Abstract
Three-dimensional (3D) bioprinting of cellular or biological components are an emerging field to develop tissue structures that mimic the spatial, mechanochemical and temporal characteristics of cardiovascular tissues. 3D multi-cellular and multi-domain organotypic biological constructs can better recapitulate in vivo physiology and can be utilized in a variety of applications. Such applications include in vitro cellular studies, high-throughput drug screening, disease modeling, biocompatibility analysis, drug testing and regenerative medicine. A major challenge of 3D bioprinting strategies is the inability of matrix molecules to reconstitute the complexity of the extracellular matrix and the intrinsic cellular morphologies and functions. An important factor is the inclusion of a vascular network to facilitate oxygen and nutrient perfusion in scalable and patterned 3D bioprinted tissues to promote cell viability and functionality. In this review, we summarize the new generation of 3D bioprinting techniques, the kinds of bioinks and printing materials employed for 3D bioprinting, along with the current state-of-the-art in engineered cardiovascular tissue models. We also highlight the translational applications of 3D bioprinting in engineering the myocardium cardiac valves, and vascular grafts. Finally, we discuss current challenges and perspectives of designing effective 3D bioprinted constructs with native vasculature, architecture and functionality for clinical translation and cardiovascular regeneration.
Graphical Abstract

1. Introduction
Cardiovascular disease is the most common cause of mortality around the world, with an estimate of 17.5 million deaths every year and an expected increase of 70% by 2030 [1]. In particular, heart failure accounts for 6.2 million adults diagnosed every year [2] and is associated with an estimated $70 billion spent on medical costs by 2030 [3]. Tissue engineering and regenerative medicine are promising approaches to develop functional de novo cardiac replacements that are capable of integration with the host tissue. However, traditional tissue engineering approaches involving cell-seeded scaffolds do not possess the desirable qualities of controlled porous structure, tissue-like mechanical properties, and biomimetic cell signaling. Cellular response to environmental signals is challenging to simulate in a traditional 2D adherent cell-culture model [4,5], and 2D culture models often fail to simulate the clinically relevant cellular phenotypes and complex architecture of native tissue. Conventional three-dimensional (3D) hydrogels can better mimic the architecture of native tissue [6,7], but lack spatial and temporal control of cell seeding.
3D printing is a novel industrial method that creates 3D objects by depositing materials layer by layer. The increased acceptance of the Additive Manufacturing (AM) system over traditional methods can be ascribed to a multitude of advantages, including high-precision manufacturing of complicated geometry, optimum material savings, design flexibility, and personal customization. Metals, polymers, ceramics, and concrete are among the materials now used in 3D printing [8]. Based on technology, cost, speed, resolution, and constraints, various approaches for completing the 3D printing procedure exist. 3D bioprinting refers to the use of AM technologies to produce functional living things from biological components such as cells, biomaterials, and growth factors [9]. 3D bioprinting has experienced significant growth in medicine and bioengineering in the recent 10 years, enabling the fabrication of tissues, organs, prosthetic devices, and drug delivery techniques [8,9].
To address the limitations of traditional tissue engineering approaches, 3D bioprinting emerged as a rapid prototyping technique that uses computer-aided design to create complex tissue constructs, such as a heart valve, myocardium, and a vascular graft [8]. Additionally, bioprinting enables the production of tailored and patient-specific equipment, which enhances its efficiency, durability, and cost-effectiveness [10,11]. 3D bioprinting employs approaches such as inkjet [12], extrusion [13] and laser-based approaches [14] to create geometrically complex and scalable tissues. 3D bioprinting offers the potential to reproducibly develop 3D structured tissue with controlled and optimized microenvironment where intrinsic cellular morphologies and structures can be reconstituted. High order assembly of the 3D functional cell-printed constructs can be developed with organized spatial pattern and tissue specific gene expression. 3D bioprinting offers the potential to fabricate multi-cellular constructs by sequential deposition of different cell types localized to specific domains. This platform is promising for fabrication of thick perfusable, endothelialized and vascularized tissues with heterogenous integration, biocompatibility and long-term stability of 3D printed constructs for human tissue and organ generation [15]. Here, we summarize the new generation of 3D bioprinting techniques, bioinks, and state-of-the-art engineered cardiovascular tissue models. We also highlight the translational applications of 3D bioprinting in engineering the myocardium, cardiac valves and vascular grafts.
2. Bioinks
3D printing involves deposition or printing of material formulations such as living cells, bioactive molecules, cell aggregates such as microtissues, biomaterials or hybrid cell constructs in a layered pattern to generate 3D structures with high reproducibility due to the automated deposition process [16]. Bioinks refer to the formulations of biomaterials and/or viable cells required to print a 3D construct [17] that are crosslinked or stabilized during or immediately after bioprinting to create the final intended tissue construct structure. Bioinks serve as a medium for delivering cells during the printing process, as well as for forming a bio-scaffold to support growth of the 3D construct. Bioinks have viscoelastic properties and higher water content to protect the cells during the printing procedure from exogenous risk factors, such as mechanical stress that occur while passing through the nozzle, drying and potential contamination. In cardiovascular tissue engineering, primary cardiac cells, cell lines and stem cell-derived cardiac cells are used as major cellular components of bioinks.
An ideal bioink must possess characteristics of biodegradability, bioprintability, mechanical stability, rigidity, shape fidelity, stimuli enhanced self-assembly and exhibit tunable gelation to exhibit extrusion, along with the potential to promote viability and cellular functionality with post-printing maturation for the culture period under specific culture conditions [18]. Other desirable qualities are crosslinking ability, industrial scalability (with minimum batch-to-batch variations), cost-effectiveness, reasonable bioprinting time and permeability to gaseous and nutrients exchange [19].
Both natural and synthetic biomaterials have gained interest for developing bioinks in cardiac tissue engineering. Natural materials are biocompatible have intrinsic properties similar to the native extracellular matrix (ECM) of a cell. Natural materials are polysaccharide based (eg., alginate, agarose, chitosan), protein based (collagen, fibrin), glycosaminoglycan based (hyaluronic acid) and decellularized ECM (dECM) [20–23]. The applications of natural materials are limited by low mechanical properties, immunogenicity, batch-batch variability and low tunability [24]. Naturally derived hydrogels have gained attraction for use as tissue scaffolds for their biocompatibility, biodegradability, ease of photo-crosslinking and specific cell-binding sites for cell attachment, spreading and differentiation [25]. Hydrogel biomaterials include collagen, fibrin/fibrinogen, dECM, hyaluronic acid (HA), agarose, chitosan and silk.
In contrast to naturally derived bioinks, the advantages of synthetic biomaterials are that they have low immunogenicity and high tunability for physical, chemical and mechanical properties. Synthetic materials can be processed with a wide range of physical and chemical modifications using pH, temp, crosslinking methods [26]. Polymeric nanoparticles have also been studied to regulate release of cells and bioactive molecules in a spatiotemporal manner [27] and to also to modulate the mechanical and rheological properties of the inks to make them compatible [27,28]
In cardiovascular tissue engineering, bioinks and materials are used to fabricate a 3D construct and to create a cardio-inductive microenvironment with respect to physicochemical and mechanical properties. Accordingly, bioinks modulate cellular growth and phenotype. Table 1 and Table 2 [29] summarize some of the natural, synthetic and commercial biomaterials used for cardiac tissue regeneration.
Table1.
Natural Bioinks and Printing Materials used in 3D Bioprinting
| Biomaterial | Printing Method | Cell culture | Cardiac function/ cellular response | Reference |
|---|---|---|---|---|
|
| ||||
| Alginate | Pressure-assisted solid freedom fabrication method | CPCs | • Cell viability (> 95%) for 7 days, printed semi-permeable microfluidic channels support mechanical integrity and fluid transport and provided an environment conducive to cell growth and function. | 30 |
| Extrusion-based | L929 murine FBs | • Cell viability (>70%) at day 7, high-strength structure was formed with high concentration of sodium alginate. | 31 | |
| Inkjet | NIH-3T3 Murine FBs | • Cell viability (>80%) at day 4. | 32 | |
| Inkjet | Rat vascular ECs and FBs | • Cell viability (>85%) at day 21. | 33 | |
| Extrusion based | CPCs | • Cell viability (>90%) at day 4, CPSs maintained the progenitor cellular functions and ECM secretion. | 34 | |
| Extrusion based | HUVSMCs | • High proliferation up to day 10; formation of ECM observed. • Higher alginate concentration resulted in higher burst pressure, young’s modulus and tensile strength. Printed conduits maintained structural integrity and ECM deposition after 6 weeks of in vitro culture. |
35 | |
| Fibrin | Laser-based | ASCs and ECFCs | • ASC-ECFC interaction resulted in stable vascular network formation for 2 weeks. | 36 |
| Hyaluronic acid | Laser-based | HAVIC | • Cell viability (>90%) for 7 days, remodeled matrix with deposition of collagen and glycosaminoglycans. HAVIC expressed αSMA and vimentin | 37 |
| Agarose | Extrusion-based | Human mesenchymal stem cells | • Cell viability (>95%) at day 21, ECM production, increased compressive strength of the printed gels in 2 weeks | 38 |
| Inkjet-based | Rat SMCs | • Cell viability (>95%) for 30 days, matrix deposition, cells expressed actin and connexin-43 over the culture period. | 39 | |
| Decellularized matrix based bioinks | Extrusion based | Human ASCs, MSCs | • High cell viability and proliferation; ECM production in printed constructs | 40 |
| Matrigel | Inkjet-based | EPCs | • VEGF-mediated high EPC proliferation. | 41 |
Abbreviations: MI (Myocardial Infarction); ECs (Endothelial cells); SMCs (Smooth muscle cells); CMs (Cardiomyocytes); MSCs (Mesenchymal stem cells); CPSs (Cardiac Progenitor Cells); HUVSMCs (Human Vascular Smooth muscle cells); ECFCs (Endothelial colony forming cells); ASCs (Adipose derived-stem cells); HAVIC (Human Aortic Valvular Interstitial cells); αSMA (smooth muscle α-actin); EPCs (Endothelial progenitor cells); VEGF (Vascular Endothelial growth factor)
Table 2.
Synthetic and Composite/Bioactive Molecule Bioinks used in 3D Bioprinting
| Bioink | Printing Method | Cell culture | Cardiac function/ cellular response | Reference |
|---|---|---|---|---|
|
| ||||
| HA/Gel (Hyaluronic acid/gelatin) | Extrusion-based | hCMPC | • hCMPC attachment and proliferation was facilitated with cardiogenic phenotype. The printed patch maintained heart function and myocardial viability. | 42 |
| GelMa-cECM (Gelatin methacrylate- decellularized cardiac extracellular matrix hydrogel) | Extrusion-based | hCPC | • hCPC maintained >90% viability and proliferation with increased cardiogenic gene expression • Improved angiogenic potentila with improved endothelial cell tube formation • In vivo vascularization of patches over 14 days. |
43 |
| Cell Aggregate | Extrusion based | Chicken CMs and Human ECs | • After 5 days, self-assembly of bio-ink particles resulted in synchronous beating, early signs of vascularization. ECs organized into vessel-like conduits. | 44 |
| Inkjet | Human ESCs | • Cell viability (>95%) at day 5, cell aggregates of uniform size and density were formed. |
45 | |
| Ultra-Short Peptides | Extrusion based | Human MSCs | • Cell elongation observed, in vivo biocompatibility and stability | 46 |
| NovoGel | Extrusion based | HASMCs, HAECs, hDFs, | • Histological analysis reveals vascular structures with cells and collagenous ECM production and organization within the vessel wall over 21 days. | 47 |
| Extrusion based | Embryonic fibroblast cells | • No cell apoptosis at 2 weeks | 48 | |
| Extrusion based | NIH-3T3 fibroblasts | • Cell viability (>80%) post 2-week printing period. | 49 | |
Abbreviations: PEG (Polyethylene glycol); ECs (Endothelial cells); SMCs (Smooth muscle cells); CMs (Cardiomyocytes); MSCs (Mesenchymal stem cells); CPSs (Cardiac Progenitor Cells); ESCs (Embryonic stem cells); ECM (Extracellular matrix); AuNP (gold nanoparticles); sECM (semi-synthetic extracellular matrix); HAECs (Human aortic Endothelial cells); HASMCs (Human Aortic Smooth muscle cells); CMs (Cardiomyocytes); MSCs (Mesenchymal stem cells); hDFs (Human dermal fibroblasts); hCMPC (Human cardiac derived progenitor cells); hCPC (Human cardiac progenitor cell).
3. 3D Bioprinting Techniques
3D bioprinting involves rapid prototyping techniques to generate functional living constructs of 3D architecture and hierarchy with high precision, high throughput, repeatability and reproducibility [50]. It provides precise control on deposition of cells and bioactive factors to mimic native tissues to guide regeneration for patient specific treatment [50]. To mimic the native organization of the myocardium, bioprinted constructs should have anisotropic structure (heart contraction), perfusion (vascularization) and mechanical strength and electrical signal propagation. Medical imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) help to provide spatial information to direct the computer-aided design (CAD) map of 3D bioprinted tissues. The generation of cardiovascular tissues using 3D bioprinting is achieved using several techniques, including inkjet-based, extrusion-based, laser-based, and scaffold-free bioprinting (Table 3). These are described in greater detail below.
Table 3.
Advantages and Limitations of Bioprinting Techniques.
| 3D Bioprinting Technique | Advantages | Limitations | Ref. |
|---|---|---|---|
|
| |||
| IBB | • Uses thermal, electromagnetic or piezoelectric technology to deposit inkjets of “ink” (materials) • Rapid printing speeds and high resolution. • Capable to print low-viscosity biomaterials. Availability and ease of replacement of bioinks. High-cell viability and relatively low cost |
• Low material viscosity (<10 Pa▪s) and low inkjet directionality. • Lack of precision with respect to inkjet size. Requirement for low viscosity bioink. • Nozzle clogging and cellular distortion due to high-cell density. • Low mechanical strength. Inability to provide continuous stream of material. |
[109] |
| EBB | • Ability to print biomaterials with high cell densities (higher than 1× 106 cells/mL) comparable to physiological cell densities. Can produce continuous stream of material. • Can successfully print high viscosity bioinks such as polymers, clay-based substrates. |
• Low printing resolution (> 100 μm) and slow printing speeds. • Loss of cellular viability and distortion of cellular structure due to the pressure to expel the bioink. |
[110] |
| LBB | • Rapid printing speeds and ability to print biomaterials with wide range of viscosities (1–300 mPa/s). High degree of precision and resolution (1 cell/inkjet). Can successfully print high density of cells 108 /mL | • Time consuming – need to prepare reservoirs/ribbons. • Lower cellular viability compared to other methods. Loss of cells due to thermal damage. • SLA requires intense UV radiation for crosslinking process. • Requires large amount of material. • High cost. • Long post processing time and fewer materials compatible with SLA. |
[111] [112,113]] |
Abbreviations: IBB (Inkjet Based Bioprinting); EBB (Extrusion-based bioprinting); LBB (Laser-based bioprinting)
3.1. Inkjet-Based Bioprinting (IBB)
IBB uses various energy sources such as laser, thermal and piezoelectric to generate bioink inkjets for deposition of cellular material and bioactive factors on a substrate [50] (Fig. 1A). This approach has the advantages of rapid fabrication and can generate tissues with high resolution. 3D constructs can be formed with concentration gradients with controlled fluid-drop densities, shapes and sizes. Multi-jet bioprinters offer the ability to deposit different types of cells and biocomponents deposited synchronously using multiple cartridges. IBB has been shown to promote 80% cellular viability [51]. On the other hand, IBB requires bioinks with lower viscosity in a range of 3.5–20 mPa▪s, such that their mechanical properties are low.
Figure 1. 3D bioprinting techniques and the resulting bioprinted tissues.
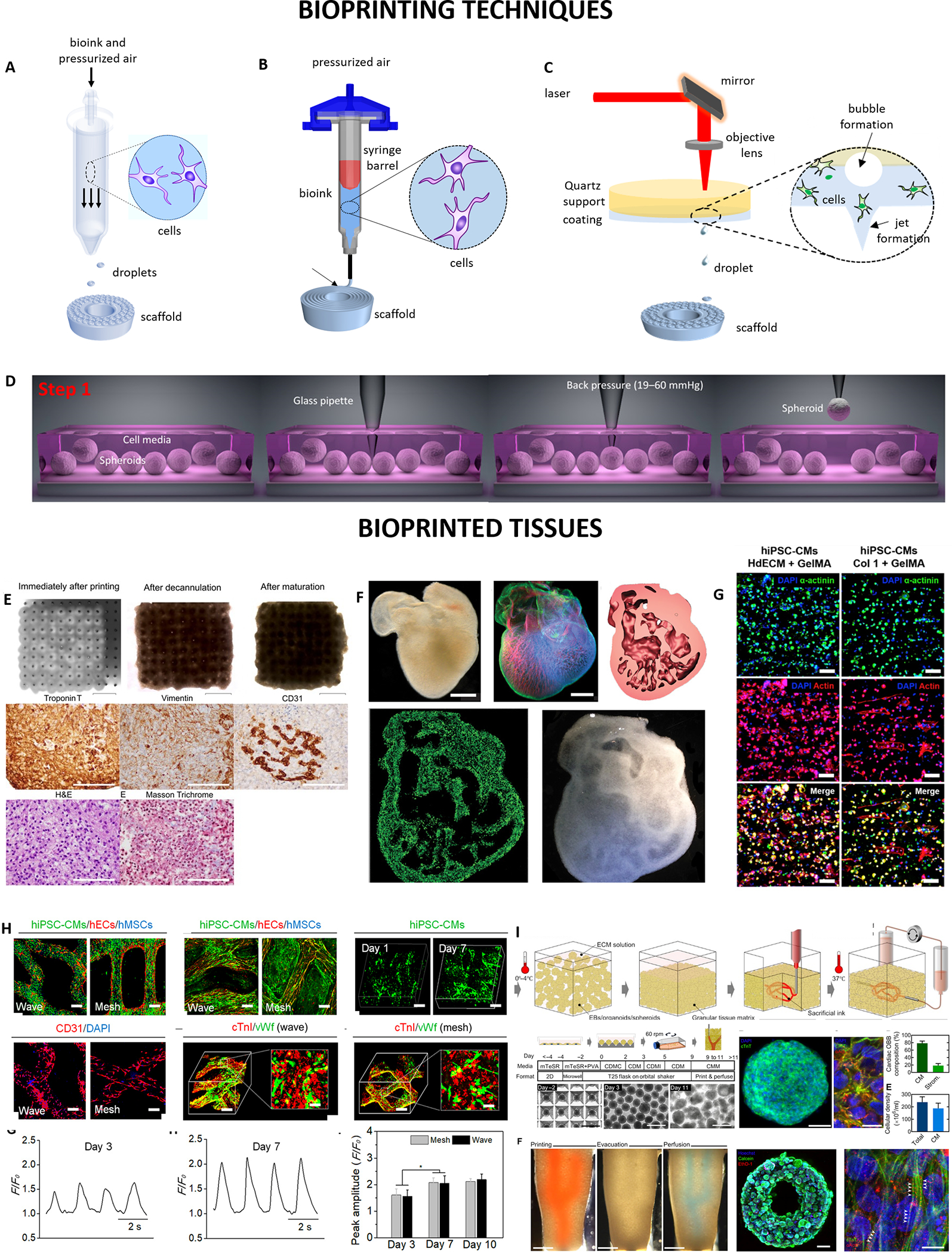
(A) Inkjet-based bioprinting, where loaded bioink is dispensed and the bioink jet breaks into small inkjets (adapted with permission from [90]), (B) Extrusion-based bioprinting, where bioink is deposited as cylindrical filaments (adapted with permission from [90], (C) Laser-based bioprinting where the bioink is transferred as jet inkjets using laser energy (adapted with permission from [90]. (D) Aspiration-based bioprinting (adapted with permission from [91]). (E) Optical images showing bioprinted cardiac patches using Kenzan technique (adapted with permission from [77]). (F) Optical and dark-field images of the 3D printed heart using FRESH method with internal structures (adapted with permission from [86]). (G) Confocal images of bioprinted dECM tissues constructs after 7 days (adapted with permission from [92]). (H) In vitro characterization of the 4D hydrogel-based cardiac patches using laser-based bioprinting (adapted with permission from [93]). (I) In vitro characterization of perfusable cardiac tissues using SWIFT (adapted with permission from [87]).
To illustrate some examples of IBB, microvascular endothelial cells (ECs) and fibrin have been bioprinted to form functional 3D microvasculature of high elastic modulus and burst pressure. The ECs proliferated and were confluent for 3 weeks of culture [51]. IBB has limitations of material viscosity, mechanical strength and cell density [52]. Low cell densities and viscosities bioinks are needed in order to diminish nozzle clogging, achieve inkjet ejection and shield from high-shear stresses that results in low structural integrity and strength.
3.2. Extrusion-Based bioprinting (EBB)
EBB uses pneumatic or mechanical induced forces to generate a 3D architecture by depositing bioinks such as cell-laden hydrogels, microcarriers, cell aggregates, decellularized matrices, microcarriers, and growth factors in a controlled manner (Fig. 1B) [53]. This technique has been most utilized for generation of cardiac tissue constructs due to the capability to deposit physiological cell densities (108-109 cells/mL) and for biocompatibility with a wide range of biomaterials and cells. High viscosities of bioinks in the range 6×107 mPa▪s could be used to ensure bioprintability and minimize cellular damage. An integrated tissue-organ printer (ITOP) has been demonstrated to for potential to fabricate mandible and calvarial bone, cartilage and skeletal muscle for clinical translation [54]. Other advantages for EBB include fast bioprinting rate, ease of procedure, wide selection of usable bioinks and relatively low cost compared to other bioprinting techniques. Some advanced features have been developed for extrusion bioprinter cartridges including temperature-controlled cartridge or stage systems, multiple nozzles and chambers direction controlled independently and coaxial nozzle systems [55]. Geometry and size of the nozzle orifice, bioprinting speed and temperature, extrusion pressure, physicochemical properties of the bioink-substrate interaction for example, rheological properties of bioink, surface tension of bioink, and wetting properties of bioprinting surface are some of the parameters that tune bioprinting precision and accuracy [56]. One of the limitations of the technique include the possibility of nozzle clogging, low cellular survival and damage to cellular function/morphology due to use of high-viscosity bioinks that are mainly used for EBB. The high viscosity materials may also impact gap junctions, contractile function and electrical conduction among bioprinted cells [57].
3.2.1. Co-Axial Bioprinting
While post-crosslinking a 3D structure necessitates a highly viscous bioink, pre-crosslinking can cause extrusion of overly gelated bioinks that expose the encapsulated cells to increased shear stresses. Recently, co-axial bioprinting has gained interest as an approach to combine various biomaterials, cells and cross-linking agents having different mechanical and biochemical properties in two distinct layers often called the shell and core. Co-axial printing of two materials with different properties can form a synergistic effect on printability, structural complexity, shape fidelity and biocompatibility. Co-axial printing can allow a stiffer material included in the core with mechanical support and relatively low strength materials located in the shell to provide microenvironment suitable for call viability and proliferation [58–60]. For example, Connell et al. developed an in situ photo-crosslinking approach using a light exposure strategy to demonstrate rapid and direct crosslinking of the bioink as it gets extruded from the nozzle obviating the need for a tubing to stabilize the filament [56]. They developed a highly stable co-axial free form extrusion system that enabled encapsulation of a liquid core within a cross-linked shell to print a human cell line Saos-2 with high viability. Coaxial bioprinting has been widely used to develop hollow or tubular structures that can mimic natural vascular networks due to its ability to deliver nutrients, oxygen and other biochemical components through the core [61–63]. Printability is an important factor in extrusion based bioprinting to develop 3D printed structures with high fidelity and precise positioning. As an example, Kim et al. developed alginate-based 3D tubular constructs with high shape fidelity using co-axial bioprinting [61].
3.2.2. Freeform Reversible Embedding of Suspended Hydrogels (FRESH) Bioprinting
One of the challenges in 3D bioprinting strategies is maintaining shape fidelity and suitability for cell-embedding, proliferation and tissue maturation. There is ongoing research on development of bioinks suitable for printability, resolution and stability to better promote cell migration, proliferation and differentiation. FRESH bioprinted cell-laden natural hydrogel approach has recently gained attention for printing low viscosity and slow polymerizing solutions with good spatial resolution [64]. The formation of functional vascular network is important for transport of nutrients to the cells especially for larger implants. Eman et. al developed a human-heart model using FRESH-printed alginate to fabricate mechanically tunable cardiac tissue construct using patient-derived magnetic resonance imaging (MRI) data sets [65]. Different strategies for in vitro vascularization have been employed such as co-culture of ECs with supporting fibroblasts (FBs) to form a delicate capillary vessel network by growth factors secreted by both cell types [66,67]. For example, Hinton et al. employed the FRESH technique for printing of cell-laden biological hydrogels with collagen, fibrinogen or alginate with an elastic modulus of less than 500kPa (Fig. 1F) [68]. This approach can fabricate highly complex 3D structures with diverse low viscosity bioinks, construct fidelity and high viability of 99.7%. Lee et al. developed a method to 3D-bioprint collagen using FRESH to engineer human heart components from capillaries to full organ [69]. They fabricated pH-driven gelation controlled a 20-μm resolution porous microstructure that enabled rapid cellular infiltration and microvascularization. he anatomical structure had mechanical strength for fabrication and perfusion of multiscale vasculature and tri-leaflet valves. Cardiac ventricles printed with human cardiomyocytes (CMs) showed synchronized contractions and directional action potential propagation. These examples highlight the advancements of FRESH 3D bioprinting for improved complexity and higher resolution than previous bioprinting approaches.
3.3. Laser-Based Bioprinting
Laser-based bioprinting (LBB) uses laser energy such as UV, visible light or near-infrared light to cross link cell-laden bioinks in a reservoir and mold high precision patterns to form 3D constructs (Fig. 1C) [70, 71]. One of the printing methods that use digital micromirror arrays to adjust the light intensity of each pixel for the bioprinting area is stereolithography (SLA). Laser light is applied in a point-by-point fashion to a liquid photosensitive substance during SLA printing to generate a solidified layer. Once the first layer has been solidified, the platform rises by a predetermined height, and a second layer is photocrosslinked. This procedure is iterated until the entire shape has been printed. SLA does not necessitate extrusion through a nozzle and is faster, more precise, and yields a greater resolution (< 100 μm) than extrusion-based printing [72]. Related to SLA bioprinting is Digital Light Processing (DLP) bioprinting technique. It is similar to SLA bioprinting with the difference in using a projector to reflect light onto photopolymerized materials [73] DLP prints especially larger objects at a higher speed than SLA does. Nevertheless, due to the limitations arising from the project area and resolution of the digital light mirrors, the printable area in DLP is less than that in SLA [74]. Varying from pico- to micro-scale properties, the resolution is impacted by a number of aspects, such as the thickness of the biological components on the film, the substrate’s wettability, their rheological features, the energy of the laser pulse, and the printing speed and structure organization. LBB can be beneficial for volumetric bioprinting, where in just a few seconds or tens of seconds, volumetric bioprinting can create whole cell-laden grafts of any size and structure with great cell survival (>85%) [75].
The advantages of this technique include the ability to generate tissue constructs with high resolution (10–100 μm) and the ability to print different biological materials with a wide viscosity range (1–2000 mPa▪s). This technique offers the ability to facilitate high CM deposition densities for it is a nozzle-free approach and has clogging or cellular damage issues due to high shear stress. However, one of the limitations of the technique is that it is only suitable for photopolymerizable bioinks. Other challenges for stereolithographic technique include long printing times, high equipment cost, low mechanical strength and distorted 3D structures and patterns use to repeated laser exposure [76].
3.4. Scaffold-Free Bioprinting
3D bioprinting techniques including inkjet-, extrusion- and laser-assisted approaches have also gained attention for bioprinting cardiac patch constructs without biomaterial/scaffolds (Fig. 1E) [77,78]. Scaffold-free 3D bioprinting of cardiac tissue has gained interest as scaffold related complications such as immunogenicity, fibrous tissue formation, biomaterial degradation and degradation product toxicity. For example, Hibino and collaborators developed “biomaterial-free” 3D bio-printed cardiac patches using aggregates of iPSC-derived CMs, FBs, and ECs forming mixed cell spheroids [78]. Cardiac patches exhibited ventricular-like action potential and uniform conduction within 3 days of printing. In vivo implantation resulted in vascularization of 3D bioprinted cardiac patches into native rat myocardium [79,80]. However, the 3D bioprinted cardiac patches were limited by low mechanical strength and electrical transmission speed.
Recent advances in development of organoids having self-assembled human embryonic stem cells and induced pluripotent stem cells (iPSCs) have displayed characteristics similar to native organs [81–82]. These organoids can serve as organ building blocks (OBBs) for biomanufacturing of organ specific tissues with needed cellular density, composition, microarchitecture and function possible with a perfusable network of vascular channels within the living matrices (Fig. 1I) [83]. Embedded 3D printing was employed by Lewis et al. who demonstrated the fabrication of a viscoelastic, sacrificial ink within acellular silicone [84] and hydrogel [85] matrices to create a 3D network of interconnected channels. Other studies have developed biopolymer and synthetic matrices that exhibit a viscoplastic response for patterning of complex 3D architectures [86]. However, their methods have only been successful to construct acellular or sparsely cellular matrices. For example, Skyler-Scott et. al developed a biomanufacturing method based sacrificial writing into functional tissue (SWIFT) made from a living densely cellular iPSC derived OBB matrix as embryoid bodies (EBs), organoids or multicellular spheroids patterned via 3D embedded bioprinting to fabricate perfusable organ specific autologous tissues with high cell density and desired functionality [87]. Additionally, Kolesky et al. developed a method for 3D bioprinting thick (>1 cm) heterogenous cell-laden long-term (> 6 weeks) perfusable vascularized tissues by co-printing multiple inks composed of human mesenchymal stem cells (hMSCs) and human neonatal dermal fibroblasts (hNDFs) with an ECM and embedded vasculature customized and lined with human umbilical vein ECs (HUVECs) [88]. These thick vascularized tissues perfused with growth factors facilitated in situ differentiation of hMSCs to osteogenic lineage tissues containing a pervasive, perfusable and endothelialized vascular network. This 3D tissue manufacturing platform holds great potential for recapitulating physiologically relevant complex microenvironments for both ex vivo and in vivo human tissue generation.
In another example, Arai and collaborators developed scaffold-free cardiac tubular constructs by placing spheroids optimized ratio (50:25:25) of iPSC-derived CMs, HUVECs and normal human dermal fibroblasts (NHDFs) on a needle array [89]. The engineered cardiac tubular constructs show cellular organization and beat rate similar to that of native cardiac tissue in vivo. This study was successful in generation of a functional scaffold-free cardiac tubular construct. One of the limitations of the study was shrinkage of the constructs after removal from the needle array because of cell-cell interactions, cell-extracellular interactions, and surface tension of spheroids. Another limitation was the use of HUVECs, which do not share the same tissue-relevant endothelial population of the myocardium. Despite the limitations, scaffold-free bioprinting has advantages of high cell density and cell-cell interaction characteristics similar to the native cardiac tissue microenvironment compared to 3D bioprinting using scaffolds.
3.5. Other Bioprinting Techniques With Translational Relevance
3.5.1. Aspiration-Assisted Bioprinting
Aspiration-assisted bioprinting (ABB) is a hybrid bioprinting method for bioprinting cellular aggregates (i.e., tissue spheroids and honeycombs) and organoids with dimensions between 80 and 800 μm into or onto hydrogels for both scaffold-free and scaffold-based applications (Fig. 1D) [94]. AAB rely on back-pressure to pick spheroids from the cell media and lift to the air then it bioprint into functional hydrogel (fibrin, collagen, GelMA) or onto sacrificial hydrogels such as alginate and agarose in higher positional precision (~11) and accuracy (~15%) with respect to tissue size. It is a unique platform to study effects of distance on angiogenic sprouting, paracrine signaling, tissue-tissue and tissue-material interactions. Various applications of AAB have been demonstrated including articular cartilage [95], bone [96], and osteochondral tissue [97] biofabrication in a scaffold-free manner. Recently, Ayan et al. demonstrated combination of AAB and FRESH methods to fabricate freeform tissues in self-healing hydrogels using tissue spheroids [98]. With this technique cardiac organoids can be bioprinted precisely (~34% with respect to the size of organoids) to fabricate cardiac patches.
3.5.2. 4D Bioprinting
4D bioprinting involves the creation of an initial 3D bioprinted product that morphs into its final form “over time” in response to external stimuli [99,100]. 4D bioprinting allows for dynamic, structural and cellular changes of a tissue overtime, adding a fourth dimension of “time” to the static nature of 3D bioprinting. An and collaborators proposed two approaches to define 4D bioprinting. The first one is the folding of tissue into a desired shape, driven by a stimulus. The second is the self-assembly of the tissue. For example, Kirillova and collaborators fabricated hollow self-folding tubes (comparable to small-diameter blood-vessels) using a shape-morphing hydrogel made of both alginate and hyaluronic acid) [101]. Cell viability was maintained for 7 days of culture. Apsite and collaborators performed spontaneous folding of multilayer scaffolds made of thermo-responsive polymers at different temperatures in an aqueous environment to form a tube with distinct layers [102]. The study also demonstrated the improvement in cell viability and adhesion by adding collagen. Self-healing hydrogels have also shown to improve the life of a functional material and exhibit properties similar to native human tissue. CMs cultured in a shape-forming hydrogel have been shown to form a three-dimensional structure over time. Neonatal CMs encapsulated in fibrin hydrogel and cultured for 2 weeks under static conditions were shown to remodel the construct and generate contractile force under electrical pacing conditions [103] (Fig. 1H). With advances in CM viability on bioprinted stimuli-responsive hydrogels, this approach can facilitate spatial arrangement of cells and internal structure of engineered myocardium.The second approach is the stimulus-driven self-assembly of tissues that can induce tissue structural and cellular changes over time. For example, cell deposition by printed microinkjets that can conform to another pattern upon stimulation [104]. Chemical factors have been shown to stimulate scaffold-free 3D bioprinted cardiac patches to facilitate better tissue organization and maturation [105,106].
Recent advancements in 4D bioprinting have been made for cardiac regeneration. Miao and collaborators developed a 4D hierarchical micropattern by employing a photolithographic-stereolithographic-tandem strategy (PSTS) for regulating human bone marrow mesenchymal stem cell (hMSC) cardiomyogenic behavior [107]. The technique was validated by printing well-defined cand consistent micro-surface featured scaffolds and growth of hMSC with highly aligned micropatterns promoting cardiomyogenesis. The advanced dynamic 4D shape change and features seems promising to provide seamless integration with the damaged tissues and organs. This study developed and demonstrated a proof-of-concept 4D micropatterned patch to have the potential to facilitate cardiac regeneration. Some of the advantages and limitations of commonly employed 3D bioprinting techniques are highlighted in Table 3 [108].
4. Translational Applications of Stem-Cell-Laden 3D Printed Tissue Constructs
In order to develop advanced bioprinted cardiovascular devices and tissue models using synthetic or biological components, incorporation of biomimetic or physiological characteristics into the structures and functionalities is required for a successful cardiovascular tissue regeneration model. Bioprinting research for cardiovascular tissue regeneration largely focuses on myocardium, vascular grafts and heart valves.
4.1. Bioprinted Myocardium
One of the key challenges in tissue reengineering and regenerative medicine is bioprinting of a functional myocardium. Myocardial infarction (MI) leads to cellular death, matrix remodeling, degradation of fibrillar collagen network and accumulation of fibrotic scar tissue. The scar tissue cannot conduct electrical and mechanical stimuli resulting in reduced pumping efficiency of the heart. This leads to disruption of ventricular wall, ventricular dilation resulting in congestive heart failure and myocardial rupture [114,115].
An approach for cardiac repair is cellular therapy which involves implanting cells at the site of damaged tissue [116]. The effectiveness of cellular therapy depends on cellular viability post implantation and integration into the heart tissue that enhances cardiac output. There are several constraints that limit the application of cellular therapy, including availability of oxygen [117]. Oxygen transport is facilitated by either: i) limiting the thickness of the cell construct (<200 μm) [118], and/or, ii) facilitating oxygen diffusion or convective mass transport all through the construct by integrating open pores. Limiting construct thickness requires multiple construct implantations in vivo and repeated surgeries to obtain vascularization and optimum clinical; outcomes [119]. Current therapies are pursued by fabricating and integrating thicker multi-layered tissues pre-implantation. Constructs of only 100 μm thickness have shown to exhibit low cellular survival [120]. Therefore, limiting cell construct thickness is not a viable strategy. The approach to introduce pores into large scaffolds allows for very low cellular densities cultured compared to in vivo tissue, that is not enough for cardiac tissue repair [121]. Increasing cell densities is also not feasible since its challenging to meet metabolic requirements of tissues for vascularization at very high cell densities, increasing the likelihood of cell death. Furthermore, due to limited proliferation potential of CMs, seeding high cell densities require stem cells culture in addition to CMs.
There has been interest in 3D bioprinting to overcome the limitations of cellular therapy for fabrication of thick 3D constructs of defined geometry and complexity and well-aligned cellular network. Ventricular function lost in myocardial infarction can be regained by augmenting or replacing the necrotic tissue with a tissue engineered “heart patch” [169, 115, 122]. Bioengineered functional cardiac tissue composed of primary CMs has been studied extensively for myocardial regeneration potential and in vitro tissue remodeling. One of the challenges in designing a functional cardiac tissue is well-defined cell alignment and contraction [123]. The ideal design requirements of cardiac patches are that they should be electrically conductive, mechanically robust and elastic and pre-vascularized for functional integration into organ architecture resulting in improved systolic and diastolic function. Some of the in vitro and in vivo studies on 3D bioprinting of a functional myocardium have been described here:
4.1.1. 3D Printing of Endothelialized Myocardium
Researchers have made advancements in 3D printing applications to generate an endothelialized myocardium. Zhang and collaborators bioprinted a scaffold using an EC-laden bioink with sodium alginate and GelMA seeded with CMs to develop an endothelialized myocardium [124]. A rigid three-dimensional structure was formed due to crosslinking of alginate with calcium ions followed by UV of the GelMA hydrogel. ECs migrated towards periphery of the bioprinted scaffold fibers and formed a confluent layer. CMs seeded into the scaffold with controlled anisotropy formed an aligned myocardium with contractions in a synchronous manner. This developed a functional myocardium with an interlacing endothelium and well-aligned CMs. Even though this approach was designed for use in a heart-on-a-chip platform, the study is a step towards fabrication of functional tissues with biomimetic function and structure. Jakeb and collaborators performed a similar study to reveal the potential of ECs to promote vascularization in 3D bioprinted cardiac constructs [125, 126]. These examples illustrate the feasibility of bioprinting endothelialized myocardium.
4.1.2. Regenerative potential of hMSCs in Myocardium
Besides using CMs within 3D bioprinted constructs, stem cells such as hMSCs have also been tested. Gaebel et al. developed an in vivo model of 3D bioprinted myocardium implanted in animal hearts with enhanced vascularization and therapeutic effects. The group developed a LIFT 3D bioprinted myocardium seeded with hMSCs and HUVECs in a systemic pattern [127]. At 10 weeks after infarction, primitive vascular networks, high density capillary networks, host vasculature integration and improved cardiac function were observed. This study was performed based on the proposed potential of hMSCs to improve angiogenesis in postinfarcted myocardium facilitating cell repair and regeneration.
4.1.3. 3D Printed Cardiac Scaffolds Composed of Stem Cell-Derived CMs
With the advances in CM differentiation from iPSCs, many bioprinted constructs utilize iPSC-derived CMs in bioprinting of cardiovascular tissues. For example, Ong et al. used Kenzan technique to bioprint iPSC-derived CMs, ECs and human fibroblasts seeded cardiac patches [128]. In vitro culture of the constructs displayed electrical conduction and ventricular-like action potentials of the whole construct. The patches implanted in nude rats demonstrated vascularization and engraftment into the native rat myocardium, which suggests clinical translation potential. In another example, Maiullari et al. constructed an alginate/PEG-fibrinogen printed cardiac tissue with HUVECs and iPSC-derived CMs [129]. The tissue displayed formation of blood vessels and well-defined cell alignment. Furthermore, implantation of the tissues into non-obese diabetic SCID mice demonstrated integration with the host vasculature signifying the potential and functionality of the bio-printed cardiac tissues. Gaetani et al. studied the functionality of a 3D-bioprinted patch composed of human cardiac-derived progenitor cells (hCMPCs) in a hyaluronic acid/gelatin matrix in post-MI murine hearts. The hCMPCs retained the cellular viability, proliferation and differentiation potential to form CM-like cells [130]. The 3D-printed patch transplanted in the murine model of MI supported in vivo survival, proliferation, maturation and engraftment of HCMPCs and increase in cardiac and vascular differentiation markers for over 4 weeks, suggesting viability of the 3D printed cardiac patches. Additionally, Gao et al. fabricated ECM scaffold using laser-based multiphoton-excited (MPE) 3D printing. Human iPSC differentiated SMCs, ECs and CMs were used to develop the cardiac patch. After a 1- and 7-day seeding period, the cardiac patch displayed enhanced electrophysical properties and gene expression for contractile behavior. After the 4-week implantation period in murine hearts, cardiac function, infarction size and vascular density were significantly superior in cell-laden cardiac patches compared to cell-free scaffolds [131]. Together these examples illustrate several 3D bioprinting strategies for the engineering and vascularization of cardiac tissue.
4.2. Cardiac Valves
Valvular diseases such as stenosis and calcification are significant causes of heart failure. Currently, there are two clinical treatments for heart valve replacement surgery, namely mechanical heart valve and biological heart valve implantation [132]. Traditional mechanical or bio-prosthetic conduits of valve replacements have limitations including need for anticoagulation, along with limited durability and non-physiological characteristics [133,134]. Therefore, advancements in 3D valve bioprinting have been explored to engineer valves with the design requirements of having structural, physicochemical, biological and functional properties that better reflect that of native cardiac valves. Tissue engineered heart valves hold the potential of using autologous cells, having improved hemocompatibility of the replacement valve, obviating anti-coagulation therapy, and improving the integration and repair of the valve with native tissue [132,135,136].
4.2.1. 3D Printed Constructs with Spatial Heterogeneity of Cardiac Valve
3D bioprinting holds the potential to enhance clinical success of tissue engineered heart valves by fabricating constructs with patient-specific valve geometry, spatial heterogeneity of valve mechanical characteristics, and spatial heterogeneity of the encapsulated cells. For example, Butcher et.al. fabricated an artificial valve by extrusion-based 3D printing of two types of photo-crosslinkable hydrogels, a rigid one (75 kPa) for the root and soft (5kPa) for the leaflet [137]. This method allowed 3D printing of an artificial valve with geometries ranging between 12 mm-22mm in diameter. In addition, printing two materials simultaneously with distinct mechanical properties to mimic the thickness of the root and leaflets of native heart valves. The group seeded porcine aortic valve interstitial cells (PAVIC) into the 3D printed heart valves and demonstrated cell viability up to 3 weeks. In another study, the Butcher group fabricated heart valve and encapsulated the root and leaflet regions with two types of cells, aortic root sinus smooth muscle cells (SMCs) and aortic valve leaflet interstitial cells (VICs) respectively [138]. The cells exhibited mechanicals properties of the alginate/gelatin mixture for over a week compared to weak mechanical strength of acellular heart valve. In a recent study, Butcher and collaborators seeded human aortic valvular interstitial cells (HAVIC) on methacrylated hydrogels (mixture of gelatin and hyaluronic acid) [139] and demonstrated that the HAVIC cells deposited ECM and remodeled the hydrogel with cellular phenotypic modulation based on the printed hydrogel stiffness. These studies illustrate the potential of 3D bioprinting to generate constructs with spatial heterogeneity of the mechanical properties of the heart valves and modulation of different types of seeded cellular behavior.
4.2.2. 3D Printed Cell-Laden Hydrogels for Generating the Aortic Valve
Besides engineering values with spatial heterogeneity, aortic valves have also been bioprinted with increasingly complex cellular compositions. For example, Lockaday et al. developed a heterogenous aortic valve scaffold using 3D photocrosslink-printing of poly (ethylene glycol)-diaacrylate (PEG-DA)/alginate hydrogels seeded with porcine aortic valve interstitial cells (PAVIC) [140]. The scaffolds had significantly high elastic modulus, shape fidelity and cellular growth and viability. In another study, Duan et al. developed 3D printed living alginate/gelatin hydrogel conduits. In vitro co-encapsulation of aortic root sinus SMCs and aortic VICs showed growth and cellular viability within 3D printed aortic valve conduits over 10 days [141]. SMCs showed contractile morphology and expression of elevated α-smooth muscle actin and VICs expressed elevated levels of protein vimentin. In another study, Duan et al. developed 3D printed hybrid hydrogels using methacrylated hyaluronic acid (Me-HA) and methacrylated gelatin (Me-Gel) [142]. The heart valve conduits encapsulating HAVICs. Optimized concentration of Me-Gel and Me-HA resulted in fabrication of a trileaflet valve with high cellular viability of HAVIC and remodeling of the initial matrix with collagen and glycosaminoglycans. This study of generating anatomically accurate bioprinted design of cardiac valves is a big step towards understanding cell-valve interactions and generation of de novo living valve replacements. In another study, van der Valk et al. engineered a three-dimensional printed calcified aortic valve disease model using methacrylated gelatin (GelMA)/methacrylated hyaluronic acid (HAMA) hydrogels encapsulated with human VIC (valvular interstitial cells) [143]. This study was successful to mimic the ECM of native tissue and maintain VIC quiescence under basal conditions. The research was helpful to understand the effect of nano and micro-calcification and pathological differentiation of naïve VIC driven by layer specific mechanical properties similar to those of disease probe fibrosa layer of the human aortic valve. Together, these studies highlight the application of 3D printing for engineering cardiac valves with increasingly complex geometry, suitability stiffness heterogeneity achieved by using multiple print heads and optimal cellular growth and function for a heart valve that usually do not require a complex and highly ordered vascular network.
With respect to limitations of bioprinted valves, there are still major areas of concern when printing cardiovascular structures such as cardiac valves such as limited availability of printing materials for representation of vessel-like properties [144] and transparent nature of the materials for visual inspection of internal structures [145]. 3D printing has limitations in using the imaging data from ultrasound that is used as the primary imaging technique in cardiac imaging. Precise representation of valve leaflets and chordae tendinae requires high spatial and temporal resolutions and any abnormalities in these structures are associated with significant hemodynamic functional consequences. Other limiting factors in applications of 3D printing in patient care include the high cost of printers, software, and printing materials, along with the time-consuming process of building structures with several layers. The post-processing is labor-intensive and requires skillful and experienced imagers.
4.2.3. Cardiac Grafts/Vasculature
Despite improvements in therapies, coronary artery disease affects 16 million adults annually in the US and accounts for one out of three deaths in the US [146]. Based on the coronary artery disease stage, different treatments are available including lifestyle changes, coronary angioplasty and coronary artery bypass grafting (CABG). For vessels with complex multiple lesions, CABG is the preferred choice that involves bypassing blood flow around a severely narrowed or blocked artery using autologous veins or vessels such as internal thoracic arteries, radial arteries and saphenous veins [147,148]. While CABG has been successful in improving the survival rate of patients with coronary artery disease, approx. 30% of the population especially elderly and patients with compromised immune system due to metabolic disorders such as diabetes are not recommended the surgery due to unavailability of healthy autologous vessels and post-surgery complications such as poor patency rates, accidental graft damage and morbidity at the donor site. Therefore, synthetic polymeric conduits gained interest for bypass graft application The most popular polymeric grafts used are expanded polytetrafluoroethylene (e-PTFE), woven polyethylene terephthalate (PET), also known as Dacron [149–152] and polyurethanes [153]. However, low patency rates of these synthetic polymeric conduits for small diameter vessels (<6mm in diameter) bypass have led to researchers moved their focus to exploring more compliant materials, pharmaceutical drug loaded conduits and tissue engineered constructs [154].
The ideal design criteria of 3D printing of vessels consist of having physiological, anatomical and biochemical characteristics to native analogues. [155–157]. One of the critical parameters that define the success of a engineered vascular conduit is formation of an endothelialized layer over the lumen [158–161]. Vascular tissue engineering has gained interest to develop coronary bypass grafts that promote endothelialization, render non-thrombogenic surface properties and exhibit patient specific geometry and comparable mechanical properties to the native blood vessel.
Current research focuses on in vitro generation of models to study endothelialization and microvascular network formation to facilitate angiogenesis and transport of nutrients and oxygen to the engineered tissue. Miller et al. developed a biocompatible sacrificial carbohydrate based material and printed 3D filament networks in engineered tissues using a custom-designed RepRap Mendel (Extrusion based) 3D printer [162]. Using this approach, the group patterned vascular channels in natural and synthetic materials and seeded living cells. They demonstrated three sections of vascularized solid tissues (i.e lumen, ECs, and interstitial zone residing cells and matrix). In another study, Li et al. developed a hybrid cell/hydrogel vascular network and seeded adipose derived stem cells (ADSCs) combined with alginate/gelatin/fibrinogen network [163]. Endothelial growth factor was provided to induce differentiation of ADSCs into endothelial-like cells. ADSCs at the vascular network-like periphery exhibited EC-like properties. In another example, Cui et. al. fabricated a microvasculature using thermal-inkjet printing and simultaneously seeded human microvascular ECs (HMVECs) and fibrin [164]. The HMVECs were shown to align inside the channels and proliferated to form a confluent layer in a week followed by formation of microvasculature. Additionally, Zhang et al. bioprinted perfusable vascular conduits of optimal mechanical properties with encapsulated human umbilical vein smooth muscle cells (HUVSMCs) [165]. Higher cell viability and ECM deposition was observed on both luminal and peripheral surfaces.
To generate vascularized thick tissues with multilevel or multi-branch channel architectures, pre-vascularized cell-layer blood vessels could be biofabricated by using cell-laden dECM as a matrix material and Pluronic F127 as a sacrificial material [166]. The construct showed improved stability and high biocompatibility. A supporting scaffold (SE1700) with a double-layer circular structure was 3D bioprinted to provide mechanical strength and structural integrity prior to the formation of the media, intima, and adventitia. Such constructed small-diameter blood vessels can be used for the in vitro pathological models such as a thrombus model to understand the effect of damaged endothelial layer on migration of fibroblasts, study of vascular cell biology mechanisms, and pharmaceutical therapeutics.
3D printing has shown potential for generation of tubular structures for CABG replacement. However, there are certain limitations in translation of a microvascular 3D printed structure as an ideal artificial coronary bypass graft. First, it is difficult to print a construct seeded with multiple cell types with distinct mechanics and functions to mimic the three-dimensional multilayered structure of a blood vessel. Second, seeding the printed structures with multiple cell types on a microscale level of a few millimeters in diameter is difficult. Third, clinical translation of these studies is also limited due to the challenge of replicating the hierarchy and multi-layered architecture of the vessels with cellular components and functionality. Transport of nutrients and oxygen within the coronary graft requires fabrication of sophisticated microvascular and macrovascular structures to mimic the native vasa vasorum (capillaries in the vessel wall) of native coronary arteries. Translational applications of 3D printing techniques for generation of a functional myocardium, cardiac valves and vasculature are presented in Table 4 and Fig. 1G.
Table 4.
Translational Applications of 3D Engineered Myocardium
| Tissue | Scaffold/Bioink/Bioprinting technique | Seeded cell culture | Cardiac function/ response | Ref |
|---|---|---|---|---|
| Myocardium | EC laden-sodium alginate and GelMA/Co-axial bioprinting | CMs | • ECs migrated towards periphery of the bioprinted scaffold fibers and formed a confluent layer. • CMs seeded into the scaffold with controlled anisotropy formed an aligned myocardium with contractions in a synchronous manner. This developed a functional myocardium with an interlacing endothelium and well-aligned CMs. |
[124] |
| Myocardium | Polyurethane polyester urea (PEUU)/Laser-Induced-Forward Transfer (LIFT) cell printing | HMSCs and HUVEC | • 10 weeks after infarction, primitive vascular networks, high density capillary networks, host vasculature integration and improved cardiac function were observed. | [127] |
| Myocardium | Biomaterial free bioprinting | Mixed cell spheroids of iPSC-CMs, human adult ventricular fibroblasts and HUVEC | • In vitro culture of the constructs displayed electrical conduction and ventricular-like action potentials of the construct. High cell density, functional cell contacts and spontaneously beating cardiac tissue was produced. | [128] |
| Myocardium | Alginate/PEG-fibrinogen/Extrusion based bioprinting | HUVECs and iPSCs-CMs | • The tissue displayed formation of blood vessels and well-defined cell alignment. Implantation of the tissues into non-obese diabetic SCID mice demonstrated integration with the host vasculature | [129] |
| Myocardium | Hyaluronic acid-gelatin matrix/Extrusion based bioprinting | HCMPC | • HCMPCs retained the cellular viability, proliferation and differentiation potential [6]. The 3D-printed patch transplanted in the murine model of myocardium infarction supported the in vivo survival, proliferation, maturation and engraftment of HCMPCs and increase in cardiac and vascular differentiation markers over 4 weeks | [130] |
| Myocardium | Gelatin hydrogel/Inkjet based bioprinting | hMSC and CM | • hMSC displayed well-defined F-actin anisotropy and elongated morphology, Well-aligned CMs and synchronized beating was observed depicting the potential of tissue to promote CM growth and contractility. | [131] |
| Myocardium | ECM scaffold/Biomaterial free bioprinting | iPSC-derived SMCs, ECs and CMs | • After a 4-week implantation period in the murine heart, cardiac function, infarction size and vascular density were significantly superior in cell-laden cardiac patches compared to cell-free scaffolds | [132] |
| Myocardium | Collagen-fibrinogen Matrigel/Extrusion-based bioprinting | C2C12 myoblasts and MC3T3 fibroblasts | • A high-density cellular network was formed with a robust mechanical structure. | [133] |
| Myocardium | Fibrin based hydrogel and poly-caprolactone (PCL) polymeric frame/Inkjet bioprinting | CMs | • These constructs exhibited cellular organization, cardiac contractile function, scalability and uniform contraction 6 weeks post MI in vivo. MI-induced fibrosis was also reduced significantly and led to increased heart function. | [134] |
| Myocardium | Methacrylated collagen (MeCol) and alginate matrix/Laser based bioprinting | HCAECs | • In vitro culture of 10 days resulted in HCAEC migration, proliferation and differentiation and lumen-like formation. The carbon nanotube (CNTs) 3D printed constructs exhibited higher viscoelastic behavior, stiffness, electrical conductivity. | [135] |
| Myocardium | dECM scaffold/Inkjet based bioprinting | iPSC-CM | • The tissue construct showed an upregulation of proteins expression of typeI/IV collagen, fibronectin, glycosaminoglycans and myosin regulatory light chain 2 (associated with actin filaments). CMs exhibited well-defined alignment and contraction: both features result in generation of directional force. | [136] |
| Myocardium | Decellularized human skin (dhUsK)/Extrusion based bioprinting | hCPCs | • hPSCs exhibited cellular engraftment and organization similar to native cardiac tissue and upregulated expression of markers for cardiac myocytes such as type I/III/IV collagen, elastin, glycosaminoglycans and connexin-43. | [137] |
| Myocardium | GelMA (methacrylated gelatin) hydrogel/Extrusion-based bioprinting | hESC-CMs | • The cells displayed a contractile and elongated morphology. This technique allows for modulating scaffold stiffness, cardiac tissue like contractility and orientation. | [138] |
| Myocardium | Decellularized ECM/Inkjet based bioprinting | Rat Myoblasts cells | • Increased expression of cardiac specific genes (Myh6 and Actn 1) and cardiac myosin heavy chain (β-MHC) compared to collagen constructs. | [139] |
| Cardiac Valves | Alginate-gelatin hydrogel/Inkjet bioprinting | Aortic root sinus SMCs and aortic valve leaflet interstitial cells (VIC) | • Cellular growth and viability within the 3D printed constructs maintained upto 10 days. SMCs showed contractile morphology and expression of elevated alpha-smooth muscle actin and VICs expressed elevated levels of protein vimentin. | [141] |
| Cardiac valves | Methacrylated hyaluronic acid(Me-HA) and methacrylated gelatin (Me-Gel)/Extrusion based bioprinting | HAVIC | • High cellular viability of HAVIC and remodeling of the initial matrix with collagen and glycosaminoglycans was observed. | [142] |
| Cardiac Valves | Methacrylated gelatin (GelMA)/methacrylated hyaluronic acid (HAMA) hydrogels/Extrusion based bioprinting | Human VIC | • Successful recapitulation of ECM of native tissue and VIC quiescence was maintained under basal conditions | [143] |
| Cardiac Valves | poly (ethylene glycol)-diacarylate (PEG-DA)/alginate hydrogels/Laser based bioprinting | PAVIC | • The scaffolds had significantly high elastic modulus, shape fidelity and cellular growth and viability. | [140] |
| Cardiac Valves | Photo-crosslinkable hydrogels, a rigid one (75 kPa) for the root and soft (5kPa) for the leaflet/Laser based bioprinting | PAVIC | • Cellular viability up to 3 weeks. | [137] |
| Cardiac Valves | Photo-crosslinkable hydrogels, a rigid one (75 kPa) for the root and soft (5kPa) for the leaflet/Laser based bioprinting | Aortic root sinus SMCs and aortic VICs respectively | • The cells exhibited mechanicals properties of the alginate/gelatin mixture for over a week compared to weak mechanical strength of acellular heart valve | [138] |
| Cardiac Valves | Methacrylated hydrogels (mixture of gelatin and hyaluronic acid)/Inkjet based bioprinting | HAVIC | • HAVIC cells deposited ECM and remodeled the hydrogel with cellular phenotypic modulation based on the printed hydrogel stiffness | [139] |
| Vascular Grafts | Hybrid cell/hydrogel/Inkjet based bioprinting | ADSCs | • ADSCs differentiated into endothelial like cells at the vascular network periphery. | [163] |
| Vascular Grafts | Micro-sized fibrin fiber/Inkjet based bioprinting | HMVECs | • HMVECs aligned inside the channels and proliferated to form a confluent layer in a week followed by formation of microvasculature. | [164] |
| Vascular Grafts | Sodium alginate hydrogel/Extrusion based bioprinting | HUVSMCs | • Higher cell viability and ECM deposition was observed on both luminal and peripheral surfaces. | [165] |
| Vascular Grafts | Pluronic F 127 & SE 1700scaffold/Inkjet based bioprinting | HUVECs, HA-VSMCs, HDF-n | • The printed scaffold exhibited an elastic modulus comparable to native aorta. The decellularized scaffold had a high cell viability and structural integrity. | [166] |
Abbreviations: MI (Myocardial Infarction); ECs (Endothelial cells); SMCs (Smooth muscle cells); CMs (Cardiomyocytes); hMSCs (human Mesenchymal stem cells); CPCs (Cardiac Progenitor Cells); HUVSMCs (Human Vascular Smooth muscle cells); ECFCs (Endothelial colony forming cells); ASCs (Adipose derived-stem cells); HAVIC (Human Aortic Valvular Interstitial cells); EPCs (Endothelial progenitor cells); VEGF (Vascular Endothelial growth factor); HUVEC (Human Vascular Endothelial cells); iPSC-CMs (induced pluripotent stem cell derived cardiomyocytes); hCMPCs (human cardiac-derived progenitor cells); HCAEC (Human coronary artery endothelial cells); hCPCs (human cardiac progenitor cells); hESC-CMs (Human embryonic stem cell derived cardiomyocytes); hVIC (human valvular interstitial cells); HUVSMCs (human umbilical vein smooth muscle cells); PAVIC (porcine aortic valve interstitial cells); ADSCs (adipose derived stem cells); HMVECs (human microvascular endothelial cells); HUVECs (Human umbilical vein endothelial cells); HA-VSMCs (Human aortic vascular smooth muscle cells); HDF-N (Human dermal fibroblasts-neonatal)
5. Bioprinting for In Vitro Modeling
Bioprinting technology has also paved the way for more advanced in vitro model development. In recent years, there have been several major advances in bioprinted constructs for in vitro disease modeling and organ scale printing for modeling of patient specific cardiac anatomy.
5.1. Disease Modeling
3D bioprinted microtissues are a promising platform for generating personalized cardiac disease models. Myocardial fibrosis in particular is a promising target, as nearly all forms of cardiac disease involve some form of fibrosis [167,168]. Althoughthe use of bioprinted constructs for disease modeling is still in its infancy, there have been advances in this application area, particularly involving modulation of construct mechanics to simulate fibrosis-prone tissue. For example, Shin and colleagues created a composite bioink enabling tunable construct stiffness from 13.4–89kPa, spanning the range from normal (5–15kPa) to fibrotic (80–100kPa) cardiac tissue [169]. The stiffness of the bioprinted construct was controlled by varying the amount of PEGDA in the bioink mixture. Despite limited cell spreading or invasion in the highest stiffness condition, stromal cells, fibroblasts, and iPSC-derived CMs were maintained in the device with high viability for up to 7 days. Langer and colleagues also used a multicomponent gel system to mimic the layer-specific mechanical properties of a human heart valve. A combination of gelatin methacrylate and hyaluronic methacrylate was used to produce gel stiffnesses of 15–37kPa, and multi-layer leaflet models showed selective microcalcification in the stiffest and most disease prone fibrosa layer [170]. These examples highlight the application of 3D bioprinting for cardiac disease modeling.
5.2. Tissue and Organ Models
Bioprinting has also been used to fabricate macroscale tissues models of patient anatomy [171]. This typically begins with clinical imaging (CT or MRI) to generate a 3D reconstruction of the area of interest. This 3D topography file is then used as a blueprint in the bioprinting software to reconstruct tissue geometry using single or multiple bioinks. This has been most successfully executed using embedded bioprinting in a support bath. Embedded bioprinting was used to generate anatomy-matched, full-thickness cardiac patches using reprogrammed iPSCs and ECM-based bioinks derived from the patient’s omentum tissue.[172]. FRESH and SWIFT bioprinting have also been used to bioprinting large scale cardiac models. SWIFT was used to bioprint anatomically-correct vasculature (left anterior descending coronary artery) in a model of a section of patient myocardium [87], while FRESH was used to print a neonatal scale human heart using a collagen ink [173]. Additionally, a novel Hyprinter has been developed to engineer a cell laden hydrogel based major vessel with a co-axially polymer shell to enable surgical anastomosis between the printed cell laden hydrogel based major vessel and the host major vessel during implantation [174]. A dual hydrogel system was also bioprinted for creating a vascular bed, comprising a relatively slow degradable hydrogel for a long-term perfusion and a relatively fast degradable hydrogel for rapid capillary network formation [175]. These examples highlight the generation of tissue and organ models using 3D bioprinting.
6. Challenges, Perspectives and Conclusions
6.1. Challenges and Opportunities in 3D Bioprinting for Cardiac Regeneration
3D bioprinted constructs for the myocardium, cardiac valves and vasculature have been successfully developed with structure and functional properties that better mimic to native analogues. However, there are several challenges that need to be addressed for the design of 3D bioprinted analogues with complex and hierarchical microarchitecture and biological functionality. Achieving physiological distribution and confluency of human-derived cardiovascular cells is difficult. Sourcing of multiple human cardiac cells using iPSCs remains a limitation due to their immaturity and potential safety issues. The potential immunogenicity of non-autologous cell sources is also a concern. Advancing 3D bioprinting also requires multiple bioinks that are biocompatible and have mechanical strength to ensure that cellular viability, biological function and healthy phenotype are maintained. 3D bioprinting with optimal bioprinting speed and resolution is important. During scaffold delivery, cells could be exposed to unavoidable effects of vibrational energy and shearing forces. Immunogenicity of the scaffold is a concern as well and more research is warranted to study the effects of by-products released as a result of short-term and long-term scaffold breakdown. Another challenge is to design perfusable vasculature with thick constructs for circulation of adequate oxygen and nutrients in physiological shear stress conditions for effective functional and biomechanical integration with the host vasculature [174], as well as allowing for surgical anastomosis for connecting major vessel during implantation for blood reperfusion [175]. It is also essential for 3D printed constructs to possess, good tensile strength to withstand forces of myocardial contractions, structural stability, controlled degradability, physiological contraction and cellular viability and proliferation for long culture periods to ensure cellular confluence, ECM deposition, and tissue integration. Optimizing ideal cellular composition of cardiac constructs is challenging as altering the composition of non-CMs such as FBs and ECs impact the viability, vascularization and function of the 3D bioprinted myocardium. In vivo long-term study of the 3D printed constructs implantation in large animal models to analyze the biological safety and efficacy is one the major limitations as well.
Despite these challenges, the translational potential of 3D bioprinting-based cardiac tissue engineering remains high. The ability to improve heart function without the requirement for donor implantation is the primary benefit of 3D cardiac tissue bioprinting. Higher efficiency and in vivo functionality of cardiac tissue constructs can be attained with continued improvements in bioprinting technologies [176]. Furthermore, cardiac 3D bioprinting presents significant potential for the micropatterning of the scaffold as well as scaffold-free techniques to recreate the native cardiac tissue’s histological architecture. It is reasonable that the bioprinting of tailored tissue constructs based on a patient’s specific anatomy can improve the positive impacts of regenerative cardiology.
The potential of 3D bioprinting to develop heterogeneous tissues with multiple cell types and biomaterials of optimal mechanical characteristics is remarkable. A major advantage of using 3D cardiac bioprinting is its potential to enhance cardiac function without donor implantation. Advancements in 3D printing for cardiovascular regeneration include enhanced vascularization, CM alignment and viability, biomimetics and mechanical properties demonstrating the potential of the 3D printing approach to design large scale functional cardiovascular tissue. Bioprinting strategies have also been used to design miniature tissue arrays to generate organ-on-chip or microphysiological systems to understand pharmacological effects of drugs and toxicological mechanisms. Cardiac 3D bioprinting offers potential for micropatterning of the scaffold and generation of scaffold -free systems to replicate the complex hierarchy and architecture of the native cardiac tissue.
6.2. Technical Challenges Tissue Vascularization Using 3D Bioprinting
A major limitation in the scalability of bioprinted cardiovascular tissues is the inability to supply sufficient essential nutrients and metabolic waste removal via well-aligned vascular networks. Since there are increased requirements for vascular supply in cell-dense tissues, transplantation of cell-dense tissues lacking a pre-formed vasculature results in necrosis due to insufficient nutrients and gaseous mass transport in the engineered tissue, inhibiting growth and cell mass [177,178]. Furthermore, other major challenges of pre-vascularized engineered cardiovascular tissues include their suturability and anastomosis to the host vasculature. Therefore, new engineering approaches to reconstruct and rapidly perfuse thick three-dimensional cell-dense functional tissues with a strong vascular network that obviates hypoxia and necrosis require further development.
The SWIFT and Hyprinting biomanufacturing methods allow for large volumes of perfusable OBB based tissues patterned with an embedded vascular network. However, there are limitations of this method for clinical applications. For instance, the current method for iPSC-derived organoids OBBs lack adequate cell maturation and microvascularization. Hence, lower observed SWIFT cardiac tissue contractility is achieved compared to adult cardiac tissue. There is need to study new approaches for SWIFT to fabricate mature microvascularized OBBs such as employing living matrices composed of primary cell spheroids harvested from adult tissues. The field of organ engineering has gained interest as recent studies have reported 3D embedded printing of heart shaped constructs using the “cells in gels” technique. However, long term in vitro perfusion of the tissues has not yet been achieved using the current approaches.
6.3. Perspectives and Outlook
With some new advancements to generate constructs with dynamic, structural and cellular changes of the tissue over time, 4D bioprinting technique is still in its infancy. 4D bioprinting allows application of stimuli-responsive materials and shape-memory polymers to generate 3D patterned biological structures that can be morphed in shape and structure driven by a stimulus. This technique provides a great opportunity to build dynamic structures that are similar to native tissue. Also, by conditioning structures to respond to stimulus, cardiac models could be build based on unique cellular cues which can greatly broaden the application of the 4D bioprinting technique for various treatments. One limitation of this technique is the cytotoxic effects of the stimulus which could hamper the cardiac construct function and hence, requires prior analysis for tuning or titration of the stimulus.
In summary, 3D printing technology holds great potential for creation of patient tailored implants that have enhanced host integration and less immune rejection. Although we have not fully reached the desirable qualities of controlled porous structure, tissue-like mechanical properties, and biomimetic cell signaling, we have made tremendous progress in recent years. Addressing the remaining challenges will advance the development of 3D printed cardiac tissues with the native vasculature, architecture and functionality for clinical translation.
Highlights.
3D bioprinting aims to develop tissue structures that mimic the spatial, mechanochemical and temporal characteristics of cardiovascular tissues.
Recent advances in 3D bioprinting techniques, bioinks and printing materials are described.
Translational applications of 3D bioprinting in engineering the myocardium, cardiac valves and vascular patches.
Current challenges and perspectives of 3D bioprinted constructs with native vasculature, architecture and functionality are discussed
ACKNOWLEDGEMENTS
This work was supported in part by grants to NFH from the US National Institutes of Health (R01 HL127113 and R01 HL142718), the US Department of Veterans Affairs (1I01BX002310, 1I01BX004259, RX001222), the National Science Foundation (1829534), California Institute for Regenerative Medicine (10603), and the American Heart Association (20IPA35360085 and 20IPA35310731).
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Virani SS, Alvonso A, Aparicio JH, Benjamin JE, Bittencourt M, Callaway CW. Clifton. et al. “Heart disease and stroke statistics—2021 update: a report from the American Heart Association.” Circulation (2021) 143(8): e254–e743. [DOI] [PubMed] [Google Scholar]
- 2.Kim S, Chang Y, Cho J, Hong SY, Zhao D, Kang J. et al. “Life’s simple 7 cardiovascular health metrics and progression of coronary artery calcium in a low-risk population: a cohort study.” Arteriosclerosis, Thrombosis, and Vascular Biology (2019) 39(4): 826–833. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC. et al. American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circulation Heart Failure 2013; 6:606–619. doi: 10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cukierman E, Pankov R, Yamada MK “Cell interactions with three-dimensional matrices.” Current Opinion in Cell Biology (2002) 14(5): 633–640. [DOI] [PubMed] [Google Scholar]
- 5.Ma T, Grayson W, Frohlic M, Vunjak G “Hypoxia and stem cell-based engineering of mesenchymal tissues.” Biotechnology progress (2009) 25(1): 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee KY and Mooney DJ “Hydrogels for tissue engineering.” Chemical Reviews (2001) 101(7): 1869–1880. [DOI] [PubMed] [Google Scholar]
- 7.Fedorovich NE, Alblas J, Wijn J, Hennink W, Verbout Ab., Dhert W. “Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing.” Tissue Engineering (2007) 13(8): 1905–1925. [DOI] [PubMed] [Google Scholar]
- 8.Shahrubudin Nurhalida, Lee Te Chuan, and Ramlan Rhaizan. “An overview on 3D printing technology: Technological, materials, and applications.” Procedia Manufacturing (2019) 35: 1286–1296. [Google Scholar]
- 9.Liaw Chya-Yan, and Guvendiren Murat. “Current and emerging applications of 3D printing in medicine.” Biofabrication (2017) 9.2: 024102. [DOI] [PubMed] [Google Scholar]
- 10.Xu T, Jin J, Gregory C, Hickman J, Boland T “Inkjet printing of viable mammalian cells.” Biomaterials (2005) 26(1): 93–99. [DOI] [PubMed] [Google Scholar]
- 11.Saunders RE, Gough J, Derby B “Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing.” Biomaterials (2008) 29(2): 193–203. [DOI] [PubMed] [Google Scholar]
- 12.Gudapati H, Dey M, Ozbolat I “A comprehensive review on inkjet-based bioprinting: Past, present and future.” Biomaterials (2016) 102: 20–42. [DOI] [PubMed] [Google Scholar]
- 13.Zhang YS, Haghiashtiani G, Hubscher T, Kelly D, Lee J, Lutolf M et al. “3D extrusion bioprinting.” Nature Reviews Methods Primers (2021) 1(1): 75. [Google Scholar]
- 14.Ventura RD “An Overview of Laser-assisted Bioprinting (LAB) in Tissue Engineering Applications.” Medical Lasers (2021) 10(2): 76–81. [Google Scholar]
- 15.Elomaa Laura and Yang Yunzhi Peter. Additive manufacturing of vascular grafts and vascularized tissue constructs. Tissue Engineering B Rev. (2017) 23(5):436–450. doi: 10.1089/ten.TEB.2016.0348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramadan Q and Zourob M. “3D Bioprinting at the Frontier of Regenerative Medicine, Pharmaceutical, and Food Industries.” Frontiers in Medical Technology (2021) 2: 607648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groll J, Burdick AJ, Cho D, Derby B, Gelinsky M, Heilshorn SC et al. “A definition of bioinks and their distinction from biomaterial inks.” Biofabrication (2018) 11(1): 013001. [DOI] [PubMed] [Google Scholar]
- 18.Ji Shen, and Guvendiren Murat. “Recent advances in bioink design for 3D bioprinting of tissues and organs.” Frontiers in Bioengineering and Biotechnology (2017) 5: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaspar VM, et al. “Advanced Bottom-Up Engineering of Living Architectures.” Advanced Materials (2020) 32(6): 1903975. [DOI] [PubMed] [Google Scholar]
- 20.Hospodiuk M, Dey M, Sosnoski D, Ozbolat T “The bioink: A comprehensive review on bioprintable materials.” Biotechnology advances (2017) 35(2): 217–239. [DOI] [PubMed] [Google Scholar]
- 21.Chouhan, D.Kaushik S, Arora D “Trends in Bio-Derived Biomaterials in Tissue Engineering.” Biomaterials in Tissue Engineering and Regenerative Medicine: From Basic Concepts to State of the Art Approaches (2021) 2: 163–213. [Google Scholar]
- 22.Gopinathan J and Noh I “Recent trends in bioinks for 3D printing.” Biomaterials Research (2018) 22(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Lee S, Cheng H, Yoo J, Atala A “3D bioprinted functional and contractile cardiac tissue constructs.” Acta Biomaterialia (2018) 70: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benwood C, Chrenek J, Kirsch R, Masri N, Richards H, Teetzen K et al. “Natural Biomaterials and Their Use as Bioinks for Printing Tissues.” Bioengineering (2021) 8: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin M, Shi J, Zhu W, Yao H, Wang D “Polysaccharide-Based Biomaterials in Tissue Engineering: A Review.” Tissue Engineering Part B: Reviews (2021) 27(6): 604–626. [DOI] [PubMed] [Google Scholar]
- 26.Rebers L, Reichsollner R, Regett S, Tovar G, Borchers K, Baudis S et al. “Differentiation of physical and chemical cross-linking in gelatin methacryloyl hydrogels.” Scientific Reports (2021) 11(1): 3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurian AG, Singh R, Patel K, Lee J, Kim H “Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics.” Bioactive Materials (2022) 8: 267–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fathi-Achachelouei M, Marques H, Ribeiro C, Barthes J, Bat E, Tezcaner A et al. “Use of Nanoparticles in Tissue Engineering and Regenerative Medicine.” Frontiers in Bioengineering and Biotechnology (2019) 7: 113–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gungor-Ozkerim PS, Zhang I, Khadamhosseini, Dockmeci M “Bioinks for 3D bioprinting: an overview.” Biomaterials Science (2018) 6(5): 915–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y,Chen Y, Ozbolat I “Characterization of printable cellular micro-fluidic channels for tissue engineering.” Biofabrication (2013) 5(2): 025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Q,He Y, Fu Jian., Lui A “Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery.” Biomaterials (2015) 61: 203–215. [DOI] [PubMed] [Google Scholar]
- 32.Gasperini L,Meniglio D, Motta A, Mignliaresi C “An electrohydrodynamic bioprinter for alginate hydrogels containing living cells.” Tissue Engineering Part C: Methods (2015) 21(2): 123–132. [DOI] [PubMed] [Google Scholar]
- 33.Khalil S,Nam J, Sun W “Multi-nozzle deposition for construction of 3D biopolymer tissue scaffolds.” Rapid Prototyping Journal (2005) 11 (1), 9–17. [Google Scholar]
- 34.Yu Y, Zhang Y, Martin J, Ozbolat I “Evaluation of cell viability and functionality in vessel-like bioprintable cell-laden tubular channels.” Journal of Biomechanical Engineering (2013) 135(9): 0910111–091011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Yu Y, Akkouch A, Dababneh A, Dolati F, Ozbolat I “In vitro study of directly bioprinted perfusable vasculature conduits.” Biomaterials Science (2015) 3(1): 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gruene M,P. Flaum M, Hess C, Diamantouros S, Schlie S, Deiwick A et al. “Laser printing of three-dimensional multicellular arrays for studies of cell–cell and cell–environment interactions.” Tissue Engineering Part C: Methods (2011) 17(10): 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duan B, Kapetanovic E, Hockaday LA, Butcher JT. “Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells.” Acta Biomaterialia (2014) 10(5): 1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campos DFD, Blaeser A, Weber M, Jakel J, Neuss S, Ficher H “Three-dimensional printing of stem cell-laden hydrogels submerged in a hydrophobic high-density fluid.” Biofabrication (2012) 5(1): 015003. [DOI] [PubMed] [Google Scholar]
- 39.Moon S, Hasan S, Song S, Xu F, Keles H, Manzur F et al. “Layer by layer three-dimensional tissue epitaxy by cell-laden hydrogel inkjets.” Tissue Engineering Part C: Methods (2010) 16(1): 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pati F, Jang J, Dong Ha., Kim Sung.,Rhie J, Shim J et al. “Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink.” Nature Communications (2014) 5(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solis LH, Ayala J, Portillo S, Ramirez A, Aguilera R, Boland T “Thermal inkjet bioprinting triggers the activation of the VEGF pathway in human microvascular endothelial cells in vitro.” Biofabrication (2019) 11(4): 045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaetani R, Feyen D, Verhage V, Slaats R, Messina E, Christman K et al. “Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction.” Biomaterials (2015) 61: 339–348. [DOI] [PubMed] [Google Scholar]
- 43.Bejleri D,Streeter B, Nachlas A, Brown M, Gaetani R, Kristman K,Davis M et al. “A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair.” Advanced Healthcare Materials (2018) 7(23): e1800672–e1800672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jakab K, Norotte C, Damon B, Marga F, Neagu A, Williford C et al. “Tissue engineering by self-assembly of cells printed into topologically defined structures.” Tissue Engineering Part A (2008) 14(3): 413–421. [DOI] [PubMed] [Google Scholar]
- 45.Faulkner-Jones A, Greenhough S, King A, Gardner J, Courtney A, Shu W “Development of a valve-based cell printer for the formation of human embryonic stem cell spheroid aggregates.” Biofabrication (2013) 5(1): 015013. [DOI] [PubMed] [Google Scholar]
- 46.Loo Y, Lakhsmanan A, Ming Ni, Toh L, Wang S, Hauser C “Peptide bioink: self-assembling nanofibrous scaffolds for three-dimensional organotypic cultures.” Nano Letters (2015) 15(10): 6919–6925. [DOI] [PubMed] [Google Scholar]
- 47.Marga F, Jakab K, Khatiwala C, Shephard B, Dorfman F, Hubbard B et al. “Toward engineering functional organ modules by additive manufacturing.” Biofabrication (2012) 4(2): 022001. [DOI] [PubMed] [Google Scholar]
- 48.Kucukgul C, Ozler S, Inci I, Karakas E, Irmak S, Gozuacik D et al. “3D bioprinting of biomimetic aortic vascular constructs with self-supporting cells.” Biotechnology and Bioengineering (2015) 112(4): 811–821. [DOI] [PubMed] [Google Scholar]
- 49.Bertassoni LE, Cardoso J, Manoharan V, Cristino A, Bhise N, Araujo W et al. “Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels.” Biofabrication (2014) 6(2): 024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui H, Nowicki M, Fisher J, Zhang L “3D bioprinting for organ regeneration.” Advanced Healthcare Materials (2017) 6(1): 1601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.da Conceicao Ribeiro R Development of bio-inks for bioprinting applications, Newcastle University; (2019). [Google Scholar]
- 52.Kjar A, McFarland B, Mecham K, Harward N, Huang Y “Engineering of tissue constructs using coaxial bioprinting.” Bioactive Materials (2021) 6(2): 460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Connell CD, Konate S, Onofrillo C, Kapsa R, Baker C, Duchi S et al. “Free-form co-axial bioprinting of a gelatin methacryloyl bio-ink by direct in situ photo-crosslinking during extrusion.” Bioprinting (2020) 19: e00087. [Google Scholar]
- 54.Zhang T, Zhao W, Xiahou Z, Wang X, Zhang X, Yin J et al. (2021). “Bioink design for extrusion-based bioprinting.” Applied Materials Today 25: 101227. [Google Scholar]
- 55.Skardal A, Zhang J, McCoard L, Xu X, Oottamasathian S, Prestwich G. “Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting.” Tissue Engineering Part A (2010) 16(8): 2675–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ouyang L, Yao R, Zhao U “Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells.” Biofabrication (2016) 8(3): 035020. [DOI] [PubMed] [Google Scholar]
- 57.Ouyang L, Highly C, Sun W, Burdick J “A generalizable strategy for the 3D bioprinting of hydrogels from nonviscous photo-crosslinkable inks.” Advanced Materials (2017) 29(8): 1604983. [DOI] [PubMed] [Google Scholar]
- 58.Dolati F, Yu Y, Zhang Y, Jesus A, Sander E, Ozbolat I “In vitro evaluation of carbon-nanotube-reinforced bioprintable vascular conduits.” Nanotechnology (2014) 25(14): 145101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Do A-V, Akkouch A, Green B, Ozbolat I, Debabneh A, Geary S et al. “Controlled and sequential delivery of fluorophores from 3D printed alginate-PLGA tubes.” Annals of Biomedical Engineering (2017) 45(1): 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jia W, Ozkorim P, Zhang Y, Yue K, Zhu K, Liu W et al. “Direct 3D bioprinting of perfusable vascular constructs using a blend bioink.” Biomaterials (2016) 106: 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim MH and Nam SY. “Assessment of coaxial printability for extrusion-based bioprinting of alginate-based tubular constructs.” Bioprinting 2020. 20: e00092. [Google Scholar]
- 62.Sasmal P, Datta P, Wu Y, Ozbolat I 3D bioprinting for modelling vasculature. Microphysiological systems (2018) 2:9 doi: 10.21037/mps.2018.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.West JL and Moon JJ “Vascularization of engineered tissues: approaches to promote angiogenesis in biomaterials.” Current Topics in Medicinal Chemistry (2008) 8(4): 300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Radia J, Yusha A, Iftekhar N, Salman P The promising rise of bioprinting in revolutionizing medical science: Advances and Possibilities. Regenerative Therapy (2021) 15(18):133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mirdamadi E, Tashman J, Shivarski D, Palchesco R, Fienberg A “FRESH 3D Bioprinting a Full-Size Model of the Human Heart.” ACS Biomaterials Science & Engineering (2020) 6(11): 6453–6459. [DOI] [PubMed] [Google Scholar]
- 66.Baldwin J, Antille M, Bonda U “In vitro pre-vascularisation of tissue-engineered constructs A co-culture perspective.” Vascular Cell (2014) 6: 13–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meng X, Xing Y, Li J, Deng C, Li Y, Ren Xi., Zhang D “Rebuilding the Vascular Network: In vivo and in vitro Approaches.” Frontiers in Cell and Developmental Biology (2021) 9: 639299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hinton T, Jallerat Q, Palchesco R, Park J, Grodzicki M, Shue H et al. “Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels.” Science Advances (2015) 1(9): e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee VK, Kim D, Ngo H, Lee Y, Seo L, Yoo S et al. “Creating perfused functional vascular channels using 3D bio-printing technology.” Biomaterials (2014) 35(28): 8092–8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang YS, Yue K, Aleman J, Bakht S, Yang J, Jia W et al. “3D bioprinting for tissue and organ fabrication.” Annals of Biomedical Engineering (2017) 45(1): 148–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Serpooshan V, Mahmoudi M, Hu D, Hu J, Wu S “Bioengineering cardiac constructs using 3D printing.” Journal of 3D Printing in Medicine (2017) 1(2): 123–139. [Google Scholar]
- 72.Kumar Hitendra, and Kim Keekyoung. “Stereolithography 3D bioprinting.” Methods Mol. Biol. 2140 (2020): 93–108. [DOI] [PubMed] [Google Scholar]
- 73.Huh JT, Yoo JJ, Atala A, Lee SJ “Three-dimensional bioprinting for tissue engineering.” Principles of Tissue Engineering. Academic Press, 2020. 1391–1415. [Google Scholar]
- 74.Mao Q, Wang Y, Li Y, Juengpanich S, Li W, Chen M, et al. “Fabrication of liver microtissue with liver decellularized extracellular matrix (dECM) bioink by digital light processing (DLP) bioprinting.” Materials Science and Engineering: C 109 (2020): 110625. [DOI] [PubMed] [Google Scholar]
- 75.Bernal P, Paul D, Damein L, Yang L, Jos M, Christophe M et al. “Volumetric bioprinting of complex living-tissue constructs within seconds.” Advanced Materials (2019) 31(42): 1904209. [DOI] [PubMed] [Google Scholar]
- 76.Ge Q, Zhiqiin L,Zhaolong W, Kavin K, Wang Z, Xiangnan H et al. “Projection micro stereolithography based 3D printing and its applications.” International Journal of Extreme Manufacturing (2020) 2(2): 022004. [Google Scholar]
- 77.Ong CS, Takuma F, Huaitao Z, Huang C, Andrew N, et al. “Biomaterial-free three-dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes.” Scientific Reports (2017) 7(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ong CS, Takuma F, Nashad A, Blazeski A, Huaitao Z, Samantha H. et al. “Creation of cardiac tissue exhibiting mechanical integration of spheroids using 3D bioprinting.” Journal of visualized experiments: JoVE (2017) 125: 55438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Atmanli A, Domian IJ “Generation of aligned functional myocardial tissue through microcontact printing.” Journal of Visualized Experiments: JoVE (2013) 73: 50288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Norotte C, Francois M, Laura N, Gabor F “Scaffold-free vascular tissue engineering using bioprinting.” Biomaterials (2009) 30(30): 5910–5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yin X, Benjamin M, Helia S, Robert L, Jeffrey K, Oren L et al. “Engineering stem cell organoids.” Cell Stem Cell (2016) 18(1): 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lancaster MA, Renner M, Martin C, Wenzel D, Bicknell L, Matthew H et al. “Cerebral organoids model human brain development and microcephaly.” Nature (2013) 501(7467): 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wehner M, Truby R, Fitzgerald D, Mosadegh B, Whitesides G, Lewis J et al. “An integrated design and fabrication strategy for entirely soft, autonomous robots.” Nature (2016) 536(7617): 451–455. [DOI] [PubMed] [Google Scholar]
- 84.Grosskopf AK, TrubY r., Kim H, Perazzo A, Lewis J, Stone H “Viscoplastic Matrix Materials for Embedded 3D Printing.” ACS Applied Materials & Interfaces (2018)10(27): 23353–23361. [DOI] [PubMed] [Google Scholar]
- 85.Wu W, Adam D, Jennifer L “Omnidirectional printing of 3D microvascular networks.” Advanced Materials (2011) 23(24): H178–H183. [DOI] [PubMed] [Google Scholar]
- 86.Hinton TJ, Quentin J, Rachlle P, Joon P, Martin G, Hao-Jan S et al. “Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels.” Science Advances (2015) 1(9): e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Skylar-Scott MA, Uzel S, Nam L, Ahrens J, Truby R, Sarita D et al. “Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels.” Science Advances (2019) 5(9): eaaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kolesky DB, Kimberly H, Mark S, Jennifer L “Three-dimensional bioprinting of thick vascularized tissues.” Proceedings of the National Academy of Sciences (2016) 113(12): 3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arai K, Daiki M, Ana V, Yosuke M, Manabu I, Anna N et al. “Fabrication of scaffold-free tubular cardiac constructs using a Bio-3D printer.” PLoS One (2018) 13(12): e0209162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Datta Pallab, Ayan Bugra, and Ozbolat Ibrahim T.. “Bioprinting for vascular and vascularized tissue biofabrication.” Acta Biomaterialia (2017) 51: 1–20. [DOI] [PubMed] [Google Scholar]
- 91.Bugra A, Dong H, Zhifeng Z, Madhuri D, Adomas P, Corina D, Ibrahim O “Aspiration-assisted bioprinting for precise positioning of biologics.” Science Advances 6.10 (2020): eaaw5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu Claire, Ma X, Zhu W, Wang P, Miller K, Stupin J et al. “Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix.” Biomaterials 194 (2019): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cui Haitao, Chengyu L, Timothy E, Yimin H, Zhou Z, San X et al. “4D physiologically adaptable cardiac patch: A 4-month in vivo study for the treatment of myocardial infarction.” Science Advances 6.26 (2020): eabb5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ayan B Aspiration-assisted Bioprinting, Pennsylvania State University. (2020). [Google Scholar]
- 95.Wu Yang, Ayan B Moncal K., Dhawan A, Koduru S, Ravnic D et al. “Hybrid Bioprinting of Zonally Stratified Human Articular Cartilage Using Scaffold-Free Tissue Strands as Building Blocks.” Advanced Healthcare Materials (2020) 9(22): 2001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buran A, Wu Y, Karuppagaounder V, Kamal F, Ozbolat I et al. “Aspiration-assisted bioprinting of the osteochondral interface.” Scientific Reports (2020) 10(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heo D, Ayan B Dey M, Banerjee D, Wee H, Lewis G et al. “Aspiration-assisted bioprinting of co-cultured osteogenic spheroids for bone tissue engineering.” Biofabrication (2020) 13(1): 015013. [DOI] [PubMed] [Google Scholar]
- 98.Bugra A, Celic N, Zhang Z, Zhao K, Kim M, Banerjee D et al. “Aspiration-assisted freeform bioprinting of pre-fabricated tissue spheroids in a yield-stress gel.” Communications Physics (2020) 3(1): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chu H, Yang W, Sun L, Cai S, Yang R, Liang W et al. “4D printing: a review on recent progresses.” Micromachines (2020) 11(9): 796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gao B, Yang Q, Zhao X, Jin G, Ma Y, Xu F et al. “4D bioprinting for biomedical applications.” Trends in Biotechnology (2016) 34(9): 746–756. [DOI] [PubMed] [Google Scholar]
- 101.Kirillova A, Makson R, Stoychev G, Gomillion C, Ionov L “4D biofabrication using shape-morphing hydrogels.” Advanced Materials (2017) 29(46): 1703443. [DOI] [PubMed] [Google Scholar]
- 102.Apsite I, Stoychev G, Zhang W, Jenichen D, Xie J, Ionov L “Porous stimuli-responsive self-folding electrospun mats for 4D biofabrication.” Biomacromolecules (2017) 18(10): 3178–3184. [DOI] [PubMed] [Google Scholar]
- 103.Haitao C, Liu C, Esworthy T, Huang Y “4D physiologically adaptable cardiac patch: A 4-month in vivo study for the treatment of myocardial infarction.” Science Advances (2020) 6(26): eabb5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.An J, Chua C, Mirinov V “A perspective on 4D bioprinting.” International Journal of Bioprinting (2016) 2(1): 3–5. [Google Scholar]
- 105.Kaji H, Takii H, Nishizawa M., Matsue T. “Pharmacological characterization of micropatterned cardiac myocytes.” Biomaterials (2003) 24(23): 4239–4244. [DOI] [PubMed] [Google Scholar]
- 106.Serpooshan V, Chen P, Wu H, Lee S, Sharma A, Hu D et al. “Bioacoustic-enabled patterning of human iPSC-derived cardiomyocytes into 3D cardiac tissue.” Biomaterials (2017) 131: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miao S, Cui H, Nowicki M, Lee S, Almeida J, Zhou X et al. “Photolithographic-stereolithographic-tandem fabrication of 4D smart scaffolds for improved stem cell cardiomyogenic differentiation.” Biofabrication (2018) 10(3): 035007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Agarwal T, Costantini M, Maiti T “Recent advances in bioprinting technologies for engineering cardiac tissue.” Materials Science and Engineering: (2021) C 124: 112057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Geckil H, Zhang F, Moon S, Demirci U “Engineering hydrogels as extracellular matrix mimics.” Nanomedicine (2010) 5(3): 469–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vega SL,Kwon Mi, Burdick J et al. “Recent advances in hydrogels for cartilage tissue engineering.” European Cells & Materials (2017) 33: 59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guo Y, Yuan T, Xiao Z, Tang P, Xiao Y, Fan Y et al. “Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering.” Journal of Materials Science. Materials in Medicine 2012, 23(9): 2267–2279. [DOI] [PubMed] [Google Scholar]
- 112.Snyder TN, Madhavan K, Intrator M, Gregalla R, Park D et al. “A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair.” Journal of Biological Engineering (2014) 8(1): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khanna A, Zamani M, Huang N “Extracellular Matrix-Based Biomaterials for Cardiovascular Tissue Engineering.” Journal of Cardiovascular Development and Disease (2021) 8(11): 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lakshmanan R, Krishnan U, Sethuraman S “Living cardiac patch: the elixir for cardiac regeneration.” Expert Opinion on Biological Therapy (2012) 12(12): 1623–1640. [DOI] [PubMed] [Google Scholar]
- 115.H van Marion M, Bax N, Spreeuwel A, Bouten C “Material-based engineering strategies for cardiac regeneration.” Current Pharmaceutical Design (2014) 20(12): 2057–2068. [DOI] [PubMed] [Google Scholar]
- 116.Cho G-S, Fernandez L, Kwon C “Regenerative medicine for the heart: perspectives on stem-cell therapy.” Antioxidants & Redox Signaling (2014) 21(14): 2018–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Curtis MW and Russell B “Cardiac tissue engineering.” The Journal of Cardiovascular Nursing (2009) 24(2): 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Radisic M, Malda J, Epping E, Geng W, Langer R, Vunjak G. “Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue.” Biotechnology and Bioengineering (2006) 93(2): 332–343. [DOI] [PubMed] [Google Scholar]
- 119.Shimizu T, Sekini H, Yang J, Isoi Y, Yamato M, Kikuchi A et al. “Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues.” The FASEB Journal (2006) 20(6): 708–710. [DOI] [PubMed] [Google Scholar]
- 120.Sakaguchi K, Shimizu T, Horaguchi S, Sekine H, Yamato M, Umezu T et al. “In vitro engineering of vascularized tissue surrogates.” Scientific Reports (2013) 3(1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miller JS “The billion cell construct: will three-dimensional printing get us there?” PLoS Biology (2014) 12(6): e1001882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gaetani R, Feyen D, Verhage V, Slaats R, Messina E, Christman K et al. “Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction.” Biomaterials (2015) 61: 339–348 [DOI] [PubMed] [Google Scholar]
- 123.Marieb EN and Hoehn K. Human Anatomy & Physiology, Pearson education. (2007). [Google Scholar]
- 124.Zhang YS, Arneri A, Bersini S, Shin S, Zhu K, Aleman J et al. “Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip.” Biomaterials (2016) 110: 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jakab K, Norotte C, Damon B, Marga F, Neagu A, Williford C et al. “Tissue engineering by self-assembly of cells printed into topologically defined structures.” Tissue Engineering Part A (2008) 14(3): 413–421. [DOI] [PubMed] [Google Scholar]
- 126.Jakab K, Norotte C, Marga F, Murphy K, Novakovic G, Forgacs G “Tissue engineering by self-assembly and bio-printing of living cells.” Biofabrication (2010) 2(2): 022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gaebel R, Liu N, Guan J, Koch L, Klopsch C, Gruene M et al. “Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration.” Biomaterials (2011) 32(35): 9218–9230. [DOI] [PubMed] [Google Scholar]
- 128.Ong CS,Fukunishi T, Zhang H, Huang C, Nashed A, Blazeski A et al. “Biomaterial-free three-dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes.” Scientific Reports (2017) 7(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maiullari F, Constantini M, Milan M,Pace V, Chirivi M, Maullari S et al. “A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes.” Scientific Reports (2018) 8(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gaetani R, Feyen D, Ferhage V, Slaats R, Messina A, Christman K et al. “Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction.” Biomaterials (2015) 61: 339–348. [DOI] [PubMed] [Google Scholar]
- 131.Gao L, Kupfer M, Jung J, Yang L, Zhang P, Sie Y et al. “Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold.” Circulation Research (2017) 120(8): 1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jana S, Teftt B, Spoon B, Simari R “Scaffolds for tissue engineering of cardiac valves.” Acta Biomaterialia (2014) 10(7): 2877–2893. [DOI] [PubMed] [Google Scholar]
- 133.Schoen FJ “Heart valve tissue engineering: quo vadis?” Current Opinion in Biotechnology (2011) 22(5): 698–705. [DOI] [PubMed] [Google Scholar]
- 134.Chambers J “Prosthetic heart valves.” International Journal of Clinical Practice (2014) 68(10): 1227–1230. [DOI] [PubMed] [Google Scholar]
- 135.Lueders C, Jastram B, Hetzer R, Shwandt H “Rapid manufacturing techniques for the tissue engineering of human heart valves.” European Journal of Cardio-thoracic Surgery (2014) 46(4): 593–601. [DOI] [PubMed] [Google Scholar]
- 136.Masoumi N, Annabi N, Assmann A, Larson B, Hjortnaes J, Alemdar N et al. “Tri-layered elastomeric scaffolds for engineering heart valve leaflets.” Biomaterials (2014) 35(27): 7774–7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hockaday L, Kang K, Colangelo N, Cheung P, Duan B, Malone E, Wu J, Girardi L et al. “Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds.” Biofabrication (2012) 4(3): 035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rajabi N, Rezaei A, Kharazhiha M, Lyo H, Ramakrishna S, Berto F “Recent Advances on Bioprinted Gelatin Methacrylate -Based Hydrogels for Tissue Repair.” Tissue Engineering- Part A, (2021) 27(11–12):679–702. [DOI] [PubMed] [Google Scholar]
- 139.Wu T and Liu W. Functional Hydrogels for the treatment of myocardial infarction. NPG Asia Materials; 14, (2022). Duan B, Kapetanovic E, Hockaday L, Butcher J. [Google Scholar]
- 140.Hockaday L, Kang K, Colangelo N, Cheung P, Duan B, Malone E et al. “Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds.” Biofabrication (2012) 4(3): 035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Duan B, Hockaday L, Kang K, Butcher J “3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels.” Journal of Biomedical Materials Research Part A (2013) 101(5): 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Duan B, Kapetanovic E, Hockaday L, Butcher J “Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells.” Acta Biomaterialia (2014) 10(5): 1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van der Valk DC, Grolman J, Van C, Blazer M,Wu P, Fenton O et al. “Engineering a 3D-Bioprinted Model of Human Heart Valve Disease Using Nanoindentation-Based Biomechanics.” Nanomaterials (Basel, Switzerland) (2018) 8(5): 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Abdel-Sayed P, Kalejs M “A new training set-up for trans-apical aortic valve replacement.” Interactive Cardiovascular and Thoracic Surgery (2009) 8(6): 599–601. [DOI] [PubMed] [Google Scholar]
- 145.Biglino G, Vercheuren P, Zegels R, Taylor A “Rapid prototyping compliant arterial phantoms for in-vitro studies and device testing.” Journal of Cardiovascular Magnetic Resonance (2013) 15(1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dani SS, Minhaas A, Arshad A, Krupica T, Goel S, Virani S et al. “Trends in Characteristics and Outcomes of Hospitalized Young Patients Undergoing Coronary Artery Bypass Grafting in the United States, 2004 to 2018.” Journal of the American Heart Association 2021. 10(17): e021361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Samano N, Souza D, Dashwood M “Saphenous veins in coronary artery bypass grafting need external support.” Asian Cardiovascular and Thoracic Annals (2021) 29(5): 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Souza DS, Bodin L, Dashwood M, Filbey D “High early patency of saphenous vein graft for coronary artery bypass harvested with surrounding tissue.” The Annals of Thoracic Surgery (2001) 71(3): 797–800. [DOI] [PubMed] [Google Scholar]
- 149.Klinkert P, Breslau P, Bockel J “Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature.” European Journal of Vascular and Endovascular Surgery (2004) 27(4): 357–362. [DOI] [PubMed] [Google Scholar]
- 150.Kannan RY, Salascinski H, Butler P, Hamilton G, Seifalian A “Current status of prosthetic bypass grafts: a review.” Journal of Biomedical Materials Research Part B: Applied Biomaterials (2005) 74(1): 570–581. [DOI] [PubMed] [Google Scholar]
- 151.Van Det R, Vriens B, Palen J, Geelkerkan R “Dacron or ePTFE for femoro-popliteal above-knee bypass grafting: short-and long-term results of a multicentre randomised trial.” European Journal of Vascular and Endovascular Surgery (2009) 37(4): 457–463. [DOI] [PubMed] [Google Scholar]
- 152.Mezzetto L, Scorsone L, Silingardi R, Gennai S, Leone N, Piffareti G et al. “Early and Long-term Results of ePTFE (Gore TAG®) versus Dacron (Relay Plus® Bolton) Grafts in Thoracic Endovascular Aneurysm Repair.” Annals of Vascular Surgery (2021) 71: 419–427. [DOI] [PubMed] [Google Scholar]
- 153.Brothers TE, Stanley J, Burkel W, Graham L “Small-caliber polyurethane and polytetrafluoroethylene grafts: a comparative study in a canine aortoiliac model.” Journal of Biomedical Materials Research (1990) 24(6): 761–771. [DOI] [PubMed] [Google Scholar]
- 154.Byrom MJ, Ng M, Bannon P “Biomechanics and biocompatibility of the perfect conduit—can we build one?” Annals of Cardiothoracic Surgery (2013) 2(4): 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Khanna A “Fabrication of Human Serum Albumin Film for Enhanced Hemocompatibility and Mitigation of Intimal Hyperplasia Under Physiologically Relevant Flow Shear Conditions.” Clemson University, 2017. [Google Scholar]
- 156.Khanna A, Luzinov I, Burtovvy R, Vatansever F, Langan I “Fabrication of Human Serum Albumin film on expanded polytetrafluoroethylene (e-PTFE) for Enhanced Hemocompatibility and Adhesion Strength.” Transactions of the 41st Annual Meeting of Society for Biomaterials (SFB) 2017. [Google Scholar]
- 157.Lu X, Khanna A, Luzinov I, Nagatomi J, Harman M “Surface modification of polypropylene surgical meshes for improving adhesion with poloxamine hydrogel adhesive.” Journal of Biomedical Materials Research Part B: Applied Biomaterials (2019)107(4): 1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Park JH, Jang J, Lee JS, Cho DW “Three-dimensional printing of tissue/organ analogues containing living cells.” Annals of Biomedical Engineering (2017) 45(1): 180–194. [DOI] [PubMed] [Google Scholar]
- 159.Visconti RP, Kasyanov V, Gentile C, Zhang J, Markwald R, Mironov V “Towards organ printing: engineering an intra-organ branched vascular tree.” Expert Opinion on Biological Therapy (2010) 10(3): 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Griffith CK, Miller C, Sainson R, Calvert J, Jeon N, Hughes C “Diffusion limits of an in vitro thick prevascularized tissue.” Tissue Engineering (2005) 11(1–2): 257–266. [DOI] [PubMed] [Google Scholar]
- 161.Phan DT, Wang X, Craver B, Sobrino A, Zhao D, Chen J et al. “A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications.” Lab on a Chip (2017) 17(3): 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Miller JS, Yang MT, Baker BM, Nguyen DH, Cohen DM, Toro C et al. “Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues.” Nature Materials (2012) 11(9): 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li S, Xiong Z, Wang X, Yan Y “Direct fabrication of a hybrid cell/hydrogel construct by a double-nozzle assembling technology.” Journal of Bioactive and Compatible Polymers (2009) 24(3): 249–265. [Google Scholar]
- 164.Cui X and Boland T “Human microvasculature fabrication using thermal inkjet printing technology.” Biomaterials (2009) 30(31): 6221–6227. [DOI] [PubMed] [Google Scholar]
- 165.Zhang Y, Yu Y, Akkouch A, Dabadneh A, Dolati F, Ozbolat I “In vitro study of directly bioprinted perfusable vasculature conduits.” Biomaterials Science (2015) 3(1): 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xu Y, Yingying H, Chnagyong L, Hongyi Y, Boxun L, Shengli M “A Novel Strategy for Creating Tissue-Engineered Biomimetic Blood Vessels Using 3D Bioprinting Technology.” Materials (Basel, Switzerland) (2018) 11(9): 1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Travers JG, Kamal F, Robbins J, Yutzey K and Blaxall B “Cardiac Fibrosis.” Circulation Research (2016) 118(6): 1021–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hinderer S and Schenke-Layland K (2019). “Cardiac fibrosis – A short review of causes and therapeutic strategies.” Advanced Drug Delivery Reviews 146: 77–82. [DOI] [PubMed] [Google Scholar]
- 169.Shin YJ, Shafranek R, Tsui J, Walcott J, Nelson A, Kim D “3D bioprinting of mechanically tuned bioinks derived from cardiac decellularized extracellular matrix.” Acta Biomaterialia (2021) 119: 75–88. [DOI] [PubMed] [Google Scholar]
- 170.Van der Valk DC, Ven C, Blaser M, Grolman J, Wu P, Fenton O, Lee L, Tibbitt Mark, Andresen J. et al. (2018). “Engineering a 3D-Bioprinted Model of Human Heart Valve Disease Using Nanoindentation-Based Biomechanics.” Nanomaterials 8(5): 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu J,He J, Liu J, Ma X,Chen Q, Lawrence N, Zhu W, Xu Y et al. “Rapid 3D bioprinting of in vitro cardiac tissue models using human embryonic stem cell-derived cardiomyocytes.” Bioprinting (2019) 13: e0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Noor N, Shapira A, Edri R, Gal I, Wetheim L, Dvir T “3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts.” Advanced Science (2019) 6(11): 190034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee A, Hudson A, Shivarski D, Tashman J, Hinton T, Yerneni S, Bliley J et al. “3D bioprinting of collagen to rebuild components of the human heart.” Science (2019) 365(6452): 482–487. [DOI] [PubMed] [Google Scholar]
- 174.Y Shanjani C, Pan C, Elomaa L, Yang Y. A novel bioprinting method and system for forming hybrid tissue engineering constructs. Biofabrication (2015) 7(4):045008. [DOI] [PubMed] [Google Scholar]
- 175.Kim Sungwoo, Pan Chichun, Yang Yunzhi. Development of a dual hydrogel model system for vascularization, Macromocular Bioscience (2020) 20(10):e2000204. [DOI] [PubMed] [Google Scholar]
- 176.Camci-Unal Gulden, Annabi N, Dokmeci M, Liao R, Khademhosseini A “Hydrogels for cardiac tissue engineering.” NPG Asia Materials (2014) 6(5): e99–e99. [Google Scholar]
- 177.Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K et al. “Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces.” Circulation Research (2002) 90(3): e40–e48 [DOI] [PubMed] [Google Scholar]
- 178.Zimmermann W-H, Didie M, Wasmeier G, Nixdorff U, Hess A, Boy O et al. “Cardiac grafting of engineered heart tissue in syngenic rats.” Circulation (2002) 106(12 suppl1): I-151–I-157. [PubMed] [Google Scholar]


