Abstract
Viral keratouveitis (VKU) could be visually debilitating owing to the intraocular inflammation causing collateral damage to the cornea and secondary elevation of intraocular pressure (IOP). In this retrospective, single-center, observational study, we analyze the clinical features and management options for VKU, with a brief review on incidence of glaucoma and its treatment outcomes. We reviewed the outpatient records at our tertiary hospital from 2015 to 2020 and found 53 eyes of 55 patients diagnosed as VKU. The main outcome measures were incidence of clinical signs, elevated IOP and glaucoma, and treatment modalities used. Sixty-four percent were males with a mean age of onset being 45.4 years. Eighty percent of the eyes were clinically diagnosed to have herpes simplex virus (HSV), 16% herpes zoster virus (HZV) and 4% cytomegalovirus (CMV). Ocular presentations most commonly noted were keratic precipitates (70.4%), corneal edema (66.7%). Associated elevation of IOP was seen in 24 eyes (44%), while glaucomatous damage was seen in 20% of the eyes. Those with fewer uveitic episodes (less than two), as opposed to those having more than two episodes (p < 0.09) posed a lesser risk of developing glaucoma. Almost all were treated with topical steroids and oral acyclovir. The need for glaucoma surgery, in our study, was only 7.2%. Majority of patients with glaucoma, as compared to those without, appeared to have a higher number of IOP spikes and uveitic episodes. CMV-associated eyes had higher risk of developing glaucoma and were more intractable, requiring more intense treatment strategies. This review of the clinical profile of an exclusive South Indian cohort of VKU with an attempt to understand the differences in presentation between the herpetic and CMV groups and its implication from a glaucoma perspective makes this study distinctive.
How to cite this article
Sudhakar P, Menon M, CK M, et al. Glaucoma in Viral Keratouveitis: A Retrospective Review at a Tertiary Eye Hospital. J Curr Glaucoma Pract 2022;16(1):65–70.
Keywords: Ahmed glaucoma valve, Antiglaucoma medication, Cytomegalovirus, Herpes simplex virus, Intraocular pressure, Ocular hypertension, Transscleral cyclophotocoagulation, Uveitic glaucoma, Varicella Zoster, Viral keratouveitis
Introduction
Elevated intraocular pressure (IOP) and secondary glaucoma are known to be common sequelae of uveitis, occurring in 10-20% and 20-40% of these patients, respectively.1 The former is termed uveitis-related ocular hypertension (OHT) and is defined as an increase in intraocular pressure (IOP) above 21 mm Hg. Whereas the latter, uveitic glaucoma (UG) applies to cases of uveitis with characteristic disk damage and/or visual field changes secondary to increased IOP. According to various surveys from the developed world, infectious uveitis accounts for relatively a minority of cases, of which herpetic anterior uveitis accounts for 4.5%–18.6%.2 This is in contrast to the developing world, where they have been reported to occur at a greater frequency of 11.9%–50%, of which herpetic anterior uveitis accounts for 0.5%.2
Keratouveitis (KU) refers to a clinical picture of active corneal disease associated with anterior chamber inflammation.3 Herpetic keratouveitis can occur in association with any form of herpetic keratitis and accounts for 5%–10% of all cases of uveitis seen at tertiary referral centers.4 While herpes simplex (HSV) and varicella zoster (VZV), have been well documented etiological agents, cytomegalovirus (CMV) has recently been recognized as a cause of keratouveitis in immunocompetent patients as well, for reasons that are yet to be understood.
The purpose of this retrospective study is to illustrate the approach to cases of viral KU (VKU) complicated by a secondary OHT or UG and their management outcomes. In addition, we have also tried to identify possible prognostic factors contributing to the success or failure of treatment of these complicated cases.
Methods
Following clearance from the Institutional Review Board at our hospital, the existing digital records of patients diagnosed to have VKU at our hospital, from January 2015 to January 2020, were reviewed. Patients aged 18 years or older were included in the study. The etiological subclassification was based on clinical presentation and included HSV, VZV and CMV. Diagnosis of herpetic KU was made by the presence of typical dendrites or stromal keratitis, signs of anterior uveitis (including iris sector atrophy developing over time), with an elevated IOP at presentation (Fig. 1). Facial skin lesions, with their typical dermatomal distribution, were considered as a strong indication of VZV-related uveitis. However, only one case of CMV associated VKU (both eyes) was identified, based on clinical suspicion with an overwhelmingly elevated IOP and viral endothelitis-like picture (characteristic round coin shaped KPs). Viral polymerase chain reaction (PCR) was done for confirmation of diagnosis, as the patient was willing to and could afford to, get tested. Patients with a superimposed infectious component of different etiology, those with pre-existing glaucoma and those complicated by other surgical interventions were excluded.
Figs 1A to C.
Clinical signs of VKU
The demographic data obtained included the age and sex, laterality, number of uveitic episodes, follow-up duration in months. The following were noted: best corrected visual acuity (BCVA)—at the time of presentation and at the final visit, biomicroscopic features as seen on slit lamp examination, intraocular pressure (IOP), cup-to-disk ratio (CDR) of the optic disk at the end of follow-up, treatment administered- medical, laser & surgical; the sequelae (elevated IOP, posterior synechiae, cataract and other ocular complications) and systemic associations if any. In patients with elevated IOP, additional data such as a total number of IOP peaks and highest recorded IOP, were also noted. IOP more than 22 mm Hg was recorded as elevated IOP, while the presence of glaucomatous optic disk changes in addition, was defined as glaucoma. Following surgical intervention, Complete success was defined as achieving IOP < 21 mm Hg without AGM; while Qualified success was defined as IOP < 21 mm Hg with additional AGM. Failure was defined as IOP > 21 mm Hg even with medication.
Results
The digital records of 55 eyes of 53 patients were analyzed. The average follow-up duration of these patients was found to be 2 years. There was a male predominance of 64%. The mean age of patients at the time of onset of viral KU was 45.4 years (range: 18–79 years) (Fig. 2). The majority were younger than 55 years (38 of 55 eyes) (69%) in the subgroup of HSV. However, HZO and CMV occurred in an older population, most of them being 55 years and older. Based on the etiological subclassification, 44 of the 55 eyes (80%) were diagnosed to have HSV, while 16% were HZO and 4% CMV keratouveitis (Fig. 3). The majority of the patients (40 of 55 eyes) had a BCVA of 20/200 or better at presentation, of which 40% had a range of 20/50 to 20/200 (Table 1). This BCVA was either maintained (40 eyes) or showed improvement at the final visit (seven eyes) and only one eye progressed to “no light perception” (Table 2).
Fig. 2.

Age distribution of VKU patients in our study
Fig. 3.

Proportion of eyes with HSV, HZO and CMV keratouveitis
Table 1.
BCVA at presentation in HSV, HZV, CMV keratouveitis
| BCVA (in LogMAR) | HSV | HZV | CMV | Total | p-value |
|---|---|---|---|---|---|
| 20/20 - 20/40 (0-0.3) | 12 | 2 | 2 | 16 | 0.115 |
| 20/50 - 20/200 (0.4-1) | 18 | 6 | 24 | ||
| CF-PL (1.3-2.5) | 14 | 1 | 15 | ||
| NPL4 | |||||
| Total | 44 | 9 | 2 | 55 |
CF, counting fingers; PL, perception of light; NPL, no perception of light
Table 2.
BCVA at final visit in HSV, HZV, CMV keratouveitis
| BCVA (in LogMAR) | HSV | HZV | CMV | Total | p-value |
|---|---|---|---|---|---|
| 20/20 - 20/40 (0-0.3) | 18 | 5 | 2 | 25 | 0.774 |
| 20/50 - 20/200 (0.4-1) | 19 | 3 | 22 | ||
| CF-PL (1.3-2.5) | 6 | 1 | 7 | ||
| NPL4 | 1 | 1 | |||
| Total | 44 | 9 | 2 | 55 |
Table 3 gives a list of the common clinical findings commonly occurring with VKU. Average CDR was 0.4, while that in the glaucoma patients was 0.7. Associated elevation of IOP was seen in 24 eyes (44%), while glaucomatous damage was seen in 20% of the eyes. Figure 4 depicts the occurrence of the associated ocular sequelae consequent to VKU. Recurrence in terms of uveitic episode or reactivation of keratitis, was seen in 100% of the cases, with only 36.4% of them being associated with ocular hypertension. Also, the incidence of ocular hypertension was higher in the HSV group (43%) and CMV group (100%) as compared to the VZV group (33.3%). The risk of developing glaucoma greatly increased with increasing number of IOP spikes (Fig. 5). Also, those with fewer uveitic episodes (less than two), as opposed to those having more than two episodes (p < 0.09) posed a lesser risk of developing glaucoma, while the requirement for an interventional filtration surgery was higher in those with five or more episodes of uveitis (p < 0.09) (Table 4).
Table 3.
Ocular findings in VKU
| Clinical signs | Number of cases | Percentage |
|---|---|---|
| Corneal edema | 34 | 64% |
| KP | 44 | 80% |
| Firbrin/hypopyon | 1 | 2% |
| Epithelial defect | 1 | 2% |
| Dendrite/ulcer | 15 | 27% |
| Iris transillumination | 8 | 15% |
| AC reaction | 36 | 65% |
| Descemet membrane folds (DMF) | 20 | 36% |
| Reduced corneal sensation | 20 | 36% |
Fig. 4.
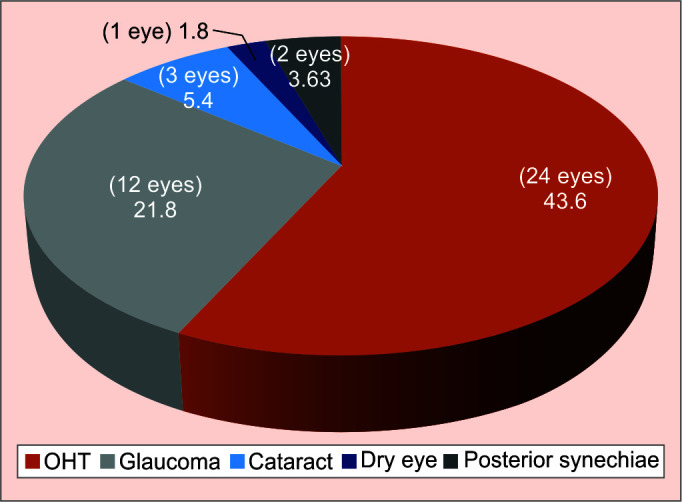
Various associated ocular features/sequelae of VKU
Fig. 5.

Association between the number of IOP spikes and development of glaucoma
Table 4.
Significance of recurrence in development of glaucoma and requirement of filtration surgery
| Recurrence (no. of episodes) | No. of eyes developing glaucoma | No. of eyes requiring trabeculectomy | Total | p-value |
|---|---|---|---|---|
| ≤2 | 4 | 1 | 5 | 0.092 |
| 2-5 | 5 | 5 | ||
| >5 | 2 | 3 | 5 | |
| Total | 11 | 4 | 15 |
Most of the patients responded well to medical treatment alone, with topical steroids (52 of 55 eyes) and oral acyclovir (45 of 55 eyes) being the most commonly used (Fig. 6). We observed that the requirement of additional antiglaucoma medications (AGMs) was necessary only in 27% of the eyes, with the topical agents being adequate in most cases. The treatment we instituted included topical Prednisolone acetate 1% in tapering dose, with oral acyclovir 400 mg, five times a day for 4 weeks, with a maintenance dose of 400 mg twice daily for severe and recurrent cases in order to prevent relapse. In addition, topical homatropine 2% was also added for cycloplegia. Topical acyclovir ointment 3% was used in some cases of typical epithelial dendritic pattern, dendro-geographic variant, or in cases of VKU with epithelial breakdown. The AGM most commonly used was aqueous suppressant—topical dorzolamide and a fixed dose combination of brimonidine and Timolol. Oral AGM in the form of Tablet Acetazolamide was indicated only in a small subset of cases (seven eyes), all of which had very high IOP uncontrolled with topical AGM alone. It was observed that the requirement of AGMs was slightly higher in CMV (two of two eyes) and HZV (three of nine eyes) groups as compared to HSV group (11 of 44 eyes).
Fig. 6.

Medical therapy in patients with VKU
Of the 20 eyes with secondary glaucoma, only five eyes required filtration surgery (trabeculectomy). Similar to the requirement of AGMs, the requirement of filtration surgery also seemed to be higher in CMV (100%) and HZV (11%) groups, as compared to the HSV (4.5%) group. Plain trabeculectomy with antimetabolite was done in four of the five eyes (2 CMV and 2 HSV) requiring filtration surgery, while the one eye with HZV required combined trabeculectomy with cataract surgery. It was observed that the five eyes undergoing trabeculectomy had a higher requirement of AGMs (3 to >5), as compared to the other eyes on AGMs. All five eyes (four plain trabeculectomy and one combined cataract with trabeculectomy) achieved qualified success (100%). Other interventions included transscleral cyclophotocoagulation (CPC) in one eye, cataract surgery in two eyes, penetrating keratoplasty (PKP) in four eyes. The one eye requiring CPC was HSV keratouveitis with a high IOP at presentation, refractory to maximal medical therapy; glaucomatous optic atrophy; progressing quickly to no light perception; and with no identifiable risk factors. However, this eye had intractable glaucoma, and the procedure ended in failure. Two of the four eyes requiring PKP, had secondary glaucoma as well, complicated by secondary fungal keratitis. While the other two eyes were HSV with no other secondary complications.
Discussion
The majority of the patients with viral KU have HSV as their etiological basis (80%). In a similar study by Hoeksema et al., where a presumable clinical diagnosis of HSV and VZV was made, 76% were former and 26% were the latter. Van der Lelij et al., also found more than 80% to be the incidence of HSV KU in a large cohort of their viral AU patients.5 However, this was based on PCR.
The BCVA in most of these patients at presentation ranged from mild to moderate impairment, which either remained the same or improved over time.
The clinical picture in these patients with viral KU was similar to viral keratitis with features of active uveitis. In addition, typically these patients have an elevated IOP as an essential feature. This corroborates with pathophysiology of the disease process in these eyes. Elevated IOP in these patients could be due to various mechanisms:
Trabeculitis and inflammatory cells clogging the trabecular meshwork
As a sequel to the secondary changes of inflammation causing distortion of angle structures
Consequent to ocular complications like peripheral anterior synechiae causing a pupil block mechanism of angle closure
These mechanisms cause an elevated IOP, which is relentless, progresses to glaucoma. Though administration of topical steroids as part of therapy, can itself be a risk factor for a secondary rise in IOP, the first recorded IOP spike was seen to coincide with the first uveitic episode at presentation. Thus, the steroid induced collateral effect was deemed unlikely.
Despite elevated IOP being a typical feature of these viral entities, results from the literature show a varying frequency of association. Our results showed a 44% incidence of ocular hypertension, which was similar to a study by Sungur et al., who reported a 47.3% incidence of ocular hypertension in the “active uveitis period.”6 Wensing et al. reported IOP > 30 mm Hg in 46%–50% of herpetic anterior uveitis (AU) eyes,7 while van der Lelij et al. an IOP > 23 mm Hg in 90% of herpetic AU eyes, and Tugal-Tutkun et al. an IOP > 22 mm Hg in 51% of herpetic AU eyes.5,8 CMV was observed to present with very high IOP values during active inflammatory episodes as compared to HSV and VZV.
The incidence of uveitic glaucoma in our study was found to be 20% (11 of 55 eyes). This was closely comparable to a study by Pohlamn et al.9 who reported a 19% incidence of glaucomatous damage. Similar studies by Sungur et al., Wensing et al. and Hoeksema et al. reported it to be 13% (10/76 eyes), 18% (7/38 eyes) and 15% (11/73 eyes) respectively.6,7,10 Interestingly, we also observed that those with less than five episodes of IOP elevation, had a 22-50% chance of developing secondary glaucoma, while the same risk increased to 80% with more than 5 hypertensive episodes (p = 0.02).
The primary goal of management includes treating the underlying cause of inflammation in addition to controlling IOP. Thus, halting or preventing the activation of viral disease is the mainstay. Traditionally, first-line therapy has included beta blockers and carbonic anhydrase inhibitors, which are advantageous in uveitic patients with concomitant cystoid macular edema. Both agents help reduce IOP via the reduction of aqueous humor production. In the setting of inflammation and high IOP, absorption of topical agents may be decreased by corneal edema, and systemic carbonic anhydrase agents should be considered if topical medications fail to have the desired effect.
Likewise, in our study also, most of the patients with VKU benefitted from medical therapy alone, with a combination of topical steroids (94.5%) and oral antivirals (81.8%). This was similar to studies by Hoeksema and Sungur et al., where medical management was predominantly beneficial.6,10
Those requiring surgical intervention formed 9% of the total VKU population. This was close to a study by Pohlman et al., who report a 10% of their study sample requiring a surgical intervention.9 Trabeculectomy augmented with mitomycin C (MMC) was the filtration surgery performed in 5 of the patients. Surgical success was achieved in the form of only qualified success in all 5 patients (100%). Hoeksema et al. found a success rate of 89% (eight of nine eyes with viral anterior uveitis with secondary glaucoma) with primary trabeculectomy. In their study of uveitic glaucoma in general, Shimzu et al. found a surgical success rate of 82.86% with trabeculectomy. The other surgical interventions included PKP and cataract surgery. CPC was necessary only in 1 patient, who had progressed to a painful blind eye.
Table 5 gives a brief overview of the results of other important studies in comparison to our present study.
Table 5.
Brief comparison of characteristics of various studies in comparison to our present study
| Our study | Hoeksema et al. | Pohlman et al. | Sungur et al. | |
|---|---|---|---|---|
| Age distribution | 18–79 years (Median: 45.4 years) | 5–85 years (Median: 50 years) | 19–87 years (Median: 56 years) | Mean: 45.6 ± 17.318-80 years |
| Gender Males Females |
n = 55 eyes 34 (64%) 19 (36%) |
n = 73 eyes 45 (62%) 28 (38%) |
n = 270 eyes 150 (56%) 120 (44%) |
n = 76 eyes 39 37 |
| Follow-up duration | 2 years | 7.9 years Eyes with glaucoma: 6 years Eyes without glaucoma 1.6 years |
Upto 2 years postoperative follow-up | 37.2 ± 18.124-120months |
| Etiopathogenic virus HSV HZV CMV |
n = 55 eyes 44 eyes (80%) 9 eyes (16%) 2 eyes (4%) |
n = 73 eyes 54 eyes (74%) 19 eyes (26%) |
n = 270 eyes 77 eyes (29%) 45 eyes (17%) 57 eyes (21%) |
n = 76 eyes 58 eyes (76.3%) 18 eyes (23.6%) |
| Clinical findings: Corneal edema KPs AC reaction Dendritic ulcer Epithelial defect Fibrin/hypopyon |
n = 55 eyes 34 (64%) 44 (80%) 36 (65%) 15 (27%) 1 (2%) 1 (2%) |
n = 73 eyes 37 (51%) 65 (89%) 8 (11%) |
n = 270 eyes 11 (4%) 205 (76%) 213 (79%) 31 (11%) |
|
| Elevated IOP | 24 of 55 eyes (44%) | 55 of 73 eyes (75%) |
120 of 270 (44.4%) | 36 of 76 eyes (47.3%) |
| Glaucoma | 12 of 55 eyes (20%) | 11 of 73 eyes (15%) | 52 of 270 eyes (19%) | 10 of 76 eyes (13%) |
| No. of patients requiring AGMs | 16 of 55 eyes (29%) | 47 of 73 eyes (64%) | 145 of 270 eyes (53.7%) | |
| Requirement of antiglaucoma surgery | Trabeculectomy: 5 of 55 eyes (9%) | Trabeculectomy: 14 of 73 eyes (19%) | Trabeculectomy: 17 of 270 eyes (6.3%) MIGS: 16 of 270 eyes (6%) |
2 of 76 eyes (2.6%) |
| Recurrence | 55 eyes (100%) | 37 of 52 eyes (75%): in glaucomatous eyes | 39 of 76 eyes (51.3%) |
Since it's a retrospective analysis, the associated limitations cannot be ignored. The smaller sample size also warrants either a longer follow-up or further advancement of the study, to be able to extrapolate the inferred data to a more universal practice. And also, the fact that most of these patients were diagnosed based on clinical characteristics and not laboratory testing. However, considering the cost to the patient in developing countries for long-term treatment and recurring follow-ups, one could consider a clinical approach alone, without the need for a PCR. This can be justified with the largely beneficial regimen of steroids and antivirals, in most cases of VKU, as evident in our study (Tables 6 to 8).
Table 6.
Significance of recurrence in development of glaucoma and requirement of trabeculectomy
| Recurrence (no. of episodes) | No. of eyes developing glaucoma | No. of eyes requiring trabeculectomy | Total | p-value |
|---|---|---|---|---|
| ≤2 | 4 | 1 | 5 | 0.092 |
| 2–5 | 5 | 5 | ||
| >5 | 2 | 3 | 5 | |
| Total | 11 | 4 | 15 |
Table 8.
Number of IOP spikes versus development of glaucoma
| Number of IOP spikes | Number of eyes | Proportion of them developing glaucoma |
|---|---|---|
| <2 | 9 | 2 eyes (22.2%) |
| 2–5 | 10 | 5 eyes (50%) |
| >5 | 5 | 4 eyes (80%) |
Conclusion
Viral keratouveitis can be a challenging entity to treat. Considering the fact that elevated IOP and glaucoma are the most common sequelae associated with VKU, a better understanding of the risk traits and prognostic factors is of paramount importance. Though the patients respond well to topical steroids and oral antivirals in the majority of the cases, oftentimes these may become complicated. A higher number of uveitic episodes are associated not only with an increased risk of developing glaucoma but also with a greater propensity for surgical intervention. Also, CMV seems to be associated with a higher risk of developing glaucoma and the requirement of more intense treatment strategies. Despite all these factors, glaucoma secondary to viral KU can be well managed and has a reasonable prognosis, especially in the more common setup of HSV. Prompt multi-specialty cross-referral especially in high-risk cases and in recurrences is important in providing better outcomes to these patients.
Table 7.
Complications of VKU
| Number of cases | Percentage | |
|---|---|---|
| Elevated IOP | 24 | 43.6% |
| PS | 2 | 3.6% |
| Glaucoma | 12 | 21.8% |
| Cataract | 3 | 5.4% |
| Dry eye | 1 | 1.8% |
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Shimizu A, Maruyama K, Yokoyama Y, et al. Characteristics of uveitic glaucoma and evaluation of its surgical treatment. Clin Ophthalmol. 2014;8:2383–2389. doi: 10.2147/OPTH.S72383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rathinam S, Namperumalsamy P. Global variation and pattern changes in epidemiology of uveitis. Indian J Ophthalmol. 2007;55(3):173. doi: 10.4103/0301-4738.31936. [DOI] [PubMed] [Google Scholar]
- 3.2012 Volume 4, Chapter 39. Keratouveitis, Duanes Lippincott Williams & Wilkins, [Google Scholar]
- 4.Cunningham ET. Diagnosing and treating herpetic anterior uveitis. Ophthalmology. 2000;107(12):2129–2130. doi: 10.1016/s0161-6420(00)00287-6. [DOI] [PubMed] [Google Scholar]
- 5.Van Der Lelij A, Ooijman FM, Kijlstra A, et al. Anterior uveitis with sectoral iris atrophy in the absence of keratitis: A distinct clinical entity among herpetic eye diseases. Ophthalmology. 2000;107(6):1164–1170. doi: 10.1016/s0161-6420(00)00115-9. [DOI] [PubMed] [Google Scholar]
- 6.Sungur GK, Hazirolan D, Yalvac IS, et al. Incidence and prognosis of ocular hypertension secondary to viral uveitis. Int Ophthalmol. 2010;30(2):191–194. doi: 10.1007/s10792-009-9305-z. [DOI] [PubMed] [Google Scholar]
- 7.Wensing B, Relvas LM, Caspers LE, et al. Comparison of rubella virus- and herpes virus-associated anterior uveitis: Clinical manifestations and visual prognosis. Ophthalmology. 2011;118(10):1905–1910. doi: 10.1016/j.ophtha.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 8.Tugal-Tutkun I, Ötük-Yasar B, Altinkurt E. Clinical features and prognosis of herpetic anterior uveitis: A retrospective study of 111 cases. Int Ophthalmol. 2010;30(5):559–565. doi: 10.1007/s10792-010-9394-8. [DOI] [PubMed] [Google Scholar]
- 9.Pohlmann D, Pahlitzsch M, Schlickeiser S, et al. Virus-associated anterior uveitis and secondary glaucoma: Diagnostics, clinical characteristics, and surgical options. PLoS One. 2020;15(2):e0229260. doi: 10.1371/journal.pone.0229260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoeksema L, Jansonius NM, Los LI. Risk Factors for Secondary Glaucoma in Herpetic Anterior Uveitis. Am J Ophthalmol. 2017;181:55–60. doi: 10.1016/j.ajo.2017.06.013. [DOI] [PubMed] [Google Scholar]



