Abstract
Cytotoxic T lymphocyte antigen-4 (CTLA-4) is an immune checkpoint molecule that is mainly expressed on activated T cells and regulatory T (Treg) cells that inhibits T-cell activation and regulates immune homeostasis. Due to the crucial functions of CTLA-4 in T-cell biology, CTLA-4-targeted immunotherapies have been developed for autoimmune disease as well as cancers. CTLA-4 is known to compete with CD28 to interact with B7, but some studies have revealed that its downstream signaling is independent of its ligand interaction. As a signaling domain of CTLA-4, the tyrosine motif plays a role in inhibiting T-cell activation. Recently, the lysine motif has been shown to be required for the function of Treg cells, emphasizing the importance of CTLA-4 signaling. In this review, we summarize the current understanding of CTLA-4 biology and molecular signaling events and discuss strategies to target CTLA-4 signaling for immune modulation and disease therapy.
Keywords: cytotoxic T lymphocyte antigen-4 (CTLA-4), immunotherapy, signaling motif, T cell, Treg cell
INTRODUCTION
Cytotoxic T lymphocyte antigen-4 (CTLA-4, CD152) is a 25 kDa molecule composed of a leader peptide, a ligand binding domain, a transmembrane domain, and a cytoplasmic domain. Three isoforms are generated through gene splicing in humans: full-length CTLA-4, soluble CTLA-4, and exon 1 and exon 4 forms (Ling et al., 1999; Valk et al., 2008). In mice, ligand-independent CTLA-4 is also present with three isoforms (Ueda et al., 2003). CTLA-4 negatively regulates T-cell activation and plays an important role in the suppressive function of regulatory T (Treg) cells (Krummel and Allison, 1995; Walunas et al., 1994; Wing et al., 2008). T cells are activated by T-cell receptor (TCR) signaling through antigen recognition and costimulatory signals such as CD28, which interacts with CD80 (B7.1) and CD86 (B7.2) expressed on antigen-presenting cells (APCs) (Linsley et al., 1990; 1991). CTLA-4 expressed on the plasma membrane of T cells has higher binding avidity to B7 molecules than CD28; thus, it competes with CD28 to regulate costimulation and induce anergy (Fig. 1A) (Linsley et al., 1994). In addition, CTLA-4 is constitutively expressed on Treg cells to sustain their suppressive functions and inhibits other T cells by interacting with B7 molecules (Read et al., 2000; Takahashi et al., 2000). As the functions of the extracellular domain of CTLA-4 have been elucidated, CTLA-4-immunoglobulin (CTLA-4-Ig) and anti-CTLA-4 antibodies that target CTLA-4 and B7 molecular interactions have been developed. However, while CTLA-4 has been intensively studied and is well understood as an important immunoregulatory molecule, CTLA-4 signaling has not received much attention. This review investigates the role of CTLA-4, its signaling mechanism, and therapeutic strategies targeting CTLA-4 signaling.
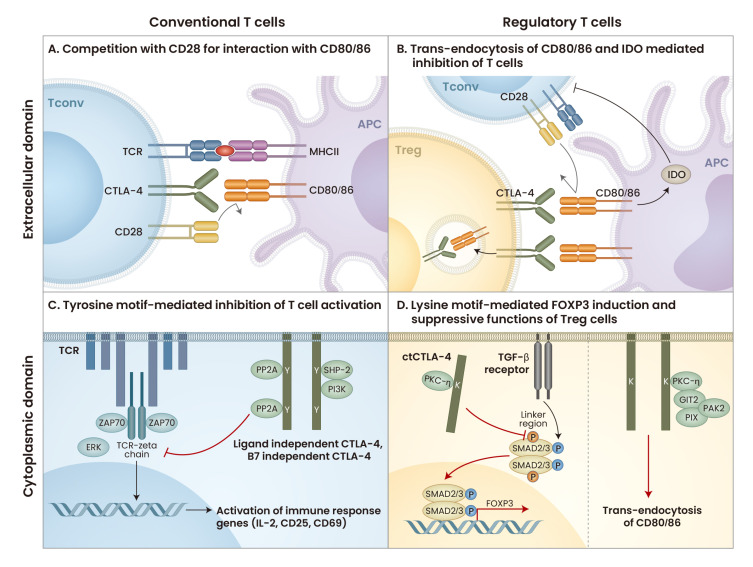
Fig. 1. The role of CTLA-4 in regulating T-cell activation and Treg-cell function.
(A) CTLA-4 interacts with CD80/86 on APCs to compete for ligands with CD28. CTLA-4 has a higher binding affinity for CD80/86 than CD28, thus blocking interaction with CD28. (B) CTLA-4 on Treg cells binds to CD80/86 on APCs, blocking costimulatory signaling in conventional T cells and depleting CD80/86 by trans-endocytosis. Therefore, CD28 of conventional T cells cannot interact with CD80/86, resulting in decreased T-cell activation. In addition, CTLA-4 induces IDO from APCs, leading to T cell inhibition. (C) CTLA-4 interacts with PP2A, SHP-2, and PI3K to transduce downstream signaling that inhibits TCR signaling to reduce T-cell activation. (D) CTLA-4 interacts with PKC-η to increase the nuclear localization of SMAD2/3, leading to FOXP3 expression. CTLA-4 in Treg cells also binds to PKC-η and recruits the GIT2, PIX, and PAK2 complex to deplete CD80/86. Tconv, conventional T cell; MHCII, major histocompatibility complex; IDO, indoleamine 2,3-dioxygenase; ZAP70, zeta chain associated protein kinase 70; ERK, extracellular signal-regulated kinase; ctCTLA-4, cytoplasmic domain of CTLA-4; TGF-β, transforming growth factor-β; GIT2, G protein-coupled receptor kinaseinteracting protein 2; PIX, PAKinteracting exchange factor; PAK2, p21 activated kinase. Diagram created with BioRender (https://biorender.com/).
HISTORY OF CTLA-4 BIOLOGY
The CTLA-4 gene sequence was discovered in 1987 and is present on chromosome 1 in mice and chromosome 2 in humans (Fig. 2) (Brunet et al., 1987). CTLA-4 is expressed on the cell surface when T cells are activated and can regulate T-cell proliferation (Krummel and Allison, 1995). CTLA-4 is transiently expressed and recycled, and most CTLA-4 is present in intracellular vesicles (Linsley et al., 1996). Membrane-expressed CTLA-4 undergoes clathrin-mediated endocytosis for recirculation (Qureshi et al., 2012).
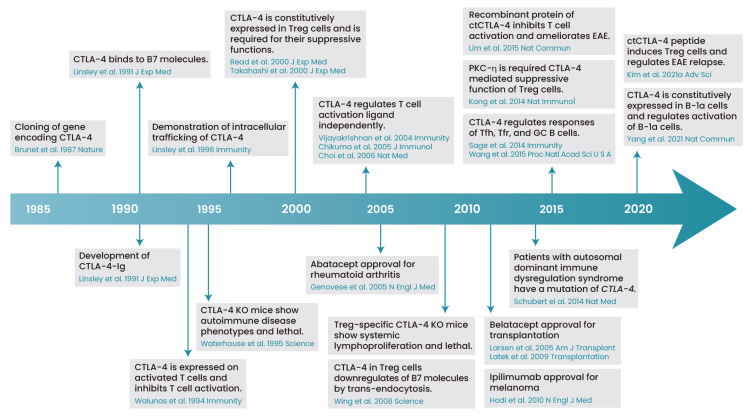
Fig. 2. History of CTLA-4 biology.
A historical overview of the understanding of CTLA-4 biology from 1987 to 2021 is provided. ctCTLA-4, Cytoplasmic domain of CTLA-4; GC B, germinal center B cells; Treg cells, regulatory T cells. Diagram created with BioRender (https://biorender.com/).
The importance of the immunomodulatory role of CTLA-4 has been highlighted by Ctla-4 knockout (KO) studies. T cells in Ctla-4 KO mice show increased proliferation and expression of activation markers such as CD25 and CD44 compared to wild-type (WT) T cells (Waterhouse et al., 1995), and Ctla-4 KO mice die at 3-4 weeks (Khattri et al., 1999). The proliferation of CD4+ T cells in Ctla-4 KO mice is suppressed by CTLA-4-Ig treatment (Chambers et al., 1997). Ctla-4 KO T cells develop more Th2 cells after CD3/CD28 stimulation than WT T cells, and CTLA-4-Ig treatment improves the mouse survival rate by reducing T-cell activation and proliferation (Khattri et al., 1999). In humans, patients with autosomal dominant immune dysregulation syndrome have a mutation in exon 1 of CTLA-4 (Schubert et al., 2014). Thus, CTLA-4 is critical for regulating T-cell activation and maintaining immune homeostasis in both mice and humans. However, adult mice with induced depletion of CTLA-4 show a protection against experimental autoimmune encephalomyelitis (EAE) progression and expansion of Treg cells, indicating that CTLA-4 may have different functions in developing T cells and peripheral T cells (Klocke et al., 2016; Paterson et al., 2015).
As described above, CTLA-4 is constitutively expressed in Treg cells and plays an important role in the suppressive function of Treg cells (Read et al., 2000; Takahashi et al., 2000). Treatment with anti-CTLA-4 antibody (Ab) in severe combined immunodeficiency (SCID) mice transplanted with CD45RBhi cells and CD25+ Treg cells showed exacerbation of diseases that would be inhibited by Τreg cells, indicating that CTLA-4 is important for the suppressive function of Treg cells in vivo (Read et al., 2000; 2006; Takahashi et al., 2000). Regarding the mechanism of CTLA-4 in Treg cells, CTLA-4 depletes CD80/86 on dendritic cells via trans-endocytosis, thereby reducing CD28 signaling in T cells and regulating T-cell activation (Fig. 1B) (Qureshi et al., 2011). CTLA-4 expressed on Treg cells also induces indoleamine 2,3-dioxygenase (IDO) from APCs, which catabolizes tryptophan and depletes the tryptophan depletion required for T-cell proliferation (Fallarino et al., 2003). In addition, Foxp3-specific Ctla-4 KO mice show splenomegaly with increased serum antibodies, and the inhibitory effect of Treg cells on APC CD80/86 depletion was decreased (Wing et al., 2008). Similar to these results, in patients with exon 1 mutations in CTLA-4, Treg cells have a reduced CD80 trans-endocytosis (Schubert et al., 2014). Therefore, CTLA-4 is highly expressed in Treg cells and is important for the suppressive function of these cells.
CTLA-4 has been reported to affect T-cell differentiation as well as conventional T-cell activation and suppressive functions of Treg cells. Mass cytometry analysis of T cells from WT mice and Ctla-4 KO mice confirmed that noncanonical CD4+ T cells were increased in Ctla-4 KO T cells, demonstrating that CTLA-4 regulates T-cell differentiation (Wei et al., 2019). As one of the mechanisms by which CTLA-4 regulates T-cell differentiation, it has been suggested that CTLA-4 engagement induces protein phosphorylation involved T-cell differentiation and mediates post-translational modification (Kim et al., 2021b; Lingel et al., 2017). There are also reports that CTLA-4 can regulate the response of follicular helper T (Tfh) cells, follicular regulatory T (Tfr) cells, and germinal center (GC) B cells. While Tfh and GC B cells are increased in Ctla-4 KO mice, Tfh and GC B cells are decreased in Cd28 KO mice (Wang et al., 2015). In another study, mice with Tfh cell-specific Ctla-4 deficiency showed increased IgG secretion from B cells and IgG1+GL7+ B cells (Sage et al., 2014). In addition, mice with Tfr cell-specific Ctla-4 deficiency showed increased Tfh cells and B cells upon NP-ovalbumin (OVA) immunization, confirming that Tfh cells and Tfr cells regulate B cells through CTLA-4. When Ctla-4 KO mice were infected with murine cytomegalovirus, Tfr cells were decreased in colonic lymph nodes (LNs) and mesorectal LNs, worsening disease progression (Chao et al., 2018). That study showed that CTLA-4 plays a role in regulating the differentiation of Tfr cells and is important in preventing viral infection. Recently, it was revealed that CTLA-4 expressed in B cells plays a role in regulating B-cell responses. CTLA-4 is highly expressed in B-1a cells, which are produced early in fetal development, and the self-replenishment of B-1a cells and autoantibodies are increased in Cd19-specific Ctla-4 KO mice (Yang et al., 2021). Therefore, CTLA-4 regulates not only T cells but also a certain subset of B cells.
Although CTLA-4 is well known to be expressed in T cells, few reports have shown that it is expressed in tumor cell lines. Human tumor cell lines, such as carcinoma, melanoma, and sarcoma cell lines, express CTLA-4 (Contardi et al., 2005). Treatment with recombinant CD80 and CD86 ligands in osteosarcoma cell lines led to transduction of CTLA-4 downstream signaling, resulting in caspase activation and tumor cell apoptosis. In another study, anti-CTLA-4 Ab induced PD-L1 expression in small-cell lung cancer cell lines and increased tumor cell growth in an in vivo xenograft tumor model, suggesting a role in promoting tumor proliferation through the intrinsic signaling pathway (Zhang et al., 2019). Therefore, CTLA-4 seems to have significant roles in some other cell types besides T cells, such as tumor cells.
TREATMENT WITH CTLA-4
To inhibit T-cell proliferation by blocking binding to B7 and interfering with B7 and CD28 interactions, CTLA-4-Ig (abatacept) was developed (Linsley et al., 1991). Abatacept was the first drug to receive U.S. Food and Drug Administration (FDA) approval among costimulatory targeting agents and was approved for rheumatoid arthritis treatment in 2005 (Genovese et al., 2005). CTLA-4-Ig was also approved by the FDA in 2017 for the treatment of active psoriatic arthritis (Mease et al., 2017) and in 2021 for the prevention of acute graft versus host disease in combination with calcineurin inhibitors and methotrexate (Watkins et al., 2021). In addition, because the binding affinity of abatacept was insufficient, belatacept, in which two amino acids of the CTLA-4 extracellular domain are mutated (leucin 104-glutamate, alanine 29-tyrosine), was developed. Belatacept was approved for use in transplantation in 2011, and the ability of belatacept to inhibit T-cell proliferation was found to be 10 times higher than that of abatacept (Larsen et al., 2005; Latek et al., 2009). However, CTLA-4-Ig has no effect in some autoimmune disorders, such as multiple sclerosis (Khoury et al., 2017), ulcerative colitis (Sandborn et al., 2012), and airway inflammation (Parulekar et al., 2013). In line with these findings, it seems that CTLA-4-Ig not only inhibits T-cell activation but also reduces the number of Treg cells (Glatigny et al., 2019; Szentpetery et al., 2017). To overcome this limitation, a phase II study in organ transplantation was conducted to study belatacept in combination with sirolimus, a drug that induces Treg-cell activation (NCT00565773). Other studies blocking CTLA-4 as a strategy to increase antitumor immunity have been conducted. The anti-CTLA-4 Ab ipilimumab was approved by the FDA in 2011 for treatment of patients with melanoma (Hodi et al., 2010). Anti-CTLA-4 increases CD28 signaling in T cells and prevents B7 trans-endocytosis mediated by Treg cells, allowing T cells to be activated (Qureshi et al., 2011; Seidel et al., 2018). In addition, exhausted T cells present in the tumor microenvironment highly express CTLA-4, and anti-CTLA-4 can induce antitumor immunity (Curran et al., 2010; Jiang et al., 2015). Similar to ipilimumab, tremelimumab is a human Ab against CTLA-4, but tremelimumab is an IgG2 isotype Ab, which minimizes antibody-dependent cellular cytotoxicity. Tremelimumab is being tested in an ongoing phase II study in pediatric cancer in combination with anti-PD-L1 therapy (NCT03837899, recruiting).
CTLA-4 SIGNALING
Although studies on the roles of the extracellular domain of CTLA-4 have been conducted, the downstream signals of CTLA-4 induced by the cytoplasmic domain remain relatively understudied. The CTLA-4 cytoplasmic domain consists of 36 amino acids and has four functional motifs: lysine, tyrosine 201, proline, and tyrosine 218 (Fig. 3). CTLA-4 agonistic Ab engagement in mouse CD4+ T cells inhibits nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), activator protein-1 (AP-1) (Olsson et al., 1999) and cluster formation of zeta chain associated protein kinase 70 (ZAP-70) (Schneider et al., 2008), which are downstream molecules of TCR signaling. In addition, CD28 and CTLA-4 interact with protein phosphatase 2A (PP2A) under different conditions, suggesting that they have distinct PP2A-mediated effects (Chuang et al., 2000). Moreover, CTLA-4 interferes with interactions with APCs by increasing T-cell motility (Schneider et al., 2006). Thus, CTLA-4 can modulate T-cell activation by regulating TCR signaling and T-cell motility.
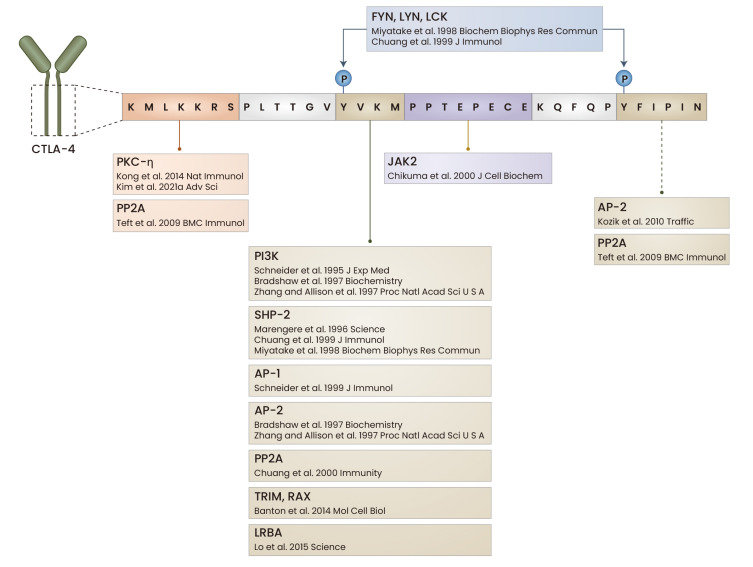
Fig. 3. Signaling motifs and interacting proteins of CTLA-4.
The cytoplasmic domain of CTLA-4 has four motifs: lysine, tyrosine 201, tyrosine 218, and proline. The two tyrosine motifs are phosphorylated by FYN, LYN, and LCK. The proteins interacting with each motif are summarized. LCK, lymphocyte specific protein tyrosine kinase; JAK2, Janus kinase 2; AP-1, activator protein-1; TRIM, T-cell receptor-interacting molecule; RAX, retina and anterior neural fold homeobox; LRBA, LPS responsive beige-like anchor protein. Diagram created with BioRender (www.biorender.com).
Compared to other motifs in the CTLA-4 signaling domain, the tyrosine motifs have been studies most (Fig. 1C). Both tyrosine motifs in CTLA-4 are phosphorylated by SRC family tyrosine kinases, such as tyrosine-protein kinase Fyn (FYN), tyrosine-protein kinase Lyn (LYN) and lymphocyte specific protein tyrosine kinase (LCK), which recruits src homology 2 domain-containing protein tyrosine phosphatase-2 (SHP-2) to CTLA-4 in a FYN-dependent manner (Chuang et al., 1999; Miyatake et al., 1998). Tyrosine 201 of CTLA-4 and the src homology 2 (SH2) domains of SHP-2 interact to dephosphorylate SRC homology/collagen, which is a Ras activator (Marengere et al., 1996). These results indicate that recruitment of SHP-2 is induced by phosphorylation of tyrosines in the CTLA-4 signaling domain and that CTLA-4 regulates TCR signaling through SHP-2. Phosphoinositide 3 kinase (PI3K) interacts with the phosphorylated YNMN motif on CD28 and tyrosine 201 on CTLA-4, confirming that PI3K is a shared downstream molecule of CTLA-4 and CD28 signaling (Hu et al., 2001; Schneider et al., 1995). Moreover, tyrosine 201 of CTLA-4 is important for TCR regulation in vivo and in human T cells. Tyrosine 201 mutant transgenic (Tg) Ctla-4 KO mice show lymphadenopathy and increased IL-4 production in LN cells (Yi et al., 2004). Costimulation of CTLA-4 with TCR in human CD4+ T cells inhibits ERK (extracellular signal-regulated kinase) activation and reduces the interaction between TCR-zeta and ZAP70 (Guntermann and Alexander, 2002). Furthermore, the tyrosine motif of CTLA-4 is required for regulation of the suppressive function of Treg cells as well as downstream TCR signaling. The induction of EAE in tyrosine 201 mutant KI (knock-in) mice results in a decreased percentage of Treg cells in the CNS (central nervous system) and a decrease in their inhibitory functions (Stumpf et al., 2014). When WT Treg cells or tyrosine 201 mutant Treg cells were transferred into recombination-activating gene (Rag) KO mice and EAE was induced, the mutant Treg cell-transferred group was unable to prevent the development of EAE compared to the WT Treg cell-transferred group (Stumpf et al., 2014). Therefore, the tyrosine motif of CTLA-4 and its signaling are involved in T-cell activation and Treg-cell functions.
The tyrosine motif of CTLA-4 is mainly involved in its recycling in T cells (Kozik et al., 2010). When tyrosine 201 is not phosphorylated, it interacts with the μ2 subunit of AP-2, and tyrosine 201 is phosphorylated by LCK and binds to PI3K (Bradshaw et al., 1997; Zhang and Allison, 1997). The AP-2 and CTLA-4 interaction results in internalization of CTLA-4 by clathrin-mediated endocytosis (Chuang et al., 1997; Shiratori et al., 1997). On the other hand, TRIM (T-cell receptor-interacting molecule), LAX (linker for activation of X cell), RAB8 and CTLA-4 form a complex in the trans-Golgi network (TGN) and traffic to the membrane (Banton et al., 2014), and exocytosis occurs in an ARF-1 (ADP-ribosylation factor 1)- and PLD (phospholipase D)-dependent manner (Mead et al., 2005). CTLA-4 interacts with AP-1 in the TGN, resulting in lysosomal degradation of CTLA-4 (Schneider and Rudd, 2014; Schneider et al., 1999). LPS-responsive beige-like anchor protein (LRBA) interacts with tyrosine 201 of CTLA-4 in the recycling endosome, preventing lysosomal degradation and increasing recycling (Lo et al., 2015). LRBA-deficient patients have the characteristics of lung inflammation and autoimmune disease, suggesting that LRBA-mediated control of CTLA-4 localization is important. A recent study revealed that FDCP6 homolog (DEF6) and RAB11 also affect the recycling of CTLA-4 (Serwas et al., 2019). T cells from DEF6 mutant patients show impaired RAB11-dependent CTLA-4 cycling and trans-endocytosis of B7 in APCs. Thus, dysregulation of CTLA-4 recycling causes autoimmune diseases, and targeting proteins involved in CTLA-4 trafficking could be an effective therapeutic strategy.
Classically, the role of tyrosines in the CTLA-4 cytoplasmic domain has been emphasized, but the importance of a lysine-containing motif has recently been highlighted (Fig. 1D). PP2A interacts with the lysine and tyrosine 218 motifs of CTLA-4 in human T cells, and this binding does not affect T-cell inhibition but is required for the inverse agonist response of CTLA-4 (Teft et al., 2009). Additionally, the lysine motif of CTLA-4 is required for the suppressive function of Treg cells because it interacts with protein kinase C-η (PKC-η) (Kong et al., 2014). This interaction between CTLA-4 and PKC-η leads to trans-endocytosis of B7 expressed on APCs by recruiting the G protein-coupled receptor kinase-interacting protein 2 (GIT2)-PAK-interacting exchange factor (αPIX)-p21 activated kinase (PAK) complex. Similarly, when Prkch KO Treg cells are transferred into B16F10 melanoma model mice, the resulting tumor size is decreased (Pedros et al., 2017). In addition, studies have shown that CTLA-4 is also involved in Treg-cell differentiation. Ctla-4-deficient mouse T cells do not express Foxp3 in the presence of TGF-β (transforming growth factor-β) (Zheng et al., 2006), a cytokine that induces Foxp3 expression. Another report showed that Ctla-4 KO naïve T cells had reduced Treg-cell differentiation compared with WT T cells (Verhagen et al., 2014). A peptide in the signaling motif of CTLA-4 increased Treg-cell differentiation in vitro and in EAE mice, whereas a lysine motif mutant peptide did not increase the differentiation of Treg cells (Kim et al., 2021a). This peptide induces nuclear translocation of mothers against decapentaplegic homolog 2/3 (SMAD2/3) by inhibiting phosphorylation of the SMAD2/3 linker region through binding to PKC-η, leading to FOXP3 induction. Moreover, CTLA-4 not only increases Treg-cell differentiation in vitro but also promotes Treg-cell accumulation in the lamina propria in a colitis model (Barnes et al., 2013). These results collectively demonstrate the importance of CTLA-4 lysine motif signaling for the differentiation and function of Treg cells.
Unlike the tyrosine and lysine motifs, other motifs have not been well studied. In 293T cells, JAK2 (Janus kinase 2) binds to the proline motif of CTLA-4 and phosphorylates tyrosine 218 of CTLA-4 (Chikuma et al., 2000). STAT5 also interacts with CTLA-4 independently of tyrosines 201 and 218. CTLA-4-transfected Jurkat cells exhibit reduced STAT5 transcriptional activity; thus, CTLA-4 negatively regulates STAT5, but the detailed mechanism has not been elucidated (Srahna et al., 2006).
The short cytoplasmic domain of CTLA-4 has multiple signaling motifs and physically interacts with various molecules, and some previous studies have revealed its importance in ligand-independent effects. Transfection of ligand-independent CTLA-4 (li-CTLA-4) into Ctla-4, Cd80, and Cd86 TKO (triple knockout) T cells resulted in reduced IFN-γ (interferon-γ) production and T-cell proliferation (Vijayakrishnan et al., 2004). Another study demonstrated that ligand-nonbinding mutant CTLA-4 Tg mice show partially rescue the sublethal phenotype of Ctla-4 KO mice (Chikuma et al., 2005). In addition, li-Ctla-4 Tg nonobese diabetic (NOD) mice show protection against autoimmune diabetes and insulitis (Stumpf et al., 2013). Therefore, CTLA-4 can transduce signals and regulate T-cell activation independent of its ligand interaction. In addition, transduction of a recombinant protein containing the CTLA-4 cytoplasmic domain conjugated to a cell-penetrating peptide inhibited T-cell activation and alleviated allergic airway inflammation (Choi et al., 2006; Lim et al., 2017), collagen-induced arthritis (Choi et al., 2008), and EAE (Lim et al., 2015). It also inhibited human T-cell activation and controlled human skin graft rejection in SCID mice (Lim et al., 2018). Moreover, a synthetic peptide containing the CTLA-4 cytoplasmic domain conjugated to a cell-penetrating peptide increased the differentiation of Treg cells and prevented disease relapse in EAE (Kim et al., 2021a). Regarding the differences between CTLA-4-Ig and the cytoplasmic domain of CTLA-4, in terms of effects on Treg-cell numbers and functions, CTLA-4-Ig has the disadvantage of sustaining immune tolerance in disease due to its reduction of Treg-cell numbers; however, CTLA-4 signaling peptide increases Treg-cell numbers, revealing a distinct mechanism for disease modulation.
CONCLUSION
The important immune checkpoint molecule CTLA-4 regulates the costimulation of T cells and is required for Treg-cell functions. Surprisingly, its signaling does not require ligand interactions, emphasizing that there are multiple mechanisms of CTLA-4 in T-cell biology. As CTLA-4 is often referred to as a “moving target” due to its recycling and transient localization in the membrane of activated T cells, the cytoplasmic domain of CTLA-4 might be more important for the intrinsic regulation of cellular signaling. In addition, due to constitutive expression of CTLA-4 in Treg cells, CTLA-4 signaling can sustain or control Treg-cell functions to cause them to function as “suppressor cells”. As a potential drug, unlike CTLA-4-Ig, strategies targeting the cytoplasmic domain function of CTLA-4 have the advantage of increasing the number of Treg cells in vivo, revealing that novel strategies are required to develop immune modulatory drugs based on CTLA-4 biology. The current understanding of CTLA-4 biology suggests that developing immune-modulatory drugs targeting CTLA-4 will be useful for disease therapy.
ACKNOWLEDGMENTS
This research was supported by grants from the Bio and Medical Technology Development Program (NRF-2017M3A9C8027972) and Basic Science Research Program (NRF-2019R1A2C3006155) of the National Research Foundation funded by the Korean government to J.-M.C.
Footnotes
AUTHOR CONTRIBUTIONS
G.-R.K. and J.-M.C. conceived and wrote the manuscript.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Banton M.C., Inder K.L., Valk E., Rudd C.E., Schneider H. Rab8 binding to immune cell-specific adaptor LAX facilitates formation of trans-Golgi network-proximal CTLA-4 vesicles for surface expression. Mol. Cell. Biol. 2014;34:1486–1499. doi: 10.1128/MCB.01331-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes M.J., Griseri T., Johnson A.M., Young W., Powrie F., Izcue A. CTLA-4 promotes Foxp3 induction and regulatory T cell accumulation in the intestinal lamina propria. Mucosal Immunol. 2013;6:324–334. doi: 10.1038/mi.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw J.D., Lu P., Leytze G., Rodgers J., Schieven G.L., Bennett K.L., Linsley P.S., Kurtz S.E. Interaction of the cytoplasmic tail of CTLA-4 (CD152) with a clathrin-associated protein is negatively regulated by tyrosine phosphorylation. Biochemistry. 1997;36:15975–15982. doi: 10.1021/bi971762i. [DOI] [PubMed] [Google Scholar]
- Brunet J.F., Denizot F., Luciani M.F., Roux-Dosseto M., Suzan M., Mattei M.G., Golstein P. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- Chambers C.A., Sullivan T.J., Allison J.P. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity. 1997;7:885–895. doi: 10.1016/S1074-7613(00)80406-9. [DOI] [PubMed] [Google Scholar]
- Chao G., Li X., Ji Y., Zhu Y., Li N., Zhang N., Feng Z., Niu M. CTLA-4 regulates T follicular regulatory cell differentiation and participates in intestinal damage caused by spontaneous autoimmunity. Biochem. Biophys. Res. Commun. 2018;505:865–871. doi: 10.1016/j.bbrc.2018.09.182. [DOI] [PubMed] [Google Scholar]
- Chikuma S., Abbas A.K., Bluestone J.A. B7-independent inhibition of T cells by CTLA-4. J. Immunol. 2005;175:177–181. doi: 10.4049/jimmunol.175.1.177. [DOI] [PubMed] [Google Scholar]
- Chikuma S., Murakami M., Tanaka K., Uede T. Janus kinase 2 is associated with a box 1-like motif and phosphorylates a critical tyrosine residue in the cytoplasmic region of cytotoxic T lymphocyte associated molecule-4. J. Cell. Biochem. 2000;78:241–250. doi: 10.1002/(SICI)1097-4644(20000801)78:2<241::AID-JCB7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Choi J.M., Ahn M.H., Chae W.J., Jung Y.G., Park J.C., Song H.M., Kim Y.E., Shin J.A., Park C.S., Park J.W., et al. Intranasal delivery of the cytoplasmic domain of CTLA-4 using a novel protein transduction domain prevents allergic inflammation. Nat. Med. 2006;12:574–579. doi: 10.1038/nm1385. [DOI] [PubMed] [Google Scholar]
- Choi J.M., Kim S.H., Shin J.H., Gibson T., Yoon B.S., Lee D.H., Lee S.K., Bothwell A.L., Lim J.S., Lee S.K. Transduction of the cytoplasmic domain of CTLA-4 inhibits TcR-specific activation signals and prevents collagen-induced arthritis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19875–19880. doi: 10.1073/pnas.0805198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang E., Alegre M.L., Duckett C.S., Noel P.J., Vander Heiden M.G., Thompson C.B. Interaction of CTLA-4 with the clathrin-associated protein AP50 results in ligand-independent endocytosis that limits cell surface expression. J. Immunol. 1997;159:144–151. doi: 10.1073/pnas.94.17.9273. [DOI] [PubMed] [Google Scholar]
- Chuang E., Fisher T.S., Morgan R.W., Robbins M.D., Duerr J.M., Vander Heiden M.G., Gardner J.P., Hambor J.E., Neveu M.J., Thompson C.B. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity. 2000;13:313–322. doi: 10.1016/S1074-7613(00)00031-5. [DOI] [PubMed] [Google Scholar]
- Chuang E., Lee K.M., Robbins M.D., Duerr J.M., Alegre M.L., Hambor J.E., Neveu M.J., Bluestone J.A., Thompson C.B. Regulation of cytotoxic T lymphocyte-associated molecule-4 by Src kinases. J. Immunol. 1999;162:1270–1277. [PubMed] [Google Scholar]
- Contardi E., Palmisano G.L., Tazzari P.L., Martelli A.M., Fala F., Fabbi M., Kato T., Lucarelli E., Donati D., Polito L., et al. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int. J. Cancer. 2005;117:538–550. doi: 10.1002/ijc.21155. [DOI] [PubMed] [Google Scholar]
- Curran M.A., Montalvo W., Yagita H., Allison J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallarino F., Grohmann U., Hwang K.W., Orabona C., Vacca C., Bianchi R., Belladonna M.L., Fioretti M.C., Alegre M.L., Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- Genovese M.C., Becker J.C., Schiff M., Luggen M., Sherrer Y., Kremer J., Birbara C., Box J., Natarajan K., Nuamah I., et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N. Engl. J. Med. 2005;353:1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- Glatigny S., Hollbacher B., Motley S.J., Tan C., Hundhausen C., Buckner J.H., Smilek D., Khoury S.J., Ding L., Qin T., et al. Abatacept targets T follicular helper and regulatory T cells, disrupting molecular pathways that regulate their proliferation and maintenance. J. Immunol. 2019;202:1373–1382. doi: 10.4049/jimmunol.1801425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntermann C., Alexander D.R. CTLA-4 suppresses proximal TCR signaling in resting human CD4(+) T cells by inhibiting ZAP-70 Tyr(319) phosphorylation: a potential role for tyrosine phosphatases. J. Immunol. 2002;168:4420–4429. doi: 10.4049/jimmunol.168.9.4420. [DOI] [PubMed] [Google Scholar]
- Hodi F.S., O'Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Rudd C.E., Schneider H. Src kinases Fyn and Lck facilitate the accumulation of phosphorylated CTLA-4 and its association with PI-3 kinase in intracellular compartments of T-cells. Biochem. Biophys. Res. Commun. 2001;288:573–578. doi: 10.1006/bbrc.2001.5814. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Li Y., Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015;6:e1792. doi: 10.1038/cddis.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattri R., Auger J.A., Griffin M.D., Sharpe A.H., Bluestone J.A. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J. Immunol. 1999;162:5784–5791. [PubMed] [Google Scholar]
- Khoury S.J., Rochon J., Ding L., Byron M., Ryker K., Tosta P., Gao W., Freedman M.S., Arnold D.L., Sayre P.H., et al. ACCLAIM: a randomized trial of abatacept (CTLA4-Ig) for relapsing-remitting multiple sclerosis. Mult. Scler. 2017;23:686–695. doi: 10.1177/1352458516662727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.R., Kim W.J., Lim S., Lee H.G., Koo J.H., Nam K.H., Kim S.M., Park S.D., Choi J.M. In vivo induction of regulatory T cells via CTLA-4 signaling peptide to control autoimmune encephalomyelitis and prevent disease relapse. Adv. Sci. (Weinh.) 2021a;8:2004973. doi: 10.1002/advs.202004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.K., Jeong M.G., Hwang E.S. Post-translational modifications in transcription factors that determine T helper cell differentiation. Mol. Cells. 2021b;44:318–327. doi: 10.14348/molcells.2021.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klocke K., Sakaguchi S., Holmdahl R., Wing K. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E2383–E2392. doi: 10.1073/pnas.1603892113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong K.F., Fu G., Zhang Y., Yokosuka T., Casas J., Canonigo-Balancio A.J., Becart S., Kim G., Yates J.R., 3rd, Kronenberg M., 3rd, et al. Protein kinase C-eta controls CTLA-4-mediated regulatory T cell function. Nat. Immunol. 2014;15:465–472. doi: 10.1038/ni.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozik P., Francis R.W., Seaman M.N.J., Robinson M.S. A screen for endocytic motifs. Traffic. 2010;11:843–855. doi: 10.1111/j.1600-0854.2010.01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel M.F., Allison J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C.P., Pearson T.C., Adams A.B., Tso P., Shirasugi N., Strobert E., Anderson D., Cowan S., Price K., Naemura J., et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am. J. Transplant. 2005;5:443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- Latek R., Fleener C., Lamian V., Kulbokas E., 3rd, Davis P.M., 3rd, Suchard S.J., 3rd, Curran M., 3rd, Vincenti F., 3rd, Townsend R., 3rd Assessment of belatacept-mediated costimulation blockade through evaluation of CD80/86-receptor saturation. Transplantation. 2009;87:926–933. doi: 10.1097/TP.0b013e31819b5a58. [DOI] [PubMed] [Google Scholar]
- Lim S., Ho Sohn J., Koo J.H., Park J.W., Choi J.M. dNP2-ctCTLA-4 inhibits German cockroach extract-induced allergic airway inflammation and hyper-responsiveness via inhibition of Th2 responses. Exp. Mol. Med. 2017;49:e362. doi: 10.1038/emm.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S., Kim W.J., Kim Y.H., Lee S., Koo J.H., Lee J.A., Yoon H., Kim D.H., Park H.J., Kim H.M., et al. dNP2 is a blood-brain barrier-permeable peptide enabling ctCTLA-4 protein delivery to ameliorate experimental autoimmune encephalomyelitis. Nat. Commun. 2015;6:8244. doi: 10.1038/ncomms9244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S., Kirkiles-Smith N.C., Pober J.S., Bothwell A.L.M., Choi J.M. Regulation of human T cell responses by dNP2-ctCTLA-4 inhibits human skin and microvessel graft rejection. Biomaterials. 2018;183:128–138. doi: 10.1016/j.biomaterials.2018.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling V., Wu P.W., Finnerty H.F., Sharpe A.H., Gray G.S., Collins M. Complete sequence determination of the mouse and human CTLA4 gene loci: cross-species DNA sequence similarity beyond exon borders. Genomics. 1999;60:341–355. doi: 10.1006/geno.1999.5930. [DOI] [PubMed] [Google Scholar]
- Lingel H., Wissing J., Arra A., Schanze D., Lienenklaus S., Klawonn F., Pierau M., Zenker M., Jansch L., Brunner-Weinzierl M.C. CTLA-4-mediated posttranslational modifications direct cytotoxic T-lymphocyte differentiation. Cell Death Differ. 2017;24:1739–1749. doi: 10.1038/cdd.2017.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P.S., Bradshaw J., Greene J., Peach R., Bennett K.L., Mittler R.S. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543. doi: 10.1016/S1074-7613(00)80480-X. [DOI] [PubMed] [Google Scholar]
- Linsley P.S., Brady W., Urnes M., Grosmaire L.S., Damle N.K., Ledbetter J.A. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P.S., Clark E.A., Ledbetter J.A. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc. Natl. Acad. Sci. U. S. A. 1990;87:5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P.S., Greene J.L., Brady W., Bajorath J., Ledbetter J.A., Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/S1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- Lo B., Zhang K., Lu W., Zheng L., Zhang Q., Kanellopoulou C., Zhang Y., Liu Z., Fritz J.M., Marsh R., et al. AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 2015;349:436–440. doi: 10.3410/f.725669971.793508623. [DOI] [PubMed] [Google Scholar]
- Marengere L.E., Waterhouse P., Duncan G.S., Mittrucker H.W., Feng G.S., Mak T.W. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science. 1996;272:1170–1173. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- Mead K.I., Zheng Y., Manzotti C.N., Perry L.C., Liu M.K., Burke F., Powner D.J., Wakelam M.J., Sansom D.M. Exocytosis of CTLA-4 is dependent on phospholipase D and ADP ribosylation factor-1 and stimulated during activation of regulatory T cells. J. Immunol. 2005;174:4803–4811. doi: 10.4049/jimmunol.174.8.4803. [DOI] [PubMed] [Google Scholar]
- Mease P.J., Gottlieb A.B., van der Heijde D., FitzGerald O., Johnsen A., Nys M., Banerjee S., Gladman D.D. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann. Rheum. Dis. 2017;76:1550–1558. doi: 10.1136/annrheumdis-2016-210724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake S., Nakaseko C., Umemori H., Yamamoto T., Saito T. Src family tyrosine kinases associate with and phosphorylate CTLA-4 (CD152) Biochem. Biophys. Res. Commun. 1998;249:444–448. doi: 10.1006/bbrc.1998.9191. [DOI] [PubMed] [Google Scholar]
- Olsson C., Riesbeck K., Dohlsten M., Michaelsson E. CTLA-4 ligation suppresses CD28-induced NF-kappaB and AP-1 activity in mouse T cell blasts. J. Biol. Chem. 1999;274:14400–14405. doi: 10.1074/jbc.274.20.14400. [DOI] [PubMed] [Google Scholar]
- Parulekar A.D., Boomer J.S., Patterson B.M., Yin-Declue H., Deppong C.M., Wilson B.S., Jarjour N.N., Castro M., Green J.M. A randomized controlled trial to evaluate inhibition of T-cell costimulation in allergen-induced airway inflammation. Am. J. Respir. Crit. Care Med. 2013;187:494–501. doi: 10.1164/rccm.201207-1205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson A.M., Lovitch S.B., Sage P.T., Juneja V.R., Lee Y., Trombley J.D., Arancibia-Carcamo C.V., Sobel R.A., Rudensky A.Y., Kuchroo V.K., et al. Deletion of CTLA-4 on regulatory T cells during adulthood leads to resistance to autoimmunity. J. Exp. Med. 2015;212:1603–1621. doi: 10.1084/jem.20141030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedros C., Canonigo-Balancio A.J., Kong K.F., Altman A. Requirement of Treg-intrinsic CTLA4/PKCeta signaling pathway for suppressing tumor immunity. JCI Insight. 2017;2:e95692. doi: 10.1172/jci.insight.95692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi O.S., Kaur S., Hou T.Z., Jeffery L.E., Poulter N.S., Briggs Z., Kenefeck R., Willox A.K., Royle S.J., Rappoport J.Z., et al. Constitutive clathrin-mediated endocytosis of CTLA-4 persists during T cell activation. J. Biol. Chem. 2012;287:9429–9440. doi: 10.1074/jbc.M111.304329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi O.S., Zheng Y., Nakamura K., Attridge K., Manzotti C., Schmidt E.M., Baker J., Jeffery L.E., Kaur S., Briggs Z., et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S., Greenwald R., Izcue A., Robinson N., Mandelbrot D., Francisco L., Sharpe A.H., Powrie F. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J. Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S., Malmstrom V., Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J. Exp. Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage P.T., Paterson A.M., Lovitch S.B., Sharpe A.H. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41:1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborn W.J., Colombel J.F., Sands B.E., Rutgeerts P., Targan S.R., Panaccione R., Bressler B., Geboes K., Schreiber S., Aranda R., et al. Abatacept for Crohn's disease and ulcerative colitis. Gastroenterology. 2012;143:62–69.e4. doi: 10.1053/j.gastro.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Schneider H., Downey J., Smith A., Zinselmeyer B.H., Rush C., Brewer J.M., Wei B., Hogg N., Garside P., Rudd C.E. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- Schneider H., Martin M., Agarraberes F.A., Yin L., Rapoport I., Kirchhausen T., Rudd C.E. Cytolytic T lymphocyte-associated antigen-4 and the TCR zeta/CD3 complex, but not CD28, interact with clathrin adaptor complexes AP-1 and AP-2. J. Immunol. 1999;163:1868–1879. [PubMed] [Google Scholar]
- Schneider H., Prasad K.V., Shoelson S.E., Rudd C.E. CTLA-4 binding to the lipid kinase phosphatidylinositol 3-kinase in T cells. J. Exp. Med. 1995;181:351–355. doi: 10.1084/jem.181.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Rudd C.E. Diverse mechanisms regulate the surface expression of immunotherapeutic target ctla-4. Front. Immunol. 2014;5:619. doi: 10.3389/fimmu.2014.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Smith X., Liu H., Bismuth G., Rudd C.E. CTLA-4 disrupts ZAP70 microcluster formation with reduced T cell/APC dwell times and calcium mobilization. Eur. J. Immunol. 2008;38:40–47. doi: 10.1002/eji.200737423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Bode C., Kenefeck R., Hou T.Z., Wing J.B., Kennedy A., Bulashevska A., Petersen B.S., Schaffer A.A., Gruning B.A., et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat. Med. 2014;20:1410–1416. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel J.A., Otsuka A., Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front. Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwas N.K., Hoeger B., Ardy R.C., Stulz S.V., Sui Z., Memaran N., Meeths M., Krolo A., Yuce Petronczki O., Pfajfer L., et al. Human DEF6 deficiency underlies an immunodeficiency syndrome with systemic autoimmunity and aberrant CTLA-4 homeostasis. Nat. Commun. 2019;10:3106. doi: 10.1038/s41467-019-10812-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori T., Miyatake S., Ohno H., Nakaseko C., Isono K., Bonifacino J.S., Saito T. Tyrosine phosphorylation controls internalization of CTLA-4 by regulating its interaction with clathrin-associated adaptor complex AP-2. Immunity. 1997;6:583–589. doi: 10.1016/S1074-7613(00)80346-5. [DOI] [PubMed] [Google Scholar]
- Srahna M., Van Grunsven L.A., Remacle J.E., Vandenberghe P. CTLA-4 interacts with STAT5 and inhibits STAT5-mediated transcription. Immunology. 2006;117:396–401. doi: 10.1111/j.1365-2567.2005.02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf M., Zhou X., Bluestone J.A. The B7-independent isoform of CTLA-4 functions to regulate autoimmune diabetes. J. Immunol. 2013;190:961–969. doi: 10.4049/jimmunol.1201362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf M., Zhou X., Chikuma S., Bluestone J.A. Tyrosine 201 of the cytoplasmic tail of CTLA-4 critically affects T regulatory cell suppressive function. Eur. J. Immunol. 2014;44:1737–1746. doi: 10.1002/eji.201343891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentpetery A., Heffernan E., Gogarty M., Mellerick L., McCormack J., Haroon M., Elmamoun M., Gallagher P., Kelly G., Fabre A., et al. Abatacept reduces synovial regulatory T-cell expression in patients with psoriatic arthritis. Arthritis Res. Ther. 2017;19:158. doi: 10.1186/s13075-017-1364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N., Mak T.W., Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teft W.A., Chau T.A., Madrenas J. Structure-Function analysis of the CTLA-4 interaction with PP2A. BMC Immunol. 2009;10:23. doi: 10.1186/1471-2172-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H., Howson J.M., Esposito L., Heward J., Snook H., Chamberlain G., Rainbow D.B., Hunter K.M., Smith A.N., Di Genova G., et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- Valk E., Rudd C.E., Schneider H. CTLA-4 trafficking and surface expression. Trends Immunol. 2008;29:272–279. doi: 10.1016/j.it.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen J., Gabrysova L., Shepard E.R., Wraith D.C. Ctla-4 modulates the differentiation of inducible Foxp3+ Treg cells but IL-10 mediates their function in experimental autoimmune encephalomyelitis. PLoS One. 2014;9:e108023. doi: 10.1371/journal.pone.0108023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakrishnan L., Slavik J.M., Illes Z., Greenwald R.J., Rainbow D., Greve B., Peterson L.B., Hafler D.A., Freeman G.J., Sharpe A.H., et al. An autoimmune disease-associated CTLA-4 splice variant lacking the B7 binding domain signals negatively in T cells. Immunity. 2004;20:563–575. doi: 10.1016/S1074-7613(04)00110-4. [DOI] [PubMed] [Google Scholar]
- Walunas T.L., Lenschow D.J., Bakker C.Y., Linsley P.S., Freeman G.J., Green J.M., Thompson C.B., Bluestone J.A. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-X. [DOI] [PubMed] [Google Scholar]
- Wang C.J., Heuts F., Ovcinnikovs V., Wardzinski L., Bowers C., Schmidt E.M., Kogimtzis A., Kenefeck R., Sansom D.M., Walker L.S. CTLA-4 controls follicular helper T-cell differentiation by regulating the strength of CD28 engagement. Proc. Natl. Acad. Sci. U. S. A. 2015;112:524–529. doi: 10.1073/pnas.1414576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse P., Penninger J.M., Timms E., Wakeham A., Shahinian A., Lee K.P., Thompson C.B., Griesser H., Mak T.W. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- Watkins B., Qayed M., McCracken C., Bratrude B., Betz K., Suessmuth Y., Yu A., Sinclair S., Furlan S., Bosinger S., et al. Phase II trial of costimulation blockade with abatacept for prevention of acute GVHD. J. Clin. Oncol. 2021;39:1865–1877. doi: 10.1200/JCO.20.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S.C., Sharma R., Anang N.A.S., Levine J.H., Zhao Y., Mancuso J.J., Setty M., Sharma P., Wang J., Pe'er D., et al. Negative co-stimulation constrains T cell differentiation by imposing boundaries on possible cell states. Immunity. 2019;50:1084–1098.e10. doi: 10.1016/j.immuni.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z., Nomura T., Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- Yang Y., Li X., Ma Z., Wang C., Yang Q., Byrne-Steele M., Hong R., Min Q., Zhou G., Cheng Y., et al. CTLA-4 expression by B-1a B cells is essential for immune tolerance. Nat. Commun. 2021;12:525. doi: 10.1038/s41467-020-20874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L.A., Hajialiasgar S., Chuang E. Tyrosine-mediated inhibitory signals contribute to CTLA-4 function in vivo. Int. Immunol. 2004;16:539–547. doi: 10.1093/intimm/dxh055. [DOI] [PubMed] [Google Scholar]
- Zhang H., Dutta P., Liu J., Sabri N., Song Y., Li W.X., Li J. Tumour cell-intrinsic CTLA4 regulates PD-L1 expression in non-small cell lung cancer. J. Cell. Mol. Med. 2019;23:535–542. doi: 10.1111/jcmm.13956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Allison J.P. Interaction of CTLA-4 with AP50, a clathrin-coated pit adaptor protein. Proc. Natl. Acad. Sci. U. S. A. 1997;94:9273–9278. doi: 10.1073/pnas.94.17.9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S.G., Wang J.H., Stohl W., Kim K.S., Gray J.D., Horwitz D.A. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J. Immunol. 2006;176:3321–3329. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]