-catalysed step is rate-limiting and that this metabolic route is responsible for the production of the majority of inorganic sulfate in vivo, since the absorption of inorganic sulfate across the gastrointestinal tract is relatively inefficient in humans [124]. The primary regulatory mechanism for CDO enzymatic activity functions at the posttranslational level. Under normal physiological conditions, high postprandial levels of Met or Cys will enhance the activity of CDO through inhibition of its degradation via the ubiquitin proteasome pathway. In the case of liver cirrhosis, however, CDO activity is significantly decreased despite high Met levels. It seems prudent that CDO expression is regulated at the mRNA level rather than the protein level. It was found that the proinflammatory cytokines, IL-1β, TNFα, and transforming growth factor-beta (TGF-β), downregulate CDO at mRNA level [98, 124]. This is evident in various health conditions with an autoimmune or inflammatory component, where high plasma levels of Cys with low plasma concentrations of inorganic sulfate are generally observed [124]. One can consequently expect reduced levels of HS sulfation during inflammatory conditions [3].
Most hospitalised COVID-19 patients in ICU are being placed on enteral nutrition [125]. Met is an essential amino acid, while Cys is conditionally essential since it is not always synthesised sufficiently, and preterm neonates have a relative inability to convert Met into Cys [126]. Most enteral and parenteral amino acid mixtures lack Cys since it is unstable in solution [126, 127]. A short-bowel syndrome rat model found that when enteral diets were supplemented with Cys and Met, improved gut mucosal and plasma cysteine/cystine redox potential and enhanced adaptive ileal mucosal growth were observed [126]. It is important to note that Met's contribution to the PAPS pool is 6–10 times lower than that of Cys [21]. Patients on enteral nutrition most likely do not get sufficient amounts of Cys [128], and during the cytokine storm seen in COVID-19, the conversion of Cys or NAC to inorganic sulfate appears to be reduced or inhibited due to the inhibitory effect of cytokines on CDO [124]. While adding Cys to enteral feed and intravenous (IV) NAC for the moderate to severely ill should be considered, IV STS should be explored in late-stage COVID-19, since it is more readily converted to inorganic sulfate. An easily digestible protein is recommended for mild to moderately ill patients, such as whey protein, high in Cys and albumin [129]. Another aspect to consider with enteral and parenteral nutrition is the very high calcium content in comparison to low levels of Cys and magnesium [126, 130, 131]. Both SARS-CoV and high levels of intracellular calcium ions would induce ER stress [120, 132]. Since high calcium levels also upregulate CSE gene expression [119], thereby increasing H2S levels and consequently membrane depolarisation [117], even more calcium ions will be released, creating a vicious cycle exacerbating inflammatory stress signaling [133] and apoptosis [120].
Cys is one of the amino acids in the hepatic precursor amino acid pool involved in both albumin and inorganic sulfate synthesis. The close arrangement between albumin synthesis and catabolic rates [134] suggests that a state of rapid equilibrium exists between the amino acid pools concerned with albumin and inorganic sulfate synthesis in the liver. It should be noted that whereas human serum albumin (HSA) is exclusively manufactured in the liver, hepatic inorganic sulfate also mixes with inorganic sulfate formed in other tissues in the extracellular fluid space. Inorganic sulfate synthesis in a healthy person mostly depends on the extent of reusing the sulfur from sulfate to synthesise new sulfur compounds. These conversions can occur through the intervention of microorganisms in the intestinal tract, although similar conversions can also occur in tissue cells [135]. Therefore, it seems probable that competitive inhibition exists in the synthesis between sulfate and albumin [134]. Pecora et al. indicated that the pathway of sulfate recruitment in the catabolism of SAAs was active in vivo [21]. They discovered that GAGs are undersulfated due to reduced extracellular sulfate uptake [134], caused by a mutation in diastrophic dysplasia sulfate transporter (Dtdst), which was reversed with the application of hypodermic NAC. This confirms that amino acid catabolism will contribute to the intracellular sulfate pool when extracellular sulfate availability is low. Therefore, the contribution of thiol compounds, such as NAC and HSA, to GAG sulfation, becomes significant by increasing the inorganic sulfate plasma concentration [21, 134]. Conversely, low levels of Cys or HSA will result in undersulfated GAGs. The dietary contribution to GAG sulfation has been reviewed extensively elsewhere [3].
Apart from NAC and HSA being sulfur donors, STS, methylsulfonylmethane (MSM) [136], and sulfated PSs [35, 137–140] will increase intracellular inorganic sulfate levels and thus exhibit antiviral and immune modulating properties. STS provides protection against ischemic brain injury and acute lung injury through inhibition of nuclear transcription factor kappa B (NF-κB) activation and TNFα-induced production of cytokines and ROS, thereby preventing upregulation of IL-6. It is well established that STS is a potent antioxidant and anti-inflammatory agent, and it has been used to treat cyanide poisoning [141, 142] and calciphylaxis [143] with a remarkably safe track record. STS also acts as an H2S donor [122]. Taken together, these observations suggest a therapeutic potential for STS in COVID-19, taken orally, inhaled or intravenously [2, 122].
As a source of organic sulfur, MSM will increase the synthesis of GSH [144] and inorganic sulfate. Amirshahrokhi showed that MSM attenuates paraquat-induced pulmonary and hepatic injury in mice, demonstrated by the reduction of TNFα, malondialdehyde (MDA), and myeloperoxidase (MPO) levels, and an increase in SOD, GSH, and catalase (CAT) levels in lung and liver tissues [144]. Several studies demonstrated that MSM inhibits lipopolysaccharide-induced release of oxidative stress biomarkers, such as nitric oxide and prostaglandin E2 in macrophages, through downregulation of NF-κB signaling [145, 146]. Kalman et al. reported that MSM potentially inhibits the translocation of the p65 subunit of NF-κB to the nucleus, therefore minimising downstream events associated with local and systemic inflammation [147–149]. Indeed, supplementation with MSM will minimise the expression of many proinflammatory cytokines [144, 147, 148, 150]. This is confirmed in a study where MSM attenuated experimental colitis by reducing IL-1β levels and protected against hepatic liver injury by decreasing TNFα and IL-6 levels [144, 150]. In another study, MSM significantly mitigated lung and pancreatic histopathological changes, decreased serum amylase and MPO activity, and inhibited caerulein-induced IL-1β expression. Moreover, MSM reduced caerulein-induced H2S levels by decreasing CSE expression in the lungs and pancreas, and increased CD34+ expression [149]. Many of these beneficial properties of MSM could be attributed to increased intracellular levels of inorganic sulfate and therefore enhanced GAG sulfation.
4.4. Albumin
Under normal physiological conditions, oncotic pressure in the vascular system is maintained by HSA [40]. The shedding of HSPGs promotes albumin leakage and therefore reduces tissue turgor [134]. Hypoalbuminemia is frequently observed in patients with DM, hypertension, and chronic heart failure, and they are statistically most vulnerable to SARS-CoV-2 infection. Hypoalbuminemia is a known factor in sepsis, ARDS, and COVID-19 [59, 151]. It has been reported that low albumin levels are seen in almost 81% of COVID-19 deaths [151].
Hypoalbuminemia, vascular disease, and coagulopathy have all been linked to COVID-19 and have been shown to predict outcomes independent of age and morbidity [151]. All these conditions can be related to the degree of GAG sulfation [3]. HSA also plays a vital role in fat metabolism by binding fatty acids and maintaining them in a soluble form in the plasma. Hyperlipemia, therefore, occurs in clinical situations of hypoalbuminemia [152], which has been associated with COVID-19 [151]. Important research questions are: does hypoalbuminemia predispose patients to COVID-19, or is it a consequence of the disease—or both?
There is no doubt that the binding of SARS-CoV-2 to ACE2 receptors influences various processes, such as vasoconstriction, kidney injury, cardiovascular disease, apoptosis, and oxidative processes [153], but the effect of the degraded GL and shedding of HS is mostly overlooked in COVID-19 research. It has been well established that proteinuria occurs when the En barrier function is compromised [154], resulting in changes in plasma protein concentration, particularly HSA [151]. In a rat model, removal of HS by HPSE led to increased permeability for both albumin and ferritin, and injection of antibodies to HS led to acute selective proteinuria [155]. Studies showed that 59% of COVID-19 patients already presented with proteinuria upon hospital admission, where 22% of nonventilated patients and 90% of ventilated patients developed acute kidney injury [156], which confirms EnGL degradation in COVID-19 patients.
Plasma proteins, such as albumin [40, 57, 64, 68, 80] and fresh frozen plasma [48, 49], may protect the GL. After cold ischemia, it was observed that albumin supplementation significantly attenuated pronounced shedding of the GL [5], and consequently adhesion of leukocytes [25], with reduced interstitial oedema [49]. Meli showed that additional albumin in the perfusion medium positively affects the formation, support, and preservation of the EnGL [40]. Since albumin is degraded into amino acids [152], it will increase Cys levels and indirectly act as a precursor to inorganic sulfate. Moreover, albumin has immunomodulatory and anti-inflammatory [49], antioxidant, anticoagulant, and antiplatelet-aggregational properties [40].
NAC and Cys play an essential role as the rate-limiting substrate in many vital physiological processes, and it is crucial to understand the homeostatic balance between the sulfur substrates to maintain physiological function [43]. It seems evident that a low level of Cys or inorganic sulfate predisposes to COVID-19 [3], and paradoxically, there is a higher demand for these sulfur substances during severe illness [3, 18]. Moreover, since NAC, H2S, GSH, and albumin demonstrate many similar physiological functions, their donation of sulfur [43, 157] for inorganic sulfate synthesis, could probably be credited for their various physiological actions.
5. NAC as a Therapeutic Agent
Various research studies indicated that NAC modulates the immune system [158], inhibits viral binding and suppresses replication [123, 159], plus reduces inflammation [22, 30, 91, 94, 105, 160], apart from its antibiofilm [123] and antioxidant properties [22, 92, 161–163]. NAC also has clinical benefits such as in cough and dry eyes, and as mucolytic [94, 106, 164, 165]. Since NAC increases GSH and inorganic sulfate production, it can modulate various smoking-related end-points. It was shown that NAC attenuated several biomarker alterations, such as inflammation and lung airspace enlargement, in heavy smokers who received NAC (600 mg twice daily) for six months [30]. It was demonstrated that NAC increased GSH levels in plasma [102] and bronchoalveolar lavage fluid in humans [166], which should subsequently lead to an increase in inorganic sulfate levels. Various studies demonstrate the beneficial role of NAC in recovery facilitation after cerebral ischemia and traumatic brain injury and in the treatment of cerebrovascular vasospasm after subarachnoid haemorrhage [42]. NAC could therefore potentially be promising in ameliorating the long-term residual neuropsychiatric and neurocognitive conditions seen in long COVID [3, 153, 159, 167, 168].
Apart from being a precursor to GSH, various other mechanisms have been ascribed to NAC's antioxidant and anti-inflammatory properties. NAC downregulates inflammasome NLRP3 mRNA expression, therefore decreasing proinflammatory cytokine expression and release from activated mononuclear phagocytes; NAC inhibits the endotoxin-induced release of IL-1β, IL-8, and TNFα; prevents systemic endotoxemia and inflammatory response by improving gut barrier dysfunction [121], where previous studies have shown that COVID-19 has been associated with gut barrier dysfunction and systemic endotoxemia; and NAC downregulates programmed cell death protein 1 expression in CD4+ and CD8+ lymphocytes, therefore increasing their counts and longevity [158]. NAC also increases IL-2 production and proliferation, thereby promoting the activation and modulation of T and B lymphocytes [169, 170]. These immunostimulatory properties of NAC further justify its use for COVID-19, since reduced levels of CD4+ and CD8+ T lymphocytes have been observed in critically ill COVID-19 patients [171]. Its immunostimulant properties could also be beneficial to prevent subsequent opportunistic bacterial infections. Unlike glucocorticoids, NAC would seem to have a more favourable effect on modulating both the inflammatory and immune responses against infection [172, 173]. Many of these properties exhibited by NAC could likely be ascribed to increased intracellular inorganic sulfate levels.
Oxidative processes enhance viral infection by promoting replication in infected cells, inhibition of cell proliferation, and cell apoptosis induction [35, 95]. Studies found that NAC treatment significantly reduced the frequency of influenza in the elderly and the severity and duration of most symptoms [31, 95, 160, 162]. To replicate, RNA-viruses need active NF-κB pathway support within host cells [158]. In a recent study, it was found that NF-κB is a mediator of SARS-CoV-2 pulmonary pathology, since it triggers the production of numerous proinflammatory cytokines [162]. It was demonstrated that the suppression of hypoxia-inducible NF-κB [92] significantly reduced the replication rate of mammalian coronaviruses. NAC has been shown to inhibit NF-κB [30, 95, 105] and replication of influenza A viruses in human lung EpCs in a dose-dependent manner. NAC also reduced the production of proinflammatory cytokines, such as IL-6, IL-8, CXCL10, and CCL5 [106, 123, 165], hence reducing chemotactic migration of monocytes [160]. In addition, it has also been demonstrated that NAC inhibits replication of other viruses [165], such as HIV and respiratory syncytial virus [30, 160]. This means that NAC and other thiol donors should potentially inhibit SARS-CoV-2 as well [91], because of its ability to downregulate NF-κB [35, 96, 160, 162]. The activity of monocyte chemotactic protein-1 (MCP-1) is upregulated by NF-kB, where MCP-1 amplifies inflammation and has been associated with the pathophysiological processes observed in COVID-19 patients [174]. Since NAC decreases both NF-κB and MCP-1 levels [175], it demonstrates another mechanism for reducing inflammation in COVID-19 patients. Moreover, Debnath et al. showed that NAC bound to SARS-CoV-2 SP and resulted in a threefold weakening of SP binding affinity with ACE2 receptors [90]. Furthermore, an interesting interrelationship exists between vitamin D deficiency and sulfur metabolism [3, 113]. In animal studies, co-supplementation of vitamin D and NAC showed a greater benefit in increasing 25(OH) vitamin D levels, and in reducing oxidative stress and inflammatory biomarkers [36].
NAC was also shown to protect GL shedding, since HS is expected to restore the GL when it is shed from EnCs [68]. Pecora et al. indicated an increase in GAG sulfation due to NAC catabolism. Therefore, the contribution of thiols to sulfation becomes significant when their plasma concentration is increased [21]. An infusion of NAC attenuated hyperglycemia-induced degradation of the EnGL and coagulation [80], indicating the ability to restore the EnGL [43]. Many experiments with various sulfur-containing compounds confirmed the inhibiting effect of Cys on coagulation [3, 176]. De Flora et al. proposed the administration of NAC as a possible strategy to preserve En function and limit micro-thrombosis in severe forms of COVID-19 [30, 168]. NAC reduces thrombotic complications by inhibiting the activity of plasminogen activator inhibitor-1, which is a procoagulant positively correlated with severe cases of COVID-19 [177, 178]. Furthermore, due to NAC's ability to break disulfide bonds, it disrupts platelet aggregation and breaks the bond between blood cells and the clotting factor, thereby maintaining blood fluidity and oxygen flow in the specific area [106, 107]. NAC could therefore reduce the activation of the characteristic coagulation cascade of severe COVID-19 [3, 168]. Apart from the effect of sulfation in coagulopathy [3], NAC may also exert its anti-coagulopathy role by interrupting the vitamin K reducing electron transfer pathway, which otherwise could result in cerebral haemorrhage if co-administered with other anticoagulants and acetaminophen. Regular monitoring of the international normalised Ratio (INR) and prothrombin time (PT) is therefore recommended for patients taking anticoagulants and NAC simultaneously [179]. However, administration of NAC alone did not worsen haemorrhagic stroke outcome, suggesting that NAC exerts thrombolytic effects without significantly impairing normal hemostasis [168]. Daid et al. successfully treated a COVID-19 patient with intrahepatic haemorrhage with the application of IV NAC [180].
Various case reports using NAC to treat COVID-19 patients successfully have recently appeared in the literature [22, 30, 91, 123, 159, 160]. In a larger cohort study, it was found that IV NAC significantly improved disease conditions in 10 severely respirator-dependent COVID-19 patients, aged between 38 and 71 years, including one with G6PD deficiency. Apart from improved lung function, IV NAC administration significantly reduced inflammatory markers, such as C-reactive protein and ferritin [91, 94, 160]. NAC administration has also been successful as a prophylactic intervention for ventilator-associated pneumonia [31]. De Flora also describes various cases where IV NAC successfully treated ARDS and increased extracellular total antioxidant power and total thiol molecules [30]. Puyo et al. also described a successful case study where IV NAC and oral hydroxychloroquine (HCQ) were used in combination. Previous work has shown that HCQ and NAC modulate the innate immune system, reduce hypercoagulability, and inhibit thrombosis [181]. NAC also potentiates the vasodilator and antiaggregatory effects of nitric oxide, which is valuable in the context of acute heart failure, myocardial ischemia, and infarction [30], while it improved renal oxygenation in acute kidney injury in a rat model by decreasing free radicals [161]. Chavarría et al. compared the effect of the antioxidants vitamin E, vitamin C, NAC, and melatonin with pentoxifylline as adjuvant therapy in COVID-19 patients with moderate to severe pneumonia, where the simultaneous use of NAC (600 mg twice daily every 12 h) and pentoxifylline demonstrated the best effect in patients with severe symptoms [104].
Even though various clinical studies reported contradicting evidence [159], NAC could serve as a first-line drug for COVID-19 due to its structural and functional characteristics [3, 35, 106, 160, 162]. NAC is widely available, inexpensive, tolerable, and safe, and has been FDA approved for many years. It could be used in an “off-label” manner to improve therapeutic strategies for COVID-19 [37, 160]. When used for acetaminophen overdose, NAC is safe at doses of up to 980 mg/kg over 48 hours [181]. It is evident that NAC administered intravenously, orally, or inhaled, should suppress SARS-CoV-2 replication and may reduce symptoms if used timely. NAC's potential therapeutic benefits include scavenging ROS radicals extracellularly, replenishing intracellular GSH and inorganic sulfate, and suppressing cytokine storm and T cell protection, thus mitigating inflammation, coagulation, and tissue injury [30, 31, 160, 181, 182]. What is more, NAC inhibits the downstream activities post TNFɑ receptor activation and gene expression of TNFɑ and IL-6 while under oxidative stress [31, 182]. In a placebo-controlled study with peritoneal dialysis patients, administration of NAC (600 mg bid for eight weeks) has been shown to reduce the plasma levels of inflammatory markers, including complement C3 [30]. Furthermore, in vivo NAC modifies the function of the renin/angiotensin system, which is probably mediated by inhibition of ACE2 activity. By blocking ACE2, NAC will potentially protect patients from the deleterious effects of angiotensin-2, which seems to be a potentially useful strategy in SARS-CoV-2 infection [30, 123, 162]. NAC administration as first-line therapy, or as an adjunct therapy combined with other antiviral agents [30], may dramatically reduce hospital admission rates, the need for ventilation and mortality [35, 94, 160]. Altay et al. had great success with the application of a mixture of combined metabolic activators, such as NAC and nicotinamide adenine dinucleotide precursors, to facilitate a more rapid symptom-free recovery in COVID-19 patients [111].
Furthermore, since proteases, such as thrombin, have been reported to support the cleavage of syndecan 1 PG ectodomains, thus facilitating shedding of the GL, the therapeutic use of protease inhibitors should be considered [57]. Among the tested inhibitors is doxycycline [25, 57, 87], an appealing candidate as a repurposed drug in the treatment of COVID-19, with an established safety track record and solid preclinical rationale [183]. In several studies, researchers demonstrated that doxycycline inhibited matrix MMP activity, which significantly reduced GL shedding and, therefore, leukocyte adhesion to EnCs in response to inflammatory and ischemic stimuli [5, 25, 183]. Apart from NAC, the early administration of doxycycline will also play an essential role in preserving the GL. It is, therefore, apparent that a comprehensive integrative protocol is needed to treat COVID-19.
6. Treatment Recommendations for COVID-19
The main goal of any COVID-19 treatment regimen should be the preservation of an intact well-sulfated GL [3, 25, 49, 82]. Therefore, for the prevention and treatment of COVID-19, we propose the treatment options and dosages summarised in Table 1, which are based on clinical observation, the science [3] and evidence outlined in this review. Please note that the proposed treatments and dosages are based on what is currently cited in the literature and could only be adopted either under accepted hospital protocols or as part of clinical trials.
Table 1.
Summary of a proposed therapeutic strategy to prevent and combat COVID-19.
| Disease state | Sulfur-donor | Protease inhibitor | Comments |
|---|---|---|---|
| Prevention | Either NAC 1200 mg/day, carbocisteine 1500 mg/day, erdosteine 600 mg/day [184], or MSM 2 g/day [136]. | Health care workers, frontline personnel, and those at high risk with comorbidities. | |
|
| |||
| Mild disease | Double up the dosages indicated above. | Adequate dietary protein intake is important; they can add whey protein to the diet [21, 129, 134]. | |
|
| |||
| Moderate to severe symptoms | IV NAC upon hospital admission (100 mg/kg/day) for 7 to 10 days [160] | Doxycycline 100 mg qid 5 to 7 days [25, 57, 87] | Add L-cysteine to enteral feed [126, 127]. |
|
| |||
| Severe to critically ill | Sodium thiosulfate—for 5 to 7 days and when symptoms subside, every 2nd or 3rd day. Adults: 100 mL (25 g) of STS (rate of 5 mL/minute). Paediatric 0–18 years: 1 mL/kg of body weight (250 mg/kg or approximately 30–40 mL/m2 of BSA) (rate of 2.5 to 5 mL/minute) not to exceed 50 mL total dose of STS [185] or IV NAC (150 mg/kg/day) for 7 to 10 days [160] |
Doxycycline 100 mg bid 7 to 10 days [25, 57, 87] | STS might be a better option than NAC to modulate the cytokine storm in the critically ill. Add L-cysteine to enteral feed [126, 127]. Give albumin [40, 57] or fresh frozen plasma [48, 49]. Avoid high tidal volume ventilation [3, 48, 69, 123]. Avoid both hypervolemia and hypernatremia [65, 68, 69]. |
Even though we propose that NAC and STS can be used as first-line therapy, their application would complement any other treatment protocol as a safe adjunct intervention. COVID-19 is a complex disease that cannot be treated with a single drug approach. Various strategies to preserve the intact sulfated GL need to be applied. It is, however, essential to prescribe a sulfur donor such as NAC or STS when drugs that are administered need sulfation for their metabolism, such as steroids, aspirin, colchicine, acetaminophen, and nonsteroidal anti-inflammatory drugs [16, 186, 187]; [19, 165, 188, 189]. Apart from preventing the depletion of inorganic sulfate when these drugs are given, sulfur-donor supplements would complement the anti-inflammatory drugs through their synergistic immune-modulatory action [3]. Even though quercetin and melatonin have been shown to be very beneficial in the early treatment of COVID-19 [190–192], they also require sulfation to be metabolised. NAC should therefore also be considered as an adjunct to these early treatment protocols.
We propose that for the prevention of COVID-19 in the general population, especially in health care workers, frontline personnel, and those at high risk with comorbidities, a cysteine derivative such as NAC [30], carbocisteine, or erdosteine [106, 184] should be taken daily. These cysteine derivatives, and MSM as sulfur-donor [136], will favourably contribute to the Cys and inorganic sulfate pool. Rogliani et al. did a meta-analysis on the effect of NAC 1200 mg/day, carbocisteine 1500 mg/day, and erdosteine 600 mg/day on chronic obstructive pulmonary disease and found erdosteine to be superior in efficacy, compared to NAC and carbocisteine [184]. Even though NAC has been labelled as “low bioavailability” for decades, oral administration of NAC given within 8 to 10 hours of acetaminophen overdose displays the same detoxification capacity compared to the IV route. It was reported that 600 mg NAC in capsule form was able to reach a level of 16 μM NAC in the peripheral blood within half an hour after administration [160]. Oral NAC at a dosage of 600 mg bid significantly decreased the frequency and severity of influenza [94, 95]. It is, therefore, expected that high dose oral NAC (1200 mg, bid) [94] can improve innate immunity through sulfation of the GL, and adaptive immunity by elevating GSH levels in lymphocytes, in addition to modulating neutrophil functions during the development of COVID-19 [3, 160]. As a preventive measure, oral NAC (600 mg bid) should be an effective and economical strategy to prevent SARS-CoV-2 infection and modulate the immune response [193]. Upon the first onset of symptoms, such as fever, sore throat, or dry cough, a higher dosage of oral NAC (1200 mg bid) should be considered to alleviate symptoms and accelerate recovery from viral infection [105, 160]. Note that high doses of oral NAC could result in intolerable gastrointestinal adverse effects such as nausea, vomiting, and diarrhoea [159]. Other micronutrients, such as zinc [194], selenium [106, 195, 196], magnesium, vitamins A, C, and D [113, 116, 197], should form part of a successful integrative protocol [110], while sulfur-donors such as MSM, allicin [157], or marine-derived sulfated PSs can also be considered. MSM is entirely safe and effective, taken at daily dosages of up to 4 g to prevent infection and modulate the immune response [136]. These self-treatment strategies to prevent viral infection with oral or inhalable NAC [22], or the use of other sulfur-donors, should help many SARS-CoV-2-infected patients to safely and cost-effectively recover at home. Since molybdenum, iron, and various B-vitamins are also important cofactor nutrients in sulfur metabolism, a balanced wholefood nutrient-dense diet should, therefore, form the cornerstone of any prevention and treatment regimen, supplying all macro- and micro-nutrients needed to prevent infection and maintain redox balance [3, 43].
In patients experiencing moderate to severe COVID-19 symptoms, IV NAC administration upon hospital admission should be considered as a standard practice of care, if allowed within the safety protocols of the hospital. The idea is to preserve and repair the GL to prevent a cytokine storm, and the sooner proper measures can be applied, the better the outcome that could be expected [22]. Shi & Puyo reported that patients with mild-to-moderate acute lung injury had significantly improved systemic oxygenation when intravenous (IV) NAC treatment (40 mg/kg/day) was given for three days, as well as reduced need for ventilatory support and lower mortality rate [160]. In another study, a 1200 mg/day oral dose of NAC was given for two weeks, which was sufficient to prevent deterioration due to severe respiratory failure requiring invasive or noninvasive mechanical ventilation in hospitalised patients with moderate or severe COVID-19 pneumonia, and reduced 14- and 28-day mortality [158]. Nonetheless, Taher et al. saw no clinical benefit with the application of NAC at a dose of 40 mg/kg/day diluted in 5% dextrose, given as a continuous intravenous infusion for 3 consecutive days, in patients with mild to moderate COVID-19-associated ARDS [159]. It should be noted that NAC was given at low dosage and in addition to standard-of-care treatment. A significant percentage of patients in both the NAC and control group received dexamethasone and other medications, which could reduce the degree of HS sulfation and therefore attenuated the GL [3, 198]. It is known that glucocorticoids may have intrinsic immunosuppressive drawbacks when applied at the wrong time, high dosages, and long term [3, 172, 173, 199, 200], while NAC is not immunosuppressive [159]. When higher concentration of IV NAC was given, better clinical outcomes could be expected. Shi & Puyo noted that high dosages of NAC will effectively reduce viral replication and significantly alleviate pneumocyte damage, as well as modulate immune responses and therefore prevent a cytokine storm [160]. NAC can, for example, be infused at a dose of 100 mg/kg for at least three days, which equals to about 1/3 of the total dose during a 3-bag regime. The aim should be to have at least an approximate NAC concentration in the blood of about 1 mM during infusion of the first bag to effectively treat virus-caused critical conditions, including pneumonia-mediated sepsis [159, 160]. However, Assimakopoulos et al. cautioned that very high doses of NAC may exert a prooxidant action, where it has been shown that the high doses may enhance Fe2+/H2O2-dependent oxidative stress and increase superoxide radical formation [158]. To offset potential ROS formation from high-dose NAC monotherapy, consider combining NAC with quercetin or vitamin C as powerful antioxidants to control free radical production [191, 201]. It seems evident that NAC as early intervention [121, 193] at the right concentration and enough exposure time is the key to an effective integrative treatment strategy. No difference has been observed between intermittent and continuous infusion of NAC regarding patient outcomes [160]. Nevertheless, when renal failure presents, an intermittent 3-bag regimen should be used. Although life-threatening anaphylactoid reactions were reported with IV administration of NAC, most of these reactions are mild in nature and easily manageable with a slower infusion rate [159]. Since HS can circulate for more than five days in the system as a result of a degraded GL, it is suggested that IV NAC should be given for at least 7 to 10 days, with doxycycline 100 mg once daily for 5 to 7 days as a protease inhibitor to attenuate shedding of the EnGL [25, 57, 87, 183]. Other antiviral medications can be added, but would be optional.
In the severe to critically ill COVID-19 patients, either IV NAC or IV STS should be considered, where the dosage and timeframe will be critical. STS is expected to be a better option to modulate the cytokine storm in the critically ill, since higher levels of cytokines will hamper the conversion of NAC to sulfate [98, 124], whereas STS will be more readily converted to inorganic sulfate (Figure 2). In a recent clinical trial, 135 severely ill late-stage COVID-19 patients received 300 mg/kg NAC over 20 hours or placebo [202]. The fact that there was no statistical difference between mortality rate and hospital stay for both groups indicates the importance of early initiation of NAC therapy [94] and considering STS application for late-stage disease. One should also consider the application of either NAC or STS at a lower dosage and for at least 7 to 10 days, since sulfated GAGs are not that easily restored [3]. A previous study demonstrated a relapse in inflammatory markers with early discontinuation of NAC treatment in COVID-19 [91]. In another study, IV NAC showed promising results in ARDS and acute lung injury patients at a loading dose of 150 mg/kg on the first day, followed by a dose of 50 mg/kg/day for three days. These patients not only showed improved oxygenation, but the mortality rate also dramatically decreased (35.7% vs. 76.9%) compared with control patients (p < 0.05) [160]. During another clinical trial, intravenous NAC (70 mg/kg body weight), given every 8 h for ten days, effectively decreased lung injury. It should be noted that NAC does not require dosage adjustments in renal or hepatic impairment [94].
We propose STS dosage to be the same as the standard treatment dosage given to patients with acute cyanide poisoning [185] or calciphylaxis [143, 203]. The recommendation is to give adults 100 mL (25 g) of STS (rate of 5 mL/minute) daily and when symptoms subside, administer every 2nd or 3rd day until there is no viral load present. In the paediatric group of 0–18 years, 1 mL/kg of body weight (250 mg/kg or approximately 30–40 mL/m2 of BSA) (rate of 2.5 to 5 mL/minute) should be given, not to exceed 50 mL of the total dose of STS [185].
The cytokine storm experienced in the critically ill will result in severe shedding of the GL that should be attenuated at all costs. One can consider the addition of dexamethasone with NAC as a combined anti-inflammatory therapy [22], as well as doxycycline at a dosage of 100 mg bid for 7 to 10 days.
6.1. Important Treatment Considerations
Steroids, aspirin, colchicine, acetaminophen, and nonsteroidal anti-inflammatory drugs appear to enhance viral replication and aggravate Ep and En dysfunction and the cytokine storm [3, 19, 172, 173, 198, 199, 204] through the depletion of inorganic sulfate for its metabolism [16, 186, 187]; [19, 165, 188, 189, 205]. These medications should therefore not be prescribed without a sulfur donor such as IV NAC or STS.
Whey protein, high in cysteine and albumin, should be added to the diet [18, 21, 129, 134] in mild conditions and add L-cysteine to enteral feed for severe to critically ill COVID-19 patients [126, 127].
Administer albumin [40, 57, 64, 68] or fresh frozen plasma [48, 49] to restore the EnGL in the critically ill.
Note that high dosages of vitamin C may cause hyperoxaluria through endogenous conversion of ascorbic acid to oxalate [125]. Both vitamin C [206] and oxalates [207] require conjugation through sulfation and contribute to Ep and En dysfunction if given at very high therapeutic dosages [3]. It appears best to give high-dose vitamin C together with IV NAC as a sulfur donor.
Seen that NAC has powerful anticoagulatory effects, adjust dosages of blood thinning medications accordingly.
Ventilation (VT) – avoid high tidal volume that could attenuate the lung EpGL [3, 87, 88, 208, 209]. However, low VT may not be the best approach for all patients with ARDS, since hypercapnia was common in patients with COVID-19-associated ARDS while using low tidal volume VT, which resulted in increased pulmonary dead space. Intermediate VT was used to correct hypercapnia efficiently in these patients while not excessively increasing driving pressure [210]. High-flow nasal oxygen remains the best option for severe COVID-19-related hypoxemic respiratory failure, particularly in settings outside the intensive care unit [211]. Excessive oxygen therapy can, however, be deleterious [112].
Avoid hypervolemia and hypernatremia, which are injurious to the EnGL [65, 68, 69, 208]. Albumin is effective for volume repletion [68].
Although Etanercept has been proposed for use in COVID-19 to reduce the mortality of toxic epidermal necrolysis (TEN) [212], Atef et al. proposed the use of 600 mg IV NAC every 8 h in surgical patients and ICU patients with TEN [213].
Check for G6PD deficiency before HCQ is administered, and if present, give IV NAC to avoid the risk of haemolysis in G6PD-deficient patients [91, 181].
Limit the use of antibiotics unless there is a known secondary infection. Gut dysbiosis seems to be an underlying risk factor for COVID-19 [3, 214].
It is best to avoid the use of statins in COVID-19 patients, since they may cause rhabdomyolysis as a complication [112].
Even though Ivermectin shows great potential as an inhibitor of SARS-CoV-2 replication [215], it is contraindicated when the blood-brain barrier (BBB) is compromised, as well as in the presence of multidrug resistance 1 (mdr-1) gene variants [216, 217] and deletions in glutathione S-transferases (GSTs) [218, 219], which can lead to increased drug accumulation in the brain, liver, and intestine [216], resulting in possible serious adverse events. Moreover, it is important to note that SARS-CoV-2 activation of cytokines, such as IL-1β, IL-16, and TNFα, cause injury to the BBB [153, 220] and decrease GSH levels [22]. Caution should therefore be taken with the use of ivermectin in late-stage COVID-19, and screening for polymorphisms in these genes should be considered.
Several clinical trials are currently evaluating the efficacy of NAC as therapeutic for COVID-19 [22, 94, 106, 123], but the various treatment considerations outlined in this review should be kept in mind with the application of NAC as therapeutic for a complex disease such as COVID-19. The timing and dosage of NAC supplementation would be the most critical factors determining the outcome, apart from the other measures needed to preserve the delicate GL layer. Even though well-designed clinical trials are needed to evaluate the effectiveness of a comprehensive NAC protocol, the use of NAC as an adjunct therapy should meanwhile be considered to ensure better clinical outcomes in the treatment of COVID-19 [106, 121, 161]. Enough evidence has been provided in the scientific literature for its safe and effective application [94]. Using a comprehensive treatment protocol with natural therapeutics, as opposed to a sole drug, would prevent the risk of highly pathological and resistant viral strains forming. Even though newly developed antiviral drugs, such as molnupiravir and paxlovid, show promising potential, they may result in more resistant viral mutations [221–223] and will not prevent GL shedding with all its pathological consequences.
After a comprehensive COVID-19 vaccine roll-out, the appearance of new variants with multiple mutations provides evidence that they are not effective; neither do they stop transmission nor infectivity, making it even more imperative to establish effective therapeutic alternatives to prevent viral infection. NAC would also appear to be a very good antidote against the possible adverse reactions experienced in susceptible individuals who received any of the COVID-19 vaccines. NAC may bind to the spike proteins, acting as a decoy to prevent binding to HS and consequent potential shedding of the GL. NAC will also facilitate detoxification of the adjuvants used in the vaccines, apart from restoring GSH levels depleted by the presence of ethylene glycol [3] and graphene oxide nanoparticles [94, 224–227].
7. Conclusion
Sulfated GAGs and various sulfur donor/thiol compounds regulate innate [3, 30] and adaptive immunity [123, 228] at various levels, as is well documented in the literature. What all sulfur donors have in common is the ability to supply sulfur for inorganic sulfate synthesis to ensure optimal GAG sulfation [3]. The degree and specific sulfation patterns of GAGs observed in a healthy GL attenuate the binding of pathogens, chemokines, and leukocytes to the EpC and EnC surfaces [156], thereby modulating immunity, inflammation and coagulation [3]. To maintain immune homeostasis, regulation of the initial inflammatory process in response to infection is crucial [22, 229]. Therefore, it is recommended that in moderate to severe COVID-19 illness, patients should be treated with IV NAC upon hospital admission. The safety of NAC was established with almost 60 years of experience in the prophylaxis and therapy of a variety of clinical conditions [123], even at very high doses and for long-term treatment. However, in the critically ill, IV STS should be considered, since the released cytokines might impede NAC oxidation into inorganic sulfate. As discussed in this review, the functions of the GL depend on an intact sulfated GL structure, with the degree of HS sulfation mainly determining biological activity [87, 230], with inorganic sulfate being the rate-limiting substrate to sulfation [3, 18, 20, 21].
Since NAC demonstrates multiple mechanisms of action, it is more likely to be effective than drugs having a single target. Moreover, since NAC addresses the underlying causes of COVID-19, it should have a beneficial effect on preventing and reducing long-COVID symptoms. Based on the herein discussed mechanistic premises, we propose using NAC in both the prevention and treatment of COVID-19. By better understanding the pathophysiological role of GAG sulfation [3], therapies can be better targeted at restoring the GL to ensure prevention and improved outcomes in COVID-19.
This review does not only emphasise the important clinical role of NAC in the prevention and treatment of COVID-19, but also highlights the physiological benefits of NAC as a precursor to inorganic sulfate. We believe that many of the physiological functions that have been attributed to NAC as a precursor to GSH in the literature to date, could in fact have been the action of inorganic sulfate, the rate-limiting precursor to sulfation. The application of NAC is therefore far-reaching beyond the treatment of COVID-19 or as an antidote to acetaminophen overdose.
Acknowledgments
The authors thank Beth Cooper for the final editing of the review article. Nicky Carlisle reproduced Figures 1 and 2 in InDesign.
Abbreviations
- ACE2:
Angiotensin converting enzyme 2
- BBB:
Blood-brain barrier
- CDO:
Cysteine dioxygenase
- COVID-19:
Coronavirus disease 2019
- CSE:
Cystathionine-γ-lyase
- Cys:
Cysteine
- ER:
Endoplasmic reticulum
- En:
Endothelial
- EnGL:
Endothelial glycocalyx
- Ep:
Epithelial
- EpGL:
Epithelial glycocalyx
- GAG:
Glycosaminoglycan
- GL:
Glycocalyx
- GSH:
Glutathione
- G6PD:
Glucose-6-phosphate dehydrogenase
- HPSE:
Heparanase
- HS:
Heparan sulfate
- HSPG:
Heparan sulfate proteoglycan
- HCQ:
Hydroxychloroquine
- HSA:
Human serum albumin
- H2S:
Hydrogen sulfide
- IL:
Interleukin
- IV:
Intravenous
- Met:
Methionine
- MPO:
Myeloperoxidase
- MSM:
Methylsulfonylmethane
- NAC:
N-acetylcysteine
- NF-κB:
Nuclear transcription factor kappa B
- PGs:
Proteoglycans
- ROS:
Reactive oxygen species
- SAA:
Sulfur amino acid
- SARS-CoV-2:
Severe acute respiratory syndrome–related coronavirus 2
- SOD:
Superoxide dismutase
- SP:
Spike protein
- STS:
Sodium thiosulfate
- PAPS:
Sulfonucleotide 3-phosphoadenosine 5-phosphosulfate
- ST or SULT:
Sulfotransferase
- TNFα:
Tumour necrosis factor α.
- VT:
Ventilation
Data Availability
All clinical recommendations made in the review article are based on published clinical trials, research, clinical experience and case reports, and are well referenced.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
HNduP conceived and researched the hypothesis proposed in this review and wrote the manuscript. She also conceptualised and produced all figures presented. Other authors reviewed and approved the final manuscript.
References
- 1.Hayden M. R. Endothelial activation and dysfunction in metabolic syndrome, type 2 diabetes and coronavirus disease 2019. Journal of International Medical Research . 2020;48(7) doi: 10.1177/0300060520939746.0300060520939746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayden M. R. Supplement repurposing of sodium thiosulfate (STS) in the treatment of COVID-19 pandemic based on a personal historical account of intravenous STS use in the treatment of calciphylaxis. 2020. https://www.researchgate.net/publication/343430633_Supplement_Repurposing_Of_Sodium_Thiosulfate_STS_In_The_Treatment_Of_COVID-19_Pandemic_Based_On_A_Personal_Historical_Account_Of_Intravenous_STS_Use_In_The_Treatment_Of_Calciphylaxis .
- 3.du Preez H. N., Aldous C., Hayden M. R., Kruger H. G., Lin J. Pathogenesis of COVID‐19 described through the lens of an undersulfated and degraded epithelial and endothelial glycocalyx. The FASEB Journal . 2022;36(1) doi: 10.1096/fj.202101100RR.e22052 [DOI] [PubMed] [Google Scholar]
- 4.Targosz-Korecka M., Kubisiak A., Kloska K., Kopacz A., Grochot-Przeczek A., Szymonski M. Endothelial glycocalyx shields the interaction of SARS-CoV-2 spike protein with ACE2 receptors. Scientific Reports . 2021;11(1):1–13. doi: 10.1038/s41598-021-91231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dogné S., Flamion B., Caron N. Endothelial glycocalyx as a shield against diabetic vascular complications. Arteriosclerosis, Thrombosis, and Vascular Biology . 2018;38(7):1427–1439. doi: 10.1161/atvbaha.118.310839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arokiasamy S., King R., Boulaghrasse H., et al. Heparanase-dependent remodeling of initial lymphatic glycocalyx regulates tissue-fluid drainage during acute inflammation in vivo. Frontiers in Immunology . 2019;10:1–20. doi: 10.3389/fimmu.2019.02316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer M., Jaspers I. Respiratory protease/antiprotease balance determines susceptibility to viral infection and can be modified by nutritional antioxidants. American Journal of Physiology—Lung Cellular and Molecular Physiology . 2015;308(12):L1189–L1201. doi: 10.1152/ajplung.00028.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clausen T. M., Sandoval D. R., Spliid C. B., et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell . 2020;183(4):1043–1057. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.HajMohammadi S., Enjyoji K., Princivalle M., et al. Normal levels of anticoagulant heparan sulfate are not essential for normal hemostasis. Journal of Clinical Investigation . 2003;111(7):989–999. doi: 10.1172/jci200315809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kazatchkine M. D., Fearon D. T., Metcalfe D. D., Rosenberg R. D., Austen K. F. Structural determinants of the capacity of heparin to inhibit the formation of the human amplification C3 convertase. Journal of Clinical Investigation . 1981;67(1):223–228. doi: 10.1172/jci110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thacker B. E., Xu D., Lawrence R., Esko J. D. Heparan sulfate 3-O-sulfation: a rare modification in search of a function. Matrix Biology . 2014;35:60–72. doi: 10.1016/j.matbio.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanpouille C., Deligny A., Delehedde M., et al. The Heparin/Heparan sulfate sequence that interacts with cyclophilin B contains a 3-O-sulfated N-unsubstituted glucosamine residue. Journal of Biological Chemistry . 2007;282(33):24416–24429. doi: 10.1074/jbc.M701835200. [DOI] [PubMed] [Google Scholar]
- 13.Delos M., Hellec C., Foulquier F., Carpentier M., Allain F., Denys A. Participation of 3-O-sulfated heparan sulfates in the protection of macrophages by herpes simplex virus-1 glycoprotein D and cyclophilin B against apoptosis. Febs Open Bio . 2017;7(2):133–148. doi: 10.1002/2211-5463.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster P. A., Mueller J. W. Sulfation pathways: insights into steroid sulfation and desulfation pathways. Journal of Molecular Endocrinology . 2018;61(2):T271–T283. doi: 10.1530/jme-18-0086. [DOI] [PubMed] [Google Scholar]
- 15.Girard J.-P., Baekkevold E. S., Amalric F. Sulfation in high endothelial venules: cloning and expression of the human PAPS synthetase 1. The FASEB Journal . 1998;12(7):603–612. doi: 10.1096/fasebj.12.7.603. [DOI] [PubMed] [Google Scholar]
- 16.Kurogi K., Sakakibara Y., Suiko M., Liu M.-C. Sulfation of vitamin D3-related compounds-identification and characterization of the responsible human cytosolic sulfotransferases. FEBS Letters . 2017;591(16):2417–2425. doi: 10.1002/1873-3468.12767. [DOI] [PubMed] [Google Scholar]
- 17.MacArthur J. M., Bishop J. R., Stanford K. I., et al. Liver heparan sulfate proteoglycans mediate clearance of triglyceride-rich lipoproteins independently of LDL receptor family members. Journal of Clinical Investigation . 2007;117(1):153–164. doi: 10.1172/jci29154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nimni M. E., Han B., Cordoba F. Are we getting enough sulfur in our diet? Nutrition and Metabolism . 2007;4(1):24–12. doi: 10.1186/1743-7075-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen I. V., Cirulli E. T., Mitchell M. W., et al. Acetaminophen (Paracetamol) use modifies the sulfation of sex hormones. EBioMedicine . 2018;28:316–323. doi: 10.1016/j.ebiom.2018.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregus Z., Oguro T., Klaassen C. D. Nutritionally and chemically induced impairment of sulfate activation and sulfation of xenobiotics in vivo. Chemico-Biological Interactions . 1994;92(1-3):169–177. doi: 10.1016/0009-2797(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 21.Pecora F., Gualeni B., Forlino A., et al. In vivo contribution of amino acid sulfur to cartilage proteoglycan sulfation. Biochemical Journal . 2006;398(3):509–514. doi: 10.1042/bj20060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourgonje A. R., Offringa A. K., van Eijk L. E., et al. N-Acetylcysteine and hydrogen sulfide in coronavirus disease 2019. Antioxidants and Redox Signaling . 2021;35(14):1207–1225. doi: 10.1089/ars.2020.8247. [DOI] [PubMed] [Google Scholar]
- 23.Klaassen C. D., Boles J. W. The importance of 3′‐phosphoadenosine 5′‐phosphosulfate (PAPS) in the regulation of sulfation. The FASEB Journal . 1997;11(6):404–418. doi: 10.1096/fasebj.11.6.91. [DOI] [PubMed] [Google Scholar]
- 24.Rosado J. O., Salvador M., Bonatto D. Importance of the trans-sulfuration pathway in cancer prevention and promotion. Molecular and Cellular Biochemistry . 2007;301(1-2):1–12. doi: 10.1007/s11010-006-9389-y. [DOI] [PubMed] [Google Scholar]
- 25.Kolářová H., Ambrůzová B., Švihálková Šindlerová L. S., Klinke A., Kubala L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediators of Inflammation . 2014;2014:17. doi: 10.1155/2014/694312.694312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker R. C. COVID-19-associated vasculitis and vasculopathy. Journal of Thrombosis and Thrombolysis . 2020;50(3):499–511. doi: 10.1007/s11239-020-02230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.da Costa D. S., Reis R. L., Pashkuleva I. Sulfation of glycosaminoglycans and its implications in human health and disorders. Annual Review of Biomedical Engineering . 2017;19:1–26. doi: 10.1146/annurev-bioeng-071516044610. [DOI] [PubMed] [Google Scholar]
- 28.Farach-Carson M. C., Warren R. C., Harrington D. A., Carson D. D. Border patrol: insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders. Matrix Biology . 2014;34:64–79. doi: 10.1016/j.matbio.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peerboom N., Block S., Altgärde N., et al. Binding kinetics and lateral mobility of HSV-1 on end-grafted sulfated glycosaminoglycans. Biophysical Journal . 2017;113(6):1223–1234. doi: 10.1016/j.bpj.2017.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Flora S., Balansky R., La Maestra S. Rationale for the use of N ‐acetylcysteine in both prevention and adjuvant therapy of COVID‐19. The FASEB Journal . 2020;34(10):13185–13193. doi: 10.1096/fj.202001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poe F. L., Corn J. N-Acetylcysteine: a potential therapeutic agent for SARS-CoV-2. Medical Hypotheses . 2020;143 doi: 10.1016/j.mehy.2020.109862.109862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clinical Immunology . 2020;215 doi: 10.1016/j.clim.2020.108427.108427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Missiry M. A., El-Missiry Z. M. A., Othman A. I. Melatonin is a potential adjuvant to improve clinical outcomes in individuals with obesity and diabetes with coexistence of Covid-19. European Journal of Pharmacology . 2020;882(882) doi: 10.1016/j.ejphar.2020.173329.173329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemkes B. A., Nieuwdorp M., Hoekstra J. B. L., Holleman F. The glycocalyx and cardiovascular disease in diabetes: should we judge the endothelium by its cover? Diabetes Technology & Therapeutics . 2012;14 doi: 10.1089/dia.2012.0011. [DOI] [PubMed] [Google Scholar]
- 35.Sansone C., Brunet C., Noonan D. M., Albini A. Marine algal antioxidants as potential vectors for controlling viral diseases. Antioxidants . 2020;9(5):p. 392. doi: 10.3390/antiox9050392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain S. K., Parsanathan P. Can Vitamin D and L-Cysteine co-supplementation reduce 25(OH)-Vitamin D deficiency and the mortality associated with COVID-19 in African Americans? Journal of the American College of Nutrition . 2020;39(8):694–699. doi: 10.1080/07315724.2020.1789518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polonikov A. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infectious Diseases . 2020;6(7):1558–1562. doi: 10.1021/acsinfecdis.0c00288. [DOI] [PubMed] [Google Scholar]
- 38.Brüssow H. COVID‐19 and children: medical impact and collateral damage. Microbial Biotechnology . 2022;15(4):1035–1049. doi: 10.1111/1751-7915.14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li P., de Vries A. C., Kamar N., Peppelenbosch M. P., Pan Q. Monitoring and managing SARS-CoV-2 evolution in immunocompromised populations. The Lancet Microbe . 2022;3(5):e325–e326. doi: 10.1016/s2666-5247(22)00061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meli I. Assessing the Role of Albumin in the Formation of the Endothelial Glycocalyx Layer Using a Microfluidic in Vitro Model . Bern, Switzerland: Master of Science Department for BioMedical Research (DBMR), Master of Science, University of Bern; 2019. [Google Scholar]
- 41.Varga Z., Flammer A. J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. The Lancet . 2020;395(10234):1417–1418. doi: 10.1016/s0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwiatkowska D. Effects of supplementation with glutathione and its precursors on athlete performance. Biomedical Journal of Scientific & Technical Research . 2019;12(4):9434–9441. doi: 10.26717/bjstr.2019.12.002293. [DOI] [Google Scholar]
- 43.Cumpstey A. F., Clark A. D., Santolini J. ., Jackson A. A., Feelisch F. COVID-19: a redox disease-what a stress pandemic can teach us about resilience and what we may learn from the reactive species interactome about its treatment. Antioxidants and Redox Signaling . 2021;35(14):1226–1268. doi: 10.1089/ars.2021.0017. [DOI] [PubMed] [Google Scholar]
- 44.Mason R. J. Pathogenesis of COVID-19 from a cell biology perspective. European Respiratory Journal . 2020;55(4) doi: 10.1183/13993003.00607-2020.2000607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyerowitz E. A., Richterman A., Bogoch I. I., Low N., Cevik M. Towards an accurate and systematic characterisation of persistently asymptomatic infection with SARS-CoV-2. The Lancet Infectious Diseases . 2021;21(6):e163–e169. doi: 10.1016/s1473-3099(20)30837-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milani G. P., Dioni L., Dioni C., et al. Serological follow-up of SARS-CoV-2 asymptomatic subjects. Scientific Reports . 2020;10(1) doi: 10.1038/s41598-020-77125-8.20048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paterson R. W., Brown R. L., Benjamin L., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain . 2020;143(10):3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uchimido R., Schmidt E. P., Shapiro N. I. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Critical Care . 2019;23(1):1–12. doi: 10.1186/s13054-018-2292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aldecoa C., Llau J. V., Nuvials X., Artigas A. Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: a review. Annals of Intensive Care . 2020;10(1):1–12. doi: 10.1186/s13613-020-00697-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varki A. Biological roles of glycans. Glycobiology . 2017;27(1):3–49. doi: 10.1093/glycob/cww086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oshima K., Haeger S. M., Hippensteel J. A., Herson P. S., Schmidt E. P. More than a biomarker: the systemic consequences of heparan sulfate fragments released during endothelial surface layer degradation (2017 Grover Conference Series) Pulmonary Circulation . 2017;8 doi: 10.1177/2045893217745786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Gemst J. J., Kouwenberg M., Rops A., et al. Differential binding of chemokines CXCL1, CXCL2 and CCL2 to mouse glomerular endothelial cells reveals specificity for distinct heparan sulfate domains. PLoS One . 2018;13(9) doi: 10.1371/journal.pone.0201560.e0201560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lortat-Jacob H., Grimaud J. A. Binding of interferon-gamma to heparan sulfaten is restricted to the heparin-like domains ad involves carboxylic - but not N-sulfated - groups. Biochimica et Biophysica Acta (BBA) - General Subjects . 1992;1117(2):126–130. doi: 10.1016/0304-4165(92)90069-7. [DOI] [PubMed] [Google Scholar]
- 54.Collins L. E., Troeberg L. Heparan sulfate as a regulator of inflammation and immunity. Journal of Leukocyte Biology . 2019;105(1):81–92. doi: 10.1002/jlb.3ru0618-246r. [DOI] [PubMed] [Google Scholar]
- 55.Huang M. L., Michalak A. L., Fisher C. J., Christy M., Smith R. A. A., Godula K. Small molecule antagonist of cell surface glycosaminoglycans restricts mouse embryonic stem cells in a pluripotent state. Stem Cells . 2018;36(1):45–54. doi: 10.1002/stem.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simeonovic C. J., Popp S. K., Starrs L. M., et al. Loss of intra-islet heparan sulfate is a highly sensitive marker of type 1 diabetes progression in humans. PLoS One . 2018;13(2) doi: 10.1371/journal.pone.0191360.e0191360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Becker B. F., Chappell D., Bruegger D., Annecke T., Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovascular Research . 2010;87(2):300–310. doi: 10.1093/cvr/cvq137. [DOI] [PubMed] [Google Scholar]
- 58.Galvis-Ramírez M. F., Quintana-Castillo J. C., Bueno-Sanchez J. C. Novel insights into the role of glycans in the pathophysiology of glomerular endotheliosis in preeclampsia. Frontiers in Physiology . 2018;9:p. 1470. doi: 10.3389/fphys.2018.01470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang L., Tang K., Levin M., et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. The Lancet Infectious Diseases . 2020;20(11):E276–E288. doi: 10.1016/s1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stopschinski B. E., Holmes B. B., Miller G. M., et al. Specific glycosaminoglycan chain length and sulfation patterns are required for cell uptake of tau versus α-synuclein and β-amyloid aggregates. Journal of Biological Chemistry . 2018;293(27):10826–10840. doi: 10.1074/jbc.ra117.000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haeger S. M., Liu X., Han X., et al. Epithelial heparan sulfate contributes to alveolar barrier function and is shed during lung injury. American Journal of Respiratory Cell and Molecular Biology . 2018;59(3):363–374. doi: 10.1165/rcmb.2017-0428OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trowbridge J. M., Gallo R. L. Dermatan sulfate: new functions from an old glycosaminoglycan. Glycobiology . 2002;12(9):117R–125R. doi: 10.1093/glycob/cwf066. [DOI] [PubMed] [Google Scholar]
- 63.Denys A., Allain F. The emerging roles of heparan sulfate 3-O-sulfotransferases in cancer. Frontiers in Oncology . 2019;9:p. 507. doi: 10.3389/fonc.2019.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marki A., Esko J. D., Pries A. R., Ley K. Role of the endothelial surface layer in neutrophil recruitment. Journal of Leukocyte Biology . 2015;98(4):503–515. doi: 10.1189/jlb.3MR0115-011R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin L., Koczera P., Zechendorf E., Schuerholz T. The endothelial glycocalyx: new diagnostic and therapeutic approaches in sepsis. BioMed Research International . 2016;2016:8. doi: 10.1155/2016/3758278.3758278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cagno V., Tseligka E. D., Jones S. T., Tapparel C. Heparan sulfate proteoglycans and viral attachment: true receptors or adaptation bias? Viruses . 2019;11(7):p. 596. doi: 10.3390/v11070596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Izvolsky K. I., Shoykhet D., Yang Y., Yu Q., Nugent M. A., Cardoso W. V. Heparan sulfate-FGF10 interactions during lung morphogenesis. Developmental Biology . 2003;258(1):185–200. doi: 10.1016/s0012-1606(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 68.Iba T., Levy J. H. Derangement of the endothelial glycocalyx in sepsis. Journal of Thrombosis and Haemostasis . 2019;17(2):283–294. doi: 10.1111/jth.14371. [DOI] [PubMed] [Google Scholar]
- 69.Collins S. R., Blank R. S., Deatherage L. S., Dull R. O. The endothelial glycocalyx. Anesthesia & Analgesia . 2013;117(3):664–674. doi: 10.1213/ane.0b013e3182975b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gordts P. L. S. M., Foley E. M., Lawrence R., et al. Reducing macrophage proteoglycan sulfation increases atherosclerosis and obesity through enhanced Type I interferon signaling. Cell Metabolism . 2014;20(5):813–826. doi: 10.1016/j.cmet.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hayden M. R. Type 2 diabetes mellitus increases the risk of late-onset alzheimer’s disease: ultrastructural remodeling of the neurovascular unit and diabetic gliopathy. Brain Sciences . 2019;9(10):p. 262. doi: 10.3390/brainsci9100262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nadanaka S., Purunomo E., Takeda N., Tamura J., Kitagawa H. Heparan sulfate containing unsubstituted glucosamine residues. Journal of Biological Chemistry . 2014;289(22):15231–15243. doi: 10.1074/jbc.M113.545343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reitsma S., Slaaf D. W., Vink H., van Zandvoort M., oude Egbrink M. The endothelial glycocalyx: composition, functions, and visualization. Pfluegers Archiv European Journal of Physiology . 2007;454(3):345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Awadasseid A., Wu Y. L., Tanaka Y., Zhang W. Initial success in the identification and management of the coronavirus disease 2019 (COVID-19) indicates human-to-human transmission in Wuhan, China. International Journal of Biological Sciences . 2020;16(11):1846–1860. doi: 10.7150/ijbs.45018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baig M. S., Alagumuthu M., Rajpoot S., Saqib U. Identification of a potential peptide inhibitor of SARS-CoV-2 targeting its entry into the host cells. Drugs in R & D . 2020;20(3):161–169. doi: 10.1007/s40268-020-00312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Odhaib S. A., Al-Aubaidy H. The physicochemical and phylogenetic properties of twelve spike glycoprotein models for SARS-CoV-2 from China, Iran, and Tunisia. Research Square . 2020;11:1–12. doi: 10.21203/rs.3.rs-63469/v1. [DOI] [Google Scholar]
- 77.Tahir ul Qamar M., Alqahtani S. M., Alqahtani M. A., Chen L.-L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. Journal of Pharmaceutical Analysis . 2020;10(4):313–319. doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suzuki K., Okada H., Takemura G., et al. Neutrophil elastase damages the pulmonary endothelial glycocalyx in lipopolysaccharide-induced experimental endotoxemia. American Journal Of Pathology . 2019;189(8):1526–1535. doi: 10.1016/j.ajpath.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 79.Veraldi N., Zouggari N., de Agostini A. The challenge of modulating heparan sulfate turnover by multitarget heparin derivatives. Molecules . 2020;25(2):p. 390. doi: 10.3390/molecules25020390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim Y.-H., Nijst P., Kiefer K., Tang W. H. Endothelial glycocalyx as biomarker for cardiovascular diseases: mechanistic and clinical implications. Current Heart Failure Reports . 2017;14(2):117–126. doi: 10.1007/s11897-017-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rizzo A. N., Haeger S. M., Oshima K., et al. Alveolar epithelial glycocalyx degradation mediates surfactant dysfunction and contributes to acute respiratory distress syndrome. JCI Insight . 2022;7(2) doi: 10.1172/jci.insight.154573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Benatti M. N., Fabro A., Miranda C. H. Endothelial glycocalyx shedding in the acute respiratory distress syndrome after flu syndrome. Journal of Intensive Care . 2020;8(1) doi: 10.1186/s40560-020-00488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Biagi S. Doctor of Philosophy, Human Health and Pathology . Rome, Italy: Università degli studi La Sapienza; 2016. A mesoscale investigation of the endothelial glycocalyx and its interaction with blood flow. [Google Scholar]
- 84.Constantinescu A. A., Vink H., Spaan J. A. E. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arteriosclerosis, Thrombosis, and Vascular Biology . 2003;23(9):1541–1547. doi: 10.1161/01.Atv.0000085630.24353.3d. [DOI] [PubMed] [Google Scholar]
- 85.Gouverneur M., Broekhuizen L., Meuwese M., Mooij H., Stroes E., Vink H. Sulfated glycosaminoglycans restore glycocalyx barrier properties of cultured endothelial cells in hyperglycemia. The FASEB Journal . 2008;22(S2):p. 83. doi: 10.1096/fasebj.22.2_supplement.83. [DOI] [Google Scholar]
- 86.Majerczak J., Grandys M., Duda K., et al. Moderate-intensity endurance training improves endothelial glycocalyx layer integrity in healthy young men. Experimental Physiology . 2017;102(1):70–85. doi: 10.1113/ep085887. [DOI] [PubMed] [Google Scholar]
- 87.Weidenfeld S., Kuebler W. M. Shedding first light on the alveolar epithelial glycocalyx. American Journal of Respiratory Cell and Molecular Biology . 2018;59(3):283–284. doi: 10.1165/rcmb.2018-0108ED. [DOI] [PubMed] [Google Scholar]
- 88.Hermans C., Bernard A. Lung epithelium-specific proteins. American Journal of Respiratory and Critical Care Medicine . 1999;159(2):646–678. doi: 10.1164/ajrccm.159.2.9806064. [DOI] [PubMed] [Google Scholar]
- 89.Ku S. K., Bae J. S. Antithrombotic activities of sulforaphane via inhibiting platelet aggregation and FIIa/FXa. Archives of Pharmacal Research . 2014;37(11):1454–1463. doi: 10.1007/s12272-014-0403-8. [DOI] [PubMed] [Google Scholar]
- 90.Debnath U., Dewaker V., Prabhakar Y. S., Bhattacharyya P., Mandal A. Conformational perturbation of SARS-CoV-2 spike protein using N-acetyl cysteine, a molecular scissor: a probable strategy to combat COVID-19. Chem . 2020:1–23. doi: 10.26434/chemrxiv.12687923.v1. [DOI] [Google Scholar]
- 91.Ibrahim H., Perl A., Smith D., et al. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clinical Immunology . 2020;219 doi: 10.1016/j.clim.2020.108544.108544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haddad R. E., Olver J. J. E., Land S. C. Antioxidant/Pro-oxidant equilibrium regulates HIF-1α and NF-κB redox sensitivity. Journal of Biological Chemistry . 2000;275(28):21130–21139. doi: 10.1074/jbc.m000737200. [DOI] [PubMed] [Google Scholar]
- 93.Sen C. K. Glutathione homeostasis in response to exercise training and nutritional supplements. Stress Adaptation, Prophylaxis and Treatment . 1999;196(1-2):31–42. doi: 10.1023/a:1006910011048. [DOI] [PubMed] [Google Scholar]
- 94.Dominari A., Hathaway III D., Kapasi A., et al. Bottom-up analysis of emergent properties of N-acetylcysteine as an adjuvant therapy for COVID-19. World Journal of Virology . 2021;10(2):34–52. doi: 10.5501/wjv.v10.i2.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Elhidsi M., Fachrucha F., Yudha Irawan R. N-Acetylcysteine for COVID-19: a potential adjuvant therapy. Journal of Health Science . 2021;11:1–6. doi: 10.17532/jhsci.2020.1156. [DOI] [Google Scholar]
- 96.Citi V., Martelli A., Brancaleone V., et al. Anti‐inflammatory and antiviral roles of hydrogen sulfide: rationale for considering H2S donors in COVID‐19 therapy. British Journal of Pharmacology . 2020;177(21):4931–4941. doi: 10.1111/bph.15230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fu M., Zhang W., Wu L., Yang G., Li H., Wang R. Hydrogen sulfide (H 2 S) metabolism in mitochondria and its regulatory role in energy production. Proceedings of the National Academy of Sciences . 2012;109(8):2943–2948. doi: 10.1073/pnas.1115634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hagiwara A., Ishizaki S., Takehana K., et al. Branched-chain amino acids inhibit the TGF-beta-induced down-regulation of taurine biosynthetic enzyme cysteine dioxygenase in HepG2 cells. Amino Acids . 2014;46(5):1275–1283. doi: 10.1007/s00726-014-1693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gout I. Coenzyme A, protein CoAlation and redox regulation in mammalian cells. Biochemical Society Transactions . 2018;46(3):721–728. doi: 10.1042/bst20170506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paramasivan S., Adav S. S., Ngan S., et al. Serum albumin cysteine trioxidation is a potential oxidative stress biomarker of type 2 diabetes mellitus. Scientific Reports . 2020;10(1):p. 6475. doi: 10.1038/s41598-020-62341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Leelarungrayub D., Khansuwan R., Pothongsunun P., Klaphajone J. N-Acetylcysteine supplementation controls total antioxidant capacity, creatine kinase, lactate, and tumor necrotic factor-alpha against oxidative stress induced by graded exercise in sedentary men. Oxidative Medicine and Cellular Longevity . 2011;2011:6. doi: 10.1155/2011/329643.329643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bartolini D., Stabile A. M., Bastianelli S., et al. SARS-CoV2 infection impairs the metabolism and redox function of cellular glutathione. Redox Biology . 2021;45 doi: 10.1016/j.redox.2021.102041.102041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mason S. A., Trewin A. J., Parker L., Wadley G. D. Antioxidant supplements and endurance exercise: current evidence and mechanistic insights. Redox Biology . 2020;35 doi: 10.1016/j.redox.2020.101471.101471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chavarría A. P., Vázquez R. R. V., Cherit J. G. D., et al. Antioxidants and pentoxifylline as coadjuvant measures to standard therapy to improve prognosis of patients with pneumonia by COVID-19. Computational and Structural Biotechnology Journal . 2021;19:1379–1390. doi: 10.1016/j.csbj.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pasini F., Stranieri M., Cominacini C., Mozzini L., Mozzini C. Potential role of antioxidant and anti-inflammatory therapies to prevent severe SARS-CoV-2 complications. Antioxidants . 2021;10(2):p. 272. doi: 10.3390/antiox10020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cazzola M., Rogliani P., Salvi S. S., Ora J., Matera M. G. Use of thiols in the treatment of COVID-19: current evidence. Lung . 2021;199(4):335–343. doi: 10.1007/s00408-021-00465-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Erel Ö., Neşeli̇oğlu S., Ergi̇n Tunçay M., et al. A sensitive indicator for the severity of COVID-19: thiol. Turkish Journal of Medical Sciences . 2021;51(3):921–928. doi: 10.3906/sag-2011-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Doğan H. O., Senol O., Bolat S., et al. Understanding the pathophysiological changes via untargeted metabolomics in COVID‐19 patients. Journal of Medical Virology . 2021;93(4):2340–2349. doi: 10.1002/jmv.26716. [DOI] [PubMed] [Google Scholar]
- 109.Khanfar A., Al Qaroot B. Could glutathione depletion be the Trojan horse of COVID-19 mortality? European Review for Medical and Pharmacological Sciences . 2020;24(23):12500–12509. doi: 10.26355/eurrev_202012_24046. [DOI] [PubMed] [Google Scholar]
- 110.Horowitz R. I., Freeman P. R. Three novel prevention, diagnostic, and treatment options for COVID-19 urgently necessitating controlled randomized trials. Medical Hypotheses . 2020;143 doi: 10.1016/j.mehy.2020.109851.109851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Altay O., Arif M., Li X., et al. Combined metabolic activators accelerates recovery in mild‐to‐moderate COVID‐19. Advanced Science . 2021;8(17):2101222–2101316. doi: 10.1002/advs.202101222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Buinitskaya Y., Gurinovich R., Wlodaver C. G., Kastsiuchenka S. Centrality of G6PD in COVID-19: the biochemical rationale and clinical implications. Frontiers of Medicine . 2020;7(612) doi: 10.3389/fmed.2020.584112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jain S. K., Parsanathan R., Levine S. N., Bocchini J. A., Holick M. F., Vanchiere J. A. The potential link between inherited G6PD deficiency, oxidative stress, and vitamin D deficiency and the racial inequities in mortality associated with COVID-19. Free Radical Biology and Medicine . 2020;161:84–91. doi: 10.1016/j.freeradbiomed.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abbas M., Verma S., Verma S., et al. Association of GSTM1 and GSTT1 gene polymorphisms with COVID‐19 susceptibility and its outcome. Journal of Medical Virology . 2021;93(9):5446–5451. doi: 10.1002/jmv.27076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saadat M. The morbidity and mortality of COVID-19 are correlated with the Ile105Val glutathione S-transferase P1 polymorphism. Egyptian Journal of Medical Human Genetics . 2020;21(1):p. 52. doi: 10.1186/s43042-020-00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karkhanei B., Talebi Ghane E. T., Mehri F. Evaluation of oxidative stress level: total antioxidant capacity, total oxidant status and glutathione activity in patients with COVID-19. New Microbes and New Infections . 2021;42 doi: 10.1016/j.nmni.2021.100897.100897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Szabo C., Ransy C., Módis K., et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. British Journal of Pharmacology . 2014;171(8):2099–2122. doi: 10.1111/bph.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Módis K., Coletta C., Erdélyi K., Papapetropoulos A., Szabo C. Intramitochondrial hydrogen sulfide production by 3‐mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. The FASEB Journal . 2013;27(2):601–611. doi: 10.1096/fj.12-216507. [DOI] [PubMed] [Google Scholar]
- 119.Marutani E., Morita M., Hirai S., et al. Sulfide catabolism ameliorates hypoxic brain injury. Nature Communications . 2021;12(1):p. 3108. doi: 10.1038/s41467-021-23363-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bahar E., Kim H., Yoon H. ER stress-mediated signaling: action potential and Ca2+ as key players. International Journal of Molecular Sciences . 2016;17(9):p. 1558. doi: 10.3390/ijms17091558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nasi A., McArdle S., Gaudernack G., et al. Reactive oxygen species as an initiator of toxic innate immune responses in retort to SARS-CoV-2 in an ageing population, consider N-acetylcysteine as early therapeutic intervention. Toxicology Reports . 2020;7:768–771. doi: 10.1016/j.toxrep.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Evgen’ev M. B., Frenkel A. Possible application of H2S-producing compounds in therapy of coronavirus (COVID-19) infection and pneumonia. Cell Stress and Chaperones . 2020;25(5):713–715. doi: 10.1007/s12192-020-01120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wong K. K., Lee S. W. H., Kua K. P. N-acetylcysteine as adjuvant therapy for COVID-19 - a perspective on the current state of the evidence. Journal of Inflammation Research . 2021;14:2993–3013. doi: 10.2147/jir.s306849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wilkinson L. J., Waring R. H. Cysteine dioxygenase: modulation of expression in human cell lines by cytokines and control of sulphate production. Toxicology in Vitro . 2002;16(4):481–483. doi: 10.1016/s0887-2333(02)00031-0. [DOI] [PubMed] [Google Scholar]
- 125.Fontana F., Cazzato S., Giovanella S., et al. Oxalate nephropathy caused by excessive vitamin C administration in 2 patients with COVID-19. Kidney International Reports . 2020;5(10):1815–1822. doi: 10.1016/j.ekir.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yarandi S. S., Zhao V. M., Hebbar G., Ziegler T. R. Amino acid composition in parenteral nutrition: what is the evidence? Current Opinion in Clinical Nutrition and Metabolic Care . 2011;14(1):75–82. doi: 10.1097/MCO.0b013e328341235a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hoffer L. Parenteral nutrition: amino acids. Nutrients . 2017;9(3):p. 257. doi: 10.3390/nu9030257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thibault R., Coëffier M., Joly F., Bohé J., Schneider S. M., Déchelotte P. How the Covid-19 epidemic is challenging our practice in clinical nutrition-feedback from the field. European Journal of Clinical Nutrition . 2021;75(3):407–416. doi: 10.1038/s41430-020-00757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Madureira A. R., Pereira C. I., Gomes A. M. P., Pintado M. E., Xavier Malcata F. X. Bovine whey proteins—overview on their main biological properties. Food Research International . 2007;40(10):1197–1211. doi: 10.1016/j.foodres.2007.07.005. [DOI] [Google Scholar]
- 130.Al-Jurf A. S., Chapman-Furr F. Magnesium balance and distribution during total parenteral nutrition: effect of calcium additives. Metabolism . 1985;34(7):658–664. doi: 10.1016/0026-0495(85)90094-0. [DOI] [PubMed] [Google Scholar]
- 131.Leder B., Silverberg S. J., Stewart A. F. High blood calcium (Hypercalcemia) Journal of Clinical Endocrinology and Metabolism . 2009;94(7):p. E2. doi: 10.1210/jcem.94.7.9987. [DOI] [PubMed] [Google Scholar]
- 132.Qaswal A. B., Suleiman A., Guzu H., Harb T.’A., Atiyat B. The potential role of lithium as an antiviral agent against SARS-CoV-2 via membrane depolarization: review and hypothesis. Scientia Pharmaceutica . 2021;89(1):p. 11. doi: 10.3390/scipharm89010011. [DOI] [Google Scholar]
- 133.Zhang K., Kaufman R. J. From endoplasmic-reticulum stress to the inflammatory response. Nature . 2008;454(7203):455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Amadi B., Fagbemi A. O., Kelly P., et al. Reduced production of sulfated glycosaminoglycans occurs in Zambian children with kwashiorkor but not marasmus. The American Journal of Clinical Nutrition . 2009;89(2):592–600. doi: 10.3945/ajcn.2008.27092. [DOI] [PubMed] [Google Scholar]
- 135.Awwad H. K., El Sheraky A. S., Helmi S. A., Shetaiwy S. K., Potchen E. J. The relationship between serum albumin and sulfate synthesis. Journal of Biological Chemistry . 1970;245(3):469–476. doi: 10.1016/s0021-9258(18)63357-5. [DOI] [PubMed] [Google Scholar]
- 136.Butawan M., Benjamin R., Bloomer R. Methylsulfonylmethane: applications and safety of a novel dietary supplement. Nutrients . 2017;9(3):p. 290. doi: 10.3390/nu9030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kwon P. S., Oh H., Kwon S. J., et al. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discovery . 2020;6(1):p. 50. doi: 10.1038/s41421-020-00192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Song S., Peng H. R., Wang Q. L., et al. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food & Function . 2020;11(9):7415–7420. doi: 10.1039/d0fo02017f. [DOI] [PubMed] [Google Scholar]
- 139.Tandon R., Sharp J. S., Zhang F. M., et al. Effective inhibition of SARS-CoV-2 entry by heparin and enoxaparin derivatives. Journal of Virology . 2021;95(3) doi: 10.1128/jvi.01987-20.e01987-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tiwari V., Tandon R., Sankaranarayanan N., et al. Preferential recognition and antagonism of SARS-CoV-2 spike glycoprotein binding to 3-O-sulfated heparan sulfate. bioRxiv . 2020;8 doi: 10.1101/2020.10.08.331751.331751 [DOI] [Google Scholar]
- 141.Bebarta V. S., Brittain M., Chan A., et al. Sodium nitrite and sodium thiosulfate are effective against acute cyanide poisoning when administered by intramuscular injection. Annals of Emergency Medicine . 2017;69(6):718–725. doi: 10.1016/j.annemergmed.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zakharov S., Vaneckova M., Seidl Z., et al. Successful use of hydroxocobalamin and sodium thiosulfate in acute cyanide poisoning: a case report with follow-up. Basic and Clinical Pharmacology and Toxicology . 2015;117(3):209–212. doi: 10.1111/bcpt.12387. [DOI] [PubMed] [Google Scholar]
- 143.Yu Z., Gu L., Pang H., Fang Y., Yan H., Fang W. Sodium thiosulfate: an emerging treatment for calciphylaxis in dialysis patients. Case Reports in Nephrology and Dialysis . 2015;5(1):77–82. doi: 10.1159/000380945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Amirshahrokhi K., Bohlooli S. Effect of methylsulfonylmethane on paraquat-induced acute lung and liver injury in mice. Inflammation . 2013;36(5):1111–1121. doi: 10.1007/s10753-013-9645-8. [DOI] [PubMed] [Google Scholar]
- 145.Kim Y. H., Kim D. H., Lim H., Baek D.-Y., Shin h.-K., Kim J.-K. The anti-inflammatory effects of methylsulfonylmethane on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biological and Pharmaceutical Bulletin . 2009;32(4):651–656. doi: 10.1248/bpb.32.651. [DOI] [PubMed] [Google Scholar]
- 146.Mohammadi S., Najafi M., Hamzeiy H., et al. Protective effects of methylsulfonylmethane on hemodynamics and oxidative stress in monocrotaline-induced pulmonary hypertensive rats. Advances in Pharmacological Sciences . 2012;2012:6. doi: 10.1155/2012/507278.507278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kalman D. S., Feldman S., Scheinberg A. R., Krieger D. R., Bloomer R. J. Influence of methylsulfonylmethane on markers of exercise recovery and performance in healthy men: a pilot study. Journal of the International Society of Sports Nutrition . 2012;9(1):p. 46. doi: 10.1186/1550-2783-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.van der Merwe M., Bloomer J., Bloomer The influence of methylsulfonylmethane on inflammation-associated cytokine release before and following strenuous exercise. The Journal of Sports Medicine . 2016;2016:9. doi: 10.1155/2016/7498359.7498359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Velusamy R. K., Tamizhselvi R. Protective effect of methylsulfonylmethane in caerulein-induced acute pancreatitis and associated lung injury in mice. Journal of Pharmacy and Pharmacology . 2018;70(9):1188–1199. doi: 10.1111/jphp.12946. [DOI] [PubMed] [Google Scholar]
- 150.Sousa-Lima I., Park S. Y., Chung M., et al. Methylsulfonylmethane (MSM), an organosulfur compound, is effective against obesity-induced metabolic disorders in mice. Metabolism . 2016;65(10):1508–1521. doi: 10.1016/j.metabol.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 151.Johnson A. S., Fatemi R., Winlow W. SARS-CoV-2 bound human serum albumin and systemic septic shock. Frontiers in Cardiovascular Medicine . 2020;7:p. 153. doi: 10.3389/fcvm.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Busher J. T. Clinical Methods: The History, Physical, and Laboratory Examinations . Boston, MA, USA: Butterworths; 1990. Serum albumin and globulin. [PubMed] [Google Scholar]
- 153.Fotuhi M., Mian A., Meysami S., Raji C. A. Neurobiology of COVID-19. Journal of Alzheimer’s Disease . 2020;76(1):3–19. doi: 10.3233/jad-200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Buijsers B., Yanginlar C., de Nooijer A., et al. Increased plasma heparanase activity in COVID-19 patients. Frontiers in Immunology . 2020;11 doi: 10.3389/fimmu.2020.575047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van Det N. F., van den Born J., Tamsma J. T., et al. Effects of high glucose on the production of heparan sulfate proteoglycan by mesangial and epithelial cells. Kidney International . 1996;49(4):1079–1089. doi: 10.1038/ki.1996.157. [DOI] [PubMed] [Google Scholar]
- 156.Buijsers B., Yanginlar C., Maciej-Hulme M. L., de Mast Q., van der Vlag J. Beneficial non-anticoagulant mechanisms underlying heparin treatment of COVID-19 patients. EBioMedicine . 2020;59 doi: 10.1016/j.ebiom.2020.102969.102969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shekh S., Amarnath Reddy K. K., Gowd K. H. In silico allicin induced S-thioallylation of SARS-CoV-2 main protease. Journal of Sulfur Chemistry . 2021;42(1):109–120. doi: 10.1080/17415993.2020.1817457. [DOI] [Google Scholar]
- 158.Assimakopoulos S. F., Aretha D., Komninos D., et al. N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: a two-center retrospective cohort study. Infectious Diseases . 2021;53(11):847–854. doi: 10.1080/23744235.2021.1945675. [DOI] [PubMed] [Google Scholar]
- 159.Taher A., Lashgari M., Sedighi L., Rahimi-bashar F., Poorolajal J., Mehrpooya M. A pilot study on intravenous N-Acetylcysteine treatment in patients with mild-to-moderate COVID19-associated acute respiratory distress syndrome. Pharmacological Reports . 2021;73(6):1650–1659. doi: 10.1007/s43440-021-00296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shi Z. C., Puyo C. A. N-acetylcysteine to combat COVID-19: an evidence review. Therapeutics and Clinical Risk Management . 2020;16:1047–1055. doi: 10.2147/tcrm.S273700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mohanty R. R., Padhy B. M., Das S., Meher B. R. Therapeutic potential of N-acetyl cysteine (NAC) in preventing cytokine storm in COVID-19: review of current evidence. European Review for Medical and Pharmacological Sciences . 2021;25(6):2802–2807. doi: 10.26355/eurrev_202103_25442. [DOI] [PubMed] [Google Scholar]
- 162.Jorge-Aarón J. A., Rosa-Ester R. E. N-acetylcysteine as a potential treatment for COVID-19. Future Microbiology . 2020;15(11):959–962. doi: 10.2217/fmb-2020-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhou N., Yang X., Huang A., Chen Z. The potential mechanism of N-acetylcysteine in treating COVID-19. Current Pharmaceutical Biotechnology . 2021;22(12):1584–1590. doi: 10.2174/1389201021999201228212043. [DOI] [PubMed] [Google Scholar]
- 164.Liu X., Liu X., Xu Y., et al. Ventilatory ratio in hypercapnic mechanically ventilated patients with COVID-19-associated acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine . 2020a;201(10):1297–1299. doi: 10.1164/rccm.202002-0373LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhan M., Doerfler R. M., Xie D. W., et al. Association of opioids and nonsteroidal anti-inflammatory drugs with outcomes in CKD: findings from the CRIC (Chronic Renal Insufficiency Cohort) study. American Journal of Kidney Diseases . 2020;76(2):184–193. doi: 10.1053/j.ajkd.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Harden L. M., Neveling N., Rossouw F., et al. The effects of an L-methionine combination supplement on symptoms of upper respiratory tract infections and performance in ultramarathon runners before, during and after ultra-endurance exercise. South African Journal of Sports Medicine . 2004;16(1):10–16. doi: 10.17159/2413-3108/2004/v16i1a188. [DOI] [Google Scholar]
- 167.Ellul M. A., Benjamin L., Singh B., et al. Neurological associations of COVID-19. The Lancet Neurology . 2020;19(9):767–783. doi: 10.1016/s1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Luo P., Liu Y., Liu D., Li J. Perspectives for the use of N-acetylcysteine as a candidate drug to treat COVID-19. Mini-Reviews in Medicinal Chemistry . 2021;21(3):268–272. doi: 10.2174/1389557520666201027160833. [DOI] [PubMed] [Google Scholar]
- 169.Jeannin P., Delneste Y., Lecoanet-Henchoz S., et al. Thiols decrease human interleukin (IL) 4 production and IL-4-induced immunoglobulin synthesis. Journal of Experimental Medicine . 1995;182(6):1785–1792. doi: 10.1084/jem.182.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kalia V., Sarkar S. Regulation of effector and memory CD8 T cell differentiation by IL-2-A balancing act. Frontiers in Immunology . 2018;9:p. 2987. doi: 10.3389/fimmu.2018.02987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lafon E., Diem G., Witting C., et al. Potent SARS-CoV-2-specific T cell immunity and low anaphylatoxin levels correlate with mild disease progression in COVID-19 patients. Frontiers in Immunology . 2021;12 doi: 10.3389/fimmu.2021.684014.684014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cheng G.-S., Evans S. E. The paradox of immunosuppressants and COVID-19. European Respiratory Journal . 2022;59(4) doi: 10.1183/13993003.02828-2021.2102828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sarzani R., Spannella F., Giulietti F., Di Pentima C., Giordano P., Giacometti A. Possible harm from glucocorticoid drugs misuse in the early phase of SARS-CoV-2 infection: a narrative review of the evidence. Internal and Emergency Medicine . 2022;17(2):329–338. doi: 10.1007/s11739-021-02860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xi X., Guo Y., Zhu M., et al. Higher expression of monocyte chemotactic protein 1 in mild COVID-19 patients might be correlated with inhibition of Type I IFN signaling. Virology Journal . 2021;18(1) doi: 10.1186/s12985-020-01478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xu C. F., Wu A. R., Shen Y. Z. Effects of N-acetylcysteine on mRNA expression of monocyte chemotactic protein and macrophage inflammatory protein 2 in acute necrotizing pancreatitis: experiment with rats. Zhonghua Yixue Zazhi . 2008;88(10):711–5. [PubMed] [Google Scholar]
- 176.Sterner J. H., Medes G. The effect of certain sulfur compounds on the coagulation of blood. American Journal of Physiology-Legacy Content . 1936;117(1):92–101. doi: 10.1152/ajplegacy.1936.117.1.92. [DOI] [Google Scholar]
- 177.Araki S., Dobashi K., Kubo K., Yamamoto Y., Asayama K., Shirahata A. N-acetylcysteine attenuates TNF-α induced changes in secretion of interleukin-6, plasminogen activator inhibitor-1 and adiponectin from 3T3-L1 adipocytes. Life Sciences . 2006;79(25):2405–2412. doi: 10.1016/j.lfs.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 178.Zuo Y., Warnock M., Harbaugh A., et al. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. medRxiv . 2020;5 doi: 10.1101/2020.08.29.20184358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hashemi S. A., Kyani A., Bathaie S. Z. The in silico mechanism of hVKOR interaction with acetaminophen and its metabolite, as well as N-acetyl cysteine: caution on application in COVID-19 patients. Journal of Biomolecular Structure and Dynamics . 2021;20:1–12. doi: 10.1080/07391102.2021.1910570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Daid S. S., Toribio A. D., Lakshmanan S., Sadda A., Epstein A. Spontaneous intraparenchymal hepatic hemorrhage as a sequela of COVID-19. Cureus . 2020;12(9) doi: 10.7759/cureus.10447.e10447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Puyo C., Kreig D., Saddi V., Ansari E., Prince O. Case Report: use of hydroxychloroquine and N-acetylcysteine for treatment of a COVID-19 patient. F1000Research . 2020;9:p. 491. doi: 10.12688/f1000research.23995.2. [DOI] [Google Scholar]
- 182.Hasan M. J. N-acetylcysteine in severe COVID-19: the possible mechanism. International Journal of Infection . 2020;7(4):1–3. doi: 10.5812/iji.106361. [DOI] [Google Scholar]
- 183.Yates P. A., Newman S. A., Oshry L. J., Glassman R. H., Leone A. M., Reichel E. Doxycycline treatment of high-risk COVID-19-positive patients with comorbid pulmonary disease. Therapeutic Advances in Respiratory Disease . 2020;14 doi: 10.1177/1753466620951053.175346662095105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rogliani P., Matera M. G., Page C., Puxeddu E., Cazzola M., Calzetta L. Efficacy and safety profile of mucolytic/antioxidant agents in chronic obstructive pulmonary disease: a comparative analysis across erdosteine, carbocysteine, and N-acetylcysteine. Respiratory Research . 2019;20(1):p. 104. doi: 10.1186/s12931-019-1078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ltd, Hope Pharmaceuticals. Sodium thiosulfate solution for injection. 2021. https://www.medicines.org.uk/emc/product/7354/smpc .
- 186.Piñeiro-Carrero V. M., Piñeiro E. O. Liver. Pediatrics . 2004;113:1097–106. [PubMed] [Google Scholar]
- 187.Thomas C. V., Sackrison J. L., Ryan U. S., Luikart S. D. Effects of colchicine on sulfated glycosaminoglycan production and cell detachment in pre-capillary pulmonary endothelial cells. Tissue and Cell . 1987;19(5):617–624. doi: 10.1016/0040-8166(87)90069-3. [DOI] [PubMed] [Google Scholar]
- 188.Rodriguez E. A., Li X., Lehmler H. J., Robertson L. W., Duffel M. W. Sulfation of lower chlorinated polychlorinated biphenyls increases their affinity for the major drug-binding sites of human serum albumin. Environmental Science and Technology . 2016;50(10):5320–5327. doi: 10.1021/acs.est.6b00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yasuda S., Idell S., Fu J., Carter G., Snow R., Liu M. C. Cigarette smoke toxicants as substrates and inhibitors for human cytosolic SULTs. Toxicology and Applied Pharmacology . 2007;221(1):13–20. doi: 10.1016/j.taap.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 190.Di Pierro F., Derosa G., Derosa P., et al. Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: a prospective, randomized, controlled, and open-label study. International Journal of General Medicine . 2021;14:2359–2366. doi: 10.2147/ijgm.s318720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Di Pierro F., Iqtadar S., Khan A., et al. Potential clinical benefits of quercetin in the early stage of COVID-19: results of a second, pilot, randomized, controlled and open-label clinical trial. International Journal of General Medicine . 2021;14:2807–2816. doi: 10.2147/ijgm.s318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lan S.-H., Lee H.-Z., Chao C.-M., Chang S.-P., Lu L.-C., Lai C.-C. Efficacy of melatonin in the treatment of patients with COVID‐19: a systematic review and meta‐analysis of randomized controlled trials. Journal of Medical Virology . 2022;94(5):2102–2107. doi: 10.1002/jmv.27595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schloss J., Leach M., Brown D., Hannan N., Kendall-Reed P., Steel A. The effects of N-acetyl cysteine on acute viral respiratory infections in humans: a rapid review. Advances in Integrative Medicine . 2020;7(4):232–239. doi: 10.1016/j.aimed.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pérez de la Lastra J. M. P., Andrés-Juan C., Plou F. J., Pérez-Lebeña E. Impact of zinc, glutathione, and polyphenols as antioxidants in the immune response against SARS-CoV-2. Processes . 2021;9(3):p. 506. doi: 10.3390/pr9030506. [DOI] [Google Scholar]
- 195.Bermano G., Méplan C., Mercer D. K., Hesketh J. E. Selenium and viral infection: are there lessons for COVID-19? British Journal of Nutrition . 2021;125(6):618–627. doi: 10.1017/s0007114520003128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Taylor E. W., Radding W. Understanding selenium and glutathione as antiviral factors in COVID-19: does the viral mpro protease target host selenoproteins and glutathione synthesis? Frontiers in Nutrition . 2020;7:p. 143. doi: 10.3389/fnut.2020.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ayseli Y. I., Aytekin N., Buyukkayhan D., Aslan I., Ayseli M. T. Food policy, nutrition and nutraceuticals in the prevention and management of COVID-19: advice for healthcare professionals. Trends in Food Science & Technology . 2020;105:186–199. doi: 10.1016/j.tifs.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pandolfi S., Simonetti V., Ricevuti G., Chirumbolo S. Paracetamol in the home treatment of early COVID‐19 symptoms: a possible foe rather than a friend for elderly patients? Journal of Medical Virology . 2021;93(10):5704–5706. doi: 10.1002/jmv.27158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Akter F., Araf Y., Hosen M. J. Corticosteroids for COVID-19: worth it or not? Molecular Biology Reports . 2022;49(1):567–576. doi: 10.1007/s11033-021-06793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ronchetti S., Ayroldi E., Ricci E., Gentili M., Migliorati G., Riccardi C. A glance at the use of glucocorticoids in rare inflammatory and autoimmune diseases: still an indispensable pharmacological tool? Frontiers in Immunology . 2021;11 doi: 10.3389/fimmu.2020.613435.613435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li C.-J., Sun L.-Y., Pang C.-Y. Synergistic protection of N-Acetylcysteine and ascorbic acid 2-phosphate on human mesenchymal stem cells against mitoptosis, necroptosis and apoptosis. Scientific Reports . 2015;5(1):p. 9819. doi: 10.1038/srep09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.De Alencar J. C. G., Moreira C. L., Müller A. D., et al. Double-blind, randomized, placebo-controlled trial with N-acetylcysteine for treatment of severe acute respiratory syndrome caused by coronavirus disease 2019 (COVID-19) Clinical Infectious Diseases . 2021;72(11):E736–E741. doi: 10.1093/cid/ciaa1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Generali J. A., Cada D. J. Sodium thiosulfate: calciphylaxis. Hospital Pharmacy . 2015;50(11):975–977. doi: 10.1310/hpj5011-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Greaves P. Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation . 4th. Amsterdam, Netherlands: Elsevier; 2012. Digestive system, histopathology of preclinical toxicity studies; pp. 325–431. [DOI] [Google Scholar]
- 205.Sestili P., Fimognari C. Paracetamol-induced glutathione consumption: is there a link with severe COVID-19 illness? Frontiers in Pharmacology . 2020;11 doi: 10.3389/fphar.2020.579944.579944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dunne J. W., Davidson L., Vandongen R., Beilin L. J., Tunney A. M., Rogers P. B. The effect of ascorbic acid on the sulphate conjugation of ingested noradrenaline and dopamine. British Journal of Clinical Pharmacology . 1984;17(3):356–360. doi: 10.1111/j.1365-2125.1984.tb02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dissayabutra T., Kalpongnukul N., Chindaphan K., et al. Urinary sulfated glycosaminoglycan insufficiency and chondroitin sulfate supplement in urolithiasis. PLoS One . 2019;14(3) doi: 10.1371/journal.pone.0213180.e0213180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dull R. O., Dinavahi R., Schwartz L., et al. Lung endothelial heparan sulfates mediate cationic peptide-induced barrier dysfunction: a new role for the glycocalyx. American Journal of Physiology - Lung Cellular and Molecular Physiology . 2003;285(5):L986–L995. doi: 10.1152/ajplung.00022.2003. [DOI] [PubMed] [Google Scholar]
- 209.Herrero R., Sanchez G., Lorente J. A. New insights into the mechanisms of pulmonary edema in acute lung injury. Annals of Translational Medicine . 2018;6(2):p. 32. doi: 10.21037/atm.2017.12.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Liu Y., Wang M. F., Luo G. S., et al. Experience of N-acetylcysteine airway management in the successful treatment of one case of critical condition with COVID-19. Medicine . 2020b;99(42) doi: 10.1097/md.0000000000022577.e22577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Calligaro G. L., Lalla U., Audley A., et al. The utility of high-flow nasal oxygen for severe COVID-19 pneumonia in a resource-constrained setting: a multi-centre prospective observational study. EClinicalMedicine . 2020;28 doi: 10.1016/j.eclinm.2020.100570.100570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen X.-Y., Yan B.-X., Man X.-Y. TNFα inhibitor may be effective for severe COVID-19: learning from toxic epidermal necrolysis. Therapeutic Advances in Respiratory Disease . 2020;14 doi: 10.1177/1753466620926800.175346662092680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Goodarzi N., Atefi E., Behrangi S., Mozafarpoor F., Seirafianpour S., Peighambari A. N-acetylcysteine and coronavirus disease 2019: may it work as a beneficial preventive and adjuvant therapy? A comprehensive review study. Journal of Research in Medical Sciences . 2020;25(1):p. 109. doi: 10.4103/jrms.JRMS_777_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yeoh Y. K., Zuo T., Lui G. C.-Y., et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut . 2021;70(4):698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wehbe Z., Wehbe M., Iratni R., et al. Repurposing Ivermectin for COVID-19: molecular aspects and therapeutic possibilities. Frontiers in Immunology . 2021;12 doi: 10.3389/fimmu.2021.663586.663586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Brinkmann U., Eichelbaum M. Polymorphisms in the ABC drug transporter gene MDR1. The Pharmacogenomics Journal . 2001;1(1):59–64. doi: 10.1038/sj.tpj.6500001. [DOI] [PubMed] [Google Scholar]
- 217.Chandler R. E. Serious neurological adverse events after ivermectin-do they occur beyond the indication of onchocerciasis? The American Journal of Tropical Medicine and Hygiene . 2018;98(2):382–388. doi: 10.4269/ajtmh.17-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Linsenmeyer M. E., Jefferson S., Wolf M., Matthews J. P., Board P. G., Woodcock D. M. Levels of expression of the mdr1 gene and glutathione S-transferase genes 2 and 3 and response to chemotherapy in multiple myeloma. British Journal of Cancer . 1992;65(3):471–475. doi: 10.1038/bjc.1992.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Senhaji N., Kassogue Y., Fahimi M., Serbati N., Badre W., Nadifi S. Genetic polymorphisms of multidrug resistance gene-1 (MDR1/ABCB1) and glutathione S-transferase gene and the risk of inflammatory bowel disease among Moroccan patients. Mediators of Inflammation . 2015;2015:8. doi: 10.1155/2015/248060.248060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sharthiya H., Seng C., Van Kuppevelt T. H., Tiwari V., Fornaro M. HSV-1 interaction to 3-O-sulfated heparan sulfate in mouse-derived DRG explant and profiles of inflammatory markers during virus infection. Journal of NeuroVirology . 2017;23(3):483–491. doi: 10.1007/s13365-017-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gordon C. J., Tchesnokov E. P., Schinazi R. F., Götte M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. Journal of Biological Chemistry . 2021;297(1) doi: 10.1016/j.jbc.2021.100770.100770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hu Y., Lewandowski E. M., Tan H., et al. 2022.
- 223. Jochmans, D., C. Liu, Kim D., A. Stoycheva, S. Boland, S. K. Stevens, C. De Vita, B. Vanmechelen, P. Maes, B. Trüeb, N. Ebert, V. Thiel, S. De Jonghe, L. Vangeel, D. Bardiot, Andreas jekle, lawrence M. Blatt, leonid beigelman, julian A. Symons, pierre raboisson, patrick chaltin, arnaud marchand, johan neyts, jerome deval, and koen vandyck. 2022.
- 224.Das C., Paul S. S., Saha A., et al. Silver-based nanomaterials as therapeutic agents against coronaviruses: a review. International Journal of Nanomedicine . 2020;15:9301–9315. doi: 10.2147/ijn.s280976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ou L., Song B., Liang H., et al. Toxicity of graphene-family nanoparticles: a general review of the origins and mechanisms. Particle and Fibre Toxicology . 2016;13(1):p. 57. doi: 10.1186/s12989-016-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pham T. A., Kim J., Kim J. S., Jeong Y. T. One-step reduction of graphene oxide with l-glutathione. Colloids and Surfaces A: Physicochemical and Engineering Aspects . 2011;384(1-3):543–548. doi: 10.1016/j.colsurfa.2011.05.019. [DOI] [Google Scholar]
- 227.Vernekar A. A., Mugesh G. Catalytic reduction of graphene oxide nanosheets by glutathione peroxidase mimetics reveals a new structural motif in graphene oxide. Chemistry - A European Journal . 2013;19(49):16699–16706. doi: 10.1002/chem.201303339. [DOI] [PubMed] [Google Scholar]
- 228.Sethia R., Prasad M., Mahapatra S., et al. Efficacy of famotidine for COVID-19: a systematic review and meta-analysis. medRxiv . 2020 doi: 10.1101/2020.09.28.20203463.20203463 [DOI] [Google Scholar]
- 229.Fruitwala S., El-Naccache D. W., Chang T. L. Multifaceted immune functions of human defensins and underlying mechanisms. Seminars in Cell & Developmental Biology . 2019;88:163–172. doi: 10.1016/j.semcdb.2018.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Smits N. C., Shworak N. W., Dekhuijzen P. N. R., van Kuppevelt T. H. Heparan sulfates in the lung: structure, diversity, and role in pulmonary emphysema. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology . 2010;293(6):955–967. doi: 10.1002/ar.20895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All clinical recommendations made in the review article are based on published clinical trials, research, clinical experience and case reports, and are well referenced.


