Abstract
目的
探讨双黄酮类药物4′-甲基醚金连木黄酮(4′-O-methylochnaflavone, MF)对棕榈酸诱导的大鼠阴茎海绵体内皮细胞(rat cavernous endothelial cells, RCECs)功能障碍的影响。
方法
将RCECs随机分为4组,分别为正常+牛血清白蛋白组(NC组)、棕榈酸(palmitic acid,PA)组、MF治疗组、淫羊藿次苷Ⅱ(icasiside Ⅱ,ICA Ⅱ)治疗组。采用蛋白印迹实验检测各组细胞的蛋白激酶B(protein kinase B, PKB/AKT)和内皮型一氧化氮合酶(endothelial nitric oxide synthase,eNOS)蛋白表达水平,使用一氧化氮荧光探针检测MF以及ICA Ⅱ对RCECs内一氧化氮含量的影响,使用CCK-8试剂盒检测MF、ICA Ⅱ对PA诱导后的RCECs增殖能力的影响。
结果
与NC组相比,MF组、ICA Ⅱ组处理后细胞内一氧化氮含量显著增加(P < 0.05),MF组的效果优于ICA Ⅱ组(P < 0.05)。与NC组相比,PA组的eNOS和AKT蛋白表达水平明显降低,提示成功构建了用于模拟高脂环境的体外RCECs内皮功能障碍模型(P < 0.05)。MF组干预后能够有效提高eNOS、AKT的表达水平,表明MF可促进恢复游离脂肪酸造成的内皮细胞损伤(P < 0.05)。CCK-8增殖实验显示PA显著降低RCECs的增殖数量(P < 0.05),而给予MF、ICA Ⅱ治疗后细胞增殖能力得到显著恢复(P < 0.05)。
结论
在RCECs中,MF与ICA Ⅱ能够有效增加细胞内一氧化氮含量,PA处理后AKT/eNOS通路的下调揭示其参与内皮细胞损伤的发生、发展,而MF的干预能够有效逆转上述变化,此外,PA诱导下RCECs的细胞增殖能力明显下降,而MF与ICA Ⅱ干预能够恢复上述变化。双黄酮类药物MF对PA诱导的RCECs细胞功能障碍有一定程度的修复作用。
Keywords: 内皮细胞功能障碍, 棕榈酸, 一氧化氮合酶, 信号转导
Abstract
Objective
To investigate the effect of biflavonoid 4′-O-methylochnaflavone (MF) on palmitic acid-induced endothelial dysfunction in rat cavernous endothelial cells (RCECs).
Methods
The isolated RCECs were commercially available and randomly divided into four groups: normal+BSA group (NC group), palmitic acid (PA) group, MF group, and icariside Ⅱ (ICA Ⅱ) group. The protein expression levels of protein kinase B (PKB/AKT) and endothelial nitric oxide synthase (eNOS) in each group were evaluated via Western blotting. The differences in the intracellular nitric oxide of RCECs treated by MF or ICA Ⅱ were detected by DAF-FM DA that served as a nitric oxide fluorescent probe. Effects of MF and ICA Ⅱ on cell proliferation of PA-stimulated RCECs were determined via CCK-8 assay.
Results
The content of nitric oxide in RCECs was significantly increased after the treatment of MF and ICA Ⅱ in comparison with the NC group (P < 0.05). Moreover, compared with ICA Ⅱ group, MF demonstrated a more obvious effect in promoting nitric oxide production (P < 0.05). Compared with the NC group, the expression levels of eNOS and AKT in the PA group were significantly decreased, indicating that a model for simulating the high-fat environment in vitro was successfully constructed (P < 0.05). Meanwhile, the intervention of MF and ICA Ⅱ could effectively increase the expression of eNOS and AKT, suggesting that MF and ICA Ⅱ could promote the recovery of endothelial dysfunction caused by high levels of free fatty acids (P < 0.05). The results of CCK-8 assays showed that PA could significantly reduce the proli-feration ability of RCECs (P < 0.05). Furthermore, the decreased cell viability induced by PA was significantly elevated by treatment with ICA Ⅱ and MF (P < 0.05).
Conclusion
In RCECs, MF and ICA Ⅱ could effectively increase the content of nitric oxide. The down-regulation of the expression of proteins associated with the AKT/eNOS pathway after PA treatment revealed that this pathway was involved in the development of endothelial dysfunction, which could be effectively reversed by MF and ICA Ⅱ. In addition, the cell proliferation ability was significantly decreased following PA treatment, but MF and ICA Ⅱ could restore the above changes. Overall, biflavonoid MF has an obvious repairing effect on PA-stimulated endothelial dysfunction.
Keywords: Endothelial dysfunction, Palmitic acid, Nitric oxide synthase, Signal transduction
勃起功能障碍(erectile dysfunction,ED)指的是男性无法获得或维持勃起以获得满意的性交[1]。近年来,众多研究人员一直致力于研究ED的发病机制,并认为ED主要是一种血管起源的疾病。在患有糖尿病、高胆固醇血症和心血管疾病的男性中,ED的发病率显著增加。内皮功能完整性的丧失和内皮功能障碍在此类ED的发生、发展中起着不可或缺的作用。心血管疾病和内皮功能障碍通过减少血液流入、动脉功能不全或动脉狭窄导致ED[2]。事实上,ED和心血管疾病密切相关,有相关症状的男性可能需要进行系统性的心脏评估。外部刺激可以改变正常的内皮功能并导致ED发作,例如氧化应激和炎症[3]。内皮功能障碍是ED的关键病理特征之一。勃起过程中血管舒张受损主要是由于内皮细胞最重要的血管舒张剂一氧化氮(nitric oxide,NO)释放减少所致。NO主要由海绵体内皮细胞的内皮型一氧化氮合酶(endothelial nitric oxide synthase,eNOS)产生释放,因此eNOS的活化调控对恢复内皮细胞损伤及修复勃起功能不可缺少且极其重要[4]。
游离脂肪酸是常见的心血管疾病危险因素,且与肥胖和2型糖尿病等代谢性疾病密切相关[5-6]。最近的研究表明,游离脂肪酸不仅是导致胰岛素抵抗的主要原因,而且还会在内皮、肝和骨骼肌等组织中靶向胰岛素诱导炎症[5, 7]。因此,目前认为血液中升高的游离脂肪酸和胰岛素抵抗、炎症、肥胖、2型糖尿病、高血压之间存在重要联系。此外,越来越多的证据表明,游离脂肪酸在内皮功能障碍中也具有重要作用。具体来说,胰岛素抵抗、氧化应激和炎症是游离脂肪酸诱导的内皮功能障碍的主要原因[8]。糖尿病和其他代谢状态会导致游离脂肪酸升高,进而引发内皮组织炎症和产生氧化应激的转录因子。此外,游离脂肪酸,尤其是棕榈酸(palmitic acid,PA),还会促进内皮细胞的凋亡并对内皮祖细胞产生诸多负面影响[9-10]。
本课题组先前的研究结果证实,单黄酮类药物淫羊藿次苷Ⅱ(icariside Ⅱ,ICA Ⅱ)在多种ED模型中能显著促进内皮细胞增殖,恢复内皮功能障碍[11-12],然而ICA Ⅱ面临着生物利用度低、溶解度低等临床转化困境。当前关于双黄酮类药物在ED中的研究甚少。本研究使用双黄酮类化合物4′-甲基醚金连木黄酮(4′-O-methylochnaflavone,MF),以PA高脂模型为切入点,比较MF和ICA Ⅱ对此条件下大鼠阴茎海绵体内皮细胞(rat cavernous endothelial cells,RCECs)增殖和内皮功能的影响,并探索蛋白激酶B(protein kinase B,PKB/AKT)及eNOS信号通路的作用机制。
1. 资料与方法
1.1. 试剂与材料
RCECs购自武汉普诺赛生命科技有限公司,PA粉末、牛血清白蛋白(bovine serum albumin,BSA)购自北京索莱宝科技有限公司,MF和ICA Ⅱ购自北京倍特仁康生物医药科技有限公司,MF和ICA Ⅱ的化学结构示意图见图 1。内皮细胞专用培养基、胎牛血清、青霉素-链霉素溶液、内皮细胞生长补充剂均购自美国ScienCell公司,胰蛋白酶购自美国Gibco公司,总蛋白提取试剂盒购自碧云天生物技术有限公司,BCA蛋白定量试剂盒购自爱必信生物技术有限公司,PAGE凝胶快速制备试剂盒购自大连美仑生物技术有限公司,Western blot所涉及的一抗和二抗分别购自美国Cell Signaling Technology公司和北京中山金桥生物技术有限公司,NO荧光探针购自碧云天生物技术有限公司,CCK-8试剂盒购自凯基生物技术股份有限公司。
图 1.
4′-甲基醚金连木黄酮和淫羊藿次苷Ⅱ的化学结构示意图
Chemical structure of 4′-O-methylochnaflavone and icariside Ⅱ
1.2. PA溶液的配置
配置10%(质量分数)的无脂肪酸BSA溶液,用涡旋法将512.8 mg粉末状PA完全溶解在10 mL无水乙醇中,55 ℃条件下将PA溶液和10%的BSA按1:19的比例混合,制得10 mmol/L的PA工作液,分别用0.45 μm、0.22 μm微孔滤膜无菌过滤,分装储存于-20 ℃。
1.3. RCECs的体外培养和模型构建
RCECs的培养环境为37 ℃、5%(体积分数)CO2的常规湿度无菌环境。每2天更换培养液1次,当细胞生长至融合度80%~90%时,按1:3比例传代。细胞干预前将一定数量的细胞接种至10 cm培养皿内,24 h后(融合度约为70%~80%)实验组更换为含有不同浓度药物的完全培养基,然后加入适量PA工作液(终浓度150 μmol/L)模拟高脂环境。具体分组如下:第一组用MF(终浓度0.1 μmol/L)预处理0.5~1.0 h,然后以PA刺激细胞;第二组用ICA Ⅱ(终浓度0.1 μmol/L)预处理0.5~1.0 h,然后以PA刺激细胞;对照组则换为含等量二甲基亚砜(dimethyl sulfoxide,DMSO)预处理0.5~1.0 h,然后加入等量BSA。继续培养24 h后,收获细胞进行后续实验。
1.4. Western blot
蛋白的提取和浓度测定均按照试剂盒说明书进行。配制10%(质量分数)SDS-PAGE分离胶,制备步骤参照PAGE凝胶快速制备试剂盒说明书。电泳条件:80 V恒压电泳30 min后,改为120 V恒压分离60~90 min。电转条件:300 mA恒流转膜0.5~1.5 h。用TBST配置5%(质量分数)脱脂牛奶,室温封闭1 h。按1:1 000~1:3 000比例(参考抗体说明书),使用TBST稀释目的蛋白一抗工作液,4 ℃孵育过夜,TBST缓冲液洗3遍,配置对应种属的二抗工作液,放置摇床室温1 h,TBST缓冲液洗3遍。经增强化学发光(enhanced chemiluminescence, ECL)后由Syngene G: BOX系列智能成像系统采集图像。采用ImageJ软件测定胶片电泳条带灰度值,分析蛋白水平变化。
1.5. 细胞增殖检测
使用CCK-8试剂盒检测MF和ICA Ⅱ对RCECs的增殖作用,参考试剂盒说明书进行实验操作:将RCECs细胞悬液以每孔3×103/mL密度接种于96孔板(100 μL/孔)。根据组别设置,不同孔内分别加入含不同浓度药物的培养基,处理24 h。暗光条件下每个孔内加入10 μL CCK-8原液,37 ℃条件下孵育1 h;使用酶标仪在450 nm波长下测量各孔光密度值(D450 nm值),计算细胞存活率,细胞活力=[(实验组D值-空白组D值)/(对照组D值-空白组D值)]×100%。
1.6. NO荧光探针检测
按试剂盒说明书贴壁培养细胞,按照1:1 000比例,用DAF-FM DA稀释液稀释DAF-FM DA原液制备工作液,终浓度为5 μmol/L。去除细胞培养液,加入适当体积稀释好的DAF-FM DA工作液以充分盖住细胞为宜,37 ℃细胞培养箱内孵育20 min,用无菌磷酸盐缓冲液(phosphate buffered saline,PBS)洗涤3次,以充分去除未进入细胞内的DAF-FM DA。
1.7. 统计学分析
实验数据采用均数±标准差表示。统计分析及作图采用GraphPad Prism 8.0软件,两组间比较采用独立样本t检验,多组间比较采用单因素方差分析(One-way ANOVA),组间两两比较采用Tukey’s多重检验,P < 0.05为差异有统计学意义。
2. 结果
2.1. MF和ICA Ⅱ对细胞内NO含量的影响
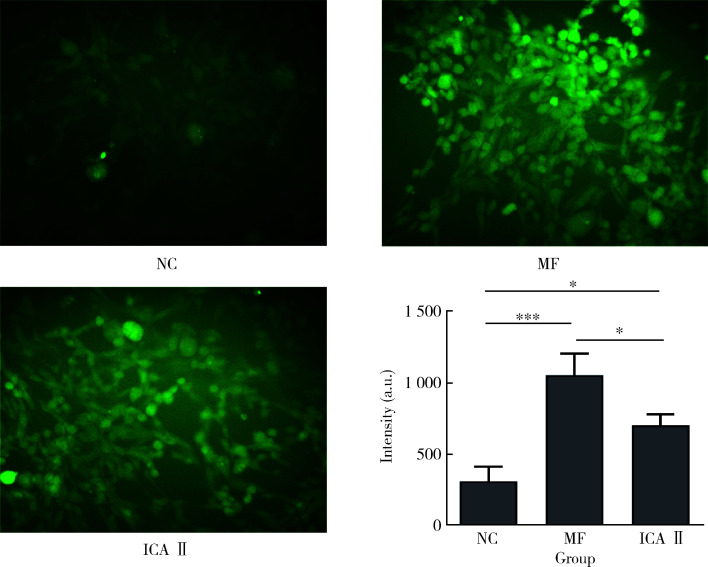
与NC组相比,0.1 μmol/L的MF和0.1 μmol/L的ICA Ⅱ干预后能够显著增加细胞内NO的含量(P < 0.05),且MF刺激NO生成的效果优于ICA Ⅱ (P < 0.05,图 2)。
图 2.
MF和ICA Ⅱ处理后细胞内NO含量的变化
The evaluation of intracellular NO following the treatment of MF and ICA Ⅱ
NC, normal control; MF, 4′-O-methylochnaflavone; ICA Ⅱ, icariside Ⅱ; NO, nitric oxide; a.u., arbitrary unit. *P < 0.05; ***P < 0.001.
2.2. PA刺激对eNOS和AKT蛋白表达的影响
使用PA刺激RCECs构建体外内皮细胞功能障碍模型,与NC组相比,PA组的eNOS和AKT蛋白水平明显降低,呈剂量依赖关系(P < 0.05,图 3),说明经PA处理过的RCECs发生AKT/eNOS信号通路的下调,RCECs出现了一定程度的细胞功能障碍,提示利用PA成功构建出内皮细胞功能障碍模型。
图 3.
PA处理后内皮细胞内eNOS和AKT蛋白的变化
The protein level of eNOS and AKT following the treatment of PA
PA, palmitic acid; AKT, protein kinase B; eNOS, endothelial nitric oxide synthase. *P < 0.05.
2.3. PA刺激下MF和ICA Ⅱ对eNOS和AKT蛋白表达的影响
利用PA诱导内皮细胞功能障碍,在PA刺激后给予MF(终浓度为0.1 μmol/L)治疗能够有效提高eNOS和AKT的蛋白表达水平(图 4),提示MF可促进内皮细胞损伤的恢复(P < 0.05);给予同等浓度的ICA Ⅱ未表现出显著的治疗效果(P>0.05),可见MF的内皮细胞保护作用优于ICA Ⅱ。
图 4.
MF和ICA Ⅱ治疗后PA刺激下内皮细胞内eNOS和AKT蛋白的变化
The protein level of eNOS and AKT in the PA-stimulated cells following the treatment of MF and ICA Ⅱ
NC, normal control; PA, palmitic acid; MF, 4′-O-methylochnaflavone; ICA Ⅱ, icariside Ⅱ; AKT, protein kinase B; eNOS, endothelial nitric oxide synthase. *P < 0.05.
2.4. MF、ICA Ⅱ对PA刺激的RCECs增殖的影响
CCK-8增殖实验发现,MF和ICA Ⅱ浓度为0.1 μmol/L和1.0 μmol/L时没有显著的细胞毒性(P>0.05,图 5A)。PA会显著降低RCECs的细胞增殖能力(P < 0.05),但使用0.1 μmol/L的MF和ICA Ⅱ治疗后细胞增殖能力得到显著恢复(P < 0.05,图 5B)。
图 5.
MF和ICA Ⅱ治疗后PA刺激下RCECs增殖能力的变化
The RCECs proliferation ability of the PA-stimulated cells following the treatment of MF and ICA Ⅱ
A, the RCECs were treated with different concentrations of MF and ICA Ⅱ for 24 h with cell viability measured via the CCK-8 assay; B, the RCECs were treated with 150 μmol/L PA in the presence or absence of MF and ICA Ⅱ for 24 h with cell viability measured via the CCK-8 assay. CCK, cell counting kit; NC, normal control; PA, palmitic acid; MF, 4′-O-methylochnaflavone; ICA Ⅱ, icariside Ⅱ; RCECs, rat cavernous endothelial cells; NS, no significant; μM, μmol/L; ***P < 0.001.
3. 讨论
作为血管内膜的重要组成部分,血管中的内皮组织由单层内皮细胞组成,排列在流动的血液和血管平滑肌细胞之间的血管腔内表面。内皮细胞具有广泛的重要功能,包括维持血管张力、血液流动性和通透性[3]。内皮细胞还负责调节炎症反应,对凝血级联激活、血栓形成、纤维蛋白溶解和血管生成有一定影响[13-14]。
内皮功能障碍是血管中内皮来源的NO的产生或生物利用度发生了失衡,损害了血管扩张等正常生理过程。正常情况下,NO作用于阴茎海绵体的血管平滑肌细胞,使血管均处于舒张状态。舒张的动脉平滑肌提高了海绵体的血流量,又促进内皮细胞合成释放NO,阴茎海绵体窦状间隙因血流量快速增加而胀大,最终启动勃起过程[4]。
目前认为,AKT/eNOS/NO信号通路对血管中NO的调控起着主要作用[15]。采用不同治疗手段激活该信号通路对恢复内皮功能障碍有促进作用,例如他汀类药物、辅酶Q10、n-3多不饱和脂肪酸等[16]。此外,很多中药单体也表现出对内皮功能障碍的改善作用,大部分为黄酮类药物,包括姜黄素、槲皮素、熊果酸[17-18]。本研究检测了双黄酮类药物MF对PA诱导的RCECs障碍后AKT/eNOS蛋白表达的影响,并与ICA Ⅱ进行比较。
内皮功能障碍可由多种危险因素诱发,如饮食、药物和衰老。高脂饮食会诱导内皮细胞中AKT/eNOS信号通路下调,进而导致ED。AKT/eNOS信号通路的下调与血浆游离脂肪酸和甘油三酯水平升高以及葡萄糖利用受损相关[19]。脂肪酸是具有长脂肪链的羧酸,其一端含有甲基,而另一端含有羧基。根据是否含有双键,它们分为没有双键的饱和脂肪酸、只有一个双键的单不饱和脂肪酸以及至少有两个双键的多不饱和脂肪酸[20]。PA是血液中含量最多的饱和游离脂肪酸,会在多种细胞类型中诱导细胞功能障碍和细胞死亡。PA的脂毒性作用主要体现在非脂肪组织的细胞暴露于高水平的饱和脂肪酸中时,会导致脂质超载,这与肥胖和2型糖尿病的病理学相关[21]。导致ED的典型危险因素包括糖尿病、心血管疾病、衰老、肥胖,这些都与脂肪代谢异常相关。我们发现PA会下调AKT/eNOS信号通路,且依赖于浓度梯度。Khan等[9]的研究发现,人脐静脉内皮细胞中PA过载会导致Ca2+依赖性自噬的发展,最终导致程序性细胞死亡。因此,PA对内皮细胞造成的损伤会导致内皮功能障碍,而其中AKT/eNOS信号通路起到重要作用。
我国具有丰富的中草药资源,提取纯化的天然小分子药物多达几十万,为人类提供了宝贵的财富。ICA Ⅱ是一种从传统中草药淫羊藿中提取的活性黄酮类化合物,具有多种生物学和药理学特性,包括抗癌、抗氧化、抗骨质疏松、抗炎、抗衰老等[22]。近些年的研究发现,ICA Ⅱ在修复内皮损伤中具有重要作用,为其在ED、心血管疾病等治疗领域的应用提供了机会。ICA Ⅱ在RCECs中能通过AKT/eNOS信号通路恢复高糖诱导和神经损伤的人海绵状血管内皮功能障碍[12, 23]。但是,许多黄酮类药物(包括ICA Ⅱ)的天然结构决定了其可溶性差、生物利用度低,临床转化前景困难重重,因此,寻找效价更好的小分子药物势在必行。
双黄酮类化合物具有许多不同的单体结构和连接方式,具有广泛的开发前景,而且当前关于双黄酮类药物在ED中的研究尚属罕见。MF是一种从金银花中分离得到的双黄酮类化合物,可抑制小鼠淋巴细胞增殖[24]。此外,双黄酮还可能用于开发针对新型冠状病毒木瓜样蛋白酶的抗病毒药物[25]。本研究发现,MF能够通过AKT/eNOS信号通路恢复PA刺激导致的内皮细胞功能障碍,且所需剂量比ICA Ⅱ少,显示出较高的治疗效价,此外,MF还能增加NO的产量,促进RCECs的增殖能力,显示了其对内皮细胞生物学功能的修复作用。未来还可进一步明确MF在内皮细胞中的作用位点,并明确其中参与的其他细胞内信号通路的传导机制。
综上所述,AKT/eNOS信号通路的变化可能是PA造成RCECs内皮功能障碍的损伤机制,而MF能够有效逆转上述变化;与ICA Ⅱ相比,MF可更有效地产生NO。由于本研究仅在细胞水平研究了MF的作用机制,具有一定局限性,未来需要进一步探索双黄酮类药物MF在治疗ED相关内皮功能障碍中的作用,包括使用ED动物模型,以期阐明其分子机制,为改善ED治疗现状和开发临床上有效的治疗手段提供全新思路。
Funding Statement
国家自然科学基金(81971379)和无锡市“太湖人才计划”医疗卫生高层次人才项目
Supported by the National Natural Science Foundation of China (81971379) and the Wuxi "Taihu Talents Program" Medical and Health High-Level Talents Project
Contributor Information
冯 宁翰 (Ning-han FENG), Email: n.feng@njmu.edu.cn.
关 瑞礼 (Rui-li GUAN), Email: guanruili@bjmu.edu.cn.
References
- 1.Bakr AM, El-Sakka AI. Erectile dysfunction among patients and health care providers during COVID-19 pandemic: A systematic review. Int J Impot Res. 2022;34(2):145–151. doi: 10.1038/s41443-021-00504-w. [DOI] [PubMed] [Google Scholar]
- 2.Yafi FA, Jenkins L, Albersen M, et al. Erectile dysfunction. Nat Rev Dis Primers. 2016;2:16003. doi: 10.1038/nrdp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh A, Gao L, Thakur A, et al. Role of free fatty acids in endothelial dysfunction. J Biomed Sci. 2017;24(1):50. doi: 10.1186/s12929-017-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, Vanhoutte PM, Leung SWS. Vascular nitric oxide: Beyond eNOS. J Pharmacol Sci. 2015;129(2):83–94. doi: 10.1016/j.jphs.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Boden G. Obesity and free fatty acids. Endocrin Metab Clin. 2008;37(3):635–646. doi: 10.1016/j.ecl.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egan BM, Greene EL, Goodfriend TL. Nonesterified fatty acids in blood pressure control and cardiovascular complications. Curr Hypertens Rep. 2001;3(2):107–116. doi: 10.1007/s11906-001-0021-y. [DOI] [PubMed] [Google Scholar]
- 7.Haus JM, Solomon TPJ, Marchetti CM, et al. Free fatty acid-induced hepatic insulin resistance is attenuated following lifestyle intervention in obese individuals with impaired glucose tolerance. J Clin Endocr Metab. 2010;95(1):323–327. doi: 10.1210/jc.2009-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durrant JR, Seals DR, Connell ML, et al. Voluntary wheel running restores endothelial function in conduit arteries of old mice: Direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587(13):3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan MJ, Rizwan Alam M, Waldeck-Weiermair M, et al. Inhibition of autophagy rescues palmitic acid-induced necroptosis of endothelial cells. J Biol Chem. 2012;287(25):21110–21120. doi: 10.1074/jbc.M111.319129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee C, Lee S, Ou H, et al. Eicosapentaenoic acid protects against palmitic acid-induced endothelial dysfunction via activation of the AMPK/eNOS pathway. Int J Mol Sci. 2014;15(6):10334–10349. doi: 10.3390/ijms150610334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Xu Y, Li H, et al. Antioxidant icariside Ⅱ combined with insulin restores erectile function in streptozotocin-induced type 1 diabetic rats. J Cell Mol Med. 2015;19(5):960–969. doi: 10.1111/jcmm.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu S J, Li M, Yuan YM, et al. A novel flavonoid derivative of icariside Ⅱ improves erectile dysfunction in a rat model of caver-nous nerve injury. Andrology. 2021;9(6):1893–1901. doi: 10.1111/andr.13065. [DOI] [PubMed] [Google Scholar]
- 13.Godo S, Shimokawa H. Endothelial functions. Arterioscler Thromb Vasc Biol. 2017;37(9):e108–e114. doi: 10.1161/ATVBAHA.117.309813. [DOI] [PubMed] [Google Scholar]
- 14.Chlopicki S. Perspectives in pharmacology of endothelium: From bench to bedside. Pharmacol Rep. 2015;67(4):vi–ix. doi: 10.1016/j.pharep.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Everaert BR, van Craenenbroeck EM, Hoymans VY, et al. Current perspective of pathophysiological and interventional effects on endothelial progenitor cell biology: Focus on Pi3K/AKT/eNOS pathway. Int J Cardiol. 2010;144(3):350–366. doi: 10.1016/j.ijcard.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Morris G, Puri BK, Olive L, et al. Endothelial dysfunction in neuroprogressive disorders-causes and suggested treatments. BMC Med. 2020;18(1):305. doi: 10.1186/s12916-020-01749-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mu H, Liu H, Zhang J, et al. Ursolic acid prevents doxorubicin-induced cardiac toxicity in mice through eNOS activation and inhibition of eNOS uncoupling. J Cell Mol Med. 2019;23(3):2174–2183. doi: 10.1111/jcmm.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt CA, Dirsch VM. Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide. 2009;21(2):77–91. doi: 10.1016/j.niox.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 19.García-Prieto CF, Hernández-Nuño F, Rio DD, et al. High-fat diet induces endothelial dysfunction through a down-regulation of the endothelial AMPK-PI3K-Akt-eNOS pathway. Mol Nutr Food Res. 2015;59(3):520–532. doi: 10.1002/mnfr.201400539. [DOI] [PubMed] [Google Scholar]
- 20.Vadivel V, Kunyanga CN, Biesalski HK. Health benefits of nut consumption with special reference to body weight control. Nutrition. 2012;28(11/12):1089–1097. doi: 10.1016/j.nut.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Brookheart RT, Michel CI, Schaffer JE. As a matter of fat. Cell Metab. 2009;10(1):9–12. doi: 10.1016/j.cmet.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M, Gao H, Li W, et al. Icariin and its metabolites regulate lipid metabolism: From effects to molecular mechanisms. Biomed Pharmacother. 2020;131:110675. doi: 10.1016/j.biopha.2020.110675. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Xu Y, Guan R, et al. Icariside Ⅱ prevents high-glucose-induced injury on human cavernous endothelial cells through Akt-eNOS signaling pathway. Andrology. 2015;3(2):408–416. doi: 10.1111/andr.303. [DOI] [PubMed] [Google Scholar]
- 24.Lee S J, Choi JH, Son KH, et al. Suppression of mouse lymphocyte proliferation in vitro by naturally-occurring biflavonoids. Life Sci. 1995;57(6):551–558. doi: 10.1016/0024-3205(95)00305-p. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Ma L, Hu Y, et al. Natural biflavones are potent inhibitors against SARS-CoV-2 papain-like protease. Phytochemistry. 2022;193:112984. doi: 10.1016/j.phytochem.2021.112984. [DOI] [PMC free article] [PubMed] [Google Scholar]