Abstract
The Klebsiella pneumoniae nitrogen fixation (nif) mRNAs are unusually stable, with half-lives of 20 to 30 min under conditions favorable to nitrogen fixation (limiting nitrogen, anaerobiosis, temperatures of 30°C). Addition of O2 or fixed nitrogen or temperature increases to 37°C or more result in the dramatic destabilization of the nif mRNAs, decreasing the half-lives by a factor of 3 to 5. A plasmid expression system, independent of nif transcriptional regulation, was used to define cis determinants required for the regulated stability of the 5.2-kb nifHDKTY mRNA and to test the model suggested by earlier work that NifA is required in trans to stabilize nif mRNA under nif-derepressing conditions. O2 regulation of nifHDKTY mRNA stability is impaired in a plasmid containing a deletion of a 499-bp region of nifH, indicating that a site(s) required for the O2-regulated stability of the mRNA is located within this region. The simple model suggested from earlier work that NifA is required for stabilizing nif mRNA under conditions favorable for nitrogen fixation was disproved, and in its place, a more complicated model involving the sensing of nitrogenase activity as a component of the system regulating mRNA stability is proposed. Analysis of nifY mutants and overexpression suggests a possible involvement of the protein in this sensing process.
In Klebsiella pneumoniae, a series of highly regulated events occur before the bacterium dedicates itself to the energy-intensive process of fixing nitrogen (reviewed in references 18 and 41). The nitrogen regulatory (ntr) system is responsible for the transcriptional regulation involved in the nitrogen regulatory cascade. Under conditions of fixed-nitrogen limitation, anaerobiosis, and temperatures at or below 30°C, the nitrogen fixation (nif) system is turned on by the transcriptional activator NtrC. NtrC, in conjunction with ς54 and RNA polymerase, is responsible for activating expression of the nifLA genes. Using an elegant regulatory scheme, the nifLA gene products are responsible for activating transcription of nif genes under conditions favorable for nitrogen fixation and for shutting down nif expression, at both the transcriptional as well as posttranscriptional levels, when conditions become unfavorable (11, 14, 15, 32). NifA is responsible for activating the ς54-dependent expression of the other nif operons by binding to an upstream activating sequence (UAS) (44), while NifL interferes with that activation (4, 25, 26, 48). glnK, also under NtrC transcriptional control, is required to relieve NifL inhibition under N-limiting conditions (24). NifL and NifA are unusual members of a two-component regulatory system in that inhibition of NifA by NifL is not mediated by the typical phosphorylation reaction (3, 36). Instead, stoichiometric levels of NifL are required for the inhibition of NifA activity, implying protein-protein interaction may be involved.
While NifL is necessary for the inhibition of nif mRNA synthesis in response to fixed nitrogen and O2, the protein is not necessary for the shutdown of synthesis in response to temperatures of 37°C or above (14). High temperature inactivates NifA in vitro (36), and it is thought that the temperature sensitivity of NifA is responsible for cessation of nif gene expression at increased temperatures.
Several reports demonstrated that nif mRNA stability is dramatically regulated in response to the same stimuli that regulate nif transcription (29, 31, 32). Research by Collins and coworkers (15) supported these claims and further demonstrated nif specificity for the regulation. By using pulse-labeling, filter hybridization, and two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) techniques, they determined that nif mRNAs under NifLA control decay with half-lives (t1/2s) of 20 to 30 min under nif-derepressing conditions. They also showed that nif mRNA (except nifLA) is rapidly destabilized, decaying with t1/2s of 4 to 6 min upon addition of fixed nitrogen or O2 or by temperatures at or above 37°C, and that functional inactivation of the mRNAs approximated chemical decay. Furthermore, they demonstrated that NifL is necessary for destabilization of the mRNA upon addition of O2 and fixed nitrogen, but is not required for the temperature effect.
Since NifL interferes with activation of nif transcription by NifA, a simple model was proposed (15) that predicted NifL also functions at the posttranscriptional level by inhibiting NifA activity. This model posited that NifA is directly or indirectly responsible for stabilizing nif mRNA under nif-derepressing conditions. The temperature-sensitive NifA would be unable to activate synthesis or stabilize nif mRNAs at or above 37°C, which would provide an explanation for the rapid destabilization of nif mRNA in nifL mutants at high temperatures.
While intriguing, this model has been difficult to test, because the overlapping roles that NifL and NifA would perform in transcription and posttranscriptional regulation make it difficult to distinguish effects due explicitly to posttranscriptional regulation. Additionally, nif mRNA stability cannot be examined in a nifA strain, because, with the exception of nifLA, no nif mRNA is expressed in such a mutant. In this work, we employed a nifHDKTY expression plasmid, pUX40, that separates nif transcription from posttranscriptional regulation without changing the wild-type mRNA sequence. We report here our studies defining the cis determinants required for the unusual anaerobic stability and for O2 regulation of stability of the nifHDKTY mRNA in K. pneumoniae. We additionally used pUX40 to test and disprove the model that NifA is sufficient to stabilize nif mRNA under nif-derepressing conditions. The data instead suggest that regulation of nif mRNA stability involves a complex interaction of a number of different nif proteins and that nitrogenase activity is a key factor in determining stability.
MATERIALS AND METHODS
Media and reagents.
The recipe for the minimal medium used for growth and derepression of strains for nitrogenase function was described previously (22).
The following antibiotics were used at the indicated concentrations: kanamycin sulfate, 50 μg/ml; chloramphenicol, 25 μg/ml; ampicillin (sodium salt), 50 μg/ml; tetracycline, 4 μg/ml; and carbenicillin (disodium salt)-ampicillin (sodium salt), 150 μg/ml each.
All chemicals, enzymes, and gases were of analytical grade or higher and were obtained from Sigma Chemical Co. (St. Louis, Mo.), Boehringer Mannheim Biochemicals (Indianapolis, Ind.), Bio-Rad Laboratories (Richmond, Calif.), Promega Corp. (Madison, Wis.), New England Biolabs (Beverly, Mass.), or Pharmacia, Inc. (Piscataway, N.J.). The [α-32P]dATP was obtained from Amersham Life Science, Inc. (Arlington Heights, Ill.).
Bacterial strains and plasmids.
The relevant strains and plasmids used in this study are listed in Table 1 and described below.
TABLE 1.
K. pneumoniae strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| UN | Wild type for nitrogen fixation (nif+) | P. W. Wilson |
| UN1696 | nifK4732 | 37 |
| UN1795 | nifH4764 | 37 |
| UN5360 | nifY6290::aphA | This report |
| UN5397 | nif+/pNF107/F′lacIQ | This report |
| UN5406 | nifL6302/pNF107/F′lacIQ | This report |
| UN5435 | ΔnifDK6292 | This report |
| UN5442 | ΔnifDK6292/pUX40/F′lacIQ | This report |
| UN5443 | nif-5000 (ΔnifJ-A)b/pUX40/F′lacIQ | This report |
| UN5445 | nif-5000 (ΔnifJ-A)b/pUX40/pVL15/F′lacIQ | This report |
| UN5448 | nif-4932 (ΔnifD-Q)/pUX40/F′lacIQ | This report |
| UN5450 | nif-4932 (ΔnifD-Q)/pUX40/pVL15/F′lacIQ | This report |
| UN5451 | inf-4934 (ΔnifD-M)/pUX40/F′lacIQ | This report |
| UN5458 | nif-2635 (ΔnifJ-A)b/pUX40/F′lacIQ | This report |
| UN5459 | nif-2635 (ΔnifJ-A)b/pUX40/pUXA1/F′lacIQ | This report |
| UN5460 | nif+/pUX201/F′lacIQ | This report |
| UN5461 | nif+/pUX202/F′lacIQ | This report |
| UN5469 | nif+/pUX214/F′lacIQ | This report |
| UN5470 | nif+/pUX215/F′lacIQ | This report |
| UN5479 | nif+/pUX203/F′lacIQ | This report |
| Plasmids | ||
| pACYC184 | Low-copy, parent plasmid | 12 |
| pJR6 | Derivative of R6K, requires pir gene product to replicate | This report |
| pNF102 | Derivative of pBR322 expressing nifTY from the Ptac promoter | This report |
| pNF107 | pBR322 derivative expressing nifY under Ptac control | This report |
| pVL15 | pBR322 derivative expressing nifA under Ptac control | 23 |
| pUXA1 | pSC101 derivative expressing nifA under Plac control | This report |
| pUX32 | Derivative of pUX25 containing nifJ′HDKTY | This report |
| pUX40 | nifHDKTY under PA1/04 control (derivative of pUX32) | This report |
| pUX50 | ΔnifDK6292 (derivative of R6K) | This report |
| pUX200 | nifHDKTY under PA1/04 control (derivative of pUX40) | This report |
| pUX201 | ΔnifD-Y6298 under PA1/04 control (derivative of pUX200) | This report |
| pUX202 | ΔnifTY6299 under PA1/04 control (derivative of pUX200) | This report |
| pUX203 | ΔnifDK6292 under PA1/04 control (derivative of pUX200) | This report |
| pUX214 | ΔnifH6300 under PA1/04 control (derivative of pUX200) | This report |
| pUX215 | ΔnifH-K6301 under PA1/04 control (derivative of pUX200) | This report |
For each strain, the nif allele is listed, followed by plasmids; chromosomal allele numbers and plasmids are separated by a “/”.
The deletion was not mapped physically, and the end falls either within nifL or nifA. The operon promoter is absent in either case, so the deletion eliminates expression through nifA.
Construction of pUX40.
pUX40, a plasmid expressing nifHDKTY from a nif-independent promoter, was constructed as follows. By oligonucleotide synthesis, the promoter PA1/04 (35) was fused to the first 15 bases at the 5′ start of nifH. PCR was performed to amplify a 724-bp partial nifH fragment fused to the PA1/04 promoter, followed by ligation into pUX32 (a plasmid containing the wild-type nifHDKTY operon, which was itself constructed by standard cloning techniques [Table 1]) to construct pUX40. The details are as follows. Two oligonucleotides were synthesized (Department of Biochemistry, University of Wisconsin—Madison) and used in the PCR to construct and amplify the 724-bp fragment: (i) the 82-mer 5′-CAGGCGAGCTCTTTTAAATAGTTTTTCTCACAACTGAACACTCGCCTATTGTTACTATGAATCTAAGCCGTTTGTGAGTTGT-3′ (the −35 and −10 regions of the promoter are underlined), identical to the sense strand and consisting of the PA1/04 promoter and the first 15 nucleotides (nt) of the nifH transcript; and (ii) the 15-mer 5′-GATCATCTGGGTACC-3′, complementary to the sense strand and hybridizing 627 nt downstream from the start of the nifH transcript. A 536-bp partial DNA fragment containing the PA1/04/nifH fusion was isolated and ligated into the EcoRV and BglII sites of pUX32 by standard cloning techniques to construct pUX40. The construct was confirmed by sequencing with the Sequenase kit (Bio-Rad; Hercules, Calif.). The sequence of the pUX40 promoter controlling nifHDKTY expression is identical to that of the 82-mer through the promoter region. The initiation site of the mRNA expressed from pUX40 was determined to be that of the wild-type nifHDKTY mRNA (7) by primer extension analysis (data not shown).
Construction of pUX40 deletion derivatives.
Various deletions were made in the nifHDKTY genes carried on the plasmid pUX40 by either conventional restriction analysis, as with pUX201, pUX202, pUX203, and pUX215, or by use of exonuclease III (Exo III), as in the case of pUX214 (see Fig. 3). All of the deletion plasmids were derived from pUX200, which was constructed from pUX40 by partial digestion with AhdI, incubation with Klenow fragment to remove the single-base 3′ overhang, and ligation, resulting in the removal of the plasmid AhdI site. Klenow fragment was employed to fill in the ends where required. NruI and SmaI were used to construct both pUX201 and pUX202. The deletion in pUX201 extends from the NruI site at nif nt 5704 (which refers to the nucleotide position by the numbering convention in reference 2) in nifD to the SmaI site at nif nt 8956 in nifY and removes 3,252 bp. The deletion was designated ΔnifD-Y6298. The deletion in pUX202 extends from the NruI site at nif nt 8233 in nifT to the SmaI site at nif nt 8956 in nifY, removing 723 bp, and was designated ΔnifTY6299. pUX203 was made by removing 2,562 bp, between nif nt 5249 in nifD and nif nt 7811 in nifK, with a single enzyme, BsiWI, creating ΔnifDK6292. pUX215 was constructed by using the AhdI site at nif nt 4184 in nifH and the HpaI site at nif nt 8140 at the end of nifK. The deletion extends from nif nt 4183 to nt 8140, removes 3957 bp, and was designated ΔnifH-K6301. pUX214 was constructed by using Exo III digestion as follows: pUX200 was digested with KpnI, which has a unique site at nt 4794, and BglII, which has a unique site at nt 4617, both in nifH. While the former site should be resistant to Exo III digestion and the latter should be susceptible, sequence analysis of several clones revealed that Exo III digestion proceeded into each end. The deletion in pUX214 extends from nif nt 4303 to nt 4802 in nifH, removes 499 bp, and was designated ΔnifH6300.
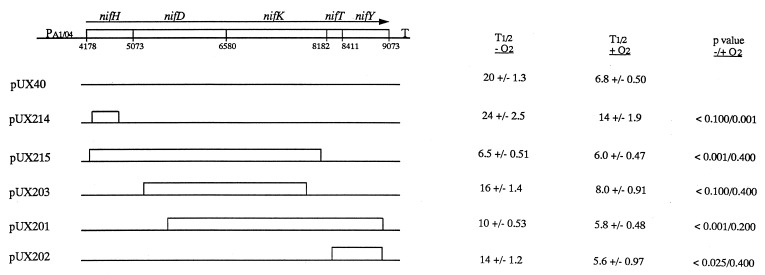
FIG. 3.
Map of deletion derivatives of pUX40 and the summary of measured t1/2s under anaerobic and aerobic conditions. A map of the intact operon is shown above. The gene designations and direction of transcription are shown above the operon; T designates the terminator. The nucleotide positions at the start of each gene (2) are given below the line. pUX40 contains the intact nifHDKTY operon, and the deletion plasmids are represented below, with the deletions depicted as boxes. Data corresponding to the results in the table are shown graphically for the deletions in Fig. 4; the data for pUX40 are those shown in Fig. 2. The t1/2 value (T1/2) was calculated from the slopes of the combined regression analyses from two independent experiments for each deletion mRNA under anaerobic and aerobic conditions, with the chromosomal nifHDKTY mRNA used as an internal control (see text).
The constructs were confirmed by restriction enzyme and, in cases where more than one enzyme was used to make the deletion, DNA sequence analysis. All deletions are in frame with the following exceptions: pUX201, in which case the 3′ end of the deletion falls near the end of the nifHDKTY transcript and the new reading frame contains a stop codon 35 nt from the end of the nifY gene; and pUX203, in which a stop codon is introduced 316 nt from the end of nifK.
Construction of strain UN5442.
ΔnifDK6292 was constructed in the plasmid pUX50 by digestion of pUX32 with BsiWI to remove a 2.6-kb fragment internal to the nifDK genes, followed by intramolecular ligation. A 3.8-kb EcoRI fragment encompassing ΔnifDK6292 was cloned into the plasmid pJR6, which had been partially digested with EcoRI to construct the plasmid pUX50. Taking advantage of the inability of pJR6 (an R6K vector derivative (43) to replicate in the absence of the pir gene product, the deletion was subsequently moved into the K. pneumoniae wild-type strain (UN) chromosome by homologous recombination. Plasmid-free deletion mutants were identified by their antibiotic-sensitive, Nif− phenotype and confirmed by Southern blot analysis (data not shown). The strain with ΔnifDK6292 was designated UN5435. An F′ plasmid with the lacIQ gene was moved by conjugation into strain UN5435 (as well as into all other strains used to harbor pUX40) to allow regulation of PA1/04, constructing strain UN5439. pUX40 was subsequently transformed into UN5439 to construct strain UN5442.
Construction of Δnif strains harboring pUX40.
pUX40 was transformed into the following recipients to create strains in Table 1: UN5408 (ΔnifJ-A), to construct UN5443; UN5446 (ΔnifD-Q), to construct UN5448; UN5447 (ΔnifD-M), to construct UN5451; and UN5457 (ΔnifJ-A), to construct UN5458. UN5408, UN5446, UN5447, and UN5457 were constructed by the introduction of an F′ with lacIQ into UN2408 (37), UN1978 (37), UN1980 (37), and UN5456 (40), respectively. UN2408, UN1978, and UN1980 are Mu-induced deletion strains; UN5456 was derived by deletion of Tn10.
UN5445 and UN5450 were constructed by transforming pVL15 (23) into UN5443 and UN5448, and UN5459 was constructed by transforming pUXA1 into UN5458 (Table 1). pVL15 is a pBR322 plasmid derivative expressing nifA from the tac promoter. pUXA1 expresses nifA from the lac promoter and was constructed by cloning a 3.0-kb SalI fragment from pSB2001 (9) containing nifA into the SalI site in pCL1920 (52). Expression of active NifA from pUXA1 was confirmed by complementation of nifA mutant strains and was observed to be similar to that of the wild-type strain, as determined by acetylene reduction analysis (data not shown).
Construction of nifY strains.
Construction of the nifY and nifY-overexpressing strains listed in Table 1 was performed as follows. UN5360 was constructed by ligating the Kanr cassette from pUC-4K (42, 46) into the SalI site of the nifY gene located on a plasmid and then using reciprocal recombination, as described by Gosink et al. (22), to move this mutation, nifY6290::aph, into the K. pneumoniae chromosome. The mutation was confirmed by Southern blot analysis (data not shown).
The nifY expression vector, pNF107, was constructed by cloning the 925-bp NruI-SspI fragment containing nifY downstream of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible Ptac promoter of pKK223-3 (10). UN5397 was constructed by transforming pNF107 into UN5350, a wild-type strain of K. pneumoniae containing an F′ with lacIQ. UN5406 was constructed by transforming pNF107 into UN5361 (nifL strain UN4357 (37) containing an F′ with lacIQ). Upon induction of pNF107, a protein is synthesized in vivo that corresponded to the wild-type NifY as determined by mobility on one- and two-dimensional SDS-PAGE gels (data not shown).
Derepression of nitrogenase.
Cell growth, nif derepression, assay for nitrogenase function, and the procedure for the addition of O2 to derepressed cultures of K. pneumoniae have been described previously (22). When appropriate, the expression of nif mRNA from plasmids was induced with 150 μM IPTG before sampling, for times ranging between 7 and 12 min, depending upon the size of the mRNA expressed.
Isolation and analysis of RNA.
Isolation of total RNA from derepressed cells and Northern blotting were carried out as described previously (22), except for the following changes. (i) Unbuffered phenol was used in place of Tris-EDTA-equilibrated phenol. (ii) After the initial ethanol precipitation, samples were treated with RNase-free DNase (Promega Corp.) for 10 min at 37°C. This digestion was stopped by the addition of 25 mM EDTA and phenol-chloroform extraction, followed by an ethanol precipitation. (iii) Samples were separated by loading 5 to 10 μg of RNA per lane onto 1 to 1.5% agarose, 0.2 M formaldehyde gels. (iv) Nytran Plus positively-charged nylon membranes (0.45 μM pore size) from Schleicher & Schuell (Keene, N.H.) were used for immobilization of RNA for hybridization.
A 0.87-kb NruI-SacII nifTY fragment and a 1.8-kb HincII nifKTY fragment were labeled with [α-32P]dATP by random hexamer labeling (20) and used separately as probes in Northern analyses. Hybridization and washes were done as described previously (22).
Determination of RNA t1/2.
Cultures that had been derepressed for nif function and induced with 150 μM IPTG when appropriate (to express the cloned nif region) were treated for 3 min with rifampin at 200 mg/liter. Samples of 2 ml were withdrawn at intervals thereafter and centrifuged at 15,000 × g for 20 s. The RNA was then extracted and analyzed by Northern blotting as described above. Radioactivity contained in RNA bands hybridizing to the 32P-labeled nif DNA probes was quantified by using the Ambis radioanalytic imaging device and Quant Probe software, version 3.0, from Ambis, Inc. (San Diego, Calif.), or the Molecular Dynamics PhosphorImager, model 445Si (Sunnyvale, Calif.). Least-squares (19) and DFFITS sensitivity (6a, 16) analyses were performed to obtain the t1/2 of mRNA decay for each experiment, and t1/2 errors were estimated from the standard error of the slope of each regression line. Comparisons between experiments and among strains were performed using a t test at the 5% significance level or, where noted, analysis of covariance (19). Data points that were <10% of the t0 point were not included in analyses.
Detection of different nif mRNA species hybridizing to probes from the nifHDKTY region.
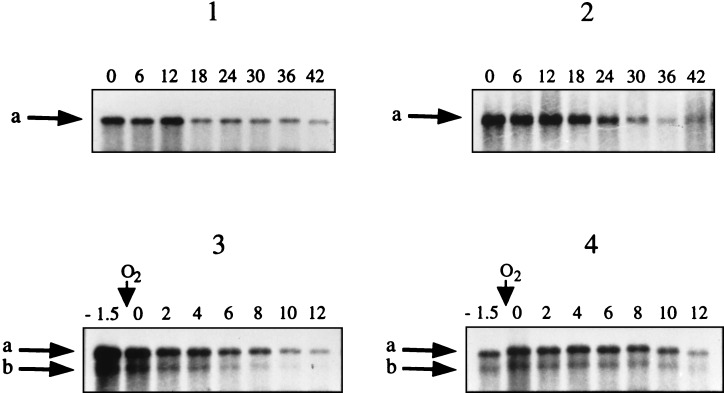
Under nif-derepressing conditions, several mRNA species that hybridize to a nifH DNA probe accumulate in UN (wild type) (13, 22). Based on hybridization patterns of the mRNAs on Northern blots, we have noted two major species of approximately 5,000 and 4,500 nt in length that correspond to nifHDKTY and nifHDK, respectively (Fig. 1) (22). Preliminary evidence demonstrated that the decay rates for these two mRNAs were similar under both stabilizing and destabilizing conditions (plus O2) (data not shown). We decided to focus our studies on the larger, 5,000-nt nifHDKTY mRNA, because it has not been determined whether the 3′ end of the shorter mRNA species arises due to transcription termination or mRNA processing. Therefore, an 874-bp NruI-SacII nifTY fragment was used for the selective detection of full-length nifHDKTY mRNA, with the exception of the Northern blots shown in panels 3 and 4 of Fig. 1, for which a 1.8-kb HincII nifKTY fragment was used for detection of nif mRNA.
FIG. 1.
Anaerobic stability and O2 regulation of stability of the chromosomal nifHDKTY mRNA in the wild-type background and the pUX40 nifHDKTY mRNA in the ΔnifDK background. Representative Northern blots of RNA isolated after rifampin addition from chromosomal (UN) (panel 1) and ΔnifDK/pUX40 (UN5442) (panel 2) anaerobic cells derepressed for nif expression and from chromosomal (panel 3) and ΔnifDK/pUX40 (panel 4) cells treated with O2. Sampling times are indicated in minutes above the lanes. The time of O2 addition relative to sampling is indicated with a downward arrow above the indicated sampling times. A nifTY-specific probe was used in panels 1 and 2, and a nifKTY-specific probe was used in panels 3 and 4. Arrows a indicate nifHDKTY mRNA, and arrows b indicate nifHDK mRNA.
RESULTS AND DISCUSSION
Construction and characterization of pUX40, which allows controlled expression of nifHDKTY mRNA independent of nif regulation.
Involvement of the nifLA gene products in both transcription and posttranscriptional regulation of nif gene expression made it essential to express the nifHDKTY mRNA from a nif-independent promoter to separate these two effects. We replaced the nifH promoter and NifA UAS in the nifHDKTY operon with PA1/04, the modified PA1 promoter which was originally derived from phage T7 (35), designating this construct pUX40. The nifHDKTY mRNA expressed from pUX40 is identical in sequence to the wild-type mRNA, as confirmed by primer extension analysis (data not shown).
Strain UN5442 contains pUX40 and a 2.6-kb deletion in the chromosomal nifDK genes. The ΔnifDK background allowed us to distinguish the pUX40-expressed nifHDKTY mRNA from the mRNA expressed from the chromosome on Northern blots. The addition of 150 μM IPTG for 12 min to strain UN5442 (ΔnifDK/pUX40) provided approximately the same accumulation of nifHDKTY mRNA as in the wild-type strain, UN, under nif-derepressing conditions, which allowed the experiments to be performed at physiologically relevant levels of mRNA. With IPTG induction, UN5442 became phenotypically Nif+, and levels of nitrogenase activity (as measured by acetylene reduction) were very similar to those of the wild-type strain (data not shown).
pUX40 and chromosomal nifHDKTY mRNA demonstrate similar anaerobic stability and O2-induced decay kinetics.
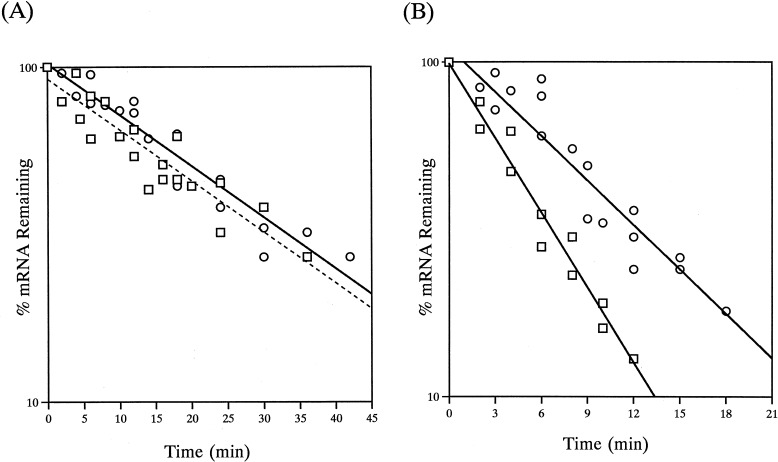
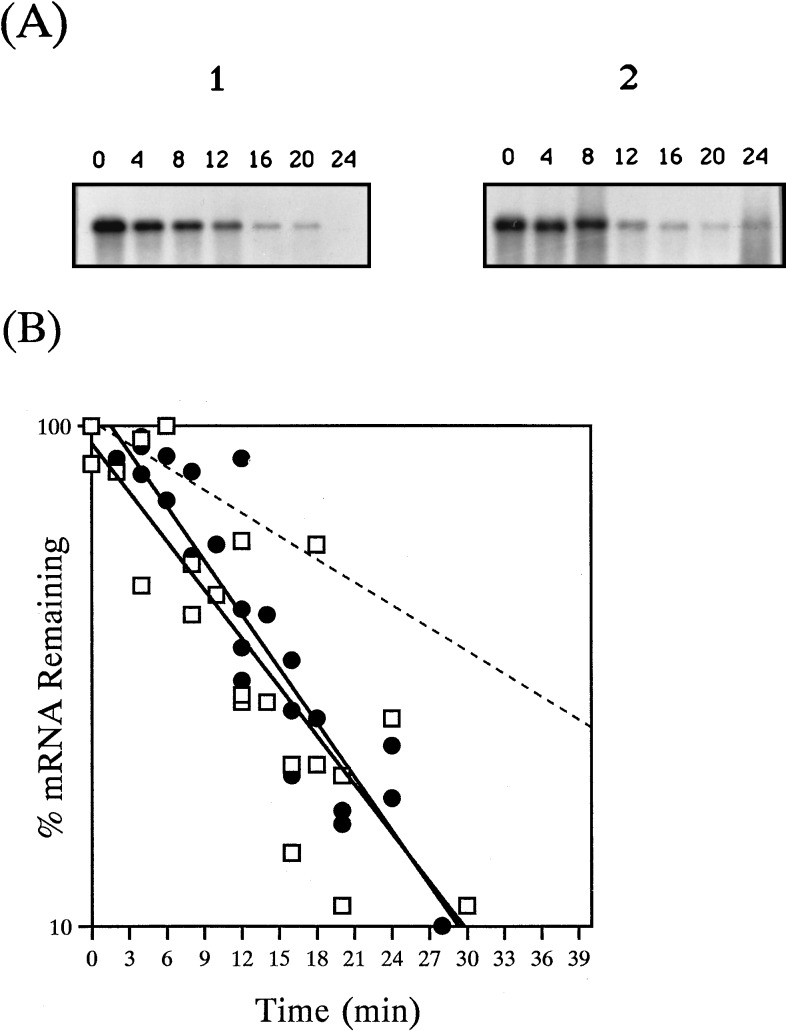
Rates of decay of pUX40 and chromosomal nifHDKTY mRNA were compared in the presence and absence of O2. The response to O2 was examined by transferring nif-derepressed cultures from anaerobic vials to baffled flasks of ≥10 sample volumes that were being shaken at 450 rpm. Figure 1 shows Northern blots of chromosomal and pUX40 nifHDKTY mRNA, isolated after the addition of rifampin, under anaerobic conditions (panels 1 and 2) or after O2 addition (panels 3 and 4). Like the chromosomal nifHDKTY mRNA, the pUX40 nifHDKTY mRNA is stable under anaerobic conditions and is destabilized upon O2 addition. Under anaerobic conditions, the t1/2 of the pUX40 nifHDKTY mRNA was 20 ± 1.3 min, compared to 20 ± 1.7 min for the chromosomal nifHDKTY mRNA (Fig. 2A). Upon addition of O2, the t1/2 of the pUX40 nifHDKTY mRNA was 6.8 ± 0.5, compared to 4.0 ± 0.2 min in the wild type (Fig. 2B). Thus, the pUX40 nifHDKTY mRNA is comparable to the chromosomal mRNA in its anaerobic stability and is regulated by O2 in a significant (P < 0.005) and dramatic manner, although the magnitude of the O2 regulation is not as striking as that for chromosomal mRNA. These data indicate that the pUX40 mRNA is a reasonable model for determining cis sites and trans-acting factors required for anaerobic stability and O2 regulation of stability. O2 addition experiments with the chromosomal nifHDKTY mRNA were also performed in the absence of rifampin, and it was found that O2 regulation of stability occurs similarly in the presence or absence of rifampin (data not shown).
FIG. 2.
Semilogarithmic plots of anaerobic and aerobic decay of pUX40 and chromosomal nifHDKTY mRNA. (A) RNA was isolated from anaerobic cells derepressed for nif expression. Time zero represents the first sample isolated after rifampin addition. □, chromosomal nifHDKTY mRNA (UN); ○, pUX40 nifHDKTY mRNA (UN5442). (B) RNA was isolated from nif-derepressed cells treated with rifampin and O2. Time zero represents the first sample isolated after O2 addition. □, chromosomal nifHDKTY mRNA (UN); ○, pUX40 nifHDKTY mRNA (UN5442). The data are from three independent experiments performed with chromosomal and pUX40 mRNAs under anaerobic conditions and pUX40 after O2 addition and two independent experiments performed with chromosomal nifHDKTY mRNA after O2 addition. (Chromosomal nifHDKTY mRNA was also examined after O2 addition without rifampin [see text].) The data from each set of experiments were collectively graphed, and least-squares analysis was used to determine the slope of the line and the t1/2 for the combined data (see Materials and Methods).
Deletion analysis implicates multiple regions as being important for anaerobic stability of nifHDKTY mRNA.
A series of deletion constructs were made starting with pUX40 (Table 1 and Fig. 3) to test the requirement of cis-acting sequences for the anaerobic stability of nifHDKTY mRNA. These constructs were examined under N-limiting, anaerobic conditions in a nif+ background. We quantified the decay rate of the chromosomal nifHDKTY mRNA as an internal standard in strains expressing the deletion mRNAs and observed that the chromosomal mRNA decayed similarly, regardless of the presence of the deletion plasmids (data not shown), indicating that the plasmids were not perturbing the analyses. Only data from those experiments in which the decay rate of the chromosomal nifHDKTY mRNA was the same as in the control experiments (Fig. 2) at a 5% significance level were considered (data not shown). Ninety-six percent of the experiments analyzed met this criterion.
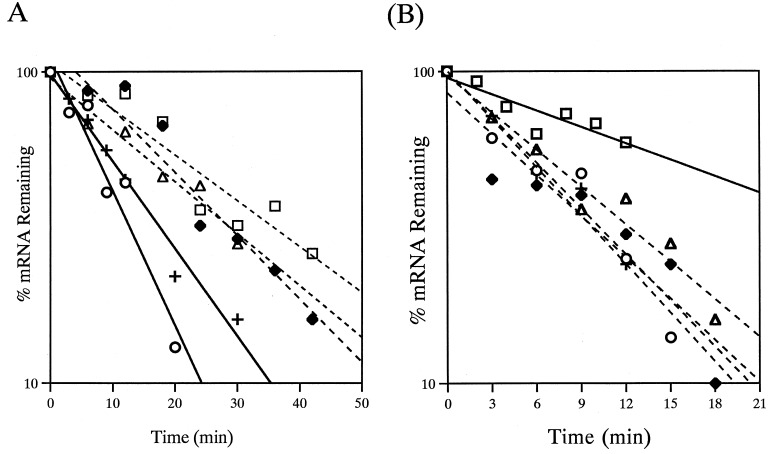
The results from experiments examining the anaerobic stability of the deletion mRNAs are summarized in Fig. 3 and displayed graphically in Fig. 4A. The largest reductions in anaerobic stability were seen with two of the deletion mRNAs, pUX201 and pUX215, although more minor reductions were also seen with pUX202 and pUX203. Examination of the extent of the deletions and their effects leads to the conclusion that no single region is responsible for the great anaerobic stability of the normal nifHDKTY mRNA, and the data cannot support identification of a specific critical region. Although the size of the mRNA might have an effect on anaerobic stability, this seems unlikely, because the deletion in pUX203 is 3.5 times larger than that in pUX202, yet the two mRNAs decay with similar t1/2s.
FIG. 4.
Anaerobic and aerobic stability of the pUX40-derived deletion mRNAs. Semilogarithmic plots are shown of the data from a representative time-course experiment (summarized in Fig. 3). The point at time zero is the first sample after rifampin addition and, in O2-treated cells, also the first sample after O2 addition. (A) Anaerobic, nif-derepressing conditions. (B) O2 treatment. Strains whose mRNA decay was significantly different (P < 0.001) from that of the control (pUX40 nifHDKTY mRNA) are shown by solid lines, while those with mRNA decay that was not significantly different from that of the control are shown by dashed lines. □, pUX214 (UN5469); ○, pUX215 (UN5470); ▵, pUX203 (UN5479); +, pUX201 (UN5460); ⧫, pUX202 (UN5461).
Sites involved in O2 regulation of nifHDKTY mRNA stability.
Results from experiments examining O2 regulation of stability of the deletion mRNAs in the nif+ background are summarized in Fig. 3 and displayed graphically in Fig. 4B. All of the deletion mRNAs except that expressed from pUX214 (ΔnifH) decay with a t1/2 similar to that of the pUX40 nifHDKTY mRNA upon O2 addition. The fact that the pUX214 mRNA is twice as stable as the pUX40 mRNA upon exposure to O2 indicates that some portion of the 499-bp region of nifH deleted in pUX214 is essential for normal O2 regulation of stability and suggests that a site required for the rate-limiting step in O2 regulation has been deleted. The pUX215 mRNA is no longer stable under anaerobic conditions, and it is our hypothesis that in addition to losing the site required for O2 regulation, the mRNA is also missing other region(s) required for its normal regulation.
The location of an O2-destabilizing determinant within the coding region of nifH (deleted in pUX214), is somewhat unexpected, given that a majority of decay determinants characterized thus far that are required for the regulation of a given mRNA have been located in the 5′ untranslated region (UTR) (5, 6, 38, 39, 45). However, regulation requiring portions of the coding region of the mRNA, and often involving cleavage within that region, has been noted in other systems (21, 30, 33, 34). The fact that most of the other deletion mRNAs appear to be normally regulated in response to O2 suggests that the majority of the nifHDKTY mRNA is not required for O2 regulation of stability. At present, the facts are consistent with a model for a rate-limiting cleavage event within nifH, although more complicated models cannot be ruled out.
nifHDKTY mRNA is unstable in large Δnif backgrounds under nif-derepressing conditions.
The pUX40 system also allowed us to ask if there is a requirement for nif-encoded trans-acting factors for the enhanced stability of the nifHDKTY mRNA under nif-derepressing conditions. The rates of decay of pUX40 nifHDKTY mRNA when expressed in several different K. pneumoniae strains that have deletions of different parts of the chromosomal nif regulon (Fig. 5) were compared. In the control strain, UN5442, the pUX40 nifHDKTY mRNA was characterized in the ΔnifDK background, which allowed it to be distinguished from the chromosomal nifHDKTY mRNA.
FIG. 5.
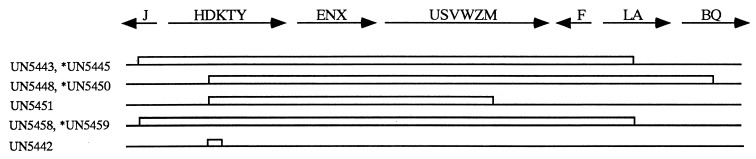
Schematic of chromosomal nif operon deletions. The nif genes and direction of transcription are shown above; the strains used in this report, with deletions depicted as boxes, are shown below. As described in the text (and in Table 1), a nifA expression system was introduced into some deletion backgrounds, and these strains are indicated with an asterisk.
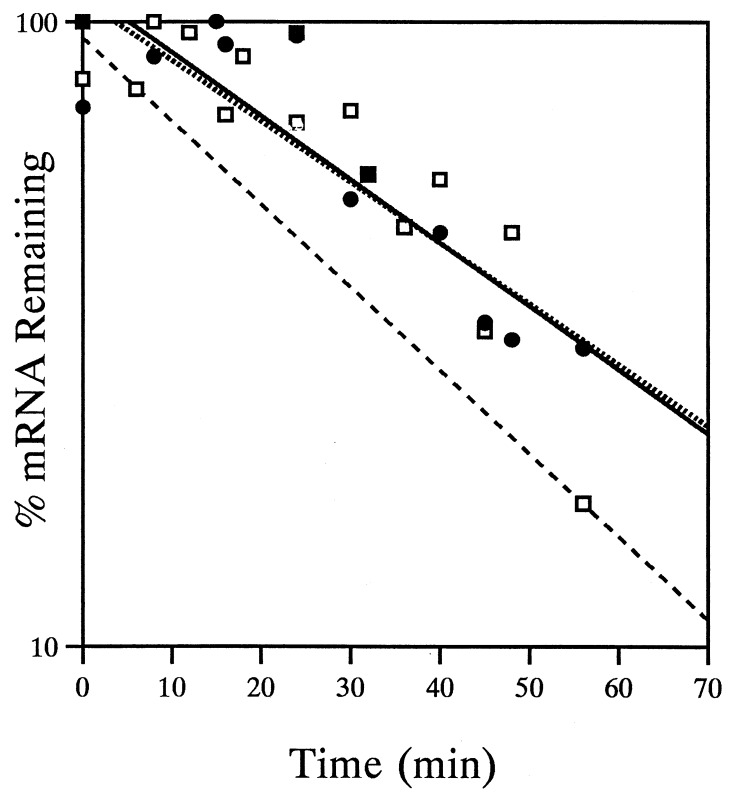
The only nif genes expressed in the strains with the largest nif deletions (Fig. 5, strains UN5443, UN5448, and UN5458) are the pUX40-encoded nifHDKTY genes (upon IPTG induction). Although these strains retain copies of one or more chromosomally encoded nif genes, the nif-specific transcriptional activator, NifA, is absent. In contrast, in the presence of IPTG, UN5442 (ΔnifDK/pUX40) expresses all of the nif genes under nif-derepressing conditions. In UN5442, pUX40 nifHDKTY mRNA decays with a t1/2 of 20 ± 1.3 min (Fig. 2). A representative Northern blot analysis of UN5443 (ΔJ-Anif/pUX40) is shown in Fig. 6A, panel 1. Analysis of covariance (19) showed that the rates of decay of pUX40 nifHDKTY mRNA were not significantly different in UN5443 (ΔnifJ-A/pUX40), UN5448 (ΔnifD-Q/pUX40), or UN5458 (ΔnifJ-A/pUX40). The data from experiments done with the three strains were thus combined for regression analysis (Fig. 6B), and the calculated t1/2 was 8.8 ± 1.0 min. Not only is pUX40 nifHDKTY mRNA significantly less stable under anaerobic conditions in the absence of the normal complement of nif proteins (P < 0.001), but its decay approximates that of the O2-destabilized pUX40 mRNA in UN5442 (t1/2 = 6.8 ± 0.50 min). These results demonstrate that a nif factor or factors are required for the exceptional stability of the nif mRNA under nif-derepressing conditions.
FIG. 6.
Effect of NifA on pUX40 nifHDKTY mRNA stability in various Δnif backgrounds under anaerobic, nif-derepressing conditions. Two sets of strains are shown: those with large chromosomal deletions that fail to express nifA (UN5443, UN5448, and UN5458) and another set in which nifA is either not deleted or is expressed from a plasmid (see text). (A) Representative Northern blots of pUX40 nifHDKTY mRNA decay in the ΔJ-Anif background in the absence (panel 1) or presence (panel 2) of NifA. The numbers above the lanes indicate the time in minutes after rifampin treatment. (B) Semilogarithmic plot of pUX40 nifHDKTY mRNA decay in various Δnif backgrounds in the absence (□) or presence (●) of NifA. The dashed line represents decay of pUX40 nifHDKTY mRNA in the presence of all of the nif proteins (strain UN5442) and has been reproduced from Fig. 2 for comparison; data points for this control are omitted for clarity.
NifA is not sufficient to stabilize the nifHDKTY mRNA under nif-derepressing conditions.
Having established the requirement for a nif gene product(s) in stabilizing the nifHDKTY mRNA under nif-derepressing conditions, we tested the model suggested earlier (15) that NifA is that factor. The medium-copy nifA expression plasmid pVL15 (23) was introduced into strains UN5443 (ΔnifJ-A/pUX40) and UN5448 (ΔnifD-Q/pUX40) to construct UN5445 and UN5450, respectively, and the low-copy nifA expression plasmid pUXA1 was introduced into UN5458 (ΔnifJ-A/pUX40) to construct UN5459 (Table 1 and Fig. 5). In the presence of IPTG, these strains express both nifHDKTY and nifA from compatible plasmids. UN5451 (ΔnifD-M/pUX40) was also included in our analysis, because nifA is expressed from the chromosome in this strain, and this would avoid any problems that might arise due to concomitant expression from the two-plasmid system. A representative Northern blot analysis of UN5445 (ΔnifJ-A/pUX40/pVL15) is shown in Fig. 6A, panel 2. The rates of decay of pUX40 nifHDKTY mRNA were not significantly different in UN5445 (ΔnifJ-A/UX40/pVL15), UN5450 (ΔnifD-Q/pUX40/pVL15), UN5451 (ΔnifD-M/pUX40), UN5459 (ΔnifJ-A/pUX40/pUXA1). A t1/2 of 8.3 ± 0.56 min, similar to what was observed in the absence of nifA expression, was obtained from analysis of covariance (19) of experiments with each of these strains and combined regression analysis (Fig. 6B). These results disprove the simple model that NifA is sufficient to stabilize nif mRNA and therefore that the nifLA gene products are solely responsible for posttranscriptional control of nif mRNA.
The results from experiments expressing nifA in addition to nifHDKTY from pUX40 in the deletion strains also allowed us to draw some conclusions concerning the ability of other nif factors to stabilize nifHDKTY mRNA. In addition to the genes expressed from the plasmid, any nif genes not deleted from the chromosome in these strains would still be expressed under NifA control. Thus, expression of nifA also results in the expression of nifBQ in UN5445 (ΔnifJ-A/pUX40/pVL15) and UN5459 (ΔnifJ-A/pUX40/pUXA1); nifJ and nifH in UN5450 (ΔnifD-Q/pUX40/pVL15); and nifJ, nifH, nifF, and nifBQ in UN5451 (ΔnifD-M/pUX40) (Fig. 5). We conclude that there was no increase in the stability of nifHDKTY mRNA expressed in the presence of NifA and any of the other nif proteins expressed in conjunction with NifA in these strains, compared to that of nifHDKTY mRNA expressed in their absence.
Nitrogenase activity regulates stability of nifHDKTY mRNA.
To discover which nif factors are required for stability of nif mRNA, we examined chromosomal nifHDKTY mRNA stability in seven strains with point mutations in individual nif genes. Strains with point mutations in the following genes (with the roles of their protein products noted) were examined: nifJ and nifF, electron transport to nitrogenase (17); nifE, biosynthesis of FeMo-co (49, 50); nifK, one of the dinitrogenase structural gene subunits; and nifH, dinitrogenase reductase, a subunit of nitrogenase that is required for electron transport to dinitrogenase (17), FeMo-co biosynthesis (47, 49), and insertion of FeMo-co into apodinitrogenase (1). Surprisingly, the stability of nifHDKTY mRNA was increased in these mutants (14 to 22%) relative to that in the wild type. Results from Northern analyses performed with UN1795 (nifH mutant) (13, 37) and UN1696 (nifK mutant) (37) are shown in Fig. 7. While nifHDKTY mRNA expressed in UN (wild type) decays with a t1/2 of 20 ± 1.3 min, the t1/2s of nifHDKTY mRNA were 31 ± 5.0 min (P < 0.025) when expressed in UN1795 (nifH) and 29 ± 4.5 min (P < 0.05) when expressed in UN1696 (nifK mutant). A strain harboring a Mu insertion in nifU was also examined and found to behave in a manner similar to that of the point mutant strains.
FIG. 7.
Stability of the chromosomal nifHDKTY mRNA in a nifK and a nifH point mutant strain under anaerobic, nif-derepressing conditions. Semilogarithmic plot of nifHDKTY mRNA decay in UN1696 (nifK) (□) and UN1795 (nifH) (●). The results from two experiments for each mutant, which were determined to be the same at a 5% significance level, are plotted. Decay of nifHDKTY mRNA in UN (wild type) under the same conditions is reproduced from Fig. 2 for comparison (dashed line), but data points for this control are omitted for clarity.
The finding that point mutations in a number of nif genes with disparate functions have a similar effect on nifHDKTY mRNA stability (stability is increased over that of the wild type) suggests that these strains are perturbed in some common function. This stabilization is statistically significant and is particularly surprising given that this mRNA is already so stable in the wild-type background. Any model of the regulated stability must therefore take this effect into account. Each of these strains is affected in at least one of the several requirements for an active nitrogenase: electron transport, FeMo-co biosynthesis, FeMo-co insertion, or nitrogenase enzymatic function. Therefore, a common property shared among these different strains is the absence of nitrogenase activity in the presence of the otherwise complete nif system. This suggests that nitrogenase activity is a component of the system that regulates nif mRNA stability. Our data also indicate that a nif factor(s) is required to stabilize nif mRNA, and thus it follows that nitrogenase activity may be sensed as a signal for stabilization. The physiological relevance of such a mechanism would be to increase gene expression through more stable mRNA when nitrogenase activity is low and to decrease gene expression when high levels of activity are achieved. Consistent with this is the observation that the accumulation of nitrogenase activity plateaus several hours after the initial derepression of nif genes (data not shown).
It is paradoxical that large deletions of nif genes have the opposite effect on nif mRNA stability from point mutations. It cannot simply be a question of “tightness” of the mutation, for example, because UN1696 (nifK) has essentially no detectable nitrogenase activity. The paradox can be explained by postulating that even though the large deletions, like the point mutations, lack nitrogenase activity, they are missing the factor or factors essential for stabilizing the mRNA. Our failure to detect a single factor required for stabilization of the mRNA suggests that this effect is achieved by the complex interaction of a number of gene products.
Our working hypothesis posits that if there is a decrease in nitrogenase activity in the presence of a mostly complete contingent of nif proteins, nif mRNA turnover is reduced and gene expression increases. However, if both nitrogenase activity and the factor(s) required for stability of nif mRNA are absent, nif mRNA is destabilized. Since the hypothesis posits that nitrogenase activity indirectly regulates nif mRNA stability, then some mechanism must exist for sensing the status of nitrogenase. We considered that NifY, which is expressed from the nifHDKTY operon, might exhibit regulatory effects on nifHDKTY mRNA accumulation. A previous report from this laboratory established that NifY functions in nitrogenase maturation (28).
Construction of a nifY strain and its effect on nitrogenase activity.
Upon nif derepression, UN5360, a K. pneumoniae strain with the Kanr (aphA) cassette from pUC-4K (42, 46) incorporated into the SalI site of nifY, possessed 50 to 70% of wild-type nitrogenase activity (as measured by acetylene reduction) and was phenotypically Nif+ (data not shown). The Nif+ phenotype of the nifY strain was surprising, given its apparent role in nitrogenase maturation (27), and it is our working model that another protein is able to substitute for NifY in its absence and fulfill its function, albeit less well.
Effect of the overexpression of nifY on nitrogenase activity and mRNA accumulation.
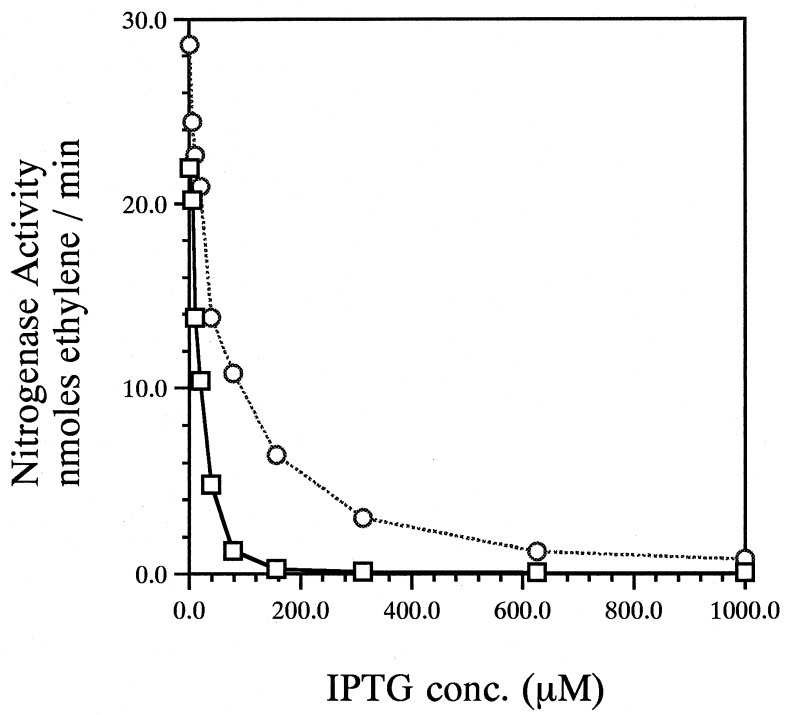
UN5397, containing the NifY expression vector pNF107, was derepressed for nif expression in the presence of IPTG (to overexpress NifY) and monitored for acetylene reduction activity. An isogenic strain containing the parent plasmid without nifY, pKK223-3 (10), was examined as a control. The addition of 1.0 mM IPTG to UN5397 (nif+/pNF107) completely blocked the appearance of acetylene reduction activity (Fig. 8), and Northern blot analysis revealed an absence of chromosomal nifHDKTY mRNA accumulation under those conditions (data not shown). In contrast, nifLA mRNA accumulation was not reduced in UN5397 compared to that of the wild type, indicating that the failure of UN5397 to accumulate nifHDKTY mRNA was not due to a reduction or absence of expression of the nifLA regulatory genes, which are under ntr control (data not shown). Examination of NifY overexpression in UN5406, a nifL mutant, demonstrated that levels of NifY expression capable of completely repressing nitrogenase activity in the wild-type strain did not do so in the nifL background (Fig. 8). These results suggest that NifY overexpression achieves its effect through interaction with the NifLA regulatory proteins.
FIG. 8.
Effect of overproduction of NifY on nitrogenase activity in the wild type and a nifL mutant. Strains were derepressed for nif function in the presence of IPTG at the concentrations shown for 4 h. Nitrogenase activity was measured as described in Materials and Methods for strains overexpressing nifY in the UN5397 (nif+; □, solid line) and UN5406 (nifL; ○, dotted line) backgrounds.
Deletion of nifY from pUX40 increases the stability of the nifHDKTY mRNA in the Δnif background under nif-derepressing conditions.
Given the regulatory effects observed with nifY overexpression, the instability of pUX40 nifHDKTY mRNA expressed in the large nif deletion strains (Fig. 6) might be the result of expressing NifY (from pUX40) in the absence of a functional nitrogenase and/or stabilizing factor. That possibility is suggested by the following. The pUX202 (ΔnifTY) mRNA is 2 to 3 times more stable than the pUX40 nifHDKTY mRNA in the Δnif background (data not shown), even though it did not have enhanced stability in the nif+ background (Fig. 3). In contrast, decay of the pUX214 (ΔnifH) mRNA in the Δnif background was similar to that of pUX40 (data not shown). This is most easily rationalized by the synthesis of NifT and/or NifY causing an instability of the mRNA under these, admittedly perturbed, conditions. The effect seen is likely due to the absence of NifY, rather than NifT, as we previously reported that the absence and overexpression of NifT alone had no discernible effect on the regulation, accumulation, and maturation of nitrogenase in K. pneumoniae (51).
Working hypothesis.
Our results demonstrate that the O2-regulated stability of nifHDKTY mRNA is controlled by a relatively small region and, furthermore, that there is no simple cis determinant for the unusual anaerobic stability of the mRNA in vivo. We have also demonstrated that there is a nif factor or factors required for the stability of the nifHDKTY mRNA under nif-derepressing conditions. We have disproved the model that NifA is sufficient for stabilizing nif mRNA and that the nifLA gene products are sufficient to achieve both transcriptional and posttranscriptional control. Our results strongly suggest that regulation of nif mRNA stability is not achieved through any simple mechanism.
The fact that the elimination of NifY, a protein involved in maturation of nitrogenase, from the pUX40 nifHDKTY mRNA increased the stability of that mRNA in the large nif deletion background, in addition to other regulatory effects discovered with NifY overexpression, is consistent with this hypothesis. NifY has been shown to associate with apodinitrogenase and dissociate upon insertion of the active site, FeMo-co (28), the final step in nitrogenase maturation. As part of the maturation process, NifY may have a role in sensing and signaling the status of nitrogenase. The purpose of sensing the status of nitrogenase in the wild type could be to serve as a feedback mechanism to regulate nitrogenase production to match the availability of various components required for nitrogen fixation, such as metals and reducing power.
A number of other observations are consistent with a role for the nitrogenase proteins themselves in the regulation of nif expression. Roberts et al. (37) reported that mutations in nifH, nifD, and nifK (encoding the nitrogenase component proteins) typically resulted in a reduction in the levels of many other Nif proteins. Chang et al. also noted differences in the accumulation of steady-state levels of nifHDKTY mRNA during nif derepression in a number of different nifH mutants compared to the wild-type strain (13). Also consistent is the evidence that nifH is required for the expression of the alternate nitrogenase transcriptional activator AnfA in Azotobacter vinelandii (8).
The fact that a non-nif protein, glnK, under NtrC control has been shown to relieve NifL inhibition of NifA activity under N-limiting conditions (24) is not inconsistent with our hypothesis of NifY sensing of nitrogenase status. NifY could interfere with the relief of inhibition by glnK under certain conditions, or there may be independent pathways for mRNA destabilization. In fact, the data for destabilization of nif mRNA in a nifL strain (15) demonstrate that destabilization still occurs in the presence of NH4+, albeit not to the same degree as in the wild-type strain. This suggests other factors (in addition to NifL) are involved in nif mRNA destabilization under those conditions.
ACKNOWLEDGMENTS
This research was supported by The College of Agricultural and Life Sciences of the University of Wisconsin—Madison and by National Science Foundation grant MCB-9604446.
We gratefully acknowledge Mike Merrick for strains, William Orme-Johnson for the plasmid pVL15, and Henry Bujard for the PA1/04 sequence. We also thank Nancy Franklin, Jon Roll, Jennifer Romanin, and Travis Jerde for strain and plasmid constructions. Statistical support was provided by the Biometry Program in the College of Agriculture and Life Sciences, and we are grateful to Brian Yandell, José Pinheiro, Tom Tabone, and Peter Crump for appreciable help.
REFERENCES
- 1.Allen R M, Homer M H, Chatterjee R, Ludden P W, Roberts G P, Shah V K. Dinitrogenase reductase- and MgATP-dependent maturation of apodinitrogenase from Azotobacter vinelandii. J Biol Chem. 1993;268:23670–23674. [PubMed] [Google Scholar]
- 2.Arnold W, Rump A, Klipp W, Priefer U B, Pühler A. Nucleotide sequence of a 24,206-base-pair DNA fragment carrying the entire nitrogen fixation gene cluster of Klebsiella pneumoniae. J Mol Biol. 1988;203:715–738. doi: 10.1016/0022-2836(88)90205-7. [DOI] [PubMed] [Google Scholar]
- 3.Austin S, Buck M, Cannon W, Eydmann T, Dixon R. Purification and in vitro activities of the native nitrogen fixation control proteins NifA and NifL. J Bacteriol. 1994;176:3460–3465. doi: 10.1128/jb.176.12.3460-3465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin S, Henderson N, Dixon R. Characterisation of the Klebsiella pneumoniae nitrogen-fixation regulatory proteins NIFA and NIFL in vitro. Eur J Biochem. 1990;187:353–360. doi: 10.1111/j.1432-1033.1990.tb15312.x. [DOI] [PubMed] [Google Scholar]
- 5.Bardwell J C A, Régnier P, Chen S M, Nakamura Y, Grunberg-Manago M, Court D. Autoregulation of RNase III operon by mRNA processing. EMBO J. 1989;8:3401–3407. doi: 10.1002/j.1460-2075.1989.tb08504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bechhofer D H, Dubnau D. Induced mRNA stability in Bacillus subtilis. Proc Natl Acad Sci USA. 1987;84:498–502. doi: 10.1073/pnas.84.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Belsley D A, Kuh E, Welsch R E. Regression diagnostics: identifying influential data and sources of collinearity. New York, N.Y: John Wiley and Sons, Inc.; 1980. [Google Scholar]
- 7.Beynon J, Cannon M, Buchanan-Wollaston V, Cannon F. The nif promoters of Klebsiella pneumoniaehave a characteristic primary structure. Cell. 1983;34:665–671. doi: 10.1016/0092-8674(83)90399-9. [DOI] [PubMed] [Google Scholar]
- 8.Bishop P E, Premakumar R. Alternative nitrogen fixation genes in free-living and symbiotic bacteria. In: Stacey G, Burris R H, Evans H J, editors. Biological nitrogen fixation. New York, N.Y: Chapman Hall; 1992. pp. 736–762. [Google Scholar]
- 9.Brooks S J, Collins J J, Brill W J. Repression of nitrogen fixation in Klebsiella pneumoniaeat high temperature. J Bacteriol. 1984;157:460–464. doi: 10.1128/jb.157.2.460-464.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brosius J, Holy A. Regulation of ribosomal RNA promoters with a synthetic lacoperator. Proc Natl Acad Sci USA. 1984;81:6929–6933. doi: 10.1073/pnas.81.22.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchanan-Wollaston V, Cannon M C, Cannon F C. The use of cloned nif (nitrogen fixation) DNA to investigate transcriptional regulation of nif expression in Klebsiella pneumoniae. Mol Gen Genet. 1981;184:102–106. doi: 10.1007/BF00271203. [DOI] [PubMed] [Google Scholar]
- 12.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang C L, Davis L C, Rider M, Takemoto D J. Characterization of nifH mutations of Klebsiella pneumoniae. J Bacteriol. 1988;170:4015–4022. doi: 10.1128/jb.170.9.4015-4022.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins J J, Brill W J. Control of Klebsiella pneumoniae nifmRNA synthesis. J Bacteriol. 1985;162:1186–1190. doi: 10.1128/jb.162.3.1186-1190.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins J J, Roberts G P, Brill W J. Posttranscriptional control of Klebsiella pneumoniae nif mRNA stability by the nifLproduct. J Bacteriol. 1986;168:173–178. doi: 10.1128/jb.168.1.173-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook R D, Weisberg W. Residuals and influence in regression. New York, N.Y: Chapman and Hall; 1982. pp. 101–156. [Google Scholar]
- 17.Dean D R, Jacobson M R. Biochemical genetics of nitrogenase. In: Stacey G, Burris R H, Evans H J, editors. Biological nitrogen fixation. New York, N.Y: Chapman Hall; 1992. pp. 763–834. [Google Scholar]
- 18.Dixon R. The oxygen-responsive NIFL-NIFA complex: a novel two-component regulatory system controlling nitrogenase synthesis in γ-Proteobacteria. Arch Microbiol. 1998;169:371–380. doi: 10.1007/s002030050585. [DOI] [PubMed] [Google Scholar]
- 19.Draper N R, Smith H. Applied regression analysis. 2nd ed. New York, N.Y: J. Wiley and Sons; 1981. pp. 1–69. [Google Scholar]
- 20.Feinberg A, Vogelstein B. A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 21.Fritsch J, Rudiger R, Rauhut R, Klug G. Identification of an mRNA element promoting rate-limiting cleavage of the polycistronic puf mRNA in Rhodobacter capsulatusby an enzyme similar to RNase E. Mol Microbiol. 1995;15:1017–1029. doi: 10.1111/j.1365-2958.1995.tb02277.x. [DOI] [PubMed] [Google Scholar]
- 22.Gosink M M, Franklin N M, Roberts G P. The product of the Klebsiella pneumoniae nifX gene is a negative regulator of the nitrogen fixation (nif) regulon. J Bacteriol. 1990;172:1441–1447. doi: 10.1128/jb.172.3.1441-1447.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris G S, White T C, Flory J E, Orme-Johnson W H. Genes required for formation of the apoMoFe protein of Klebsiella pneumoniae nitrogenase in Escherichia coli. J Biol Chem. 1990;265:15909–15919. [PubMed] [Google Scholar]
- 24.He L, Soupene E, Ninfa A, Kustu S. Physiological role for the GlnK protein of enteric bacteria: relief of NifL inhibition under nitrogen-limiting conditions. J Bacteriol. 1998;180:6661–6667. doi: 10.1128/jb.180.24.6661-6667.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson N, Austin S A, Dixon R A. Role of metal ions in negative regulation of nitrogen fixation by the nifL gene product from Klebsiella pneumoniae. Mol Gen Genet. 1989;216:484–491. [Google Scholar]
- 26.Hill S, Austin S, Eydmann T, Jones T, Dixon R. Azotobacter vinelandiiNIFL is a flavoprotein that modulates transcriptional activation of nitrogen-fixation genes via a redox-sensitive switch. Proc Natl Acad Sci USA. 1996;93:2143–2148. doi: 10.1073/pnas.93.5.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Homer M J, Dean D R, Roberts G P. Characterization of the gamma protein and its involvement in the metallocluster assembly and maturation of dinitrogenase from Azotobacter vinelandii. J Biol Chem. 1995;270:24745–24752. doi: 10.1074/jbc.270.42.24745. [DOI] [PubMed] [Google Scholar]
- 28.Homer M J, Paustian T D, Shah V K, Roberts G P. The nifY product of Klebsiella pneumoniaeis associated with apodinitrogenase and dissociates upon activation with the iron-molybdenum cofactor. J Bacteriol. 1993;175:4907–4910. doi: 10.1128/jb.175.15.4907-4910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houmard J, Bogusz D. Kinetic studies of the expression of Klebsiella pneumoniae nitrogen fixation (nif) genes under conditions of inhibited transcription. Biochem Biophys Res Commun. 1981;100:1237–1244. doi: 10.1016/0006-291x(81)91956-2. [DOI] [PubMed] [Google Scholar]
- 30.Jain C, Belasco J G. RNase E autoregulates its synthesis by controlling the degradation rate of its own mRNA in Escherichia coli: unusual sensitivity of the rnetranscript to RNase E activity. Genes Dev. 1995;9:84–96. doi: 10.1101/gad.9.1.84. [DOI] [PubMed] [Google Scholar]
- 31.Kahn D, Hawkins D M, Eady R R. Metabolic control of Klebsiella pneumoniaemRNA degradation by the availability of fixed nitrogen. J Gen Microbiol. 1982;128:3011–3018. doi: 10.1099/00221287-128-12-3011. [DOI] [PubMed] [Google Scholar]
- 32.Kaluza K, Hennecke H. Regulation of nitrogenase messenger RNA synthesis and stability in Klebsiella pneumoniae. Arch Microbiol. 1981;130:38–43. doi: 10.1007/BF00527069. [DOI] [PubMed] [Google Scholar]
- 33.Klug G. Endonucleolytic degradation of puf mRNA in Rhodobacter capsulatusis influenced by oxygen. Proc Natl Acad Sci USA. 1991;88:1765–1769. doi: 10.1073/pnas.88.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulkarni R D, Golden S S. mRNA stability is regulated by a coding-region element and the unique 5′ untranslated leader sequences of the three Synechococcus psbAtranscripts. Mol Microbiol. 1997;24:1131–1142. doi: 10.1046/j.1365-2958.1997.4201768.x. [DOI] [PubMed] [Google Scholar]
- 35.Lanzer M, Bujard H. Promoters largely determine the efficiency of repressor action. Proc Natl Acad Sci USA. 1988;85:8973–8977. doi: 10.1073/pnas.85.23.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee H-S, Narberhaus F, Kustu S. In vitro activity of NifL, a signal transduction protein for biological nitrogen fixation. J Bacteriol. 1993;175:7683–7688. doi: 10.1128/jb.175.23.7683-7688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacNeil T, MacNeil D, Roberts G P, Supiano M A, Brill W J. Fine-structure mapping and complementation analysis of nif (nitrogen fixation) genes in Klebsiella pneumoniae. J Bacteriol. 1978;136:253–266. doi: 10.1128/jb.136.1.253-266.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melefors Ö, von Gabain A. Site-specific endonucleolytic cleavages and the regulation of stability of E. coli ompAmRNA. Cell. 1988;52:893–901. doi: 10.1016/0092-8674(88)90431-x. [DOI] [PubMed] [Google Scholar]
- 39.Melin L, Friden H, Dehlin E, Rutberg L, von Gabain A. The importance of the 5′-region in regulating the stability of sdh mRNA in Bacillus subtilis. Mol Microbiol. 1990;4:1881–1889. doi: 10.1111/j.1365-2958.1990.tb02037.x. [DOI] [PubMed] [Google Scholar]
- 40.Merrick M, Filser M, Dixon R, Elmerich C, Sibold L, Houmard J. The use of translocatable genetic elements to construct a fine-structure map of the Klebsiella pneumoniae nitrogen fixation (nif) gene cluster. J Gen Microbiol. 1980;117:509–520. doi: 10.1099/00221287-117-2-509. [DOI] [PubMed] [Google Scholar]
- 41.Merrick J J. Regulation of nitrogen fixation genes in bacteria. In: Stacey G, Burris R H, Evans H J, editors. Biological nitrogen fixation. New York, N.Y: Chapman and Hall; 1992. pp. 835–876. [Google Scholar]
- 42.Messing J, Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest and restriction fragments. Gene. 1982;19:269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- 43.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of enteric membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morett E, Buck M. NifA-dependent in vivo protection demonstrates that the upstream activator sequence of nifpromoters is a protein binding site. Proc Natl Acad Sci USA. 1988;85:9401–9405. doi: 10.1073/pnas.85.24.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mudd E A, Prentki D B, Krisch H M. Processing of unstable bacteriophage T4 32 mRNAs into a stable species requires Escherichia coliribonuclease E. EMBO J. 1988;7:3601–3607. doi: 10.1002/j.1460-2075.1988.tb03238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oka A, Sugisaki H, Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981;147:217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- 47.Robinson A C, Burgess B K. Iron-molybdenum cofactor biosynthesis in Azotobacter vinelandii requires the uribprotein of nitrogenase. J Biol Chem. 1987;262:14327–14332. [PubMed] [Google Scholar]
- 48.Santero E, Hoover J, Kustu S. In vitroactivity of the nitrogen fixation regulatory protein NifL. Proc Natl Acad Sci USA. 1989;86:7346–7350. doi: 10.1073/pnas.86.19.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah V K, Hoover T R, Imperial J, Paustian T D, Roberts G P, Ludden P W. Role of nif gene products and homocitrate in the biosynthesis of the iron-molybdenum cofactor. In: Bothe E, Je de Bruijn F, Newton W E, editors. Nitrogen fixation: hundred years after. Stuttgart, Germany: Gustav Fischer; 1988. pp. 115–120. [Google Scholar]
- 50.Shah V K, Imperial J, Ugalde R A, Ludden P W, Brill W J. In vitrosynthesis of the iron-molybdenum cofactor of nitrogenase. Proc Natl Acad Sci USA. 1986;83:1636–1640. doi: 10.1073/pnas.83.6.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simon H M, Homer M J, Roberts G P. Perturbation of nifT expression in Klebsiella pneumoniaehas limited effect on nitrogen fixation. J Bacteriol. 1996;178:2975–2977. doi: 10.1128/jb.178.10.2975-2977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequence of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]