Abstract
How to cite this article: Dada T, Verma S, Bukke AN, et al. Aqueous Angiography-guided Minimally Invasive Glaucoma Surgery. J Curr Glaucoma Pract 2022;16(1):1-3.
Introduction
Aqueous angiography (AA) is a new imaging technique that allows real-time, physiological visualization of aqueous outflow pathways beyond Schlemm's canal (SC). Recent studies with AA have shown the segmental nature of these outflow pathways, which can significantly alter our understanding of the success and failure of minimally invasive glaucoma surgeries (MIGS) and help us in customizing as well as devising new treatment strategies which can reduce intraocular pressure (IOP) with minimal tissue damage. The main limitation of AA is that it is an invasive procedure which limits its use in the follow-up period after surgery.
Aqueous Humor Outflow
Aqueous humor is secreted by ciliary processes in the pars plicata, passes from the posterior to the anterior chamber of the eye through the pupil, and exits the eye through the trabecular meshwork (TM) and uveoscleral pathways. The TM pathway is responsible for up to 90% of outflow and thus resistance in this pathway is what usually leads to an increase in IOP.1 Aqueous humor outflow (AHO) through TM is guided by a pressure gradient, which in turn seems to be guided primarily by aqueous outflow pathway distal to SC. In normal eyes, most resistance to AHO is localized to the inner wall region comprising of juxtacanalicular connective tissue and the inner wall of SC.2,3 From SC, aqueous further passes through collector channels (CC), intrascleral venous plexus, aqueous veins and finally into the episcleral venous system. AHO through TM is not circumferentially uniform but instead has preferential outflow adjacent to the location of CC.4,5
Imaging Distal Outflow Pathways with Aqueous Angiography
AA provides for real-time imaging of aqueous pathways. It is based on the introduction of a fluorescent tracer in the anterior chamber and repeated imaging using relevant excitation and barrier filters to track the movement of the tracer through the outflow pathways.
Saraswathy et al. described this real-time, physiological AHO imaging technique in enucleated human eyes using fluorescein dye as a tracer.6 To confirm the AHO pathway lumens as the source of angiographic signal, anterior segment optical coherence tomography (ASOCT) was simultaneously performed on angiographically positive and negative areas. Intrascleral lumens, reminiscent of the AHO pathway, were visible only in angiographically positive areas. This was followed by perfusion of eyes with fluorescent dextran, which could bind to the walls of AHO lumens, and subsequent fluorescent microscopy of frozen sections showed greater tracer deposition in the angle adjacent to angiographically positive areas.
Huang et al. were then the first to perform AA in living human subjects.7 Indocyanine green (ICG; diluted with a balanced salt solution to 0.4%) was introduced in the anterior chamber for capsular staining during phacoemulsification while simultaneously serving as a tracer for AA. The angiographic camera (Spectralis HRA + OCT Flex module; Heidelberg Engineering GmbH, Heidelberg, Germany) was placed on a customized arm and centered over the eye. Using ICG capture mode (excitation wavelength 796 nm; transmission filter >800 nm), fluorescent images were first obtained to establish the black pre-injection background for comparison. The procedure was repeated after the introduction of ICG and change images were acquired with subjects looking in different directions to obtain angiographic images of each ocular quadrant. Their study confirmed segmental patterns of outflow in living subjects. In addition, the pulsatile and dynamic flow was observed in the imaging videos. Comparative analysis with simultaneously taken ASOCT images again confirmed the presence of larger intrascleral lumens in angiographically positive rather than negative regions.
Huang et al. later showed the viability of fluorescein as a tracer for AA in live human subjects using the same instruments.8 They also performed sequential angiography wherein ICG AA was followed by fluorescein angiography because, in the presence of ICG, fluorescein could show some emission even when ICG capture mode was being used, likely due to concentration-related effects. Though largely similar, a subtle difference in angiography pattern obtained by using these tracers was due to differences in their protein binding and molecular characteristics. ICG being more protein-bound showed more intraluminal retention, and fluorescein being less protein-bound could leak and stain surrounding tissue. The use of a longer excitation wavelength with ICG could also theoretically lead to a better imaging of deeper pathways when compared with fluorescein.
Huang and Saraswathy et al. also demonstrated the ability of trabecular micro bypass stents to improve AHO in regions initially without angiographic flow using sequential AA in enucleated human eyes and later in the nasal region of glaucoma subjects.9,10 Their study provided proof of concept for a surgical application of AA in MIGS.
Surgical Application
The segmental nature of AHO is an important finding and has the potential for guiding the future of ab interno MIGS. Mapping areas of functioning and non-functioning distal drainage and customizing surgeries to accentuate existing outflow or recruiting non-functioning outflow tracts can open up new avenues in glaucoma surgeries (Fig. 1).
Figs 1A to D.
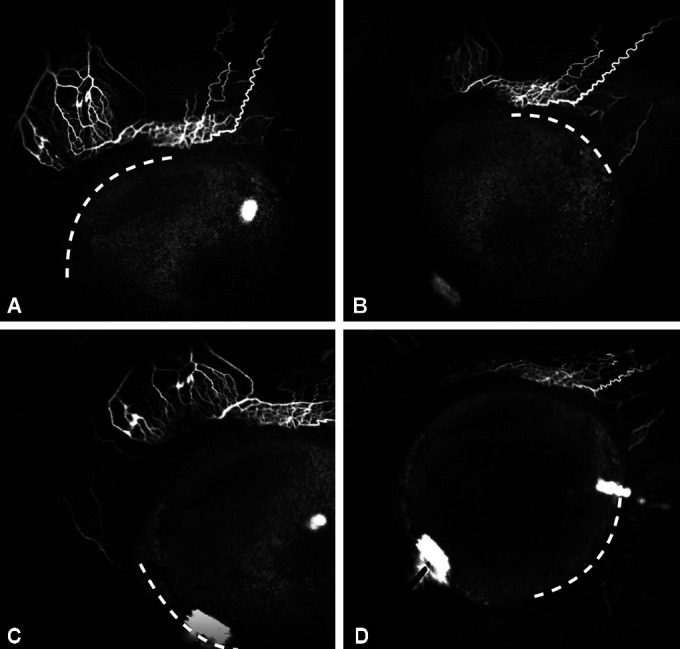
Aqueous angiography in (A) Superonasal, (B) Superotemporal, (C) Inferonasal and (D) Inferotemporal quadrant (respective quadrant marked by white dotted line). Note maximum density of outflow channels in superonasal followed by superotemporal quadrant
We recently described a novel technique wherein AA-guided focal ab interno goniectomy was performed in a patient with primary open-angle glaucoma wherein only a small strip of the TM was removed in the region identified with maximal AHO signal on AA.11 MIGS placement can thus be customized in areas where the maximum angiographic signal is obtained since the anatomy in these areas is supportive of outflow. This technique spares the rest of the TM from unnecessary surgery.
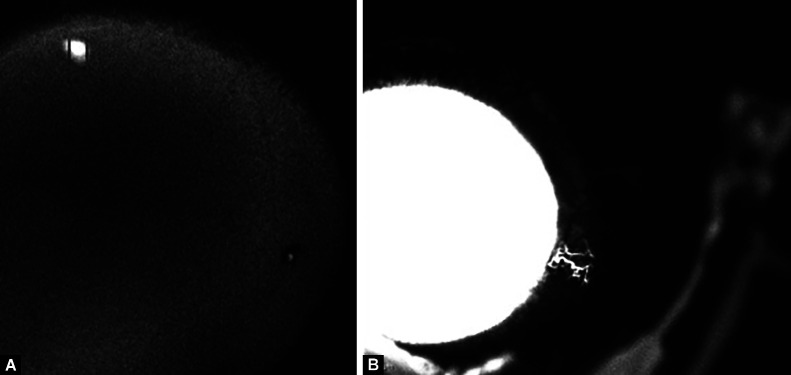
An alternative approach is to perform MIGS in areas of poor baseline AA signal in an attempt to recruit previously low aqueous flow areas rather than operating on high aqueous flow zones. Figures 2A and B show an AA image that initially revealed no drainage in the temporal quadrant. Ab interno goniectomy then created new outflow pathways where none previously existed. This approach is further unique because not only are low-flow regions being targeted but the temporal side of the eye is being operated on. The temporal side of the eye generally has lower outflow; thus, temporal surgery is an easier way to find low-flow regions. Temporal MIGS are currently rarely done with limited study, and reasons for this include difficulty in accessing this region. However, if the clinical data suggests potential benefits, surgical innovation would surely follow to overcome challenges in operating in any location of the eye.
Figs 2A and B.
(A) ICG-assisted aqueous angiography showing no outflow temporally; (B) Repeat aqueous angiography using fluorescein demonstrated two new channels visible temporally after goniectomy
Multiple studies have demonstrated that minimally invasive trabecular ablation/bypass surgeries have very variable outcomes and are often not able to achieve optimal IOP reduction. One hypothesis to explain this, is the segmental nature of AHO, as the success and failure of such surgeries is influenced by where in the eye (superior, nasal, inferior, or temporal) the surgery is being performed. It stands to reason that the outcome of ab interno surgeries depends on the status of these distal outflow pathways, and identification of functioning and non-functioning areas of drainage may help in guiding as well as predicting the outcomes of these surgeries.
Future studies comparing MIGS outcomes with the aforementioned approaches, that is, AA-guided enhancement in areas with maximum angiographic signal vs recruitment of areas with no angiographic signal can help in determining which approach is better suited for IOP control.
Thus, AA-assisted ab interno glaucoma surgery opens up new avenues for improving the safety and efficacy of MIGS. This targeted approach reduces unwanted tissue damage and has the potential to direct future strategies and decision-making to improve results in surgeries targeting the trabecular pathways.
Current Limitations
Application and pressure from the eyelid speculum and ocular anesthesia have the potential to alter the aqueous outflow. Outflow might also be altered by use of mydriatics during imaging, which warrants comparative studies in the dilated and undilated states. When used in pseudophakic eyes, staining of the intraocular lens, posterior capsule and vitreous is a potential complication that needs to be evaluated. The staining with the use of fluorescein is much more than that with ICG and using two separate dyes for documenting the increase in outflow after MIGS remains a challenge. Imaging is only done at the time of surgery as follow-up repeat AA is not generally possible due to ethical concerns as it is an invasive procedure and possible tracer toxicity due to repeat exposure.12
Conclusion
Mechanics of AHO is extremely complex and influenced by a myriad of factors. The outflow tract distal to SC seems to be much more important in determining resistance to outflow than historically credited for, and correct determination of its anatomical and functional distribution in a clinical setting provides a unique opportunity to develop minimally invasive targeted therapies in tackling glaucoma. AA is the first step in studying the actual dynamics of aqueous outflow in glaucoma patients and using imaging to target specific regions of the TM. The IOP-lowering efficacy of AA guided MIGS versus MIGS alone would be an interesting topic for future studies. We look forward to new technologies that would permit the non-invasive evaluation of AHO akin to the current era of OCT angiography of the retinal circulation.
Declarations
Presentation at a meeting: None
Consent to participate—Informed consent was obtained from all the patients.
Consent for publication—Obtained from all the authors and the patients.
Availability of data and material—All pertaining data have been provided.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Toris CB, Koepsell SA, Yablonski ME, et al. Aqueous humor dynamics in ocular hypertensive patients. J Glaucoma. 2002;11(3):253–258. doi: 10.1097/00061198-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Fautsch MP, Johnson DH. Aqueous humor outflow: what do we know? Where will it lead us? Invest Ophthalmol Vis Sci. 2006;47(10):4181–4187. doi: 10.1167/iovs.06-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dada T, Mahalingam K, Bhartiya S. Minimally invasive glaucoma surgery—to remove or preserve the trabecular meshwork: that is the question? J Curr Glaucoma Pract. 2021;15(2):47–51. doi: 10.5005/jp-journals-10078-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hann CR, Fautsch MP. Preferential fluid flow in the human trabecular meshwork near collector channels. Invest Ophthalmol Vis Sci. 2009;50(4):1692–1697. doi: 10.1167/iovs.08-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battista SA, Lu Z, Hofmann S, et al. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49(12):5346–5352. doi: 10.1167/iovs.08-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saraswathy S, Tan JCH, Yu F, et al. Aqueous angiography: real-time and physiologic aqueous humor outflow imaging. PLoS ONE. 2016;11(1):e0147176. doi: 10.1371/journal.pone.0147176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang AS, Camp A, Xu BY, et al. Aqueous angiography: aqueous humor outflow imaging in live human subjects. Ophthalmology. 2017;124(8):1249–1251. doi: 10.1016/j.ophtha.2017.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang AS, Penteado RC, Saha SK, et al. Fluorescein aqueous angiography in live normal human eyes. J Glaucoma. 2018;27(11):957–964. doi: 10.1097/IJG.0000000000001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang AS, Saraswathy S, Dastiridou A, et al. Aqueous angiography–mediated guidance of trabecular bypass improves angiographic outflow in human enucleated eyes. Invest Ophthalmol Vis Sci. 2016;57(11):4558–4565. doi: 10.1167/iovs.16-19644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang AS, Penteado RC, Papoyan V, et al. Aqueous angiographic outflow improvement after trabecular microbypass in glaucoma patients. Ophthalmol Glaucoma. 2019;2(1):11–21. doi: 10.1016/j.ogla.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dada T, Bukke AN. Aqueous angiography guided ab interno trabecular surgery for open-angle glaucoma. BMJ Case Rep. 2022;15(1):e248261. doi: 10.1136/bcr-2021-248261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tandogan T, Khoramnia R, Uwe Auffarth G, et al. Impact of indocyanine green concentration, exposure time, and degree of dissolution in creating toxic anterior segment syndrome: evaluation in a rabbit model. J Ophthalmol. 2016;2016:1–9. doi: 10.1155/2016/3827050. [DOI] [PMC free article] [PubMed] [Google Scholar]