Abstract
Objectives
When diagnosing Coronavirus disease 2019(COVID‐19), radiologists cannot make an accurate judgments because the image characteristics of COVID‐19 and other pneumonia are similar. As machine learning advances, artificial intelligence(AI) models show promise in diagnosing COVID-19 and other pneumonias. We performed a systematic review and meta-analysis to assess the diagnostic accuracy and methodological quality of the models.
Methods
We searched PubMed, Cochrane Library, Web of Science, and Embase, preprints from medRxiv and bioRxiv to locate studies published before December 2021, with no language restrictions. And a quality assessment (QUADAS-2), Radiomics Quality Score (RQS) tools and CLAIM checklist were used to assess the quality of each study. We used random-effects models to calculate pooled sensitivity and specificity, I2 values to assess heterogeneity, and Deeks' test to assess publication bias.
Results
We screened 32 studies from the 2001 retrieved articles for inclusion in the meta-analysis. We included 6737 participants in the test or validation group. The meta-analysis revealed that AI models based on chest imaging distinguishes COVID-19 from other pneumonias: pooled area under the curve (AUC) 0.96 (95 % CI, 0.94–0.98), sensitivity 0.92 (95 % CI, 0.88–0.94), pooled specificity 0.91 (95 % CI, 0.87–0.93). The average RQS score of 13 studies using radiomics was 7.8, accounting for 22 % of the total score. The 19 studies using deep learning methods had an average CLAIM score of 20, slightly less than half (48.24 %) the ideal score of 42.00.
Conclusions
The AI model for chest imaging could well diagnose COVID-19 and other pneumonias. However, it has not been implemented as a clinical decision-making tool. Future researchers should pay more attention to the quality of research methodology and further improve the generalizability of the developed predictive models.
Abbreviations: AI, artificial intelligence; AUC, area under the curve; COVID-19, Coronavirus disease 2019; CT, Computed tomography; CXR, Chest X-Ray; CNN, Convolutional neural network; CRP, C-reactive protein; SARS, severe acute respiratory syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; GGO, ground-glass opacities; KNN, K-nearest neighbor; LASSO, least absolute shrinkage and selection operator; ML, machine learning; MEERS-COV, Middle East respiratory syndrome coronavirus; PLR, positive likelihood ratio; PLR, negative likelihood ratio; RT-PCR, Reverse transcriptase polymerase chain reaction; ROI, regions of interest; SROC, summary receiver operating characteristic; SVM, Support vector machine; 2D, two-dimensional; 3D, three-dimensional
Keywords: COVID-19, Pneumonia, Artificial Intelligence, Diagnostic Imaging, Machine learning
1. Introduction
Beginning in 2020, the coronavirus disease 2019 (COVID-19) has spread widely around the world. As of July 15, 2022, there have been more than 557,917,904 confirmed cases of COVID-19 and 6,358,899 deaths worldwide [1]. Based on the estimated viral reproduction number (R0), the average number of infected individuals who transmit the virus to others in a completely non-immune population is about 3.77 [2], indicating that the disease is highly contagious. Therefore, It is crucial to identify infected individuals as early as possible for quarantine and treatment procedures.
The diagnosis of COVID-19 relies on the following criteria: clinical symptoms, epidemiological history, chest imaging, and laboratory tests [3], [4]. The most common clinical symptoms were: fever, cough, dyspnea, malaise, fatigue, phlegm/discharge, among others [5]. However, these symptoms are nonspecific, and non-COVID-19 pneumonia will have similar symptoms [6]. Reverse transcriptase polymerase chain reaction (RT-PCR) is the gold standard for diagnosing COVID-19, however, it has been reported that RT-PCR may not be sensitive enough for early detection of suspected patients, and in many cases the test must be repeated multiple times to confirm the results [7], [8], [9].
Another major diagnostic tool for COVID-19 is chest imaging. Chest CT of COVID-19 is characterized by ground-glass opacities (GGO) (including crazy‐paving) and consolidation [10], [11], [12]. While typical CT images may be useful for early screening of suspected cases, images of various viral pneumonias are highly similar and overlap with image features of other lung infections [13]. For example, GGOs are common in other atypical pneumonia and viral pneumonia diseases such as influenza, severe acute respiratory syndrome (SARS), and Middle East respiratory syndrome (MERS) [14], making it difficult for radiologists to diagnose COVID-19. The results of a meta-analysis showed that chest CT can be used to rule out COVID-19 pneumonia, but cannot distinguish COVID-19 from other lung infections; The fact that both types of pneumonia can appear on chest CT as exudative lesions, GGOs, implies that CT cannot differentiate SARS-CoV-2 infection from other respiratory diseases [15].
Radiomics is an emerging field that can extract high-throughput imaging features from biomedical images and convert them into mineable data for quantitative analysis. The underlying assumption is that changes and heterogeneity of lesions at the microscopic scale (such as at the cellular or molecular level) can be reflected in the images [16], offering hope for distinguishing COVID-19 from other pneumonias. In the past 3 years, there have been many studies on the diagnosis of COVID-19 based on radiomics methods. However, there has not been any research systematically summarizing the current research on artificial intelligence(AI) models for distinguishing COVID-19 from other pneumonias on images, and the overall efficacy of this predictive model is still unknown.Additionally, because radiomics research is a multi-step, complicated process, it is crucial to evaluate the method's quality before applying it to clinical applications to assure dependable and repeatable models.
Our systematic review aimed to (1) provide an overview of radiomics studies identifying COVID-19 from other pneumonias and evaluate the efficacy of prediction models; (2) Assess methodological quality and risk of bias in radiomics workflows; (3) Determine which algorithms are most commonly used to distinguish COVID-19 from other pneumonias.
2. Materials and methods
We followed the STARD (Standards for the Reporting of Diagnostic Accuracy Studies) [17] and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [18]. The registration number CRD 42021272433.
2.1. Search strategy
We searched from the databases of Pubmed, Web of Science, Embase, and Cochrane Library, for studies conducted before November 30, 2021. We searched for preprints from medRxiv and bioRxiv, using the method of combining subject words and free words. The main subject words were"COVID-19", "Artificial Intelligence", and "Diagnostic Imaging". We aimed to identify all relevant studies, regardless of language or publication status, with no language restrictions. We also filtered through the search to identify relevant systematic reviews for inclusion. Meetings, letters, short communication, opinion article were excluded. Details of the search are provided in the Table S1.
2.2. Eligibility criteria
Two doctors independently screened articles that were retrieved electronically. Articles that met all the following criteria were included: (1) the index test was studied with chest CT, chest X-ray, or lung ultrasound (2) only tests of metrics interpreted by algorithms, not human interpretations, were included. We included studies involving human interpretations if they provided data related to the diagnostic accuracy of algorithmic interpretations, (3) they had information that distinguished between COVID-19 and other pneumonia, including (community-acquired pneumonia, bacterial pneumonia, viral pneumonia, influenza, interstitial pneumonia, etc.). Exclusion criteria were as follows: (1) the included cases had normal, lung cancer, lung nodules, or other non-pneumonic cases, (2) only the training group model was included and there was no validation group or data regarding diagnostic accuracy, (3) the validation group accepted the index test and reference standard studies with less than 10 participants, (4) no exact number of cases of COVID-19 was provided or other pneumonias, and the data related to the diagnostic accuracy were calculated by the number of CT image layers.
2.3. Data extraction
We extracted the following items: date of the study, number of participants and demographic information about participants, type of common pneumonia, type of images used in the model, interest in the selection basis of the area, the diagnostic performance of the training group model, the diagnostic accuracy data of the verification model, whether there was external verification, detailed information regarding the AI algorithm, technical parameters of the index test, reference standard results, and detailed information.
Two reviewers independently assessed and extracted relevant information from each included study. For each study, we extracted 2 × 2 data (true positive (TP), true negative (TN), false positive (FP), false negative (FN)) for the validation group. If a study reported accuracy data for more than one model, we took the 2 × 2 contingency table for the model with the largest Youden index. If a study reported accuracy data for one or more radiologists and AI accuracy data, we extracted only the 2 × 2 contingency table corresponding to AI accuracy. If a study reported a combined model of clinical information and radiomics signature data and accuracy data for a separate radiomics data model, we only extracted the 2 × 2 contingency table corresponding to the radiomic model data. If both internal and external validation were reported in a study, we only extracted the 2 × 2 contingency table corresponding to the external validation accuracy data; if the training group, the validation group, and the test group were reported in a study, and we only extracted the test group accuracy data corresponding to the 2 × 2 contingency table. If a study reported accuracy data for more than one external validation, we extracted the 2 × 2 contingency table for the accuracy data for the validation group with the largest number of participants.
2.4. Quality assessment
The Radiomics Quality Score (RQS) [19], Checklist for Artificial Intelligence in Medical Imaging (CLAIM) checklist [20] and Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) [21] were used to assess the methodological quality and study-level risk of bias of the included studies, respectively. Studies based on machine learning(ML) methods were evaluated using the Radiomics Quality Score (RQS) (Table S2), while studies relying on deep learning(DL) methods were evaluated using the CLAIM checklist (Table S3). The Diagnostic Accuracy Study Quality Assessment 2 (QUADAS-2) standard consists of four parts: patient selection, index test, reference standard, and flow and timing (Table S4). Two graduate students independently assessed the quality and discussed disagreements with the evidence-based medicine teacher to reach a consensus.
2.5. Statistical analysis
We created a 2 × 2 table for each study based on data extracted directly from the article and calculated the accuracy of diagnostic tests [22] (sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio (PLR), and negative likelihood ratio (PLR) with 95 % CI of each study. We analyzed the data at the participant level, rather than the image level and lesion level, which is related to the treatment of pneumonia, and which is how most studies report data.
We performed meta-analyses using a bivariate random-effects model, taking into account any correlations that may exist between sensitivity and specificity [23]. We did not perform meta-analyses if only two or three studies (less than four) were assessed for a given study. analysis, because the number of studies was too small for a reliable assessment.We assessed sensitivity and specificity with 95 % confidence intervals(CI) by plotting forest plots, and we performed meta-analyses using midas in Stata14.
We explored heterogeneity between studies by visually examining the sensitivity and specificity of forest plots and summary receiver operating characteristic (SROC) plots. If sufficient data and information were available, we plan to perform subgroup analyses to explore study heterogeneity. We considered some sources of heterogeneity, including: comparisons between different imaging methods (CT vs. CRX), modeling methods (radiomics models vs. DL models), comparisons between different sample sizes, Comparisons between different regions of interest (Infection regions vs. Others) and different segmentation methods (2D vs. 3D). Also allow us to assess the impact of various factors on the model's diagnostic performance.
We assessed publication bias because we included more than ten studies in this systematic review. We initially assessed reporting bias using funnel plot visual asymmetry, plotting measures of effect size with measures of study precision. We then conducted a formal evaluation using Deeks' test and diagnostic odds ratio (DOR) as a measure of test accuracy [24].
3. Results
3.1. Literature search
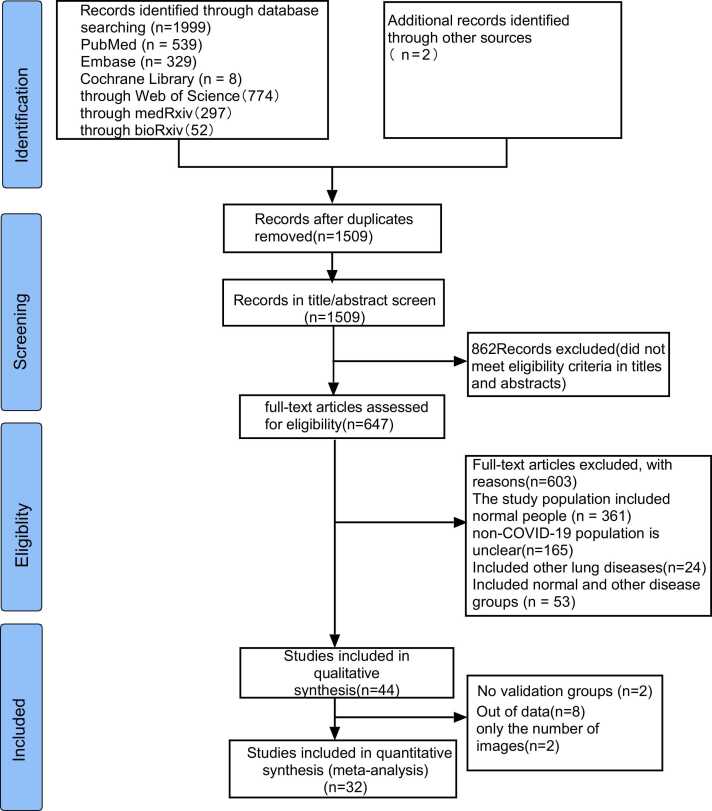
As of November 30, 2021, we have retrieved a total of 2001 articles, and after removing duplicates, there are 1509 articles left. Two reviewers independently browsed the titles and abstracts and removed 862 articles that did not match the research topic. After evaluating 647 AI-assisted imaging studies conducted to diagnose, classify, and detect the full text of COVID-19, we excluded 603 articles (including 165 non-new coronary pneumonia types that did not specifically account for the participants; 279 articles including COVID-19, common pneumonia, and healthy individuals; 8 articles including COVID-19, pneumonia, and other non-inflammatory lung diseases for participants; 53 articles including participants with COVID-19, pneumonia, healthy people, and other lung diseases; participants with COVID-19, 16 articles of other lung diseases; participants with COVID-19, 82 articles including healthy individuals); in the final 44 articles, we ultimately included 32 articles, because 8 articles lacked sufficient data to construct a 2 × 2 table; 2 articles lacked a validation group; 2 studies were conducted at the image level. The selection process is shown in Fig. 1.
Fig. 1.
Flow diagram of the study selection process for this meta-analysis.
We included 32 studies with a total of 6737 participants, of whom 4076 (60.5 %) were diagnosed with COVID-19, other pneumonias, including viral pneumonia (MEERS-COV, adenovirus, respiratory syncytial virus, influenza virus), bacterial pneumonia, fungal pneumonia, pneumonia caused by atypical pathogens (mycoplasma, chlamydia and legionella), pulmonary mycosis, interstitial pneumonia, and community-acquired pneumonia. The number of participants ranged from 105 to 5372. The mean age of the participants ranged from 40.92 ± 20.41 years to 61.45 ± 15.04 years. The percentage of male participants with COVID-19 ranged from 40.7 % to 62.0 %, and the percentage of male participants in other pneumonias ranged from 36.8 % to 64.0 %. The characteristics of the included studies are summarized in Table 1 and Table 2.
Table 1.
Summary of general study characteristics.
| Training validation/ Testing | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID |
Country of corresponding author | Study type | Index test | Date source | Eligibility criteria | Reference standard | Common type of pneumonia | Number of COVID-19 vs. other pneumonias | AUC | Type of validation | Number of COVID-19 vs. other pneumonias | SEN | SPC |
| Ardakani 2020 | Iran | R | CT | Single hospital | Yes | RT-PCR | Atypical, viral pneumonia | 86 vs. 69 | 0.999 | Random split | 22 vs.17 | 1.00 | 0.99 |
| Ardakani 2021 | Iran | R | CT | Single hospital | Yes | RT-PCR | Atypical and viral pneumonia | 244 vs.244 | 0.988 | Random split | 62 vs.62 | 0.935 | 0.903 |
| Ali 2021 | turkey | R | CXR | Single database | No | NA | Viral pneumonia |
146 vs.901 | NR | 3fold CV | 73 vs.444 | 0.973 | NR |
| Han2021 | Korea | R | CT | 2datasets | No | NA | Viral pneumonia, bacterial pneumonia, fungal pneumonia |
164 vs.320 | NR | External validation | 21 vs.40 | 0.997 | 0.959 |
| Di2020 | China | R | CT | 5hospitals | No | RT-PCR | CAP | 1933 vs. 1064 | NR | 10 fold CV | 215 vs.118 | 0.932 | 0.840 |
| Bai 2020 | China | R | CT | 10hospitals | Yes | RT-PCR | Pneumonia of other origin |
377 vs.453 | NR | Random split | 42 vs.77 | 0.950 | 0.960 |
| Panwar 2020 | Mexico | R | CXR | 3datasets | No | NA | Pneumonia | 133 vs.231 | NR | Random split | 29 vs.85 | 0.966 | 0.953 |
| Kang 2020 | China | R | CT | 3hospitals | No | RT-PCR | CAP | 1046 vs. 719 | NR | Random split | 449 vs.308 | 0.966 | 0.932 |
| Liu 2021 | China | R | CT | 2hospitals | Yes | RT-PCR | Viral infections, mycoplasma infections, chlamydia infections, fungus infections, co-infections |
66 vs.313 | 1.000 | External validation | 20 vs.20 | 0.850 | 0.900 |
| Chen 2021 | China | R | CT | Single hospital | Yes | RT-PCR | Other types of pneumonia | 54 vs.60 | 0.984 | Random split | 9 vs.11 | 0.816 | 0.923 |
| Song 2020 | China | R | CT | 2hospitals | Yes | RT-PCR | CAP | 66 vs.66 | 0.979 | External validation | 15 vs.20 | 0.800 | 0.750 |
| Sun 2020 | China | R | CT | 6hospitals | No | RT-PCR | CAP | 1196 vs. 822 | NR | 5fold CV | 299 vs.205 | 0.931 | 0.899 |
| Wang 2021 | China | R | CT | 3hospitals | Yes | RT-PCR | Other types of viral pneumonia |
74 vs.73 | 0.970 | External validation | 17 vs.17 | 0.722 | 0.751 |
| Zhou 2021 | China | R | CT | 12hospitals | Yes | RT-PCR | Influenza pneumonia | 118 vs.157 | NR | External validation | 57 vs.50 | 0.860 | 0.772 |
| Azouji2021 | Switzerland | R | CXR | 7datasets | No | NA | MERS, SARS | 338 vs.222 | NR | 5fold CV | 85 vs.56 | 0.989 | NR |
| Cardobi 2021 | Italy | R | CT | Single hospital | No | swab test | Interstitial pneumonias | 54 vs.30 | 0.830 | Random split | 14 vs.17 | 0.570 | 0.930 |
| Yang 2021 | China | R | CT | Single hospital | No | RT-PCR | Other pneumonias | 70 vs.70 | NR | 10fold CV | 20 vs.20 | 0.942 | 0.854 |
| Chikontwe 2021 | Korea | R | CT | Single hospital | No | RT-PCR | Bacterial pneumonia | 38 vs.49 | NR | Random split | 30 vs.39 | 1.000 | 0.975 |
| Zhu 2021 | China | R | CT | 6hospitals | No | RT-PCR | CAP | 1345 vs. 924 | NR | 10fold CV | 150 vs.103 | 0.913 | 0.910 |
| Xie 2020 | China | R | CT | 5hospitals | Yes | RT-PCR | Bacterial infection, Viral infection |
227 vs.153 | NR | prospective RWD | 243 vs.73 | 0.810 | 0.820 |
| Qi 2021 | China | R | CT | 3hospitals+dataset | Yes | RT-PCR | CAP | 127 vs.90 | NR | 10fold CV | 14 vs.10 | 0.972 | 0.940 |
| Wang 2020 | China | R | CT | 7hospitals | Yes | RT-PCR | Bacterial pneumonia, Mycoplasma pneumonia, Viral pneumonia, Fungal pneumonia | 560 vs.149 | 0.900 | External validation | 102 vs.124 | 0.804 | 0.766 |
| Yang 2020 | China | R | CT | 8hospitals | No | RT-PCR | CAP | 960 vs.628 | 0.976 | External validation | 1605 vs. 452 | 0.869 | 0.901 |
| Wu 2020 | China | R | CT | 3hospitals | No | RT-PCR | Other pneumonia | 294 vs.101 | 0.767 | Random split | 37 vs.13 | 0.811 | 0.615 |
| Zhang 2021 | China | R | CT | 3hospitals | No | RT-PCR | CAP, influenza, mycoplasma pneumonia | 72 vs.127 | 0.987 | 5fold CV | 31 vs.62 | 0.879 | 0.887 |
| Xin 2021 | China | R | CT | 2hospitals | Yes | swab tests | CAP | 34 vs.48 | NR | 5fold CV | 9 vs.12 | 0.957 | 0.984 |
| Guo 2020 | China | R | CT | 2hospitals | No | RT-PCR | Seasonal flu,CAP | 8 vs.42 | 0.970 | External validation | 11 vs.44 | 0.889 | 0.935 |
| Fang2020 | China | R | CT | 2hospitals | Yes | nucleic acid detection | Viral pneumonia | 136 vs.103 | 0.959 | Random split | 56 vs.34 | 0.929 | 0.971 |
| Xia 2021 | China | R | CXR | 2hospitals | Yes | nucleic acid | Influenza A/B pneumonia |
246 vs.44 | NR | Random split | 266 vs.62 | 0.869 | 0.742 |
| Huang2020 | China | R | CT | 15hospitals | Yes | RT-PCR | Viral pneumonia | 62 vs.64 | 0.849 | 5fold CV | 27 vs.28 | 0.778 | 0.786 |
| Wu 2021 | China | R | CT | Single hospital | Yes | nucleic acid | Other infectious pneumonia |
76 vs.77 | NR | 5fold CV | 19 vs.19 | 0.809 | 0.842 |
| Chen2021 | China | R | CT | 2hospitals | No | RT-PCR | Viral pneumonia | 81 vs.81 | 0.807 | Random split | 27 vs.19 | 0.733 | 0.822 |
Abbreviations: AUC: area under the curve; CAP: community acquired pneumonia;CT: Computed tomography; CV: cross-validation;CXR: Chest X-Ray; R: retrospective; RT-PCR: Reverse transcriptase polymerase chain reaction; RWD: Real-world dataset; SEN: sensitivity;SPC: specificity
Table 2.
Summary of artificial intelligence-based prediction model characteristics described in included studies.
| Study ID |
ROI | Segmentation Style |
AI Method | Labeling Procedure |
Pre-Processing | Augmentations | Model Structure |
Loss Function |
Comparison between algorithms | AI vs. Radiologist |
|---|---|---|---|---|---|---|---|---|---|---|
| Ardakani 2020 | Regions of infections | 2D | DL | by a radiologist with more than 15 years of experience in thoracic imaging | Manual ROI extraction by cropping, Normalization, transfer-learning |
NA | Ten well-known CNN |
NA | Ten well-known CNN |
Yes |
| Ardakani 2021 | CT chest | 2D | ML | By two radiologists | feature extraction | random scaling shearing horizontal flip |
ensemble method | NA | DT, KNN, Naïve Bayes, SVM | Yes |
| Ali 2021 | Whole image | 2D | DL | NA | Normalization, transfer-learning | Horizontal, vertical flip, Zoom, Shift |
ResNet50, ResNet101, Res Net 152 | NA | ResNet50, ResNet101, Res Net 152 | No |
| Han2021 | CT slices | 2D | DL | using the labeled COVID-19 dataset | both labeled and unlabeled data can be used | random scaling random translation, random shearing, horizontal flip |
a semi-supervised deep neural network | standard cross entropy loss | Supervised learning | No |
| Di2020 | Infected lesions | 2D | ML | NA | extracted both regional and radiomics features, Segmentation | NA | UVHL | cross- entropy |
SVM, MLP, iHL, tHL | No |
| Bai 2020 | Lung regions |
2D | DL | Lesions (COVID-19 or pneumo- nia) were manually labeled by2 radiologists |
Normalized, Segmentation | flips, scaling, rotations, random brightness and contrast manipulations, random noise, and blurring |
DNN | NA | No | Yes |
| Panwar 2020 | Whole image | 2D | DL | NA | Filter, dimension reduction, deep transfer learning | Shear, Rotation Zoom, shift |
A DL and Grad-CAM | binary cross-entropy loss | No | No |
| Kang 2020 | Lesion region | 3D | ML | NA | Segmentation, Feature Extraction, Normalization |
NA | Structured Latent Multi-View Representation Learning |
Ross-entropy loss | LR,SVM,GNB, KNN, NN | No |
| Liu 2021 | Each pneumonia lesion | 3D | ML | By three experienced radiologists | Feature Extraction, Filters |
NA | LASSO regression | NA | No | Yes |
| Chen 2021 | Consolidation and ground- glass opacity lesions |
3D | ML | By fifteen radiologists | Feature Extraction, wavelet filters, Laplacian of Gaussian filters, Feature selection |
NA | SVM | NA | No | No |
| Song 2020 | CT images | 2D | DL | NA | semantic feature extraction | NA | BigBiGAN | NA | SVM, KNN | Yes |
| Sun 2020 | Infected lung regions |
3D | DL | NA | Feature extraction |
NA | AFS-DF | NA | LR, SVM, RF, NN | No |
| Wang 2021 | Pneumonia lesions | 3D/2D | ML | By four radiologists | manual segmentation, Feature extraction |
NA | Linear, LASSO, RF, KNN | NA | Linear, LASSO, RF, KNN | Yes |
| Zhou 2021 | Lesion regions | 2D | DL | annotated by 2 radiologists | Segmentation | randomly flipped, cropped | Trinary scheme(DL) | Binary cross- entropy loss |
Plain scheme(DL) | Yes |
| Azouji2021 | X-ray images | 2D | DL | NA | Resizing x-ray images, Contrast limited adaptive histogram equalization, Deep feature extraction, Deep feature fusion | Rotation, translation | LMPL classifier | hinge loss function | NaiveBayes, KNN, SVM,DT, AdaBoostM2, TotalBoost,RF, SoftMax,VGG-Net | No |
| Cardobi 2021 | Lung area | 3D | ML | NA | Segmentation, features extraction | NA | LASSO model | NA | No | No |
| Yang 2021 | Pneumonia lesion | 3D | ML | artificially delineated | Segmentation, features extraction | spatially resampled | SVM | NA | Sigmoid-SVM, Poly-SVM, Linear-SVM, RBF-SVM | No |
| Chikontwe 2021 | CT slices | 3D | DL | NA | Segmentation | random transformations, flipping |
DA-CMIL | NA | DeCoVNet, MIL, DeepAttentionMIL, JointMIL | No |
| Zhu 2021 | CT images | 3D | DL | NA | Segmentation, features extraction |
NA | GACDN | Binary cross entropy | SVM,KNN,NN | No |
| Xie 2020 | CT slices | 3D | DL | NA | Segmentation, extract 2D local features and 3D global features |
random horizontal flip, random rotation, random scale, random translation, and random elastic transformation | DNN | NA | No | Yes |
| Qi 2021 | Lung field | 3D | DL | NA | segmentation of the lung field, Extraction of deep features, Feature representation | Image rotation, reflection, and translation | DR-MIL | NA | MResNet-50-MIL, MmedicalNet, MResNet-50-MIL-max-pooling, MResNet-50-MIL-Noisy-AND-pooling, MResNet-50-Voting, MResNet-50-Montages | Yes |
| Wang 2020 | Lung area | 3D | DL | NA | fully automatic DL model to segment, normalization, convolutional filter | NA | DL | NA | No | No |
| Yang 2020 | Infection regions |
3D | DL | NA | Class Re-Sampling Strategies, Attention Mechanism | scaling | Dual-Sampling Attention Network | binary cross entropyloss | RN34 + US, Attention RN34 + US Attention RN34 + SS Attention RN34 + DS |
No |
| Wu 2020 | CT slices | 3D | DL | NA | segmentation | NA | Multi-view deep learning fusion model |
NA | Single-view model | No |
| Zhang 2021 | Major lesions | 3D | DL | NA | Segmentation Feature extraction, Feature selection, |
scaling | DL-MLP | NA | DL-SVM,DL-LR, DL-XGBoost | Yes |
| Xin 2021 | Lungs, lobes, and detected opacities | 2D | DL | Confirmed by 3 experienced radiologists and human auditing | Segmentation Feature extraction |
NA | LR, MLP, SVM, XGboost |
NA | LR, MLP, SVM, XGboost |
No |
| Guo 2020 | NR | NA | ML | by two radiologists | Segmentation Feature extraction |
NA | RF | NA | No | No |
| Fang2020 | Primary lesion | 3D/2D | ML | by two chest radiologists | Segmentation feature extraction, feature reduction and selection |
NA | LASSO regression | NA | No | No |
| Xia 2021 | Lung areas | 2D | DL | NA | Segmentation feature extraction |
random rotation, scale, transmit |
DNN | Categorical cross- entropy |
No | Yes (pulmonary physicians) |
| Huang2020 | Pneumonia lesion | 3D | ML | by two chest radiologists | Segmentation feature extraction, filter |
NA | Logistic model | NA | No | No |
| Wu 2021 | Maximal regions Involving inflammatory lesions | 2D | ML | by two radiologists | feature extraction, manually delineating | NA | RF | NA | No | No |
| Chen2021 | Lesion region |
2D | ML | by two radiologists | Segmentation feature extraction,feature dimensionality reduction |
NA | WSVM | NA | RF, SVM LASSO |
Yes |
Abbreviations:AFS-DF:adaptive feature selection guided deep forest;AI:artificial intelligence;BigBiGAN: bi-directional generative adversarial network; CT: Computed tomography; CXR: Chest X-Ray; CNN: Convolutional neural network;DA-CMIL: Dual Attention Contrastive multiple instance learning; DT: Decision tree; DNN: Deep Neural Networks; DR-MIL: deep represented multiple instance learning; DL: deep learning; RF: Random Forests; GNB: Gaussian-Naive-Bayes; Grad-CAM: Gradient Weighted Class Activation Mapping; GACDN: generative adversarial feature completion and diagnosis network; IHL:Inductive Hypergraph Learning; KNN: K-nearest neighbor; LR: Logistic-Regression; LASSO: least absolute shrinkage and selection operator; LMPL: large margin piecewise linear; ML: machine learning; MLA: Machine learning algorithms; MLP: Multilayer Perceptron; MERS: Middle East respiratory syndrome; NN: Neural-Networks; ROI: Region of interest; SVM: Support vector machine; THL: Transductive Hypergraph Learning; 2D: two-dimensional;3D: three-dimensional;UVHL: Uncertainty Vertex-weighted Hypergraph Learning; WSVM: weighted support vector machine
Most studies (20/32) included participants selected from two or more hospitals, 7 studies included participants from only one hospital, 4 studies used image data from public databases, and one study had participants from both hospitals and public database [25]. Most studies (28/32) used CT scans, and the remaining four studies used X-rays. Most of the studies (28/32) used RT-PCR as the diagnostic criteria for diagnosing SARS-CoV-2, and the diagnostic criteria of the remaining four studies were unknown [26], [27], [28], [29].
Sixteen studies performed automatic segmentation, 12 studies performed manual segmentation, and the remaining four studies input full-slice images. Fourteen studies performed two-dimensional(2D) segmentation, 15 studies performed three-dimensional(3D) segmentation, two studies performed both 2D segmentation and 3D segmentation [30], [31], and the remaining one study did not describe the segmentation method [32]. Fifteen studies used the infected lesions as regions of interest (ROI), 10 studies used the entire image level as the ROI input models, 6 studies used the entire lung region as ROI, and the remaining one study ROI was not described [32].
Pyradiomics (6/32) was the most often used software for extracting image characteristics, followed by MatLab (4/32), PyTorch (3/32) and Python (3/32).
With 13 studies using radiomics models and 19 employing DL models, feature selection and dimensionality reduction are essential to prevent overfitting when developing radiomics models since radiomics characteristics typically exceed the sample size [33]. Least Absolute Shrinkage and Selection Operator (LASSO) regression is the most used algorithm.
Twenty studies used two or more models, and 12 studies used a single model. The three most common models include convolutional neural network(CNN), support vector machine (SVM), K-nearest neighbor (KNN). Twenty studies only calculated the diagnostic performance of the AI model, 11 studies compared the AI model with the diagnostic performance of radiologists, and one study compared the AI model with the diagnostic level given by pulmonary physicians [34], The results of these 12 studies all showed that the diagnostic performance of the AI model in distinguishing other pneumonias of the SARS-CoV-2 was higher than that of radiologists or pulmonary physicians.
3.2. Risk of bias assessment
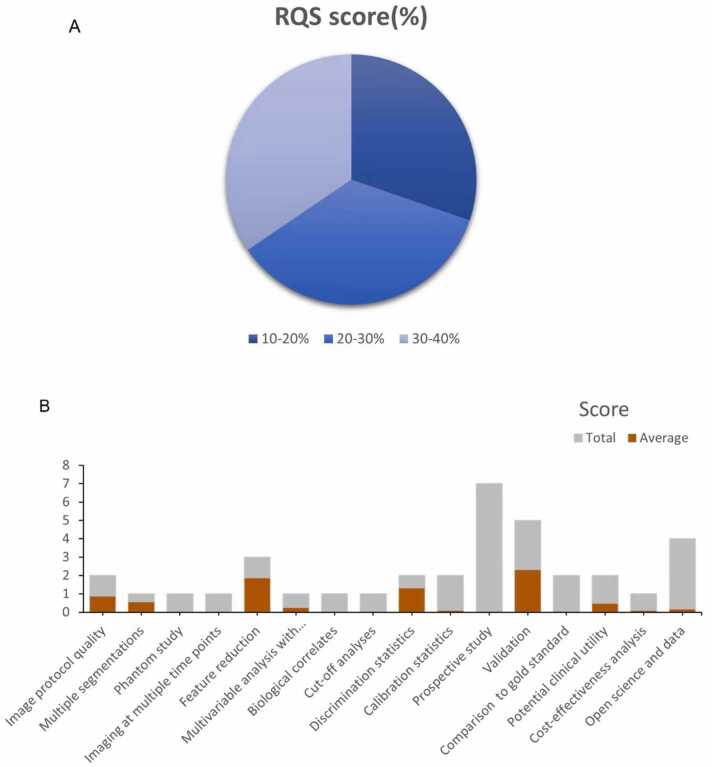
The mean RQS score of the included 13 studies was 7.8, accounting for 22 % of the total score. The highest RQS score was 13 (full score was 36), seen in only one study [35], and the lowest RQS score was 4 [32], [36]. Since no study considered the six items "Phantom study", "Imaging at multiple time points", "Biological correlates", "Cut-off analyses", "Prospective study" and "Comparison to 'goldstandard", these six items received a score of zero. Other underperforming items included "Multivariable analysis with nonradiomics features", "Calibration statistics" and "Potential clinical utility", "Cost-effectiveness analysis", "Open science and data", where each item had an average score below 15 % (Fig. 2). Table S5 provides a detailed description of the RQS scores.The average CLAIM score of the 19 included studies using the DL approach was 20, slightly less than half (48.24 %) of the ideal score of 42.00, the highest score was 29 [37] and the lowest was 14 [28] (Fig. 3, Table S6).
Fig. 2.
Methodological quality evaluated by using the Radiomics Quality Score (RQS) tool. (A). Proportion of studies with different RQS percentage score. (B). Average scores of each RQS item (gray bars stand for the full points of each item, and red bars show actual points).
Fig. 3.
CLAIM items of the 19 included studies expressed as percentage of the ideal score according to the six key domains. CLAIM, Checklist for Artificial Intelligence in Medical Imaging.
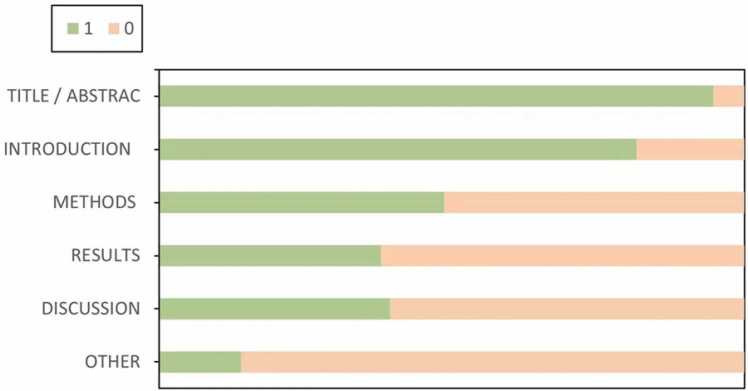
Risk of bias and applicability issues for 32 diagnostic-related studies according to QUADAS-2 are shown in Fig. S1. Overall, the methods of the 32 selected studies were of poor quality. Most studies showed unclear risk or high of bias in each domain (Table S7). Regarding patient selection, 22 studies were considered to be at high or unclear risk of bias due to unclear how participants were selected and/or unclear detailed exclusion criteria. With regard to the index test, 30 studies were considered to be at high or unclear risk of bias, because it was unclear whether a threshold was used or the threshold was not pre-specified. Regarding reference standards, 5 studies were considered to be at high or unclear risk of bias because reference standards were not described. Regarding the flow and timing, 30 studies were considered to be at high or unclear risk of bias, due to unclear time intervals between indicator tests and reference standards and/or to clarify whether all participants received the same reference standards.
3.3. Data analysis
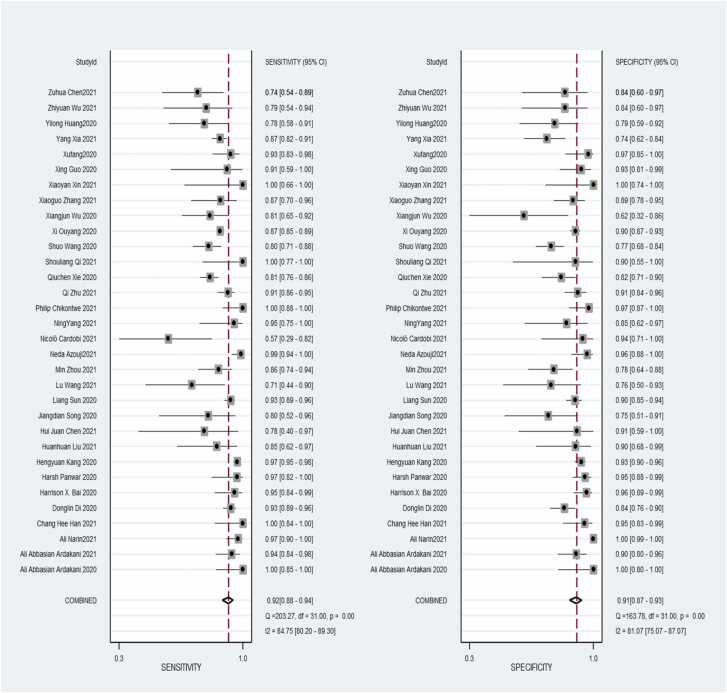
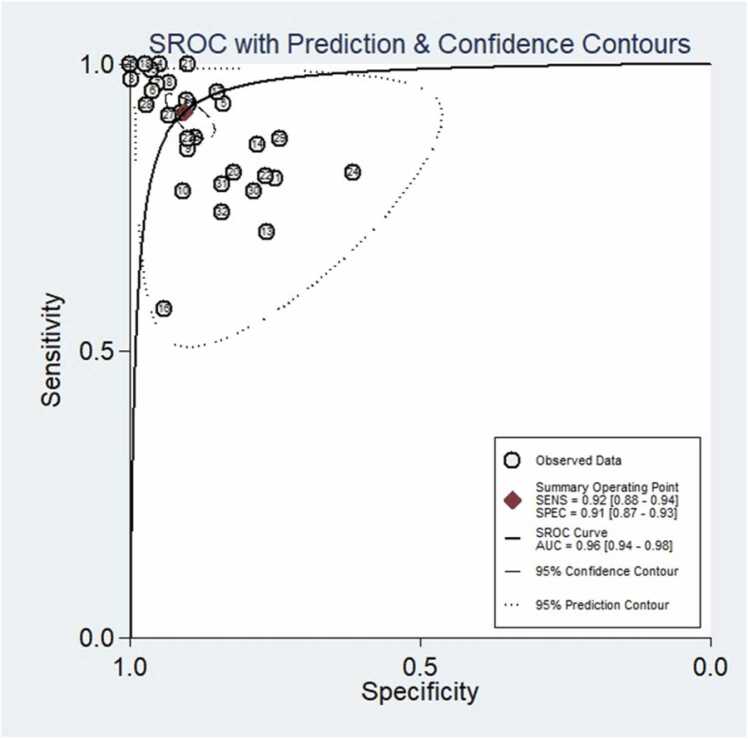
A total of 32 studies were included in the meta-analysis, and for the validation or test group of all studies, the pooled values and 95 % CI for sensitivity, specificity, PLR, NLR, and AUC were 0.92 (95 % CI, 0.88–0.94), 0.91, (95 % CI, 0.87–0.93), 9.7 (95 % CI, 6.8–13.9), 0.09 (95 % CI, 0.06–0.13), 0.96 (95 % CI, 0.94–0.98), respectively. When calculating pooled estimates, We observed great heterogeneity between studies in terms of sensitivity (I2 = 84.7 %), specificity (I2 = 81.1 %). The forest plot is shown in Fig. 4, and we can also see the obvious difference between the 95% confidence and 95 % prediction regions from the SROC curve in Fig. 5, indicating a high possibility of heterogeneity across the studies.
Fig. 4.
Coupled forest plots of pooled sensitivity and specificity of diagnostic performance of chest imaging for distinguished COVID-19 and other pneumonias. The numbers are pooled estimates with 95 % CIs in parentheses; horizontal lines indicate 95 % CIs.
Fig. 5.
Diagnostic performance of SROC curve of an artificial intelligence model for distinguishing COVID-19 from other pneumonias on chest imaging. There was an obvious difference between the 95 % confidence and 95 % prediction regions, indicating a high possibility of heterogeneity across the studies.
3.4. Subgroup analysis
We performed subgroup analyses including five different conditions and ten subgroups. Different imaging methods (CT, CRX), modeling methods (radiomics and deep learning), sample size (whether greater than 100), regions of interest (infection and others) and segmentation methods (2D and 3D) moderate to high diagnostic value was shown in each subgroup. The results are shown in Table 3.
Table 3.
The results of subgroup analysis.
| Subgroup | Number of study | Sensitivity (95 % CI) |
I2 (%) |
Specificity | I2 (%) |
PLR | I2 (%) |
NLR | I2 (%) |
AUC |
|---|---|---|---|---|---|---|---|---|---|---|
| Imaging modality | ||||||||||
| CRX | 4 | 0.91(0.88,0.94) | 85.6 | 0.96(0.95,0.98) | 95.3 | 26.04(3.73,181.94) | 93.3 | 0.04(0.00,0.41) | 92.6 | 0.9914 |
| CT | 28 | 0.89(0.88,0.90) | 78.9 | 0.89(0.87,0.90) | 62.1 | 6.92(5.35,8.96) | 69.5 | 0.14(0.11,0.19) | 80.0 | 0.9427 |
| Modeling methods | ||||||||||
| Radiomic algorithm | 13 | 0.92(0.90,0.94) | 78.4 | 0.90(0.87,0.92) | 36.8 | 7.16(4.96,10.33) | 53.0 | 0.15(0.08,0.28) | 85.6 | 0.9446 |
| Deep learning | 19 | 0.88(0.87,0.89) | 78.0 | 0.91(0.90,0.92) | 88.5 | 8.32(5.69,12.18) | 82.5 | 0.12(0.09,0.17) | 76.9 | 0.9702 |
| sample size | ||||||||||
| <100 | 18 | 0.87(0.83,0.90) | 65.4 | 0.89(0.86,0.92) | 47.8 | 6.50(4.42,9.58) | 49.3 | 0.18(0.12,0.28) | 59.0 | 0.9371 |
| >100 | 14 | 0.89(0.88,0.90) | 87.0 | 0.91(0.90,0.92) | 90.8 | 8.81(6.02,12.89) | 86.2 | 0.10(0.07,0.14) | 88.6 | 0.9725 |
| ROI | ||||||||||
| Infection regions | 15 | 0.89(0.88,0.90) | 81.0 | 0.89(0.88,0.91) | 48.8 | 6.89(5.20,9.12) | 58.0 | 0.14(0.09,0.20) | 81.3 | 0.9409 |
| others | 16 | 0.88(0.86,0.90) | 80.4 | 0.92(0.90,0.94) | 89.5 | 9.33(5.64,15.45) | 83.3 | 0.11(0.07,0.19) | 83.2 | 0.9691 |
| segmentation | ||||||||||
| 2D | 14 | 0.91(0.89,0.93) | 71.6 | 0.93(0.91,0.95) | 88.9 | 9.71(5.78,16.33) | 79.3 | 0.10(0.06,0.17) | 77.3 | 0.9740 |
| 3D | 15 | 0.88(0.87,0.90) | 85.1 | 0.89(0.87,0.90) | 64.8 | 6.77(4.79,9.57) | 76.6 | 0.15(0.10,0.22) | 85.9 | 0.9386 |
Abbreviations: AUC: area under the curve; CT: Computed tomography; CXR: Chest X-Ray; NLR: negative likelihood ratio; PLR:positive likelihood ratio; ROI: Region of interest;2D: two-dimensional;3D: three-dimensional
3.5. Publication bias
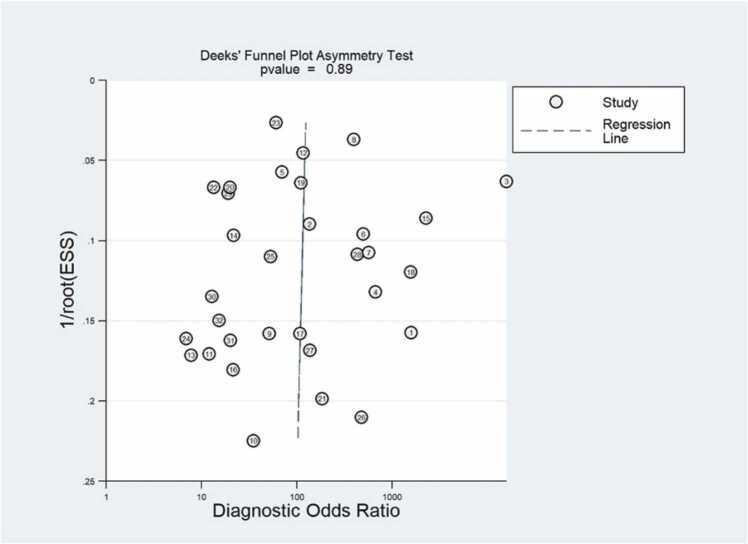
We assessed publication bias for the 3 included studies, first observing that the funnel plots (Fig. 6) were symmetric and uniformly distributed along the x and y axes. Second, we formally assessed using Deeks' test and observed that the slope coefficients were not statistically significant, (P = 0.89) indicating that the data were symmetric, suggesting a low possibility of publication bias.
Fig. 6.
Effective sample size (ESS) funnel plots and the associated regression test of asymmetry, as reported by Deeks et al. A p value < 0.10 was considered evidence of asymmetry and potential publication bias.
4. Discussion
In this systematic review, we aimed to determine the diagnostic accuracy of chest imaging-based AI models in distinguishing COVID-19 from other pneumonias, using the QUADAS-2, RQS tool, and the CLAIM checklist assess the quality of included studies. Furthermore, our meta-analysis is the first to quantitatively combine and interpret data from different independent surveys, potentially providing key clues for its clinical application and further research. Despite the favorable results, pooled sensitivity, specificity, and AUC were 0.92 (95 % CI, 0.88–0.94), 0.91 (95 % CI, 0.87–0.93), and 0.96 (95 % CI, 0.94–0.98), but due to the immature stage and relatively poor methodological quality, these imaging studies did not provide clear conclusions for clinical implementation and widespread use.
In this review, the combination of the complete RQS tool, CLAIM checklist, and QUADAS-2 assessments revealed several common methodological limitations, some of which apply to both DL and ML studies.
The majority of studies (13/32) did not have images segmented by multiple radiologists, however, due to inter-observer heterogeneity, unavoidable even among experienced radiologists [38], this also limits the generalizability of the developed predictive models. Some studies applied automatic segmentation, which overcomes the differences introduced by human factors. However, models created utilizing various segmentations would undoubtedly perform differently even when trained on the same dataset and using the same AI techniques, adding another level of heterogeneity to the field.
More than half of the studies did not describe algorithms and software in sufficient detail to replicate the study. Only six percent of the studies published the codes for the models, indicating that readers have access to the full protocol, i.e., code availability. Open data and code facilitate independent researchers using the same methodology and same/different datasets to validate results, with the aim of making research findings more robust. However, only two studies published small amounts of data [37], [39]. Therefore, it is hypothesized that some practical issues, such as reproducibility and generalizability of AI models, should be well resolved before translating these models into routine clinical applications.
We know that the typical imaging manifestations of SARS-CoV-2 are ground-glass opacities and consolidation foci, GGO is an indistinct increase in attenuation that occurs in various interstitial and alveolar processes while sparing bronchial [40] and vascular margins, while consolidation is an area of opacity obscuring the margins of the vessel and airway walls [41]. However, other types of pneumonia may share some similar CT imaging features with SARS-CoV-2, especially other viral pneumonias [42], [43], [44], This confuses radiologists when diagnosing SARS-CoV-2, unable to correctly diagnose whether it is SARS-CoV-2 or other pneumonia. A total of 11 studies in our systematic review also assessed the diagnostic performance of radiologists, and one study assessed the diagnostic performance of pulmonologists [34]. Then compared it with the diagnostic accuracy of AI models. all studies have shown that the diagnostic performance of AI models is higher than that of radiologists/pulmonologists. Shows that AI models have great potential in diagnosing SARS-CoV-2 and other pneumonias.
We performed a subgroup analysis using five key factors, and in the subgroup analysis of different imaging modalities, the diagnostic performance of the chest X-rays -based AI models were better than that of the CT-based models, but only four studies focusing on chest X-rays (including 453 COVID-19 patients out of 1100 subjects) were included, and all studies used deep learning models. Therefore, the pooled results showing that chest X-rays is superior to chest CT are not entirely convincing. Another subgroup analysis showed that studies using DL models were slightly more valuable than those using ML. The main disadvantage of ML algorithm is that the method is based on hand-crafted feature extractors, which requires a lot of manpower and effort [45]. Furthermore, radiomic signatures are contrived and rely on domain-specific expertize [46]. The advantage of DL is that it does not need to manually extract features during the learning process, avoiding the defects of artificially designed features in radiomics analysis [47]. Since the classifier training, feature selection, and classification of DL model occur simultaneously, researchers only need to input images, not clinical data, or radiomics features. The most commonly used DL model in research is CNN,which inspired by the biological natural visual cognition mechanism, build by convolutional layer, rectified linear units layer, pooling layer and fully-connected layer [48], [49]. For example, VGG and ResNet are adjusted and combined by simple CNN [50]. In addition, the results showed that studies with large sample sizes had better diagnostic accuracy than studies with small sample sizes. Therefore, in future studies, increasing the sample size will improve the ability to diagnose SARS-CoV-2 and various other pneumonias.
Limitations of this review. First, many articles published in authoritative journals using AI models to diagnose COVID-19 were not included because the models were not validated. Unvalidated models have limited value, and validation is an integral part of a complete radiomics analysis [19]. Models must be validated internally or externally. Second, the heterogeneity of studies was evident, we performed subgroup analyses to explore sources of heterogeneity, but this was limited, and in fact, heterogeneity is a recognized feature in a review of diagnostic test accuracy [23], and it is impossible to know the source of all the heterogeneity.
To date, no systematic review or meta-analysis has been performed that includes all types of imaging techniques to diagnose COVID-19 and other pneumonias. Kao et al.[51] evaluated the CT-based radiomics signature model to successfully distinguish COVID-19 from other viral pneumonias, and came to similar conclusions as ours, with high study heterogeneity. They assessed studies up to February 26, 2021, so only 6 studies were included, and all studies were conducted in China. However, there are several systematic reviews on the diagnosis of COVID-19 based on AI models [52], [53], [54], [55]. The participants in their studies included a series of non-pneumonic participants including lung cancer patients, lung nodules patients, and normal healthy people. These non-pneumonic chest images each have their own typical features. The imaging features are significantly different from those of COVID-19, and radiologists can easily distinguish them, so we did not include such articles in our study.
In conclusion, the artificial intelligence approach shows potential for diagnosing COVID-19 and other pneumonias. However, the immature stage and unsatisfactory quality of the research means that the proposed model cannot currently be used for clinical implementation. Before the AI models can be successfully introduced into the clinical environment of COVID-19, we need further large-sample multi-center research, open science and data, to increase the universality of the model. Furthermore, there are some technical hurdles that should be faced when considering the application of image mining tools into daily practice. Persistent efforts are required to make this tool widely available in clinical practice.
Ethical statement
This manuscript has not been published or presented elsewhere and is not under consideration for publication elsewhere. All the authors have approved the manuscript and agree with submission to your esteemed journal. There are no conflicts of interest to declare.
Funding
This study was supported by the Health Commission of Gansu Province, China [GSWSKY2020–15]. The funder has no role in the initial plan of the project, designing, implementing, data analysis, interpretation of data and in writing the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ejro.2022.100438.
Contributor Information
Lu-Lu Jia, Email: wenjiu20212021@163.com.
Jian-Xin Zhao, Email: zjx121415@163.com.
Ni-Ni Pan, Email: pnn18394130729@163.com.
Liu-Yan Shi, Email: sly461461@126.com.
Lian-Ping Zhao, Email: 370013496@qq.com.
Jin-Hui Tian, Email: tianjh@lzu.edu.cn.
Gang Huang, Email: keen0999@163.com.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.World Health Organization. https://covid19.who.int/
- 2.T.P. Velavan, C.G.J.Tm Meyer, i. health, The COVID‐19 epidemic, 25(3) (2020) 278. [DOI] [PMC free article] [PubMed]
- 3.Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19. N.R.M. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kevadiya B.D., Machhi J., Herskovitz J., Oleynikov M.D., Blomberg W.R., Bajwa N., Soni D., Das S., Hasan M., Patel M.J.Nm. Diagnostics for SARS-CoV-2 infections. Nat. Mater. 2021;20(5):593–605. doi: 10.1038/s41563-020-00906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Struyf T., Deeks J.J., Dinnes J., Takwoingi Y., Davenport C., Leeflang M.M., Spijker R., Hooft L., Emperador D., Domen J. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID‐19. Cochrane Database Syst. Rev. 2022;5 doi: 10.1002/14651858.CD013665.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.K. McIntosh , S.J.M. Perlman , Douglas , b.s . principles, p.o.i. diseases, Coronaviruses, including severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), (2015) 1928.
- 7.Winichakoon P., Chaiwarith R., Liwsrisakun C., Salee P., Goonna A., Limsukon A., Kaewpoowat Q. Negative nasopharyngeal and oropharyngeal swabs do not rule out COVID-19. J. Clin. Microbiol. 2020;58(5) doi: 10.1128/JCM.00297-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Z., Li Y., Wu B., Hou Y., Bao J., Deng X. A patient with COVID-19 presenting a false-negative reverse transcriptase polymerase chain reaction result. Korean J. Radiol. 2020;21(5):623–624. doi: 10.3348/kjr.2020.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323(22):2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 10.Pan F., Ye T., Sun P., Gui S., Liang B., Li L., Zheng D., Wang J., Hesketh R.L., Yang L.J.R. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295(3) doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D. Caruso, M. Zerunian, M. Polici, F. Pucciarelli, T. Polidori, C. Rucci, G. Guido, B. Bracci, C. De Dominicis, A.J.R. Laghi, Chest CT features of COVID-19 in Rome, Italy, (2020). [DOI] [PMC free article] [PubMed]
- 12.T. Ai, Z. Yang, H. Hou, C. Zhan, C. Chen, W. Lv, Q. Tao, Z. Sun, L.J.R. Xia, Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases, (2020). [DOI] [PMC free article] [PubMed]
- 13.Hui D.S., Azhar E.I., Memish Z.A., Zumla A. Human coronavirus infections—severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and SARS-CoV-2. Encycl. Respir. Med. 2022;2:146. [Google Scholar]
- 14.Hosseiny M., Kooraki S., Gholamrezanezhad A., Reddy S., Myers L. Radiology perspective of coronavirus disease 2019 (COVID-19): lessons from severe acute respiratory syndrome and middle east respiratory syndrome. AJR Am. J. Roentgenol. 2020;214(5):1078–1082. doi: 10.2214/AJR.20.22969. [DOI] [PubMed] [Google Scholar]
- 15.Islam N., Ebrahimzadeh S., Salameh J.P., Kazi S., Fabiano N., Treanor L., Absi M., Hallgrimson Z., Leeflang M.M., Hooft L., van der Pol C.B., Prager R., Hare S.S., Dennie C., Spijker R., Deeks J.J., Dinnes J., Jenniskens K., Korevaar D.A., Cohen J.F., Van den Bruel A., Takwoingi Y., van de Wijgert J., Damen J.A., Wang J., McInnes M.D. Thoracic imaging tests for the diagnosis of COVID-19, The. Cochrane Database Syst. Rev. 2021;3(3):Cd013639. doi: 10.1002/14651858.CD013639.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillies R.J., Kinahan P.E., Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2) doi: 10.1148/radiol.2015151169. 563-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.P.M. Bossuyt, J.B. Reitsma, D.E. Bruns, C.A. Gatsonis, P.P. Glasziou, L. Irwig, J.G. Lijmer, D. Moher, D. Rennie, H.C. de Vet, H.Y. Kressel, N. Rifai, R.M. Golub, D.G. Altman, L. Hooft, D.A. Korevaar, J.F. Cohen, STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies, BMJ (Clinical research ed.) 351 (2015) h5527. [DOI] [PMC free article] [PubMed]
- 18.McInnes M.D.F., Moher D., Thombs B.D., McGrath T.A., Bossuyt P.M., Clifford T., Cohen J.F., Deeks J.J., Gatsonis C., Hooft L., Hunt H.A., Hyde C.J., Korevaar D.A., Leeflang M.M.G., Macaskill P., Reitsma J.B., Rodin R., Rutjes A.W.S., Salameh J.P., Stevens A., Takwoingi Y., Tonelli M., Weeks L., Whiting P., Willis B.H. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018;319(4):388–396. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 19.Lambin P., Leijenaar R.T.H., Deist T.M., Peerlings J., de Jong E.E.C., van Timmeren J., Sanduleanu S., Larue R., Even A.J.G., Jochems A., van Wijk Y., Woodruff H., van Soest J., Lustberg T., Roelofs E., van Elmpt W., Dekker A., Mottaghy F.M., Wildberger J.E., Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017;14(12):749–762. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]
- 20.Mongan J., Moy L., Kahn C.E., Jr. Checklist for artificial intelligence in medical imaging (CLAIM): a guide for authors and reviewers. Radiol. Artif. Intell. 2020;2(2) doi: 10.1148/ryai.2020200029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whiting P.F., Rutjes A.W., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B., Leeflang M.M., Sterne J.A., Bossuyt P.M. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155(8) doi: 10.7326/0003-4819-155-8-201110180-00009. 529-36. [DOI] [PubMed] [Google Scholar]
- 22.Jaeschke R., Guyatt G.H., Sackett D.L., Guyatt G., Bass E., Brill-Edwards P., Browman G., Cook D., Farkouh M., Gerstein H.J.J. Users' guides to the medical literature: III. How to use an article about a diagnostic test B. What are the results and will they help me in caring for my patients? JAMA. 1994;271(9):703–707. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 23.J.B. Reitsma, A.S. Glas, A.W. Rutjes, R.J. Scholten, P.M. Bossuyt, A.H.J.Joce. Zwinderman, Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews, 58(10) (2005) 982–990. [DOI] [PubMed]
- 24.W.A. van Enst, E. Ochodo, R.J. Scholten, L. Hooft, M.M.J.Bmrm. Leeflang, Investigation of publication bias in meta-analyses of diagnostic test accuracy: a meta-epidemiological study, 14(1) (2014) 1–11. [DOI] [PMC free article] [PubMed]
- 25.S. Qi, C. Xu, C. Li, B. Tian, S. Xia, J. Ren, L. Yang, H. Wang, H.J.Cm Yu, p.i. biomedicine, DR-MIL: deep represented multiple instance learning distinguishes COVID-19 from community-acquired pneumonia in CT images, 211 (2021) 106406. [DOI] [PMC free article] [PubMed]
- 26.A. Narin, Y.J.JotFo.E.. Isler, A.o.G. University, Detection of new coronavirus disease from chest x-ray images using pre-trained convolutional neural networks, 36(4) (2021) 2095–2107.
- 27.Han C.H., Kim M., Kwak J.T. Semi-supervised learning for an improved diagnosis of COVID-19 in CT images. PLoS One. 2021;16(4) doi: 10.1371/journal.pone.0249450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.H. Panwar, P. Gupta, M.K. Siddiqui, R. Morales-Menendez, P. Bhardwaj, V.J.C. Singh, Solitons, Fractals, A deep learning and grad-CAM based color visualization approach for fast detection of COVID-19 cases using chest X-ray and CT-Scan images, 140 (2020) 110190. [DOI] [PMC free article] [PubMed]
- 29.N. Azouji, A. Sami, M. Taheri, H.J.Ci.B. Müller, Medicine, A large margin piecewise linear classifier with fusion of deep features in the diagnosis of COVID-19, 139 (2021) 104927. [DOI] [PMC free article] [PubMed]
- 30.L. Wang, B. Kelly, E.H. Lee, H. Wang, J. Zheng, W. Zhang, S. Halabi, J. Liu, Y. Tian, B.J.Ejor. Han, Multi-classifier-based identification of COVID-19 from chest computed tomography using generalizable and interpretable radiomics features, 136 (2021) 109552. [DOI] [PMC free article] [PubMed]
- 31.X. Fang, X. Li, Y. Bian, X. Ji, J.J.Er Lu, Radiomics nomogram for the prediction of 2019 novel coronavirus pneumonia caused by SARS-CoV-2, 30(12) (2020) 6888–6901. [DOI] [PMC free article] [PubMed]
- 32.Guo X., Li Y., Li H., Li X., Chang X., Bai X., Song Z., Li J., Li K. An improved multivariate model that distinguishes COVID-19 from seasonal flu and other respiratory diseases. Aging. 2020;12(20):19938–19944. doi: 10.18632/aging.104132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayerhoefer M.E., Materka A., Langs G., Häggström I., Szczypiński P., Gibbs P., Cook G. Introduction to radiomics. J. Nucl. Med.: Off. Publ., Soc. Nucl. Med. 2020;61(4):488–495. doi: 10.2967/jnumed.118.222893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia Y., Chen W., Ren H., Zhao J., Wang L., Jin R., Zhou J., Wang Q., Yan F., Zhang B., Lou J., Wang S., Li X., Zhou J., Xia L., Jin C., Feng J., Li W., Shen H. A rapid screening classifier for diagnosing COVID-19. Int. J. Biol. Sci. 2021;17(2):539–548. doi: 10.7150/ijbs.53982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H., Ren H., Wu Z., Xu H., Zhang S., Li J., Hou L., Chi R., Zheng H., Chen Y., Duan S., Li H., Xie Z., Wang D. CT radiomics facilitates more accurate diagnosis of COVID-19 pneumonia: compared with CO-RADS. J. Transl. Med. 2021;19(1):29. doi: 10.1186/s12967-020-02692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbasian Ardakani A., Acharya U.R., Habibollahi S., Mohammadi A. COVIDiag: a clinical CAD system to diagnose COVID-19 pneumonia based on CT findings. Eur. Radiol. 2021;31(1):121–130. doi: 10.1007/s00330-020-07087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai H.X., Wang R., Xiong Z., Hsieh B., Chang K., Halsey K., Tran T.M.L., Choi J.W., Wang D.C., Shi L.B., Mei J., Jiang X.L., Pan I., Zeng Q.H., Hu P.F., Li Y.H., Fu F.X., Huang R.Y., Sebro R., Yu Q.Z., Atalay M.K., Liao W.H. Artificial intelligence augmentation of radiologist performance in distinguishing COVID-19 from pneumonia of other origin at chest CT. Radiology. 2020;296(3):E156–e165. doi: 10.1148/radiol.2020201491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellini D., Panvini N., Rengo M., Vicini S., Lichtner M., Tieghi T., Ippoliti D., Giulio F., Orlando E., Iozzino M.J.Er. Diagnostic accuracy and interobserver variability of CO-RADS in patients with suspected coronavirus disease-2019: a multireader validation study. 2021;31(4):1932–1940. doi: 10.1007/s00330-020-07273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song J., Wang H., Liu Y., Wu W., Dai G., Wu Z., Zhu P., Zhang W., Yeom K.W., Deng K. End-to-end automatic differentiation of the coronavirus disease 2019 (COVID-19) from viral pneumonia based on chest CT. Eur. J. Nucl. Med. Mol. Imaging. 2020;47(11):2516–2524. doi: 10.1007/s00259-020-04929-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X., Cui J., Xu W., Yang Y., Fayad Z.A., Jacobi A., Li K., Li S., Shan H., Imaging C.T. Features of 2019 Novel Coronavirus (2019-nCoV) Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 42.Koo H.J., Lim S., Choe J., Choi S.-H., Sung H., Do K.-H.J.R. Radiographic and CT features of viral pneumonia. 2018;38(3):719–739. doi: 10.1148/rg.2018170048. [DOI] [PubMed] [Google Scholar]
- 43.P. Reittner, S. Ward, L. Heyneman, T. Johkoh, N.L.J.Er Müller, Pneumonia: high-resolution CT findings in 114 patients, 13(3) (2003) 515–521. [DOI] [PubMed]
- 44.Shiley K.T., Van Deerlin V.M., Miller W.T., Jr. Chest CT features of community-acquired respiratory viral infections in adult inpatients with lower respiratory tract infections. J. Thorac. Imaging. 2010;25(1):68–75. doi: 10.1097/RTI.0b013e3181b0ba8b. [DOI] [PubMed] [Google Scholar]
- 45.Kumar V., Gu Y., Basu S., Berglund A., Eschrich S.A., Schabath M.B., Forster K., Aerts H.J., Dekker A., Fenstermacher D.J.Mri. Radio.: Process Chall. 2012;30(9):1234–1248. doi: 10.1016/j.mri.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.P. Lambin, E. Rios-Velazquez, R. Leijenaar, S. Carvalho, R.G. Van Stiphout, P. Granton, C.M. Zegers, R. Gillies, R. Boellard, A.J.Ejoc. Dekker, Radiomics: extracting more information from medical images using advanced feature analysis, 48(4) (2012) 441–446. [DOI] [PMC free article] [PubMed]
- 47.Wainberg M., Merico D., Delong A., Frey B.J. Deep learning in biomedicine. Nat. Biotechnol. 2018;36(9):829–838. doi: 10.1038/nbt.4233. [DOI] [PubMed] [Google Scholar]
- 48.Yan L.C., Yoshua B., Geoffrey H.Jn. Deep Learn. 2015;521(7553):436–444. [Google Scholar]
- 49.L. Alzubaidi, J. Zhang, A.J. Humaidi, A. Al-Dujaili, Y. Duan, O. Al-Shamma, J. Santamaría, M.A. Fadhel, M. Al-Amidie, L.J.JobD. Farhan, Review of deep learning: Concepts, CNN architectures, challenges, applications, future directions, 8(1) (2021) 1–74. [DOI] [PMC free article] [PubMed]
- 50.H.-C. Shin, H.R. Roth, M. Gao, L. Lu, Z. Xu, I. Nogues, J. Yao, D. Mollura, R.M.J.Itomi. Summers, Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning, 35(5) (2016) 1285–1298. [DOI] [PMC free article] [PubMed]
- 51.Kao Y.S., Lin K.T. A meta-analysis of computerized tomography-based radiomics for the diagnosis of COVID-19 and viral pneumonia. Diagnostics. 2021;11(6) doi: 10.3390/diagnostics11060991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouchareb Y., Moradi Khaniabadi P., Al Kindi F., Al Dhuhli H., Shiri I., Zaidi H., Rahmim A. Artificial intelligence-driven assessment of radiological images for COVID-19. Comput. Biol. Med. 2021;136 doi: 10.1016/j.compbiomed.2021.104665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi F., Wang J., Shi J., Wu Z., Wang Q., Tang Z., He K., Shi Y., Shen D. Review of artificial intelligence techniques in imaging data acquisition, segmentation, and diagnosis for COVID-19. IEEE Rev. Biomed. Eng. 2021;14:4–15. doi: 10.1109/RBME.2020.2987975. [DOI] [PubMed] [Google Scholar]
- 54.Gudigar A., Raghavendra U., Nayak S., Ooi C.P., Chan W.Y., Gangavarapu M.R., Dharmik C., Samanth J., Kadri N.A., Hasikin K., Barua P.D., Chakraborty S., Ciaccio E.J., Acharya U.R. Role of artificial intelligence in COVID-19 detection. Sensors. 2021;21(23) doi: 10.3390/s21238045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suri J.S., Agarwal S., Gupta S.K., Puvvula A., Biswas M., Saba L., Bit A., Tandel G.S., Agarwal M., Patrick A., Faa G., Singh I.M., Oberleitner R., Turk M., Chadha P.S., Johri A.M., Miguel Sanches J., Khanna N.N., Viskovic K., Mavrogeni S., Laird J.R., Pareek G., Miner M., Sobel D.W., Balestrieri A., Sfikakis P.P., Tsoulfas G., Protogerou A., Misra D.P., Agarwal V., Kitas G.D., Ahluwalia P., Teji J., Al-Maini M., Dhanjil S.K., Sockalingam M., Saxena A., Nicolaides A., Sharma A., Rathore V., Ajuluchukwu J.N.A., Fatemi M., Alizad A., Viswanathan V., Krishnan P.K., Naidu S. A narrative review on characterization of acute respiratory distress syndrome in COVID-19-infected lungs using artificial intelligence. Comput. Biol. Med. 2021;130 doi: 10.1016/j.compbiomed.2021.104210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material