Abstract
With planned deep space and commercial spaceflights, gaps remain to address health risks in astronauts. Multiple studies have shown associations between clonal expansion of hematopoietic cells with hematopoietic malignancies and cardiometabolic disease. This expansion of clones in the absence of overt hematopoietic disorders is termed clonal hematopoiesis (CH) of indeterminate potential (CHIP). Using deep, error-corrected, targeted DNA sequencing we assayed for somatic mutations in CH-driver genes in peripheral blood mononuclear cells isolated from de-identified blood samples collected from 14 astronauts who flew Shuttle missions between 1998–2001. We identified 34 nonsynonymous mutations of relatively low variant allele fraction in 17 CH-driver genes, with the most prevalent mutations in TP53 and DNMT3A. The presence of these small clones in the blood of relatively young astronaut cohort warrants further retrospective and prospective investigation of their clinical relevance and potential application in monitoring astronaut’s health.
Subject terms: Risk factors, Predictive markers
Deep targeted DNA sequencing of peripheral blood mononuclear cells isolated from blood samples from 14 astronauts who flew Shuttle missions between 1998–2001 identifies 34 non-silent mutations, predominantly in TP53 and DNMT3A.
Introduction
With the advent of NASA’s deep space Gateway and Artemis missions and the expansion of commercial spaceflights, there is an increased need to understand, counteract, and mitigate health risks associated with spaceflight. Specific acute and long-term adverse effects and risks inherent to space missions are defined not only by duration, but also the course of flight, i.e., remaining in low Earth orbit (LEO) where Earth’s magnetic field provides shielding from space radiation or going beyond this protective shield into deep space. Some of these adverse effects include bone-density loss, neurovestibular changes and muscle atrophy. In addition, there are degenerative risks, including space radiation (IR)-induced cancer, cardiovascular and neurodegenerative diseases1,2, and all three are priority risk reduction areas identified by NASA’s Human Research Roadmap. Thus, quality health assessments for astronauts that account for an individual’s susceptibility are critical for understanding and addressing risks before, during and after future exploration-type, deep-space missions. Considering baseline genetic and extrinsic variability, the development of tools that permit the assessment of individual genetic susceptibility would improve risk stratification and long-term clinical management.
Somatic mutations occur randomly and in response to various systemic and environmental stressors. While most mutations do not affect cell phenotype, others alter the cell’s fitness, conferring a selective proliferative and survival advantage leading to clonal events in normal tissues. Clonal hematopoiesis (CH) is the expansion of such clones in hematopoietic stem/progenitor cells (HSPCs), resulting in a relatively benign precursor state. The presence of these clonal mutations in the absence of neoplasia at a >2% variant allele frequency (VAF) is designated as CH of indeterminate potential (CHIP)3. The accumulation of somatic mutations occurs as a function of aging and can be facilitated by inflammatory alterations in the microenvironment. However, other mechanisms promoting clonal progression remain unclear. Most mutations associated with age-related CHIP occur in epigenetic modifier genes (DNMT3A, TET2, ASXL1)4,5. In contrast to age-related CHIP, there is an accelerated form of CH that is found in cancer survivors who have been exposed to therapy with genotoxic agents. This is referred to as therapy-associated clonal hematopoiesis (t-CH) and mutations occur predominantly in genes involved in DNA damage response (DDR) pathway (TP53, PPM1D)6. Studies have associated CHIP with an increased risk of hematological and solid malignancies, cardiovascular disease (CVD), cardiometabolic and neurodegenerative disease, and overall higher mortality4,5,7. Notably, the size of mutant clones is associated with increased risk; however, additional factors such as comorbidities or cell-extrinsic factors, are likely to impact the degree of risk for such outcomes. Additionally, experimental studies have shown that CH can contribute to chronic diseases by promoting inflammation8–10.
Spaceflights are associated with exposure to various stressors, including IR, microgravity, and other harmful space environmental factors. NASA’s Twin study reported hematopoietic mutations in DNMT3A and TET2, consistent with the presence of CHIP11. While the Twin studies employed conventional next generation, whole genome sequencing, the current study employed deep, error-corrected DNA sequencing to detect somatic mutations in CH-driver genes using de-identified blood samples collected from 14 astronauts who flew Space Transportation System (STS), aka Space Shuttle, missions between 1998–2001. We identified 34 non-silent mutations, predominantly in TP53 and DNMT3A, consistent with the presence of CH in this relatively young astronaut cohort.
Results
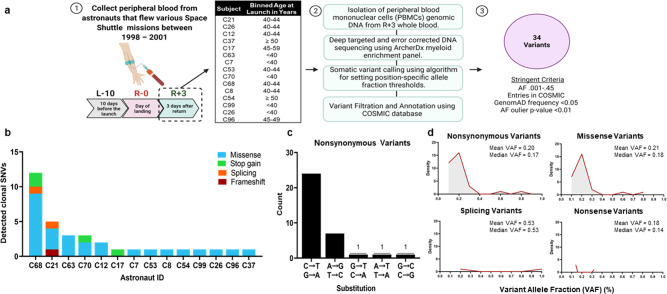
We obtained de-identified whole blood samples from 14 astronauts who flew relatively short Space Shuttle missions (median 12 days) between 1998–2001. These samples were stored at −80 °C for ~20 years. Blood samples were collected 10 days before flight, the day of landing, and 3 days after landing12. However, for this specific study, only samples from 3 days after landing (R + 3) were collected as buffy coats (peripheral blood mononuclear cells - PBMCs). The median age of the Shuttle mission crew (including individuals who did not donate blood) at sample collection was approximately 42 years (IQR = 39–45) (Fig. 1a). Six of the 14 (43%) astronauts were on their first mission at time of sample collection. Approximately 85% of crew members were male during missions conducted between 1998–2001. An average of two extravehicular activities (EVAs) occurred per Shuttle mission, with two astronauts participating per EVA (an average of four astronauts per mission). Per the Lifetime Surveillance of Astronaut Health (LSAH) at NASA Johnson Space Center, the astronauts who participated in the following study did not have any prior exposures to chemotherapy or radiation therapy. To detect somatic mutations in CH-driver genes in PBMCs, we performed deep, error-corrected DNA sequencing using a myeloid panel to enrich 37 candidates frequently mutated in myeloid malignancies. Mutational analysis of PBMCs identified 34 somatic nonsynonymous single nucleotide variants (SNVs) in 17 genes at 0.10–0.95% (median 0.18%) VAF (Fig. 1a, d). Five out of 14 astronauts (36%) harbored two or more mutations. Subjects C68 and C21 harbored the largest number of clonal SNVs, 12 and 5, respectively (Fig. 1b). We detected 27 missense SNVs (median VAF 0.18%), 4 nonsense SNVs (median VAF 0.14%), 2 splicing SNVs (median VAF 0.53%), and 1 frameshift SNV (Fig. 1b, d). The most common exonic substitution was cytosine to thymine (C → T) transition, followed by guanine to adenine (G → A) transition (Fig. 1c), which is considered a mutational signature of aging13 and is common in variants associated with CHIP.
Fig. 1. Characteristics of Clonal Hematopoiesis (CH) Mutations.
a We identified somatic mutations in known clonal hematopoiesis of indeterminate potential (CHIP) driver genes using peripheral blood mononuclear cells isolated from 14 astronauts who flew short space Shuttle missions lasting a median of 12 days between 1998–2001. Created with BioRender.com. b Number of somatic nonsynonymous single nucleotide variants (SNVs) in CHIP-driver genes harbored per subject. c Rates of different substitution types observed in clonal SNVs. Only one guanine to thymine transition was observed. d Density of mutations by VAF for each mutation type.
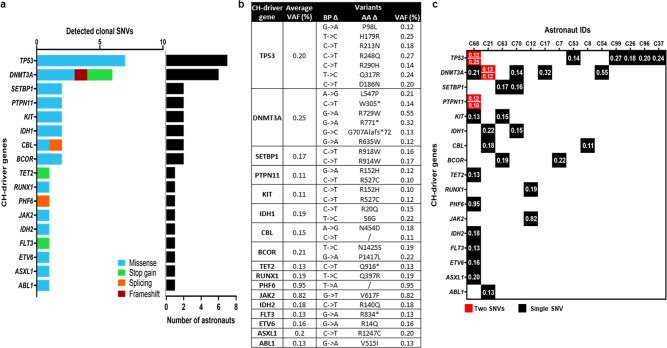
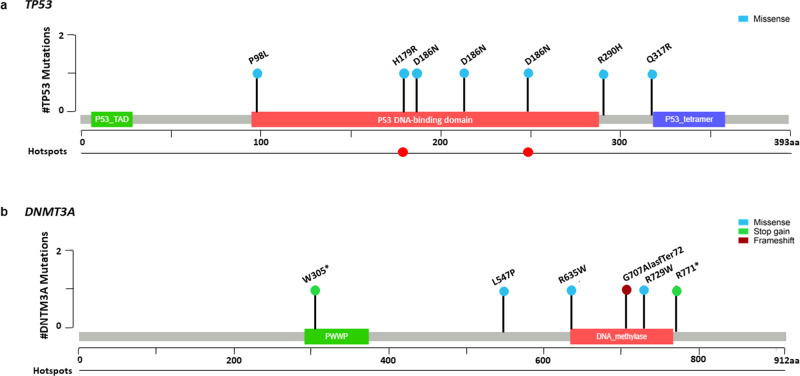
We detected variants in 17 of the 37 genes represented in the panel. The most commonly mutated gene was TP53 (7 variants) followed by DNTM3A (6 variants) accounting for 38% of mutations detected (Fig. 2a). One subject, C12, harbored the JAK2 V617F mutation (VAF 0.82%) (Fig. 2b, c), a clinically relevant variant identified in myeloproliferative neoplasms (MPNs) that has also been associated with ~12-fold increased risk of CVD and thrombosis risk5,14–17. Subject C68 had a single PHF6 SNV with a VAF of 0.95% (Fig. 2b, c), representing the largest variant clone detected by this analysis. While the pathogenicity of this mutation is unclear, PHF6 variants are frequently detected in patients with hematopoietic malignancies18. Multiple mutations in TP53 and PTPN11 were also identified in subject C68, and four other subjects harbored co-mutations in other CH-driver genes (Fig. 2c). All TP53 SNVs missense mutations occurred in the DNA binding domain. Two mutations were located in the TP53 mutation hotspots: Arg248Gln (VAF 0.27%) and His179Arg (VAF 0.25%) (Fig. 3a), which are implicated in various cancers19–21. Six out of 14 astronauts (43%) had at least 1 TP53 SNV (average VAF 0.20%), of which subject C68 had two missense mutations (Fig. 2c). In comparison, 5/14 (36%) of astronauts had at least one DNMT3A SNV, which were predominantly missense mutations in the methyltransferase domain, thus likely impacting enzyme function, or nonsense mutations (Arg771Ter, Trp305Ter) that had been identified in patients with aplastic anemia and angioimmunoblastic T Cell lymphoma22,23 (Fig. 3b). Subject C21 had two DNMT3A SNVs with 1 missense and 1 frameshift variant (Fig. 2c).
Fig. 2. Clonal Hematopoiesis (CH) Profile of Astronauts.
a Mutational profile of 17 known mutated CHIP-driver genes and number of astronauts with mutations in each gene. b Table with average VAF, noted nucleotide (BPΔ) and amino acid (AAΔ) changes for each nonsynonymous mutation. Asterix (*) refers to terminated sequencing for stop gain mutations while backslash (/) corresponds to splicing mutations where AAΔ is not applicable. c Mutation plot for each individual is represented as columns. Each rectangle represents number of mutations identified in each gene, with black being 1 mutation and red 2 mutations, along with individual VAF (%) for each variant.
Fig. 3. Profile of nonsynonymous mutations for TP53 and DNMT3A.
Mapping of nonsynonymous mutations in (a) TP53 and (b) DNMT3A through Loliplot visualization. Clinically relevant hotspots are identified (red dots). Asterix (*) in mutations corresponds to sequence termination in stop gain mutations. P53_TAD: p53 transactivation domain; PWWP: Pro-Trp-Trp_Pro domain.
Discussion
The advancement of error-corrected DNA sequencing technology permits the assessment of somatic mutations (at lower VAF) in CH-driver genes associated with increased risk of hematologic and non-hematologic cancers as well as cardiovascular diseases24. This study used PBMCs collected ~20 years ago from 14 relatively young astronauts who flew space Shuttle missions between 1998–2001 to identify, retrospectively, somatic mutations in CH-driver genes.
We identified 34 nonsynonymous SNVs in 17 known CH-driver genes, of which TP53 and DNMT3A were the most frequent. Notably, clone size was small, ranging from 0.10% to 0.95% VAF, and thus did not achieve the technical threshold to be considered as CHIP. In contrast, a population-based CH study of middle-aged participants (median age 58 years, range 19 to 108) showed that the most frequent variants occur in epigenetic modifier genes DNMT3A, followed by TET2, ASXL1, and TP534. However, the DDR gene TP53 was the most frequently mutated in this astronaut cohort (median age 44 years, range 37–67), reflecting a potential difference compared to the civilian population4,5. Somatic TP53 mutations are uncommon in patients without a history of cancer therapy25. TP53 encodes tumor suppressor p53 that is induced by various stress stimuli, including UV IR, DNA double-stranded breaks, and oxidative stress. TP53 coordinates the transcription of genes involved in DNA damage repair, apoptosis, growth arrest, or senescence, and the dysregulation of p53 poses a risk for developing cancers, CVD, and other metabolic disorders26,27. While the clones identified in this astronaut cohort are relatively small, there is a possibility for increased clonal instability with aging that may be facilitated by co-mutations in other driver genes. Indeed, 5/14 astronauts harbored mutations in at least two CH-driver genes, and subject C68 had variants in the four mutated genes. While we cannot assess the pathogenic risk and the clinical value for each small clone mutation, it provides a rationale for longitudinal studies to determine which clones are at high risk of transformation and then to develop strategies to inhibit their expansion at early stages.
Due to the lack of longitudinal samples and small sample size, conclusions regarding the implications of observed lesions remain limited, and further studies are required to assess the penetrance of these clones. Van Egeren et al. showed single HSPCs acquired JAK2-V617F mutations decades before the development of MPNs, with clonal growth and/or extinction often fluctuating years after mutation acquisition28. While the initial occurrence of these CH lesions within our astronaut cohort cannot be ascertained, the spaceflight environment introduces considerable physiological stress, which could temporally alter clonal dynamics. Our team recently showed plasma cell-free mitochondrial DNA (cf-mtDNA) and 8-OHdG levels were elevated in these astronauts 3 days post-flight (R + 3) and found inflammatory (TNFα, IL-1α, IL-1β), oxidative stress (SOD1, GPX1, NOX4, SERPINE1, HMOX1), and DNA damage (OGG1, PARP-1) markers were significantly upregulated at R + 3 compared to baseline (10 days before flight) in isolated PBMCs29, suggesting spaceflight contributes to significant physiological stress, which may be an impetus for clonal selection and expansion. While we cannot ascertain which isolated space environment factor may pose considerable risks for CH development, considering the clonal landscape in the following astronaut cohort bares closer resemblance to t-CH, it is possible space IR may be a primary driver of clonal selection. Space IR comprises of high charge and energy (HZE) ions with a high linear energy transfer (LET) and provides different energy deposition patterns in cells creating densely ionizing tracks that generate clustered DNA damage compared to low LET terrestrial IR. Prior studies examining the risk of cataracts following space IR exposure during Shuttle missions noted several thousand high-LET particles (LET > 30 keV/µm) pass through the lens with an average scaled mission lens dose of 73.5 mSv (0.07 Gy) for missions of 10 to 14-day duration30,31. Additionally, astronauts within the studied cohort embarked on EVAs during their Shuttle mission which also modifies degree of stressor exposure. While most EVAs are scheduled around solar activity, most galactic cosmic rays (GCRs) are unpredictable, and spacesuit radiation shielding alone may not be enough to mitigate radiation exposure and its associated risks32. Considering cancer patients receiving external beam radiation therapy exhibit pronounced CH mutations in DDR genes (TP53, CHEK2, PPM1D) along with faster clone growth following cumulative exposure33, it can be postulated that exposure to space IR may alter the fitness landscape of observed CH mutations and warrants further investigation. Additionally, the effect of sex on CH dynamics should be considered as approximately 85% of crew members in Shuttle Missions from 1998–2001 were male. Prior studies have shown the male sex acquired more somatic mutations with higher allele burdens in known CH-drivers such as JAK2, ASXL1, SRSF2, U2AF1, and IDH1/2 and were associated with overall poorer clinical outcomes34,35. Thus, more comprehensive studies are required to ascertain whether sex modifies space environment-induced CH risk using a more diverse cohort.
Overall, further longitudinal studies are required to characterize CH and somatic mutational profiles in the context of space flight-associated stressors and their associated clinical impact. To date, there is no evidence of relevant CVD, cancer, or neurodegenerative diagnoses associated with this given astronaut cohort (current median age 62.5 years (IQR 60–67)). The lack of longitudinal samples from these same astronauts limits the assessment of clone stability, pathogenic potential, and prognostic value. Studies of CH in astronauts would provide a new tool for assessing individual risk and potentially optimize long-term health monitoring. Additional analyses would also offer an opportunity to address the paucity of longitudinal CH studies using a unique cohort of physically fit individuals and provide more insights into factors that influence CH in the general population. Thus, this study serves to address the feasibility of using bio-banked astronaut samples and demonstrate the importance of collaborations between NASA’s Human Research Program, Translational Research Institute for Space Health, Space Biology Program, NASA’s clinical support teams and corresponding data and biorepository branches, and NASA IRB to facilitate retrospective and prospective longitudinal studies by increasing sample availability (while protecting health information (PHI) and maintaining HIPAA regulations). This integration of clinical data could optimize the value of CHIP studies for the assessment of lifetime risks before and after spaceflight.
Methods
Subjects and samples
We obtained de-identified whole blood samples from 14 astronauts who flew relatively short (median 12 day long) space Shuttle missions, between 1998–2001. Identifying information related to these samples is limited to the crew’s binned age in order to ensure privacy of involved personnel (Fig. 1a). De-identified aliquots of 14 peripheral blood mononuclear cell (PBMC) samples, isolated from blood samples 3 days after return (R + 3; during original study in 1998–2001; note, the buffy coats to obtain PBMCs were collected only at this time point), were prepared and shipped overnight to the University of Virginia School of Medicine for detection of somatic mutations involved in known CHIP-driver genes (Fig. 1a). Given that samples are de-identified and targeted DNA sequencing would not permit individual identification, NASA’s Institutional Review Board (IRB) deemed this study non-human research (STUDY00000075) and the Icahn School of Medicine at Mount Sinai’s IRB deemed this study as exempt (HSM19-00367). Blood samples were collected during a NASA flight study; astronauts provided written informed consent to participate in that study and have blood stored and used for residual analyses12.
Sample preparation and targeted DNA sequencing
Genomic DNA was isolated from PBMCs from each sample using the QIAamp DNA Blood Mini kit (Cat #51104). DNA quality was assessed using the PreSeq QC kit (Cat # SA0597 and SA0598) provided by ArcherDX (now Invitae) using real-time polymerase chain reaction (RT-PCR). ArcherDX VariantPlex core myeloid kit with 37 genes panel (Supplementary Table 1) was used to perform target specific PCR using Anchored Multiplex PCR (AMP™) chemistry to add specific Molecular Barcodes (MBC) and indexes. DNA libraries were prepared using Archer Library Preparation Reagents for Illumina (Cat #AB0101) by following the manufacturer’s protocol. Library sequencing was performed using Illumina NextSeq platform with high output kits as per manufacturer guidelines to generate 150 bp; paired end data. Sequencing was performed to 15000X average coverage. NGS data was analyzed using the Archer Analysis software (https://analysis.archerdx.com/) including the following steps. Raw paired end FASTQ files were subjected to read cleaning and adaptor trimming to remove adapter sequences and low-quality portions of reads at 5’ and 3’ ends. Then unique reads / read pairs were grouped together into “molecular bins”, according to molecular barcode and index sequences. Bins were subjected to deduplication and error correction to create a consensus read for each bin. A combination of bowtie2 and BWA-MEM was used to map reads against the reference human genome (hg19/GCRh37). Generated variants were annotated with Ensembl Variant Effect Predictor.
Variants (SNP/ InDel) generated with this method were compared with a normal dataset using Archer’s analysis pipeline to distinguish noise from a true call. The normal dataset was created with sequencing data from seven young, healthy individuals. Based on the frequency at which a variant is observed in the normal dataset, the noise distribution at each variant call was calculated. Then the probability was calculated for the observed variant AF that could have been generated by noise given that noise distribution. Low normal data set AF outlier p value indicated that a variant was unlikely to have been generated by noise.
A maximum VAF threshold of 0.40 was used to exclude potential germline mutation from variant calling. Below this threshold, variants were further filtered if (1) AF outlier p ≤ 0.01; (2) GenomeAD frequency ≤ 0.05; (3) no previous report of the variant identified in Catalog of Somatic Mutations in Cancer or ClinVar; (4) duplicate variant per sample. The final data set is available in Supplementary Data 1.
Statistics and reproducibility
Due to limited sample availability, there is no replicate for the sequencing. Strict criteria for sequencing data quality check were applied to each sample. All the samples passed the QC status that is determined by the Invitae (Archer) data analysis pipeline (https://analysis.archerdx.com/). Statistical evaluation for sequencing data from each sample is presented in Supplementary Data 2. As stated above, only true somatic variants were selected by applying the strict filtering criteria of AF outlier p ≤ 0.01, present in COSMIC and GenomeAD AF ≤ 0.05. Binomial distribution was used to model the noise at each position from the sequencing data of the normal data set to generate the AF outlier p-value. The background noise model was established using these steps: (i) Identified base changes with unusually high AF and exclude them using Inter Quantile Range (IQR) filtering. (ii) For remaining variants: (a) Background noise = Sum of all AOs (Alternate observations) (across all samples)/Sum of depth (across all samples) at a given position, on per base change basis. If AO = 0, background noise was assumed to be 1/Sum of the depth (across all samples). (b) The background noise calculated in (A) became the expected rate of AO observations per unique molecule at a given position. This rate was used as the “p” term in a binomial distribution. (c) For a given variant, analysis can calculate a p value which expresses the probability that the noise model for that variant would generate the observed AF or greater. This assumes a binomial distribution with p = background noise and n = depth of coverage for that variant, which computes the probability of the null hypothesis (p-value). The null hypothesis was that the number of alternate observations seen are due to the background error that was estimated. (d) Any variant that had a low p-value was considered a significant variant. (The recommended default p-value cutoff, or alpha, is 0.01, but this can be adjusted based on tolerance for false positives and false negatives).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Data
Acknowledgements
We would like to thank the Lifetime Surveillance of Astronaut Health (LSAH) at NASA Johnson Space Center for their assistance with mission information and manuscript review. This work is supported by the Translational Research Institute for Space Health through NASA Cooperative Agreement NNX16AO69A – FIP0005, National Aeronautics and Space Administration Human Research Program Space Biology Element grant 80NSSC19K1079 (to Dr. Goukassian), and National Aeronautics and Space Administration Human Research Program Space Biology Element grant 80NSSC21K0549 (to Drs. Goukassian and Walsh).
Author contributions
A.B., A.K., K.W., P.M., D.A.G. are responsible for conceptualization of this study. A.B., A.K., M.C.T., E.P. are responsible for study design, carried out experiments, and data analysis. A.B., A.K., M.C.T., E.P., K.W., D.A.G. carried out data interpretation. A.B., M.B., D.A.G. were responsible for data visualization. A.B., D.A.G. wrote the original draft. A.B., A.K., M.C.T., E.P., P.M., M.B., V.N.S.G., L.H., K.W., D.A.G. contributed to paper preparation. All authors have given approval to the final version of the paper.
Peer review
Peer review information
Communications Biology thanks Mrinal Patnaik, Juan Bautista Menendez-Gonzalez and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Kaoru Ito and Christina Karlsson-Rosenthal.
Data availability
All data derived from analysis of clinical sequencing data (CH mutations) for all astronauts necessary to replicate the findings in the article are available within the main text and supplementary materials. The raw sequencing data for the astronaut cohorts are protected and are not broadly available due to privacy laws. The LSDA provides an appropriate process for archival of experimental data and dissemination, which complies with policies to govern sensitive data in accordance with NASA Human Research Program and Johnson Space Center (JSC) Institutional Review Board direction. Raw data elements may be requested from david.goukassian@mssm.edu with appropriate institutional approvals.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Agnieszka Brojakowska, Anupreet Kour.
Change history
10/10/2022
A Correction to this paper has been published: 10.1038/s42003-022-04071-8
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-022-03777-z.
References
- 1.Cucinotta FA. Space radiation risks for astronauts on multiple International Space Station missions. PLoS One. 2014;9:e96099. doi: 10.1371/journal.pone.0096099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stepanek J, Blue RS, Parazynski S. Space Medicine in the Era of Civilian Spaceflight. N. Engl. J. Med. 2019;380:1053–1060. doi: 10.1056/NEJMra1609012. [DOI] [PubMed] [Google Scholar]
- 3.Steensma DP. Clinical Implications of Clonal Hematopoiesis. Mayo Clin. Proc. 2018;93:1122–1130. doi: 10.1016/j.mayocp.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Jaiswal S, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaiswal S, et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coombs CC, et al. Therapy-Related Clonal Hematopoiesis in Patients with Non-hematologic Cancers Is Common and Associated with Adverse Clinical Outcomes. Cell Stem Cell. 2017;21:374–382.e374. doi: 10.1016/j.stem.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa H, Horitani K, Izumiya Y, Sano S. Somatic Mosaicism in Biology and Disease. Annu Rev. Physiol. 2022;84:113–133. doi: 10.1146/annurev-physiol-061121-040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuster JJ, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sano S, et al. Tet2-Mediated Clonal Hematopoiesis Accelerates Heart Failure Through a Mechanism Involving the IL-1beta/NLRP3 Inflammasome. J. Am. Coll. Cardiol. 2018;71:875–886. doi: 10.1016/j.jacc.2017.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuster JJ, et al. TET2-Loss-of-Function-Driven Clonal Hematopoiesis Exacerbates Experimental Insulin Resistance in Aging and Obesity. Cell Rep. 2020;33:108326. doi: 10.1016/j.celrep.2020.108326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mencia-Trinchant N, et al. Clonal Hematopoiesis Before, During, and After Human Spaceflight. Cell Rep. 2020;33:108458. doi: 10.1016/j.celrep.2020.108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mills PJ, Perez CJ, Adler KA, Ziegler MG. The effects of spaceflight on adrenergic receptors and agonists and cell adhesion molecule expression. J. Neuroimmunol. 2002;132:173–179. doi: 10.1016/S0165-5728(02)00313-2. [DOI] [PubMed] [Google Scholar]
- 13.Alexandrov LB, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, et al. Macrophage Inflammation, Erythrophagocytosis, and Accelerated Atherosclerosis in Jak2 (V617F) Mice. Circ. Res. 2018;123:e35–e47. doi: 10.1161/CIRCRESAHA.118.313283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muendlein A, et al. Occurrence of the JAK2 V617F mutation in patients with peripheral arterial disease. Am. J. Hematol. 2015;90:E17–E21. doi: 10.1002/ajh.23874. [DOI] [PubMed] [Google Scholar]
- 16.Cordua S, et al. Prevalence and phenotypes of JAK2 V617F and calreticulin mutations in a Danish general population. Blood. 2019;134:469–479. doi: 10.1182/blood.2019001113. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen C, Birgens HS, Nordestgaard BG, Kjaer L, Bojesen SE. The JAK2 V617F somatic mutation, mortality and cancer risk in the general population. Haematologica. 2011;96:450–453. doi: 10.3324/haematol.2010.033191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurzer JH, Weinberg OK. PHF6 Mutations in Hematologic Malignancies. Front Oncol. 2021;11:704471. doi: 10.3389/fonc.2021.704471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2010;2:a001008. doi: 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabapathy K, Lane DP. Therapeutic targeting of p53: all mutants are equal, but some mutants are more equal than others. Nat. Rev. Clin. Oncol. 2018;15:13–30. doi: 10.1038/nrclinonc.2017.151. [DOI] [PubMed] [Google Scholar]
- 21.Chang MT, et al. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nat. Biotechnol. 2016;34:155–163. doi: 10.1038/nbt.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulasekararaj AG, et al. Somatic mutations identify a subgroup of aplastic anemia patients who progress to myelodysplastic syndrome. Blood. 2014;124:2698–2704. doi: 10.1182/blood-2014-05-574889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M, et al. Angioimmunoblastic T cell lymphoma: novel molecular insights by mutation profiling. Oncotarget. 2017;8:17763–17770. doi: 10.18632/oncotarget.14846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans MA, Sano S, Walsh K. Cardiovascular Disease, Aging, and Clonal Hematopoiesis. Annu Rev. Pathol. 2020;15:419–438. doi: 10.1146/annurev-pathmechdis-012419-032544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makishima H, et al. Dynamics of clonal evolution in myelodysplastic syndromes. Nat. Genet. 2017;49:204–212. doi: 10.1038/ng.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Tavana O, Gu W. p53 modifications: exquisite decorations of the powerful guardian. J. Mol. Cell Biol. 2019;11:564–577. doi: 10.1093/jmcb/mjz060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sano, S. et al. TP53-mediated therapy-related clonal hematopoiesis contributes to doxorubicin-induced cardiomyopathy by augmenting a neutrophil-mediated cytotoxic response. JCI Insight6, (2021). [DOI] [PMC free article] [PubMed]
- 28.Van Egeren D, et al. Reconstructing the Lineage Histories and Differentiation Trajectories of Individual Cancer Cells in Myeloproliferative Neoplasms. Cell Stem Cell. 2021;28:514–523.e519. doi: 10.1016/j.stem.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bisserier M, et al. Cell-Free Mitochondrial DNA as a Potential Biomarker for Astronauts’ Health. J. Am. Heart Assoc. 2021;10:e022055. doi: 10.1161/JAHA.121.022055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cucinotta FA, et al. Space radiation and cataracts in astronauts. Radiat. Res. 2001;156:460–466. doi: 10.1667/0033-7587(2001)156[0460:SRACIA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 31.Chylack LT, Jr, et al. NASA study of cataract in astronauts (NASCA). Report 1: Cross-sectional study of the relationship of exposure to space radiation and risk of lens opacity. Radiat. Res. 2009;172:10–20. doi: 10.1667/RR1580.1. [DOI] [PubMed] [Google Scholar]
- 32.Belobrajdic B, Melone K, Diaz-Artiles A. Planetary extravehicular activity (EVA) risk mitigation strategies for long-duration space missions. NPJ Microgravity. 2021;7:16. doi: 10.1038/s41526-021-00144-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolton KL, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat. Genet. 2020;52:1219–1226. doi: 10.1038/s41588-020-00710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moliterno AR, Braunstein EM. The roles of sex and genetics in the MPN. Int Rev. Cell Mol. Biol. 2022;366:1–24. doi: 10.1016/bs.ircmb.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Karantanos T, et al. Sex determines the presentation and outcomes in MPN and is related to sex-specific differences in the mutational burden. Blood Adv. 2020;4:2567–2576. doi: 10.1182/bloodadvances.2019001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Data
Data Availability Statement
All data derived from analysis of clinical sequencing data (CH mutations) for all astronauts necessary to replicate the findings in the article are available within the main text and supplementary materials. The raw sequencing data for the astronaut cohorts are protected and are not broadly available due to privacy laws. The LSDA provides an appropriate process for archival of experimental data and dissemination, which complies with policies to govern sensitive data in accordance with NASA Human Research Program and Johnson Space Center (JSC) Institutional Review Board direction. Raw data elements may be requested from david.goukassian@mssm.edu with appropriate institutional approvals.