Abstract
Aim
“Consensual ophthalmotonic reaction” refers to changes in intraocular pressure (IOP) in one eye, which is accompanied by a corresponding change in IOP in the contralateral eye. This study evaluates whether monocular administration of selective laser trabeculoplasty (SLT) leads to a consensual ophthalmotonic reaction and how long this effect lasts.
Materials and methods
A retrospective chart review was performed on patients receiving SLT at Kresge Eye Institute in Detroit, MI, from January 2015 to August 2016. Patients were excluded if they had previous history of glaucoma incisional and/or laser procedures; required additional laser trabeculoplasty; had glaucoma medication changes during the follow-up period; experienced no decrease in IOP during the follow-up period; or had a diagnosis of angle closure on gonioscopy. Various demographic, clinical, and surgical data were collected. IOP measurements were collected at baseline and postoperatively at 1–3 months, 4–9 months, and 12–15 months.
Results
At all follow-up periods, the IOP of the treated eye was decreased from baseline IOP (p ≤ 0.05, paired t-test). For the fellow eye, there was a statistically significantly decrease from baseline up to the 4–9 months follow-up period (p ≤ 0.05, paired t-test). Linear regression analysis of the percent reduction in IOP from baseline in the SLT-treated eye with the fellow eye shows a mild correlation at all-time points: R2 = 0.284 (p < 0.001) at 1–3 months; R2 = 0.348 (p < 0.001) at 4–9 months; R2 = 0.118 (p = 0.054) at 12–15 months.
Conclusion
This study showed that monocular administration of SLT results in a consensual ophthalmotonic reaction. The consensual ophthalmotonic reaction appears to last for up to 4–9 months.
Clinical significance
Therefore, although SLT does lead to a consensual ophthalmotonic reaction, monocular administration of SLT is not a reliable method of long-term IOP control for the contralateral non-SLT-treated eye.
How to cite this article
Nassiri N, Mei F, Tokko H, et al. Consensual Ophthalmotonic Reaction Following Selective Laser Trabeculoplasty. J Curr Glaucoma Pract 2022;16(1):36–40.
Keywords: Consensual ophthalmotonic reaction, Glaucoma treatment, Intraocular pressure, Retrospective study, Selective laser trabeculoplasty
Introduction
The term “consensual ophthalmotonic reaction” was first coined by Weekers in 1924 to describe how the alteration of intraocular pressure (IOP) in one eye is accompanied by a corresponding change in IOP in the contralateral eye.1 The consensual ophthalmotonic reaction has been shown to occur with monocular application of timolol and pilocarpine.2 One theory is that the medications are capable of systemic distribution as noted in previous studies.3,4 However, Newman et al. found that the application of timolol gel, which is known to have a lower systemic distribution, also resulted in a consensual ophthalmic reaction, suggesting the possibility of other methods of IOP control.5,6
Selective laser trabeculoplasty (SLT) uses short bursts of energy directed at the trabecular meshwork in order to lower the IOP. Previous studies on argon laser trabeculoplasty (ALT) have suggested that laser trabeculoplasties have a mechanical mechanism of action via a thermal-burn of the trabecular meshwork.7 However, studies of SLT in particular have shown that SLT is only cytotoxic to melanin-challenged cells, and not to nonpigmented cells.8,9 SLT is therefore proposed to have a biologic mechanism of action as opposed to a mechanical one. One theory proposes that the thermal energy of the laser stimulated recruitment of macrophages in the trabecular meshwork and remodeling of the extracellular matrix, allowing increased aqueous outflow from the eye.8 Given a possible biological mechanism of action, a consensual ophthalmotonic reaction is possible in patients treated with unilateral SLT. Our study seeks to determine whether a consensual ophthalmotonic reaction occurs in this scenario and how long this effect lasts.
Methods
A retrospective chart review was conducted on all patients that received unilateral SLT treatment at the Kresge Eye Institute, Detroit, MI from January 2015 to August 2016. The Institutional Review Board at Wayne State University approved the study protocol.
Patients in this study were diagnosed with glaucoma or ocular hypertension and SLT was determined to be a method to control IOP. The decision to treat with SLT was at clinician's discretion. We included adult patients (>18 years old) who received monocular SLT treatment. Patients were excluded if they: had a previous history of glaucoma incisional and/or laser procedures, including peripheral iridotomies, in either eye; required additional laser trabeculoplasty after the initial treatment; had medication changes for either eye during the follow-up period; experienced no decrease in IOP of the treated eye during the follow-up period; or had a Grade 0 or Grade I angle configuration by Shaffer criteria on gonioscopy.
Various demographic, clinical and surgical data were collected from patients’ electronic medical records. IOPs were measured by Goldmann applanation tonometry. IOP measurements were collected for both the SLT–treat eye and the fellow eye at baseline and postoperatively during the three follow-up periods: 1–3 months, 4–9 months, and 12–15 months. The most recent measurement of IOP before treatment was considered baseline.
All surgeries were performed using standard SLT protocol by glaucoma faculty members or glaucoma fellows at the Kresge Eye Institute. Eyes were pretreated with topical anesthesia and an alpha-adrenergic agonist. The Coherent Selecta 7000 laser, a frequency-doubled q-switched neodymium:ytrium-aluminum-garnet laser, was used to treat these patients. The laser was emitting at 532 nm with a pulse duration of 3 nanoseconds and a spot size of 400 um. The pigmented trabecular meshwork was targeted and patients received 180° or 360° treatment. The initial energy level of the SLT was set at 0.8 mJ. The energy was decreased or increased until minimal cavitation bubbles within the trabecular meshwork was noted. The total number of pulses delivered at each energy level and the total amount of energy delivered, as indicated on the laser control panel, were recorded. The IOP was checked 1 hour after treatment for an IOP spike. An IOP spike was defined as ≥5 mm Hg rise in IOP. If an IOP spike was detected, the eye was treated with appropriate antiglaucoma medications. After treatment, some patients were prescribed a topical steroid four times a day for 1 week.
Statistical analyses were performed using SAS Studio 3.5 (SAS Institute, Inc, Cary, North Carolina, USA). A p value of <0.05 was considered statistically significant. The Kolmogorov-Smirnov test was used to check the normal distribution of the study variables. Different parametric and nonparametric statistics were used to compare study variables between the study groups. Linear regression analysis was performed to determine the association between study variables.
Results
Eighty-five patients were enrolled in our study. Demographic and clinical characteristics of patients in our study are summarized in Table 1. The average age was 64.87 years (±12.19). Most patients were African American (85.9%) with two patients with an undocumented ethnic origin. The majority of patients were diagnosed with primary open angle glaucoma (POAG) (89.4%).
Table 1.
Demographic and clinical characteristics of the study participants
| Parameters | Values | p-value |
|---|---|---|
| Total number of patients (eyes) | 85100 | -- |
| Race, n (%) | -- | |
| African American | 73 (85.9) | |
| Caucasian | 7 (8.2) | |
| Asian | 1 (1.2) | |
| Hispanic | 1 (1.2) | |
| Other | 1 (1.2) | |
| Undefined | 2 (2.3) | |
| Sex, n (%) | -- | |
| Male: | 40 (47.1) | |
| Female: | 45 (52.9) | |
| Diagnosis, n (%) | -- | |
| POAG | 76 (89.4) | |
| Low-tension glaucoma | 6 (7.1) | |
| Pigmentary glaucoma | 2 (2.3) | |
| Congenital glaucoma | 1 (1.2) | |
| Vertical C: D ratio, average ± SD | <0.01 | |
| SLT eye | 0.80 ± 0.15 | |
| Fellow Eye | 0.71 ± 0.17 | |
| Corneal thickness, average ± SD | 0.89 | |
| SLT eye | 543.81 ± 48.26 | |
| Fellow eye | 533.70 ± 46.17 | |
| Baseline IOP, average ± SD | <0.01 | |
| SLT eye | 20.17 ± 4.57 | |
| Fellow eye | 17.71 ± 5.17 | |
| Baseline number of IOP lowering medications, average ± SD | <0.01 | |
| SLT eye | 1.83 ± 1.27 | |
| Fellow eye | 1.65 ± 1.27 |
p < 0.05 was considered as statistically significant; C:D, cup to disk; IOP, intraocular pressure
Seventy three (85.89%) patients received 360° SLT with an average of 91.73 ± 10.53 applications and an average energy of 0.72 ± 0.12 mJ. Four (4.71%) patients received 180 SLT with an average of 46.25 ± 4.5 applications and an average energy of 0.88 ± 0.25 mJ. Eight (9.41%) patients did not have documentation of the power or number of applications used. Three (3.53%) patients experienced an IOP spike after SLT—that returned to baseline after appropriate intervention.
At baseline, the eyes receiving SLT had a statistically significant greater cup to disk ratio than the fellow eye, 0.80 ± 0.15 vs 0.71 ± 0.17, respectively (p = 0.001, Student t-test). The baseline IOP in the eye being treated with SLT was also statistically significant greater than the fellow eye, 20.15 ± 4.62 mm Hg vs 17.71 ± 5.17 mm Hg, respectively (p = 0.0001, Student t-test).
Average follow-up was 9.34 ± 6.10 months. After treatment, at all-time points, the IOP of the treated eye had a statistically significant decrease from baseline IOP (p < 0.01 for 1–3 months, p < 0.01 for 4–9 months, p < 0.01 for 12–15 months, paired t–test) (Fig. 1). For the fellow eye, there was a statistically significantly decrease from baseline up to the 4–9 months’ follow-up period (p < 0.01 for 1–3 months, p < 0.01 for 4–9 months, paired t-test). At the 12–15 months’ follow-up period, the decrease in IOP from baseline was not statistically significant (p = 0.05, paired t-test) (Fig. 1).
Fig. 1.

Average of intraocular pressures (IOPs) before and after SLT in the treated eye and the fellow eye in different follow-up periods; * = p < 0.05 when compared to pretreatment (Student paired t-test)
Figure 2 shows that the average percentage of IOP change from baseline decreased over time in both groups and the reduction was more pronounced in the fellow eye, though not statistically significant in SLT group (p = 0.53) and the untreated group (p = 0.31) (ANOVA). The average percent IOP changes from baseline in the SLT-treated eyes vs untreated fellow eyes were 24.08% vs 12.64% at 1–3 months (p < 0.01), 18.24% vs 8.71% at 4–9 months (p = 0.02) and 17.82% vs 3.95% at 12–15 months (p = 0.01).
Fig. 2.
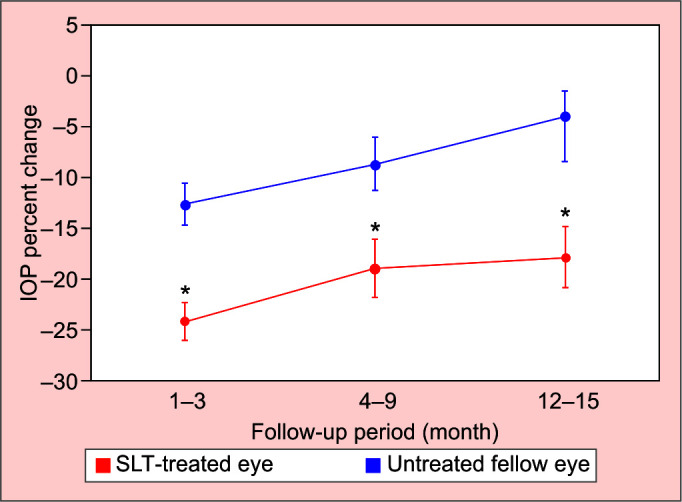
Average percentage of IOP change from baseline after SLT administration in the treated eye and the fellow eye in different follow-up periods. * shows statistically significant difference between SLT-treated eyes and untreated fellow eyes with regard to average percentage of IOP change from baseline (p < 0.05; Student t-test)
Linear regression analysis (Fig. 3) of the percent reduction in IOP from baseline in the SLT-treated eye with the fellow eye shows a mild correlation at all-time points: R2 = 0.28 and p < 0.01 at 1–3 months; R2 = 0.35 and p < 0.01 at 4–9 months; R2 = 0.12 and p = 0.05 at 12–15 months.
Figs 3A to C.
Scatter plot of percentage of IOP change from baseline between the SLT-treated and the fellow eye at (A) 1–3 months (R2 = 0.284; p < 0.001), (B) 4–9 months (R2 = 0.348; p < 0.001), and (C) 12–15 months (R2 = 0.118; p = 0.054)
Discussion
In recent years, the use of SLT has grown, especially for patients with uncontrolled IOPs at maximum medication burden or patients that struggle with compliance.10 The use of SLT is expected to increase even more after studies have shown the efficacy of SLT in newly diagnosed glaucoma patients being similar to drops.11 Clinically, we have observed that patient receiving monocular administration of SLT would often have a IOP drop in the fellow untreated eye but this response was not analyzed till now. This study builds on previous studies on the consensual ophthalmotonic reaction in SLT by looking at a larger patient population and excluding any patients with previous IOP-lowering surgical or laser treatments in either eye.
There have been several theories proposed to explain the consensual ophthalmotonic response. One common theory is that monocular administration of drops is distributed systemically. However, a study by Newman et al. found that the application of 0.1% timolol gel, which is known to have a lower systemic distribution, resulted in an IOP reduction in the untreated eye similar to that of patients receiving 0.5% timolol drops.5 Other studies have shown a consensual ophthalmotonic reaction in patients receiving trabeculotomy and even tonometry.12,13
In our study, the IOP statistically significantly decreased from baseline at all-time points in the SLT-treated group (Fig. 1). For the untreated fellow eyes, there was a statistically significantly decrease in IOP from baseline up to the 4–9 month follow-up period. We did not find any statistically significant difference in IOP from baseline at the 12–15 months in the untreated fellow eyes (Fig. 1). We also found that the average percentage of IOP change from baseline decreased over time in both groups and the reduction was more pronounced in the fellow eye, though not statistically significant (Fig. 2). The percentage of reduction in IOP remained more stable in the SLT-treated group after 4–9 months (Fig. 2). Our findings are in consistent with results of previous studies. Latina et al. reported an average decrease in IOP of 4.6 mm Hg (18.7%) in the SLT-treated eye and 2.1 mm Hg (9.7%) in the contralateral untreated eye at 26 weeks after SLT therapy (n = 44).14 Rhodes et al. also found that patients had a mean IOP reduction of 3.9 ± 0.6 mm Hg (18.8%) in the SLT-treated eye and 2.1 ± 0.5 mm Hg (12.2%) in the contralateral eye (n = 33) at 6 months after monocular treatment with SLT.15 Linear regression analysis by Rhodes et al. found that at 6 months there is correlation between IOP reduction in the SLT-treated eye and the IOP reduction in the fellow eye (R = 0.65). Our study expands on previous studies14,15 by the inclusion of more patients, a longer follow-up period, and the exclusion of patients who have had previous ALT-treatments or peripheral iridotomies.
Interestingly, the consensual ophthalmotonic reaction was not seen in our study at the 12–15 month follow-up period, with a IOP reduction of 1.6 mm Hg (6.45% reduction) from baseline (p = 0.05, paired t-test). A study by McIlraith et al. found that 23 patients that underwent SLT had a 1.6 mm Hg decrease (8% reduction) of IOP from baseline in the untreated fellow eye at the 1-year follow-up.16 The possible difference in findings could be due to the inclusion of patients with longer follow-up times, as SLT is known to have a decrease in efficacy with increasing time post-treatment.17
A theory as to why SLT would result in a consensual ophthalmotonic reaction is the production of prostaglandins, which can then cross systemically to the other eye. Latina et al. previously showed that SLT application induces an upregulation of IL-1a, IL-1b, and TNF-a in the trabecular meshwork of the treated eye.18 This increase in vasoactive and chemotactic agents results in local macrophage recruitment and gelatinase release, ultimately leading to improved aqueous humor outflow.18 In support of this theory are several studies that investigated factors associated with efficacy of SLT.19–21 Those studies showed that prior administration of latanoprost decreases the efficacy of SLT.19–21
Limitations to this study include its retrospective nature. Another limitation to this study is that African Americans comprise a majority of the study population (85.9%), therefore the results may not be generalized to other races. A confounding variable in our study is that the administration of SLT can cause an increase in compliance with drops in patients, shown by Novak et al.22 If administration of SLT does increase compliance in patients, that could explain some of the consensual ophthalmotonic reaction. However, this increase in compliance is usually short-lived, typically only 1–2 months. Another source of error is that the patients with continued follow-up may have increased medication compliance in general.
Conclusion
In summary, our study shows that monocular administration of SLT results in an IOP reduction in the treated eye as well as in the fellow untreated eye, a consensual ophthalmotonic reaction. The consensual ophthalmotonic reaction appears to last for up to 4–9 months. Future prospective studies are warranted with the focus on increasing the sample size, longer follow-up times and standardization of factors that may influence the extent of IOP reduction due to the ophthalmotonic reaction.
This is to certify that:
The article has not been presented in a meeting.
The authors did not receive any financial support from any public or private sources.
The authors have no financial or proprietary interest in a product, method, or material described herein.
Clinical Significance
Although SLT does result in a consensual ophthalmotonic reaction, its effect is not a reliable long-term means to control IOP in the contralateral untreated eye.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Weekers L. Modifications experimentales de l'ophtalmotonus. Reaction ophtalmotonique consensuelle. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.879.7259&rep=rep1&type=pdf Arch Ophtalmol (Paris) 1924;41:641–658. [Google Scholar]
- 2.Gibbens MV. The consensual ophthalmotonic reaction. Br J Ophthalmol. 1988;72(10):746–749. doi: 10.1136/bjo.72.10.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piltz J, Gross R, Shin DH, et al. Contralateral effect of topical beta-adrenergic antagonists in initial one-eyed trials in the ocular hypertension treatment study. Am J Ophthalmol. 2000;130(4):441–453. doi: 10.1016/s0002-9394(00)00527-4. [DOI] [PubMed] [Google Scholar]
- 4.Saari KM, Ali-Melkkila T, Vuori ML, et al. Absorption of ocular timolol: drug concentrations and beta-receptor binding activity in the aqueous humour of the treated and contralateral eye. Acta Ophthalmol (Copenh) 1993;71(5):671–676. doi: 10.1111/j.1755-3768.1993.tb04659.x. [DOI] [PubMed] [Google Scholar]
- 5.Newman H, Kurtz S, David R. Intraocular pressure changes in the contralateral eye after topical treatment: does an “ophthalmotonic consensual reaction” exist? https://www.ima.org.il/FilesUploadPublic/IMAJ/0/40/20185.pdf. Isr Med Assoc J. 2010;12(9):568–571. [PubMed] [Google Scholar]
- 6.Rouland JF, Morel-Mandrino P, Elena PP, et al. Timolol 0.1% gel (Nyogel 0.1%) once daily versus conventional timolol 0.5% solution twice daily: a comparison of efficacy and safety. Ophthalmologica. 2002;216(6):449–454. doi: 10.1159/000067548. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 7.Van Buskirk EM. Pathophysiology of laser trabeculoplasty. Surv Ophthalmol. 1989;33(4):264–272. doi: 10.1016/0039-6257(82)90152-7. [DOI] [PubMed] [Google Scholar]
- 8.Kagan DB, Gorfinkel NS, Hutnik CM. Mechanisms of selective laser trabeculoplasty: a review. Clin Exp Ophthalmol. 2014;42(7):675–681. doi: 10.1111/ceo.12281. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 9.Latina MA, Park C. Selective targeting of trabecular meshwork cells: in vitro studies of pulsed and CW laser interactions. Exp Eye Res. 1995;60(4):359–371. doi: 10.1016/s0014-4835(05)80093-4. [DOI] [PubMed] [Google Scholar]
- 10.Rachmiel R, Trope GE, Chipman ML, et al. Laser trabeculoplasty trends with the introduction of new medical treatments and selective laser trabeculoplasty. J Glaucoma. 2006;15(4):306–309. doi: 10.1097/01.ijg.0000212233.11287.b3. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 11.Francis BA, Ianchulev T, Schofield JK, et al. Selective laser trabeculoplasty as a replacement for medical therapy in open-angle glaucoma. Am J Ophthalmol. 2005;140(3):524–525. doi: 10.1016/j.ajo.2005.02.047. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 12.Vysniauskiene I, Shaarawy T, Flammer J, et al. Intraocular pressure changes in the contralateral eye after trabeculectomy with mitomycin C. Br J Ophthalmol. 2005;89(7):809–811. doi: 10.1136/bjo.2004.050294. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stocker FW. On changes in intraocular pressure after application of the tonometer; in the same eye and in the other eye. Am J Ophthalmol. 1958;45(2):192–196. doi: 10.1016/0002-9394(58)90643-3. [DOI] [PubMed] [Google Scholar]
- 14.Latina MA, Sibayan SA, Shin DH, et al. Q-switched 532-nm Nd:YAG laser trabeculoplasty (selective laser trabeculoplasty): a multicenter, pilot, clinical study. Ophthalmology. 1998;105(11):2082–2090. doi: 10.1016/S0161-6420(98)91129-0. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes KM, Weinstein R, Saltzmann RM, et al. Intraocular pressure reduction in the untreated fellow eye after selective laser trabeculoplasty. Curr Med Res Opin. 2009;25(3):787–796. doi: 10.1185/03007990902728316. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 16.McIlraith I, Strasfeld M, Colev G, et al. Selective laser trabeculoplasty as initial and adjunctive treatment for open-angle glaucoma. J Glaucoma. 2006;15(2):124–130. doi: 10.1097/00061198-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Juzych MS, Chopra V, Banitt MR, et al. Comparison of long-term outcomes of selective laser trabeculoplasty versus argon laser trabeculoplasty in open-angle glaucoma. Ophthalmology. 2004;111(10):1853–1859. doi: 10.1016/j.ophtha.2004.04.030. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 18.Latina MA, de Leon JM. Selective laser trabeculoplasty. Ophthalmol Clin North Am. 2005;18(3):409–419. vi. doi: 10.1016/j.ohc.2005.05.005. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 19.Bruen R, Lesk MR, Harasymowycz P. Baseline factors predictive of SLT response: a prospective study. J Ophthalmol. 2012;2012:642869. doi: 10.1155/2012/642869. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latina MA, de Leon JM. The effect of topical glaucoma medication on the efficacy of SLT. Glaucoma Today. 2004. https://glaucomatoday.com/articles/2004-nov-dec/1104_01.html pp. 31–33.https://glaucomatoday.com/articles/2004-nov-dec/1104_01.html
- 21.Kara N, Altan C, Satana B, et al. Comparison of selective laser trabeculoplasty success in patients treated with either prostaglandin or timolol/dorzolamide fixed combination. J Ocul Pharmacol Ther. 2011;27(4):339–342. doi: 10.1089/jop.2011.0015. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 22.Novack GD, David R, Lee PF, et al. Effect of changing medication regimens in glaucoma patients. Ophthalmologica. 1988;196(1):23–28. doi: 10.1159/000309870. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]



