Abstract
Purpose
The management of patients with advanced/metastatic adrenocortical carcinoma (ACC) is challenging, EDP-M (etoposide, doxorubicin, cisplatin combined with mitotane) is the standard regimen. However, it is quite toxic, so an adequate supportive therapy is crucial to reduce as much as possible the side effects and maintain the dose intensity of cytotoxic agents.
Methods
We describe the main side effects of the EDP-M scheme and the best way to manage them based on the experience of the Medical Oncology Unit of the Spedali Civili of Brescia. We also deal with the administration of EDP-M in specific frail patients, such as those with huge disease extent and poor performance status (PS) and those with mild renal insufficiency.
Results
In patients with hormone secreting ACC the rapid control of Cushing syndrome using adrenal steroidogenesis inhibitors such as metyrapone or osilodrostat is mandatory before starting EDP-M. Primary prophylaxis of neutropenia with Granulocyte-Colony Stimulating Factors is crucial and should be introduced at the first chemotherapy cycle. Possible mitotane induced hypoadrenalism should be always considered in case of persistent nausea and vomiting and asthenia in the interval between one cycle to another. In case of poor PS. A 24 h continuous infusion schedule of cisplatin could be an initial option in patients with poor PS as well as to reduce the risk of nefrotoxocity in patients with mild renal impairment.
Conclusion
A careful and accurate supportive care is essential to mitigate EDP-M side effects as much as possible and avoid that, due to toxicity, patients have to reduce doses and or postpone cytotoxic treatment with a negative impact on efficacy of this chemotherapy regimen.
Introduction
EDP-M (etoposide, doxorubicin, cisplatin combined with mitotane) is the standard first-line regimen in the management of patients with advanced adrenocortical carcinoma (ACC), an extremely rare disease [1, 2]. The efficacy of this regimen is notoriously limited [3, 4], however, it is still the most efficacious regimen we currently have in the management of this disease, leading to complete pathological remission in a small proportion of patients.
Other cytotoxic therapies [5–8], molecular target therapies [9], and immunotherapies [10, 11] administered to patients with disease progression to EDP-M, did not show remarkable efficacy. Based on this premise we should administer the EDP-M regimen in the best possible way in order to obtain the maximum benefit in each patient. Strong evidence shows that cancer patients benefit from the delivery of full-dose cancer chemotherapy. Reducing the delivery of full chemotherapy dose intensity through treatment delays, dose reduction, or early termination of cancer treatment may increase the risk for recurrence and death [12].
EDP-M is quite toxic, but ACC patients are often young and could tolerate full doses. ACC hypersecretion of androgens may promote tolerance to EDP-M although to our knowledge there are no comparative tolerability data in patients stratified by androgen hypersecretion or not, Certainly, maintaining the dose exposes patients to a greater risk of side effects. Moreover, in the EDP-M scheme chemotherapy is administered in association with mitotane, a drug with adrenolytic activity. This association poses problems in interpreting the origin of symptoms and on the best supportive therapy to be administered. In this paper, we will discuss the main side effects of the EDP-M scheme and the best way to manage them based on the experience gained in the Medical Oncology of the Spedali Civili of Brescia, one of the reference centers for ACC in Italy. In addition, we will deal with specific aspects in the management of patients with advanced ACC treated with EDP-M, such as patients with huge disease extent and mild renal insufficiency.
Management EDP-related toxicities
As previously reported, the most common EDP-M-related toxicities are neutropenia, nausea/vomiting, and asthenia. Cisplatin-related neuropathy also frequently occurs in these patients. The management of these toxicities is necessary to preserve patients’ quality of life and avoid chemotherapy discontinuations.
Neutropenia
Neutropenia has been reported to occur in 53% of patients at treatment recycle and 77% of patients at nadir [3]. To prevent neutropenia, primary prophylaxis is crucial: Granulocyte-Colony Stimulating Factors should be administered in all patients treated with EDP-M from the first cycle of therapy; G-CSF (Peg-filgrastim) 6 mg/0.6 mL 1 prefilled syringe is usually injected 24–48 h after the end of every cycle. In the management of EDP-M related neutropenia, particular attention should be given to patients with Cushing’s syndrome, which is notoriously associated with infectious diseases and sepsis [13].
In these cases, EDP-related neutropenia is particularly dangerous. Therefore, the rapid control of Cushing syndrome in ACC-eligible EDP-M patients is therefore a priority. Surgery on the primary adrenal mass is undoubtedly a rapid and effective therapeutic procedure to obtain a reduction in circulating levels of cortisol. However, it should be emphasized that debulking surgery of a very aggressive disease can involve a risk of rapid growth of the residual tumor favored by the immunosuppression of the post-surgical period [14]. Furthermore, this therapeutic strategy implies a delay in the initiation of an effective systemic treatment. Several adrenally directed medical therapies are currently available including ketoconazole, metyrapone, osilodrostat, mitotane, and etomidate. These drugs inhibit one or several enzymes involved in adrenal steroidogenesis [15].
Mitotane is highly effective in the long-term management of ACC-induced Cushing’s syndrome and its mechanism of action prevents the escape phenomenon, even after treatment withdrawal, because the drug is stored in adipose tissue and has a long half-life. However, the efficacy of mitotane is often delayed so the association with adrenal steroid inhibitors with a greater rapidity of action is often necessary.
Metyrapone metabolism and elimination are not altered by concomitant mitotane, which is known to be a strong hepatic enzyme inducer and a recent paper has shown that the combination of EDP-M with metyrapone is effective and leads to rapid control of Cushing’s syndrome induced by cortisol-secreting ACC [16]. The addition of ketoconazole to the combination of metyrapone and mitotane may increase its efficacy, as well as osilodrostat can be a useful option in cases where metyrapone is contraindicated or could be not prescribed [17, 18].
Cortisol inhibition after metyrapone and osilodrostat is associated with increased androgen levels, so these drugs are not effective in controlling hyperandrogenism in patients with concomitant cortisol and androgen hypersecretion. For these patients abiraterone, a drug inhibiting 17 alpha hydroxylase and 11–20 lyase, used in the treatment of patients with prostate cancer, could be the most suitable drug as demonstrated by a preclinical in vitro and in vivo experience and confirmed in a published case report. However, Abiraterone is not currently approved in the management of Cushing syndrome [19, 20].
Due to the availability of adrenally directed drugs, ACC patients with Cushing syndrome should be initially treated with these drugs in association with mitotane and EDP regimen should be delayed for 10–15 days when serum cortisol levels are consistently decreased.
Nausea and vomiting
Nausea and vomiting are extremely frequent in EDP-M treated patients. This side effect was observed in 90% of treated patients. Emesis therefore should be effectively managed both during the EDP-M administration and the following days. Standard antiemetic therapies for cisplatin containing regimen should be administered in patients undergoing EDP-M therapy, following the international guidelines [21]. These includes highly selective serotonin 5-HT(3) receptor antagonists such as palonosetron 0.25 mg intravenously from day 1 to day 4 of chemotherapy infusion; dexamethasone 8 mg/daily from day 1 to day 4. In addition, due to the highly emetogenic effect of EDP-M, aprepitant, a substance P/neurokinin 1 (NK1) receptor antagonist, 125 mg on day 1 and 80 mg on days 2 and 3 of the cycle should be carefully considered. Granisetron transdermal patch is another therapeutic option to be considered for inadequate emesis control between one cycle and the subsequent one. In the experience of the Medical Oncology of Brescia, patients undergoing EDP are at risk of symptoms of hypoadrenalism on the 5th and 6th day when the administration of high doses of dexamethasone is interrupted. These symptoms should be recognized immediately and an extra dose of oral or parenteral glucocorticoids should be given. Our suggestion is that, in ACC patients who are eligible for EDP-M, this therapy should be given preferentially on an inpatient basis in order to better manage supportive care and possible acute hypoadrenalism [22] after cessation of administration of the cytotoxic agents and dexamethasone.
In the period of time between one cycle and the next, particular attention must be given to the management of nausea and, more rarely, vomiting as they may be due to either the effect of cytotoxic drugs or consequent to hypoadrenalism. An extra dose of glucocorticoids should be considered in case of persistent nausea.
Asthenia
As for nausea, even asthenia is frequently included among EDP-M toxicities occurring in 70% of patients [3]. Also with regard to this symptom, similarly to what previously stated for nausea and vomiting, it is necessary to consider the possibility that it can be due to concomitant hypoadrenalism and extradoses of glucocorticoids should be taken into consideration in case of persistent asthenia.
Neuropathy
Patients submitted to treatments for advanced ACC often experience neurological toxicities; differentiate neuropathy due to mitotane from the one caused by cisplatin is important, since they are central and peripheral, respectively. Because of the different mechanism of nerval damage, mitotane should not be withdrawn if cisplatin-related peripheral neuropathy occurs during EDP administration.
Until now, no effective agent exists to prevent cisplatin-induced peripheral neuropathy (CIPN). Efficacious pharmacological therapeutic options for patients with established CIPN are limited and CIPN recovery is in general partial with residual deficits in most patients.
Management of patients with mild renal impairment
Cisplatin is notoriously nephrotoxic and nephrotoxicity is caused by the drug accumulation in renal proximal tubules and typically start approximately 10 days after treatment. In some ACC patients who underwent surgery, there was a need to remove the kidney as it was macroscopically involved. These patients may have mild renal impairment and this can hinder the administration of cisplatin. A nephrologist consultation is crucial before starting cisplatin administration in a patient with renal impairment.
Independent predictors of cisplatin nephrotoxicity are hypoalbuminemia, smoking, female sex, and old age [23].
Consistent hydration (3 L/day) during cisplatin administration associated with sodium bicarbonate infusion and magnesium is recommended for mitigating nephrotoxicity.
Particular attention should be paid on the management of hypoalbuminemia since it leads to an increased concentration of unbound cisplatin which could enhance drug nephrotoxicity [24].
We preferentially hospitalize patients with mild renal impairment who require EDP-M therapy and adopt a 24-hour cisplatin-based infusion on the basis of the results of a study on pediatric cancer patients that reported a less frequent nephrotoxicity with continuous drug administration than with divided doses [25] although the significance of this practice has not been fully established. Many experiences of using carboplatin instead of cisplatin are available in other types of cancers, aiming to benefit through different toxicity profiles [26–29]. However, the use of carboplatin instead of cisplatin is not usually adopted by us as we do not have data on the efficacy and tolerability of EDP with carboplatin in adrenocortical carcinoma.
Management of patients with poor performance status
Patients with ACC in whom the diagnosis was late, may present with extensive disease and compromised general conditions. In view of the toxicity, EDP-M may not be advisable. In these cases, our strategy is to start with mitotane combined with a single administration of cisplatin, 45 mg/m2, administered in a 24-hour continuous infusion. Previous studies have shown that this administration schedule is well tolerated.
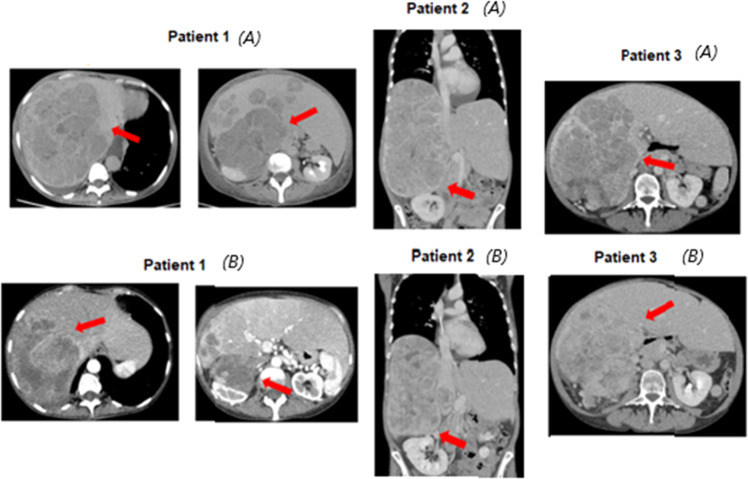
This therapy is also associated with strong nutritional support and drugs that counteract asthenia and improve appetite such as progestins (i.e., megestrol acetate). Patients are subsequently followed up and, if in the following days there is an improvement in their performance status, full EDP doses are administered after 10–15 days. Figure 1 shows the results of this approach in 3 patients with huge disease extent and poor PS who obtained a response to this therapeutic strategy that lasted 8, 7, and 9 months, respectively.
Fig. 1.
Patients with poor performance status before A and after (B) chemotherapy (3 cycles) preceded by a single administration of cisplatin (45 mg/m2) in a 24-hour continuous infusion
Table 1 shows the toxicity of cisplatin continuous infusion (45 mg/m2 daily) in 5 ACC patients in which it was administered for renal insufficiency (2 patients) and poor performance status (3 patients).
Table 1.
Adverse events related to 24-hour cisplatin in continuous infusion (45 mg/m2 daily)
| Adverse Events | N = 5 patients (%) | |
|---|---|---|
| Any grade | ≥Grade 3 | |
| Nausea | 0 | 0 |
| Vomiting | 0 | 0 |
| Diarrhea | 0 | 0 |
| Asthenia | 2 (40) | 0 |
| Constipation | 0 | 0 |
| Hematological toxicity | 4 (80) | 0 |
| Neutropenia | 0 | 0 |
| Anemia | 4 (80) | 0 |
| Thrombocytopenia | 0 | 0 |
We also collected data about five patients affected by ACC and renal disease that underwent standard EDP-M regimen after 24-hour administration (Table 2): as expected the most common chemotherapy-induced toxicities were hematological toxicity (5 patients, 100%), especially anemia (5 patients, 100%), asthenia (3 patients, 60%) and nausea (3 patients, 60%). These side effects, however, were mild to moderate. and, Interestingly, no ≥grade 3 adverse events were reported after the first administration with EDP standard schedule, suggesting that the 24-hour cisplatin administration could increase the patient tolerability of the following EDP. This strategy was successfully adopted in 3 patients (Fig. 1) with huge disease extension and poor PS at presentation who obtained a significant improvement in their general conditions after administration of 24 hour cisplatin infusion plus megestrol acetate and nutritional support. In these patients it was possible to administer EDP-M at full doses after 10–15 days and the results after 3 cycles show disease control in all 3 and an objective response in all of them.
Table 2.
Adverse events related to 24-hour cisplatin and following EDP-M after first cycle
| Adverse events | N = 5 patients (%) | |
|---|---|---|
| Any grade | ≥Grade 3 | |
| Nausea | 3 (60) | 0 |
| Vomiting | 0 | 0 |
| Diarrhea | 0 | 0 |
| Asthenia | 3 (60) | 0 |
| Constipation | 3 (60) | 0 |
| Hematological toxicity | 5 (100) | 0 |
| Neutropenia | 0 | 0 |
| Anemia | 5 (100) | 0 |
| Thrombocytopenia | 0 | 0 |
Conclusion
In conclusion, EDP-M is an aggressive chemotherapy scheme burdened with significant toxicity. Patients with ACC are however generally young and can tolerate the full doses that must be pursued to achieve the maximum possible efficacy. Supportive therapies aiming to prevent most common chemotherapy toxicities and therapies to rapidly control hormone hypersecretion are crucial. Many recent papers have underlined the benefits obtained combining early supportive therapies with oncologic active therapies for patients with advanced cancer to relieve their symptoms and improve the efficacy of the therapies. The association with mitotane to EDP regimen poses difficulties of interpretation between the typical side effects of chemotherapy (i.e., nausea, vomiting, asthenia) and mitotane-induced hypoadrenalism. ACC patients undergoing mitotane must be carefully followed by a team of expert endocrinologists and oncologists in order to mitigate side effects as much as possible and avoid that, due to toxicity, patients have to reduce doses and or postpone cytotoxic treatment with an impact. negative on efficacy.
In general endocrinologists and oncologists should not have a pessimistic attitude when treating patients with advanced ACC as this leads to the administration of suboptimal treatment delivery. We are aware that the EDP-M scheme has limited efficacy however it can achieve long-lasting disease control in a minority of patients. Such results are not obtained if therapy is inadequately administered for an ineffective management of toxicity.
Funding
Open access funding provided by Università degli Studi di Brescia within the CRUI-CARE Agreement.
Footnotes
These authors contributed equally: Antonella Turla, Marta Laganà, Deborah Cosentini, Alfredo Berruti
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fassnacht M, Dekkers OM, Else T, Baudin E, Berruti A, de Krijger R, Haak HR, Mihai R, Assie G, Terzolo M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018;179(4):G1–G46. doi: 10.1530/EJE-18-0608. [DOI] [PubMed] [Google Scholar]
- 2.Fassnacht M, Assie G, Baudin E, Eisenhofer G, de la Fouchardiere C, Haak HR, de Krijger R, Porpiglia F, Terzolo M, Berruti A. ESMO Guidelines Committee: Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(11):1476–1490. doi: 10.1016/j.annonc.2020.08.2099. [DOI] [PubMed] [Google Scholar]
- 3.Berruti A, Terzolo M, Sperone P, Pia A, Della Casa S, Gross DJ, Carnaghi C, Casali P, Porpiglia F, Mantero F, Reimondo G, Angeli A, Dogliotti L. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: a large prospective Phase II trial. Endocr Relat Cancer. 2005;12(3):657–666. doi: 10.1677/erc.1.01025. [DOI] [PubMed] [Google Scholar]
- 4.Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A, Jarzab B, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366:2189–2197. doi: 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 5.Cosentini D, Badalamenti G, Grisanti S, Basile V, Rapa I, Cerri S, Spallanzani A, Perotti P, Musso E, Laganà M, Ferrari VD, Luppi G, Dalla Volta A, Incorvaia L, Sigala S, Russo A, Volante M, Terzolo M, Berruti A. Activity and safety of temozolomide in advanced adrenocortical carcinoma patients. Eur J Endocrinol. 2019;181(6):681–689. doi: 10.1530/EJE-19-0570. [DOI] [PubMed] [Google Scholar]
- 6.Henning JEK, Deutschbein T, Altieri B, Steinhauer S, Kircher S, Sbiera S, Wild V, Schlötelburg W, Kroiss M, Perotti P, Rosenwald A, Berruti A, Fassnacht M, Ronchi CL. Gemcitabine-based chemotherapy in adrenocortical carcinoma: a multicenter study of efficacy and predictive factors. J Clin Endocrinol Metab. 2017;102(11):4323–4332. doi: 10.1210/jc.2017-01624. [DOI] [PubMed] [Google Scholar]
- 7.Grisanti S, Cosentini D, Laganà M, Morandi A, Lazzari B, Ferrari L, Volta AD, Ambrosini R, Ferrari VD, Sigala S, Berruti A. Clinical prognostic factors in patients with metastatic adrenocortical carcinoma treated with second line gemcitabine plus capecitabine chemotherapy. Front Endocrinol. 2021;12:624102. doi: 10.3389/fendo.2021.624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laganà M, Grisanti S, Ambrosini R, Cosentini D, Abate A, Zamparini M, Ferrari VD, Gianoncelli A, Turla A, Canu L, Terzolo M, Tiberio GAM, Sigala S, Berruti A. Phase II study of cabazitaxel as second-third line treatment in patients with metastatic adrenocortical carcinoma. ESMO Open. 2022;7(2):100422. doi: 10.1016/j.esmoop.2022.100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grisanti S, Cosentini D, Laganà M, et al. Are we failing in treatment of adrenocortical carcinoma? Lights and shadows of molecular signatures. Curr Opin Endocr Metab Res. 2019;8:80–87. doi: 10.1016/j.coemr.2019.07.007. [DOI] [Google Scholar]
- 10.Fiorentini C, Grisanti S, Cosentini D, Abate A, Rossini E, Berruti A, Sigala S. Molecular drivers of potential immunotherapy failure in adrenocortical carcinoma. J Oncol. 2019;1(2019):6072863. doi: 10.1155/2019/6072863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grisanti S, Cosentini D, Laganà M, Volta AD, Palumbo C, Massimo TGA, Sigala S, Berruti A. The long and winding road to effective immunotherapy in patients with adrenocortical carcinoma. Future Oncol. 2020;16(36):3017–3020. doi: 10.2217/fon-2020-0686. [DOI] [PubMed] [Google Scholar]
- 12.Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Canc Netw. 2009;7(1):99–108. doi: 10.6004/jnccn.2009.0009. [DOI] [PubMed] [Google Scholar]
- 13.Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A. Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4(7):611–629. doi: 10.1016/S2213-8587(16)00086-3. [DOI] [PubMed] [Google Scholar]
- 14.Cosentini, D., Laganà, M., Turla, A., Tiberio, G.A.M., Grisanti, S., Berruti, A.: Letter to the editor from Berruti et al: “Cytoreductive Surgery of the Primary Tumor in Metastatic Adrenocortical Carcinoma: Impact on Patients’ Survival”. J Clin Endocrinol Metab.: dgac169 (2022). 10.1210/clinem/dgac169 [DOI] [PubMed]
- 15.Tritos NA. Adrenally directed medical therapies for cushing syndrome. J Clin Endocrinol Metab. 2021;106(1):16–25. doi: 10.1210/clinem/dgaa778. [DOI] [PubMed] [Google Scholar]
- 16.Claps M, Cerri S, Grisanti S, Lazzari B, Ferrari V, Roca E, Perotti P, Terzolo M, Sigala S, Berruti A. Adding metyrapone to chemotherapy plus mitotane for Cushing’s syndrome due to advanced adrenocortical carcinoma. Endocrine. 2018;61(1):169–172. doi: 10.1007/s12020-017-1428-9. [DOI] [PubMed] [Google Scholar]
- 17.Kamenický P, Droumaguet C, Salenave S, Blanchard A, Jublanc C, Gautier JF, Brailly-Tabard S, Leboulleux S, Schlumberger M, Baudin E, Chanson P, Young J. Mitotane, metyrapone, and ketoconazole combination therapy as an alternative to rescue adrenalectomy for severe ACTH-dependent Cushing’s Syndrome. J Clin Endocrinol Metab. 2011;ume 96(9):2796–2804. doi: 10.1210/jc.2011-0536. [DOI] [PubMed] [Google Scholar]
- 18.Haissaguerre M, Puerto M, Nunes ML, Tabarin A. Efficacy and tolerance of osilodrostat in patients with severe Cushing’s syndrome due to non-pituitary cancers. Eur J Endocrinol. 2020;183(4):L7–L9. doi: 10.1530/EJE-20-0557. [DOI] [PubMed] [Google Scholar]
- 19.Claps M, Lazzari B, Grisanti S, Ferrari V, Terzolo M, Sigala S, Vezzoli S, Memo M, Castellano M, Berruti A. Management of severe cushing’s syndrome induced by adrenocortical carcinoma with abiraterone acetate: a case report. AACE Clin Case Rep. 2016;2:e337–e341. doi: 10.4158/EP151104.CR. [DOI] [Google Scholar]
- 20.Fiorentini C, Fragni M, Perego P, Vezzoli S, Bonini SA, Tortoreto M, Galli D, Claps M, Tiberio GA, Terzolo M, Missale C, Memo M, Procopio G, Zaffaroni N, Berruti A, Sigala S. Antisecretive and antitumor activity of abiraterone acetate in human adrenocortical cancer: a preclinical study. J Clin Endocrinol Metab. 2016;101(12):4594–4602. doi: 10.1210/jc.2016-2414. [DOI] [PubMed] [Google Scholar]
- 21.Roila F, Molassiotis A, Herrstedt J, Aapro M, Gralla RJ, Bruera E, Clark-Snow RA, Dupuis LL, Einhorn LH, Feyer P, Hesketh PJ, Jordan K, Olver I, Rapoport BL, Roscoe J, Ruhlmann CH, Walsh D, Warr D, van der Wetering M. participants of the MASCC/ESMO Consensus Conference Copenhagen 2015.: 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. Sep. 2016;27(suppl 5):v119–v133. doi: 10.1093/annonc/mdw270. [DOI] [PubMed] [Google Scholar]
- 22.Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, Torpy DJ. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. Feb. 2016;101(2):364–389. doi: 10.1210/jc.2015-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Jongh FE, van Veen RN, Veltman SJ, de Wit R, van der Burg ME, van den Bent MJ, Planting AS, Graveland WJ, Stoter G, Verweij J. Weekly high-dose cisplatin is a feasible treatment option: analysis on prognostic factors for toxicity in 400 patients. Br J Cancer. 2003;88(8):1199–1206. doi: 10.1038/sj.bjc.6600884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horie S, Oya M, Nangaku M, Yasuda Y, Komatsu Y, Yanagita M, Kitagawa Y, Kuwano H, Nishiyama H, Ishioka C, Takaishi H, Shimodaira H, Mogi A, Ando Y, Matsumoto K, Kadowaki D, Muto S. Guidelines for treatment of renal injury during cancer chemotherapy 2016. Clin Exp Nephrol. 2018;22(1):210–244. doi: 10.1007/s10157-017-1448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erdlenbruch B, Pekrum A, Roth C, Grunewald RW, Kern W, Lakomek M. Cisplatin nephrotoxicity in children after continuous 72-h and 3x1-h infusions. Pediatr Nephrol. 2001;16(7):586–593. doi: 10.1007/s004670100610. [DOI] [PubMed] [Google Scholar]
- 26.Griesinger F, Korol EE, Kayaniyil S, Varol N, Ebner T, Goring SM. Efficacy and safety of first-line carboplatin-versus cisplatin-based chemotherapy for non-small cell lung cancer: A meta-analysis. Lung Cancer. 2019;135:196–204. doi: 10.1016/j.lungcan.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto H, Watanabe K, Kunikane H, Yokoyama A, Kudoh S, Asakawa T, Shibata T, Kunitoh H, Tamura T, Saijo N. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer. 2007;97(2):162–169. doi: 10.1038/sj.bjc.6603810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.du Bois A, Lück HJ, Meier W, Adams HP, Möbus V, Costa S, Bauknecht T, Richter B, Warm M, Schröder W, Olbricht S, Nitz U, Jackisch C, Emons G, Wagner U, Kuhn W, Pfisterer J. Arbeitsgemeinschaft Gynäkologische Onkologie Ovarian Cancer Study Group.; A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95(17):1320–1329. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- 29.Alberts DS. Carboplatin versus cisplatin in ovarian cancer. Semin Oncol. 1995;22(5 Suppl 12):88–90. [PubMed] [Google Scholar]