Abstract
Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) is a highly pathogenic and transmissible virus. Infection caused by SARS-CoV-2 known as Coronavirus disease 2019 (COVID-19) can be severe, especially among high risk populations affected of underlying medical conditions. COVID-19 is characterized by the severe acute respiratory syndrome, a hyper inflammatory syndrome, vascular injury, microangiopathy and thrombosis. Antiviral drugs and immune modulating methods has been evaluated. So far, a particular therapeutic option has not been approved for COVID-19 and a variety of treatments have been studied for COVID-19 including, current treatment such as oxygen therapy, corticosteroids, antiviral agents until targeted therapy and vaccines which are diverse in each patient and have various outcomes. According to the findings of different in vitro and in vivo studies, some novel approach such as gene editing, cell based therapy, and immunotherapy may have significant potential in the treatment of COVID-19. Based on these findings, this paper aims to review the different strategies of treatment against COVID-19 and provide a summary from traditional and newer methods in curing COVID-19.
Keywords: Coronavirus, Cell based therapy, Immunotherapy, Vaccine
1. Introduction
In December 2019 in Wuhan, the capital of central China’s Hubei province began an outbreak of viral pneumonia symptom, which spread to almost all countries. It has led to a great concern of the potential for not only an epidemic but a pandemic in all over the world [1], [2]. At first the cause of the pneumonia was unknown and then it was determined that the contagious infection caused by a new human pathogen that belongs to the Coronavirus (CoV) family. The infection caused by this CoV referred as the CoV disease 2019 (COVID-19) or 2019 novel Coronavirus (nCoV) [3].
CoVs are a family of viruses that belong to the family Coronaviridae and subfamily Coronaviridae [4]. The family Coronaviridae classified as a type of RNA virus includes the genus CoV and torovirus that cause disease in human beings and animals [5]. About seven types of CoVs known to be associated with human included the alpha-CoVs HCoV-NL63, HCoV-229E, beta-CoVs HCoV-OC43, HCoV-HKU1, severe acute respiratory syndrome-CoV (SARS-CoV), and middle east respiratory syndrome-CoV (MERS-CoV). Types 229E and OC43 are a major cause of the common cold [6]. Although most human CoV infections are mild, two epidemics have occurred by CoVs included 1) severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). SARS has originated in Guangdong state of China and expanded to 29 countries from November 2002 through July 2003 and resulted in 774 deaths out of 8,098 infected individuals while Middle East MERS has began in Saudi Arabia and expanded to 27 countries through July 2019 that was responsible for 848 deaths among 2,458 individuals. In addition, SARS and MERS source of origin were identified as civet cats and camels, respectively. As a result, COVID-19 is a third CoV that can cause severe respiratory illness that has 79 % nucleotide identity to SARS-CoV and about 50 % to MERS-CoV [7].
Since, SARS coronavirus 2 (SARS-CoV-2) is a highly pathogenic and transmissible, it spread between family homes, hospitals and cities. In about 2 to 14 days, a person infected with the SARS‐CoV‐2 can be asymptomatic; during which this period, the infected person can involve other people as a carrier [8], [9].
CoVs are positive-sense RNA viruses with an enveloped, nonsegmented and single-stranded ribonucleic acid whose genome structure is best known among all RNA viruses. CoV due to 9–12 nm-long surface spikes has solar corona like appearance. Genomes of these viruses are about 30 kb, enclosed by a 5′-cap and 3′-poly-A tail. The genome of 2019-nCoV is of 29.891 kb long, with a G + C content of 38 %. Unlike some β-CoVs, the 2019-nCoV has not hemagglutinin-esterase gene (HE) which is characteristic of this category of viruses [10], [11], [12]. In addition, the four major structural proteins encoded by other one-third of the genome included spike (S), envelope (E), membrane or matrix (M), and nucleocapsid (N) protein as well as the non-structural proteins, such as proteases (nsp3 and nsp5) and RdRp (nsp12). Interaction of spike (S) protein to angiotensin-converting enzyme 2 (ACE2) receptor is first step for virus attachment that causes viral entry into the host cell [13], [14], [15].
SARS-CoV-2 infection can be severe, especially among high risk populations affected of underlying conditions such as elderly, hypertension, cardiovascular disease, diabetes, cancer, end-stage renal disease, chronic obstructive pulmonary disease (COPD), and asthma [16], [17].
So far, completely specific treatment for COVID-19 is not approved and a variety of treatments have been considered for COVID-19. The aim of this article is to describe the treatment strategies that have been used for COVID-19 patients and review all the available literature.
2. Gene editing or approaches based on nucleic acid
Gene-editing technology has a broad spectrum of applications. Efficiency of such approaches has been demonstrated against viral infections including SARS and respiratory syncytial virus (RSV) [18], [19], [20].
Introduction of the CRISPR-Cas9 has received considerable attention for viral diagnosis and antiviral therapies [21]. In contrast to CRISPR-Cas9 that only targets double-stranded DNA (dsDNA), CRISPR-Cas13 is capable of targeting single-stranded RNA (ssRNA) such as influenza A virus, lymphocytic choriomeningitis virus, and SARS-CoV-2 [22], [23], [24]. Recently, Prophylactic Antiviral CRISPR in human cells (PAC-MAN) has been introduced against COVID 19, which was a novel strategy based on Cas13d. PAC-MAN entails an endonuclease with a pool of CRISPR-associated RNA (crRNA), targeting conserved sequences of viral genomes. PAC-MAN merits recognition as it is able to simultaneously eliminate multiple strains of coronaviruses by using a panel of crRNA [23]. However, PAC-MAN harbors shortcomings restricting its application, including off-target affects, immunogenicity, delivery, and toxicity. Moreover, the function of Cas13 may further be hindered by the notorious formations of RNA secondary structures in the host cells [20], [25].
3. Cell based therapy
Cell therapy is a cell-based therapy in which human cells as therapeutic agents are injected, grafted or implanted into blood stream, tissue, and organ patients to replace or repair of injured tissue and/or cells [26], [27], [28], [29], [30]. Cell therapy can be the allogeneic which transplanted cells are taken from donor of the same species, and the autologous that transplanted cells are taken from the donor himself, and or the xenogeneic which transplanted cells are taken from one species to another. Cell therapy may also be classified according to the target tissue and type of cells [31], [32].
3.1. Stem cells therapy
Stem cells have the ability to product every cell and tissue in the human body. In recent years, different studies have been aimed to investigate of the stem cells as a therapeutic agent for treat or prevent of diseases and it uses in regenerative medicine, gene therapy, immunotherapy, tissue regeneration and repair that have leads to promising findings [29], [33], [34], [35], [36].
Mesenchymal stem cells or mesenchymal stromal cells (MSCs) are multipotent stem cells that can differentiate into a variety of cell types [37], [38]. MSCs referred as immune-privileged cells, because they express low levels of human leucocyte antigen (HLA) class I, and do not express HLA class II, CD40, CD40L, CD80 and CD86 that cause escape the cytotoxic activities of lymphocytic T cells, B cells and NK cells [39]. After intravenous injection, MSCs are primarily trapped in capillary beds of the liver and lungs, and then bind to receptors, including chemokine receptors, adhesion proteins, and matrix metalloproteinase (MMPs) [40].
As previously mentioned, in COVID 19, after entrance of SARS-CoV-2 into the lungs, an immune response induced and then beginning a cytokine storm by attracting immune cells to the region attacked by the virus [41]. Since, the main functions of MSCs are immune regulation, as a result can be a potential treatment for patients with COVID-19 which registered the some of them in Clinical trial.gov. The treatment of acute respiratory distress syndrome (ARDS) induced by epidemic influenza A (H7N9) via allogeneic menstrual-blood-derived MSCs compare to the control group has indicated that MSC transplantation significantly decreased the mortality, inflammatory effects and cytokine storm, as a result, suggested this type of treatment for COVID-19 patients which have shown similar complications [42].
The use of menstrual-blood-derived MSCs transplantation for the treatment of two confirmed cases of COVID-19 in Wuhan from China with ARDS demonstrated that increases the immune indicators levels such as CD4+ and lymphocytes and decreases the inflammation indicators levels including IL-6 and C-reactive protein (CRP) without a considerable side effects [43]. The human umbilical cord-derived MSCs (hUC-MSCs) to use for transplantation to COVID-19 patients has been shown be therapeutically effective which significantly decreased the level of TNF-α, IL-6 and CRP and increase IL-10 (as an anti-inflammatory protein activating regulatory cells such as Tregs) and returned the lymphocytes count to the normal range faster in compare to control [43], [44]. It is also shown that the use of hUCMSCs transplantation has reduced the seral levels of CRP, ALT, AST, bilirubin, the white blood cell count and neutrophil count and increase the lymphocyte count, CD3+ T cell, CD4+ T cell, and CD8+ T cell [45].
It has been shown that exogenous insulin use significantly reduced in patients with COVID-19 after hUC-MSC injection [46]. Results of phase I clinical trial study to evaluate the safety of hUC-MSCs infusions in the treatment of 18 patients (n = 9 moderate and 9 severe) with COVID-19 pulmonary disease indicated IgG and IgM anti-SARS-CoV-2 antibodies were not statistically decreased in the UC-MSCs treatment group compared with the control group [47]. The angiotensin I converting enzyme 2 (ACE2) receptors required to COVID-19 distributed on the human cells surface especially the alveolar type II cells (AT2), but it not expresses in some cells such as bone marrow, lymph nodes, thymus, and the spleen, immune cells [48]. It was indicated that MSCs have barely express ACE2 and transmembrane serine protease 2 (TMPRSS2) [49], two receptors needed for the virus endocytosis. Therefore, MSCs are free from SARS-CoV-2 infection. of MSCs in patients with COVID-19 has induced of IL-10, and CD14 + CD11c + CD11bmid regulatory dendritic cells. It has decreased the level TNF-α, and CRP, and disappeared T cells (CXCR3 + CD4+, CXCR3 + CD8 + ) and activated CXCR3 + NK cells over activated. Moreover, the induced IL-10 and VEGF triggered the lung repair. lymphopenia which is observed in these patients was significantly improved after the cell transplantation and increased the lymphocyte count [50], [51].
3.2. Immune cell therapy
Immune cell therapy, known as cellular immunotherapy, uses the cells of immune system to improving the condition of the disease. The allogeneic source has a more primacy due to costs, and emergency conditions of COVID-19 [39], [52].
3.2.1. Natural killer (NK) and chimeric antigen receptor (CAR) T-Cell therapy
Natural killer (NK) cells which are another candidate for cell-based therapy to treatment of COVID-19 patients, to be a type of cytotoxic lymphocyte critical to the innate immune system by protecting against tumorigenic cells and viral invaders and contain 10–15 % of total peripheral blood leukocytes in humans [53]. In CAR-T therapy, T cells that have been genetically modified to express CAR [4] are proliferated in vitro and eventually transplanted to the patients. CAR-T therapy uses CARs that target different antigens including, CD19, CD30, CD33, CD123, FLT3, and BCMA. In the cytokine storm, after binding of CAR-T receptor and its specific antigen the large amounts of IFN-γ and TNF-α are released which finally induce the produce of cytokines such as IL-1, IL-6, and IL-10 by monocytes and macrophages [54], [55]. Although NK cell and CAR T cell therapy is mostly used to treat cancer, but similar mechanisms could provide guidance in the fight against viruses. Of course, COVID-19 clinical trials studies (https://www.clinicaltrials.gov) are underway by NK cell and CAR T cell therapy (Table 1 ) [56].
Table 1.
List of COVID-19 clinical trials using immune cell therapies.
| Author | Therapeutic | Status | Locations | Phase | Participants | Ref |
|---|---|---|---|---|---|---|
| Min Liu, A.B | NKG2D-ACE2, CAR-NK cells | Recruiting | China | I/II | 90 | NCT04324996 |
| Lucia Silla | Natural Killer Cells | Not yet Recruiting | Brazil | I | 24 | NCT04634370 |
| ZHU | Natural Killer Cells | Recruiting | China, Henan | I | 30 | NCT04280224 |
| Erica Rave | Natural Killer Cells (CYNK-001) | Recruiting | United States | I/II | 86 | NCT04365101 |
| David A Bernal E | Natural Killer Cells | Not yet recruiting | Colombia | I/II | 10 | NCT04344548 |
| Antonio Pérez Martinez | T memory cells and NK cells | Recruiting | Spain | I/II | 58 | NCT04578210 |
| Jae Park | CART Cell | Recruiting | United States, New York | I/II | 90 | NCT04148430 |
4. Immunotherapy
Immunotherapy has indicated considerable outcomes in the treatment of different diseases. Several immunotherapy attempts have been successful to fight against CoV such as SARS-CoV and MERS-CoV [57], [58].
Insufficient antiviral immune response and intensive inflammation induced by inefficient immune response are the two main challenges in COVID‐19. Developing novel immune‐based therapeutics that aim viral infection and dysfunctional immune response can improve the clinical result of patients with COVID‐19. This new approach is named as “immunotherapy of COVID‐19″ and is among the novel treatments being developed. Several methods can be used as immunotherapeutic treatment for COVID‐19, which are discussed below [59].
Studies shown that in the pathogenesis of SARS, a cytokine storm occurred, involving a remarkable release of proinflammatory cytokine such as IL-6, TNF-α, and IL-12, IL-2, IL-7, IL-10, granulocyte-colony stimulating factor, IFN-γ and TNF-α, which implied that a cytokine storm occurred and related to the severity and prognostic of COVID-19. Prevention and suppression of the cytokine storm may be the critical to save patients with severe COVID‐19 [60], [61], [62].
4.1. Interferon-based immunotherapy
Type I of IFNs as components of the immune system, has a vital role in defending the body against viruses. SARS‐CoV‐2 can prevent the creation of IFN‐1 by impairing IFN‐signaling pathways [63]. IFN-based immune defense are applied against the virus; anyway, the threshold of the incitement for the manufacture of IFN is different based on age. The low mortality rate of the COVID‐19 in kids has been dependent on the low IFN-producing threshold of IFN, which causes the early production of IFN, and can finally control the SARS‐CoV‐2 infection. Interestingly, the higher death rate in aged patients is at least partly related to the higher threshold for IFN production, leading to the delay in IFN production and insufficient immune response. The restraint of the IFN-producing pathway by the virus and insufficient IFN‐production is one of the primary challenges of the immune response against COVID‐19 [64].
In the clinical setting, accompanying IFN-β1b in the early treatment of COVID-19 with lopinavir and ritonavir is more effective than lopinavir-ritonavir alone, effectively inhibiting triple therapy of infection and reducing disease progression to severe stages [65], [66]. In vitro and clinical results have supported the efficacy of treatment with type I IFNs. IFN-based immunotherapy has been considered in clinical trials of COVID‐19 patients [67]. IFN-lambda (IFN-λ) is a member of the type III IFN family. IFN-λ is attractive in the antiviral immune response against viral infections [68]. Treatment of COVID‐19 by IFN-λ could stimulate an effective immune response against the virus. Thus, IFN‐λ has been used in COVID‐19‐ treating clinical trials.
At the first stages of the disease, IFNs are critical for inducing a sufficient immune response against the virus. At late and severe stages, however, the main immune response against the virus is mediated by immune cells. The uncontrolled activation of the macrophages leads to the production of excess amounts of proinflammatory cytokines. Therefore, in IFN-dependent treatment of the COVID‐19, the stage of the disease must be considered. Use of IFNs in the earlier stages of the COVID‐19 could induce a stronger antiviral response and inhibit the infection. However, at later stages of the disease, use of the IFNs could induce a hyperactivated immune response and worsen the cytokine storm [64].
Although IFNs is suggested to treat the disease [67] but some studies demonstrated that IFNs may enhance ACE2 expression and thus viral entry [69]. On the other hand, favorable results were reported by using iINF type I, including INF-β-1a in clinical trial [70]. As a reason for the diverse effects INFs, the difference in the route of administration (either subcutaneous (s.c.) and intravenous (i.v.)) in some studies was proposed reported [71].
4.2. Antibodies-based immunotherapy
Monoclonal antibodies designed against SARS-CoV-2 can be classified in three main groups based on their target: 1) antibodies that inhibit the virus attachment and entry through both target the virus shape or host receptors, 2) antibodies that interfere with the virus replication and transcription, 3) antibodies that prevent various steps of the immune system response [72]. Monoclonal antibodies, which are proposed to date against SARS-CoV-2, mainly target immune responses [42], [73], [74], [75].
The significant increase in the level of chemokines and cytokines, which is called the cytokine storm, can be inhibited by antibodies therapy [76]. The preliminary studies of severe COVID-19 have shown that IL-6 and GMCSF are the key cytokines leading to inflammatory storm, which result in gas exchange dysfunction and impaired oxygen diffusion. These cytokines can lead to the pulmonary fibrosis, organ failure and ARDS [77]. Control of the cytokine storm can improve respiratory failure in severe cases with hyper-inflammation. One way to achieve this management is the inhibition of the inflammatory pathways. To inhibit the inflammatory pathways, one could block the release of the inflammatory cytokines [78].
Tocilizumab (TCZ), an IL‐6 receptor (IL‐6R) antagonist, can repress cytokine storms by impeding the IL‐6 signal transduction pathway [79]. IL-6 receptor (IL-6R) has two structures: membrane-bound IL-6 R (mIL-6R) and soluble IL-6 R (sIL-6R). IL-6 and sIL-6R binds together to form a complex, then binds to gp130 on the cell membrane to complete trans-signal transduction and induce a proinflammatory role [80], [81], [82], [83]. As a recombinant humanized against human IL-6R monoclonal antibody, TCZ can specifically bind sIL-6R and mIL-6R and inhibit signal transduction. It is at the moment used predominantly for rheumatoid arthritis [77]. The utilization of TCZ has demonstrated promising clinical advantages in some COVID‐19 patients [84]. In a 21-understanding clinical examination selected in China, TCZ brought about the decrease of oxygen need in 75 % of patients, lung lesion opacity absorption in 90.5 % of patients, and correction of lymphocyte and CRP levels [77].
It has been shown that patients with severe COVID-9, 90 % recovered after a few days of treatment by TCZ, therefore TCZ improve survival outcomes [77], [85]. In this regard, there are similar studies that confirmed the positive effects of TCZ in COVID‐19 patients [77], [86], [87], [88], [89], [90], [91], [92], [93], [94].
TCZ inhibits the damaging effects of the SARS‐COV‐2 on the respiratory system; nevertheless, TCZ does not have a direct antiviral function on SARS‐CoV‐2. Like other immune‐restraining drugs, uncontrolled or unnecessary application of TCZ could be associated with some adverse effects, such as an increase in hepatic enzymes, hypercholesterolemia, skin allergy, and opportunistic fungal infections [93], [95].
4.3. Polyclonal antibodies against SARS-CoV-2
The design of monoclonal antibodies and their testing at a clinical stage is a long pathway and the massive production of monoclonal antibodies might be costly, time-consuming; that are factors cannot be at least taken in the time of a pandemic outbreak and an urgent need to effective therapeutics. The administration of multivalent antibodies (cocktail of monoclonal antibodies) for the neutralization of SARS-CoV-2 also seems more logical. Because the risk of contamination and host reactions will be decline compared with their plasma equivalents and in addition to, targeting more than one epitope can cause synergistic effects in neutralization and would limit the formation of escape-mutants. for example, a recent study revealed that a cocktail of antibody noticeably enhanced SARSCoV-2 neutralization compared with the use of one monoclonal antibody [96], [97].
5. Vaccination
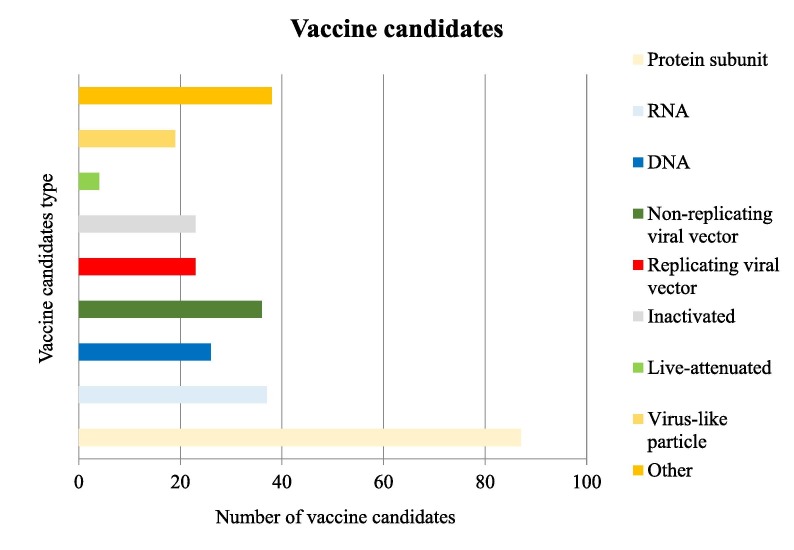
The recent outbreak of the COVID-19 endangered public health and further imposed a substantial economic burden on societies worldwide; thus, the development of therapeutic drugs and vaccines was urgently required. Reports have been cited four types of vaccines being under development which were based on whole virus, viral vector, nucleic acid (DNA or RNA), and recombinant protein [98]. According to the world health organization (WHO) records, 283 COVID 19 candidate vaccines were reported until December 28, 2020; in which 66 of the potential vaccines had entered the clinical trial testing. Fig. 1 illustrates further details on the aforementioned vaccines (WHO; COVID-19 Vaccine tracker).
Fig. 1.
Number of vaccine candidates and types of them.
In the case of the SARS-CoV-2, there have been several immunogenic antigens serving as targets for vaccine developments, with the S protein being the most prominent earmark. By the grace of high throughput sequencing methods, scientific communities were able to identify high conservancy with the N protein, thus serving as a useful target for vaccine development.
Of note, vaccine’s efficiency is directly correlated with the induction of a robust innate and adaptive immune response. One of the common strategies is the employment of natural or synthetic adjuvants. The live-attenuated vaccines inherently possess the adjuvant necessary for prompt yet prolonged, induction of immune response [99].
In contrast, vaccines designed from inactivated virus particles or protein-based vaccines harbor limited immunogenicity, therefore external adjuvants should be provided to efficiently promote immune responses [99], [100]. There are repertoires of adjuvants commonly used by scientists, among which the Aluminium salts (alum) are of popular choices. Although that it produces a poor T cell response, Alum has been one of the primary choices among scientists, merely due to the safety observed throughout decades of experiments, furthermore, Alum enhances B cell stimulation resulting in a higher antibody-mediated immunity. Moreover, the alum aids in the stabilization and longevity of antigens and it further improves safety levels by reducing the adverse effect of sudden exposure of immune cells to high titers of antigens [99]. Additionally, other candidates such as AS03 (GSK) and CpG 1018 (Dynavax) have been proposed to enhance vaccines [101].
5.1. Inactivated and live-attenuated vaccines
Given the fact that the complete inactivation of the virus, reduces the possibility of infection to great extent with minimum alteration in the immunogenic antigens, has brought attention to the approach [102]. Several companies mainly originated in China, such as Sinovac Biotech are attempting to develop live-attenuated based vaccines exploiting the whole virion particles. The production of such vaccines involve exploiting the effects of chemical excipients such as hydrogen peroxide, formaldehyde, and β-propiolactone; or the use of gamma and ultra-violate irradiation, and heat inactivation of the infectious agent [103]. Due to the high conservancy of the structural proteins namely the S, N, E, and M of SARS-CoV-2, such proteins function as suitable immunogenic antigens and act as the primary immune cells’ recognition target. Although adjuvant supplementation may increase the immune response to inactivated vaccines [104], the approach is faced with the limitation of administrating reminder shots for efficient memory cell development and long-lasting immunity [105].
The live-attenuated vaccines display several advantages that merit recognition, including providing a vast and diverse repertoire of proteins serving as immunogenic antigens, namely the conserved structural proteins, biologically important non-structural proteins, and accessory proteins; capable of triggering an efficient immune response. However, such traditionally produced therapeutics harbor the risk of infection which could be alleviated through genetic manipulations, rendering the pathogenic agents deficient and with long-lasting immunogenicity [106]. Nevertheless, concerning the safety, the approach may still bear disadvantages brought about by genetic back mutations which may lead to illness and development of infection in individuals with a compromised immune system. [104]. The Chinese Sinovac Biotech Company has developed a vaccine using the traditional live-attenuated system and was further branded the product PiCoVacc. The vaccine demonstrated a high rate of neutralizing antibody generation against S, (receptor binding domain) RBD, and N protein among 10 strains of the SARS-CoV-2 virus. Interestingly, as opposed to SARS and MERS inactivated vaccines, no pulmonary immunopathology was witnessed [100]. However, the capability to elicit T cell-mediated responses was impaired which deems further investigation [107]. Alongside the Chinese, The USA in collaboration with the Institute of India is working on a product named CDX-CoV. The vaccine is based on both structural and non-structural proteins of the virus [108].
5.2. Viral-vector vaccines
Viral-vector vaccines are a relatively new approach to vaccine development. The strategy encompasses a viral particle posing as a carrier of genes related to the target pathogen. This leads to the generation of viral particles that carry the treats and antigenic properties of the selected pathogen. The viral particles may actively reproduce and replicate in cells to efficiently manifest the immunopathogenic treats such as measles and pox or may be genetically modified and non-replicating such as adeno associated viruses, to preclude viral infection. The strategy has illustrated long-term innate and adaptive immune responses [109]. Studies have demonstrated that such vectors bear the capacity of carrying large genetic fragments which is a major advantage, however, the approach has raised some safety and economic concerns, as there is a risk of random insertion due to the inherent properties of virus-based vectors and the manufacturing is quite cumbersome and economically unfavorable [110].
Focusing on SARS-CoV-2, a replication defected simian adenovirus vector entitled ChAdOx1, encoding the complete sequence of S protein has developed by Oxford University. Preliminary results from studies on mice models has demonstrated that the vaccine triggered neutralizing antibodies (nAbs), IgG, and high levels of Th1 response and a low level of Th2 response. Further study unveiled a similar amount of nAbs in patients and convalescent control samples [111]. The Chinese company, CanSinoBIO, has focused on exploiting the adenovirus type-5 (Ad-5) to carry the immunogens derived from the S gene. The study elucidated that immune response in the form of IgG against the RBD and nAbs was observed, furthermore, cellular immunity mediated by the Th1 subset was witnessed. However, the approach harbored downsides including pre-existing immunity against the Ad-5 vector that may adversely affect B cell and T cell responses [109].
Although non-replicating viral vaccines have proven somewhat safe, the fact that every viral particle tends to infect only a single cell imposes a major drawback on its application; to circumvent the issue, a high dosage of vaccine should be commenced to generate strong and long-lasting immune responses[112].
5.3. Nucleic acid vaccines
DNA vaccines are the first derivative of nucleic acid vaccines, consisting of a circular expression vector empowered by strong promoters to transcribe immune antigens once absorbed by the cells [102]. DNA vaccines are prompted the induction of IgG nAbs and T cell response. However, their application has faced a major limitation; the vector must be able to traverse the cytoplasm and enter the nucleus environment to ignite the transcription process, moreover, the risk of random insertion of part of the plasmid into chromosomes is not negligible, and measures must be taken into account to mitigate the shortcomings [102].
The incorporations adopting mRNA-based vaccines are Pfizer–BioNTech and Moderna, in which a synthetic mRNA encoding for the entire S gene was produced. The novelty of the method brought the scientific attention to the companies. According to the scientific reports, the mRNA-1273 vaccine developed by the Moderna Inc was able to trigger sufficient immune response including IgG nAbs and Th1 based cellular immunity [113]. Furthermore, the BNT162b2 vaccine designed by Pfizer–BioNTech has induced a higher IgG nAbs compared to convalescent plasma samples [100]. The Pfizer–BioNTech further introduced the BNT162b1 product, which was emerged from the RBD subunit of the S protein. The vaccine was able to elicit sufficient Th1 immune response as well as exhibiting high levels of IgG nAbs against the RBD.
The synthetic mRNA encoding the target immunogen is subjected to translation once entered into the recipient cells; subsequently, the antigen triggers an immune response. The stimulation of the immune response is a complicated effect that requires adjuvants including protamines and nanoparticles to augment and hasten the process. Of note, such mRNA vaccines often are supplemented with lipid nanoparticles, serving as both a carrier vector and an adjuvant, boosting the immune response [109], [114]. The mRNA vaccines bear some advantages over the counterpart DNA-based, as the mRNA does not integrate into the host chromosome and further activates the INF-mediated immune response upon administration leading to a halt in the translation process. Moreover, mRNAs are inherently unstable and degrade ensuing translation, improving their safety and efficiency [115].
The nucleic acid vaccines possess a myriad of benefits including economically favorable and cost-effective, easy to manipulate the design and the manufacturing process, high stability at the ambient temperature which considerably facilitates the transportation process, and perhaps above all, they give rise to sufficient and sustainable immune response [102], [109].
5.4. Protein based vaccines
Protein-based vaccines include subunit and virus-like particles (VLP); in the first approach, the key structural proteins such as the S gene of the SARS-CoV-2 which hold a crucial role in the disease etiology, are the main focus. Such vaccines have gained popularity particularly in the case of the COVID 19 outbreak. The recombinant proteins necessitate the usage of an adjuvant to raise the immune response [101]. The second approach entails recombinant production of viral capsids devoid of any genetic material and therefore eliminating the risk of pathogenesis while evoking a strong immune response [116].
Notably, more than 80 scientific research groups have been striving to develop a vaccine based on the subunit and VLP approaches. Reports indicate that either the complete sequence of the S protein or the smaller portion of the protein designated as the RBD region, are of higher immunogenicity importance and therefore, more suitable for vaccine development [117], [118].
5.5. Vaccines and outbreak of new variants
As a RNA virus, one mutation naturally occurs in the genetic material of SARS-CoV-2 every-two weeks. These changes in the genome have not mostly great effect in the structure of main protein. But some mutations may alter the main strain of virus's protein, and then they may alter viral infectivity, transmissibility, and disease severity. As reported by the US Centers for Disease Control and Prevention (CDC), the original virus genome has undergone some deletion, nonsynonymous and synonymous mutations in the spike segment and has created new variants. Predicting these new variants and presented vaccines efficiency is difficult. Based on US-CDC reporting, some vaccines could generate polyclonal antibodies that recognize different epitopes of spike proteins and consequently could respond to new variants. Moreover, even if these vaccines are not completely match or effective to new variants, it can still be enough impact to lessen the severity of the disease. However, there are some concerns about the accumulation of new mutated versions which could evade antibody responses. Furthermore, many countries have not been reported any data about the sequence of virus circulated in their population. Therefore, these are hurdles to control the pandemic using present vaccines. In order to evaluate the effectiveness of available vaccines, it is necessitating to document and characterized any alterations in different COVID-19 sequences in variety of nations [119].
This chart depicts that attention is majorly inclined toward the Protein Subunit Vaccines which encompass 87 of total candidates. Thus far, 4 candidates have registered for the live-attenuated type. According to the WHO reports, following the Protein Subunit Vaccines, RNA based, non-replicating viral vectors, DNA based, replicating viral vectors, inactivated, and virus-like particle are the most popular vaccine candidates against the COVID 19, respectively.
6. Survey signaling pathways
Some studies have been stated that the pathogenesis of COVID-19 is caused by dysregulation of several cell signaling pathways. The mammalian target of rapamycin (mTOR) and the Janus Kinase/Signal Transducers and Activators of Transcription (JAK/STAT) pathway have significant roles among them [120]. For this reason, in recent studies, mTOR and JAK inhibitors have been noticed to reduce the hyper-reactivity of the immune system in patients with COVID-19.
6.1. mTOR inhibition
mTOR, a serine/threonine protein kinase, is highly conserved during evolution and forms two main complexes including mTORC1 and mTORC2 [121]. mTOR has an important role in the proliferation and metabolism of cells and the regulation of immune response and tolerance. Any defects in the mTOR signaling pathway can take part in various pathological circumstances [122], [123], [124]. Recent studies have been demonstrated the role of mTOR in regulating immune system activity to control the severe immune response in patients with COVID-19. One study has expressed that rapamycin as an mTOR inhibition drug, can prevent the cell growth of conventional T cells while it can increase Tregs proliferation and maintain its activity. Thus, in COVID-19 patients, the use of mTOR inhibitors such as rapamycin and everolimus - a second-generation rapamycin derivative that specifically inhibits mTORC1- can alleviate the cytokine storm by reducing the proliferation of conventional T cells and also mitigate the hyperactivity of the immune system in the critical phase of the disease, by maintained Treg growth and activity [124]. A paper also has been stated that elevated levels of mTOR expression are probably facilitated virus replication in coronavirus infection. Therefore, inhibitors of mTOR such as rapamycin and everolimus or specific microRNAs could help to inhibition of virus replication in the human respiratory system [123]. Since approved mTOR inhibitor drugs are available in the market, so, targeting the mTOR pathway may be a promising therapeutic way to combat COVID-19, alleviate symptoms, and reduce mortality rates.
6.2. Janus kinase inhibition
The JAK/STAT signaling pathway is an intracellular signal transduction pathway that is involved in many critical biological processes. Following stimulation of the cytokine receptor, phosphorylation of STAT by JAK leads to dimerization and transfer of STAT into the nucleus. Finally, dimer STAT in the nucleus, regulates the expression of relevant genes [125]. As mentioned before, some COVID-19 patients show cytokine release syndrome in the critical phase of the disease. Because the increased activation of the JAK/STAT pathway can lead to cytokine release syndrome, therefore the use of JAK inhibitors may help treat COVID-19 patients. A recent small pilot series have shown that short-term treatment of COVID-19 patients with hyper inflammation by ruxolitinib (Rux; Jakavi®) - an inhibitor of JAK1 and 2 - is safe and it is efficient to prevent multi-organ failure [126]. In China, a randomized controlled phase II trial which is conducted on patients with severe COVID-19has demonstrated that although there was no statistically significant difference between the ruxolitinib receiving group and the placebo group, nevertheless the ruxolitinib receiving group has shown numerically faster recovery without a significant side effect. Also, this study found that administration of ruxolitinib along with standard-of-care treatment could remarkably release cytokine storm featured in severe COVID-19 [127]. In a case study, three patients with COVID-19, two of whom also had hematologic neoplasms, were examined. In addition to chemotherapy and immunosuppressants for cancer treatment, these patients also received the ruxolitinib to treat COVID-19. The results of the study showed that the ruxolitinib treated the hyperinflammatory state, released the cytokine storm, and had no conflict with viral clearance [128]. Recently, a double-blind, randomized, placebo-controlled trial appraised the combination treatment of baricitinib (a selective inhibitor of JAK 1 and 2) with remdesivir in hospitalized COVID-19 adult patients. Based on the results, the combination treatment in comparison remdesivir treatment alone reduced the recovery time and accelerated the improvement of clinical status in particular among coronavirus patients, who received high-flow oxygen or noninvasive ventilation. It has also shown fewer serious adverse events [129].
7. Plasma therapy
Since the early 20th century, convalescent plasma (CP) therapy has been used as an emergency method in the treatment of patients with viral infections and has had significant positive effects in the treatment of patients with Spanish influenza, SARS, MERS and more recently Ebola [130], [131], [132], [133]. However, there are concerns about the side effects of plasma therapy and the possibility of transmitting autoantibodies or secondary infections to the plasma receptor [134], [135]. These apheresis blood products should be screened for possible infections and blood transfusion protocols before transfusion to patients or other recipients. [136], [137], [138]. The mechanism of plasma therapy is not completely understood [138]. Whole blood plasma from COVID-19 survivors contains nAbs, anti-inflammatory cytokines, coagulation and anticoagulant factors, albumin and other proteins [131]. In addition to stimulating the innate immune system in the recipient, chronic phase seems to increase phagocytosis, activate complement, and neutralize viral spikes, which are involved in virus entry and proliferation [139]. CP has also been reported to be effective in reducing or neutralizing cytokine storms in the recipient, leading to damage to lung tissue [140], [141]. Various studies have been conducted to evaluate the effects of plasma therapy in the prevention and treatment of COVID-19. Some case studies have been reported that plasma therapy is a rapid and reliable way to reduce the severity of disease and prevent disease progression in COVID-19 patients [142], [143], [144]. Although examining the true effects of CP in the treatment and prevention of COVID-19 requires randomized clinical trials, the results of clinical trial studies are different and confusing. Randomized controlled trial does not agree with the positive and definitive effect of plasma therapy on the prevention and control of COVID-19 [145], [146], [147]. Moreover, it seems that confounding factors in different randomized controlled trials have affected the final results of these studies. Of course, a review of randomized clinical trials shows a 57 % reduction in mortality in plasma therapy groups compared to control groups [148]. However, differences in techniques and injected CP dose, sample size, inclusion criteria, and nAbs level raise the question of whether a reliable conclusion can be drawn on the effectiveness of plasma therapy in COVID-19.
Table 2 provides some details and results of clinical trial studies on the effectiveness of CP in the treatment and prevention of COVID-19. The primary and secondary outcomes and the protocols selected are different in randomized controlled trials. Also, some details such as dosage and exact time of CP injection and sometimes NAbs concentration are unknown.
Table 2.
Randomized controlled trials in convalescent plasma COVID-19.
| Authors | Study | Patients Number | Age | Gender | Severity of Disease Intervention Group | Severity of Disease Control Group | Time of Administration | Dosage | Antibodies Concentration | Standard of Care | Primary Outcomes | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R. Libster et al. [149] | randomized, double-blind, placebo-controlled trial | 80 were assigned to receive convalescent plasma and 80 were control | 77.2 ± 8.6 years | 100 patients (62 %) were women | unknown | unknown | Convalescent plasma or placebo was administered less than 72 h after the onset of symptoms | unknown | 1/3200 | no mention | severe respiratory disease developed in 13 of 80 patients (16 %) who received convalescent plasma and in 25 of 80 patients (31 %) who received placebo (relative risk, 0.52; 95 % confidence interval [CI], 0.29 to 0.94; P = 0.03) | Plasma with IgG titers of 1:3200 or higher reduced the risk of severe respiratory disease by 73 %; this exploratory result directly implicates antibodies as the active therapeutic ingredient in convalescent plasma. |
| Ling Lir, et al. [145] | Open-label, multicenter, randomized clinical trial | 52in intervention group and 51 in control group | 70 years | 60 [58.3 %] male | Total 52 14 in WHO-7 21 in WHO-5 15 in WHO-4 2 in WHO-3 |
Total 51 11 in WHO-7 23 in WHO-5 15 in WHO-4 1 in WHO- 3 1excluded |
Unknown schedule of administration | 4–13 mL/kg | Unknow exactly concentration of NAbs. | Antivirals, antibiotics, steroids, human immunoglobulin, Chinese herbal medicines, or interferon | There was no significant difference in 28-day mortality (15.7 % vs 24.0 %; OR, 0.59 [95 % CI, 0.22–1.59]; P = 0.30) or time from randomization to discharge (51.0 % vs 36.0 % discharged by day 28 | Among patients with severe or life-threatening COVID-19, convalescent plasma therapy added to standard treatment, compared with standard treatment alone, did not result in a statistically significant improvement in time to clinical improvement within 28 days. |
| Hassan Abolghasemi, et al. [150] | nonrandomized clinical trial | 115 convalescent plasma treatment group and 74 control group | 54.41 ± 13.71 in plasma group 56.83 ± 14.98 in control group | 67 males (58.3 %) in plasma group, 37 males (50.0 %) in control group. 48 females (41.7 %) in plasma group, 37 females (50.0 %) in control group. |
unknown | unknown | The first 500 cc (one unit) plasma nwas infused within four hours and if the patient did not show any improvement after 24 h, based on the decision of responsible physician, another unit of plasma was administrated. | unknown | unknown | routine antiviral therapy including Lopinavir/Ritonavir, Hydroxychloroquine and an anti-inflammatory agent | Total of 98 (98.2 %) of patients who received convalescent plasma were discharged from hospital which is substantially higher compared to 56 (78.7 %) patients in control group. Length of hospital stay was significantly lower (9.54 days) in convalescent plasma group compared with that of control group (12.88 days). Only 8 patients (7 %) in convalescent plasma group required intubation while this value was 20 % in control group. | convalescent plasma treatment should be considered as a safe and effective therapy for COVID-19 patients. Convalescent plasma therapy substantially improved patients’ survival, significantly reduced hospitalization period and needs for intubation in COVID-19 patients in comparison with control group. |
| Agarwal, et al. [151] | open label, parallel arm, phase II, multicentre, randomised controlled trial | Total 235 in intervention group & 229 in control group | 52 (42–60) in intervention group, 52 (41–60) in control group. | 177 Men (75) in intervention group, 177 Men (77) in control group | unknown | unknown | 200 mL in two doses separated by 24 h | unknown | NAbs 1/20 | Hydroxychloroquine, Remdesivi, Lopinavir/ Ritonavir, Oseltamivir, broad spectrum antibiotics, steroids, Tocilizumab, Heparin,Azithromycin, or Intravenous immunoglobulin | Progression to severe disease: OR: 1.09 (95 % CI, 0.67–1.77) Mortality:OR 1.06 (95 % CI, 0.61–1.83) | Convalescent plasma was not associated with a reduction in progression to severe covid-19 or all cause mortality. |
| Eric Salazar, et al[152] | cohort | total 25 patients | 51 years | 14 were female | unknown | unknown | unknown | unknown | unknown | Most patients received concomitant anti-inflammatory treatments within 5 days of the plasma transfusion, including tocilizumab and steroids. | Of the 25 patients, 9 had improvement by day 7, and an additional 12 patients (for a total of 19) had improvement by day 14. | Outcomes from this case series of 25 patients indicate that administration of convalescent plasma is a safe treatment option for those with severe COVID-19 disease. |
| Ventura A. Simonovich,[153] | double-blind, placebo-controlled, multicenter trial | total of 228 patients were assigned to receive convalescent plasma and 105 to receive placebo | 62.5 (53–72.5) in intervention group, 62 (49–71) in control group. | female: 29/4% in intervention group, 39 % in control group | unknown | unknown | The median time from the onset of Covid-19 symptoms to enrollment was 8 days | unknown | median titer of 1:3200 | Patients were allowed to receive antiviral agents, glucocorticoids, or both according to the standard of care at the provider health care institution. | Overall mortality was 10.96 % in the convalescent plasma group and 11.43 % in the placebo group, for a risk difference of − 0.46 percentage points (95 % CI, −7.8 to 6.8) | No significant differences were observed in clinical status or overall mortality between patients treated with convalescent plasma and those who received placebo. |
| Gharbharan et al[146] | clinical trial | Total 86 (43 cases in each group) |
63 (IQR 56 – 74) years of age | Patients were mostly males | 7 in WHO-3 31 in WHO-4 or 5 8 in WHO-6 or 7 |
1 in WHO- 3 34 in WHO-4 or 5 5 in WHO- 6 or 7 |
2 receive an additional dose in five days | 300 mL single dose. Subjects without improvement of clinical status or persistently positive RT-PCR for SARS CoV-2 |
Unknow exactly concentration of NAbs. Authors argued for an approximately concentration of 1/ 80. IgG 1/640 | Chloroquine, Azithromycin, Lopinavir/Ritonavir, Tocilizumab, or Anakinra | Clinical improvement: OR 1.30 (95 % CI, 0.52–3.32) Mortality: OR 0.95 (95 % CI, 0.20–4.67, P ¼ 0.95) Time to discharge: HR 0.88 (95 % CI, 0.49–1.60, P ¼ 0.68) | |
| Balcells et al.[147] | Open-label, single-center, randomized clinical trial | Total 58(21 in plasma contol and 24 in control group) | 65.8 years | 50 % male | in different stages | in different stages | unknown | 200 mL in two doses separated by 24 h | Nabs 1/160 IgG titers 1/400 |
Steroids, Tocilizumab, Hydroxychloroquine, Lopinavir/Ritonavir, Thromboprophylaxis, or Heparin | Immediate addition of CP therapy in early stages of COVID-19 -compared to its use only in case of patient deterioration-did not confer benefits in mortality, length of hospitalization or mechanical ventilation requirement. |
The US Food and Drug Administration (FDA) has set the minimum concentration of nAbs for CP effectiveness in COVID-19 patients at 1/160 [154]. However, in many studies, this level is much lower than the recommended concentration. The first completed randomized controlled trial conducted in India by Agarwal et al., CP with low NAbs concentration (i.e., 1/20) was transfused to some patients, whereas others received CP containing a higher concentration of antibodies [151]. It seems that the concentration of NAbs can affect the results of studies, although there is no evidence that higher concentration of NAbs improves clinical outcomes. It is suggested that randomized controlled trials use standardized doses of NAbs and different concentrations to better compare treatment regimens in different intervention groups. On the other hand, the time of receiving the first dose of CP and the number of injections can also affect the outputs. Case studies have shown that CP injection in the first 72 h of hospitalization can increase a patient's chances of survival [152]. However, most randomized controlled trials did not pay attention to this data or it was performed at different times in intervention and control groups. Therefore, the design methods of clinical trial studies and the determination of input variables should be reviewed and matched.
One of the factors that complicate the analysis of the effects of CP on COVID-19 is the admission of patients with different degrees of disease severity. It is also very difficult to predict the effect of oxygen intake and the patient's requirement for mechanical ventilation (MCV) on the CP and control groups. For example, 24.3 % of patients in the study of Li et al were on MCV at the moment of the inclusion [145]. Since CP may be helpful in patients with moderate disease or healthy individuals, the choice of patients by severity of disease can be critical [155]. Also, placing all patients with different disease intensities in one group can increase the risk of bias in studies [156]. In addition, the variable volume of samples in different groups and the poor matching in the groups receiving CP, in terms of gender, age and disease severity are among the confounding factors of the study. Thus, further randomized controlled trials should estimate the efficacy of CP in every stage of disease with an appropriate sample size.
Most studies considered mortality and early discharge from the hospital as primary and secondary outcomes in the final conclusion, while variables such as hospitalization in intensive care units (ICU) and patients' requirement for mechanical ventilation were not noticed.
In addition to all of the above, the lack of proper blinding and the presence of multiple confounding variables have led to the fact that existing randomized controlled trials have high bias that make it difficult to judge the true effect of convalescent plasma on improving the condition of COVID-19 patients. Lack of homogeneous plasma therapy regimens, different sample sizes, different primary and secondary data, selection of patients with different disease intensities, possible complications from plasma injection and ethical limitations in selecting control groups all lead to errors in the organized analysis of the clinical trials in this area. Perhaps in the immediate future, with the effectiveness of vaccines and control the current pandemic, it will be possible to design broader and more organized studies on CP therapy in patients with COVID-19.
8. Phage therapy
8.1. Mechanism of COVID-19 entry to cell
Although the invasive mechanisms of the SARS-Cov-2 are still in doubt, based on the many similarities between SARS-Cov-2 and other CoVs, especially SARS-Cov-1 (SARS), it is possible to predict how the virus enters the cell [157], [158]. The new coronavirus, like the SARS-Cov-1, binds to human ACE2 through spike S proteins, allowing the virus to enter its genome and replicate it in host cells [159], [160]. Unlike the SARS-Cov, the new one does not use aminopeptidase N (APN) and dipeptidyl peptidase (DPP) as receptors [161]. Examining ACE2-expressing and non– ACE2-expressing HeLa cells from different animals and human in vitro, Zhou et al found that SARS-Cov-2 infected all ACE2-expressing cell lines except mice, while it could not enter ACE2 non-expressing cells [161].
High expression of ACE2 in various cellular tissues such as respiratory tract, bronchial, kidney, tongue epithelial cells, and heart confirms why these organs play an important role in the pathogenesis of the disease [157]. The virus binds to ACE2 by making use of its spike (S) protein which has two functional domains S1 and S2; S1 contains RBD which interacts with ACE2, and S2 is needed for membrane fusion [162], [163].
X-ray crystallography studies and protein–protein binding assays using surface plasmon resonance has appeared the difference in several amino acids in the N-terminal region of the domain has caused much higher affinity between ACE2 and the SARS-Cov-2 RBD than other similar viruses, which explains the direct relationship between ACE2 expression and disease severity [164], [165]. Not only ACE2 but also the TMPRSS2 suggest that protease expressions are likely to also contribute to disease severity [160], [164]. TMPRSS2 is widely expressed in respiratory epithelial cells and plays an important role in the early activation of CoVs spike S proteins to enter cells [160], [166]. TMPRSS2 mediates S1/S2 cleavage which is required S protein priming to bind ACE2 [166]. Since there is no effective drug in the treatment of COVID-19, it is possible to prevent the progression of the disease by inhibiting or neutralizing any of the effective components in the process of virus entry into host cells.
8.2. Receptor binding domain inhibitor and ACE2 blockers (anti-ACE2 monoclonal antibodies)
The S protein of SARS-CoV-2 serves as an essential component of the virus for cellular attachment, fusion, and viral entry. RBD is located in middle of S1 subunit and directly binds to the peptidase domain (PD) of ACE2 [167]. SARS-CoV-2 RBD has a greater affinity to bind to ACE2 than its cousins, which has led to greater infectivity and consequent greater mortality of the new coronavirus. So, one hypothesis is that if we inhibit RBD with monoclonal antibodies or molecular components before binding to ACE2, we will probably prevent more cells from becoming infected with the virus. Some hypertension drugs such as N-(2-aminoethyl)-1 aziridine-ethanamine, captopril, perindopril, ramipril, lisinopril, benazepril, and moexipril as a novel ACE2 inhibitor might be effective treatment for covid-19 to block the RBD/ACE2 interaction [162], [168].
On the other hand, monoclonal Ab targeted SARS-CoV-2 RBD or ACE2 can be a worthy drug to inhibit RBD/ACE2 interaction. Also, the CR3022 is a SARS-CoV-1 specific human monoclonal antibody to block RBD and the human 47D11 antibody binds to cells expressing the full-length spike proteins of SARS-CoV and SARS-CoV-2 [169], [170]. The 47D11 antibody was found to potently inhibit infection of VeroE6 cells with SARS-S and SARS2-S pseudotyped VSV with IC50 values of 0.061 and 0.061 μg/mL [169]. These antibodies, alone or in combination with other antiviral drugs, can inhibit the binding and fusion of the virus and thus provide good investment options as SARS-CoV-2 drugs.
8.3. Soluble ACE2
One possible hypothesis for the prevention and treatment of COVID-19 is the use of the extracellular portion of ACE2 in solution. The human soluble ACE2 can act as a competitor to the main receptor, which is located on the cell surface, thus binding to virus spikes to neutralize the virus [171]. Human recombinant soluble ACE2 (rhACE2) has showed an inhibitory effect on SARS-CoV-2 infection in vitro condition [172]. Although rhACE2 alone requires a high dose to be effective, studies show that the combination of rhACE2 with other drugs, while increasing the effectiveness of rhACE2 and the drug, also leads to further neutralization of the virus. Vanessa Monteil et al hypothesized that combining two different modalities of anti-viral activity using remdesivir and targeting SARSCoV-2 entry into cells by hrsACE2 might show additive effects. Testing on Vero6, they approved that concomitant use of remdesivir and hrsACE2 significantly reduced the infectivity of the virus [173]. Sun et al. also found that the use of cyclodextrin in combination with hrsACE2 increased its water solubility and could be used as an inhalable drug against SARS-CoV-2 [174]. However, these methods need to be studied in further studies, including clinical trials.
8.4. Host proteases inhibitors
SARS-CoV-2 spike S protein is dependent on host cell proteases as TMPRSS2 and endosomal cysteine proteases cathepsin B and L (CatB/L) for activation and attachment [160], [166].
Stoptek et al. suggested that not only CoVs but also influenza viruses use this primary activation mechanism [166]. These proteases are widely expressed in the airways and bronchial epithelial cells and are also affected by the body's androgen signaling, which results in increased TMPRSS2 expression, which may explain the increased COVID-19 mortality in men [175], [176].Thus, TMPRSS2 inhibitors could have promising effects in inhibiting viral entry. However, CatB/L and TMPRSS2 inhibitors could not totally prohibit SARS-CoV-2 entry in an in vitro study [160]. Other in vitro studies reported MI-432, and MI-1900, and aprotinin (a broad range serine protease inhibitor) attenuate TMPRSS2 protease activity. Moreover, MI-1851, a synthetic furin inhibitor, reduced viral replication significantly. These TMPRSS2 inhibitors are shown more effective result when combined [166], [177], [178]. Although all molecular inhibitors and monoclonal antibodies have shown appropriate inhibitory properties in cell lines and molecular studies to combat the CoVs and can be considered as suitable drug and vaccination options, but there were no clinical trials studies and the lack of knowledge of the side effects of these drugs have created many limitations for use in coronavirus drug regimens.
9. Conventional therapy
Although there are insufficient clinical data to approve the clinical effectiveness of drugs for COVID-19, different studies suggest some agents can serve as potential drug candidates for COVID-19 management (Table 3 ). However, data on the safety and efficacy of these therapies are limited [179].
Table 3.
List of studies on COVID-19 treatment using conventional therapy.
| Country | Study (ClinicalTrials.gov number) | Drug | Patients Number | Dose | Duration of treatment (day) | Aims of study | Main results | Ref. |
|---|---|---|---|---|---|---|---|---|
| France | Observational | HCQ | 84 received HCQ and 89 control | 600 mg/day | 2 | Effectiveness of HCQ in COVID-19 | This study do not HCQ in patients who require oxygen | [215] |
| Italy | Retrospective observational | HCQ | Out of 3,451, 2634 received HCQ | 400 mg/day1 | 10 | HCQ effect on and COVID-19 in-hospital mortality | HCQ was associated with a 30 % lower risk of death | [216] |
| France | Non-randomized clinical trial | HCQ and AZT | 26 received HCQ, 16 controls. Among HCQ group, 6 received AZT | 600 mg HCQ/day AZT (500 mg on day1 followed by 250 mg/day | 5 | HCQ and AZT as a treatment of COVID-19 | HCQ is reduce viral load and its effect is reinforced by AZT | [217] |
| Brazil | Clinical trial (NCT04322123) | HCQ and AZT | 217 received HCQ plus AZT, 221 received HCQ, and 229 control | HCQ 400 mg twice daily AZT 500 mg/day |
7 | Effect of HCQ and AZT | HCQ, alone or with AZT, did not improve clinical status | [218] |
| United States | Clinical trial (NCT04308668) | HCQ | HCQ group (414), placebo group (407) | 800 mg once, followed by 600 mg in 6 to 8 h, then 600 mg/day for 4 days | 5 | HCQ as pos-texposure prophylaxis | HCQ did not prevent illness | [219] |
| United States | Observational study | HCQ | 811 received HCQ, 565 control | 600 mg twice on day 1, then 400 mg/day | 5 | HCQ use would be associated with a lower risk of a composite end point of intubation or death | HCQ was not associated with lowered or an increased risk of the composite end point of intubation or death | [220] |
| Brazil | Clinical Trial (NCT04323527) | CQ | 81 patients were randomized low-dosage group and 41 high-dosage group | high-dosage 600 mg twice daily for 10 days or low-dosage 450 mg twice daily on day 1 and once daily for 4 days | – | Safety and efficacy of CQ | The higher CQ dosage not be recommended | [221] |
| China | Retrospectively | Adjuvant corticosteroid therapy | Out of 244, 151 given adjuvant corticosteroid | hydrocortisone-equivalent dosage 200 [range 100–800] mg/day | Corticosteroids in critically COVID-19 | Corticosteroid not associated with mortality. Increased corticosteroid dosage is significantly associated with elevated mortality risk |
[222] | |
| United Kingdom | Clinical Trial (NCT04381936) | Dexamethasone | Of the 11,303, 9355 received dexamethasone | 6 mg/ day | Median 7 days (3 to 10) | Glucocorticoids reduce respiratory failure and death | Dexamethasone decreased mortality among patient received invasive mechanical ventilation or oxygen alone but not among those receiving no respiratory support | [223] |
| China | Retrospective | Heparin | Out of 449, 99 received heparin (mainly LMWH) | 94 received LMWH (40–60 mg enoxaparin/d) and five received unfractionated heparin (10 000–15 000 U/d), | 7 | Heparin decreased mortality in severe COVID-19 | LMWH is associated with better prognosis in severe COVID-19 | [198] |
| Italy | Retrospective | Fondaparinux vs Enoxaparin | 74 received enoxaparin and 46 received fondaparinux | Enoxaparin (4,000 or 6,000 units/day), fondaparinux (2.5 units/day). | – | Safety and effectiveness of VTE prophylaxis with fondaparinux and enoxaparin in COVID-19 | Fondaparinux is appropriate for VTE prophylaxis of COVID-19. | [224] |
| United States, Europe, Canada, Japan | Cohort | Remdesivir | 61 patients received remdesivir | 200 mg on day 1, followed by 100 mg/day | 10 | Outcomes of severe COVID-19 patients received remdesivir | Clinical improvement in 68 % of patients | [225] |
| China | Clinical Trial (NCT04257656) | Remdesivir | 158 receive remdesivir, 79 receive placebo | 200 mg on day 1 followed by 100 mg /day | 10 | remdesivir in patients with severe COVID-19 | This dose of remdesivir has sufficiently tolerated but had not offer significant antiviral effects in severe COVID-19 | [226] |
| United States, Europe, Asia. | Clinical Trial (NCT04292730) | Remdesivir | 197 received a 10-day course of remdesivir, 199 a 5-day course of remdesivir and 200 received standard care | 200 mg on day 1 followed by 100 mg/day | 5 or 10 | evaluate the efficacy and adverse events of remdesivir administered for 5 or 10 days in COVID-19. | Patients randomized to a 5-day course of remdesivir had a statistically significant difference in clinical status | [211] |
| United States, Denmark, United Kingdom, Greece, Germany, Korea, Mexico, Spain, Japan, Singapore | Clinical Trial (NCT04280705) | Remdesivir | 541 assigned to remdesivir and 521 to placebo | 200 mg on day 1 followed by 100 mg/day | 10 | To evaluate the clinical efficacy and safety of remdesivir in hospitalized adults COVID-19 | Remdesivir was superior to placebo in shortening the recovery time in adults | [227] |
1: In one center it has used at the dose of 600 mg/day and in another at the dose of 600 mg/day but only in patients younger than 65 years.
Abbrevation: AZT: Azetromycin, CQ: Chloroquine, HCQ: Hydroxychloroquine, LMWH: low molecular weight heparin, VTE: Venous thromboembolism.
9.1. Chloroquine and hydroxychloroquine
Chloroquine and hydroxychloroquine (HCQ) have been shown to have anti-viral effects [180]. The worldwide, attention to CHQ as a promising drug for COVID-19 was made by studies that suggested it antiviral activity at both arrivals and at post-arrival stages of COVID-19. According to the previous studies, these effects might include inhibiting terminal phosphorylation and glycosylation of ACE2, enhanced the pH of the endosome, inhibiting replication of SARS-CoV-2, and reducing induction of cytokines [180], [181], [182].
HCQ has been studied more than chloroquine, probably due to its lower toxicity and higher antiviral efficacy for COVID-19. These parameters along with, oral administration, cheaper cost, and safety history in cure of malaria and systemic lupus erythematosus [183], [184] led to the approval of HCQ for COVID-19 and On March 28, 2020, the FDA issued an Emergency Use permission for HCQ e to cure adults hospitalized with COVID-19 [185].
A recent large cohort study conducted in 33 hospitals and clinical centers in Italy with 3,451 patients with confirmed SARS-CoV-2 infection demonstrated that treatment with HCQ was associated with a significant better survival [186]. Moreover, an open-label non-randomized clinical trial has asserted that HCQ (with or without azithromycin) is useful in treating COVID-19 [187]. In recently, there has been an increasing amount of literature on the lack of effectiveness of these drugs [39], [73], [184], [188], [189], [190].
A blinded, placebo-controlled randomized trial of low dose HCQ n patients with COVID-19 in 34 hospitals in the US, reported no difference clinical status between groups treated with, compared with placebo [73]. Another randomized double-blind, placebo-controlled clinical trial of high dose HCQ in high-risk or moderate-risk exposure patients with COVID-19, showed HCQ had no prophylaxis effect in the incidence of new COVID-19 infection [189]. Moreover, an observational controlled study of patients with COVID-19 pneumonia in French, reported that treatment at 600 mg/day of HCQ was not associated with a reduction of admissions to the ICU or survival at 21 days after hospital admission compared with standard care eight of 84 patients receiving hydroxychloroquine had the drug stopped due to electrocardiogram changes [184]. QT corrected for heart rate (QTc) prolongation in patients treated with HCQ was reported that in some cases led to stop the remedy [184], [188], [191]. Furthermore, increasing liver-enzyme levels, headache, dizziness, nausea, vomiting, and stomach pain, loss of appetite, skin rash were other side effect of HQC [188].
The evidence presented to date suggested this drug did not have much effect at different times from the onset of the COVID-19 and in different doses, and due to the possible side effects of its, researchers claim that HCQ was not useful, maybe even adverse. Finally, the FDA revoked the permission for HCQe to cure adults hospitalized with COVID-19 on June 15, 2020.
9.2. Glucocorticoids
Corticosteroids have been utilized to prevent excessive lung damage and respiratory distress during the SARS and MERS outbreak [192]. It was considered that cytokine storm in the second week of disease would extend COVID-19 pneumonia and respiratory symptoms [42]. Therefore, anti-inflammatory drugs such as corticosteroids have been raised as a promising target for the cure of COVID-19.
The results of the some observational study reported that corticosteroid prescribe did not considerable effect on symptoms duration, term of hospitalization and virus clearance in COVID- 19 patients [42]. In this regard, other studies contribute additional evidence that suggests the corticosteroids probably associated with elevated ICU admission, mortality risk, and prolonged viral clearance [46], [193], [194]. However, with a small sample size, caution must be applied, as the finding not be transferable to public. Currently, the evidence from the controlled, open-label study was suggested that dexamethasone (6 mg given once daily for ten days) attenuated the mortality in hospitalized patients that receiving respiratory support (mechanical ventilation or oxygen), while, in COVID-19 patients with a mild condition and receiving no oxygen support, the drug was not effect [195]. However, corticosteroids act as a double-edged sword in complication of COVID-19 due to its immunomodulation and immunosuppression effect that can elevated risk of secondary infections [182]. According to the controversial results and adverse effects of corticosteroid, for use of this drug in the remedy of COVID-19 must be careful in assign the dose, timing, and duration of prescribing. It is recommended that glucocorticoids are being prescribed for a limited time (3–5 days), with a dosage not more than the equivalent of methylprednisolone 1–2 mg/kg/day for severe COVID-19 patients. Given the immunomodulatory effects of corticosteroid, the upper dose can delay the removal of the virus for a long time [196]. WHO permitted the consumption of dexamethasone in severe to critical conditions only, not be routinely [197]. Finally, corticosteroid therapy in COVID-19 patients may be case-based and the drug can be used for the correct patient at the right time.
9.3. Anticoagulant (Heparin, Enoxaparin, Fondaparinux)
Thromboinflammation or COVID-19-associated coagulopathy is a condition that inflammatory cytokines, stimulate the coagulation system and counteract anticoagulant mechanisms, result in defective coagulation activity, small-vessel vasculitis, and wide microthrombosis [198]. Therefore, it was suggested consumption of anticoagulation discounted these complications.
Primitive data proposes that heparin and enoxaparin revealed promising effect and can instate novel therapeutic chance in the cure of COVID-19 [198], [199]. The WHO approved the utilization of heparin, as a prophylaxis drug for venous thromboembolism (VTE) in COVID-19 patients [200].
A retrospective study reported that in the heparin group, the incidence of death was lower than in the non-heparin group, (40.0 % vs 64.2 %). although, not statistically significant. Moreover, d-dimer, prothrombin time, and age were directly, and platelet count was inverse, associate with 28-day mortality in multivariate analysis [198]. The association between heparin and survival is agree with the findings of an observational study where among the 1734 Spanish patients who received heparin and mortality was significantly lower [201]. Another recent observational cohort study from Italy examined the effect of enoxaparin with the median dose of 40 (40–80 mg) per day and in 6 (3–9) days duration of therapy. Enoxaparin therapy was correlated with improvement of COVID-19 prognosis in patients, and reduced in-hospital mortality, and risk of intensive care admission [199]. Furthermore, according to Russo, fondaparinux has a greater net clinical advantage compared with enoxaparin with a numerically lower number of both ARDS and death events. The preliminary evidence of this study demonstrates, safety and effectiveness of fondaparinux for VTE prophylaxis in hospitalized COVID-19 patients. [202]. Of note, larger multicenter prospective studies specially randomized controlled trial) are needed to confirm these finding.
9.4. Remdesivir
Remdesivir (RDV) is a broad-spectrum antiviral drug that inhibits viral RNA polymerases. It was shown, by a competitive inhibition, incorporated into the generating RNA strand instead of triphosphate (ATP) and caused preventing viral replication [203], [204]. According to, safety and clinical efficacy of RDV against Ebola, and RNA virus families (SARS-CoV and MERS-CoV) infection, it may become a suitable drug target for the treatment of COVID-19 [205]. In the same vein, in vitro efficacy of RDV against COVID-19 infection has been tested and suggested remdesivir as an extremely effective agent against COVID-19 infection [206]. Intravenous administration of RDV r was performed in the first case of COVID-19 in the US on day 7 of diseases, and improvement was observed followed by discontinuation of supplemental oxygen and improvement in oxygen saturation [207].
A study based on results from 53 patients who received RDV during 10-day, showed a clinical improvement in 68 % of patients, by significant betterment oxygen-support status, reduction in the need for mechanical ventilation and a reduction in mortality in those [208]. However, in a recent placebo controlled randomized trial of RDV in patients with severe COVID-19, reported that intravenous RDV use was not associated with a significant clinical improvement, mortality or time to clearance of virus [209]. Notwithstanding, patients treatment with RDV had a numerically faster time to clinical improvement than those receiving placebo [210]. In the open-label, randomized study of remdesivir in hospitalized patients with moderate-severity COVID-19, patients who received RDV for 5 days had more favorable clinical outcome than those receiving standard care. This efficacy was not seen with the 10-day course [211]. A without placebo randomized phase 3 clinical trial evaluated the efficacy and safety of treatment with remdesivir for 5 or 10 days in patients with severe COVID-19. Two- hundred patients were assigned to receive a 5-day course of remdesivir and 197 a 10-day course (200 mg of RDV on day 1 and 100 mg once daily on subsequent days). There were no significant differences in efficacy of RDW between two groups [212]. Moreover, in another, randomized double-blind, placebo-controlled trials, was reported RDV effect was superior in shortening the time to recovery than placebo (11 days versus 15 days) [213]. In accordance with the experimental evidence on RDV, the FDA issued an Emergency Use Authorization on May 1, 2020 (modified on August 28, 2020), to permit the use of RDV for treatment in adults and children hospitalized with suspected, or laboratory confirmed COVID-19. RDV can cause GI symptoms (e.g., nausea, vomiting, and constipation), elevated transaminases and renal failure, and prothrombin time elevation [208], [212], [214] that possible side‐effects of treatment with RDV are valuable for further research.
10. Conclusion
Clinical result about the safety and efficacy of agent therapy used remains scanty and the search for a magic drug against COVID-19 continues. To date, protocols developed and studies have been done have included the utilization of chloroquine and HCQ with negative results, glucocorticoids with promising results in case-based, anticoagulant with promising results, and RDV with promising results. Taken together, treatment with one drug alone is not likely to be sufficient for all patients, and a variety of therapeutic approaches and combination of drugs and approaches are needed to evaluating, and identifying safe and effective therapy against COVID-19.
CRediT authorship contribution statement
Moein Shirzad: Writing – original draft. Marjan Nourigorji: Writing – original draft. Atefe Sajedi: Writing – original draft. Maryam Ranjbar: . Faeze Rasti: . Zahra Sourani: Writing – review & editing. Mona Moradi: Writing – review & editing. Seyed Mostafa Mir: . Mohammad Yousef Memar: .
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to Golestan and Tabriz University of Medical Sciences, for providing all kinds of facilities to prepare this manuscript. The Ethics Committee of Golestan University of Medical Sciences (IR.GOUMS.REC.1401.174) approved this study.
Data availability
No data was used for the research described in the article.
References
- 1.Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N., Diao K., Lin B., Zhu X., Li K. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A., Dai J., Sun Q., Zhao F., Qu J. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): A multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 2020 doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J., Wu X., Zeng W., Guo D., Fang Z., Chen L., Huang H., Li C. Chest CT findings in patients with coronavirus disease 2019 and its relationship with clinical features. Invest. Radiol. 2020;55(5):257. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.K. Dhama, K. Sharun, R. Tiwari, S. Sircar, S. Bhat, Y.S. Malik, K.P. Singh, W. Chaicumpa, D.K. Bonilla-Aldana, A.J. Rodriguez-Morales, Coronavirus disease 2019–COVID-19, 2020. [DOI] [PMC free article] [PubMed]
- 5.Peiris J., Lai S., Poon L., Guan Y., Yam L., Lim W., Nicholls J., Yee W., Yan W., Cheung M. Coronavirus as a possible cause of severe acute respiratory syndrome. The Lancet. 2003;361(9366):1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukhtar F., Mukhtar N. Coronavirus (COVID-19): Let’s Prevent Not Panic. Journal of Ayub Medical College Abbottabad. 2020;32(1):141–144. [PubMed] [Google Scholar]
- 8.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 9.X.-W. Xu, X.-X. Wu, X.-G. Jiang, K.-J. Xu, L.-J. Ying, C.-L. Ma, S.-B. Li, H.-Y. Wang, S. Zhang, H.-N. Gao, Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series, bmj 368 (2020). [DOI] [PMC free article] [PubMed]
- 10.A.R. Fehr, S. Perlman, Coronaviruses: an overview of their replication and pathogenesis, Coronaviruses, Springer, 2015, pp. 1–23. [DOI] [PMC free article] [PubMed]
- 11.Chan J.-F.-W., Kok K.-H., Zhu Z., Chu H., To K.-K.-W., Yuan S., Yuen K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D. Brian, R. Baric, Coronavirus genome structure and replication, Coronavirus replication and reverse genetics, Springer, 2005, pp. 1-30. [DOI] [PMC free article] [PubMed]
- 13.Sahin A.R., Erdogan A., Agaoglu P.M., Dineri Y., Cakirci A.Y., Senel M.E., Okyay R.A., Tasdogan A.M. 2019 novel coronavirus (COVID-19) outbreak: a review of the current literature. EJMO. 2020;4(1):1–7. [Google Scholar]
- 14.Kirchdoerfer R.N., Cottrell C.A., Wang N., Pallesen J., Yassine H.M., Turner H.L., Corbett K.S., Graham B.S., McLellan J.S., Ward A.B. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531(7592):118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., Zhong W., Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson B.S., Laloraya M. A cytokine super cyclone in COVID-19 patients with risk factors: the therapeutic potential of BCG immunization. Cytokine Growth Factor Rev. 2020 doi: 10.1016/j.cytogfr.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L., Mao Y., Chen G. Risk factors for 2019 novel coronavirus disease (COVID-19) patients progressing to critical illness: a systematic review and meta-analysis. Aging (Albany NY) 2020;12(12):12410. doi: 10.18632/aging.103383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mock U., Machowicz R., Hauber I., Horn S., Abramowski P., Berdien B., Hauber J., Fehse B. 565. CCR5-Uco-TALEN–A Novel Transcription Activator-Like Effector Nuclease That Mediates High-Efficiency Knockout of HIV Co-Receptor CCR5 in Primary T Cells After mRNA Transfection. Mol. Ther. 2015;23:S225–S226. [Google Scholar]
- 19.Brunger J.M., Zutshi A., Willard V.P., Gersbach C.A., Guilak F. CRISPR/Cas9 editing of murine induced pluripotent stem cells for engineering inflammation-resistant tissues. Arthritis Rheumatol. 2017;69(5):1111–1121. doi: 10.1002/art.39982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aquino-Jarquin G. Recent progress on rapid SARS-CoV-2/COVID-19 detection by CRISPR-Cas13-based platforms. Drug Discovery Today. 2021;26(8):2025–2035. doi: 10.1016/j.drudis.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin X., Liu Y., Chemparathy A., Pande T., La Russa M., Qi L.S. A comprehensive analysis and resource to use CRISPR-Cas13 for broad-spectrum targeting of RNA viruses. Cell Reports Med. 2021;2(4):100245. doi: 10.1016/j.xcrm.2021.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinoda H., Taguchi Y., Nakagawa R., Makino A., Okazaki S., Nakano M., Muramoto Y., Takahashi C., Takahashi I., Ando J. Amplification-free RNA detection with CRISPR–Cas13. Commun. Biol. 2021;4(1):1–7. doi: 10.1038/s42003-021-02001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nalawansha D.A., Samarasinghe K.T.G. Double-Barreled CRISPR Technology as a Novel Treatment Strategy For COVID-19. ACS Pharmacol. Transl. Sci. 2020;3(5):790–800. doi: 10.1021/acsptsci.0c00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbaszadeh-Goudarzi K., Nematollahi M.H., Khanbabaei H., Nave H.H., Mirzaei H.R., Pourghadamyari H., Sahebkar A. Targeted delivery of CRISPR/Cas13 as a promising therapeutic approach to treat SARS-CoV-2. Curr. Pharm. Biotechnol. 2021;22(9):1149–1155. doi: 10.2174/1389201021666201009154517. [DOI] [PubMed] [Google Scholar]
- 25.Mahas A., Wang Q., Marsic T., Mahfouz M.M. A Novel Miniature CRISPR-Cas13 System for SARS-CoV-2 Diagnostics. ACS Synth. Biol. 2021;10(10):2541–2551. doi: 10.1021/acssynbio.1c00181. [DOI] [PubMed] [Google Scholar]
- 26.Lipsitz Y.Y., Timmins N.E., Zandstra P.W. Quality cell therapy manufacturing by design. Nat. Biotechnol. 2016;34(4):393–400. doi: 10.1038/nbt.3525. [DOI] [PubMed] [Google Scholar]
- 27.N. Sher, R. Ofir, Clinical Development and Commercialization of Placenta-Derived Cell Therapy, Perinatal Stem Cells, Elsevier, 2018, pp. 357-375.
- 28.S.I. Savitz, K. Parsha, Enhancing Stroke Recovery with Cellular Therapies, Stroke, Elsevier, 2016, pp. 981-991.
- 29.Biehl J.K., Russell B. Introduction to stem cell therapy. J. Cardiovasc. Nursing. 2009;24(2):98. doi: 10.1097/JCN.0b013e318197a6a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen R., Li L., Feng L., Luo Y., Xu M., Leong K.W., Yao R. Biomaterial-assisted scalable cell production for cell therapy. Biomaterials. 2020;230:119627. doi: 10.1016/j.biomaterials.2019.119627. [DOI] [PubMed] [Google Scholar]
- 31.R. Champlin, Selection of autologous or allogeneic transplantation, Cancer Medicine. 6th. BC Decker, 2003.
- 32.Yamasaki S., Mera H., Itokazu M., Hashimoto Y., Wakitani S. Cartilage repair with autologous bone marrow mesenchymal stem cell transplantation: review of preclinical and clinical studies. Cartilage. 2014;5(4):196–202. doi: 10.1177/1947603514534681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zakrzewski W., Dobrzyński M., Szymonowicz M., Rybak Z. Stem cells: past, present, and future. Stem Cell Res. Ther. 2019;10(1):1–22. doi: 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nautiyal M. A Review on the Discovery of Different Stem Cells and the Potential Therapies based on these Cells. Int. J. Health Biol. Sci. 2019;2(4):06–12. [Google Scholar]
- 35.Fleifel D., Rahmoon M.A., AlOkda A., Nasr M., Elserafy M., El-Khamisy S.F. Recent advances in stem cells therapy: A focus on cancer, Parkinson’s and Alzheimer’s. J. Genet. Eng. Biotechnol. 2018;16(2):427–432. doi: 10.1016/j.jgeb.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brignier A.C., Gewirtz A.M. Embryonic and adult stem cell therapy. J. Allergy Clin. Immunol. 2010;125(2):S336–S344. doi: 10.1016/j.jaci.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 37.Shetty A.K. Mesenchymal stem cell infusion shows promise for combating coronavirus (COVID-19)-induced pneumonia. Aging Dis. 2020;11(2):462. doi: 10.14336/AD.2020.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lukomska B., Stanaszek L., Zuba-Surma E., Legosz P., Sarzynska S., Drela K. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. 2019;2019 doi: 10.1155/2019/9628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Group R.C., Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R., Wiselka M., Ustianowski A., Elmahi E., Prudon B., Whitehouse T., Felton T., Williams J., Faccenda J., Underwood J., Baillie J.K., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Lim W.S., Montgomery A., Rowan K., Tarning J., Watson J.A., White N.J., Juszczak E., Haynes R., Landray M.J. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl. J. Med. 2020;383(21):2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kavianpour M., Saleh M., Verdi J. The role of mesenchymal stromal cells in immune modulation of COVID-19: focus on cytokine storm. Stem Cell Res. Ther. 2020;11(1):1–19. doi: 10.1186/s13287-020-01849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respiratory Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang L., Jiang Y., Zhu M., Chen L., Zhou X., Zhou C., Ye P., Chen X., Wang B., Xu Z. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front. Med. 2020;14(5):664–673. doi: 10.1007/s11684-020-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao R.C. Stem Cell-Based Therapy for Coronavirus Disease 2019. Stem Cells Dev. 2020 doi: 10.1089/scd.2020.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang B., Chen J., Li T., Wu H., Yang W., Li Y., Li J., Yu C., Nie F., Ma Z. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine. 2020;99(31) doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., Shi J., Zhou M., Wu B., Yang Z. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meng F., Xu R., Wang S., Xu Z., Zhang C., Li Y., Yang T., Shi L., Fu J., Jiang T. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduction Targeted Therapy. 2020;5(1):1–7. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salamanna F., Maglio M., Landini M.P., Fini M. Body localization of ACE-2: On the trail of the keyhole of SARS-CoV-2. Front. Med. 2020;7:935. doi: 10.3389/fmed.2020.594495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Avanzini M.A., Mura M., Percivalle E., Bastaroli F., Croce S., Valsecchi C., Lenta E., Nykjaer G., Cassaniti I., Bagnarino J. Human mesenchymal stromal cells do not express ACE2 and TMPRSS2 and are not permissive to SARS-CoV-2 infection. Stem Cells Transl. Med. 2021;10(4):636–642. doi: 10.1002/sctm.20-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao Y., Wu H., Zhai W., Wang Y., Li M., Li M., Yang L., Tian Y., Song Y., Li J. A safety consideration of mesenchymal stem cell therapy on COVID-19. Stem Cell Res. 2020;49:102066. doi: 10.1016/j.scr.2020.102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong R., Zhao H., Wang Y., Chen Y., Cai H., Hu Y., Wei G., Huang H. Clinical characterization and risk factors associated with cytokine release syndrome induced by COVID-19 and chimeric antigen receptor T-cell therapy. Bone Marrow Transplant. 2020:1–11. doi: 10.1038/s41409-020-01060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golchin A. Cell-based therapy for severe COVID-19 patients: clinical trials and cost-utility. Stem Cell Rev. Reports. 2020:1–7. doi: 10.1007/s12015-020-10046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yadav P., Vats R., Bano A., Bhardwaj R. Mesenchymal stem cell immunomodulation and regeneration therapeutics as an ameliorative approach for COVID-19 pandemics. Life Sci. 2020:118588. doi: 10.1016/j.lfs.2020.118588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Eeden C., Khan L., Osman M.S., Cohen Tervaert J.W. Natural killer cell dysfunction and its role in COVID-19. Int. J. Mol. Sci. 2020;21(17):6351. doi: 10.3390/ijms21176351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Market M., Angka L., Martel A.B., Bastin D., Olanubi O., Tennakoon G., Boucher D.M., Ng J., Ardolino M., Auer R.C. Flattening the COVID-19 curve with natural killer cell based immunotherapies. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.AminJafari A., Ghasemi S. The possible of immunotherapy for COVID-19: A systematic review. Int. Immunopharmacol. 2020:106455. doi: 10.1016/j.intimp.2020.106455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Finelli C. Obesity, COVID-19 and immunotherapy: the complex relationship! Future Med. 2020 doi: 10.2217/imt-2020-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esmaeilzadeh A., Elahi R. Immunobiology and immunotherapy of COVID-19: A clinically updated overview. J. Cell. Physiol. 2020 doi: 10.1002/jcp.30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y., Chen M., Cao H., Zhu Y., Zheng J., Zhou H. Extraordinary GU-rich single-strand RNA identified from SARS coronavirus contributes an excessive innate immune response. Microbes Infect. 2013;15(2):88–95. doi: 10.1016/j.micinf.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blanco-Melo D., Nilsson-Payant B.E., Liu W.-C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020 doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.P. Mosaddeghi, M. Negahdaripour, Z. Dehghani, M. Farahmandnejad, M. Moghadami, N. Nezafat, S.M. Masoompour, Therapeutic approaches for COVID-19 based on the dynamics of interferon-mediated immune responses, 2020.
- 65.Hung I.-F.-N., Lung K.-C., Tso E.-Y.-K., Liu R., Chung T.-W.-H., Chu M.-Y., Ng Y.-Y., Lo J., Chan J., Tam A.R. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. The Lancet. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Negahdaripour M. The rise and fall in therapeutic candidates for COVID-19. Iranian J. Med. Sci. 2020;45(4):231. doi: 10.30476/ijms.2020.46689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.E. Sallard, F.-X. Lescure, Y. Yazdanpanah, F. Mentre, N. Peiffer-Smadja, A. Florence, Y. Yazdanpanah, F. Mentre, F.-X. Lescure, N. Peiffer-Smadja, Type 1 interferons as a potential treatment against COVID-19, Antiviral Res. (2020) 104791. [DOI] [PMC free article] [PubMed]
- 68.Phillips S., Mistry S., Riva A., Cooksley H., Hadzhiolova-Lebeau T., Plavova S., Katzarov K., Simonova M., Zeuzem S., Woffendin C. Peg-interferon lambda treatment induces robust innate and adaptive immunity in chronic hepatitis B patients. Front. Immunol. 2017;8:621. doi: 10.3389/fimmu.2017.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ziegler C.G., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020 doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dastan F., Nadji S.A., Saffaei A., Marjani M., Moniri A., Jamaati H., Hashemian S.M., Shiva P.B., Abedini A., Varahram M. Subcutaneous administration of Interferon beta-1a for COVID-19: A non-controlled prospective trial. Int. Immunopharmacol. 2020:106688. doi: 10.1016/j.intimp.2020.106688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jalkanen J., Hollmén M., Jalkanen S. Interferon beta-1a for COVID-19: critical importance of the administration route. Crit. Care. 2020;24(1):1–3. doi: 10.1186/s13054-020-03048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020:1–12. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Self W.H., Semler M.W., Leither L.M., Casey J.D., Angus D.C., Brower R.G., Chang S.Y., Collins S.P., Eppensteiner J.C., Filbin M.R., Files D.C., Gibbs K.W., Ginde A.A., Gong M.N., Harrell F.E., Jr., Hayden D.L., Hough C.L., Johnson N.J., Khan A., Lindsell C.J., Matthay M.A., Moss M., Park P.K., Rice T.W., Robinson B.R.H., Schoenfeld D.A., Shapiro N.I., Steingrub J.S., Ulysse C.A., Weissman A., Yealy D.M., Thompson B.T., Brown S.M., National Heart L., Blood Institute P.C.T.N., Steingrub J., Smithline H., Tiru B., Tidswell M., Kozikowski L., Thornton-Thompson S., De Souza L., Hou P., Baron R., Massaro A., Aisiku I., Fredenburgh L., Seethala R., Johnsky L., Riker R., Seder D., May T., Baumann M., Eldridge A., Lord C., Shapiro N., Talmor D., O'Mara T., Kirk C., Harrison K., Kurt L., Schermerhorn M., Banner-Goodspeed V., Boyle K., Dubosh N., Filbin M., Hibbert K., Parry B., Lavin-Parsons K., Pulido N., Lilley B., Lodenstein C., Margolin J., Brait K., Jones A., Galbraith J., Peacock R., Nandi U., Wachs T., Matthay M., Liu K., Kangelaris K., Wang R., Calfee C., Yee K., Hendey G., Chang S., Lim G., Qadir N., Tam A., Beutler R., Levitt J., Wilson J., Rogers A., Vojnik R., Roque J., Albertson T., Chenoweth J., Adams J., Pearson S., Juarez M., Almasri E., Fayed M., Hughes A., Hillard S., Huebinger R., Wang H., Vidales E., Patel B., Ginde A., Moss M., Baduashvili A., McKeehan J., Finck L., Higgins C., Howell M., Douglas I., Haukoos J., Hiller T., Lyle C., Cupelo A., Caruso E., Camacho C., Gravitz S., Finigan J., Griesmer C., Park P., Hyzy R., Nelson K., McDonough K., Olbrich N., Williams M., Kapoor R., Nash J., Willig M., Ford H., Gardner-Gray J., Ramesh M., Moses M., Ng Gong M., Aboodi M., Asghar A., Amosu O., Torres M., Kaur S., Chen J.T., Hope A., Lopez B., Rosales K., Young You J., Mosier J., Hypes C., Natt B., Borg B., Salvagio Campbell E., Hite R.D., Hudock K., Cresie A., Alhasan F., Gomez-Arroyo J., Duggal A., Mehkri O., Hastings A., Sahoo D., Abi Fadel F., Gole S., Shaner V., Wimer A., Meli Y., King A., Terndrup T., Exline M., Pannu S., Robart E., Karow S., Hough C., Robinson B., Johnson N., Henning D., Campo M., Gundel S., Seghal S., Katsandres S., Dean S., Khan A., Krol O., Jouzestani M., Huynh P., Weissman A., Yealy D., Scholl D., Adams P., McVerry B., Huang D., Angus D., Schooler J., Moore S., Files C., Miller C., Gibbs K., LaRose M., Flores L., Koehler L., Morse C., Sanders J., Langford C., Nanney K., MdalaGausi M., Yeboah P., Morris P., Sturgill J., Seif S., Cassity E., Dhar S., de Wit M., Mason J., Goodwin A., Hall G., Grady A., Chamberlain A., Brown S., Bledsoe J., Leither L., Peltan I., Starr N., Fergus M., Aston V., Montgomery Q., Smith R., Merrill M., Brown K., Armbruster B., Harris E., Middleton E., Paine R., Johnson S., Barrios M., Eppensteiner J., Limkakeng A., McGowan L., Porter T., Bouffler A., Leahy J.C., deBoisblanc B., Lammi M., Happel K., Lauto P., Self W., Casey J., Semler M., Collins S., Harrell F., Lindsell C., Rice T., Stubblefield W., Gray C., Johnson J., Roth M., Hays M., Torr D., Zakaria A., Schoenfeld D., Thompson T., Hayden D., Ringwood N., Oldmixon C., Ulysse C., Morse R., Muzikansky A., Fitzgerald L., Whitaker S., Lagakos A., Brower R., Reineck L., Aggarwal N., Bienstock K., Freemer M., Maclawiw M., Weinmann G., Morrison L., Gillespie M., Kryscio R., Brodie D., Zareba W., Rompalo A., Boeckh M., Parsons P., Christie J., Hall J., Horton N., Zoloth L., Dickert N., Diercks D. Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19: A Randomized Clinical Trial. JAMA. 2020;324(21):2165–2176. doi: 10.1001/jama.2020.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y., Ying T. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 2020;9(1):382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou G., Zhao Q. Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2. Int. J. Biol. Sci. 2020;16(10):1718. doi: 10.7150/ijbs.45123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhaskar S., Sinha A., Banach M., Mittoo S., Weissert R., Kass J.S., Rajagopal S., Pai A.R., Kutty S. Cytokine storm in COVID-19—immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front. Immunol. 2020;11:1648. doi: 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.R. Davies, E. Choy, Clinical experience of IL-6 blockade in rheumatic diseases—implications on IL-6 biology and disease pathogenesis, Seminars in immunology, Elsevier, 2014, pp. 97-104. [DOI] [PubMed]
- 81.Wolf J., Rose-John S., Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70(1):11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 82.Jones S.A., Scheller J., Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J. Clin. Investig. 2011;121(9):3375–3383. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaly L., Rosner I. Tocilizumab–A novel therapy for non-organ-specific autoimmune diseases. Best Pract. Res. Clin. Rheumatol. 2012;26(1):157–165. doi: 10.1016/j.berh.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 84.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2019;2020:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.N. Wadud, N. Ahmed, M.M. Shergil, M. Khan, M.G. Krishna, A. Gilani, S. El Zarif, J. Galaydick, K. Linga, S. Koor, Improved survival outcome in SARs-CoV-2 (COVID-19) Acute Respiratory Distress Syndrome patients with Tocilizumab administration, medRxiv (2020).
- 86.E.J. Giamarellos-Bourboulis, M.G. Netea, N. Rovina, K. Akinosoglou, A. Antoniadou, N. Antonakos, G. Damoraki, T. Gkavogianni, M.-E. Adami, P. Katsaounou, Complex immune dysregulation in COVID-19 patients with severe respiratory failure, Cell Host & Microbe 2020. [DOI] [PMC free article] [PubMed]
- 87.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: A single center experience. J. Med. Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guo C., Li B., Ma H., Wang X., Cai P., Yu Q., Zhu L., Jin L., Jiang C., Fang J. Tocilizumab treatment in severe COVID-19 patients attenuates the inflammatory storm incited by monocyte centric immune interactions revealed by single-cell analysis. BioRxiv. 2020 [Google Scholar]
- 89.Cellina M., Orsi M., Bombaci F., Sala M., Marino P., Oliva G. Favorable changes of CT findings in a patient with COVID-19 pneumonia after treatment with tocilizumab. Diagn. Interventional Imaging. 2020;101(5):323. doi: 10.1016/j.diii.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Di Giambenedetto S., Ciccullo A., Borghetti A., Gambassi G., Landi F., Visconti E., Zileri Dal Verme L., Bernabei R., Tamburrini E., Cauda R. Off-label use of tocilizumab in patients with SARS-CoV-2 infection. J. Med. Virol. 2020 doi: 10.1002/jmv.25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sciascia S., Aprà F., Baffa A., Baldovino S., Boaro D., Boero R., Bonora S., Calcagno A., Cecchi I., Cinnirella G. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in severe patients with COVID-19. Clin. Exp. Rheumatol. 2020;38(3):529–532. [PubMed] [Google Scholar]
- 92.Klopfenstein T., Zayet S., Lohse A., Balblanc J.-C., Badie J., Royer P.-Y., Toko L., Mezher C., Bossert M., Bozgan A.-M. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Médecine et Maladies Infectieuses. 2020 doi: 10.1016/j.medmal.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.M. Roumier, R. Paule, G. Matthieu, A. Vallée, F. ACKERMANN, Interleukin-6 blockade for severe COVID-19, medrxiv (2020).
- 94.Alattar R., Ibrahim T.B., Shaar S.H., Abdalla S., Shukri K., Daghfal J.N., Khatib M.Y., Aboukamar M., Abukhattab M., Alsoub H.A. Tocilizumab for the Treatment of Severe COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.P. Mehta, D.F. McAuley, M. Brown, E. Sanchez, R.S. Tattersall, J.J. Manson, H.A.S. Collaboration, COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet (London, England) 395(10229) (2020) 1033. [DOI] [PMC free article] [PubMed]
- 96.Pinto D., Park Y.-J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020:1–6. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 97.Owji H., Negahdaripour M., Hajighahramani N. Immunotherapeutic approaches to curtail COVID-19. Int. Immunopharmacol. 2020:106924. doi: 10.1016/j.intimp.2020.106924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rehman M., Tauseef I., Aalia B., Shah S.H., Junaid M., Haleem K.S. Therapeutic and vaccine strategies against SARS-CoV-2: past, present and future. Future Virol. 2020;15(7):471–482. [Google Scholar]
- 99.Lee S., Nguyen M.T. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw. 2015;15(2):51–57. doi: 10.4110/in.2015.15.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dai L., Gao G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaur S.P., Gupta V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020;288:198114. doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haque A., Pant A.B. Efforts at COVID-19 Vaccine Development: Challenges and Successes. Vaccines. 2020;8(4):739. doi: 10.3390/vaccines8040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Durante M., Schulze K., Incerti S., Francis Z., Zein S., Guzmán C.A. Virus Irradiation and COVID-19 Disease. Front. Phys. 2020;8(415) [Google Scholar]
- 104.Wang J., Peng Y., Xu H., Cui Z., Williams R.O., 3rd The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation. AAPS PharmSciTech. 2020;21(6):225. doi: 10.1208/s12249-020-01744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rawat K., Kumari P., Saha L. COVID-19 vaccine: A recent update in pipeline vaccines, their design and development strategies. Eur. J. Pharmacol. 2020;892:173751. doi: 10.1016/j.ejphar.2020.173751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang L., Rong Y., Pan Q., Yi K., Tang X., Zhang Q., Wang W., Wu J., Wang F. SARS-CoV-2 vaccine research and development: Conventional vaccines and biomimetic nanotechnology strategies. Asian J. Pharm. Sci. 2020 doi: 10.1016/j.ajps.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., Li Y., Zhu L., Wang N., Lv Z., Gao H., Ge X., Kan B., Hu Y., Liu J., Cai F., Jiang D., Yin Y., Qin C., Li J., Gong X., Lou X., Shi W., Wu D., Zhang H., Zhu L., Deng W., Li Y., Lu J., Li C., Wang X., Yin W., Zhang Y., Qin C. Development of an inactivated vaccine candidate for SARS-CoV-2. Science (New York, N.Y.) 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sampath Kumar N.S., Chintagunta A.D., Jeevan Kumar S.P., Roy S., Kumar M. Immunotherapeutics for Covid-19 and post vaccination surveillance, 3. Biotech. 2020;10(12):527. doi: 10.1007/s13205-020-02522-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dong Y., Dai T., Wei Y., Zhang L., Zheng M., Zhou F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduction Targeted Therapy. 2020;5(1):237. doi: 10.1038/s41392-020-00352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Y.-D., Chi W.-Y., Su J.-H., Ferrall L., Hung C.-F., Wu T.C. Coronavirus vaccine development: from SARS and MERS to COVID-19. J. Biomed. Sci. 2020;27(1):104. doi: 10.1186/s12929-020-00695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., Avanzato V.A., Bushmaker T., Flaxman A., Ulaszewska M., Feldmann F., Allen E.R., Sharpe H., Schulz J., Holbrook M., Okumura A., Meade-White K., Pérez-Pérez L., Edwards N.J., Wright D., Bissett C., Gilbride C., Williamson B.N., Rosenke R., Long D., Ishwarbhai A., Kailath R., Rose L., Morris S., Powers C., Lovaglio J., Hanley P.W., Scott D., Saturday G., de Wit E., Gilbert S.C., Munster V.J. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586(7830):578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Robert-Guroff M. Replicating and non-replicating viral vectors for vaccine development. Curr. Opin. Biotechnol. 2007;18(6):546–556. doi: 10.1016/j.copbio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Corbett K.S., Edwards D.K., Leist S.R., Abiona O.M., Boyoglu-Barnum S., Gillespie R.A., Himansu S., Schäfer A., Ziwawo C.T., DiPiazza A.T. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586(7830):567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., Baum A., Pascal K., Quandt J., Maurus D., Brachtendorf S., Lörks V., Sikorski J., Hilker R., Becker D., Eller A.-K., Grützner J., Boesler C., Rosenbaum C., Kühnle M.-C., Luxemburger U., Kemmer-Brück A., Langer D., Bexon M., Bolte S., Karikó K., Palanche T., Fischer B., Schultz A., Shi P.-Y., Fontes-Garfias C., Perez J.L., Swanson K.A., Loschko J., Scully I.L., Cutler M., Kalina W., Kyratsous C.A., Cooper D., Dormitzer P.R., Jansen K.U., Türeci Ö. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 115.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics–developing a new class of drugs. Nat. Rev. Drug Discovery. 2014;13(10):759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 116.Fuenmayor J., Gòdia F., Cervera L. Production of virus-like particles for vaccines. N Biotechnol. 2017;39(Pt B):174–180. doi: 10.1016/j.nbt.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martínez-Flores D., Zepeda-Cervantes J., Cruz-Reséndiz A., Aguirre-Sampieri S., Sampieri A., Vaca L. SARS-CoV-2 vaccines based on the spike glycoprotein and implications of new viral variants. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.701501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kleanthous H., Silverman J.M., Makar K.W., Yoon I.-K., Jackson N., Vaughn D.W. Scientific rationale for developing potent RBD-based vaccines targeting COVID-19. npj Vaccines. 2021;6(1):1–10. doi: 10.1038/s41541-021-00393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.F. Rahimi, A. Talebi Bezmin Abadi, Implications of the Emergence of a New Variant of SARS-CoV-2, VUI-202012/01, Archives of medical research (2021). [DOI] [PMC free article] [PubMed]
- 120.Yarmohammadi A., Yarmohammadi M., Fakhri S., Khan H. Targeting pivotal inflammatory pathways in COVID-19: A mechanistic review. Eur. J. Pharmacol. 2020;173620 doi: 10.1016/j.ejphar.2020.173620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maiese K. The mechanistic target of rapamycin (mTOR): novel considerations as an antiviral treatment. Curr. Neurovasc. Res. 2020;17(3):332–337. doi: 10.2174/1567202617666200425205122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cai Z., Chen G., He W., Xiao M., Yan L.-J. Activation of mTOR: a culprit of Alzheimer’s disease? Neuropsychiatr. Dis. Treat. 2015;11:1015. doi: 10.2147/NDT.S75717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ramaiah M.J. mTOR inhibition and p53 activation, microRNAs: The possible therapy against pandemic COVID-19. Gene Reports. 2020:100765. doi: 10.1016/j.genrep.2020.100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Terrazzano G., Rubino V., Palatucci A.T., Giovazzino A., Carriero F., Ruggiero G. An open question: is it rational to inhibit the mTor-dependent pathway as COVID-19 therapy? Front. Pharmacol. 2020;11:856. doi: 10.3389/fphar.2020.00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xin P., Xu X., Deng C., Liu S., Wang Y., Zhou X., Ma H., Wei D., Sun S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020;80:106210. doi: 10.1016/j.intimp.2020.106210. [DOI] [PubMed] [Google Scholar]
- 126.La Rosée F., Bremer H., Gehrke I., Kehr A., Hochhaus A., Birndt S., Fellhauer M., Henkes M., Kumle B., Russo S. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia. 2020;34(7):1805–1815. doi: 10.1038/s41375-020-0891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cao Y., Wei J., Zou L., Jiang T., Wang G., Chen L., Huang L., Meng F., Huang L., Wang N. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J. Allergy Clin. Immunol. 2020;146(1):137–146. doi: 10.1016/j.jaci.2020.05.019. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rojas P., Sarmiento M. JAK/STAT pathway inhibition may be a promising therapy for COVID-19-related hyperinflammation in hematologic patients. Acta Haematol. 2020:1–5. doi: 10.1159/000510179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., Marconi V.C., Ruiz-Palacios G.M., Hsieh L., Kline S. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marano G., Vaglio S., Pupella S., Facco G., Catalano L., Liumbruno G.M., Grazzini G. Convalescent plasma: new evidence for an old therapeutic tool? Blood transfusion = Trasfusione del sangue. 2016;14:152–157. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garraud O., Heshmati F., Pozzetto B., Lefrere F., Girot R., Saillol A., Laperche S. Plasma therapy against infectious pathogens, as of yesterday, today and tomorrow. Transfusion clinique et biologique : journal de la Societe francaise de transfusion sanguine. 2016;23:39–44. doi: 10.1016/j.tracli.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.-M., Lim W.S., Makki S., Rooney K.D., Nguyen-Van-Tam J.S., Beck C.R. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J. Infect. Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Planitzer C.B., Modrof J., Kreil T.R. West Nile virus neutralization by US plasma-derived immunoglobulin products. J. Infect. Dis. 2007;196:435–440. doi: 10.1086/519392. [DOI] [PubMed] [Google Scholar]
- 134.Sharun K., Tiwari R., Iqbal Yatoo M., Patel S.K., Natesan S., Dhama J., Malik Y.S., Harapan H., Singh R.K., Dhama K. Antibody-based immunotherapeutics and use of convalescent plasma to counter COVID-19: advances and prospects. Expert Opin. Biol. Ther. 2020;20:1033–1046. doi: 10.1080/14712598.2020.1796963. [DOI] [PubMed] [Google Scholar]
- 135.Hendrickson J.E., Hillyer C.D. Noninfectious serious hazards of transfusion. Anesth. Analg. 2009;108:759–769. doi: 10.1213/ane.0b013e3181930a6e. [DOI] [PubMed] [Google Scholar]
- 136.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Korkmaz M.F., Türe E., Dorum B.A., Kılıç Z.B. The epidemiological and clinical characteristics of 81 children with COVID-19 in a pandemic hospital in Turkey: an observational cohort study. J. Korean Med. Sci. 2020;35 doi: 10.3346/jkms.2020.35.e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yeh K.-M., Chiueh T.-S., Siu L.K., Lin J.-C., Chan P.K.S., Peng M.-Y., Wan H.-L., Chen J.-H., Hu B.-S., Perng C.-L. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J. Antimicrob. Chemother. 2005;56:919–922. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lünemann J.D., Nimmerjahn F., Dalakas M.C. Intravenous immunoglobulin in neurology—mode of action and clinical efficacy. Nat. Rev. Neurol. 2015;11:80. doi: 10.1038/nrneurol.2014.253. [DOI] [PubMed] [Google Scholar]
- 140.S. Wan, Q. Yi, S. Fan, J. Lv, X. Zhang, L. Guo, C. Lang, Q. Xiao, K. Xiao, Z. Yi, Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP), MedRxiv (2020).
- 141.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang B., Liu S., Tan T., Huang W., Dong Y., Chen L., Chen Q., Zhang L., Zhong Q., Zhang X. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020;158:e9–e13. doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cheng Y., Wong R., Soo Y.O.Y., Wong W.S., Lee C.K., Ng M.H.L., Chan P., Wong K.C., Leung C.B., Cheng G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Soo Y.O.Y., Cheng Y., Wong R., Hui D.S., Lee C.K., Tsang K.K.S., Ng M.H.L., Chan P., Cheng G., Sung J.J.Y. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin. Microbiol. Infect. 2004;10:676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., Kong Y., Ren L., Wei Q., Mei H., Hu C., Tao C., Yang R., Wang J., Yu Y., Guo Y., Wu X., Xu Z., Zeng L., Xiong N., Chen L., Wang J., Man N., Liu Y., Xu H., Deng E., Zhang X., Li C., Wang C., Su S., Zhang L., Wang J., Wu Y., Liu Z. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.A. Gharbharan, C.C.E. Jordans, C. GeurtsvanKessel, J.G. den Hollander, F. Karim, F.P.N. Mollema, J.E. Stalenhoef, A. Dofferhoff, I. Ludwig, A. Koster, Convalescent Plasma for COVID-19. A randomized clinical trial, MEDRxiv (2020).
- 147.M.E. Balcells, L. Rojas, N. Le Corre, C. Martínez-Valdebenito, M.E. Ceballos, M. Ferrés, M. Chang, C. Vizcaya, S. Mondaca, Á. Huete, Early anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: a randomized phase II clinical trial, medRxiv (2020). [DOI] [PMC free article] [PubMed]
- 148.Joyner M.J., Klassen S.A., Senefeld J., Johnson P.W., Carter R.E., Wiggins C.C., Shoham S., Grossman B.J., Henderson J.P., Musser J.M. Evidence favouring the efficacy of convalescent plasma for COVID-19 therapy. J. medRxiv. 2020 [Google Scholar]
- 149.Libster R., Pérez Marc G., Wappner D., Coviello S., Bianchi A., Braem V., Esteban I., Caballero M.T., Wood C., Berrueta M., Rondan A., Lescano G., Cruz P., Ritou Y., Fernández Viña V., Álvarez Paggi D., Esperante S., Ferreti A., Ofman G., Ciganda Á., Rodriguez R., Lantos J., Valentini R., Itcovici N., Hintze A., Oyarvide M.L., Etchegaray C., Neira A., Name I., Alfonso J., López Castelo R., Caruso G., Rapelius S., Alvez F., Etchenique F., Dimase F., Alvarez D., Aranda S.S., Sánchez Yanotti C., De Luca J., Jares Baglivo S., Laudanno S., Nowogrodzki F., Larrea R., Silveyra M., Leberzstein G., Debonis A., Molinos J., González M., Perez E., Kreplak N., Pastor Argüello S., Gibbons L., Althabe F., Bergel E., Polack F.P. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. New Engl. J. Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Abolghasemi H., Eshghi P., Cheraghali A.M., Imani Fooladi A.A., Bolouki Moghaddam F., Imanizadeh S., Moeini Maleki M., Ranjkesh M., Rezapour M., Bahramifar A., Einollahi B., Hosseini M.J., Jafari N.J., Nikpouraghdam M., Sadri N., Tazik M., Sali S., Okati S., Askari E., Tabarsi P., Aslani J., Sharifipour E., Jarahzadeh M.H., Khodakarim N., Salesi M., Jafari R., Shahverdi S. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: Results of a multicenter clinical study. Transfusion Apheresis Sci.: Off. J. World Apheresis Assoc. : Off. J. Eur. Soc. Haemapheresis. 2020;59 doi: 10.1016/j.transci.2020.102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ (Clinical research ed.) 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Salazar E., Perez K.K., Ashraf M., Chen J., Castillo B., Christensen P.A., Eubank T., Bernard D.W., Eagar T.N., Long S.W., Subedi S., Olsen R.J., Leveque C., Schwartz M.R., Dey M., Chavez-East C., Rogers J., Shehabeldin A., Joseph D., Williams G., Thomas K., Masud F., Talley C., Dlouhy K.G., Lopez B.V., Hampton C., Lavinder J., Gollihar J.D., Maranhao A.C., Ippolito G.C., Saavedra M.O., Cantu C.C., Yerramilli P., Pruitt L., Musser J.M. Treatment of Coronavirus Disease 2019 (COVID-19) Patients with Convalescent Plasma. Am. J. Pathol. 2020;190:1680–1690. doi: 10.1016/j.ajpath.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vázquez C., Savoy N., Giunta D.H., Pérez L.G., Sánchez M.D.L., Gamarnik A.V., Ojeda D.S., Santoro D.M., Camino P.J., Antelo S., Rainero K., Vidiella G.P., Miyazaki E.A., Cornistein W., Trabadelo O.A., Ross F.M., Spotti M., Funtowicz G., Scordo W.E., Losso M.H., Ferniot I., Pardo P.E., Rodriguez E., Rucci P., Pasquali J., Fuentes N.A., Esperatti M., Speroni G.A., Nannini E.C., Matteaccio A., Michelangelo H.G., Follmann D., Lane H.C., Belloso W.H. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. New Engl. J. Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yiğenoğlu T.N., Hacıbekiroğlu T., Berber İ., Dal M.S., Baştürk A., Namdaroğlu S., Korkmaz S., Ulas T., Dal T., Erkurt M.A. Convalescent plasma therapy in patients with COVID-19. J. Clin. Apheresis. 2020;35:367–373. doi: 10.1002/jca.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rojas M., Rodríguez Y., Monsalve D.M., Acosta-Ampudia Y., Camacho B., Gallo J.E., Rojas-Villarraga A., Ramírez-Santana C., Díaz-Coronado J.C., Manrique R. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun. Rev. 2020:102554. doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rojas M., Anaya J.-M. Why will it never be known if convalescent plasma is effective for COVID-19. J. Transl. Autoimmunity. 2020:100069. doi: 10.1016/j.jtauto.2020.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.X. Zou, K. Chen, J. Zou, P. Han, J. Hao, Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection, (2020). [DOI] [PMC free article] [PubMed]
- 158.Y.-r. Guo, Q.-d. Cao, Z.-s. Hong, Y.-y. Tan, S.-d. Chen, H.-j. Jin, K.-s. Tan, D.-y. Wang, Y. Yan, The origin , transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status, (2020) 1-10. [DOI] [PMC free article] [PubMed]
- 159.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stopsack K.H., Mucci L.A., Antonarakis E.S., Nelson P.S., Kantoff P.W. TMPRSS2 and COVID-19: Serendipity or Opportunity for Intervention? Cancer Discovery. 2020;10:779–782. doi: 10.1158/2159-8290.CD-20-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 168.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. ChemBioChem. 2020;21:730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang C., Li W., Drabek D., Okba N.M.A., van Haperen R., Osterhaus A.D.M.E., van Kuppeveld F.J.M., Haagmans B.L., Grosveld F., Bosch B.-J. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020;11:1–6. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Giron C.C., Laaksonen A., da Silva F.L.B. On the interactions of the receptor-binding domain of SARS-CoV-1 and SARS-CoV-2 spike proteins with monoclonal antibodies and the receptor ACE2. Virus Res. 2020;285:198021. doi: 10.1016/j.virusres.2020.198021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Batlle D., Wysocki J., Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin. Sci. 2020;134:543–545. doi: 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- 172.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Del Pozo C.H., Prosper F. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Monteil V., Dyczynski M., Lauschke V.M., Kwon H., Wirnsberger G., Youhanna S., Zhang H., Slutsky A.S., Hurtado Del Pozo C., Horn M. Human soluble ACE2 improves the effect of remdesivir in SARS-CoV-2 infection. EMBO Mol. Med. 2021;13:e13426. doi: 10.15252/emmm.202013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sun P., Lu X., Xu C., Wang Y., Sun W., Xi J. CD-sACE2 inclusion compounds: An effective treatment for coronavirus disease 2019 COVID-19. J. Med. Virol. 2019;92(2020):1721–1723. doi: 10.1002/jmv.25804. [DOI] [PubMed] [Google Scholar]
- 175.Kawase M., Shirato K., van der Hoek L., Taguchi F., Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J. Virol. 2012;86:6537–6545. doi: 10.1128/JVI.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mikkonen L., Pihlajamaa P., Sahu B., Zhang F.-P., Jänne O.A. Androgen receptor and androgen-dependent gene expression in lung. Mol. Cell. Endocrinol. 2010;317:14–24. doi: 10.1016/j.mce.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 177.Lukassen S., Chua R.L., Trefzer T., Kahn N.C., Schneider M.A., Muley T., Winter H., Meister M., Veith C., Boots A.W. SARS-CoV-2 receptor ACE2 and TMPRSS2 are predominantly expressed in a transient secretory cell type in subsegmental bronchial branches. BioRxiv. 2020 doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bestle D., Heindl M.R., Limburg H., Pilgram O., Moulton H., Stein D.A., Hardes K., Eickmann M., Dolnik O., Rohde C. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci. Alliance. 2020;3 doi: 10.26508/lsa.202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lai C.C., Liu Y.H., Wang C.Y., Wang Y.H., Hsueh S.C., Yen M.Y., Ko W.C., Hsueh P.R. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. J. Microbiol. Immunol. Infect. 2020;53(3):404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71(15):732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.McKee D.L., Sternberg A., Stange U., Laufer S., Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol. Res. 2020;157:104859. doi: 10.1016/j.phrs.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.J. Uttamani, G. Fernandes, S. Pandey, V. Surabathula, D. Bhat, (2020).
- 183.Zhou D., Dai S.M., Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J. Antimicrobial Chemother. 2020;75(7):1667–1670. doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mahevas M., Tran V.T., Roumier M., Chabrol A., Paule R., Guillaud C., Fois E., Lepeule R., Szwebel T.A., Lescure F.X., Schlemmer F., Matignon M., Khellaf M., Crickx E., Terrier B., Morbieu C., Legendre P., Dang J., Schoindre Y., Pawlotsky J.M., Michel M., Perrodeau E., Carlier N., Roche N., de Lastours V., Ourghanlian C., Kerneis S., Menager P., Mouthon L., Audureau E., Ravaud P., Godeau B., Gallien S., Costedoat-Chalumeau N. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844. doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Guo Y.-J., Long T., Chen W., Ning C.-Q., Zhu Z.-A., Guo Y.-P. Bactericidal property and biocompatibility of gentamicin-loaded mesoporous carbonated hydroxyapatite microspheres. Mater. Sci. Eng., C. 2013;33(7):3583–3591. doi: 10.1016/j.msec.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 186.Covid R., Treatments C. Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study. Eur. J. Int. Med. 2020;82:38–47. doi: 10.1016/j.ejim.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Tissot Dupont H., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A., Damiani L.P., Marcadenti A., Kawano-Dourado L., Lisboa T., Junqueira D.L.M., de Barros E.S.P.G.M., Tramujas L., Abreu-Silva E.O., Laranjeira L.N., Soares A.T., Echenique L.S., Pereira A.J., Freitas F.G.R., Gebara O.C.E., Dantas V.C.S., Furtado R.H.M., Milan E.P., Golin N.A., Cardoso F.F., Maia I.S., Hoffmann Filho C.R., Kormann A.P.M., Amazonas R.B., Bocchi de Oliveira M.F., Serpa-Neto A., Falavigna M., Lopes R.D., Machado F.R., Berwanger O. I.I. Coalition Covid-19 Brazil, Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl. J. Med. 2020;383(21):2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C., Skipper C.P., Nascene A.A., Nicol M.R., Abassi M., Engen N.W., Cheng M.P., LaBar D., Lother S.A., MacKenzie L.J., Drobot G., Marten N., Zarychanski R., Kelly L.E., Schwartz I.S., McDonald E.G., Rajasingham R., Lee T.C., Hullsiek K.H. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl. J. Med. 2020;383(6):517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., Labella A., Manson D.K., Kubin C., Barr R.G., Sobieszczyk M.E., Schluger N.W. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl. J. Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., Mourão M.P.G., Brito-Sousa J.D., Baía-da-Silva D., Guerra M.V.F., Hajjar L.A., Pinto R.C., Balieiro A.A.S., Pacheco A.G.F., Santos J.D.O., Jr, Naveca F.G., Xavier M.S., Siqueira A.M., Schwarzbold A., Croda J., Nogueira M.L., Romero G.A.S., Bassat Q., Fontes C.J., Albuquerque B.C., Daniel-Ribeiro C.-T., Monteiro W.M., Lacerda M.V.G. f.t.C.-Team, Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Network Open. 2020;3(4):e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 192.Jiang S., Liu T., Hu Y., Li R., Di X., Jin X., Wang Y., Wang K. Efficacy and safety of glucocorticoids in the treatment of severe community-acquired pneumonia: a meta-analysis. Medicine. 2019;98(26) doi: 10.1097/MD.0000000000016239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lu X., Chen T., Wang Y., Wang J., Yan F. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Crit Care. 2020;24(1):241. doi: 10.1186/s13054-020-02964-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xu K., Chen Y., Yuan J. Factors associated with prolonged viral RNA shedding in patients with COVID-19 [published online ahead of print, 2020 Apr 9] Clin. Infect. Dis. 2020:ciaa351. doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Commission N.H. Diagnosis and treatment Protocol for novel coronavirus pneumonia (trial version 7) Chinese Med. J. 2020;133:1087–1095. doi: 10.1097/CM9.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.W.D.-G.s.o.r.a.t.m.b.o.C.-. June, https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re, m.-a.-t.-m.-b.-o.-c.-.-.-j.-A.J. 2020).
- 198.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Albani F., Sepe L., Fusina F., Prezioso C., Baronio M., Caminiti F., Di Maio A., Faggian B., Franceschetti M.E., Massari M., Salvaggio M., Natalini G. Thromboprophylaxis with enoxaparin is associated with a lower death rate in patients hospitalized with SARS-CoV-2 infection. A cohort study. EClinicalMedicine. 2020;27:100562. doi: 10.1016/j.eclinm.2020.100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.McCloskey B., Heymann D.L. SARS to novel coronavirus - old lessons and new lessons. Epidemiol. Infect. 2020;148:e22. doi: 10.1017/S0950268820000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ayerbe L., Risco C., Ayis S. The association between treatment with heparin and survival in patients with Covid-19. J. Thromb. Thrombolysis. 2020;50(2):298–301. doi: 10.1007/s11239-020-02162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Russo V., Cardillo G., Viggiano G.V., Mangiacapra S., Cavalli A., Fontanella A., Agrusta F., Bellizzi A., Amitrano M., Iannuzzo M., Sacco C., Lodigiani C., Castaldo G., Di Micco P. Thromboprofilaxys With Fondaparinux vs. Enoxaparin in Hospitalized COVID-19 Patients: A Multicenter Italian Observational Study. Front. Med. 2020;7 doi: 10.3389/fmed.2020.569567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cao Y.C., Deng Q.X., Dai S.X. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: An evaluation of the evidence. Travel Med. Infect. Dis. 2020;35:101647. doi: 10.1016/j.tmaid.2020.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hashemian S.M., Farhadi T., Velayati A.A. A Review on Remdesivir: A Possible Promising Agent for the Treatment of COVID-19. Drug Des. Devel. Ther. 2020;14:3215–3222. doi: 10.2147/DDDT.S261154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rauf A., Abu-Izneid T., Olatunde A., Ahmed Khalil A., Alhumaydhi F.A., Tufail T., Shariati M.A., Rebezov M., Almarhoon Z.M., Mabkhot Y.N., Alsayari A., Rengasamy K.R.R. COVID-19 Pandemic: Epidemiology, Etiology, Conventional and Non-Conventional Therapies. Int. J. Environ. Res. Public Health. 2020;17(21) doi: 10.3390/ijerph17218155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D'Arminio Monforte A., Ismail S., Kato H., Lapadula G., L'Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hossain M., Jannat T., Brishty S.R., Roy U., Mitra S., Rafi M., Islam M., Nesa M., Emran T.B. Clinical Efficacy and Safety of Antiviral Drugs in the Extended Use against COVID-19: What We Know So Far. Biologics. 2021;1(2):252–284. [Google Scholar]
- 210.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Y., Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Spinner C.D., Gottlieb R.L., Criner G.J., López J.R.A., Cattelan A.M., Viladomiu A.S., Ogbuagu O., Malhotra P., Mullane K.M., Castagna A. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Goldman J.D., Lye D.C.B., Hui D.S., Marks K.M., Bruno R., Montejano R., Spinner C.D., Galli M., Ahn M.Y., Nahass R.G., Chen Y.S., SenGupta D., Hyland R.H., Osinusi A.O., Cao H., Blair C., Wei X., Gaggar A., Brainard D.M., Towner W.J., Munoz J., Mullane K.M., Marty F.M., Tashima K.T., Diaz G., Subramanian A., Investigators G.-U. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N. Engl. J. Med. 2020;383(19):1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.D., Ruiz-Palacios G.M., Benfield T., Fatkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C., Members A.-S.-G. Remdesivir for the Treatment of Covid-19 - Final Report. N. Engl. J. Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.R. Perveen, M. Nasir, M. Murshed, R. Naznin, S. Ahmad, Remdesivir and Favipiravir Changes Hepato-Renal Profile in COVID-19 Patients: A Cross Sectional Observation in Bangladesh, (2021).
- 215.Mahévas M., Tran V.-T., Roumier M., Chabrol A., Paule R., Guillaud C., Fois E., Lepeule R., Szwebel T.-A., Lescure F.-X. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369 doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Di Castelnuovo A., Costanzo S., Antinori A., Berselli N., Blandi L., Bruno R., Cauda R., Guaraldi G., Menicanti L., My I. Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study. Eur. J. Int. Med. 2020;82:38–47. doi: 10.1016/j.ejim.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gautret P., Lagier J.-C., Parola P., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C., Veiga V.C., Avezum A., Damiani L.P., Marcadenti A., Kawano-Dourado L., Lisboa T. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N. Engl. J. Med. 2020;383(21):2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C., Skipper C.P., Nascene A.A., Nicol M.R., Abassi M. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N. Engl. J. Med. 2020;383(6):517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., Labella A., Manson D.K., Kubin C., Barr R.G. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., Mourão M.P.G., Brito-Sousa J.D., Baía-da-Silva D., Guerra M.V.F. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Network Open. 2020;3(4):e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 222.Lu X., Chen T., Wang Y., Wang J., Yan F. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Crit. Care. 2020;24(1):1–4. doi: 10.1186/s13054-020-02964-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Group R.C. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Russo V., Cardillo G., Viggiano G.V., Mangiacapra S., Cavalli A., Fontanella A., Agrusta F., Bellizzi A., Amitrano M., Iannuzzo M. Thromboprofilaxys with fondaparinux vs. enoxaparin in hospitalized COVID-19 patients: a multicenter Italian observational study. Front. Med. 2020;7:569567. doi: 10.3389/fmed.2020.569567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S. Remdesivir for the treatment of Covid-19. N. Engl. J. Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.