Abstract
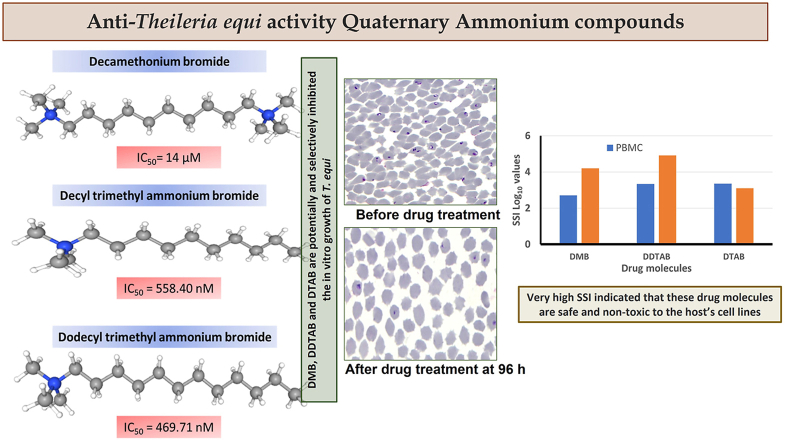
Equine piroplasmosis has become a global problem of the equine husbandry sector. Haemoprotozoans evolved very quickly and developed resistance against most of the current available drugs. Phospholipid membrane synthesis by choline kinase enzyme is vital for propagation of intra-erythrocytic protozoa parasites. This pathway was targeted in the present study. Quaternary ammonium salts (QAS) and their analogues act against choline and hamper the biosynthesis process for phosphatidylcholine. We analysed anti-T. equi activity of three QAS - decamethonium bromide (DMB), decyl trimethyl ammonium bromide (DTAB) and dodecyl trimethyl ammonium bromide (DDTAB). Theileria equi parasites in vitro treated with different concentrations of DMB, DDTAB and DTAB. Drug treated T. equi failed to multiply further in the viability test. The IC50 value of DMB, DDTAB and DTAB for growth inhibition of T. equi was 14.0 μM, 469.51 nM and 558.40 nM, respectively. DMB, DDTAB and DTAB treated T. equi parasites were observed to be devoid of internal structures, showing pyknotic and degenerative appearances. Various concentration of DMB, DDTAB and DTAB were analysed for their cytotoxicity and haemolytic activity on horse's PBMCs and RBCs. DMB was less than 10% cytotoxic to PBMCs, while DDTAB and DTAB were 40%–50% cytotoxic at 1000 μM concentrations. The respective CC50 values were 7202.96 μM, 1026.26 μM and 1263.95 μM. DMB and DTAB showed least haemolytic activity (<3%); whereas DDTAB was more haemolytic to RBCs at highest concentration of 2000 μM. The respective CC50 values of these drugs were 224495.3 μM, and 39101.35 μM; 713.54 μM. Specific selective index for DMB, DDTAB and DTAB values with respect to host's PBMC and RBC cells, were 514.50, 2185.81, 2263.52 and 16035.38, 1519.75, 70023.91, respectively. These data indicated its non-toxicity to host's cells and selective potential of anti-T. equi in vitro activity.
Keywords: Theileria equi, Quaternary ammonium salts, Cytotoxicity assay, Haemolytic assay, Decamethonium bromide, Decyl trimethyl ammonium bromide, Dodecyl trimethyl ammonium bromide
Graphical abstract
Highlights
-
•
In vitro anti-Theileria equi efficacy of QAS - DMB, DDTAB and DTAB as choline kinase inhibitor was investigated.
-
•
DMB, DDTAB and DTAB potentially and selectively arrested the in vitro growth of T. equi.
-
•
These drug molecules have very high SSI values and are non-toxic to host's PBMC and RBC cell lines.
Abbreviations
- QAC
Quaternary ammonium compounds
- QAS
Quaternary ammonium salts
- DMB
Decamethonium bromide
- DTAB
Decyl trimethyl ammonium bromide
- DDTAB
Dodecyl trimethyl ammonium bromide
- h
Hours
- PBMCs
Peripheral blood mononuclear cells
- RBCs
Red blood cells
- IC50
50% Inhibitory concentration
- CC50
50% Cytotoxic concentration
- SSI
Specific selective index
- MASP
Micro-aerophilous stationary phase
- VYM
Vega Y Martinez phosphate buffered saline
- NPPs
New permeation pathways
1. Introduction
Global climate change has affected the distribution pattern of vector-borne diseases and disease conditions by apicomplexan parasites have emerged. Theileriosis is the utmost prevailing haemoprotozoan disease of animals (Rashid et al., 2018) and is responsible for huge economic losses to livestock owners. Theileriosis in equines is caused by Theileria equi an intra-erythrocytic protozoa and movement of the infected horses has been restricted across the international border (Mehlhorn and Schein, 1998). Development of drug resistance in theileriosis necessitates the search for new target-based drug molecules (Kumar et al., 2003; Vial and Gorenflot, 2006).
During an asexual erythrocytic development of Theileria equi, numerous vital components are synthesized viz phospholipid, DNA, heme etc, which are essential in maintaining its life cycle in the natural host. Erythrocyte needs an immense amount of the phospholipids for its membrane synthesis (Holz and Bull, 1977; Vial and Ancelin, 1992, 1998). A majority of phospholipid metabolism (up to 85%) are governed by two key components - Phosphatidyl-choline and phosophatidyl-ethanolamine (Vial and Ancelin, 1992). Phosphatidyl-choline is the most abundant phospholipid synthesized by Kennedy or CDP - choline pathway (Vial and Ancelin, 1998; Pessi et al., 2005). Choline kinase is the first key enzyme in the Kennedy pathway and its inhibition arrested in vitro and in vivo growth of Theileria parasites (Choubey et al., 2007; Ancelin et al., 1998, 2003; Ancelin et al., 2003a, Ancelin et al., 2003b; Roggero et al., 2004). Lehane et al. (2004) investigated the uptake of choline kinase by Plasmodium parasitized erythrocytes. They reported that choline influx was inhibited competitively by quinine. Quinine (established anti-malarial drug) inhibited the uptake of choline via the competitive inhibition of the choline transporter, indicating that this pathway is essential for the survival of malarial parasites.
Earlier studies indicated that quaternary ammonium salts and their analogues act against choline (by mimicking its structure), impeding the biosynthesis process for phosphatidylcholine (Ancelin et al., 1991). Mono and bis quaternary ammonium salts were found highly effective against P. falciparum at IC50 values of as low as 0.33 nM and 3 pM, respectively (Calas et al., 2000). Bis-quaternary ammonium salts also can selectively accumulate in Plasmodium-infected erythrocytes as compared to unparasitized erythrocytes (Staines et al., 2000; Stead et al., 2001; Biagini et al., 2003; Ancelin et al., 2003a, Ancelin et al., 2003b). Bis-quaternary ammonium compounds compartmentalize themself in the parasite's food vacuole and prevent formation of free heme (hemozoin) contributing to its antimalarial activity (Biagini et al., 2003). Theileria and Babesia are malaria-like protozoans infecting host-erythrocytes. These haemo-protozoans lack a central food vacuole but may be able to synthesize phospholipids de novo and as such do not produce hemozoin. Existence of these contrasting pathobiology among animals Theileria, Babesia and Plasmodium species protozoa prompted us to investigate anti-theilerial activities of quaternary ammonium compounds. We aimed to explore the growth inhibition potential of decamethonium bromide, decyl trimethyl ammonium bromide and dodecyl trimethyl ammonium bromide against in vitro cultured Theileria equi parasite.
2. Materials and methods
2.1. In vitro MASP culture of Theileria equi
Theileria equi was in vitro cultured by micro-aerophilous stationary phase (MASP) technique. Theileria equi negative and positive horses (reared at ICAR-National Research Centre on Equines animal shed) were identified by performing ELISA (Kumar et al., 2013). Defibrinated whole blood was collected aseptically from a T. equi negative horse (as above) and centrifuged at 1500 g for 5 min. The supernatant plasma and the top white cells layer were discarded. Sedimented erythrocytes were washed with 1:1 volume of Vega Y Martinez phosphate buffered saline (VYM) by centrifuging at 1500 g for 5 min. Erythrocytes washing procedure with VYM was repeated three-times. Final pelleted erythrocytes were suspended in 1:1 volume of VYM buffer and stored at 4 °C for further use in MASP. The culture medium M 199 (Sigma-Aldrich, India) was used for MASP culture of T. equi and supplemented with 40% defibrinated equine serum, antibiotic solution (containing 60 IU/ml penicillin and 60 mg/ml streptomycin) and 200 μM hypoxanthine solution. Theileria equi MASP cultures were maintained at a temperature of 37 °C with micro-aerophilic atmosphere of 5% CO2, 3% O2, and 95% N. Erythrocytes collected from ELISA positive horse (as above) were seeded in the MASP culture for propagation of T. equi parasite and used for in vitro drug trial studies.
2.2. In vitro growth inhibitory assay
Theileria equi parasitized RBCs were collected from the MASP culture and adjusted to 1% parasitaemia by diluting with un-infected RBCs (ELISA T. equi negative horse, as above). Theileria equi in vitro growth inhibitory assay was performed in 48 well culture plates. Decamethonium bromide (DMB), Decyl trimethyl ammonium bromide (DTAB) and dodecyl trimethyl ammonium bromide (DDTAB) were obtained commercially (Sigma-Aldrich, India). Stock solutions (1000 μM) of these drugs were prepared in deionized distilled water. Working concentrations of DMB (1, 5, 10, 20, 50, 100 and 200 μM) and DTAB and DDTAB (125, 250, 500, 750, 1000 nM) were obtained by diluting their respective stock concentration with T. equi complete culture medium. Fifty microliter of T. equi parasitized RBCs (at 1% parasitaemia) were dispensed per well (in triplicate) together with 500 μl of the culture complete medium containing the indicated drug concentrations (as above). These in vitro cultures with or without drug molecules concentrations were incubated at 37 °C in an atmosphere of 5% CO2, 3% O2, and 95% N, for a period of 96 h. The overlaid culture medium was replaced with fresh medium containing indicated drug molecule concentration after every 24 h. IC50 value was calculated by standard curve fitting technique (Bork et al., 2004).
2.3. Viability test
After 96 h of in vitro treatment with different drugs (as per individual concentration), 20 μL of drug-treated/un-treated parasitized RBCs were collected and transferred to a fresh 48 well culture plate containing 30 μL of parasite-free normal horse RBCs in 500 μl of T. equi complete growth medium (without any drug molecule). The overlaid growth medium was replaced after every 24 h for the next 72 h, and T. equi parasite recrudescence/viability was determined by examining its Giemsa-stained blood smears (Bork et al., 2004).
2.4. In vitro cytotoxicity assay
In vitro cytotoxicity of different concentrations of drug was assessed on PBMCs by resazurin-based cell viability assay (Gopalakrishnan et al., 2016). PBMCs were separated on histopaque-1077 (Sigma-Aldirch, India) from the whole blood collected from a healthy horse. These PBMCs were resuspended in 1 ml complete growth medium consisting of RPMI-1640 supplemented with 2 mM L-glutamine, 60 μg/ml penicillin, 100 μg/ml streptomycin and 10% foetal bovine serum (Sigma Aldrich, India). Final cells concentration was adjusted to 3 × 105 cells/100 μL and 100 μL volume was distributed to each well of the 96 well culture plate. Simultaneously, 50 μL phytohaemagglutinin (PHA @ 10 μg/ml) was also added to each of these well. The culture plate was incubated at 37 °C having 5% CO2 in air for 48 h. Further, these PBMCs were treated with 100 μl volume of the different respective concentration of drugs (as above). Negative (without drug complete culture medium) and positive (complete culture medium with 1% triton-X100 solution) control were also maintained during cytotoxicity drug trial. Culture plate was further incubated for 24 h at 37 °C in an incubator (5% CO2 in air). A 25 μL volume of resazurin dye (150 μg/ml) was added to each well and culture plate was again incubated for next 4 h. The change of dye colour was monitored by measuring optical density (OD) at 570 nm and 650 nm. The effective OD value for each well was calculated by deducting OD570 value from its respective OD650 value. Effect of different drug molecules on PBMCs in terms of per cent viable cell population was determined as below:
The IC50 of each drug molecule on PBMCs was also calculated from a regression equation.
2.5. In vitro haemolytic assay
Haemolytic activity of each drug was assessed on horse RBCs as per standard haemolytic assay (Raghava et al., 1994). Fresh RBCs were separated from the whole blood collected from a healthy horse by centrifuging at 1200 g for 10 min. RBCs pellet was washed three times with PBS (phosphate buffer saline) by centrifugation. Different concentrations of each drug molecules (DMB, DTAB and DDTAB) were prepared in solubilising buffer (10% dimethylformamide in PBS). Twenty microliters of RBCs suspension were added to each well of 96 well culture plate containing 180 μL of different concentrations of drug molecules. Positive (RBCs suspended in distilled water) and negative (RBCs suspended in PBS) control were also maintained in the assay. The 96 well plate was incubated further at 37 °C for 90 min. The contents of each well after incubation was transferred into 2 ml micro-centrifuge tube, followed by centrifugation at 3000g for 5 min. Supernatants from each micro-centrifuge tubes were transferred to new 96 well plates and the OD was measured at 543 nm in UV spectrophotometer. Percentage of haemolysis was determined as below:
2.6. Specific selectivity index (SSI)
The extent of selectivity of different drug molecules against T. equi in comparison to horse PBMCs or RBCs at respective IC50 concentration was calculated using the below mentioned standard formula:
2.7. Statistical analysis
Statistical analysis was performed using GraphPad Prism version 6.00 software (San Diego California, USA). Two-way ANOVA followed by Bonferroni post-hoc test (p < 0.05) was computed to know the anti-T. equi activity of these drug molecules. The ‘p’ values < 0.05 were considered statistically significant differences between the treated groups and control cultures. The correlation between drug molecule concentrations, cytotoxicity and haemolytic activity was also evaluated.
3. Result
3.1. In vitro growth inhibitory assay
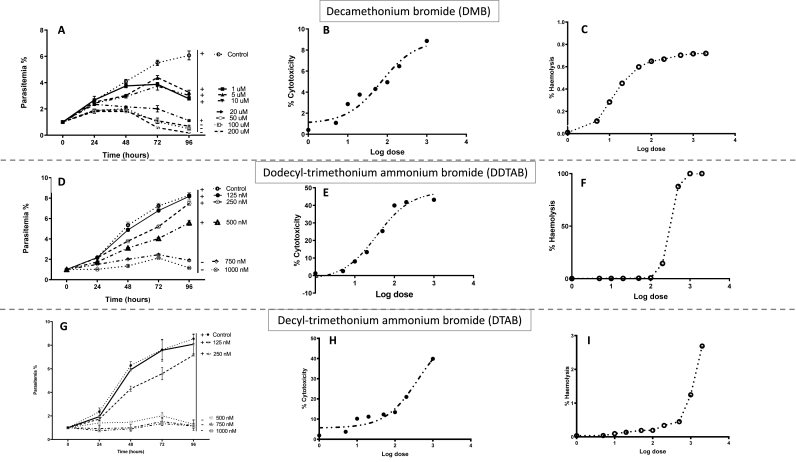
Theileria equi in vitro growth inhibition observation at 24 h exhibited significant difference from its respective control well (p < 0.05) at higher concentrations of DMB (100 μM and 200 μM; Fig. 1A); DDTAB (750 and 1000 nM; Fig. 1D); DTAB (500, 750 and 1000 nM; Fig. 1G). While, at 48 h, 72 h and 96 h of in vitro drug treatment with different concentration of DMB (5 μM–200 μM), DDTAB (250 nM–1000 nM), DTAB (250 nM–1000 nM) displayed significant (p < 0.05) growth inhibition from respective control well. The IC50 value of DMB, DDTAB and DTAB for growth inhibition of T. equi on 96 h of culture was 14.0 μM, 469.51 nM and 558.40 nM, respectively.
Fig. 1.
Liner in vitro growth inhibition curves of Theileria equi (A, D, G) and cytotoxicity assay on horse's peripheral blood mononuclear cells (PBMCs; B, E, H) and red blood cells (RBCs; C, F, I) after treatment with different concentrations of decamethonium bromide (DMB), dodecyl trimethyl ammonium bromide (DDTAB) and decyl trimethyl ammonium bromide (DTAB). Each value in the individual graph (A, D, G) represents percent parasitaemia (Mean ± SEM) observed at different time interval. ‘+’; ‘- ‘numeral on right hand side of the graph represented viability status of the T. equi parasite after 96 h of viability test.
Theileria equi parasites treated in vitro (for 96 h) with different concentrations of DMB (50 μM–200 μM), DDTAB (500 nM–1000 nM) and DTAB (750 nM and 1000 nM) failed to multiply further in the viability test (Fig. 1A, D, 1G). While T. equi parasites were live and showed recrudescence after 96 h of in vitro treatment with remaining concentrations of these drugs.
3.2. Morphological changes observed in parasites
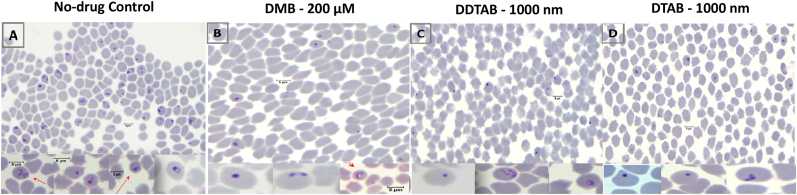
Decamethonium bromide, DDTAB and DTAB treated T. equi parasites were observed. They were devoid of internal structures, showing pycnotic and degenerative appearances (Fig. 2). Moreover, the drug treated parasites were showing no demarcation between cytoplasm and nucleus (Fig. 2). Changes in the morphology of treated parasite were indicating the efficacy of these drug molecules.
Fig. 2.
Microphotographs depicting morphological changes observed in T. equi parasites after in vitro treatment (at 96 h) with decamethonium bromide (DMB; B), dodecyl trimethyl ammonium bromide (DDTAB; C) and decyl trimethyl ammonium bromide (DTAB; D). Control (no-drug, at 96 h; A) culture showed pyriform shaped T. equi merozoites, whereas the drug treated parasites were degenerated or with condensed nucleus and appeared pyknotic. Giemsa X 1000. Bars 5 μM.
3.3. In vitro cytotoxicity and haemolytic assay
Various concentration of DMB, DDTAB and DTAB were analysed for their cytotoxicity (1 μM–1000 μM) on PBMCs cell lines and haemolytic activity (10 μM–2000 μM) on horse's RBCs. Decamethonium bromide was less than 10% cytotoxic to horse PBMCs, while DDTAB and DTAB were 40%–50% cytotoxic to horse PBMCs at highest concentrations (1000 μM; Fig. 1B, E, 1H). The respective cytotoxic concentration (CC50) as deduced by regression analysis of these drugs were 7202.96 μM, 1026.26 μM and 1263.95 μM.
Decamethonium bromide and DTAB showed least haemolytic activity (<3%; Fig. 1C and I); whereas DDTAB was more (Fig. 1F) haemolytic to horse RBCs at highest concentration of 2000 μM. The respective CC50 as deduced by regression analysis of these drugs were 224495.3 μM, 39101.35 μM and 713.54 μM.
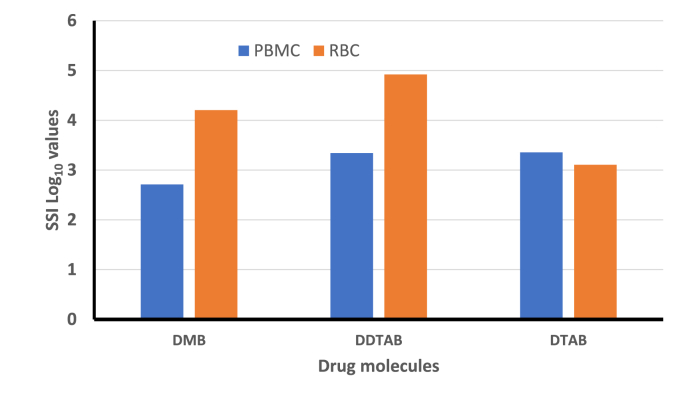
Specific selective index (SSI) for DMB, DDTAB and DTAB values with respect to host's PBMC and RBC cells, were 514.50, 2185.81, 2263.52 (respective Log10 values: 2.71, 3.33, 3.35) and 16035.38, 1519.75, 70023.91 (respective Log10 values: 4.20, 4.92, 3.10), respectively (Fig. 3).
Fig. 3.
Specific selectivity index (SSI) of decamethonium bromide (DMB), dodecyl trimethyl ammonium bromide (DDTAB) and decyl trimethyl ammonium bromide (DTAB) with respect to horse PBMCs and RBCs.
4. Discussion
The plasma membrane of the host erythrocytes has abundance of membrane transport proteins which are responsible for the flow of nutrients into and out of the cells. Plasmodium parasites, require some essential nutrients (after some hour of invasion) which are not sufficiently available within the infected erythrocytes. These are accomplished through the creation of new permeation pathways (NPPs) that facilitate the transport of a broad range of substrates across the erythrocyte membrane (Ginsburg et al., 1985; Desai et al., 1993). The mature host-erythrocyte lacks the pathways for phospholipid synthesis; however, the intra-erythrocytic parasite synthesises a range of phospholipids de novo – phosphatidylcholine from choline. Parasitized erythrocytes from Plasmodium vinckei – infected erythrocytes exhibited a significant influx of choline via NPP (Staines et al., 2000). Bis-quaternary ammonium compounds had been shown to enter the P. falciparum-infected erythrocyte via the NPP and inhibit in vitro growth of the parasite (Biagini et al., 2003). In this perspective, compounds which mimic choline structure have been developed to target phospholipid metabolism for possible use as novel class of antimalarials or apicomplexan parasites.
The DMB, DDTAB and DTAB are quaternary ammonium salt and have shown promising in vitro anti-plasmodial activities at lower concentrations. In our in vitro studies DMB, DDTAB and DTAB successfully inhibited the growth of T. equi with IC50 value of 14.0 μM, 469.51 nM and 558.40 nM, respectively. The T. equi parasites were completely dead in in vitro culture at ≥ 50 μM or ≥500 nM concentration of these drug molecules. Hexadecyltrimethylammonium bromide (HDTAB – a quaternary ammonium compound) has efficiently inhibited the in vitro growth of T. equi and Plasmodium falciparum at IC50 value of 14 μM (Gopalakrishnan et al., 2016) and 10 μM (Choubey et al., 2007), respectively. Ancelin and Vial (1986) also reported in vitro anti-P. falciparum activities of DMB, DDTAB and DTAB at respective IC50 value of 1 μM, 500 nM and 700 nM concentrations.
Quaternary ammonium salts are cationic compounds containing alkyl groups. The alkyl chain with 10–12 methylene groups were found to be more active against P. falciparum (Calas et al., 2000). The DDTAB drug molecule has 12 carbon atoms, whereas DTAB has 10 carbon atoms. Quaternary ammonium compounds with longer alkyl chain would exist with a particle size of 60–110 nm at low concentration resulting more affinity with target (Cheng and Ran, 2014). The coexistence of small compacted (size) particles and larger aggregates (large alkyl chain), initiate drug-target aggregation and enhanced efficacy of the drug molecules. We evaluated lowest anti-T. equi efficacy of DDTAB in nano-molar concentration. DDTAB has more carbon atoms than DTAB attributing to its target-specific efficacy at lower concentration.
It is imperative to know the selectivity of the tested drug candidate between host cells and target parasite for its future applicability. Respective drug candidates may show promising in vitro efficacy trial and simultaneously may be significant cytotoxic to the host cells. In the present experiment we also intended to investigate the cytotoxic and haemolytic activity of tested drug molecules. The results of the present study showed that these three drug molecules (DMB, DDTAB and DTAB) have no significant toxicity against host's PBMC and erythrocytes cell line. However more toxicity of these drug molecules in the natural host biological system needs to be analysed before accepting them as therapeutic drug. Evaluation of SSI value for a drug molecule is very critical for documenting its bioactivity specifically against the pathogen of interest. Awouafack et al. (2013) recommended an acceptance criterion of SSI ≥10 for a selective drug molecule. In this study, the tested drug molecules were observed to be very safe and non-toxic to the host's cell lines as very high SSI values were recorded and these were - 500 to 2200 (PBMCs cell lines) and 1.2 × 103 to 83.0 × 103 (RBC cell lines). QAC have prominent surface-activity and widely used in bactericidal and fungicidal preparation. Bioavailability of these compounds was observed to be less upon oral and subcutaneous administration in mouse model (Ancelin et al., 2003a, Ancelin et al., 2003b). This might forbid its anti-plasmodial usage, but it can be improved by modifying these compounds for systemic usage.
5. Conclusions
It can be concluded that DMB, DDTAB and DTAB are the quaternary ammonium salts and potential, selective T. equi in vitro growth inhibitors. These drug molecules have very high SSI values and are non-toxic to host's PBMC and RBC cell lines. These drugs molecules may be taken up further for their in-vivo anti-T. equi potential in horses.
Credit authors’ statement
AS and AG performed the whole experiments. CM performance in vitro cytotoxicity trials. RKD performed haemolytic trials, SK conceptualized, designed, and supervised the whole study. Also drafted the final version of manuscript. RK performed statistical calculations and prepared the graphs. All authors read and approved the final manuscript.
Funding
The financial support from ICAR, New Delhi funded Consortia Research Platform on Vaccine and Diagnosis for Equine Piroplasmosis is duly acknowledged.
Ethics declarations
Prior approval was taken for equine sampling in the present study from the Institutional Animal Ethics Committee of ICAR-NRCE, Hisar.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
This manuscript is a part of the Master's Thesis of the first author. The authors wish to acknowledge their gratitude to the Director, ICAR-National Research Centre on Equines (Indian Council of Agricultural Research), Hisar, Haryana, India for providing all the necessary facilities for conducting this study and to the Head, Division of Veterinary Medicine, ICAR-Indian Veterinary Research Institute, Izatnagar for managing the administrative matters of the first author as a student of the division.
References
- Ancelin M.L., Vial H.J. Quaternary ammonium compounds efficiently inhibit Plasmodium falciparum growth in vitro by impairment of choline transport. Antimicrob. Agents Chemother. 1986;29:814–820. doi: 10.1128/aac.29.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancelin M.L., Calas M., Bompart J., Cordina G., Martin D., Ben Bari M., Jei T., Druilhe P., Vial H.J. Antimalarial activity of 77 phospholipid polar head analogues: close correlation between inhibition of phospholipid metabolism and in vitro Plasmodium falciparum growth. Blood. 1998;91:1426–1437. [PubMed] [Google Scholar]
- Ancelin M.L., Calas M., Bonhoure A., Herbute S., Vial H.J. In vivo antimalarial activities of mono- and bis quaternary ammonium salts interfering with Plasmodium phospholipid metabolism. Antimicrob. Agents Chemother. 2003;47:2598–2605. doi: 10.1128/AAC.47.8.2598-2605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancelin M.L., Calas M., Vidal-Sailhan V., Herbuté S., Ringwald P., Vial H.J. Potent inhibitors of Plasmodium phospholipid metabolism with a broad spectrum of in vitro antimalarial activities. Antimicrob. Agents Chemother. 2003;47:2590–2597. doi: 10.1128/AAC.47.8.2590-2597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancelin M.L., Parant M., Thuet M.J., Philippot J.R., Vial H.J. Increased permeability to choline in simian erythrocytes after Plasmodium knowlesi infection. Biochem. J. 1991;273:701–709. doi: 10.1042/bj2730701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awouafack M.D., McGaw L.J., Gottfried S., Mbouangouere R., Tane P., Spiteller M., Eloff J.N. Antimicrobial activity and cytotoxicity of the ethanol extract, fractions and eight compounds isolated from Eriosema robustum (Fabaceae) BMC Compl. Alternative Med. 2013;13:289. doi: 10.1186/1472-6882-13-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagini G.A., Richier E., Bray P.G., Calas M., Vial H.J., Ward S.A. Heme binding contributes to antimalarial activity of bis-quaternary ammoniums. Antimicrob. Agents Chemother. 2003;47:2584–2589. doi: 10.1128/AAC.47.8.2584-2589.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork S., Yokoyama N., Ikehara Y., Kumar S., Sugimoto C., Igarashi I. Growth inhibitory effect of heparin on Babesia parasites. Antimicrob. Agents Chemother. 2004;48 doi: 10.1128/AAC.48.1.236-241.2004. 236–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calas M., Ancelin M.L., Cordina G., Portefaix P., Piquet G., Vidal- Sailhan V., Vial H.J. Antimalarial activity of compounds interfering with Plasmodium falciparum phospholipid metabolism: comparison between mono- and bisquaternary ammonium salts. J. Med. Chem. 2000;43:505–516. doi: 10.1021/jm9911027. [DOI] [PubMed] [Google Scholar]
- Cheng C., Ran S.Y. Interaction between DNA and trimethyl-ammonium bromides with different alkyl chain lengths. Sci. World J. 2014;2014 doi: 10.1155/2014/863049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choubey V., Maity P., Guha M., Kumar S., Srivastava K., Kumar S.P., Bandyopadhyay U. Inhibition of Plasmodium falciparum choline kinase by hexadecyltrimethylammonium bromide: a possible antimalarial mechanism. Antimicrob. Agents Chemother. 2007;51:696–706. doi: 10.1128/AAC.00919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai S.A., Krogstad D.J., McCleskey E.W. A nutrient-permeable channel on the intraerythrocytic malaria parasite. Nature. 1993;362:643–646. doi: 10.1038/362643a0. [DOI] [PubMed] [Google Scholar]
- Ginsburg H., Kutner S., Krugliak M., Cabantchik Z.I. Characterization of permeation pathways appearing in the host membrane of Plasmodium falciparum infected red blood cells. Mol. Biochem. Parasitol. 1985;14:313–322. doi: 10.1016/0166-6851(85)90059-3. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan A., Maji C., Dahiya R.K., Suthar A., Kumar R., Gupta A.K., Dimri U., Kumar S. In vitro growth inhibitory efficacy of some target specific novel drug molecules against Theileria equi. Vet. Parasitol. 2016;217:1–6. doi: 10.1016/j.vetpar.2015.12.024. [DOI] [PubMed] [Google Scholar]
- Holz G., Bull G. Lipids and the malarial parasite. Wld. Hlth. Organiz (WHO). 1977;55:237–248. [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Gupta A.K., Pal Y., Dwivedi S.K. In-vivo therapeutic efficacy trial with artemisinin derivative, buparvaquone and imidocarb dipropionate against Babesia equi infection in donkeys. J. Vet. Med. Sci. 2003;65:1171–1177. doi: 10.1292/jvms.65.1171. [DOI] [PubMed] [Google Scholar]
- Kumar S., Kumar R., Gupta A.K., Yadav S.C., Goyal S.K., Khurana S.K., Singh R.K. Development of EMA-2 recombinant antigen-based enzyme-linked immunosorbent assay for seroprevalence studies of Theileria equi infection in Indian equine population. Vet. Parasitol. 2013;198:10–17. doi: 10.1016/j.vetpar.2013.08.030. [DOI] [PubMed] [Google Scholar]
- Lehane A.M., Saliba K.J., Allen R.J., Kirk K. Choline uptake into the malaria parasite is energized by the membrane potential. Biochem. Biophys. Res. Commun. 2004;320:311–317. doi: 10.1016/j.bbrc.2004.05.164. [DOI] [PubMed] [Google Scholar]
- Mehlhorn H., Schein E. Redescription of Babesia equi laveran 1901 as Theileria equi. Parasitol. Res. 1998;84:467–475. doi: 10.1007/s004360050431. [DOI] [PubMed] [Google Scholar]
- Pessi G., Choi J.Y., Reynolds J.M., Voelker D.R., Mamoun C.B. In vivo evidence for the specificity of Plasmodium falciparum phosphor-ethanolamine methyltransferase and its coupling to the Kennedy pathway. J. Biol. Chem. 2005;13:12461–12466. doi: 10.1074/jbc.M414626200. [DOI] [PubMed] [Google Scholar]
- Raghava G.P., Goel A., Singh A.M., Varshney G.C. A simple microassay for computing the haemolytic potency of drugs. Biotechniques. 1994;17:1148–1153. [PubMed] [Google Scholar]
- Rashid M., Akbar H., Rashid I., Saeed K., Ahmad L., Ahmad A.S., Shehzad W., Islam S., Farooqi S. Economic significance of tropical theileriosis on a Holstein Friesian dairy Farm in Pakistan. J. Parasitol. 2018;104:310–312. doi: 10.1645/16-179. [DOI] [PubMed] [Google Scholar]
- Roggero R., Zufferey R., Minca M., Richier E., Calas M., Vial H., Mamoun C.B. Unraveling the mode of action of the antimalarial choline analogue G25 in Plasmodium falciparum and Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 2004;48:2816–2824. doi: 10.1128/AAC.48.8.2816-2824.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staines H.M., Rae C., Kirk K. Increased permeability of the malaria-infected erythrocyte to organic cations. Biochim. Biophys. Acta. 2000;1463:88–98. doi: 10.1016/s0005-2736(99)00187-x. [DOI] [PubMed] [Google Scholar]
- Stead A.M., Bray P.G., Edwards I.G., DeKoning H.P., Elford B.C., Stocks P.A., Ward S.A. Diamidine compounds: selective uptake and targeting in Plasmodium falciparum. Mol. Pharmacol. 2001;59:1298–1306. doi: 10.1124/mol.59.5.1298. [DOI] [PubMed] [Google Scholar]
- Vial H.J., Gorenflot A. Chemotherapy against babesiosis. Vet. Parasitol. 2006;138:147–160. doi: 10.1016/j.vetpar.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Vial H.J., Ancelin M.L. In: Malaria: Parasite Biology, Biogenesis, Protection. Sherman I.W., editor. ASM Press; Washington DC: 1998. Malarial lipids; pp. 159–175. [Google Scholar]
- Vial H.J., Ancelin M.L. vol. 18. Plenum Subcell. Biochem.; New York, NY: 1992. pp. 259–306. (Malarial Lipids, Intracellular Parasites). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.