Abstract
The regulator of sigma D (Rsd) was identified as an RNA polymerase ς70-associated protein in stationary-phase Escherichia coli with the inhibitory activity of ς70-dependent transcription in vitro (M. Jishage and A. Ishihama, Proc. Natl. Acad. Sci. USA 95:4953–4958, 1998). Primer extension analysis of rsd mRNA indicated the presence of two promoters, ςS-dependent P1 and ς70-dependent P2 with the gearbox sequence. To get insight into the in vivo role of Rsd, the expression of a reporter gene fused to either the ς70- or ςS-dependent promoter was analyzed in the absence of Rsd or the presence of overexpressed Rsd. In the rsd null mutant, the ς70- and ςS-dependent gene expression was increased or decreased, respectively. On the other hand, the ς70- or ςS-dependent transcription was reduced or enhanced, respectively, after overexpression of Rsd. The repression of the ςS-dependent transcription in the rsd mutant is overcome by increased production of the ςS subunit. Together these observations support the prediction that Rsd is involved in replacement of the RNA polymerase ς subunit from ς70 to ςS during the transition from exponential growth to the stationary phase.
The survival of bacterial cells in various environments depends on their abilities to sense the external conditions and adopt their internal metabolic systems by turning on or off the expression of specific sets of genes (17). Bacteria employ several different systems for switching the global pattern of gene expression. The specificity control of the transcription apparatus is a powerful mechanism with which to change the gene expression pattern. In Escherichia coli, the RNA polymerase core enzyme with the subunit composition α2ββ′ has the ability to transcribe the genetic information on DNA into RNA. For initiation of transcription at specific promoter sites on DNA, an additional component, the ς subunit, is required (7). The promoter recognition specificity of RNA polymerase is conferred by one of the multiple species of ς subunit (21). Replacement of the ς subunit on RNA polymerase is an efficient way of switching the transcription pattern.
Up to the present time, seven different molecular species of the ς subunit have been identified in E. coli (21, 25). The major ς subunit, ς70, is responsible for transcription of most, if not all, genes expressed during exponential cell growth (21, 25, 51). The other six species of the ς subunit are required only during certain growth stages or under specific growth conditions. In agreement with their functional roles, the levels of these alternative ς subunits vary, depending on the cell growth conditions (31, 34). In addition to the level control, the activity of at least some E. coli ς subunits is also subject to control in various ways (26, 27). For instance, the unused ς subunits are stored in inactive forms by forming complexes with another set of proteins, often designated as anti-ς factors, with the regulatory activity of ς functions (24, 27).
Bacteria use flagella to move away from stressful areas into microenvironments favorable for growth. Subunit ς28 (ςF) is involved in transcription of the genes required for the formation of flagella. The flgM gene product is an anti-ςF factor that acts by directly binding ςF and thereby preventing its interaction with the core RNA polymerase (40). Subunit ς24 (ςE) is a member of the ECF (extracytoplasmic function) family of ς subunits for transcription of the genes for proteins involved in extracytoplasmic functions (44) as well as those required for survival at high temperature, or thermotolerance (14). The ςE activity is regulated by the rseA (regulator of sigma E, or anti-ςE factor) gene product, which is associated with the inner membrane and inhibits the activity of ςE by directly interacting with ςE (12, 48). FecI, which belongs to the ECF family, is involved in transcription activation of the ferric-citrate transport genes (fec) (4). Genetic studies revealed that FecR, an inner membrane protein, negatively regulates the activity of FecI (59). Likewise, DnaK associates with and possibly controls the activity of the heat shock ς32 (ςH) subunit (24), which is induced following heat shock, and is involved in transcription of the genes encoding heat shock proteins, including DnaK, DnaJ, and GrpE (18). After returning from heat shock to normal growth conditions, unused ςH is temporarily stored as DnaJ-DnaK-ςH complexes (43), which are then dissociated by the action of GrpE, and the released ςH is finally degraded by FtsH protease (16).
Recently we discovered a novel E. coli protein, referred to Rsd (regulator of sigma D), which forms a complex with ς70 and prevents its function (33). Purified Rsd protein formed complexes in vitro with ς70, but not with other ς subunits, and inhibited ς70-dependent transcription in vitro to various extents, depending on the promoters used. Since Rsd is induced in the stationary phase of cell growth, unused excess ς70 subunit, without being involved in the transcription cycle, should be trapped by Rsd. Thus, the possibility has arisen that Rsd is an anti-ς factor for the major ς70 subunit for its storage in the stationary phase. In order to clarify the in vivo function of Rsd, we analyzed the influence of both depletion and overproduction of Rsd on ς70- and ςS-dependent transcription in vivo. On the basis of the results herewith described, we propose that Rsd is a regulator that facilitates the switching of ς subunit on RNA polymerase from ς70 to ςS during the transition from exponential growth to stationary phase.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The E. coli strains used in this work are listed in Table 1. Cells were grown at 37°C with shaking in either Luria-Bertani (LB) broth or medium M9 (46). For cultures of cells carrying antibiotic resistance markers, the media were supplemented with ampicillin (100 μg/ml), chloramphenicol (20 μg/ml), or kanamycin (50 μg/ml). For induction of the cloned genes under the control of the arabinose-regulated promoter, arabinose was added at a final concentration of 0.02%.
TABLE 1.
Characteristics of bacterial strains used in this study
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| W3110 | Type A | 32 |
| MC4100 | F−araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR | National Institute of Genetics stock ME8286 |
| JC7623 | argE3 his4 leu6 proA2 thr1 thi1 rpsL31 galK2 lacY1 ara14 xyl15 mtl1 supE44 kdgK51 recB15 recC22 sbcB15 | 50 |
| ZK126 | W3110 ΔlacU169 tna2 | 6 |
| ZK1000 | ZK126 rpoS::Km | 6 |
| CF1946 | CF1943 ΔrelA251::Km ΔspoT207::Cm | 62 |
| MH20 | F− ΔlacU169 rpsL relA thiA flbB | 20 |
| MH513 | MH20 λ(ompF-lacZ) 16-13 | 20 |
| MJ57 | MH513 rsd::Km | This work |
| MJ83 | MH513 rpoS::Km | This work |
| MJ31 | MC4100 λ(bolA-lacZ) | This work |
| MJ6 | MC4100 λ(rsdI-lacZ) | This work |
| MJ39 | MJ6 spoT::Cm | This work |
| MJ19 | MC4100 λ(rsdIII-lacZ) | This work |
| MJ30 | MC4100 rsd::Km | This work |
| MJ35 | MJ30 λ(bolA-lacZ) | This work |
| MJ23 | MC4100 rpoS::Km | This work |
| MJ27 | MJ23 λ(rsdI-lacZ) | This work |
Plasmids.
The plasmids used in this work are listed in Table 2. To create an Rsd expression plasmid, pUCRsd33, carrying the entire rsd gene from its own promoters to terminator(s), an 820-bp-long DNA fragment was PCR amplified by using primers Rsd-5 (5′-CGCGGATCCAACCAAACAGGTTCCCCCTGCCAT-3′; BamHI site underlined) and Rsd-6 (5′-AACTGCAGCTCGAGCTCAGCCAGTTAAGGCACTCC-3′; PstI site underlined) (see Fig. 1F for the locations of primer sequences on the rsd gene), and the resulting PCR product was cloned into pUC18 between the BamHI and PstI sites to construct pUCRsd33. The BamHI-SphI fragment was isolated from pUCRsd33 and recloned into pACYC184 (9) to create pACYCRsd. The cloned rsd gene carries the entire rsd sequence downstream from nucleotide −261 (as counted from the ATG initiation codon of Rsd) (see Fig. 1E for the rsd sequence).
TABLE 2.
Characteristics of plasmids used in this study
| Plasmid | Vector | Gene to be expressed | Source or reference |
|---|---|---|---|
| pUJC-1 | pUC18 | Truncated rsd | This work |
| pUCRsd33 | pUC18 | Complete rsd | This work |
| pACYCRsd | pACYC184 | Complete rsd | This work |
| pBADRsd31-1 | pBAD22A | Complete rsd | This work |
| pBF1 | pBAD22A | rpoS | 57a |
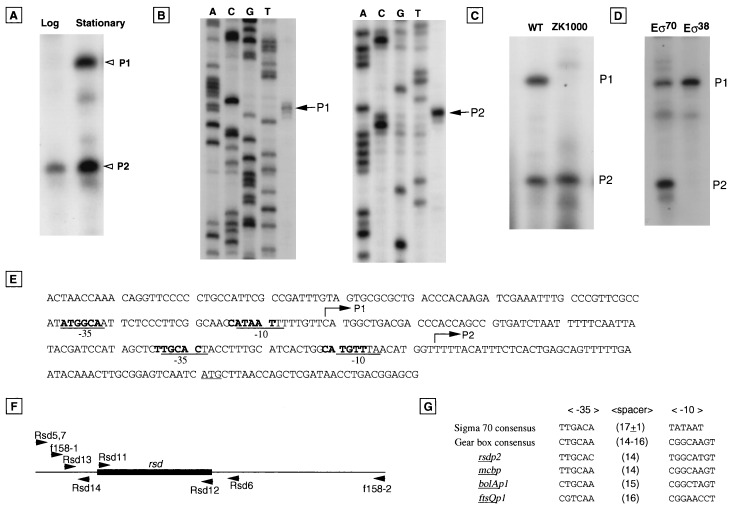
FIG. 1.
Identification of the rsd promoters. (A) Primer extension analysis was carried out for RNA extracted from the exponentially growing cells (Log lane) and the stationary-phase cells (Stationary lane). (B) Primer extension products were analyzed by electrophoresis on a denatured polyacrylamide gel together with sequence ladders. P1, transcript from rsdP1 promoter; P2, transcript from rsdP2 promoter. (C) Primer extension analysis was carried out for RNA extracted from stationary-phase cells of wild-type (WT) strain ZK126 (rpoS+) and mutant strain ZK1000 (rpoS). (D) In vitro transcription of the rsd promoters by Eς70 or EςS holoenzymes. Transcription products were analyzed by polyacrylamide gel electrophoresis in the presence of 8 M urea. (E) Nucleotide sequence of the upstream region of rsd. (F) Primers used in this study. (G) Sequence comparison of rsdP2 with the known gearbox promoters.
To construct pBADRsd31-1 for high-level expression of Rsd, an rsd fragment containing only the Rsd coding sequence without the promoters was PCR amplified with primers Rsd11 (5′-CCGGAATTCACCATGCTTAACCAGCTCGATAAC-3′; EcoRI site and the rsd initiation codon, underlined) and Rsd12 (5′-CATGCATGCTCAAGCAGGATGTTTGACGCGGGC-3′) (see Fig. 1F for the locations of the primer sequences), and cloned into pBAD22A (19) between the EcoRI and SphI sites.
Construction of the rsd promoter-lacZ transcriptional fusions.
Two species of rsd promoter fragment were PCR amplified with two pairs of primers, Rsd7 (5′-CCGGAATTCAACCAAACAGGTTCCCCCTGCCAT-3′; EcoRI site underlined) plus Rsd14 (5′-CGCGGATCCCAGTGAGAAATGTAAAAACCATGT-3′; BamHI site underlined), and Rsd13 (5′-CCGGAATTCACGACCCACCAGCCGTGATCTAAT-3′; EcoRI site underlined) plus Rsd14 (see Fig. 1F for the locations of these sequences on rsd). The PCR products were cloned into pRS551 between the EcoRI and BamHI sites, generating pRsd1 or pRsd3, respectively. pRsd1 and pRsd3 contain the rsd promoter region sequence from either −261 or −138, respectively, to −34. The sequences of these rsd inserts were confirmed by dideoxynucleotide sequencing. The rsd gene fusions were then integrated onto phage λRS45, and the recombinant phages were used to lysogenize MC4100 as described previously (56).
As a control, strain MC4100 carrying the bolA-lacZ transcriptional fusion on the genome was constructed with λ(bolAP1-lacZ) (2).
Disruption of the rsd gene.
Strain MJ30 carrying an internal deletion of the rsd gene was constructed as follows. A 1.4-kbp rsd gene fragment was PCR amplified with primers f158-1 (5′-CATGCATGCCACAAGATCGAAATTTGCCCGTTC-3′) and f158-2 (5′-CCGGAATTCCATTTCCGGCGTGATGATGCCCTG-3′), which were used for the cloning of rsd (33), and subcloned into pUC18 between the SphI and EcoRI sites. The rsd coding region between the BsmI and SnaBI sites was replaced by a HincII fragment of pUC4K (Pharmacia) carrying the kanamycin resistance gene. The resulting plasmid, pUJC-1, was digested with a mixture of BsmI and SnaBI, and the BsmI-SnaBI fragment was purified by SUPREC01 (Takara Shuzo Co.). Two micrograms of this linear DNA fragment was transformed into E. coli JC7623 (50). Kanamycin-resistant transformants were isolated, which carried the rsd deletion mutation integrated in the chromosome. Phage P1vir transduction was used to transfer the rsd mutation to strain MC4100 for construction of MJ30.
Primer extension analysis.
Cells of E. coli W3110 type A (32) were grown in LB medium at 37°C. At both the exponentially growing phase and the transition phase from exponential growth to stationary phase, total RNA was prepared by phenol extraction according to the method of Aiba et al. (1). For primer extension reactions, a 25-nucleotide-long primer with the sequence 5′-TGACGCGCTCCGTCAGGTTATCGAG-3′, corresponding to the rsd coding sequence between +13 and +37 (as counted from the ATG initiation codon), was 32P-labeled by using MEGALABEL (Takara Shuzo). The reaction mixture, containing 2 pmol of the end-labeled primer and 50 μg of total RNA, was heated for 5 min at 80°C, followed by incubation on ice for 5 min. After addition of 12.5 U of avian myeloblastosis virus reverse transcriptase (Takara Shuzo) in 50 mM Tris-HCl (pH 7.6), 60 mM KCl, 10 mM MgCl2, 1 mM deoxynucleoside 5′-triphosphates, and 1 mM dithiothreitol in a total volume of 20 μl, the mixture was incubated at 42°C for 60 min. The reaction was terminated by adding 180 μl of a stop solution (0.15 M NaOH and 5 mM EDTA), followed by incubation at 70°C for 20 min. After precipitation with ethanol, the samples were resuspended in 15 μl of formamide loading buffer and analyzed by electrophoresis on a 6% polyacrylamide gel containing 8 M urea. Dideoxy sequencing reactions were carried out with the appropriate plasmid DNA as the template and the primer used for primer extension. Reaction products were run in parallel with the sequence ladder obtained with a 7-DEAZA sequencing kit (Takara Shuzo, Japan) to determine the end point of extension products.
β-Galactosidase assay.
The activity of β-galactosidase was assayed according to the procedure of Miller (46), by using cells which were made permeable by treatment with sodium dodecyl sulfate and CHCl3. The activity assay was repeated at least twice for each sample. The activity is expressed as Miller units: 1,000 × [(A420 − 1.75 × A550)/(A600 × reaction time × volume)].
In vitro single-round transcription assay.
RNA polymerase core enzyme was purified from E. coli W3350 by passage of the purified RNA polymerase at least three times through phosphocellulose columns (39). Holoenzymes were reconstituted by mixing the core enzyme and threefold molar excess of each ς subunit. Single-round transcription by the reconstituted holoenzymes was carried out under the standard reaction conditions described previously (35).
Quantitative Western blot analysis.
A quantitative Western blot analysis was carried out according to the method of Jishage and Ishihama (31). Polyclonal antibodies against purified ς70, ςS, and Rsd were raised in rabbits as described previously (31, 33).
RESULTS
Identification of the transcriptional start sites of rsd.
The intracellular level of Rsd protein increases during the transition from exponential growth to the stationary phase (33). In order to get insight into the regulatory mechanism underlying growth-dependent expression of the Rsd protein, we determined the transcription start site(s) of the rsd gene. For this purpose, total RNA was isolated from both exponentially growing and stationary-phase cells of the type A W3110 strain, which carries the rpoS gene in its intact form (32), and was subjected to primer extension analysis. Only one major product (P2) was observed for RNA from the exponential-phase cells (Fig. 1A). Besides the P2 transcript, another product (P1) was identified for RNA from the stationary-phase cells (Fig. 1A). The transcription start point of P1 RNA is located at 148 bp upstream of the translation initiation codon (Fig. 1B), while the start site of P2 transcript is located at 54 bp upstream of the initiation codon, the two promoters being separated by 94 bp (Fig. 1E).
The P1 start site at −148 is preceded by a sequence, CATAAT, with a reasonable similarity to the consensus sequence (TATAAT) of ς70-dependent promoter −10 (Fig. 1E). Separated from this −10-like sequence by a 17-bp spacer is an ATGGCA sequence with a reasonable similarity to the consensus sequence (TTGACA) of ς70-dependent promoter −35 (Fig. 1E). On the other hand, the transcription of P2 is initiated at G at −54 bp upstream from ATG (Fig. 1B). Although the −10 hexamer of P2 (CATGTT) is not in good agreement with the ς70 promoter −10 consensus sequence, the presence of upstream TG characterizes it as an extended −10 promoter (TGGCATGTT) (36, 47). Such a −10 sequence alignment is a common feature of several gearbox promoters (Fig. 1G) (3, 60). The −35 hexamer (TTGCAC) is separated from the −10 sequence by a 17-bp spacer and is in agreement with the −35 sequence associated with the gearbox promoters (Fig. 1E). Two gearbox promoters, bolAp1 and ftsQp1, are known to be recognized by the ςS subunit (5, 6, 40). To determine whether ςS is responsible for transcription from the rsd P1 and P2 promoters, total RNA was isolated from strain ZK1000, which lacks rpoS, and analyzed by primer extension. As shown in Fig. 1C, the P1 product was not detected in the absence of rpoS. The results suggest that the P1 promoter is dependent on ςS, whereas the P2 promoter is transcribed by Eς70. This conclusion was confirmed by using an in vitro transcription assay. As shown in Fig. 1D, EςS can transcribe only from the P1 promoter, whereas Eς70 is able to initiate transcription from both the P1 and P2 promoters. (Note that the ςS-dependent promoters are recognized in vitro by both Eς70 and EςS under the conditions employed [58].)
Influence of growth rate and growth phase on the expression of rsd.
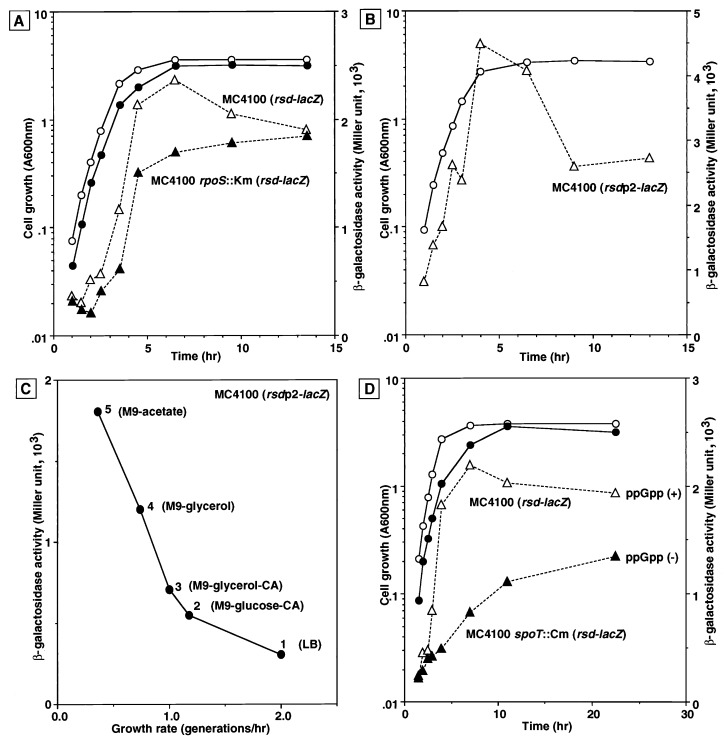
Transcription from the gearbox promoters bolAp1 and ftsQp1 increases in the stationary phase of cell growth, and the level of transcription at the growth phase is inversely related to growth rate (3). To determine whether the expression of rsd is also dependent on growth phase or growth rate, we constructed an rsd-lacZ transcriptional fusion on phage λ and inserted the transducing phage at its normal attachment site on the E. coli genome. By using the rsd-lacZ transductant thus constructed, the β-galactosidase activity was measured at various time points during the transition from exponential growth to the stationary phase in LB medium at 37°C. The β-galactosidase activity increased seven- to eightfold in the stationary phase compared to that at the exponential phase (Fig. 2A).
FIG. 2.
Growth-dependent expression of the rsd-lacZ fusions. (A) Strain MJ6 [MC4100 λ(rsdI-lacZ); open symbols] and an isogenic rpoS mutant, MJ27 [MC4100 rpoS::Km λ(rsdI-lacZ); solid symbols], both carrying the rsdP1P2-lacZ fusion integrated in the genome (Table 1), were grown in LB medium. Cell growth (circles) was monitored by measuring turbidity, while rsd promoter activity was determined by measuring β-galactosidase activity (triangles). (B) Strain MJ19 [MC4100 λ(rsdIII-lacZ)] carrying the rsdP2-lacZ fusion was grown in LB medium. The cell growth (circles) and the β-galactosidase activity (triangles) were measured at the indicated times. (C) MJ6 [MC4100 λ(rsdI-lacZ)] was grown in LB medium (medium 1), M9–0.4% glucose–0.4% Casamino Acids (medium 2), M9–0.4% glycerol–0.4% Casamino Acids (medium 3), M9–0.4% glycerol (medium 4), and M9–0.4% acetate (medium 5). Exponentially growing cells at A600 of 0.4 to 0.5 were used for the assay of β-galactosidase activity. (D) Strain MJ6 [MC4100 λ(rsdI-lacZ); open symbols] and its isogenic spoT mutant, MJ39 [MC4100 rpoT::Cm λ(rsdI-lacZ); solid symbols], were grown in LB medium. The β-galactosidase activity (triangles) and cell growth (circle) were measured at the indicated times.
To examine whether this activation of the rsd promoter in the stationary phase is dependent on ςS, the rsd(P1-P2)-lacZ fusion gene was transduced into an rpoS null mutant, and the β-galactosidase activity was measured at different growth phases. The results, shown in Fig. 2A, indicated that the β-galactosidase activity in the rpoS null mutant also increased during the transition from the exponential growth phase to the stationary phase, but the maximum level of expression in the rpoS mutant was about 70% the level of the wild-type transductant in the early stationary phase (3 to 4 h after the cessation of cell growth). After prolonged culture in the stationary phase, however, the β-galactosidase activity in the rpoS mutant reached the same level as that observed with the wild type, suggesting that the basal level of rsd transcription is maintained by using the downstream P2 promoter recognized by Eς70 RNA polymerase, and transcription from the upstream P1 promoter by EςS takes place only in the early stationary phase.
To measure the β-galactosidase activity solely from the P2 promoter, another rsd(P2)-lacZ transcriptional fusion without the P1 promoter was constructed and transduced into the wild-type strain. Again the maximum activity was observed during the transition from exponential growth to stationary phase (Fig. 2B). Moreover, the maximum level of β-galactosidase activity driven by the P2 promoter alone was twofold higher than the activity from both P1 and P2. One possible explanation for this unexpected observation is that a regulatory signal repressing rsd transcription is located upstream of the P2 promoter.
We next investigated the effect of growth rate on rsd expression. The expression levels of the rsd(P1-P2)-lacZ fusion were compared among the exponentially growing cultures in media supplemented with various carbon sources. As shown in Fig. 2C, the β-galactosidase activity was low in cells growing at high rates (media 1 to 3), but substantially increased in cells growing at lower rates (media 4 and 5). This result indicates that rsd expression is inversely related to the growth rate. Genes whose expression increase with decreasing growth rate are often under the positive control of ppGpp, the mediator of stringent control (for details see Discussion). To determine whether ppGpp also affects rsd expression, we measured the β-galactosidase activity in a relA1 spoT strain which does not produce ppGpp. As shown in Fig. 2D, the β-galactosidase activity in the mutant strain MJ39 devoid of ppGpp was reduced to 38% the level of the relA1 spoT+ strain at the maximum expression, and then the expression increased to 70% in the late stationary phase. These observations suggest that ppGpp is partly involved in stimulation of rsd transcription, but the full expression of rsd may require an additional factor(s) or condition(s).
Effect of rsd mutation on ςS-dependent transcription.
Upon entry into the stationary phase, ςS begins to be produced (31, 34) and allows the core polymerase to recognize and transcribe the genes required for stationary-phase survival (22). Previously we showed that Rsd interacts in vitro preferentially with free ς70, but not the core enzyme-bound ς70 (Eς70) (33). If Rsd interacts in vivo with free ς70, the level of functional ς70 available for use in the transcription cycle should be reduced, depending on the concentration of Rsd, ultimately leading to the switching of the global transcription pattern from the ς70-dependent genes to those which carry ςS-dependent promoters.
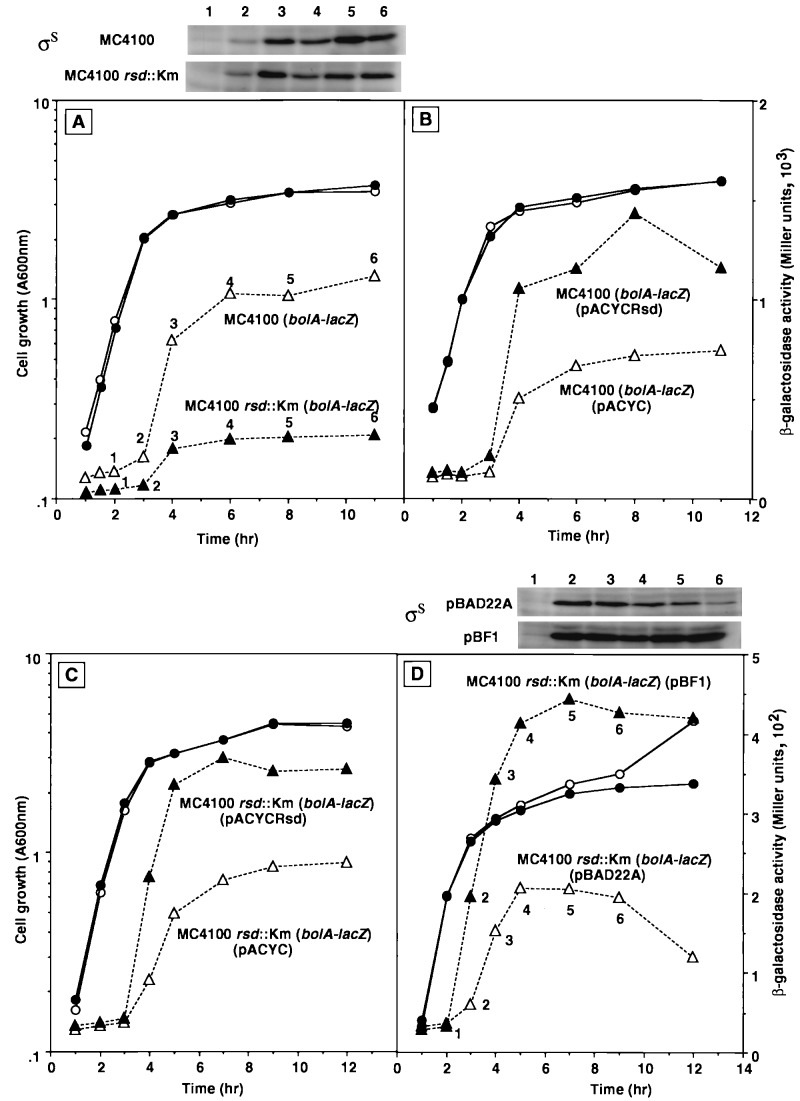
To test the above possibility, we constructed a mutant E. coli strain lacking the rsd gene and measured the expression of a ςS-dependent bolAp1-lacZ transcription fusion under the exogenous supply of various levels of Rsd. As shown in Fig. 3A, the expression of bolAp1-lacZ in the rsd mutant strain was reduced to about 30% the level of wild-type strain. However, Western blotting analysis indicated that this reduction in β-galactosidase synthesis was not caused by a decrease or increase in the levels of ςS and ς70 proteins, respectively (for ςS, see the Western blot pattern above Fig. 3A [data not shown for the ς70 pattern]). Thus, the decrease in Rsd level led to a reduction in the utilization of ςS for expression of the ςS-dependent bolAp1-lacZ fusion gene. We then tested the opposite case, i.e., the effect of increased expression of Rsd on ςS-dependent gene expression. Expression of Rsd in wild-type cells by using an rsd expression vector, constructed with plasmid pACYC, resulted in a transient but significant (about 1.5-fold) increase in the expression level of bolAp1-lacZ (Fig. 3B). Western blot analysis indicated that the maximum level of Rsd expression was higher than that of ς70 (data not shown). To confirm that the observed decrease or increase in bolAp1-lacZ expression was due to the direct effect of a decrease or increase, respectively, in the Rsd level, we introduced an Rsd expression vector, pACYCRsd, into the rsd mutant. As expected, the β-galactosidase activity increased by about twofold after induction of Rsd (Fig. 3C).
FIG. 3.
Effect of the rsd mutation on bolAP1-directed transcription. Cells were grown in LB medium. Growth (circles) was monitored by measuring turbidity, while β-galactosidase activity (triangles) was determined at the indicated times. Aliquots containing the same cell numbers were subjected to a quantitative Western blot assay for measurement of ςS levels. (A) Strain MJ31 [MC4100 λ(bolA-lacZ); open symbols] and its isogenic rsd mutant, MJ35 [MC4100 rss::Km λ(bolA-lacZ); solid symbols]. (B) MJ31 [MC4100 λ(bolA-lacZ)] carrying either pACYC184 (open symbols) or pACYCRsd (solid symbols). (C) MJ35 [MC4100 rsd::Km λ(bolA-lacZ)] carrying either pACYC184 (open symbols) or pACYCRsd (solid symbols). (D) MJ35 [MC4100 rsd::Km λ(bolA-lacZ)] carrying either pBAD22A (open symbols) or pBF1 (solid symbols). Arabinose was added at 2 h of culture (time point 1).
The increase in Rsd should lead to a decrease in the concentration of functional ς70 subunit, and as a result, the relative amount of EςS holoenzyme may increase because the intracellular level of core enzyme stays constant at a level characteristic of the rate of cell growth (29). Likewise the decrease in Rsd level may result in an increase in Eς70 holoenzyme, ultimately leading to the reduction in EςS level. In order to further confirm this hypothesis, we next examined possible effect of the exogenous supply of ςS on the expression of bolAp1-lacZ fusion. For this purpose, ςS was overexpressed by using the ςS expression vector under the control of an arabinose-inducible promoter (see the Western blot pattern shown above Fig. 3D). The activity of bolAp1-lacZ indeed increased more than twofold (Fig. 3D), reaching a level as high as that observed when Rsd was expressed in the rsd mutant strain (Fig. 3C).
Altogether, these phenomena support the prediction that the decrease in intracellular level of functional ς70 by forming complexes with Rsd leads to the increase in EςS level and activation of transcription from ςS-dependent promoters.
Effect of rsd mutation on ς70-dependent transcription.
The total number of core enzyme molecules, which are not involved in the transcription cycle, is close to the combined number of seven species of the ς subunit (25, 27). Thus, the intracellular concentrations of seven species of the ς subunit should be the major determinant of the relative amount of the seven forms of the holoenzyme. If this is the case, an increase or decrease in the amount of one ς subunit should affect not only the level of the holoenzyme containing that particular ς subunit, but also the levels of other holoenzymes containing different ς subunits. To monitor the change in the intracellular level of ς70 with a high sensitivity, we used the ompF promoter as a test promoter. The ompF gene encoding an outer membrane porin protein is transcribed by Eς70 and regulated by OmpR. The level of ompF transcription is known to be directly correlated to the level of Eς70 (15, 54). Thus, the rsd null mutation may lead to an increase in functional ς70 (and a decrease in EςS level) and ultimately to induction of ompF transcription. To test this possibility, we next measured the β-galactosidase activity encoded by the ompF-lacZ transcriptional fusion.
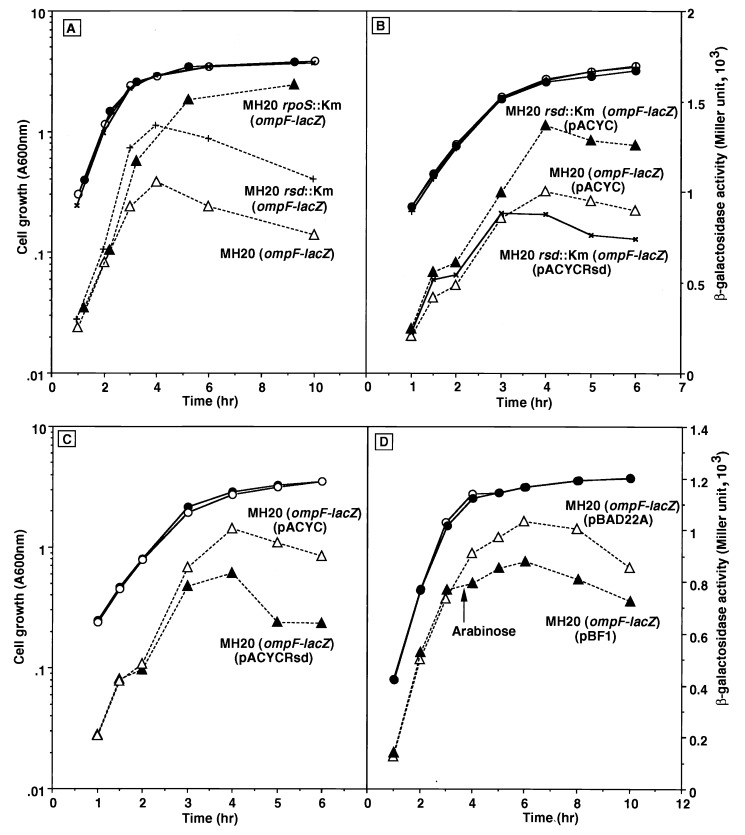
As shown in Fig. 4A, the expression of the ompF-lacZ fusion significantly increased in the mutant E. coli strain lacking the rsd gene (Fig. 4A), but the high-level expression of ompF-lacZ in the rsd mutant was suppressed by the supply of Rsd protein by an expression plasmid (Fig. 4B). The inhibitory effect on β-galactosidase synthesis by the overexpressed Rsd was also observed with the wild-type E. coli (Fig. 4C). All of these observations are consistent with the prediction that the Rsd protein forms complexes with ς70, and thereby the concentration of holoenzyme Eς70 decreases, leading to the reduction in ς70-dependent transcription.
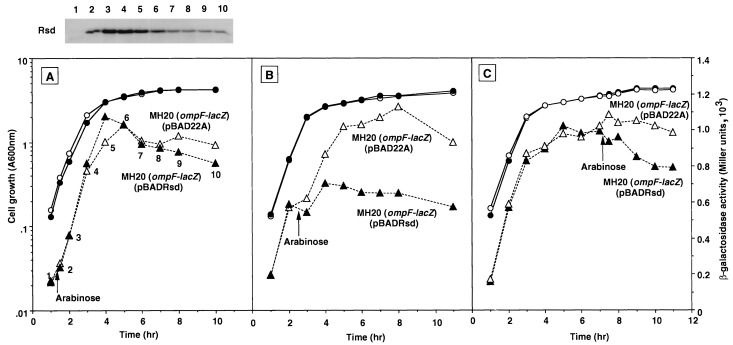
FIG. 4.
Effect of the rsd mutation on ompF promoter-directed transcription. Cells were grown in LB medium. Growth (circles) was monitored by measuring turbidity, while β-galactosidase activity (triangles) was determined at the indicated times. Expression of ςS was induced by adding arabinose (0.02%) at the middle of the exponential phase. All E. coli strains carried the ompF-lacZ fusion integrated in the genomes. (A) Strain MH513 [MH20 λ(ompF-lacZ); open symbols], its isogenic rpoS mutant, MJ83 [MH20 rpoS::Km λ(ompF-lacZ); solid symbols], and an rsd mutant, MJ57 [MH20 rsd::Km λ(ompF-lacZ); crosses]. (B) MJ513(pACYC184; open symbols), MJ57(pACYC184; solid symbols), and MJ57(pACYCRsd; crosses). (C) MH513(pACYC184; open symbols) and MH513(pACYCRsd; solid symbols). (D) MH513(pBAD22A; open symbols) and MH513(pBF1; solid symbols).
A decrease in Eς70 may also take place with an increase in other ς subunits. To test this possibility, attempts were made to change the level of the ςS subunit. In the rpoS mutant, the level of ompF-lacZ expression increased about twofold compared with that in wild-type cells (Fig. 4A). On the other hand, the expression of ompF-lacZ was significantly reduced upon induction of the expression of the ςS subunit under control of the arabinose-inducible promoter (Fig. 4D). In agreement with our observations, it has been reported that rpoS mutations result in overproduction of OmpF (49), whereas the expression of ςS leads to repression of ompF expression (54).
Influence of Rsd expression on ς70-dependent transcription.
If the competition in core binding among various ς subunits is so critical for the determination of the global pattern of transcription, the level of ς70-dependent transcription should be influenced by the presence or absence of other minor ς subunits. The influence of the high-level expression of Rsd on ς70-dependent ompF transcription was examined in the presence and absence of the ςS subunit. The high-level expression of Rsd protein could be achieved by inserting the rsd gene into an expression vector under the control of the arabinose-inducible promoter. When Rsd was induced in the early exponential phase (in the absence of ςS), the expression level of ompF-lacZ fusion, as measured by β-galactosidase activity, was essentially the same as that in the absence of Rsd induction (Fig. 5A). On the other hand, the expression of Rsd in the late exponential phase or the early stationary phase (in the presence of ςS) significantly inhibited the expression of the ompF-lacZ fusion, down to about 60% of the level in the absence of Rsd expression (Fig. 5B). In the late stationary phase, the inhibitory effect of Rsd on the ompF-lacZ induction again became weaker, giving 80% of the activity without Rsd expression (Fig. 5C).
FIG. 5.
Effect of Rsd production on ompF-directed transcription. Cells of MH513 [MH20 λ(ompF-lacZ)] carrying either pBAD22A (open symbols) or pBADRsd (closed symbols) were grown in LB medium. Growth (circles) was monitored by measuring turbidity, while β-galactosidase activity (triangles) was determined at the indicated times. Overproduction of Rsd was induced by adding arabinose (0.02%) at the early exponential phase (A), at the middle of the exponential phase (B), and after entry into the stationary phase (C). Aliquots containing the same cell numbers were subjected to quantitative Western blotting for measurement of the Rsd level.
The fluctuation of the inhibition level of ompF-lacZ expression by the expressed Rsd could be observed if the level of Rsd expression is altered, depending on the cell growth phase. By checking the expression level of plasmid-encoded Rsd by quantitative Western blotting, however, the level of Rsd expression was found to stay almost constant, at least during the growth transition period analyzed in this study (data not shown). Since the pattern of growth phase-dependent variation in the inhibition of ompF-lacZ expression by the overexpressed Rsd correlates with the change in ςS production level, we assumed that the effect of Rsd is observed only in the situation in which the ςS subunit is present for competition with ς70 in binding to the core enzyme. This is consistent with the expectation deduced from our proposal that the presence of functional ςS represses the expression of ς70-dependent genes such as ompF.
DISCUSSION
The rsd gene was found to carry two transcriptional start sites, rsdP1 and rsdP2. Transcription from the upstream P1 promoter is dependent on ςS, while the downstream P2 is driven by ς70. The sequence of P2 shows a strong similarity to that of the gearbox promoters, such as bolAP1 and ftsQP1, which are both induced in the stationary phase (2, 3, 45) and are transcribed by the EςS holoenzyme (5, 6, 41). On the basis of sequence similarity and unique expression patterns, such as the inverse relationship with the growth rate (Fig. 2C), the rsdP2 promoter can be classified as a member of the gearbox family promoters, but it cannot be transcribed by EςS in vivo (Fig. 1C) and in vitro (Fig. 1D). The sequence TGGCATGT of the rsdP2 promoter −10 region is slightly different from the gearbox consensus sequence, CGGCAAGT (Fig. 1G). The gearbox promoter for mcb also does not require ςS for its stationary-phase induction (6, 42), but instead, the ς70-dependent mcb transcription requires transcription factors such as OmpR and EmrR (45). Thus, the possibility should be tested that an as yet unidentified transcription factor(s) is involved in ς70-dependent transcription from rsdP2. The mediator ppGpp for stringent control is one candidate for the transcription factor for transcription activation from the rsdP2 promoter (see below).
Even though the rsd gene carries the ςS-dependent P1 and ς70-dependent P2 promoters, the contribution of ςS in the expression of rsd seems to be transient for a short period during the transition from exponential growth to the stationary phase (Fig. 2A and 5). Under certain circumstances, the upstream sequence including the P1 promoter exerts an inhibitory effect on the P2 function, because the deletion of the P1 region resulted in an increase in transcription from the downstream P2 promoter (Fig. 2B). The mechanism of inhibition by the upstream sequence remains unsolved.
The expression of several genes encoding stationary-phase proteins or stress-response proteins, including rmf (63), cspD (64), lrp (41), and sspA (61), shows an inverse relationship to the growth rate, even though the promoters of these genes do not share a common sequence. The rmf gene encoding a protein associated with 100S ribosome dimers is expressed in the stationary phase, but does not require ςS (63). Disruption of rmf results in loss of the formation of ribosome dimers and reduction in cell viability during the stationary phase (63). A stationary-phase protein, CspD, with the predicted function of an RNA and/or DNA chaperone has a high level of sequence similarity to the cold shock protein CspA, but cspD expression is not induced by cold shock (64). Stringent starvation protein A (SspA) is one of the RNA polymerase-associated proteins (28). The synthesis of SspA is induced by starvation for glucose, nitrogen, phosphate, or amino acids (61). Lrp is also induced under starvation conditions and plays an enhancing role in transcription of some stress-response genes (41). The stress-response genes under the control of gearbox promoters are also inversely proportional to the growth rate. Detailed analysis is needed to define the promoter element(s) that is present in these promoters, including rsdP2, and is critical for the inverse relationship with the growth rate.
After entry into the stationary phase or under carbon source starvation, the cellular level of ppGpp is known to increase (8). Direct interaction of ppGpp with the RNA polymerase has been demonstrated both in vitro (11, 55) and in vivo (8, 23). The ppGpp-associated RNA polymerase loses transcription activity of the growth-related genes such as those for rRNA, ribosomal proteins, and tRNA. The promoter activity of the genes, rsd (this study), cspD (64), lrp (41), and sspA (61), all showing an inverse relationship with the growth rate, is positively regulated by ppGpp, suggesting that the putative DNA signal must be recognized by the ppGpp-bound RNA polymerase. At present, however, an indirect effect of ppGpp has not been excluded.
To gain insight into the function of Rsd in vivo, we constructed an rsd null mutant strain. However, the mutant showed apparently no distinct phenotype from the wild-type parental strain, as analyzed by measuring its growth curve and its viability in various media. Several lines of evidence, however, indicated that the variation in Rsd level influenced the level of ς70- and ςS-dependent transcription of at least some specific genes. (i) The level of ς70-dependent ompF-lacZ and ςS-dependent bolA-lacZ fusions increases and decreases, respectively, in the absence of Rsd (Fig. 3), but (ii) the expression of ompF-lacZ and bolA-lacZ decreases and increases, respectively, in the presence of overexpression of Rsd (Fig. 4).
The intracellular concentration of the RNA polymerase core enzyme stays constant (25, 29), while the levels of seven species of the ς subunit vary, depending on the rate and phase of cell growth (see references 30 and 34 and also reviewed in references 26 and 27). In exponentially growing E. coli cells, only three species of the ς subunit, ς70, ςN, and ςF, are present at detectable levels (34), but upon entry into the stationary phase, the levels of both ςS and ςH increase markedly, while the levels of the other ς do not change significantly (30, 34). Under the steady state of cell growth, the vast majority of core enzyme is associated with the nucleoid and is involved in the dynamic cycle of transcription (29, 53, 57). The level of free core enzyme, not involved in transcription, is considered to be 10 to 30% of the total number of RNA polymerase molecules (29). Thus, a competition must take place between the seven species of the ς subunit for binding to a fixed number of core enzyme molecules. The observations herewith described support the prediction that Rsd binds to free ς70 subunit and thereby affects the relative level of Eς70 and EςS holoenzymes. The prediction is supported by the observations that (i) the reduction of ςS-dependent gene expression by the rsd null mutation is suppressed by the expression of ςS (Fig. 3D), (ii) overexpression of ς70 results in a reduction in ςS-dependent transcription (15), and (iii) an rpoS mutant strain exhibits increased expression of the ς70-dependent genes (15, 54). Similar situations have been observed between ς70 and ςH (ς32). For instance, the decrease in the intracellular level of ς70 results in superinduction of ςH-dependent genes (51). On the other hand, overexpression of ς70 leads to a reduction in the expression of some ςH-dependent genes (65).
The inhibitory effect of ς70-dependent transcription by the overexpressed Rsd was observed only at certain stages of the cell growth, although the levels of Rsd expression were not much different between the early and the late exponential phases (see the Western blot pattern above Fig. 5A). Possible mechanisms to explain the apparent lack of inhibitory effect of Rsd on the ς70 function in the early exponential growth phase include the following. (i) The reduction in the concentration of functional ς70 by Rsd might not affect the concentration of Eς70 holoenzyme if the level of ς70 subunit exceeds that of free core enzyme available for binding of the ς subunit. (ii) The affinity of various ς subunits to the core enzyme may vary depending on the cytoplasmic conditions. Under the conditions favorable for transcription in vitro by the Eς70 holoenzyme, the core enzyme-binding affinity of ς70 is stronger than that of the ςS subunit (38). However, transcription in vitro by the EςS holoenzyme is markedly enhanced in the presence of high concentrations of glutamate (13), trehalose (37), and polyphosphate (38). (iii) Under the steady-state growth conditions, Rsd is not synthesized, but the artificially expressed Rsd might be inactivated by an as yet unidentified mechanism. (iv) It has also not been excluded that an additional factor present only in the stationary-phase cells is required for Rsd binding to ς70.
A number of anti-ς factors have been discovered in both Bacillus subtilis and E. coli (for a review, see reference 24). The T4 AsiA protein binds to the E. coli ς70 subunit and inhibits ς70-dependent transcription, but AsiA is a positive factor for transcription of the middle class of T4 genes (52). E. coli FlgM can interact with EςF and induces the dissociation of ςF from the core enzyme (9). The ECF anti-ς factors of E. coli are inner membrane proteins which have the sensor domains in the periplasm and the ς subunit-binding domains in the cytoplasm. Extracytoplasmic signals are likely involved in the interaction between the ECF ς subunits and their cognate anti-ς factors (24). By definition, the anti-ς factors have the activity of not only binding to the cognate ς subunits but also inducing the dissociation of target ς subunits from the core enzyme (24). Rsd has binding activity with ς70, but appears to lack the activity of dissociating ς70 from Eς70. The putative accessory factor or some specific reaction conditions may be required for the enhancement of Rsd activity or the modulation of its specificity. Along this line, the possibility remains that Rsd is a different type of regulatory protein which promotes ς switching from ς70 to other minor ς subunits under stress conditions.
ACKNOWLEDGMENTS
We thank K. Tanaka (University of Tokyo) for providing pBF1 and T. Mizuno (Nagoya University) for providing strain MH513. We also thank N. Fujita for discussion.
This work was supported by grants-in-aid from the Ministry of Education, Science and Culture of Japan, and CREST (Core Research for Evolutional Science and Technology) of Japan Science and Technology Corporation (JST).
REFERENCES
- 1.Aiba H, Adhya S, de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia colicells. J Biol Chem. 1981;256:11905–11910. [PubMed] [Google Scholar]
- 2.Aldea M, Garrido T, Hernandez-Chico C, Vicente M, Kushner S R. Induction of a growth-phase-dependent promoter triggers transcription of bolA, an Escherichia colimorphogene. EMBO J. 1989;8:3923–3931. doi: 10.1002/j.1460-2075.1989.tb08573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldea M, Garrido T, Pla J, Vicente M. Division genes in Escherichia coliare expressed coordinately to cell septum requirements by gearbox promoters. EMBO J. 1990;9:3787–3794. doi: 10.1002/j.1460-2075.1990.tb07592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angerer A, Enz S, Ochs M, Braun V. Transcriptional regulation of ferric citrate transport in Escherichia coli K-12. FecI belongs to a new subfamily of ς70-type factors that respond to extracytoplasmic stimuli. Mol Microbiol. 1995;18:163–174. doi: 10.1111/j.1365-2958.1995.mmi_18010163.x. [DOI] [PubMed] [Google Scholar]
- 5.Ballesteros M, Kusano S, Ishihama A, Vicente M. The ftsQ1p gearbox promoter of Escherichia coli is a major sigma S-dependent promoter in the ddlB-ftsAregion. Mol Microbiol. 1998;30:419–430. doi: 10.1046/j.1365-2958.1998.01077.x. [DOI] [PubMed] [Google Scholar]
- 6.Bohannon D E, Connell N, Keener J, Tormo A, Espinosa-Urgel M, Zambrano M M, Kolter R. Stationary-phase-inducible “gearbox” promoters: differential effects of katF mutations and the role of ς70. J Bacteriol. 1991;173:4482–4492. doi: 10.1128/jb.173.14.4482-4492.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgess R R, Travers A A. Factor stimulating transcription by RNA polymerase. Nature. 1969;221:43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- 8.Cashel M, Gentry D R, Hernandez V J, Vinella D. The stringent response. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C: American Society for Microbiology; 1996. pp. 1458–1496. [Google Scholar]
- 9.Chadsey M S, Karlinsey J E, Hughes K T. The flagellar anti-ς factor FlgM actively dissociates Salmonella typhimurium ς28RNA polymerase holoenzyme. Genes Dev. 1998;12:3123–3136. doi: 10.1101/gad.12.19.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterji D, Fujita N, Ishihama A. The mediator for stringent control, ppGpp, binds to the β-subunit of Escherichia coliRNA polymerase. Genes Cells. 1998;3:279–287. doi: 10.1046/j.1365-2443.1998.00190.x. [DOI] [PubMed] [Google Scholar]
- 12.De Las Penas A, Connolly L, Gross C A. The ςE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of ςE. Mol Microbiol. 1997;24:373–385. doi: 10.1046/j.1365-2958.1997.3611718.x. [DOI] [PubMed] [Google Scholar]
- 13.Ding Q, Kusano S, Villarejo M, Ishihama A. Promoter selectivity control of Escherichia coli RNA polymerase by ionic strength: differential recognition of osmoregulated promoters by ED and ESholoenzymes. Mol Microbiol. 1995;16:649–656. doi: 10.1111/j.1365-2958.1995.tb02427.x. [DOI] [PubMed] [Google Scholar]
- 14.Erickson J W, Gross C A. Identification of the ςEsubunit of Escherichia coli RNA polymerase: a second alternate ς factor involved in high-temperature gene expression. Genes Dev. 1989;3:1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- 15.Farewell A, Kvint K, Nyström T. Negative regulation by RpoS: a case of sigma factor competition. Mol Microbiol. 1998;29:1039–1051. doi: 10.1046/j.1365-2958.1998.00990.x. [DOI] [PubMed] [Google Scholar]
- 16.Gamer J, Multhaup G, Tomoyasu T, McCarty J S, Rudiger S, Schönfeld H, Schirra C, Bujard H, Bukau B. A cycle of binding and release of the DnaK, DnaJ and GrpE chaperones regulates activity of the Escherichia coli heat shock transcription factor ς32. EMBO J. 1996;15:607–617. [PMC free article] [PubMed] [Google Scholar]
- 17.Gottesman S. Bacterial regulation: global regulatory networks. Annu Rev Genet. 1984;18:415–441. doi: 10.1146/annurev.ge.18.120184.002215. [DOI] [PubMed] [Google Scholar]
- 18.Gross C A. Function and regulation of the heat shock proteins. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C: American Society for Microbiology; 1996. pp. 1382–1399. [Google Scholar]
- 19.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBADpromoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall M N, Shilhavy T J. Genetic analysis of the ompB locus in Escherichia coliK-12. J Mol Biol. 1981;151:1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- 21.Helmann J D, Chamberlin M J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- 22.Hengge-Aronis R. Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell. 1993;72:165–168. doi: 10.1016/0092-8674(93)90655-a. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez V J, Cashel M. Changes in conserved region 3 of synthesis in Escherichia colistrains devoid of ppGpp. J Biol Chem. 1995;268:10851–10862. [PubMed] [Google Scholar]
- 24.Hughes K T, Mathee K. The anti-sigma factors. Annu Rev Microbiol. 1998;52:231–286. doi: 10.1146/annurev.micro.52.1.231. [DOI] [PubMed] [Google Scholar]
- 25.Ishihama A. Promoter selectivity control of RNA polymerase. Nucleic Acids Mol Biol. 1997;11:53–70. [Google Scholar]
- 26.Ishihama A. Adaptation of gene expression in stationary phase bacteria. Curr Opin Genet Dev. 1997;7:582–588. doi: 10.1016/s0959-437x(97)80003-2. [DOI] [PubMed] [Google Scholar]
- 27.Ishihama A. Modulation of the nucleoid, the transcription apparatus, and the translation machinery in bacteria for stationary phase survival. Genes Cells. 1998;4:135–143. doi: 10.1046/j.1365-2443.1999.00247.x. [DOI] [PubMed] [Google Scholar]
- 28.Ishihama A, Saitoh T. Subunits of RNA polymerase in function and structure. IX. Regulation of RNA polymerase activity by stringent starvation protein (SSP) J Mol Biol. 1979;129:517–530. doi: 10.1016/0022-2836(79)90466-2. [DOI] [PubMed] [Google Scholar]
- 29.Ishihama A, Taketo M, Saitoh T, Fukuda R. Control of formation of RNA polymerase in Escherichia coli. In: Camberlin M, Losick R, editors. RNA polymerase. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1976. pp. 475–502. [Google Scholar]
- 30.Jenkins D E, Auger E A, Matin A. Role of RpoH, a heat shock regulator protein, in Escherichia colicarbon starvation protein synthesis and survival. J Bacteriol. 1991;173:1992–1996. doi: 10.1128/jb.173.6.1992-1996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jishage M, Ishihama A. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of ς70 and ς38. J Bacteriol. 1995;177:6832–6835. doi: 10.1128/jb.177.23.6832-6835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jishage M, Ishihama A. Variation in RNA polymerase sigma subunit composition within different stocks of Escherichia coliW3110. J Bacteriol. 1997;179:959–963. doi: 10.1128/jb.179.3.959-963.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jishage M, Ishihama A. A stationary phase protein in Escherichia coliwith binding activity to the major ς subunit of RNA polymerase. Proc Natl Acad Sci USA. 1998;95:4953–4958. doi: 10.1073/pnas.95.9.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jishage M, Iwata A, Ueda S, Ishihama A. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of four species of sigma subunit under various growth conditions. J Bacteriol. 1996;178:5447–5451. doi: 10.1128/jb.178.18.5447-5451.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kajitani M, Ishihama A. Determination of the promoter strength in the mixed transcription system: promoters of lactose, tryptophan and ribosomal protein L10 operons from Escherichia coli. Nucleic Acids Res. 1983;11:671–686. doi: 10.1093/nar/11.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar A, Malloch R A, Fujita N, Smillie D A, Ishihama A, Hayward R S. The minus 35-recognition region of Escherichia colisigma 70 is inessential for initiation of transcription at an “extended minus 10” promoter. J Mol Biol. 1993;232:406–418. doi: 10.1006/jmbi.1993.1400. [DOI] [PubMed] [Google Scholar]
- 37.Kusano S, Ishihama A. Stimulatory effect of trehalose on formation and activity of Escherichia coli RNA polymerase Eς38holoenzyme. J Bacteriol. 1997;179:3649–3654. doi: 10.1128/jb.179.11.3649-3654.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kusano S, Ishihama A. Functional interaction of Escherichia coliRNA polymerase with inorganic polyphosphate. Genes Cells. 1997;2:433–441. doi: 10.1046/j.1365-2443.1997.13203301320330.x. [DOI] [PubMed] [Google Scholar]
- 39.Kusano S, Ding Q, Fujita N, Ishihama A. Promoter selectivity control of Escherichia coli RNA polymerase EςD and EςSholoenzymes: effect of DNA supercoiling. J Biol Chem. 1996;271:1998–2004. doi: 10.1074/jbc.271.4.1998. [DOI] [PubMed] [Google Scholar]
- 40.Kutsukake K, Iino T. Role of the FliA-FlgM regulatory system on the transcriptional control of the flagellar regulon and flagellar formation in Salmonella typhimurium. J Bacteriol. 1994;176:3598–3605. doi: 10.1128/jb.176.12.3598-3605.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landgraf J R, Wu J, Calvo J M. Effects of nutrition and growth rate on Lrp levels in Escherichia coli. J Bacteriol. 1996;178:6930–6936. doi: 10.1128/jb.178.23.6930-6936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lange R, Hengge-Aronis R. Growth phase-regulated expression of bolA and morphology of stationary-phase Escherichia coli cells are controlled by the novel sigma factor ςS. J Bacteriol. 1991;173:4474–4481. doi: 10.1128/jb.173.14.4474-4481.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Libereck K, Wall D, Georgopoulos C. The DnaJ chaperone catalytically activates the DnaK chaperone to preferentially bind the ς32heat shock transcriptional regulator. Proc Natl Acad Sci USA. 1995;92:6224–6228. doi: 10.1073/pnas.92.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lonetto M A, Brown K L, Rudd K E, Buttner M J. Analysis of the Streptomyces coelicolor sigEgene reveals the existence of a subfamily of eubacterial RNA polymerase ς factors involved in the regulation of extracytoplasmic functions. Proc Natl Acad Sci USA. 1994;91:7573–7577. doi: 10.1073/pnas.91.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao W, Siegele D A. Genetic analysis of the stationary phase-induced mcb operon promoter in Escherichia coli. Mol Microbiol. 1998;27:415–424. doi: 10.1046/j.1365-2958.1998.00690.x. [DOI] [PubMed] [Google Scholar]
- 46.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 47.Minchin S, Busby S. Location of close contacts between Escherichia coliRNA polymerase and guanine residues at promoters either with or without consensus −35 region sequences. Biochem J. 1993;289:771–775. doi: 10.1042/bj2890771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Missiakas D, Raina S, Georgopoulos C. Modulation of the Escherichia coli ςE(RpoE) heat-shock transcription-factor activity by the RseA, RseB, and RseC proteins. Mol Microbiol. 1996;24:355–371. doi: 10.1046/j.1365-2958.1997.3601713.x. [DOI] [PubMed] [Google Scholar]
- 49.Nyström T. Role of guanosine tetraphosphate in gene expression and survival of glucose or seryl-tRNA starved cells of Escherichia coliK-12. Mol Gen Genet. 1994;245:355–362. doi: 10.1007/BF00290116. [DOI] [PubMed] [Google Scholar]
- 50.Oishi M, Cosloy S D. The genetic and biochemical basis of the transformability of Escherichia coliK-12. Biochem Biophys Res Commun. 1972;49:1568–1572. doi: 10.1016/0006-291x(72)90520-7. [DOI] [PubMed] [Google Scholar]
- 51.Osawa T, Yura T. Effects of reduced amounts of RNA polymerase sigma factor on gene expression and growth of Escherichia coli: studies of the rpoD40 (amber) mutation. Mol Gen Genet. 1981;184:166–173. doi: 10.1007/BF00272900. [DOI] [PubMed] [Google Scholar]
- 52.Ouhammouch M, Orsini G, Brody E N. The asiAgene product of bacteriophage T4 is required for middle mode RNA synthesis. J Bacteriol. 1994;176:3956–3965. doi: 10.1128/jb.176.13.3956-3965.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pettijohn D, Clarkson K, Kossman C, Stonington J. Synthesis of ribosomal RNA on a protein-DNA complex isolated from bacteria: a comparison of ribosomal RNA synthesis in vitro and in vivo. J Mol Biol. 1970;52:281–300. doi: 10.1016/0022-2836(70)90031-8. [DOI] [PubMed] [Google Scholar]
- 54.Pratt L A, Silhavy T J. The response regulator SprE controls the stability of RpoS. Proc Natl Acad Sci USA. 1996;93:2488–2492. doi: 10.1073/pnas.93.6.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reddy P S, Raghavan A, Chatterji D. Evidence for a ppGpp binding site on the β-subunit of Escherichia coliRNA polymerase: proximity relationship with the rifampicin-binding domain. Mol Microbiol. 1995;15:255–265. doi: 10.1111/j.1365-2958.1995.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 56.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusion. Gene. 1987;53:85–89. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 57.Stonington O G, Pettijohn D E. The folded genome of Escherichia coliisolated in a protein-DNA-RNA complex. Proc Natl Acad Sci USA. 1971;68:6–9. doi: 10.1073/pnas.68.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57a.Tanaka, K. Unpublished observations.
- 58.Tanaka K, Fujita N, Ishihama A, Takahashi H. Heterogeneity of the principal sigma factor in Escherichia coli: the rpoS gene product, ς38, is a principal sigma factor of RNA polymerase in stationary phase Escherichia coli. Proc Natl Acad Sci USA. 1993;90:3511–3515. doi: 10.1073/pnas.90.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Hove B, Staudenmaier H, Braun V. Novel two-component transmembrane transcription control: regulation of iron dicitrate transport in Escherichia coliK-12. J Bacteriol. 1990;172:6749–6758. doi: 10.1128/jb.172.12.6749-6758.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vicente M, Kushner S R, Garrido T, Aldea M. The role of the ‘gearbox’ in the transcription of essential genes. Mol Microbiol. 1991;5:2085–2091. doi: 10.1111/j.1365-2958.1991.tb02137.x. [DOI] [PubMed] [Google Scholar]
- 61.Williams M D, Ouyang T X, Flickinger M C. Starvation-induced expression of SspA and SspB: the effects of a null mutation in sspA on Escherichia coliprotein synthesis and survival during growth and prolonged starvation. Mol Microbiol. 1994;11:1029–1043. doi: 10.1111/j.1365-2958.1994.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 62.Xiao H, Kalman M, Ikehara K, Zemel S, Glaser G, Cashel M. Residual guanosine 3′,5′-bisphosphate synthetic activity of relA null mutations can be eliminated by spoTnull mutations. J Biol Chem. 1991;266:5980–5990. [PubMed] [Google Scholar]
- 63.Yamagishi M, Matsushima H, Wada A, Sakagami M, Fujita N, Ishihama A. Regulation of the Escherichia coli rmfgene encoding the ribosome modulation factor: growth phase- and growth rate-dependent control. EMBO J. 1993;12:625–630. doi: 10.1002/j.1460-2075.1993.tb05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamanaka K, Inouye M. Growth-phase-dependent expression of cspD, encoding a member of the CspA family in Escherichia coli. J Bacteriol. 1997;179:5126–5130. doi: 10.1128/jb.179.16.5126-5130.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou Y N, Walter W A, Gross C A. A mutant ς32with a small deletion in conserved region 3 of ς has reduced affinity for core RNA polymerase. J Bacteriol. 1992;174:5005–5012. doi: 10.1128/jb.174.15.5005-5012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]