Abstract
Uncertainty regarding the development of postoperative metastatic disease is highly prevalent. Here we assert that numerous processes that occur during the immediate perioperative period (IPP) markedly affect the probability of post-operative metastatic disease, and that these processes can be manipulated to improve cancer survival. Specifically, tumor excision facilitates both pro-metastatic and anti-metastatic processes, which, within each domain, are often synergistic and self-propagating. Consequently, minor perioperative dominance of either pro-metastatic or anti-metastatic processes can trigger a “snowball-like effect”, leading to either accelerated progression of minimal residual disease (MRD), or to its dormancy/elimination, establishing the “surgical metastatic roulette”. Thus, the IPP should become a significant anti-metastatic therapeutic arena, exploiting feasible approaches including immunotherapies and manipulations/modifications of inflammatory-stress responses, surgical procedures, and hormonal status.
Keywords: perioperative, cancer, metastases, surgery, beta-adrenergic blocker, COX2 inhibitor
Uncertainty regarding post-operative metastatic disease
In many operated cancer patients, despite all known prognostic factors and the specific treatments used, there is a high level of uncertainty regarding whether a patient at risk will develop post-operative metastatic disease. In this Opinion article I argue that the immediate perioperative period (IPP) significantly contribute to this uncertainty, and that specific pro-metastatic and anti-metastatic processes during this period can be manipulated to potentially improve patients’ chances of remaining disease-free.
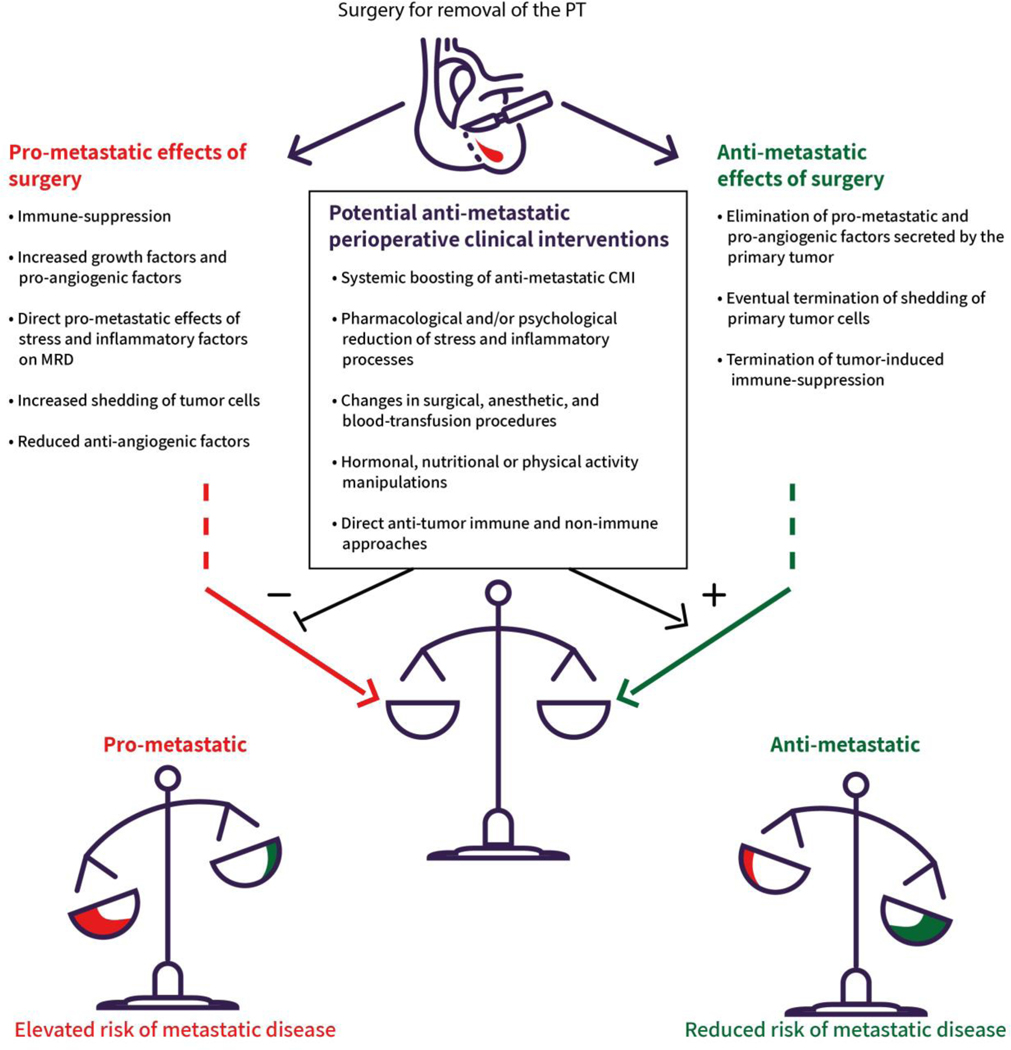
For most solid cancers, surgery for the removal of the primary tumor (PT) is an essential life-saving procedure. Unfortunately, various aspects of surgery and of the IPP (defined as days before to days-weeks after surgery) often increase the risk for progression of preexisting micrometastases and for the initiation of new metastases through (i) directly affecting malignant tissue, (ii) suppressing anti-tumor CMI or protecting MRD, and through (iii) affecting the microenvironment of tumor/MRD (also see Fig 1, Key Figure) [1–3]. Mechanisms underlying these deleterious effects have been implicated or speculated upon by numerous translational and clinical studies and include: (i) potential excess shedding of tumor cell and increased number of circulating tumor cells (CTC) as a result of the surgical manipulation of the malignant tissue, its blood vessels, and/or adjacent tissue [4, 5]; (ii) a drop in anti-angiogenic factors (e.g., endostatin & angiostatin) as a result of the removal of the PT [6]; (iii) local and systemic increase in levels of growth factors and pro-angiogenic factors, physiologically aimed to promote post-operative tissue healing, but inadvertently facilitate growth of minimal residual disease (MRD) [7]; (iv) protection of CTC from immunocytes lysis by “platelet cloaking”, which also promote CTC capacity to extravasate and establish new organ metastases [1, 8]; and (v) marked suppression of anti-metastatic cell-mediated immunity (CMI) (e.g., CTL & NK cells) caused by tissue damage, anesthetic and analgesic agents, hypothermia, blood transfusion, and other perioperative events [2, 9–11]. It is also acknowledged that inflammation, a hallmark of cancer, and adrenergic-stress responses, which collectively are mediated by the release prostaglandins (PGs, e.g. PGE2) and catecholamines (CAs, i.e., epinephrine and norepinephrine), are prominent factors driving cancer progression through their direct and indirect effects on malignant tissue [2, 3, 12, 13]. Both PGs and CAs are abundantly released during the perioperative period [3], and excess release of these factors synergistically mediate many of the abovementioned prometastatic processes, and triggers additional process to do so [2, 3, 14] (see Box 1).
Figure 1, Key Figure. Surgery for the removal of a primary tumor (PT) induces both pro- and anti-metastatic processes.
A minor imbalance between these opposing processes during the immediate perioperative period can determine whether minimal residual disease (MRD) will progress toward accelerated growth, or revers toward dormancy/regression. In either case, the effect is often self-propagating, leading to a “snowball-like effect” that has the power to determine long-term cancer outcomes. Several perioperative interventions can be used during this critical, yet un-exploited, window of opportunity to shift the balance toward an anti-metastatic balance and potentially save the lives of operated cancer patients.
Box 1 – Catecholamines and prostaglandins promote metastasis: Mechanisms and prevention.
Catecholamines (CAs) and prostaglandins (PGs ) suppress anti-metastatic CMI, directly by deactivating NK and CTL cells, and indirectly by reducing levels of pro-CMI Th1 cytokines [9]. Additionally, both CAs and PGs directly affect malignant cells, making them more aggressive and improving their metastasizing capacity through various mechanisms, including increase tumor cell survival, proliferation, motility, and resistance to anoikis[2, 3, 53]. CAs and PGs increase tumor release of VEGF, MMP2, MMP9, IL-6 and IL-8, factors which assist the malignant tissue in acquiring new blood vessels, penetrating the extracellular matrix, and proliferating [2, 3, 53]. CAs and PGs were each shown to induce an epithelial-to-mesenchymal transition (EMT) in malignant tissue [54, 55], another pro-metastatic process with well-established negative predictive value for disease-free-survival in several cancer types [56–60]. Last, CAs and PGs induce a M2macrophage shift within metastatic foci, which support metastatic growth [53, 61].
Therefore, it would be expected that perioperative inhibition of CAs and PGs signaling would reduce post-operative metastatic disease. Indeed, a short perioperative use of a β-blocker (propranolol) and COX2 inhibitor (etodolac) was shown to counteract many deleterious effects of surgery, and to reduce metastasis and long-term cancer mortality in several animal models [22–24, 62–67]. For example, a single administration of propranolol and etodolac on the day of tumor excision of a spontaneously metastasizing human PT in nude mice, prevented a post-operative eruption of metastatic foci, keeping them in a dormant/non-progressing state [66].
In addition to having pro-metastatic effects, the removal of the PT also triggers processes that exert anti-metastatic effects. Most healthy adults bear micro foci of malignant tissue, which are apparently not progressing or slowly progressing (e.g., in the prostate, breast, or thyroid)[15]. A single malignant cell is believed to initiate each micro-malignancy, but at some undefined time point the malignant mass halts its exponential growth, probably due to limiting interactions with its microenvironment (including immune cells) [16]. Thus the progression of micro-malignancies can naturally be limited or terminated by the host. Here we suggest that the removal of the PT (rather than the surgical procedure) may present an opportunity to halt progression of MRD and to prevent the initiation of new metastatic foci. Specifically, removal of the PT eventually stops or reduces the shedding and spread of tumor cells [17], which are necessary for creating new metastases. Additionally, the removal of the PT terminates PT secretion of a variety of factors that (i) suppress anti-metastatic immunity, (ii) promote the establishments of metastatic niches [18, 19], and (iii) support the growth of already established micrometastases that are not yet self-sufficient. For example, PGs and IL-8, which are often secreted by PTs, are known to cause systemic suppression of NK cells and of intra-tumoral anti-metastatic immune activity [9], as well as to directly support the growth of malignant foci[20]. It is our belief that the cumulative effects of removing the PT, which terminates these pro-metastatic processes, are anti-metastatic to a degree that prevents postoperative progression of metastatic disease in a substantial portion of operated cancer patients, despite postoperative existence of MRD (see Box 2 for mechanisms and examples) (also see Fig 1).
Box 2 – Mechanism of anti-metastatic effects of tumor removal.
Many single tumor cells are susceptible to lysis by CTLs, macrophages, or NK cells, especially by specialized hepatic and pulmonary marginating-NK cells that are strategically located to lyse circulating tumor cells and have the capacity to kill “resistant” tumor cells [48, 62, 63, 68]. Once immune suppression is eased by elimination of PT-derived immune-suppressive factors, such as TGFβ and IL-6 [69–72], the lysis of those last remaining CTC after removal of the PT can markedly reduce the chances of post-operative initiation of new metastatic foci.
Additionally, pre-existing growing micrometastases may regress to a dormant state or may be eradicated following a drop in PT-secreted factors. Growth of micrometastases is restricted by immunocytes’ lysis, by lack of blood supply, and/or by lack of growth factors. The elimination of immuno-suppressive factors released by the PT, and/or induced by stress and surgery [9], may assist tumor-infiltrating-lymphocytes (TILs) (e.g., NK cells and CTLs) to eliminate tumor cells in established micrometastases[71–74]. Additionally, pro-angiogenic, pro-growth, and pro-invasion factors are abundantly secreted by the PT, including IL-6, IL-8, VEGF, EGF, PDGFaa, MIF, and SerpinE1 [19, 66, 67, 75]. These factors may be critical for maintenance and progression of micrometastases [20, 76–79], especially at an early stage when these microscopic malignant foci are not yet self-sufficient [67]. Thus, the removal of the PT and the elimination of its secreted factors is expected to halt the progression of micrometastases. Indeed, we recently found that (i) the secretome of a human PT supports the growth of its spontaneous metastases in nude mice, and that (ii) the removal of the PT causes reliable regression and dormancy of its micrometastases, but not of larger metastases that are apparently self-sufficient [67]. In cancer patients, post-operative regression of metastases is a well-documented phenomenon in several types of cancer, but is a very rare event [80, 81]. However, in patients this phenomenon can be potentially recognized only regarding detectable (large) metastases, which often contain 106-109 cells, unlike in the aforementioned animal studies that employ labeled tumor cells and imaging techniques recognizing micrometastases containing as few as 102-103 tumor cells. Thus, postoperative halt of MRD progression or their regression may be markedly more prevalent clinically in unrecognized metastases than is currently assumed.
Multifaceted biological processes, such as progression of metastases, are hard to predict, given known and expected interactions between the many factors that affect them. We assert that both the pro-metastatic and the anti-metastatic processes induced by surgery are often synergistic within each category, and/or are self-propagating. For example, EMT together with high MMP2/MMP9 levels, can lead to excess release of tumor cells into the circulation, that, when combined with immune suppression and growth factors, can markedly increase the chances of establishing new metastatic foci. Existing metastatic foci become more effective in inducing local immune suppression and angiogenic signals due to increasing numbers of secreting malignant cells and facilitation of such secretion by high CA levels. Conversely, elimination of PT-secreted growth factors may cause regression in existing micrometastases, which will then become even less self-sufficient and will further regress or remain dormant. If anti-metastatic immunity will simultaneously recover from immune-suppression, some dormant or regressing metastases may actually be eliminated. Consequently, if the balance between the pro- and anti-metastatic processes is significantly leaning towards one direction, beyond a certain threshold, it may create a snowball effect, either leading to accelerated progression of MRD, or to regression/dormancy of MRD (Fig 1).
Clinically, it would be advantageous to know whether a patient currently identified as at-risk for metastatic disease would benefit from perioperative interventions, as any potential intervention entails medical risks or financial costs. Currently, however, despite the use of multiple biomarkers, including tumor stage, grade, receptor status, proliferation markers, lymph node status, leukocytes infiltration profile, malignant genomic composition, number of CTC, etc., there is still uncertainty whether a patient at-risk will eventually show disease recurrence. This uncertainty similarly exists in patients who receive neo-adjuvant and/or adjuvant therapy. This state resembles a roulette whose outcome is practically unpredictable, although completely based on multiple physical properties of the roulette play. One may even consider it a Russian roulette, as the outcome may depend on processes activated by tumor excision, and eventually be life or death. I propose that a significant level of this uncertainty is explained by the numerous perioperative processes described above, leading to either progression or regression/dormancy of MRD following PT removal (Fig 1). These multiple processes are not assessed nor manipulated clinically, and their combined integrative impact is hard to consider. It therefore seems unlikely that one could successfully predict whether these factors collectively cause a self-propagating process that promotes metastatic progression, or that causes metastatic regression.
Most importantly, I believe that addressing even some of the unattended perioperative factors described above would suffice to markedly reduce the risk of developing metastatic disease by tipping the scale toward an anti-metastatic dominance.
Immediate perioperative interventions can significantly impact long-term cancer outcomes
Given existing uncertainty in the occurrence of post-operative metastatic disease, the critical empirical question is whether short interventions or events during the critical perioperative period can tilt the balance between pro- and anti-metastatic processes, leading to either metastatic dormancy/regression or to metastatic progression. I assert that there are ample pre-clinical and clinical evidence supporting this claim.
Translational studies
Animal studies employing models of spontaneous metastasis, where survival and/or metastatic growth were assessed following the excision of a metastasizing PT, directly indicated beneficial effects of short perioperative interventions, including immune-stimulation [21], blockade of CAs and PGs signaling [22–24], or the use of specific anesthetic regiments [25] or perioperative nutrition regimens [26]. For example, in a study where a spontaneously metastasizing orthotopic PTs were removed surgically in mice, the combined inhibition of CAs & PGs signaling (i.e., the use of propranolol and etodolac), given only on the day of tumor excision, prevented metastatic disease and doubled long-term survival rate in two syngeneic tumor models [2, 22].
Human clinical trials
More convincing are evidences from human studies, and specifically randomized clinical trials (RCT). As exemplified below, few short perioperative interventions or randomized modifications in surgical procedures were shown to improve long-term cancer outcomes or biomarkers of DFS. Unfortunately, none of these approaches has been integrated into standard clinical routine.
First, a 3-day pre-operative low-dose IL-2 treatment, ending 36h prior to colorectal resection, significantly reduced 5-year cancer progression rate [27]. Even more impressive, pancreatic cancer patients showed significant improvements in 3-year DFS and OS following this immediate pre-operative treatment [28]. Although low fever was evident in nearly all treated patients, no interference with the surgical treatment and no increase in short- or long-term complications were evident [27].
Another line of studies addresses the controversial claim that levels of female sex hormone during surgery for breast cancer impact long-term cancer outcomes [29–31]. One hypothesis is that high estrogen levels concurrently with low progesterone levels is a perioperative risk factor for metastatic progression [30], potentially because this hormonal pattern promotes a greater perioperative immunosuppression [32] and other pro-metastatic processes. A pivotal RCT conducted in 1,000 women with operable breast cancer showed that a single pre-operative administration of a synthetic progesterone (hydroxyprogesterone), which disrupts this potentially disadvantageous hormonal pattern, reduced recurrence rates in lymph-node-positive patients (which are at risk for metastatic disease), but not in lymph-node-negative patients, irrespective of tumor hormonal receptor status [33].
Last, recent studies targeted excess perioperative release of CAs and PGs in two biomarker RCTs [34–36]. Breast and colorectal cancer patients received 11–20 days of treatment with a β-adrenergic antagonist and a COX-2 inhibitor (propranolol and etodolac), or were treated with placebo, beginning 5 days prior to tumor excision. Molecular analyses of the excised tumor indicated a significant reduction in EMT status and in the activity of several pro-metastatic/pro-inflammatory transcription factors, including GATA-1, GATA-2, EGR3 (early-growth-response-3), and STAT-3 (signal transducer and activator of transcription-3) [34–36]. Additionally, a change in tumor infiltrating WBC milieu towards an improved immunological response against the malignant tissue was evident [34, 36], and reduced levels of the proliferation marker Ki67 was evident in breast cancer patients [35]. The treatment also reduced serum prometastatic and pro-inflammatory indices and improved immune-anti-metastatic indices [36]. Last, although these studies were not powered to study long-term cancer outcome, an exploratory analysis of 3-year DFS in colorectal cancer patients indicated a statistically non-significant trend for improved DFS from 33.3% in placebo patients to 12.5% in treated patients (intent-to-treat analysis, p =.239) [34], suggesting the long-term safety of the treatment and its potential efficacy. The treatment was well tolerated in both trials, with adverse event rates comparable to placebo [34, 36]. In translational studies, this treatment had no adverse effects on wound healing, anastomosis strength, and abdominal wall wounds [37], and improved post-operative long-term survival rates [22]. Overall, these RCTs clearly show that short perioperative interventions that are safe and easy to administer can improve anti-metastatic characteristics of the malignant tissue and/or improve long-term cancer outcomes in patients bearing various cancer types.
Human retrospective studies
Numerous retrospective clinical studies reported adverse or beneficial long-term cancer outcomes of various immediate perioperative events or of modifications in surgical procedures. For example, the intraoperative use of the anesthetic agent dexmedetomidine [38], blood transfusion, the occurrence of hypothermia, wound infection [39, 40], or anastomotic leak [41, 42], were all shown to be associated with decreased OS in cancer patients, even when all known risk factors were matched to control patients [1, 2]. Conversely, the use of propofol anesthesia, compared to the common use of volatile anesthesia, significantly improved 5-year OS rate [43, 44]. These studies, although retrospective and only statistically controlling for known risk factors, suggest that immediate perioperative events and processes that are often temporary and seems innocuous (e.g., the intraoperative use of dexmedetomidine or propofol) can have significant long-term cancer consequences.
Perioperative use of anti-metastatic interventions and practical considerations
Contraindications to surgery are the main reason for not using anti-metastatic treatments during the short perioperative timeframe. These include jeopardizing post-operative tissue healing and suppressing immunity, which are common adverse effects of chemo- and radio-therapies [45]. With respect to immune-therapies, their common inflammatory-pyrogenic effects are (i) often indistinguishable from signs of infections, which would usually lead to postponing surgery, and (ii) may theoretically increase the risk for SIRS (systemic inflammatory response syndrome), which is a post-operative life-threatening complication. Last, some pre-operative interventions, such as immuno-nutritional, physical-activity, or psychosocial preparations for surgery, may require postponing surgery for a few days or a few weeks, potentially increasing the risk of metastatic disease. However, various existing interventions can be used perioperatively with minimal risks that are manageable, and other interventions may be adjusted to enable their perioperative use [46]. Interventions that require a brief postponement of surgery should be considered against potential benefits. Combination of interventions may be most effective, given their independent-complementary nature or synergistic effects, and given that they could prevent adverse effects of each other. For example, the perioperative use of the immune-stimulating TLR9 agonist, CpG-C, which is self-limiting in terms of its inflammatory-pyrogenic effects, simultaneously with blockade of inflammatory-stress responses through propranolol and etodolac, was found in translational studies to have synergistic effects without noticeable adverse effects [47].
Overall, our current understanding and empirical evidence indicate that several anti-metastatic approaches should be considered and/or tested perioperatively, some without any modification. These include: (i) Systemic boosting of anti-metastatic CMI through immune-stimulating agents (e.g., CpG-C or low doses IL-2/IL-12) [46, 48]; (ii) Reduction of stress and inflammatory processes, which could prevent immune suppression and the direct promoting effects of CAs and PGs on progression of MRD [3, 34–36, 49]; (iii) Changes in surgical, anesthetic, and blood-transfusion procedures, which were shown or suggested to improve postoperative survival rates in cancer patients [1, 50]; (iv) Various perioperative hormonal [33], nutritional [51], physical activity [52], and psychological manipulations [2, 3, 46]; and (v) Various anti-tumor approaches that may be adjusted to the perioperative period, including immune-checkpoint modification therapies and other anti-metastatic approaches.
Concluding remarks
The short perioperative period is characterized by many pro-metastatic and anti-metastatic processes that can either lead to accelerated progression of MRD or to its dormancy and regression. Thus, relatively minor interventions during this sensitive and largely unexploited period may have large impacts on long-term cancer outcomes. Empirical clinical evidence supports this claim, yet currently anti-metastatic approaches are rarely part of perioperative clinical routine, forfeiting a major potential anti-metastatic approach due to our complacency with uncertainty that stem from perioperative processes. It is time to make the immediate perioperative period a major focus for anti-metastatic interventions by clinically testing feasible existing approaches, and by modifying other approaches for use in this timeframe (see Outstanding Questions box). Exploiting this short window of opportunity may improve the odds of the surgical metastatic roulette for the benefit of cancer patients. Perioperative intervention may also be less aversive compared to the post-operative use of standard adjuvant therapies or experimental immune therapies, which, unfortunately, need to target metastases at their more advanced and resistant phase, aiming to arrest a speeding growing snowball.
Outstanding Questions Box:
Would a potential perioperative intervention jeopardize or improve tissue healing?
Would perioperative pyrogenic effects of immunotherapy increase or decrease shortterm risks of surgery, including postoperative infections and systemic inflammatory response syndrome (SIRS)?
Could pre-operative nutritional and/or physical-exercise interventions reduce the likelihood/severity of deleterious effects of surgery, including immune suppression and excessive stress-inflammatory responses?
If several perioperative approaches are found feasible, should they be used simultaneously or sequentially, and do specific approaches act synergistically or are contra-indicated to each other?
Highlights:
The immediate perioperative period (IPP), although spanning only a few days before and after surgery, has a disproportionately large impact on the probability of the occurrence of post-operative metastatic disease
Primary tumor excision induces both pro-metastatic and anti-metastatic processes, which, within each category, can act synergistically and in a self-propagating manner (snowball-like effect)
Excess perioperative release of inflammatory and stress factors (and specifically prostaglandins and catecholamines) often (i) suppress anti-metastatic immunity, and (ii) directly facilitate pro-metastatic and progrowth characteristics in the primary tumor and in minimal residual disease
Several anti-metastatic approaches are feasible and effective during the IPP, with minimal adverse effects, including some immunotherapies and anti-stress-inflammatory approaches, but none has been integrated into standard clinical routine
Acknowledgement:
I am thankful for my students and colleagues for critical discussions of the issues presented herein, and for our collaborative empirical work that set the basis for this Opinion article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Hiller JG, et al. (2018) Perioperative events influence cancer recurrence risk after surgery. Nature reviews. Clinical oncology 15, 205–218 [DOI] [PubMed] [Google Scholar]
- 2.Horowitz M, et al. (2015) Exploiting the critical perioperative period to improve long-term cancer outcomes. Nature reviews. Clinical oncology 12, 213–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricon I, et al. (2019) Perioperative biobehavioral interventions to prevent cancer recurrence through combined inhibition of beta-adrenergic and cyclooxygenase 2 signaling. Cancer 125, 4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peach G, et al. (2010) Prognostic significance of circulating tumour cells following surgical resection of colorectal cancers: a systematic review. Br J Cancer 102, 1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papavasiliou P, et al. (2010) Circulating tumor cells in patients undergoing surgery for hepatic metastases from colorectal cancer. Proceedings 23, 11–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Reilly MS, et al. (1997) Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88, 277–285 [DOI] [PubMed] [Google Scholar]
- 7.Abramovitch R, et al. (1999) Stimulation of tumour growth by wound-derived growth factors. Br J Cancer 79, 1392–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma D, et al. (2014) Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. Journal of cellular physiology 229, 1005–1015 [DOI] [PubMed] [Google Scholar]
- 9.Neeman E. and Ben-Eliyahu S. (2013) Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav Immun 30 Suppl, S32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiller J, et al. (2013) Understanding clinical strategies that may impact tumour growth and metastatic spread at the time of cancer surgery. Best practice & research. Clinical anaesthesiology 27, 427–439 [DOI] [PubMed] [Google Scholar]
- 11.Dubowitz JA, et al. (2018) Implicating anaesthesia and the perioperative period in cancer recurrence and metastasis. Clinical & experimental metastasis 35, 347–358 [DOI] [PubMed] [Google Scholar]
- 12.Yap A, et al. (2018) Effect of beta-blockers on cancer recurrence and survival: a metaanalysis of epidemiological and perioperative studies. Br J Anaesth 121, 45–57 [DOI] [PubMed] [Google Scholar]
- 13.Karpisheh V, et al. (2019) Prostaglandin E2 as a potent therapeutic target for treatment of colon cancer. Prostaglandins & other lipid mediators, 106338 [DOI] [PubMed] [Google Scholar]
- 14.Nagaraja AS, et al. (2016) Sustained adrenergic signaling leads to increased metastasis in ovarian cancer via increased PGE2 synthesis. Oncogene 35, 2390–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin M, et al. (2008) Prevalence of incidental prostate cancer in the general population: a study of healthy organ donors. The Journal of urology 179, 892–895; discussion 895 [DOI] [PubMed] [Google Scholar]
- 16.Haldar R. and Ben-Eliyahu S. (2018) Reducing the risk of post-surgical cancer recurrence: a perioperative anti-inflammatory anti-stress approach. Future oncology 14, 1017–1021 [DOI] [PubMed] [Google Scholar]
- 17.Patel H, et al. (2002) Clearance of circulating tumor cells after excision of primary colorectal cancer. Ann Surg 235, 226–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan RN, et al. (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S, et al. (2009) Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. nature 457, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waugh DJ and Wilson C. (2008) The interleukin-8 pathway in cancer. Clin Cancer Res 14, 6735–6741 [DOI] [PubMed] [Google Scholar]
- 21.Goldfarb Y, et al. (2011) Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg 253, 798–810 [DOI] [PubMed] [Google Scholar]
- 22.Glasner A, et al. (2010) Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol 184, 2449–2457 [DOI] [PubMed] [Google Scholar]
- 23.Inbar S, et al. (2011) Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin-E2 through reduced NK activity. PLoS One 6, e19246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorski L, et al. (2016) Reducing liver metastases of colon cancer in the context of extensive and minor surgeries through beta-adrenoceptors blockade and COX2 inhibition. Brain Behav Immun 58, 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman J, et al. (2019) Effect of Perioperative Lidocaine, Propofol and Steroids on Pulmonary Metastasis in a Murine Model of Breast Cancer Surgery. Cancers 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldfarb Y, et al. (2012) Fish oil attenuates surgery-induced immunosuppression, limits post-operative metastatic dissemination and increases long-term recurrence-free survival in rodents inoculated with cancer cells. Clin Nutr 31, 396–404 [DOI] [PubMed] [Google Scholar]
- 27.Brivio F, et al. (2006) Pre-operative immunoprophylaxis with interleukin-2 may improve prognosis in radical surgery for colorectal cancer stage B-C. Anticancer Res 26, 599–603 [PubMed] [Google Scholar]
- 28.Caprotti R, et al. (2008) Free-from-progression period and overall short preoperative immunotherapy with IL-2 increases the survival of pancreatic cancer patients treated with macroscopically radical surgery. Anticancer Research 28, 1951–1954 [PubMed] [Google Scholar]
- 29.Hrushesky WJ, et al. (1989) Menstrual influence on surgical cure of breast cancer. Lancet 2, 949–952 [DOI] [PubMed] [Google Scholar]
- 30.Badwe RA, et al. (1991) Timing of surgery during menstrual cycle and survival of premenopausal women with operable breast cancer. Lancet 337, 1261–1264 [DOI] [PubMed] [Google Scholar]
- 31.Samuel M, et al. (2011) Timing of breast surgery in premenopausal breast cancer patients. The Cochrane database of systematic reviews, CD003720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben-Eliyahu S, et al. (1996) Increased susceptibility to metastasis during pro-oestrus/oestrus in rats: possible role of oestradiol and natural killer cells. British Journal of Cancer 74, 1900–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badwe R, et al. (2011) Single-injection depot progesterone before surgery and survival in women with operable breast cancer: a randomized controlled trial. J Clin Oncol 29, 2845–2851 [DOI] [PubMed] [Google Scholar]
- 34.Haldar R, et al. (in press) Perioperative COX2 and beta-adrenergic blockade improves biomarkers of tumor metastasis, immunity, and inflammation in colorectal cancer: A randomized controlled trial. Cancer [DOI] [PubMed] [Google Scholar]
- 35.Haldar R, et al. (2018) Perioperative inhibition of beta-adrenergic and COX2 signaling in a clinical trial in breast cancer patients improves tumor Ki-67 expression, serum cytokine levels, and PBMCs transcriptome. Brain Behav Immun 73, 294–309 [DOI] [PubMed] [Google Scholar]
- 36.Shaashua L, et al. (2017) Perioperative COX-2 and beta-Adrenergic Blockade Improves Metastatic Biomarkers in Breast Cancer Patients in a Phase-II Randomized Trial. Clin Cancer Res 23, 4651–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hazut O, et al. (2011) The effect of beta-adrenergic blockade and COX-2 inhibition on healing of colon, muscle, and skin in rats undergoing colonic anastomosis. Int J Clin Pharmacol Ther 49, 545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavon H, et al. (2018) Dexmedetomidine promotes metastasis in rodent models of breast, lung, and colon cancers. Br J Anaesth 120, 188–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murthy BL, et al. (2007) Postoperative wound complications and systemic recurrence in breast cancer. Br J Cancer 97, 1211–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beecher SM, et al. (2016) Influence of complications following immediate breast reconstruction on breast cancer recurrence rates. The British journal of surgery 103, 391–398 [DOI] [PubMed] [Google Scholar]
- 41.Lu ZR, et al. (2016) Anastomotic Leaks After Restorative Resections for Rectal Cancer Compromise Cancer Outcomes and Survival. Diseases of the colon and rectum 59, 236–244 [DOI] [PubMed] [Google Scholar]
- 42.Mirnezami A, et al. (2011) Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 253, 890–899 [DOI] [PubMed] [Google Scholar]
- 43.Wigmore TJ, et al. (2016) Long-term Survival for Patients Undergoing Volatile versus IV Anesthesia for Cancer Surgery: A Retrospective Analysis. Anesthesiology 124, 69–79 [DOI] [PubMed] [Google Scholar]
- 44.Lee JH, et al. (2016) Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: a retrospective study. Korean journal of anesthesiology 69, 126–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matzner P, et al. (2020) Harnessing cancer immunotherapy during the unexploited immediate perioperative period. Nature reviews. Clinical oncology [DOI] [PubMed] [Google Scholar]
- 46.Matzner P, et al. (2020) Harnessing cancer immunotherapy during the unexploited immediate perioperative period. Nature reviews. Clinical oncology 17, 313–326 [DOI] [PubMed] [Google Scholar]
- 47.Matzner P, et al. (2019) Deleterious synergistic effects of distress and surgery on cancer metastasis: Abolishment through an integrated perioperative immune-stimulating stressinflammatory-reducing intervention. Brain Behav Immun [DOI] [PubMed] [Google Scholar]
- 48.Sorski L, et al. (2020) Prevention of liver metastases through perioperative acute CpG-C immune stimulation. Cancer Immunol Immunother [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hiller JG, et al. (2020) Preoperative beta-Blockade with Propranolol Reduces Biomarkers of Metastasis in Breast Cancer: A Phase II Randomized Trial. Clin Cancer Res 26, 1803–1811 [DOI] [PubMed] [Google Scholar]
- 50.Cata JP, et al. (2017) Intraoperative use of dexmedetomidine is associated with decreased overall survival after lung cancer surgery. Journal of anaesthesiology, clinical pharmacology 33, 317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, et al. (2012) Perioperative immunonutrition for gastrointestinal cancer: a systematic review of randomized controlled trials. Surgical oncology 21, e87–95 [DOI] [PubMed] [Google Scholar]
- 52.Meyerhardt JA, et al. (2006) Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 24, 3527–3534 [DOI] [PubMed] [Google Scholar]
- 53.Cole SW, et al. (2015) Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer 15, 563–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cole SW and Sood AK (2012) Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res 18, 1201–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hugo HJ, et al. (2015) New Insights on COX-2 in Chronic Inflammation Driving Breast Cancer Growth and Metastasis. Journal of mammary gland biology and neoplasia 20, 109–119 [DOI] [PubMed] [Google Scholar]
- 56.Aiello NM and Kang Y. (2019) Context-dependent EMT programs in cancer metastasis. The Journal of experimental medicine 216, 1016–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaianigo N, et al. (2017) EMT and Treatment Resistance in Pancreatic Cancer. Cancers 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Otsuki Y, et al. (2018) Prospects for new lung cancer treatments that target EMT signaling. Developmental dynamics : an official publication of the American Association of Anatomists 247, 462–472 [DOI] [PubMed] [Google Scholar]
- 59.Saitoh M. (2018) Involvement of partial EMT in cancer progression. J Biochem 164, 257–264 [DOI] [PubMed] [Google Scholar]
- 60.Vu T. and Datta PK (2017) Regulation of EMT in Colorectal Cancer: A Culprit in Metastasis. Cancers 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim R, et al. (2006) Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer research 66, 5527–5536 [DOI] [PubMed] [Google Scholar]
- 62.Benish M, et al. (2008) Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol 15, 2042–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melamed R, et al. (2005) Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun 19, 114–126 [DOI] [PubMed] [Google Scholar]
- 64.Lee JW, et al. (2009) Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res 15, 2695–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sloan EK, et al. (2010) The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer research 70, 7042–7052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haldar R, et al. (2019) Sympathetic-inflammatory responses in operated nude mice prevent transformation into dormancy of human breast cancer metastases: Multiple mediating mechanisms through immunity and tumor secretion of IL-6, IL-8, and VEGF. Brain, Behavior, and Immunity 76, e13 [Google Scholar]
- 67.Shaashua L, et al. (2020) Spontaneous regression of micro-metastases following primary tumor excision: a critical role for primary tumor secretome. bioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Melamed R, et al. (2010) The marginating-pulmonary immune compartment in rats: characteristics of continuous inflammation and activated NK cells. J Immunother 33, 16–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frey AB (2015) Suppression of T cell responses in the tumor microenvironment. Vaccine 33, 7393–7400 [DOI] [PubMed] [Google Scholar]
- 70.Sinha P, et al. (2005) Tumor immunity: a balancing act between T cell activation, macrophage activation and tumor-induced immune suppression. Cancer immunology, immunotherapy : CII 54, 1137–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ostrand-Rosenberg S, et al. (2004) Antagonists of tumor-specific immunity: tumor-induced immune suppression and host genes that co-opt the anti-tumor immune response. Breast disease 20, 127–135 [DOI] [PubMed] [Google Scholar]
- 72.Baxevanis CN, et al. (1994) Abnormal cytokine serum levels correlate with impaired cellular immune responses after surgery. Clin Immunol Immunopathol 71, 82–88 [DOI] [PubMed] [Google Scholar]
- 73.Danna EA, et al. (2004) Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res 64, 2205–2211 [DOI] [PubMed] [Google Scholar]
- 74.Kusmartsev S. and Gabrilovich DI (2002) Immature myeloid cells and cancer-associated immune suppression. Cancer immunology, immunotherapy : CII 51, 293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh RK, et al. (1994) Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer research 54, 3242–3247 [PubMed] [Google Scholar]
- 76.Im JH, et al. (2004) Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer research 64, 8613–8619 [DOI] [PubMed] [Google Scholar]
- 77.Chen H, et al. (2015) Silencing of plasminogen activator inhibitor-1 suppresses colorectal cancer progression and liver metastasis. Surgery 158, 1704–1713 [DOI] [PubMed] [Google Scholar]
- 78.Hwang RF, et al. (2003) Inhibition of platelet-derived growth factor receptor phosphorylation by STI571 (Gleevec) reduces growth and metastasis of human pancreatic carcinoma in an orthotopic nude mouse model. Clinical Cancer Research 9, 6534–6544 [PubMed] [Google Scholar]
- 79.Simpson KD, et al. (2012) Macrophage migration inhibitory factor promotes tumor growth and metastasis by inducing myeloid-derived suppressor cells in the tumor microenvironment. The Journal of Immunology 189, 5533–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DAO TL (1962) Regression of Pulmonary Metastases of a Breast Cancer: Report of a Case of Spontaneous and Temporary Regression After Radical Mastectomy. Archives of Surgery 84, 574–577 [DOI] [PubMed] [Google Scholar]
- 81.Lekanidi K, et al. (2007) Spontaneous regression of metastatic renal cell carcinoma: case report. Journal of medical case reports 1, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]