Abstract
Rationale
Oral tobacco–derived nicotine products include on!® nicotine pouches (NPs) which are tobacco-leaf free and available in multiple flavors and nicotine levels. Switching completely to NPs from cigarettes and moist smokeless tobacco (MST) has the potential to reduce harm for adult tobacco consumers. However, the dependence potential of NPs is not established. Therefore, we characterized the abuse potential of NPs with different nicotine levels compared to cigarettes and MST.
Objectives
To evaluate nicotine pharmacokinetics (PK) and subjective effects of NPs (ranging from 1.5 to 8 mg nicotine) compared to own brand cigarettes (OBCs) and MST (OBMST).
Methods
We used a randomized, in-clinic, partial single-blind, 7-way crossover design to assess nicotine PK and subjective effects in dual users of cigarettes and MST.
Results
The mean nicotine Cmax for NPs increased with nicotine level, ranging from 3.5 ng/mL (1.5 mg NP) to 15.4 ng/mL (8 mg NP), compared with 12.2 ng/mL for OBCs and 9.8 ng/mL for OBMST. Nicotine tmax was much longer for all NPs and OBMST (32.5–34.4 min) compared to OBCs (8.5 min). Reductions in urges to smoke after use of the 2 mg, 3.5 mg, and 8 mg NPs were not statistically different (p > 0.05) relative to OBC. Also, NPs resulted in lower ratings of positive subjective effects relative to OBCs and OBMST.
Conclusions
Overall, based on the study results and literature reported nicotine PK values for cigarettes and MST, the abuse potential of NPs is not likely to be higher than OBCs and OBMST. NPs may be potentially acceptable switching products for users of cigarettes and MST products.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00213-022-06172-y.
Keywords: Novel oral tobacco products, Oral tobacco–derived nicotine products, Nicotine pouches, Abuse potential, Dependence potential, Urges to smoke, Subjective measures, Nicotine pharmacokinetics
Introduction
Cigarette smoking remains the leading cause of preventable premature death and disease in the USA. Smoking-related diseases are caused by harmful and potentially harmful constituents (HPHCs) and other compounds in cigarettes that are inhaled in the smoke (US Department of Health and Human Services 2014). Many in public health (Gottlieb and Zeller 2017; Hatsukami et al. 2007; Zeller et al. 2009) have acknowledged that a continuum of risk exists among tobacco products, with conventional, combustible cigarettes at the higher end and non-combustible products on the lower end. In recent years, there has been a rapid growth of a variety of innovative, non-combustible products, including oral tobacco–derived nicotine (OTDN) products. Nicotine pouches (NPs) are one example of OTDN products that contain pharmaceutical grade nicotine derived from tobacco, flavors, and other ingredients used in foods. NPs are tobacco-leaf free and contain far fewer HPHCs than cigarette smoke (Wagner et al. 2020). Switching to NPs, therefore, presents a potential harm reduction opportunity among the ~ 34.1 million (Cornelius et al. 2020) adult cigarette smokers (AS) and ~ 5.9 million (Cornelius et al. 2020) adult moist smokeless tobacco (MST) users, particularly those that are unable or unwilling to quit tobacco products.
To date, there are only two published studies that inform the abuse potential of NPs (Lunell et al. 2020; Rensch et al. 2021). Lunell et al. (2020) compared Zyn® NPs containing 3 and 6 mg nicotine with 8 mg General snus, and Zyn® NPs containing 8 mg nicotine with 18 mg Longhorn moist snuff in a study of snus users. The authors observed that the two higher levels of Zyn® (6 and 8 mg) delivered nicotine as quickly and to a similar extent as the comparator MST. The authors had also measured subjective effects (“head buzz”) and observed no obvious correlation between nicotine levels and the maximum score for “head buzz.” Rensch et al. recently reported nicotine pharmacokinetics (PK) and subjective effects of six flavors of on!® NPs containing 4 mg nicotine in AS compared to own brand cigarettes (2021). This study was specifically designed to assess abuse potential of the NPs investigated relative to cigarettes. The authors concluded that based on the PK profiles and subjective responses, the NPs were likely to be associated with lower abuse potential than cigarettes. The findings from the study also indicated that flavor does not appear to influence nicotine PK or subjective responses, and the NPs may be potentially acceptable switching products for AS and adult MST users (Rensch et al. 2021). We present here results from a randomized, controlled, clinical study with varying nicotine levels to further inform the abuse potential of the on!® NPs. The rationale for this study is to add to the limited scientific knowledge regarding the abuse potential of NP products.
This study evaluated the nicotine PK and subjective effects of five mint-flavored NPs with different nicotine levels relative to participants’ own brand cigarettes (OBCs) and own brand MST (OBMST) in adult dual users of cigarettes and MST. Evaluation of nicotine PK, including the time-course and amount of nicotine delivery, is used to assess tobacco products’ abuse potential; whereby, tobacco products with a greater rate and extent of nicotine delivery are more likely to be used repeatedly (Henningfield and Keenan 1993). The subjective responses to tobacco products are measured using well-established questionnaires, which can serve as proxy measures of the positive, rewarding effects as well as the negative, adverse effects of tobacco products that may influence subsequent use behavior and inform abuse potential (Carter et al. 2009; Cobb et al. 2010; Cox et al. 2001; Gray et al. 2008; Hanson et al. 2009; Vansickel et al. 2010). The purpose of this study was to assess the nicotine PK profiles and subjective measure responses from use of NPs which will inform the abuse potential of the NPs relative to adult smokers’ OBCs and smokeless tobacco (ST) users’ OBMST. We hypothesize that the abuse potential of the NPs tested will not be higher than OBCs and OBMST products.
Materials and methods
Participants
Participants were recruited and screened at three different study sites: Celerion (Lincoln, NE), Bio-Kinetic Clinical Applications, LLC (Springfield, MO), and Midwest Clinical Research Center, LLC (Dayton, OH). The recruitment utilized the database of the potential study population of AS and MST users at each site, as well as social media videos, radio, print, and digital advertising. Healthy adult dual users (smokers who also used MST) age 21 to 65 years who fulfilled all inclusion criteria and met none of the exclusion criteria were eligible to participate. The eligible participants checked in to the clinic at Celerion in Lincoln, NE, where the study was conducted from September to November in 2019. Participants were self-affirmed dual users of cigarettes (consumption of at least 10 cigarettes per day [CPD]) and MST (consumption of at least one can per week) for at least 12 months and had urine cotinine levels ≥ 500 ng/mL at screening. Potential participants were excluded if they had used any OTDN pouch products within 30 days prior to the screening visit or reported any plans or attempts to quit smoking or using MST in the past 3 months.
Study products
The test products were mint-flavored on!® NPs, which contain tobacco-derived nicotine bitartrate dihydrate at five different nicotine levels (1.5 mg, 2 mg, 3.5 mg, 4 mg, and 8 mg), microcrystalline cellulose, sodium carbonate, flavoring ingredients, and binders within a permeable, non-dissolving pouch. Participants’ OBCs and OBMST were used as the reference products. The products investigated in the study are commercially available products. We note that the 1.5 mg and 3.5 mg test products described in this manuscript were identified as 1 mg and 3 mg products, respectively, in the study documents.
Study design
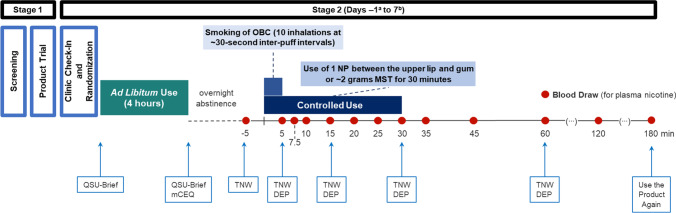
This study used a randomized, partial single-blind, 7-way crossover design. Participants who passed screening completed an ambulatory 5-day (consecutive or non-consecutive) product trial (Stage 1), in which they were provided with one can (20 pouches) of each of the five nicotine levels of the NPs. During this product trial period, participants were instructed to use at least one NP of each nicotine level, starting from the lowest on the first day and moving to the higher levels sequentially in the subsequent product trial days, concluding with use of at least one 8 mg NP for at least 30 min to confirm tolerability. Product use behavior (e.g., the number and nicotine level of NPs used per day, the number used each time, and the number of days used) was documented in a diary by participants.
Following the product trial, eligible participants checked in to the clinic and were randomized to one of seven product sequences and were only allowed to use assigned study products at scheduled times (Stage 2). During the 8-day, in-clinic product use, participants knew whether the study product was an NP, OBC, or OBMST, but were blinded to the nicotine level of the NP (partial single-blind). The randomized, 7-way, crossover design allowed each participant to use all five nicotine levels and their OBCs and OBMST products in this within-subject design. Participants used their assigned product ad libitum for 4-h periods approximately 15 h before the controlled product use period. The 15-h overnight abstinence from use of any tobacco- or nicotine-containing products was monitored by the clinic staff. Participants completed subjective measure questionnaires at pre-determined timepoints (see Supplementary Table 1). During the controlled product use periods, participants used the assigned product under the following conditions: smoked one cigarette with 10 inhalations at ~ 30-s inter-puff intervals, used one NP by placing the pouch between the upper lip and gum for 30 min, or used ~ 2 g (± 0.01 g) of OBMST for 30 min. The 30-min use of the NP reflects an “extreme” condition of use (the marketed product indicates “Enjoy for up to 20 min” on the packaging). Participants completed the controlled use subjective measures questionnaires at pre-determined timepoints (see Fig. 1 and Supplementary Table 1).
Fig. 1.
Study design. aThere was no controlled product use period on day − 1; participants completed the ad libitum use period followed by overnight abstinence on day − 1. The participants stayed in the clinic for a total of 8 days (day − 1 through day 7). bThere was no afternoon ad libitum use period after the controlled use period in the morning on the last study day, day 7. DEP, Direct Effects of Product Questionnaire; mCEQ, Modified Cigarette Evaluation Questionnaire; MST, moist smokeless tobacco; NP, nicotine pouch; OBC, own brand cigarette; QSU, Questionnaire on Smoking Urges; TNW, Tobacco/Nicotine Withdrawal Questionnaire
Over each 4-h ad libitum product use period, clinic staff documented the start and stop time for each product use, the total number of NPs or cigarettes used, the number of NPs used each time, the number of OBMST quids (a quid refers to a pinch of OBMST that a participant placed in the mouth at the time of use) used, the amount of time in the mouth during each NP or OBMST use, and the total weight of OBMST used, as applicable.
Nicotine pharmacokinetics
Plasma nicotine concentrations were measured from blood samples collected at ~ 5 min prior to and at 5, 7.5, 10, 15, 20, 25, 30, 35, 45, 60, 120, and 180 min following the start of each controlled product use period. PK parameters (including area under the curve [AUC(0–180)], maximum nicotine concentration [Cmax], time of the maximum measured plasma concentration [tmax], apparent first-order terminal elimination rate constant [kel], and apparent first-order elimination half-life [t½]) were calculated from the baseline-adjusted plasma nicotine concentration–time data using Phoenix® WinNonlin® version 7.0.
Subjective measures
A summary of the items contained in and administration timing of the previously published subjective measures questionnaires completed during the ad libitum and controlled product use periods is provided in Supplementary Table 1. Participants completed the subjective measures questionnaires using a tablet with preloaded sequences programed by Clinical Ink (Horsham, PA). During ad libitum in-clinic use, the Questionnaire on Smoking Urges-Brief (QSU-Brief) was administered before and after each product use period to assess desire and intention to smoke and anticipation of relief from negative affects (Cox et al. 2001). The Modified Cigarette Evaluation Questionnaire (mCEQ; further modified for use with NPs and OBMST) was completed after each ad libitum product use period to assess satisfaction, enjoyment of sensations, psychological reward, craving, and aversion (Rose et al. 2010; St. Helen et al. 2016).
To assess the magnitude, onset, and offset of product reinforcing effects during controlled product use, an in-the-moment response to a visual analog scale (VAS) of the Tobacco/Nicotine Withdrawal (TNW) Questionnaire was collected before, during, and after each use period and response to the Direct Effects of Product (DEP) Questionnaire was captured during and after each controlled use period. Evidence from Hanson et al. (2009) indicates that the items included in the TNW and DEP Questionnaires are sensitive enough to detect differences between tobacco products. As a measure of the overall likelihood of subsequent use behavior, response to the Use the Product Again (adapted from Griffiths et al. 2003) bipolar VAS, anchored with “definitely would” and “definitely would not” at either end and “don’t care” in the middle, was also assessed after each controlled use period.
Safety assessments
Clinical safety evaluations included clinical laboratory testing (serum chemistry, hematology, and urinalysis), drug and alcohol screens, pregnancy tests (females), confirmation of tobacco use, physical examinations, and electrocardiograms. Vital signs (blood pressure, pulse/respiration rates, and body temperature) were measured at screening, check in, and at the end of the study. Adverse events (AEs) were monitored and reported from the first NP use until the end of study.
Statistical analysis
The primary outcome variables were nicotine PK parameters (Cmax and AUC(0–180)), maximum reduction in response, under controlled use conditions, relative to pre-use for TNW items (Emax_TNW) and the maximum response on the DEP items following the product use under controlled use conditions (Emax_DEP). The hypothesis, based on the primary outcome variables, was that the Cmax, Emax_TNW, and Emax_DEP following the controlled use of NPs tested are not statistically different (α level of 0.05) from that observed for OBCs or OBMST. Data were analyzed using the statistical methods described in a previous publication (Rensch et al. 2021). SAS software (version 9.4, Cary, NC) was used for all data presentation and summarization including statistical analyses, summary tables, graphs, and data listings. A linear mixed model for analysis of variance was performed on the natural log-transformed AUC and Cmax. The model included sequence, study product, and period as fixed effects and subject-nested-within-sequence as a random effect.
Sample size estimation
Based on a literature search, typical sample sizes range from 10 to 32 participants for studies examining the PK and subjective effects across different tobacco/nicotine conditions (Carter et al. 2009; Cobb et al. 2010; Cox et al. 2001; Gray et al. 2008; Hatsukami et al. 2004; Kotlyar et al. 2007; Lunell and Curvall 2011; Perkins et al. 1997). The sample size of 30 participants was considered adequate for the current study design.
Results
Out of 66 people screened for this study, 36 people failed screening procedures. A total of 30 participants (29 males and 1 female) were enrolled, completed the 5-day ambulatory product trial, and checked in to the clinic on day − 1. All 30 participants were randomized to one of seven study product sequences, and 28 participants completed the study. One male participant chose to withdraw from the study on day 2 due to unrelated AEs (body aches, chills, and fatigue), and one male participant withdrew on day 5 due to a family emergency.
The study population was predominantly white (90%) and male (97%) with an average age of 35 years (Table 1). All participants were cigarette smokers and concurrent MST users at screening, had smoked an average of about 15 CPD for 14 years, and used ~ 3.5 cans of MST per week for 12 years. Two-thirds of study participants (67%) reported using non-menthol cigarettes and the majority (97%) reported using long-cut MST.
Table 1.
Demographics and product use history
| Parameter | N = 30 |
|---|---|
| Sex, n (%) | |
|
Female Male |
1 (3) 29 (97) |
| Race, n (%) | |
|
White Black |
27 (90) 3 (10) |
| Ethnicity, n (%) | |
|
Hispanic or Latino Not Hispanic or Latino |
1 (3) 29 (97) |
| Age, years | 34.9 (9.63) |
| BMI, kg/m2 | 28.5 (5.08) |
| Number of cigarettes smoked per day | 15.3 (4.12) |
| Number of years of smoking | 14.3 (9.49) |
| Number of cans of MST product used per week | 3.5 (2.28) |
| Number of years of MST product use | 11.9 (8.31) |
Data are presented as mean (standard deviation) unless otherwise noted
BMI body mass index, MST moist smokeless tobacco
During the product trial period, participants reported using about five NPs per day. Overall, during each 4-h ad libitum product use period, the average pouch consumption ranged from ~ 6 pouches (8 mg) to ~ 18 pouches (1.5 mg). The mean cigarette consumption was ~ 10 cigarettes, and OBMST use was ~ 3 quids/pinches. The average length of time that the NPs were used in the mouth ranged from ~ 29 (1.5 mg) to ~ 56 (2 mg) min. OBMST quids were used for ~ 42 min per use during the 4-h ad libitum product use period.
Nicotine pharmacokinetics
Plasma nicotine PK parameters were baseline-adjusted (Supplementary Table 2) because measurable nicotine levels (≥ 0.2 ng/mL) were observed at baseline prior to product use under controlled conditions, in most study participants (Supplementary Table 2). The tmax of nicotine uptake from all five NPs (range: 32.5 to 33.9 min) was slower than OBCs (8.5 min) and was similar to OBMST (34.4 min; Supplementary Table 2). The shape of the nicotine PK profiles was similar among all the NPs and OBMST products. The geometric least squares mean Cmax and AUC(0–180) values increased with the increasing nicotine level of the NPs (Table 2).
Table 2.
Summary of statistical comparisons of baseline-adjusted plasma nicotine pharmacokinetic parameters
| NP nicotine level or OBMST | LS mean Cmax, ng/mL and AUC, ng min/mL (n) |
Comparison with OBCs | Comparison with OBMST | ||
|---|---|---|---|---|---|
| Geometric LS mean ratio (test/Refa), % (95% CI) |
p-value | Geometric LS mean ratio (test/OBMST), % (95% CI) | p-value | ||
| 1.5 mg NP | |||||
|
Cmax AUC |
3.2 (30) 306.0 (29) |
30.8 (25.5, 37.1) 38.1 (31.7, 45.8) |
< 0.0001 < 0.0001 |
35.4 (29.3, 42.7) 31.0 (25.8, 37.2) |
< 0.0001 < 0.0001 |
| 2 mg NP | |||||
|
Cmax AUC |
4.6 (29) 426.6 (29) |
43.7 (36.2, 52.8) 53.1 (44.2, 63.8) |
< 0.0001 < 0.0001 |
50.3 (41.6, 60.7) 43.2 (36.0, 51.9) |
< 0.0001 < 0.0001 |
| 3.5 mg NP | |||||
|
Cmax AUC |
7.1 (28) 699.0 (28) |
67.0 (55.4, 81.1) 87.0 (72.3, 104.7) |
< 0.0001 0.1405 |
77.1 (63.7, 93.3) 70.8 (58.8, 85.2) |
0.0077 0.0003 |
| 4 mg NP | |||||
|
Cmax AUC |
8.4 (28) 796.0 (28) |
80.1 (66.2, 96.9) 99.1 (82.4, 119.3) |
0.0227 0.9251 |
92.1 (76.1, 111.4) 80.6 (67.0, 97.0) |
0.3925 0.0226 |
| 8 mg NP | |||||
|
Cmax AUC |
14.5 (28) 1441 (27) |
137.4 (113.5, 166.2) 179.4 (148.8, 216.3) |
0.0013 < 0.0001 |
157.9 (130.5, 191.1) 145.9 (121.0, 175.9) |
< 0.0001 0.0001 |
| OBMST | |||||
|
Cmax AUC |
9.2 (29) 987.7 (29) |
87.0 (72.0, 105.1) 123.0 (102.4, 147.7) |
0.1465 0.0271 |
— — |
— — |
AUC area under the nicotine concentration–time curve from time 0 to 180 min, CI confidence interval, Cmax maximum measured plasma concentration, LS least squares, NP nicotine pouch, OBC own brand cigarette, OBMST own brand moist smokeless tobacco
aReference (OBC): Cmax = 10.5 ng/mL; AUC = 803.1 ng min/mL; n = 29
The Cmax geometric mean values ranged from 3.2 ng/mL (1.5 mg NP) to 14.5 ng/mL (8 mg NP). The AUC(0–180) ranged from 306 ng*min/mL (1.5 mg NP) to 1441 ng*min/mL (8 mg NP). The 1.5, 2, 3.5, and 4 mg NPs resulted in statistically significantly lower (p < 0.05) Cmax values, while the 8 mg NP resulted in a statistically significantly (p < 0.05) higher Cmax relative to participants’ OBCs. The AUC(0–180) values were statistically significantly lower (p < 0.0001) for 1.5 mg and 2 mg NPs relative to participants’ OBC AUC(0–180). No statistically significant differences were observed for AUC(0–180) for the 3.5 and 4 mg NPs, and the 8 mg NP’s AUC(0–180) was significantly higher (p < 0.0001) relative to participants’ OBC.
Relative to participants’ OBMST, the Cmax for the 1.5, 2, and 3.5 mg NPs was significantly lower (p < 0.0001). The 4 mg NP resulted in similar Cmax, and the 8 mg NP resulted in significantly higher Cmax (p < 0.0001) relative to participants’ OBMST. The 1.5, 2, 3.5, and 4 mg NPs resulted in significantly lower (p < 0.05 for all comparisons) AUC(0–180), while the 8 mg NP resulted in a significantly higher (p < 0.0001) AUC relative to participants’ OBMST. No indications of non-linear pharmacokinetics were observed based on the estimated nicotine elimination half-life values at the nicotine levels examined.
Subjective responses
While there was a reduction in the QSU-Brief factor scores (Factor 1 — desire and intention to smoke and Factor 2 — anticipation of relief from negative affect) after 4-h ad libitum use of the NPs, the magnitude of reduction relative to baseline was much smaller than with participants’ OBCs. Additionally, no association was apparent between the nicotine levels and the factor scores. The mean Factor 1 scores ranged from 5.9 to 6.5 before the ad libitum use period; scores decreased to a range of 4.8 to 5.2 for NPs, and 2.5 for OBCs, and 4.2 for OBMST at the end of the ad libitum use period. Similarly, mean Factor 2 scores reduced after use from 3.9 to 4.4 at baseline to a range of 2.8 to 3.0 for NPs, and 2.0 and 2.5 for OBCs and OBMST, respectively.
The mean factor scores for satisfaction, psychological reward, enjoyment of sensation, and craving reduction from the mCEQ after 4-h ad libitum use were generally similar with the use of NPs among all nicotine levels and were lower than mean scores for participants’ OBCs and OBMST (Supplementary Fig. 1). The aversion factor scores (average values ranging from 1.2 to 1.5) were similar between the 1.5, 2, 3.5, and 4 mg NPs, OBCs, and OBMST. The 8 mg NP exhibited the highest aversion score (average 2.3).
Based on responses to the TNW Questionnaire under controlled use conditions, the magnitude of the maximum reduction in urges to smoke was significantly larger for OBC relative to the 1.5 and 4 mg NPs (p < 0.05). The magnitude of the reduction in urges to smoke was not statistically significantly different between the OBC and after use of the other NPs (2, 3.5, and 8 mg; p > 0.05; Table 3 and Supplementary Table 3). The magnitude of reduction in craving a cigarette was statistically significantly lower (p < 0.05) for the 1.5, 2, 3.5, and 4 mg NPs and not statistically different for the 8 mg NP compared to OBCs. Compared with OBMST, responses to craving a cigarette on the TNW Questionnaire were significantly lower for the 1.5, 2, 3.5, and 4 mg NPs and responses to urges to smoke were significantly lower for the 1.5 and 4 mg NPs (p < 0.05).
Table 3.
Mean (SD) and median Emax VAS subjective ratings of the of NPs, OBCs, and OBMST
| 1.5 mg NP | 2 mg NP | 3.5 mg NP | 4 mg NP | 8 mg NP | OBC | OBMST | ||
|---|---|---|---|---|---|---|---|---|
| Tobacco/Nicotine Withdrawal Questionnaire | ||||||||
| Urges to smoke |
Mean (SD) Median |
17.7 (20.1) 12.0 |
22.6 (22.4) 18.0 |
23.1 (28.1) 18.0 |
18.8 (24.4) 15.5 |
27.1 (28.2) 17.5 |
29.9 (26.2) 22.0 |
29.6 (31.8) 17.0 |
| Craving a cigarette |
Mean (SD) Median |
19.3 (17.7) 17.0 |
19.6 (21.0) 17.0 |
19.8 (26.8) 14.0 |
21.6 (24.2) 19.0 |
28.0 (26.7) 22.5 |
30.9 (26.7) 25.0 |
32.8 (29.1) 27.0 |
| Direct Effects of Product Questionnaire | ||||||||
| Is the product “Pleasant” right now? |
Mean (SD) Median |
48.0 (24.1) 51.0 |
54.1 (21.6) 57.0 |
53.3 (21.4) 52.5 |
52.1 (23.9) 52.5 |
51.3 (24.5) 50.5 |
69.3 (28.3) 77.0 |
65.7 (22.7) 68.0 |
| Is the product “Satisfying” right now? |
Mean (SD) Median |
46.3 (23.4) 46.5 |
53.7 (21.5) 58.0 |
53.2 (22.6) 52.5 |
50.8 (22.8) 55.5 |
50.9 (25.3) 55.5 |
70.8 (26.2) 75.0 |
67.0 (24.1) 67.0 |
| Is the product making you feel “Calm” right now? |
Mean (SD) Median |
41.8 (23.9) 41.5 |
49.0 (21.9) 49.0 |
47.8 (22.8) 51.5 |
43.2 (27.6) 47.0 |
46.9 (28.6) 48.5 |
63.6 (28.4) 65.0 |
62.0 (29.5) 74.0 |
| Is the product helping you “Concentrate” right now? |
Mean (SD) Median |
34.9 (23.6) 33.5 |
43.9 (25.4) 50.0 |
42.0 (23.9) 45.5 |
38.8 (25.9) 42.5 |
39.1 (27.6) 45.0 |
49.9 (29.5) 56.0 |
49.3 (26.6) 47.0 |
| Is the product making you feel more “Awake” right now? |
Mean (SD) Median |
40.7 (27.9) 33.0 |
44.0 (25.3) 53.0 |
44.4 (25.8) 50.0 |
37.5 (26.3) 41.5 |
40.4 (26.9) 46.0 |
49.6 (30.1) 52.0 |
50.9 (28.9) 51.0 |
| Is the product making you feel “Sick” right now? |
Mean (SD) Median |
16.7 (18.5) 7.0 |
14.3 (18.2) 5.0 |
14.7 (18.2) 7.5 |
18.0 (20.4) 11.0 |
26.2 (30.1) 11.5 |
21.2 (26.0) 10.0 |
20.0 (27.5) 6.0 |
| Is the product reducing your “Hunger” for food right now? |
Mean (SD) Median |
25.4 (24.0) 21.5 |
27.9 (24.7) 28.0 |
27.8 (27.3) 19.0 |
29.4 (25.2) 28.0 |
32.9 (28.0) 30.5 |
38.1 (30.6) 38.0 |
36.6 (31.4) 44.0 |
| Would you like “More” of the product right now? |
Mean (SD) Median |
65.2 (28.0) 69.0 |
65.4 (29.6) 70.0 |
63.2 (26.7) 67.0 |
61.7 (27.7) 61.5 |
51.0 (31.8) 51.5 |
73.1 (22.9) 74.0 |
67.5 (27.5) 68.0 |
The Tobacco/Nicotine Withdrawal (TNW) Questionnaire and the Direct Effects of Product (DEP) Questionnaire are described in Supplementary Table 1. Emax represents the maximum reduction from pre-use in the score on the TNW assessment; Emax represents the maximum score on the DEP assessment
NP nicotine pouch, OBC own brand cigarette, OBMST own brand moist smokeless tobacco, SD standard deviation, VAS visual analog scale
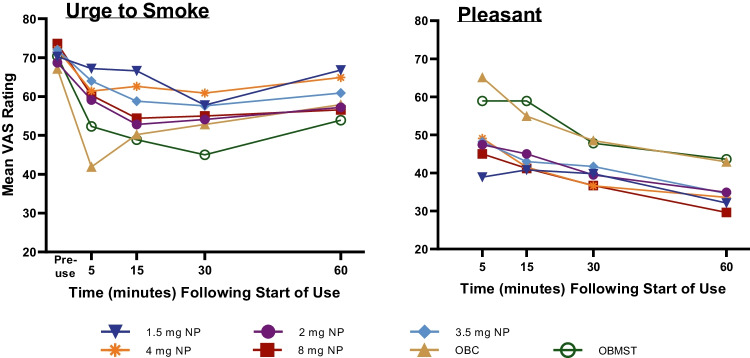
Subjective ratings for the NPs on the DEP Questionnaire were either statistically significantly lower (p < 0.05) than or similar to participants’ OBCs (Table 3; Supplementary Table 3. More participants responded positively to the Use the Product Again Questionnaire for OBCs (77%) or OBMST (93%) than they did for NPs (range: 46% [8 mg] to 66% [1.5 mg]; Supplementary Fig. 2). Among the NPs tested, the 8 mg NP engendered the lowest percentage of positive (46%) responses, the highest percentage of negative (36%) and neutral (18%) responses, relative to OBC and OBMST. The time-course of changes in “urges to smoke” suggests that the maximum reduction during use of the NPs occurred later than for OBCs (15 to 30 min vs. 5 min) and at a similar time to OBMST (30 min; Fig. 2).
Fig. 2.
Mean visual analog scale scores over time for subjective ratings before and/or during controlled use. Items assessed in the Tobacco/Nicotine Withdrawal and the Direct Effects of Product Questionnaires are described in Supplementary Table 1. NP, nicotine pouch; OBC, own brand cigarette; OBMST, own brand moist smokeless tobacco; VAS, visual analog scale
Adverse event reports
No serious adverse events (AEs) were reported, and no participants were discontinued due to AEs. After study product randomization on day − 1, 61 AEs were reported by 20 participants (67%), with 59 of the events being mild in severity and two events (headache in the OBC group and nausea in the 8 mg NP group) being moderate in severity. Headache was the most frequently reported event, experienced by eight participants (27%), followed by nausea, experienced by six participants (20%). All remaining events were reported by four or fewer participants (≤ 13%) each. Seven events were considered to be likely related to study products and 13 events possibly related. The likely/possibly related events occurred across study products and included, but were not limited to, nausea, vomiting, dizziness, and headache, and were as typically expected with use of oral nicotine products.
Discussion
Results of this partial single-blind, randomized, 7-way crossover study suggest that, among current AS and MST users, the abuse potential of NPs tested is not likely to be higher than cigarette or MST. The study results and literature reported PK values for cigarettes (D’Ruiz et al. 2015; Goldenson et al. 2020; O’Connell et al. 2019; Phillips-Waller et al. 2021; Picavet et al. 2016; Rensch et al. 2021; Stiles et al. 2018; Voos et al. 2019; Yuki et al. 2017) and MST (Benowitz et al. 1988; Digard et al. 2013; Fant et al. 1999; Kotlyar et al. 2007; Lunell et al. 2020; Pickworth et al. 2014) indicate that the nicotine delivery and subjective effects of NPs tested are not likely to be greater than cigarette or MST. NPs may be potentially acceptable switching products for AS and adult MST users. A previous study demonstrated that nicotine PK and subjective responses are comparable across different flavor varieties for the 4 mg on!® NP; thus, findings from this study of mint-flavored on!® NPs extend to other on!® flavor varieties (Rensch et al. 2021).
The NPs, regardless of nicotine level, delivered nicotine far slower (higher tmax values) than participants’ OBCs and similar to OBMST. Additionally, the 1.5, 2, 3.5, and 4 mg NPs delivered lower peak nicotine concentrations (lower Cmax values) than participants’ OBCs. The slower onset of nicotine delivery and lower peak concentration, along with the lower ratings of positive subjective effects relative to participants’ OBCs and/or OBMST, suggests likely lower reinforcing effects (Carter et al. 2009; Henningfield and Keenan 1993), and therefore, likely lower abuse potential for the NPs with nicotine levels of 1.5, 2, 3.5, and 4 mg. Our findings demonstrate that the NPs delivered nicotine in a manner consistent with their nicotine level (increased nicotine delivery with increasing nicotine level). These findings suggest that range of nicotine levels may allow individualized product use based on the specific needs of an AS or MST user.
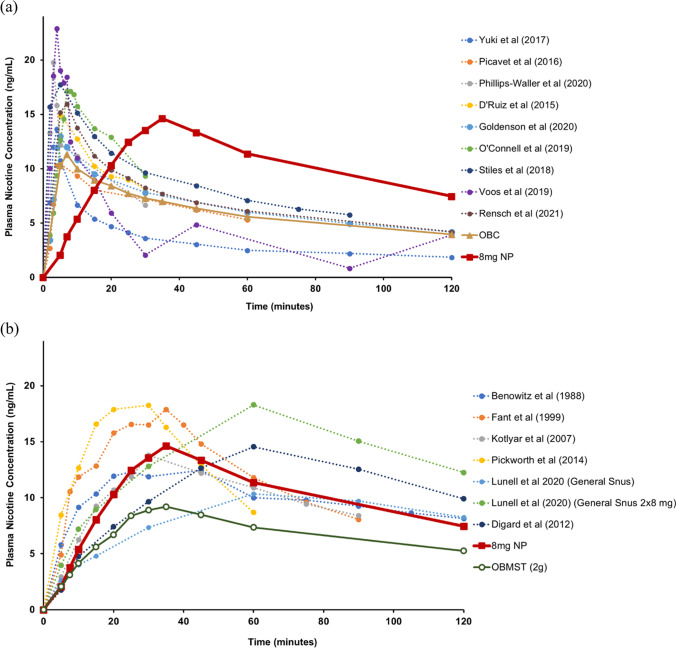
While we observed a higher Cmax for the 8 mg NPs compared to OBC and OBMST used in this study, the mean nicotine Cmax measured during use of the 8 mg NP (15.4 ng/mL) was within the range reported in published literature (Benowitz et al. 1988; D’Ruiz et al. 2015; Digard et al. 2013; Fant et al. 1999; Goldenson et al. 2020; Kotlyar et al. 2007; Lunell and Curvall 2011; Lunell et al. 2020; O’Connell et al. 2019; Phillips-Waller et al. 2021; Picavet et al. 2016; Pickworth et al. 2014; Stiles et al. 2018; Voos et al. 2019; Yuki et al. 2017) for cigarettes (11.8 to 23 ng/mL; Fig. 3a) and MST products (10.6 to 21.4 ng/ml; Fig. 3b). Also, in a previous study, we observed a higher mean Cmax for OBCs was 17.7 ng/mL (Rensch et al. 2021), than that observed in this study (12.2 ng/mL). Similarly, the mean Cmax values observed with OBMST (9.8 mg/mL) were lower than those reported in published literature (range of 12 to 19 ng/mL; Benowitz and Gourlay 1997; Benowitz et al. 1988; Fant et al. 1999; Kotlyar et al. 2007; Pickworth et al. 2014). Overall, based on the study results and literature reported PK values for cigarettes and MST, the abuse potential for the NPs is not likely to be higher than cigarettes or MST currently available in the US market.
Fig. 3.
Plasma nicotine values over time during use of the 8 mg NP and representative published data for a cigarettes and b smokeless tobacco products. The plasma pharmacokinetic profiles from the published literature (dotted lines) are replotted from estimated values based on figures in the publications. For consistency, all data has been baseline adjusted. Results from this study (solid lines) are presented; only the 8 mg NP nicotine PK profile is presented because the 8 mg NP exhibited the highest nicotine PK relative to the lower nicotine level NPs. NP, nicotine pouch; OBC, own brand cigarette; OBMST, own brand moist smokeless tobacco
Several factors could account for the lower-than-expected nicotine delivery from OBCs and OBMST in this study. While we have no direct evidence, the product use behavior may be different among exclusive smokers or exclusive MST users vs. dual users of cigarettes and MST products. There is some evidence of differential product use behavior; for example, Felicione et al. (2020) observed significantly lower cotinine levels among dual users on days when they only smoked cigarettes as opposed to when they dual used both cigarettes and MST. Additional factors likely contributing to the lower-than-expected nicotine delivery could be due to differences in own brand products or inherent inter-subject variability, including variations in use behavior, such as the size of the MST quid used in the ad libitum vs. controlled use periods. For example, the percent coefficient of variation was relatively higher for OBC (75.4%) in this study (Supplementary Table 2) compared to that reported for OBC (42.4%) by Rensch et al. (2021).
During the ad libitum use, participants reported that the NPs were satisfying, pleasant, and reduced craving a cigarette and urges to smoke (based on the QSU-Brief scores). However, the magnitude of change was relatively smaller compared to that observed with OBCs. Similar results were observed based on the responses to mCEQ related to satisfaction, psychological reward, enjoyment of sensation, and craving reduction.
Controlled use of the NPs resulted in a reduction in scores on similar items (e.g., urges to smoke, craving a cigarette, pleasantness, and satisfaction) addressed in the TNW and DEP Questionnaires. However, the magnitude of change was lower relative to participants’ OBCs and OBMST. The maximum change in cigarette craving and urges to smoke were not statistically significantly different for the 8 mg NP relative to OBC and OBMST (Supplementary Table 3). The maximum change in these subjective outcomes occurred later for 8 mg NP (15 min) than for OBC (5 min) and at a similar time to OBMST. These data suggest that the 8 mg NP can provide some cigarette craving relief, despite lower positive subjective ratings of the product’s effects. Nonetheless, the aversion factor score was highest for the 8 mg NP relative to OBC and OBMST (Supplementary Fig. 1).
The time-course of maximum reduction in nicotine withdrawal symptoms was similar among all NPs and OBMST and occurred 10 to 25 min later than with OBCs. Lower reinforcing efficacy can be expected from the NPs, regardless of nicotine level, because despite the positive subjective effects and some cigarette craving relief, the magnitude of change was lower than participants’ OBCs or OBMST. These observations suggest that, regardless of nicotine level, the NPs relieve nicotine withdrawal symptoms, but not to the same extent as smoking.
Potential limitations should be considered when drawing broad conclusions from this study. First, we did not directly measure dependence potential of the NPs because validated methods do not exist for such products. Additionally, even if such a measurement tool existed, it would be difficult to distinguish dependence resulting solely from the NPs because of the confounding effects of pre-existing nicotine dependence inherent to the study population since they are current tobacco users. Second, the study design involved measurement of subjective responses after a relatively brief duration of a 4-h period of in-clinic ad libitum product use. The lower subjective response results seen with the NPs compared to OBCs could possibly be due to NPs being novel and different, whereas the study participants are more familiar with their OBCs. Product familiarity may play an important role in the favorable subjective responses. Nonetheless, the in-clinic product use allowed for obtaining reliable observations since the product use took place in the presence of the clinic staff. Third, the controlled use condition of 30-min duration for a single pouch may not reflect typical real-world usage behavior. Vansickel et al. observed that typical use behavior for these pouches under actual use conditions is around 15 min (Vanickel et al. 2021); thus, the 30-min controlled use condition may reflect an extreme condition. Finally, the relatively small sample size and lack of sufficient gender balance may be a potential limitation as well. However, as described in the “Materials and methods” section, the sample size is reasonable and is typical of other studies examining PK and subjective effects across different tobacco/nicotine conditions (Carter et al. 2009; Cobb et al. 2010; Cox et al. 2001; Gray et al. 2008; Hatsukami et al. 2004; Kotlyar et al. 2007; Lunell and Curvall 2011; Perkins et al. 1997). Additionally, gender balance in this study reflects the population of ST product users, as it is well established that the vast majority of ST users are predominantly male. According to Centers for Disease Control and Prevention, non-Hispanic whites have the highest prevalence of ST use, and the number of women who use ST products is so small that statistically reliable estimates could not even be calculated (CDC 2021). Nonetheless, these limitations may restrict the generalizability to all tobacco/nicotine product users, particularly since differences in male and female nicotine PK have been reported by Benowitz et al. (2006). Despite these limitations, the nicotine PK analysis and the subjective measures used in this study are well-established methods for evaluating the abuse potential of tobacco/nicotine products (Carter et al. 2009; Cobb et al. 2010; Cox et al. 2001; Gray et al. 2008; Hanson et al. 2009; Henningfield and Keenan 1993; Lunell and Curvall 2011; Lunell et al. 2020; Vansickel et al. 2010, 2021a, b).
Overall, results from this study demonstrated that the 1.5, 2, 3.5, and 4 mg NPs likely have a lower abuse potential relative to OBCs and OBMST and the 8 mg NP is not likely to exhibit higher abuse potential than OBCs and OBMST. The abuse liability or dependence potential of a tobacco product must be considered in the context of that product’s harm reduction potential. Assessment of the abuse potential of the NPs informs the likelihood of switching from cigarettes and MST. In an actual use study, a modest proportion of AS and MST product users (who did not intend to quit using tobacco) switched completely to NPs when allowed open access to NPs under near real-world conditions over 6 weeks (Becker et al. 2021). In that actual use study, approximately 28% of AS and 72% of ST product users switched completely to NPs at the end of 6 weeks. These data demonstrate that the NPs are likely to be adopted by a portion of AS and MST users. Reduction in harm can only be achieved if adult tobacco users are willing to switch products. Therefore, for a tobacco/nicotine product to have the potential for reducing risk, the associated abuse potential should be sufficiently high enough, but not higher, than that of cigarettes (Institute of Medicine 2012). The abuse potential for the NPs, assessed in this study, is not likely to be higher than cigarettes or MST products and the NPs may be potentially acceptable substitutes for cigarettes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Scientific writing support was provided by Meghan L. Thompson, PharmD, PhD, of Altria Client Services LLC.
Declarations
This study was funded by Altria Client Services LLC, and all authors are employees of Altria Client Services LLC. The Advarra Institutional Review Board (IRB) (Columbia, MD) reviewed and approved this study. The study was conducted in accordance with Food and Drug Administration (FDA) regulations as described in the Code of Federal Regulations (CFR) 21 Parts 50 and 56, Department of Health and Human Services regulations as described in 45 CFR 46, guidelines resulting from the International Council for Harmonisation (ICH) E6 Good Clinical Practice (GCP), the European Union (EU) directive 2001/20/EC, and the ethical principles set forth in the Declaration of Helsinki. In addition, the IRB operates in compliance with the portions of the Health Insurance Portability and Accountability Act of 1996 (HIPAA Privacy Rule) that apply to research, as described in 45 CFR Parts 160 and 164. All participants in this study reviewed, signed, and dated the informed consent form prior to study initiation.
Conflict of interest
The authors are employees of Altria Client Services LLC.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Becker E, McCaffrey S, Larson EH, Sarkar M (2021) Impact of using an oral tobacco derived nicotine pouch product on cigarette consumption among adult smokers under near real-world conditions over six-weeks 27th Society for Research on Nicotine and Tobacco (SRNT), Virtual Conference
- Benowitz N, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29:1422–1431. doi: 10.1016/s0735-1097(97)00079-x. [DOI] [PubMed] [Google Scholar]
- Benowitz N, Porchet H, Sheiner L, Jacob P., 3rd Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther. 1988;44(1):23–28. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79(5):480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Carter LP, Stitzer ML, Henningfield JE, O’Connor RJ, Cummings KM, Hatsukami D. Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiol Biomark Prev. 2009;18(12):3241–3262. doi: 10.1158/1055-9965.EPI-09-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2021) Smokeless tobacco product use in the United States. Retrieved 31 Jan 2022 from
- Cobb CO, Weaver MF, Eissenberg T. Evaluating the acute effects of oral, non-combustible potential reduced exposure products marketed to smokers. Tob Control. 2010;19(5):367–373. doi: 10.1136/tc.2008.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults - United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(46):1736–1742. doi: 10.15585/mmwr.mm6946a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-Brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- D’Ruiz CD, Graff DW, Yan XS. Nicotine delivery, tolerability and reduction of smoking urge in smokers following short-term use of one brand of electronic cigarettes. BMC Public Health. 2015;15:991. doi: 10.1186/s12889-015-2349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digard H, Proctor C, Kulasekaran A, Malmqvist U, Richter A. Determination of nicotine absorption from multiple tobacco products and nicotine gum. Nicotine Tob Res. 2013;15(1):255–261. doi: 10.1093/ntr/nts123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fant RV, Henningfield JE, Nelson RA, Pickworth WB. Pharmacokinetics and pharmacodynamics of moist snuff in humans. Tob Control. 1999;8(4):387–392. doi: 10.1136/tc.8.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felicione NJ, Ozga-Hess JE, Ferguson SG, Dino G, Kuhn S, Haliwa I, Blank MD. Cigarette smokers’ concurrent use of smokeless tobacco: dual use patterns and nicotine exposure. Tob Control. 2020 doi: 10.1136/tobaccocontrol-2019-055345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration (2015) Premarket tobacco application technical project lead review. Swedish Match North America, Inc. Retrieved from https://www.fda.gov/media/94582/download
- Goldenson NI, Buchhalter AR, Augustson EM, Rubinstein ML, Henningfield JE. Abuse liability assessment of the JUUL system in four flavors relative to combustible cigarette, nicotine gum and a comparator electronic nicotine delivery system among adult smokers. Drug Alcohol Depend. 2020;217:108395. doi: 10.1016/j.drugalcdep.2020.108395. [DOI] [PubMed] [Google Scholar]
- Gottlieb S, Zeller M. A nicotine-focused framework for public health. N Engl J Med. 2017;377(12):1111–1114. doi: 10.1056/NEJMp1707409. [DOI] [PubMed] [Google Scholar]
- Gray JN, Breland AB, Weaver M, Eissenberg T. Potential reduced exposure products (PREPs) for smokeless tobacco users: clinical evaluation methodology. Nicotine Tob Res. 2008;10(9):1441–1448. doi: 10.1080/14622200802323258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Ator NA. Principles of initial experimental drug abuse liability assessment in humans. Drug Alcohol Depend. 2003;70(3 Suppl):S41–54. doi: 10.1016/s0376-8716(03)00098-x. [DOI] [PubMed] [Google Scholar]
- Hanson K, O’Connor R, Hatsukami D. Measures for assessing subjective effects of potential reduced-exposure products. Cancer Epidemiol Biomark Prev. 2009;18(12):3209–3224. doi: 10.1158/1055-9965.EPI-09-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, Lemmonds C, Tomar SL. Smokeless tobacco use: harm reduction or induction approach? Prev Med. 2004;38(3):309–317. doi: 10.1016/j.ypmed.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Joseph AM, Lesage M, Jensen J, Murphy SE, Pentel PR, Kotlyar M, Borgida E, Le C, Hecht SS. Developing the science base for reducing tobacco harm. Nicotine Tob Res. 2007;9 Suppl 4(0 4):S537–S553. doi: 10.1080/14622200701679040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61(5):743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- Holm H, Jarvis MJ, Russell MA, Feyerabend C. Nicotine intake and dependence in Swedish snuff takers. Psychopharmacology. 1992;108(4):507–511. doi: 10.1007/BF02247429. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (2012) Scientific standards for studies on modified risk tobacco products. The National Academies Press. http://www.nap.edu/catalog/13294/scientific-standards-for-studies-on-modified-risk-tobacco-products
- Kotlyar M, Mendoza-Baumgart MI, Li ZZ, Pentel PR, Barnett BC, Feuer RM, Smith EA, Hatsukami DK. Nicotine pharmacokinetics and subjective effects of three potential reduced exposure products, moist snuff and nicotine lozenge. Tob Control. 2007;16(2):138–142. doi: 10.1136/tc.2006.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunell E, Curvall M. Nicotine delivery and subjective effects of Swedish portion snus compared with 4 mg nicotine polacrilex chewing gum. Nicotine Tob Res. 2011;13(7):573–578. doi: 10.1093/ntr/ntr044. [DOI] [PubMed] [Google Scholar]
- Lunell E, Fagerstrom K, Hughes J, Pendrill R. Pharmacokinetic comparison of a novel non-tobacco-based nicotine pouch (ZYN) with conventional, tobacco-based Swedish snus and American moist snuff. Nicotine Tob Res. 2020 doi: 10.1093/ntr/ntaa068. [DOI] [PubMed] [Google Scholar]
- Lunell E, Lunell M. Steady-state nicotine plasma levels following use of four different types of Swedish snus compared with 2-mg Nicorette chewing gum: a crossover study. Nicotine Tob Res. 2005;7(3):397–403. doi: 10.1080/14622200500125468. [DOI] [PubMed] [Google Scholar]
- O’Connell G, Pritchard JD, Prue C, Thompson J, Verron T, Graff D, Walele T. A randomised, open-label, cross-over clinical study to evaluate the pharmacokinetic profiles of cigarettes and e-cigarettes with nicotine salt formulations in US adult smokers. Intern Emerg Med. 2019;14(6):853–861. doi: 10.1007/s11739-019-02025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Caggiula A, Wilson AS, Stiller RL. Acute reinforcing effects of low-dose nicotine nasal spray in humans. Pharmacol Biochem Behav. 1997;56(2):235–241. doi: 10.1016/s0091-3057(96)00216-x. [DOI] [PubMed] [Google Scholar]
- Phillips-Waller A, Przulj D, Smith KM, Pesola F, Hajek P. Nicotine delivery and user reactions to Juul EU (20 mg/ml) compared with Juul US (59 mg/ml), cigarettes and other e-cigarette products. Psychopharmacology. 2021;238(3):825–831. doi: 10.1007/s00213-020-05734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picavet P, Haziza C, Lama N, Weitkunat R, Ludicke F. Comparison of the pharmacokinetics of nicotine following single and ad libitum use of a tobacco heating system or combustible cigarettes. Nicotine Tob Res. 2016;18(5):557–563. doi: 10.1093/ntr/ntv220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickworth WB, Rosenberry ZR, Gold W, Koszowski B. Nicotine absorption from smokeless tobacco modified to adjust pH. J Addict Res Ther. 2014;5(3):1000184. doi: 10.4172/2155-6105.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensch J, Liu J, Wang J, Vansickel A, Edmiston J, Sarkar M. Nicotine pharmacokinetics and subjective response among adult smokers using different flavors of on!® nicotine pouches compared to combustible cigarettes. Psychopharmacology. 2021 doi: 10.1007/s00213-021-05948-y. [DOI] [PubMed] [Google Scholar]
- Rensch S, Vansickel AR, Edmiston J, Wang J, Liu J, Sarkar M (2020) Characterization of nicotine pharmacokinetics from on!® nicotine pouches in adult smokers SRNT 26th Annual Meeting New Orleans, LA
- Rose JE, Salley A, Behm FM, Bates JE, Westman EC. Reinforcing effects of nicotine and non-nicotine components of cigarette smoke. Psychopharmacology. 2010;210(1):1–12. doi: 10.1007/s00213-010-1810-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Helen G, Havel C, Dempsey DA, Jacob P, Benowitz NL. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111:535–544. doi: 10.1111/add.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles MF, Campbell LR, Jin T, Graff DW, Fant RV, Henningfield JE. Assessment of the abuse liability of three menthol Vuse Solo electronic cigarettes relative to combustible cigarettes and nicotine gum. Psychopharmacology. 2018;235(7):2077–2086. doi: 10.1007/s00213-018-4904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services (2014) The health consequences of smoking–50 years of progress: a report of the surgeon general. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, & Office on Smoking and Health
- Vansickel A, Baxter S, Sherwood N, Kong M, Campbell L. Human abuse liability assessment of tobacco and nicotine products: approaches for meeting current regulatory recommendations. Nicotine Tob Res. 2021 doi: 10.1093/ntr/ntab183. [DOI] [PubMed] [Google Scholar]
- Vansickel A, Nguyen N, Edmiston J, Sarkar M (2021b) Pharmacokinetic modeling and simulation of single and multiple uses of an oral tobacco-derived nicotine product compared to moist smokeless tobacco products and combustible cigarettes under actual use conditions SRNT 27th Annual Meeting
- Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19:1945–1953. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos N, Kaiser L, Mahoney MC, Bradizza CM, Kozlowski LT, Benowitz NL, O’Connor RJ, Goniewicz ML. Randomized within-subject trial to evaluate smokers’ initial perceptions, subjective effects and nicotine delivery across six vaporized nicotine products. Addiction. 2019;114(7):1236–1248. doi: 10.1111/add.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KA, Ballentine RM, Brown AP, Jin XC, Lopez VF, M S, CB M, S MM, MJ M, Danielson TL (2020) Characterization of on! nicotine pouches – Part 1: HPHCs CORESTA, Online Congress
- Yuki D, Sakaguchi C, Kikuchi A, Futamura Y. Pharmacokinetics of nicotine following the controlled use of a prototype novel tobacco vapor product. Regul Toxicol Pharmacol. 2017;87:30–35. doi: 10.1016/j.yrtph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Zeller M, Hatsukami D, Strategic Dialogue on Tobacco Harm Reduction G The Strategic Dialogue on Tobacco Harm Reduction: a vision and blueprint for action in the US. Tob Control. 2009;18(4):324–332. doi: 10.1136/tc.2008.027318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.