Abstract
目的
用环磷酰胺腹腔注射的方法建立SD大鼠膀胱疼痛综合征模型,从尿动力学与组织学层面评估模型的有效性,为膀胱疼痛综合征的临床研究奠定动物学基础,并进一步指导临床治疗。
方法
将32只8周龄SD大鼠随机分为4组,包括急性实验组、急性对照组、慢性实验组、慢性对照组,每组8只。急性实验组在第1天测完尿动力学数据后即刻行腹腔注射环磷酰胺150 mg/kg,第3天再次行尿动力学检查,之后处死大鼠,获取膀胱组织。慢性实验组第1天测量尿动力基线数据后,在第1、4、7天腹腔注射环磷酰胺75 mg/kg,第8天再次测量尿动力学数据后处死大鼠,得到膀胱组织。急性对照组与慢性对照组在腹腔注射等量生理盐水,尿动力学测量时间点与对应的实验组一致。苏木精-伊红(hematoxylin-eosin staining,HE)染色评估膀胱组织病理学改变。
结果
急性与慢性每组的对照组和实验组的尿动力学基线水平差异无统计学意义。急性实验组给药后尿动力学最大膀胱容量显著减小(t=-2.961, P < 0.05),组织学可看到严重的间质水肿、明显的炎性细胞浸润、黏膜水肿和黏膜下出血,部分尿路上皮缺失,符合急性膀胱炎表现。慢性实验组给药后可看到尿动力学最大膀胱容量显著减小(t=-3.886, P < 0.05),膀胱顺应性较对照组降低,但差异无统计学意义,慢性实验组组织学表现为尿路上皮剥脱、间质水肿、黏膜下出血和淋巴细胞等炎性细胞浸润,血管分布密集。
结论
急性实验组单次腹腔注射环磷酰胺可诱导大鼠产生膀胱疼痛综合征急性发作的膀胱炎症表现,慢性实验组反复注射环磷酰胺可诱导大鼠产生慢性膀胱疼痛综合征慢性炎症的组织学改变,但急性与慢性实验组膀胱功能并未出现明显受损。
Keywords: 膀胱疼痛综合征, 间质性膀胱炎, 环磷酰胺, 动物模型, 尿动力学
Abstract
Objective
To establish a model of bladder pain syndrome in SD rats by cyclophosphamide intraperitoneal injection, to evaluate the effectiveness of the model from the urodynamic and histological levels, to lay a zoological foundation for the clinical study of bladder pain syndrome, and to further guide clinical treatment.
Methods
Thirty-two 8-week-old SD rats were randomly divided into 4 groups, including acute test group, acute control group, chronic test group, and chronic control group, with 8 rats in each group. The acute test group received intraperitoneal injection of cyclophosphamide 150 mg/kg immediately after the measurement of urodynamic data on the first day, and urodynamic examination was performed again 2 days later. After that, the rats were sacrificed to obtain bladder tissue. In the chronic test group, after measuring the baseline data of urodynamics on the first day, cyclophosphamide 75 mg/kg was intraperitoneally injected on the first, fourth, and seventh days, and the rats were sacrificed after measuring the urodynamic data again on the eighth day to obtain bladder tissue. The acute control group and the chronic control group were injected with the same amount of normal saline during intraperitoneal injection, and the urodynamic testing time point were consistent with the corresponding test groups. Histopathological changes of the bladder were assessed by HE staining.
Results
In each acute and chronic group, there were no intragroup differences in baseline urodynamic levels between the test and control groups. The urodynamic maximum bladder volume was significantly reduced in the acute test group after administration(t=-2.961, P < 0.05), histologically, severe interstitial edema, obvious inflammatory cell infiltration, mucosal edema and submucosal hemorrhage, and partial urothelium were absent could be seen, which were consistent with acute cystitis performance. The urodynamic maximum bladder capacity was significantly reduced in the chronic test group after administration (t=-3.886, P < 0.05), and the bladder compliance was lower than that in the control group, but not significant, the histological manifestations were urothelial exfoliation, interstitial edema, submucosal hemorrhage, infiltration of inflammatory cells such as lymphocytes, and dense vascular distribution.
Conclusion
In the acute test group, a single intraperitoneal injection of cyclophosphamide could induce acute bladder inflammation in the rats. In the chronic test group, repeated injections of cyclophosphamide could induce histological changes in chronic inflammation of chronic bladder pain syndrome in the rats. But the bladder function was not significantly impaired.
Keywords: Bladder pain syndrome, Interstitial cystitis, Cyclophosphamide, Animal model, Urodynamics
尿频、尿急、夜尿、盆腔疼痛这些临床症状极大地影响着膀胱疼痛综合征患者的生活质量,患者大多数为女性,患病后工作效率低下,睡眠严重不足,经常伴发焦虑、抑郁、性功能障碍等精神心理疾病,在后续治疗中还需要占用许多社会医疗资源及投入较大的医疗服务成本[1]。为了解决患者的病痛,改善生活质量,缓解社会医疗资源的压力,规范膀胱疼痛综合征的诊断与治疗方法成为当前亟待解决的难题,然而目前尚未有统一的临床诊断与治疗标准。
国际尿失禁协会膀胱疼痛综合征分会指出,间质性膀胱炎(interstitial cystitis,IC)又称膀胱疼痛综合征(bladder pain syndrome,BPS),是一种与膀胱有关的不悦感,包括疼痛、压力、不适感,与下尿路症状相关,持续时间超过6周,无感染或其他可识别原因[2]。东亚国家泌尿外科医生提出BPS/IC分为有Hunner溃疡的BPS/IC患者(BPS/IC with Hunner lesions,HIC)和无Hunner溃疡的BPS/IC患者(BPS/IC without Hunner lesions, NHIC), HIC与NHIC膀胱组织学差异有统计学意义,这种分型对于诊断、疾病预后及治疗策略的选择具有重要意义[3-4]。膀胱镜检查和组织活检仍是确诊的可靠手段,各地区诊断方面各有侧重,但逐渐趋归统一。
BPS/IC的发病机制尚不明确,多数认为与尿路上皮的损伤有关,糖胺聚糖层的缺失导致膀胱黏膜屏障破坏,膀胱壁渗透性增加,尿液刺激感觉神经引起疼痛[5],还有学说认为肥大细胞浸润、感染、神经系统异常、肌肉功能紊乱及精神心理等因素均与BPS/IC相关[3, 6]。
目前临床常规治疗主要有健康教育、口服药物治疗、灌注或注射治疗、手术治疗等,健康教育包括改善饮食及行为习惯、减轻压力、理疗等[7];口服药物治疗有M受体拮抗剂、α受体阻滞剂、戊聚糖多硫酸酯、环孢素A[8]、抗菌药物、镇痛药物、精神类药物等;灌注或注射治疗有膀胱灌注二甲基亚砜、透明质酸或硫酸软骨素、膀胱壁注射肉毒素或类固醇等;手术治疗包括膀胱镜下水扩张术、经尿道膀胱电灼术、骶神经刺激术等,膀胱扩大术、膀胱切除术、尿流改道术是治疗BPS/IC最终的手段[3, 9]。
为了深入研究BPS/IC的发病机制及治疗方法,建立简便有效的动物模型是不可或缺的。Ryu等[10]通过膀胱内滴注硫酸鱼精蛋白以及灌注脂多糖建立大鼠模型,Lv等[11]通过向大鼠膀胱灌注透明质酸酶来模拟BPS/IC膀胱疼痛的症状。腹腔内注射环磷酰胺也是诱导BPS/IC较为有效的方法,环磷酰胺代谢物丙烯醛在膀胱特异性蓄积,产生膀胱毒性[12],诱导BPS/IC表型。de Oliveira等[13]对C57小鼠单次高剂量腹腔注射环磷酰胺,24 h后即可诱导BPS/IC的疼痛、组织水肿炎症等典型表现,Augé等[14]评估了环磷酰胺诱导的SD大鼠BPS/IC模型内脏疼痛以及膀胱炎症反应水平,指出该模型与人类NHIC非常相似。
以往研究重点关注动物模型的疼痛表型特征,没有对于BPS/IC动物模型的尿动力学特征与组织学的相关性进行分析和评估。本研究用环磷酰胺腹腔注射的方法建立SD大鼠BPS/IC模型,从尿动力学与组织学层面评估模型的有效性,为BPS/IC的临床研究奠定动物学基础,进一步指导临床治疗。
1. 资料与方法
1.1. 实验动物及分组
选取8周龄雌性SPF级SD大鼠32只,体质量在250~350 g,购自北京维通利华实验动物技术有限公司,饲养在SPF级动物实验室,控制室温在21 ℃± 3 ℃,给予12 h光照及12 h黑暗规律交替循环,自由饮水和进食,所有动物实验均经北京大学人民医院医学伦理委员会批准(批准号:2019PHE060)。
32只SD大鼠随机分为4组,包括急性实验组、急性对照组、慢性实验组、慢性对照组,每组8只。急性实验组在第1天测完尿动力学数据后即刻行腹腔注射环磷酰胺150 mg/kg,第3天后再次行尿动力学检查,之后处死大鼠,获取膀胱组织。慢性实验组第1天测量尿动力基线数据后,在第1、4、7天腹腔注射环磷酰胺75 mg/kg,第8天再次测量尿动力学数据后处死大鼠,得到膀胱组织。急性对照组与慢性对照组在腹腔注射等量生理盐水,尿动力学测量时间点与对应的实验组一致。苏木精-伊红(hematoxylin-eosin staining,HE)染色评估膀胱炎组织病理学变化。
1.2. SD大鼠尿动力学检测方法
(1) 准备物品:鼠板、气麻机、异氟烷、50 mL注射器、防咬手套、输液泵、碘伏、棉签、胶布、100 mL无菌注射盐水、微型镊、BL-420N生物信号采集与分析系统、电脑、压力传感器、三通管、泵管等; (2)连接仪器:将电脑与生物信号采集与分析系统相连接,连接三通管,一端通向19G膀胱测压导管,一端通向输液泵泵管,一端通向压力传感器。连接输液泵与泵管,排空三通管、传感器、泵管内空气,设定输液泵速度为6 mL/h; (3)麻醉:用异氟烷吸入性麻醉诱导SD大鼠后,将其仰卧位固定于鼠板,再持续动物面罩吸入性麻醉; (4)插管:将大鼠尿道口用碘伏消毒3次,用微型镊提起尿道,将19G测压导管插入尿道进入膀胱,插入深度大约3 cm; (5)固定:测压管用胶布固定于鼠台,防止自行脱出;(6)采集记录:开始灌注生理盐水,可观察电脑软件中采集到尿动力曲线图像,同时测压仪与大气压校零后进行测压,并记录相关数据。
1.3. 统计学分析
采用R (3.6.3版本) 软件进行统计学分析与可视化,其中R包(主要为ggplot2,3.3.3版本)用于可视化,符合正态分布计量资料的描述采用均数±标准差的形式。急性组与慢性组尿动力学基线数据的组间比较采用独立样本t检验,单组注射药物前后尿动力学指标比较用配对t检验,数据不符合正态性分布时使用Wilcoxon符号秩和检验,P < 0.05为差异有统计学意义。
2. 结果
2.1. 急性组与慢性组SD大鼠尿动力学指标基线
基线数据如表 1,急性对照组1只大鼠在麻醉测量尿动力时意外死亡,因此急性对照组剩余7只大鼠。
表 1.
尿动力学指标基线数据
Baseline of urodynamic data
| Groups | Bladder capacity/mL | t | P | Max bladder pressure/cmH2O | t | P | Bladder compliance/ (mL/cmH2O) | t | P |
| 1 cmH2O=0.098 kPa. | |||||||||
| Acute control (n=7) | 1.62±0.77 | -1.41 | 0.18 | 36.57±15.02 | -0.37 | 0.72 | 0.05±0.02 | -1.08 | 0.30 |
| Acute test (n=8) | 1.14±0.55 | 33.57±16.08 | 0.04±0.02 | ||||||
| Chronic control (n=8) | 0.99±0.44 | -1.28 | 0.22 | 38.58±11.53 | -1.29 | 0.22 | 0.03±0.01 | -0.42 | 0.68 |
| Chronic test (n=8) | 0.75±0.28 | 31.08±11.03 | 0.03±0.01 | ||||||
2.2. 急性实验组与急性对照组大鼠尿动力学指标变化
急性组给药前后最大膀胱容量比较结果显示,在急性对照组中,给药后低于给药前的平均水平,差异无统计学意义(t=-0.292, P>0.05,图 1A);在急性实验组中,给药后低于给药前的平均水平,差异具有统计学意义(t=-2.961, P=0.021,图 1A)。给药前后最大膀胱压力结果显示,在急性对照组中,给药后低于给药前的平均水平,差异具有统计学意义(t=-6.554, P=0.001,图 1B);在急性实验组中,给药后低于给药前的平均水平,差异无统计学意义(t=-0.681, P>0.05,图 1B)。给药前后膀胱顺应性结果显示,在急性对照组中,给药后高于给药前,变化的差值中位数为0.013(-0.002, -0.057),差异无统计学意义(P>0.05,图 1C);在急性实验组中,给药后低于给药前,变化的差值中位数为-0.004(-0.03, -0.013),差异无统计学意义(P>0.05,图 1C)。
图 1.
给药前后急性对照组与急性实验组尿动力学指标比较
Comparison of urodynamic data between acute control group and acute test group before and after administration
A, comparison of maximum bladder capacity; B, comparison of maximum bladder pressure; C, comparison of bladder compliance; * P < 0.05; △P < 0.001.
2.3. 慢性对照组与慢性实验组大鼠尿动力学指标
慢性对照组给药前后的最大膀胱容量比较显示,在慢性对照组中,给药后低于给药前的平均水平,差异无统计学意义(t=-1.029, P>0.05,图 2A);在慢性实验组中,给药后低于给药前的平均水平,差异具有统计学意义(t=-3.886, P=0.006,图 2A)。最大膀胱内压力比较结果显示,在慢性对照组中,给药后低于给药前的平均水平,差异无统计学意义(t=-1.958, P>0.05,图 2B);在慢性实验组中,给药后低于给药前的平均水平,差异无统计学意义(t=-0.544, P>0.05,图 2B)。膀胱顺应性比较结果显示,在慢性对照组中,给药后高于给药前,差异无统计学意义(P>0.05,图 2C);在慢性实验组中,给药后低于给药前,差异无统计学意义(P>0.05,图 2C)。
图 2.
给药前后慢性对照组与慢性实验组尿动力学指标比较
Comparison of urodynamic data between chronic control group and chronic test group before and after administration
A, comparison of maximum bladder capacity; B, comparison of maximum bladder pressure; C, comparison of bladder compliance; # P < 0.01.
2.4. 组织学评估
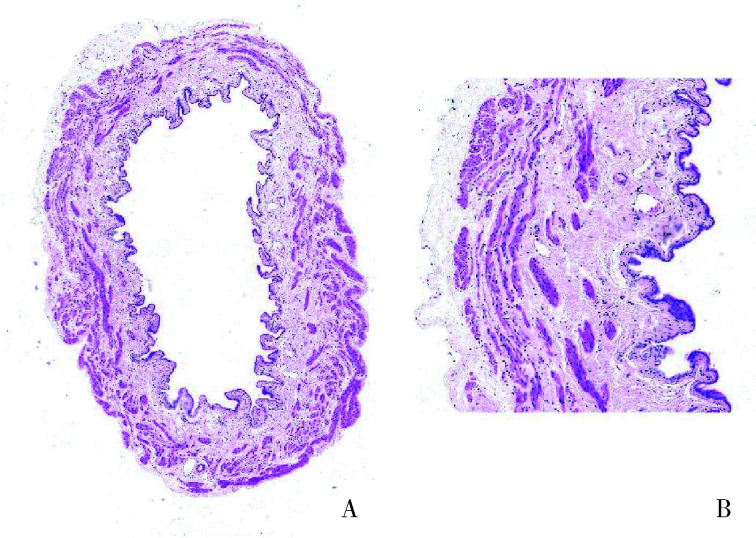
对急性组SD大鼠膀胱组织进行HE染色,镜下观察到急性实验组膀胱组织有膀胱炎症表现,出现严重的间质水肿、明显的炎性细胞浸润、黏膜水肿和黏膜下出血、部分尿路上皮缺失,而急性对照组并未观察到这种改变(图 3、4)。
图 3.
急性对照组膀胱切片
Bladder tissue sections in acute control group
A, bladder cross section (HE staining ×4); B, normal bladder mucosa (HE staining ×20).
图 4.
急性实验组膀胱切片
Bladder tissue sections in acute test group
A, bladder cross section (HE staining ×4); B, absence of the urothelium with dense submucosal small blood vessels (HE staining ×20); C, interstitial edema (HE staining ×20).
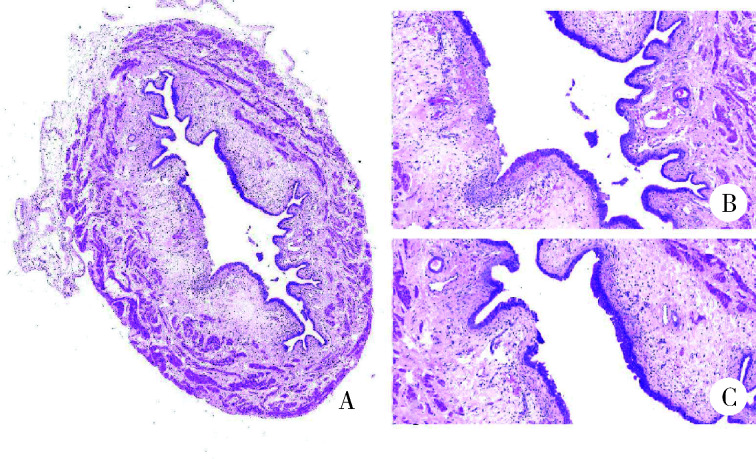
慢性组大鼠膀胱组织HE染色显示,慢性实验组较慢性对照组出现尿路上皮剥脱现象、间质水肿、黏膜下出血明显、炎性细胞浸润、黏膜下小血管分布密集,而慢性对照组并无这种改变(图 5、6)。
图 5.
慢性对照组膀胱切片
Bladder tissue sections in chronic control group
A, bladder cross section (HE staining ×4); B, normal bladder mucosa (HE staining ×10); C, normal urothelium (HE staining ×20).
图 6.
慢性实验组膀胱切片
Bladder tissue sections in chronic test group
A, bladder cross section (HE staining ×4); B, submucosal hemorrhage and interstitial edema (HE staining ×10); C, exfoliated urothelium and inflammatory cell infiltration (HE staining ×20).
3. 讨论
BPS/IC的病理生理学机制一直没有明确统一的定论,这对于BPS/IC的治疗树立提出了极大的挑战,探究一种能够有效模拟BPS/IC的动物模型,对于BPS/IC的机制研究有着重要的意义。
已知的动物模型有以膀胱疼痛为中心的用环磷酰胺诱导的,有通过尾静脉注射假狂犬病毒激活中枢神经系统导致神经源性膀胱改变的[15],以及通过束缚等应激情形或注射肾上腺素调节环境温度、光照等造成动物膀胱过度敏感的等[16]。近年来,出现了用膀胱尿路上皮抗原或组织匀浆或转基因等方式诱导的BPS/IC的自身免疫性膀胱炎模型(experimental autoimmune cystitis, EAC)[17],但该模型仍存在较多局限性:(1)所有模型都是由已知或未知的膀胱组织抗原诱导的,但仍然缺乏膀胱组织抗原参与人类BPS/IC的直接证据;(2)HIC膀胱尿路上皮出现剥脱、糜烂或变薄,然而,大多数EAC模型显示尿路上皮增生,这不是具有Hunner病变的BPS/IC的特征性组织学特征。还有学者通过膀胱灌注透明质酸酶或脂多糖等方法进行造模[10-11],这种方式需要多次插尿管,对插尿管的技术要求较高,且容易造成尿道损伤。这些模型的造模流程并不统一,结果也不尽相同,且多为探究神经递质相关通路,并没有对BPS/IC的发病机制进行模拟探究和验证。
既往研究已表明,环磷酰胺的活性代谢产物丙烯醛具有膀胱毒性,通过腹腔注射能够诱导膀胱炎模型,使啮齿类动物出现许多BPS/IC的典型疼痛相关行为,如膀胱逼尿肌不稳定以及炎症表现[18]。环磷酰胺诱导BPS/IC模型运用的是腹腔注射的方法,操作简便,避免了造模过程中膀胱及尿道的损伤性炎症,并且诱导周期较短,诱导试剂可以根据体质量定量并且容易获取,疼痛表型也已经有过学者进行验证[16]。虽然这种化学试剂诱导的膀胱炎症性改变与BPS/IC的病因及发病机制仍具有一定的差异,但目前来看,该模型诱导快、性价比高,疼痛症状及病理改变较为相似,仍不失为BPS/IC一种理想的动物模型。目前,大量研究着重于该动物模型的疼痛评估及神经通路的病理变化,而对于临床常用的尿动力学指标与组织学特征的关系却没有深入的研究。本研究通过单次腹腔注射及3次腹腔注射环磷酰胺诱导SD大鼠的急性、慢性膀胱炎模型,探究其尿动力学及组织病理学特征改变,为BPS/IC的机制研究奠定了基础。
本研究结果表明,单次腹腔注射环磷酰胺第3天后,大鼠即出现膀胱容量缩小、尿路上皮脱失、黏膜水肿及小血管增生等急性膀胱炎改变,对于BPS/IC急性发作的机制模拟可能具有一定的意义。反复注射环磷酰胺的慢性膀胱炎模型中,SD大鼠尿动力学也出现了膀胱容量缩小,但膀胱顺应性与对照组差异无统计学意义,在组织学上可看到明显的尿路上皮剥脱、黏膜下出血、黏膜水肿、淋巴细胞等多种炎性细胞浸润。慢性膀胱炎大鼠模型在一定程度上可以模拟BPS/IC的慢性炎症组织学表现,但膀胱功能还没有受到明显的损害。总之,急性和慢性大鼠模型均更接近于HIC的特征性改变,对于NHIC的研究应采用其他更接近的动物模型。
Funding Statement
国家自然科学基金(81970660)
Supported by the National Natural Science Foundation of China (81970660)
References
- 1.Hakimi Z, Houbiers J, Pedersini R, et al. The burden of bladder pain in five European countries: a cross-sectional study. Urology. 2017;99(1):84–91. doi: 10.1016/j.urology.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 2.Hanno P, Lin A, Nordling J, et al. Bladder pain syndrome committee of the International Consultation on Incontinence. Neurourol Urodyn. 2010;29(1):191–198. doi: 10.1002/nau.20847. [DOI] [PubMed] [Google Scholar]
- 3.Homma Y, Akiyama Y, Tomoe H, et al. Clinical guidelines for interstitial cystitis/bladder pain syndrome. Int J Urol. 2020;27(7):578–589. doi: 10.1111/iju.14234. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama Y, Hanno P. Phenotyping of interstitial cystitis/bladder pain syndrome. Int J Urol. 2019;26(Suppl 1):17–19. doi: 10.1111/iju.13969. [DOI] [PubMed] [Google Scholar]
- 5.Chai TC, Russo A, Yu S, et al. Mucosal signaling in the bladder. Auton Neurosci. 2016;200(10):49–56. doi: 10.1016/j.autneu.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Karamali M, Shafabakhsh R, Ghanbari Z, et al. Molecular pathogenesis of interstitial cystitis/bladder pain syndrome based on gene expression. J Cell Physiol. 2019;234(8):12301–12308. doi: 10.1002/jcp.28009. [DOI] [PubMed] [Google Scholar]
- 7.Lee MH, Wu HC, Tseng CM, et al. Health education and symptom flare management using a video-based health system for caring women with BPS/IC. Urology. 2018;119(9):62–69. doi: 10.1016/j.urology.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 8.Crescenze IM, Tucky B, Li J, Moore C, et al. Efficacy, side effects, and monitoring of oral cyclosporine in interstitial cystitis-bladder pain syndrome. Urology. 2017;107(9):49–54. doi: 10.1016/j.urology.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.杨 进益, 魏 伟, 叶 林, et al. 膀胱水扩张后透明质酸钠灌注治疗间质性膀胱炎疗效分析. 中华泌尿外科杂志. 2012;33(3):219–222. doi: 10.3760/cma.j.issn.1000-6702.2012.03.019. [DOI] [Google Scholar]
- 10.Ryu CM, Yu HY, Lee HY, et al. Longitudinal intravital imaging of transplanted mesenchymal stem cells elucidates their functional integration and therapeutic potency in an animal model of interstitial cystitis/bladder pain syndrome. Theranostics. 2018;8(20):5610–5624. doi: 10.7150/thno.27559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lv YS, Yao YS, Rong L, et al. Intravesical hyaluronidase causes chronic cystitis in a rat model: a potential model of bladder pain syndrome/interstitial cystitis. Int J Urol. 2014;21(6):601–607. doi: 10.1111/iju.12358. [DOI] [PubMed] [Google Scholar]
- 12.Mills KA, Chess-Williams R, McDermott C. Novel insights into the mechanism of cyclophosphamide-induced bladder toxicity: chloroacetaldehyde's contribution to urothelial dysfunction in vitro. Arch Toxicol. 2019;93(11):3291–3303. doi: 10.1007/s00204-019-02589-1. [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira MG, Mónica FZ, Calmasini FB, et al. Deletion or pharmacological blockade of TLR4 confers protection against cyclophosphamide-induced mouse cystitis. Am J Physiol Renal Physiol. 2018;315(3):460–468. doi: 10.1152/ajprenal.00100.2018. [DOI] [PubMed] [Google Scholar]
- 14.Augé C, Gamé X, Vergnolle N, et al. Characterization and validation of a chronic model of cyclophosphamide-induced interstitial cystitis/bladder pain syndrome in rats. Front Pharmacol. 2020;11(8):1305. doi: 10.3389/fphar.2020.01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang W, Yaggie RE, Jiang MC, et al. Acyloxyacyl hydrolase modulates pelvic pain severity. Am J Physiol Regul Integr Comp Physiol. 2018;314(3):353–365. doi: 10.1152/ajpregu.00239.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee UJ, Ackerman AL, Wu A, Zhang R, et al. Chronic psychological stress in high-anxiety rats induces sustained bladder hyperalgesia. Physiol Behav. 2015;139(2):541–548. doi: 10.1016/j.physbeh.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama Y, Luo Y, Hanno PM, et al. Interstitial cystitis/bladder pain syndrome: the evolving landscape, animal models and future perspectives. Int J Urol. 2020;27(6):491–503. doi: 10.1111/iju.14229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birder L, Andersson KE. Animal modelling of interstitial cystitis/bladder pain syndrome. Int Neurourol J. 2018;22(Suppl 1):3–9. doi: 10.5213/inj.1835062.531. [DOI] [PMC free article] [PubMed] [Google Scholar]