Abstract
The Escherichia coli genome carries seven rRNA (rrn) operons, each containing three rRNA genes. The presence of multiple operons has been an obstacle to many studies of rRNA because the effect of mutations in one operon is diluted by the six remaining wild-type copies. To create a tool useful for manipulating rRNA, we sequentially inactivated from one to all seven of these operons with deletions spanning the 16S and 23S rRNA genes. In the final strain, carrying no intact rRNA operon on the chromosome, rRNA molecules were expressed from a multicopy plasmid containing a single rRNA operon (prrn). Characterization of these rrn deletion strains revealed that deletion of two operons was required to observe a reduction in the growth rate and rRNA/protein ratio. When the number of deletions was extended from three to six, the decrease in the growth rate was slightly more than the decrease in the rRNA/protein ratio, suggesting that ribosome efficiency was reduced. This reduction was most pronounced in the Δ7 prrn strain, in which the growth rate, unlike the rRNA/protein ratio, was not completely restored to wild-type levels by a cloned rRNA operon. The decreases in growth rate and rRNA/protein ratio were surprisingly moderate in the rrn deletion strains; the presence of even a single operon on the chromosome was able to produce as much as 56% of wild-type levels of rRNA. We discuss possible applications of these strains in rRNA studies.
In Escherichia coli, the number of ribosomes per cell is proportional to the growth rate to satisfy the cell’s demand for protein synthesis (23). At fast doubling times there are as many as 70,000 ribosomes per E. coli cell, while at lower growth rates this number is reduced to 20,000 or less (5). Control of ribosome content is exerted at the level of transcription of the seven rRNA (rrn) operons on the chromosome (18, 29). Expression of rRNA is gene dosage independent; when the number of rRNA operons in the cell is increased by the presence of plasmid-borne operons, total rRNA synthesis rates remain constant (feedback control) (10, 19, 21, 23). Conversely, inactivation of up to four rrn operons on the chromosome leads to a compensatory increase in expression of the remaining intact copies (7). Regulation of rrn expression occurs at the level of transcription initiation (23), and one effector of both growth rate-dependent and feedback control is thought to be the intracellular concentrations of ATP and GTP, the initiating nucleotides of the rrn P1 promoters (14).
Although it is generally assumed that the redundancy of rRNA operons in E. coli has evolved to support the high levels of ribosome production necessary for rapid growth rates (22, 28), there is also evidence suggesting that E. coli requires all of its operons for optimal adaptation to changing physiological conditions (8). rRNA operon multiplicity among the best-studied eubacteria has, however, significantly impeded the genetic study of rRNA structure, function, and evolution in these organisms. Besides the 7 rrn operons present in E. coli (24), Bacillus subtilis (4, 26) and Clostridium perfringens (15) have 10 each and Lactococcus lactis (2, 34) has 6 copies of each of the rRNA genes. As a means of overcoming the multiplicity problem, we sequentially inactivated rrn operons in E. coli until we ultimately succeeded in constructing a strain containing a single exchangeable operon on a plasmid.
We had previously inactivated up to four of the rRNA operons by a deletion-insertion mutagenesis scheme in which each deletion site was filled in with a different antibiotic resistance gene (7). While this technique provided a facile means of operon inactivation, there was an insufficient number of suitable antibiotic resistance genes to inactivate all seven operons, and we were concerned that the accumulation of antibiotic resistance mechanisms would influence the physiology of the cell. In the study reported here, we therefore employed a different approach, in which many of the operons were inactivated without the introduction of antibiotic resistance cassettes, and succeeded in inactivating all seven chromosomal rRNA operons. The survival of this strain is ensured by the presence of a plasmid-encoded rRNA operon. In a separate publication (1), we have demonstrated one important use of this strain by successfully exchanging the wild-type plasmid-borne E. coli rrn operon for operons from Salmonella typhimurium and Proteus vulgaris as well as a hybrid operon in which the GTPase center of the E. coli 23S rRNA had been replaced by the corresponding domain from Saccharomyces cerevisiae. Here we describe in detail the construction of the rrn deletion series and an initial study of their physiological properties, as much to answer questions about the effect of rrn multiplicity on bacterial physiology as to characterize a set of strains we believe will be useful to the scientific community.
MATERIALS AND METHODS
Bacterial growth conditions.
The bacterial growth conditions were described previously (1).
Exchange of alleles with a polA strain and the sacB gene.
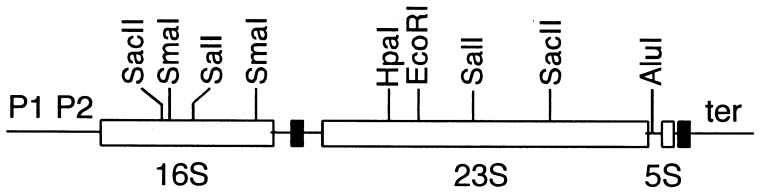
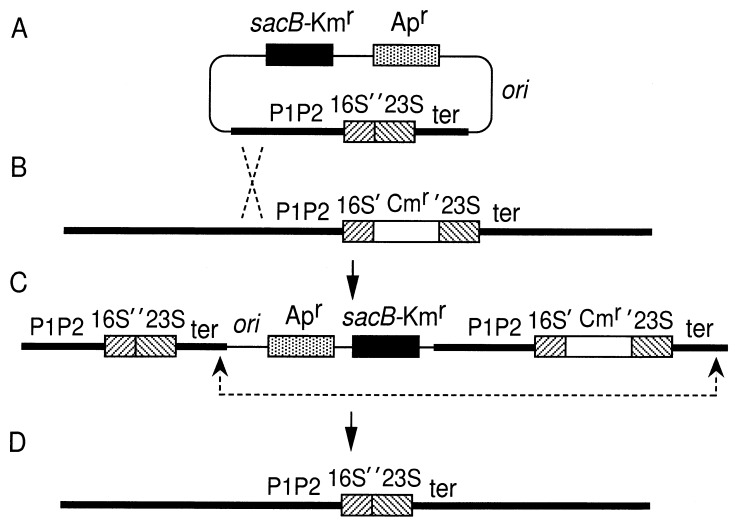
We have developed an effective method for allele exchange between chromosomal and plasmid-encoded rRNA operons by modifying previously reported techniques (20, 31). DNA fragments containing each of the seven rRNA operons (Fig. 1) and their flanking regions were first cloned into ColE1-type plasmid vectors carrying the ampicillin resistance (Apr) gene. Deletion mutations inactivating both the 16S and 23S rRNA genes were then introduced into each operon. A cassette containing the B. subtilis sacB gene and the kanamycin resistance marker (sacB-Kmr) was then prepared from pBIP3 (31) and inserted into the plasmid within the vector sequence (Fig. 2A). Expression of sacB in E. coli is lethal in the presence of sucrose (16). Thus, the cassette allows both positive (Kmr) and negative (sucrose-sensitive [Sucs]) selection of the resulting plasmid. The plasmid was then electroporated into polA1 (Am) mutant cells in which the corresponding rRNA operon on the chromosome had been inactivated with the chloramphenicol resistance (Cmr) gene (the cat gene [Fig. 2B]). We took advantage of previous work from our laboratory (9) in which each rRNA operon on the chromosome was inactivated by this gene. Initiation of DNA replication from the ColE1-type origin requires the polA gene product, DNA polymerase I. Thus, polA mutant cells transformed to Apr and Kmr are likely to contain the entire plasmid integrated into the chromosome by a single crossover event (Fig. 2C). All integrants showed sucrose sensitivity. Since the rRNA genes encoded in the seven operons have essentially identical primary structures, we relied on flanking sequences to direct recombination with the desired operon, and by Southern blot analysis (rrnB) or P1 transduction (rrnH, rrnG, and rrnA) we confirmed that integration had occurred in the correct operon. In the latter case, P1 lysates were prepared on each integrant and the cotransduction frequency of antibiotic resistance markers was analyzed. If the plasmid integrated into the correct operon, the Cmr marker of the inactivated chromosomal operon and the Apr and Kmr markers introduced by the plasmid cotransduced with a high frequency.
FIG. 1.
The common structure of the rRNA operons in E. coli. Open and filled rectangles represent rRNA (16S, 23S, and 5S) and tRNA genes, respectively. rrnB, rrnC, rrnE, and rrnG contain the spacer tRNA gene for Glu-2, and the other operons (rrnA, rrnD, and rrnH) contain the spacer tRNA genes for Ile-1 and Ala-1B (25). Distal tRNA genes are encoded by only three operons: rrnC contains the tRNA genes for Asp-1 and Trp, and rrnD and rrnH contain the tRNA genes for Thr-1 and Asp-1, respectively. The figure also indicates the relative positions of promoters (P1 P2), terminators (ter), and relevant restriction sites.
FIG. 2.
The basic strategy for allele exchange. Thick and thin lines represent chromosomal and plasmid sequences, respectively. The hatched rectangles indicate the 16S and 23S rRNA genes. The 5S rRNA and tRNA genes are not shown. Stippled and open rectangles represent the ampicillin and chloramphenicol resistance genes, respectively, and closed rectangles the sacB-Kmr cassette. ori indicates the relative position of the ColE1-type replication origin. Broken lines indicate possible crossover sites for a successful allele exchange. In panels B, C, and D, only a part of the chromosome is shown. See Fig. 1 for definitions of the other symbols.
If a second crossover occurs, as indicated in Fig. 2C, the vector DNA, which includes the Apr gene and the sacB-Kmr cassette, and the cat-containing operon are excised, leaving the operon with the deletion mutation on the chromosome (Fig. 2D). Cells that had undergone such an excision event were effectively isolated by selecting the integrants for sucrose resistance followed by screening the Sucr cells for sensitivity to ampicillin, kanamycin, and chloramphenicol. In practice, we grew several Sucs Cmr integrants independently at 37°C overnight in Luria-Bertani (LB) broth without antibiotics. The cultures were then diluted and plated on salt-free LB plates containing 8% sucrose (3). The plates were incubated at 30°C (for rrnB, rrnH, and rrnG) or 37°C (for rrnA) for 20 h, and colonies were picked for a chloramphenicol sensitivity test. Typically ∼1% of the cells grown overnight in the absence of antibiotics were Sucr, and ∼5% of these Sucr cells were Cms. All Cms cells were sensitive to ampicillin and kanamycin.
Construction of rrn deletion strains.
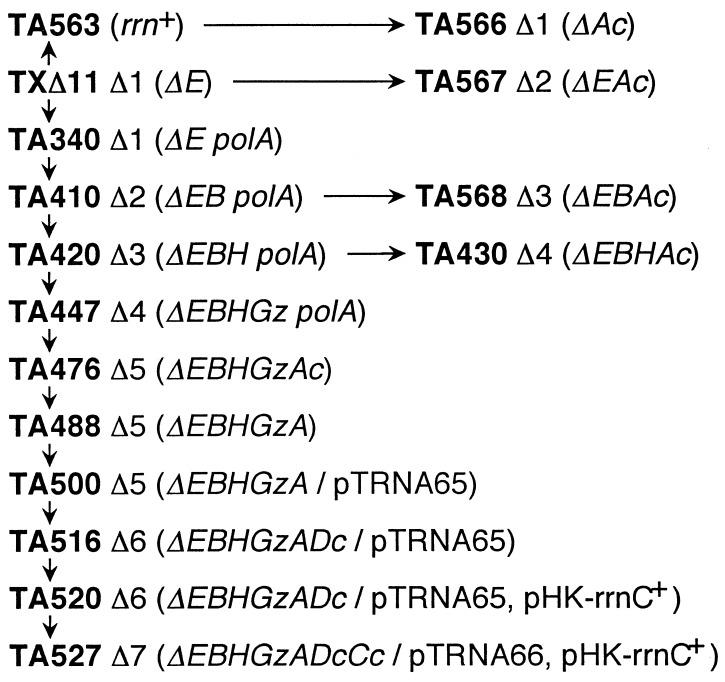
As a starting point for rrn operon inactivation, we used Ellwood and Nomura’s TXΔ11 strain (12), which carries a large chromosomal deletion spanning the rrnE operon. Before the first allele exchange, the polA1 mutation was introduced into TXΔ11 by P1 transduction by virtue of its linkage to zih::Tn10 (encoding tetracycline resistance), generating TA340 (Fig. 3; Table 1). The presence of the mutation in this strain was verified by its sensitivity to methyl methanethiosulfonate.
FIG. 3.
The pedigree of rrn deletion strains. Inactivated rRNA operons are indicated by uppercase letters derived from their specific operon names (for example, A for rrnA). When the inactivation was carried out by a deletion-insertion mutation, an uppercase letter is followed by a lowercase c or z, representing the inserted gene cat+ or lacZ+, respectively (for example, Ac for rrnA::cat+). See Table 1 for precise genotypes. polA mutant strains are resistant to tetracycline since these mutations are linked to Tn10. pTRNA and pHK-rrnC+ contain spectinomycin (Spcr) and Kmr markers, respectively.
TABLE 1.
E. coli strains used in this study
| Strain | Relevant genotype | Source, reference, or construction |
|---|---|---|
| TXΔ11b | Δ(purDH-rrnE-metA) | 12 |
| Strains of TXΔ11 background | ||
| TA340 | TXΔ11 polA1 zih::Tn10 | TXΔ11 × P1.AQ8809, with selection for Tcr, PolA− |
| TA405 | TA340 Δ(rrsB-gltT-rrlB)1::kan+ | TA340 × P1.CC164, with selection for Kmr |
| TA406 | TA340 Δ(rrsB-gltT-rrlB)1::kan+zij::pMA101 | TA405 transformed with pMA101; selection for Apr, Sucs |
| TA410 | TA340 Δ(rrsB-gltT-rrlB)101 | Sucr derivative of TA406; Aps Kms |
| TA415 | TA410 Δ(rrsH-ileV-alaV-rrlH)37::cat+ | TA410 × P1.JP37, with selection for Cmr |
| TA418 | TA410 Δ(rrsH-ileV-alaV-rrlH)37::cat+zaf::pMA103 | TA415 transformed with pMA103; selection for Apr, Kmr, Sucs |
| TA420 | TA410 Δ(rrsH-ileV-alaV-rrlH)103 | Sucr derivative of TA418; Aps Kms Cms |
| TA430 | TA420 Δ(rrsA-ileT-alaT-rrlA)1::cat+polA+ | TA420 × P1.CC164, with selection for Cmr, Tcs, PolA+ |
| TA443 | TA420 Δ(rrsG-gltW-rrlG)33::cat+ | TA420 × P1.JP33, with selection for Cmr |
| TA445 | TA420 Δ(rrsG-gltW-rrlG)33::cat+zfg::pNY30 | TA443 transformed with pNY30; selection for Apr, Kmr, Sucs |
| TA447 | TA420 Δ(rrsG-gltW-rrlG)30::lacZ+ | Sucr derivative of TA445; Aps Kms Cms |
| TA472 | TA410 Δ(rrsA-ileT-alaT-rrlA)1::cat+ | TA410 × P1.CC164, with selection for Cmr, Tcr, PolA− |
| TA476 | TA447 polA+ Δ(rrsA-ileT-alaT-rrlA)1::cat+ | TA447 × P1.CC164, with selection for Cmr, Tcs, PolA+ |
| TA480 | TA410 Δ(rrsA-ileT-alaT-rrlA)1::cat+zih::pNY34 | TA472 transformed with pNY34; selection for Apr, Kmr, Sucs |
| TA485 | TA447 polA+ Δ(rrsA-ileT-alaT-rrlA)1::cat+zih::pNY34 | TA476 × P1.TA480, with selection for Cmr, Kmr, Apr, Tcs, PolA+, Sucs |
| TA488 | TA447 polA+ Δ(rrsA-ileT-alaT-rrlA)34 | Sucr derivative of TA485; Aps Kms Cms |
| TA500 | TA488/pTRNA65 | TA488 transformed with pTRNA65; selection for Spcr |
| TA516 | TA488 Δ(rrsD-ileU-alaU-rrlD)25::cat+/pTRNA65 | TA500 × P1.JP25, with selection for Cmr |
| TA520 | TA488 Δ(rrsD-ileU-alaU-rrlD)25::cat+/pTRNA65 pHK-rrnC+ | TA516 transformed with pHK-rrnC+; selection for Kmr |
| TA520.5 | TA488 Δ(rrsD-ileU-alaU-rrlD)25::cat+/pTRNA66 pHK-rrnC+ | tRNA2Glu deletion from pTRNA65 in TA520, generating pTRNA66 |
| TA525 | TA520.5 Δ(rrsC-gltU-rrlC)15::cat+ilv500::Tn10 | TA520.5 × P1.TA575, with selection for Tcr, rrnC::cat+ |
| TA527 | TA520.5 Δ(rrsC-gltU-rrlC)15::cat+ilv+ | TA525 × P1.JP15, with selection for Ilv+, Tcs |
| TA559.5 | TXΔ11/pBEU49 | TXΔ11 transformed with pBEU49; selection for Apr, Kmr, Tsa |
| TA560c | pur+ rrnE+ metA+ metB1 btuB3191::Tn10/pBEU49 | TA559.5 × Hfr CAG5052, with selection for Tcr Apr, Kmr, Pur+, rrnE+, Met− |
| TA563c | pur+ rrnE+ metA+ metB1 btuB3191::Tn10 | Temperature-resistant derivative of TA560; Aps Kms |
| TA566 | TA563 Δ(rrsA-ileT-alaT-rrlA)1::cat+ | TA563 × P1.CC164, with selection for Cmr |
| TA567 | TXΔ11 Δ(rrsA-ileT-alaT-rrlA)1::cat+ | TXΔ11 × P1.CC164, with selection for Cmr |
| TA568 | TA410 Δ(rrsA-ileT-alaT-rrlA)1::cat+polA+ | TA410 × P1.CC164, with selection for Cmr, Tcs, PolA+ |
| Intermediates in strain constructions | ||
| AQ8809 | polA1 zih::Tn10 | T. Kogoma |
| CAG5052 | KL227 btuB3191::Tn10 | 30 |
| CAG18431 | MG1655 ilv500::Tn10 | 30 |
| CC164 | W1485 Δ(rrsB-gltT-rrlB)1::kan+ Δ(rrsA-ileT-alaT-rrlA)1::cat+ Δ(rrsG-gltW-rrlG)1::spc+ | BAG1 in reference 7 |
| JP15 | W1485 Δ(rrsC-gltU-rrlC)15::cat+ | W1485 ΔC in reference 9 |
| JP25 | W1485 Δ(rrsD-ileU-alaU-rrlD)25::cat+ | W1485 ΔD in reference 9 |
| JP33 | W1485 Δ(rrsG-gltW-rrlG)33::cat+ | W1485 ΔG in reference 9 |
| JP37 | W1485 Δ(rrsH-ileV-alaV-rrlH)37::cat+ | W1485 ΔH in reference 9 |
| TA575 | W1485 Δ(rrsC-gltU-rrlC)15::cat+ilv500::Tn10 | JP15 × P1.CAG18431, with selection for Tcr, Cmr |
Ts, temperature sensitive.
The remaining genotypes are F− ara Δlac thi.
The presence of metA+ metB1 in these strains has not been verified (see Materials and Methods).
(i) Inactivation of rrnB.
The rrnB operon in TA340 was first inactivated by introducing the Δ(rrsB-gltT-rrlB)1::kan+ mutation (7). (For rrnB, a cat-containing operon was not used in this step.) The resulting strain (TA405) was then transformed to ampicillin resistance (TA406) with pMA101 (Table 2), and the Δ(rrsB-gltT-rrlB)1::kan+ allele was removed from the chromosome as described above except that Sucr cells were screened for sensitivity to ampicillin and kanamycin. The presence of the Δ(rrsB-gltT-rrlB)101 deletion mutation on the chromosome of one of the Aps Kms clones (TA410) was confirmed by PCR amplification of ribosomal DNA followed by agarose gel analysis. The primers used for the PCR were 5′-GGCCTAACACATGCAAGTCGAA-3′ and 5′-GCTTACACACCCGGCCTATCAA-3′, which hybridize near the 5′ end of the 16S gene and the 3′ end of the 23S gene, respectively. With these primers, the rrnB operon carrying the Δ(rrsB-gltT-rrlB)101 deletion gives a 2,287-bp PCR fragment whereas the wild-type and kan-containing operons give 4,791- and 4,026-bp fragments, respectively.
TABLE 2.
Plasmids used in this study
| Plasmid designation | Relevant characteristics | Source or reference |
|---|---|---|
| Plasmids used for inactivation of rrnB | ||
| pSTL102 | pBR322 carrying a mutant (Spcr Eryr) rrnB operon and its flanking regions in the tet gene | 33 |
| pMA100 | pSTL102 carrying the SalI-SalI deletion in rrnB | This work |
| pMA101 | pMA100 carrying the sacB-Kmr cassette in the BamHI site | This work |
| Plasmids used for inactivation of rrnH | ||
| pLC7-21 | A ColE1 plasmid carrying the rrnH operon and its flanking regions | 6 |
| pC5 | pBR322 carrying rrnH+ (EcoRV fragment of pLC7-21) in the EcoRV site | This work |
| pMA102 | pC5 carrying the SacII-SacII deletion in rrnH | This work |
| pMA103 | pMA102 carrying the sacB-Kmr cassette in the SalI site | This work |
| Plasmids used for inactivation of rrnG | ||
| pLC23-30 | A ColE1 plasmid carrying the rrnG operon and its flanking regions | 6 |
| pC14 | pBR322 carrying rrnG+ (BamHI fragment of pLC23-30) | 7 |
| pNY2 | The SmaI-HpaI region of rrnG in pC14 was replaced with a lacZ+ fragment | This worka |
| pNY30 | pNY2 carrying the sacB-Kmr cassette in the HindIII site | This worka |
| Plasmids used for inactivation of rrnA | ||
| pLC19-3 | A ColE1 plasmid carrying the rrnA operon and its flanking regions | 6 |
| pC1 | pBR322 carrying rrnA+ (BamHI fragment of pLC19-3) in the BamHI site | This work |
| pC1ΔSacII | pC1 carrying the SacII-SacII deletion in rrnA | This work |
| pNY34 | pC1ΔSacII carrying the sacB-Kmr cassette in the EcoRV site | This work |
| Other plasmids | ||
| pBEU49 | A runaway-replication mutant plasmid carrying the recAo281 gene (Apr Kmr) | 35 |
| pBIP3 | A source of the sacB-Kmr cassette (4.7-kb BamHI fragment) | 31 |
| pC4 | pBR322 carrying rrnC+ (EcoRV fragment of pLC22-36) in the EcoRV site | This work |
| pC8 | pBR322 carrying rrnD+ (AflII-PstI fragment of pLC16-6) in the EcoRV site | This work |
All cloning experiments for these plasmids were carried out at 30°C with M9-glycerol medium.
(ii) Inactivation of rrnH.
TA410 (ΔEB polA) was first transduced to Cmr (TA415) with Δ(rrsH-ileV-alaV-rrlH)37::cat+ (ΔrrnH in reference 9) and then transformed to Apr and Kmr (TA418) with pMA103 (Table 2). The Δ(rrsH-ileV-alaV-rrlH)37::cat+ allele was removed from TA418 as described above. The presence of the new deletion mutation in one of the Cms Aps Kms clones (TA420) was confirmed by PCR with the primers described above. We detected a PCR fragment of the expected size (1,290 bp).
(iii) Inactivation of rrnG.
TA420 (ΔEBH polA) was first transduced to Cmr (TA443) with Δ(rrsG-gltW-rrlG)33::cat+ (ΔrrnG in reference 9) and then transformed to Apr and Kmr (TA445) with pNY30 (Table 2). This plasmid carries an rrnG allele inactivated by an internal deletion and the concomitant insertion of the lacZ coding region into the site of the deleted operon. The Δ(rrsG-gltW-rrlG)33::cat+ allele was removed from TA445 as described above except that 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 60 μg/ml) was added to sucrose-containing plates. Sucr, blue colonies were then screened for sensitivity to ampicillin, kanamycin, and chloramphenicol. Cells that were sensitive to these antibiotics should have contained only the Δ(rrsG-gltW-rrlG)30::lacZ+ allele on the chromosome and were designated TA447.
(iv) Inactivation of rrnA.
The standard inactivation procedure was slightly modified as described below to restore the polA+ genetic background. The Δ(rrsA-ileT-alaT-rrlA)1::cat+ mutation (ΔrrnA in reference 9) was first introduced into TA410 (ΔEB polA) by P1 transduction. The TA410 transductants were selected for Cmr and Tcr and screened for PolA− by assaying the transformation efficiency of pBR322. This strain was named TA472. Next, pNY34 (Table 2) was integrated into the chromosome of TA472 near rrnA. Plasmid pNY34 contains an internally deleted rrnA operon. A P1 lysate was prepared on the resulting strain (TA480) and used to transduce integrated pNY34 (zih::pNY34) into TA476. TA476 was generated by transducing the Δ(rrsA-ileT-alaT-rrlA)1::cat+ mutation into TA447 (ΔEBHGz polA) and is polA+ due to cotransduction of the polA+ allele. The TA476 transductants were selected for resistance to ampicillin, kanamycin, and chloramphenicol and screened for sensitivity to tetracycline (Tcs). The presence of polA+ in Apr Kmr Cmr Tcs cells was confirmed by assaying the transformation efficiency of pACYC184, which also requires DNA polymerase I for replication. The resulting cells (TA485) were grown to saturation, and Sucr cells were obtained as described above. Finally, the Sucr cells were screened for sensitivity to ampicillin, kanamycin, and chloramphenicol, thereby obtaining TA488. The presence of the rrnA deletion in this strain was verified by Southern blot analysis.
(v) Inactivation of rrnD.
TA500 is the same as TA488 (ΔEBHGzA) but carries the tRNA-containing plasmid pTRNA65 (1). This strain was transduced to Cmr with Δ(rrsD-ileU-alaU-rrlD)25::cat+ (ΔrrnD in reference 9), generating TA516.
(vi) Inactivation of rrnC.
pHK-rrnC+ (Kmr) (1) was first introduced into TA516 (ΔEBHGzADc/pTRNA65), generating TA520. A spontaneous deletion of the gene for tRNA2Glu from pTRNA65 in TA520 resulted in TA520.5 carrying pTRNA66 (1). Restriction mapping indicated that pTRNA65 suffered a spontaneous deletion, likely by recombination between redundant parts of the 23S gene surrounding tRNA2Glu. The Δ(rrsC-gltU-rrlC)15::cat+ mutation (ΔrrnC in reference 9) was then introduced into TA520.5 by P1 transduction by virtue of its linkage to ilv500::Tn10 in strain TA575. The presence of Δ(rrsC-gltU-rrlC)15::cat+ in Tcr transductants (TA525) was verified by PCR with the following primers: 5′-CTTCCATGTCGGCAGAATGCTT-3′ and 5′-GCCTGCATACCGTTGTCGATAG-3′. These primers hybridize near the ends of the cat gene and the rrnC operon, respectively, and amplify an 850-bp fragment. Finally, the ilv+ allele was introduced into TA525 (ΔEBHGzADcCc ilv/pTRNA66/pHK-rrnC+) by P1 transduction, generating TA527. The lack of intact 16S and 23S rRNA genes on the chromosome of this strain was confirmed by Southern blot analysis (1).
Construction of an rrn+ strain, TA563.
An rrn+ strain was constructed from TXΔ11 by introducing rrnE+ by Hfr mating. The donor and recipient strains were CAG5052 and TA559.5, respectively. TA559.5 was constructed from TXΔ11 by introducing pBEU49 (35). This plasmid was used only to provide convenient counterselection (Apr Kmr) of the donor strain. The mating was carried out at 30°C for 20 min, and the cells were plated on LB plates containing tetracycline, ampicillin, and kanamycin. The plates were incubated at 30°C for 16 h, and the exconjugants were screened for Pur+ and rrnE+. The screening for rrnE+ was carried out by PCR. The primers used for the reaction were 5′-GAATTCGACGATACCGGCTTTG-3′ and 5′-CCACTCGTCAGCAAAGAAGCAA-3′, which hybridize to the purH and 16S sequences, respectively. These primers amplify a 787-bp fragment from the wild-type rrnE region. Finally, pBEU49 was removed from one of the Pur+ rrnE+ exconjugants (TA560) by using its runaway replication property, generating TA563. Although metA+ was most likely introduced into TA560 with pur+-rrnE+ by Hfr mating, the strain remains Met− since the metB1 mutation is located close to btuB::Tn10 in the donor chromosome. The presence of all seven rRNA operons in TA563 was confirmed by Southern blot analysis (1).
Total RNA/total protein and tRNA/rRNA ratios.
Total RNA/total protein and tRNA/rRNA ratios were determined as described previously (1).
RESULTS AND DISCUSSION
Construction of rrn deletion strains.
The starting material for the construction of our rrn deletion series was TXΔ11, an E. coli strain constructed by Ellwood and Nomura (12), which contains a deletion mutation encompassing the entire rrnE operon (Table 1). The six remaining rrn operons in this strain were sequentially inactivated by deletion mutations spanning the 16S and 23S rRNA genes as summarized in Fig. 3. The details of the inactivation procedures are described in Materials and Methods. Briefly, the rrnB and rrnH operons were inactivated by removal of internal SalI-SalI and SacII-SacII fragments, respectively (Fig. 1). The rrnG operon was inactivated by a deletion-insertion mutation (ΔGz in Fig. 3) in which the SmaI-HpaI region of the operon (Fig. 1) was replaced by the lacZ coding region. The rrnA operon was initially inactivated with one of the deletion-cat insertion mutations constructed previously in our laboratory (9). This deletion-cat insertion mutation in rrnA (ΔAc in TA476 [Fig. 3]) was then replaced by a simple deletion mutation (ΔA in TA488) that removed the SacII-SacII region of the operon. The rrnD and rrnC operons were also inactivated with deletion-cat insertion mutations (ΔDc and ΔCc, respectively) (9). In the final strain (TA527), carrying no intact rRNA operons on the chromosome (Δ7 prrn), rRNA molecules were expressed from a multicopy plasmid, pHK-rrnC+ (prrn). This plasmid is a derivative of pSC101 containing only the wild-type rrnC operon (1).
Each rRNA operon contains at least one tRNA gene between the 16S and 23S rRNA genes (Fig. 1); rrnA, rrnD, and rrnH contain the tRNA genes for Ile-1 and Ala-1B, and the rest (rrnB, rrnC, rrnE, and rrnG) contain the tRNA gene for Glu-2 (25). These tRNA (spacer tRNA) genes are encoded only in the rRNA operons. Since the introduction of our rrn deletions ultimately removes all spacer tRNA genes, we have cloned these genes into a derivative of pACYC184, resulting in pTRNA65 and -66 (1) (Fig. 3). In these plasmids, the tRNA genes are transcribed from the tac promoter. Expression of tRNAs for Ile-1 and Ala-1B from the cloned genes was essential for the viability of Δ6 strains, in which only the rrnC operon carrying the gene for tRNA2Glu was left on the chromosome. The same tRNA genes were also required in the Δ7 prrn strain that contained pHK-rrnC+.
In addition to the spacer tRNA genes, rrnC, rrnD, and rrnH encode different tRNA genes near the end of the operons (distal tRNA genes) (Fig. 1). Although none of the above-described rrn deletions blocks the expression of distal tRNAs, their genes were also cloned in pTRNA65 and -66. This was done to ensure sufficient expression of the distal tRNAs in Δ7 prrn strains carrying a high-copy-number rRNA plasmid (1), in which the chromosomal rrn operons are likely to be severely feedback repressed.
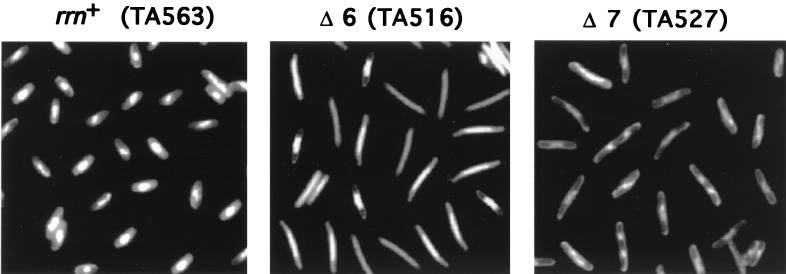
Cell morphology.
We first carried out a microscopic examination of cells from rrn+ (TA563) and rrn deletion strains. We found that cells with inactivated rRNA operons showed a pronounced morphological change during exponential growth; the cells became more and more elongated, with this change being very apparent in a Δ6 strain (TA516 [Fig. 4]). Using a vital stain, we saw no indication that dead cells accumulated in the deletion strains (data not shown). The elongated cell morphology was not completely reversed in a Δ7 prrn strain (TA527) containing the rRNA and tRNA plasmids (Fig. 4), suggesting that cellular processes other than rrn gene dosage are still perturbed in this strain (see below).
FIG. 4.
Microscopic examination. Cells from exponential-phase cultures were stained with BacLight (Molecular Probes Inc., Eugene, Oreg.) and analyzed by fluorescence microscopy.
Growth rate.
We next measured the growth rates of rrn+ and rrn deletion strains in a rich nutrient medium (1), in which a large number of ribosomes are needed for short cell division times and in which the effects of rrn inactivation should be most pronounced (7, 8). For this and the other physiological studies described below, we constructed new Δ1 to Δ4 strains (TA566, -567, -568, and -430, respectively) (Fig. 3; Table 1) that contained the polA+ allele and the deletion-cat insertion mutation in rrnA) (ΔAc). The Δ5 strain used for physiological studies was TA476, which also contained polA+ and ΔAc. For a Δ6 strain, we used TA516, which contained the deletion-cat insertion mutation in rrnD but not in rrnA. Unlike these strains, the Δ7 prrn strain, TA527, contains two deletion-cat insertion mutations on the chromosome, within rrnD and rrnC.
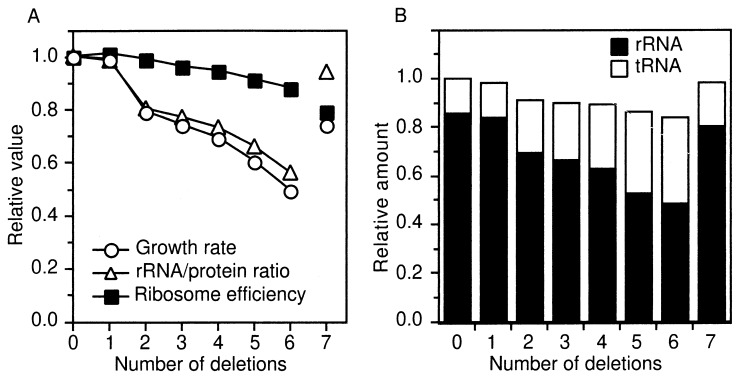
As shown in Fig. 5A, a Δ1 strain, TA566, grew at a rate that was indistinguishable from that of the rrn+ strain, TA563. Normal growth of Δ1 strains has been previously reported by us and others (8, 12). Inactivation of two operons, however, significantly reduced the growth rate (Fig. 5A). In a previous study, in which up to four rRNA operons were inactivated, a significant growth rate decrease was observed when the third operon was inactivated (8). This difference is likely because of the different E. coli strains used in the previous and present studies. In the present study, the growth rate continued to decrease gradually as the number of deletions was extended from three to six. The Δ7 prrn strain, TA527, grew slower than the rrn+ and Δ1 strains, which, like the elongated cell morphology, may reflect the persistence of defects in other cellular processes.
FIG. 5.
Physiological effects of rrn inactivation. (A) Relative growth rates, rRNA/protein ratios, and ribosome efficiencies. Growth rates (doublings per hour) were determined by monitoring the turbidity of each culture with a Klett-Summerson photoelectric colorimeter and are presented relative to the rrn+ strain values. The maximum standard error of growth rate measurements was 0.07. The actual growth rate of the rrn+ strain, TA563, was 2.0 doublings/h. rRNA/protein ratios were determined from the data presented in panel B. Ribosome efficiencies were calculated from growth rates and rRNA/protein ratios as described by Bremer and Dennis (5). (B) Relative total RNA/total protein and tRNA/rRNA ratios. The total RNA/total protein and tRNA/rRNA ratios in each total RNA sample were determined as described previously (1). These parameters were normalized to the total amount of RNA in the rrn+ strain. Closed and open bars represent the amounts of rRNA and tRNA, respectively. The maximum standard error of RNA measurements was 0.05. Note that the Δ6 strain contains only the tRNA plasmid while the Δ7 strain contains both the tRNA and rRNA plasmids.
It was originally proposed that multiple rRNA operons are necessary to support the high levels of ribosome production required for rapid bacterial growth (22, 27, 28). However, more-recent data suggest that the role of high-level ribosome production in achieving a maximum growth rate is not as important as the ability to rapidly adapt to better nutritional environments by having a substantial burst in ribosome synthesis (8). The rate of rRNA synthesis in exponentially growing rrn+ cells in rich medium is considerably lower than the maximum attainable rate (perhaps only one-sixth of the maximum rate [see below]). Furthermore, strains lacking multiple rrn operons require considerably longer periods of time to respond to environmental shifts that demand an upshift in the growth rate (8). It is therefore likely that the higher multiplicity of operons in rrn+ cells is necessary for rapid recovery when environmental conditions vary (8).
RNA/protein and tRNA/rRNA ratios.
Ninety-eight percent of the total RNA is stable RNA in wild-type E. coli (5). We therefore expected that the total RNA/total protein ratio would be one of the physiological parameters most profoundly affected in the rrn deletion strains. We measured total RNA and total protein from rapidly growing cells and determined the RNA/protein ratio. Contrary to our expectations, the ratio remained relatively constant, with the Δ6 strain’s RNA/protein ratio being approximately 84% of that of the rrn+ strain (Fig. 5B).
tRNA accounts for about 14% of the stable RNA, and the tRNA/rRNA ratio is essentially invariant and growth rate independent (5). We wondered, however, whether the rrn deletion strains might deviate from this generalization. We have previously shown that inactivation of rRNA operons on the chromosome leads to increased expression of the remaining intact copies (7). While this phenomenon could account for the relatively constant RNA/protein ratio, it might also lead to an increase in the amount of tRNA in the cell, since the control of many tRNA operons is thought to be similar to that of rRNA (23). We therefore measured the proportion of tRNA in the stable RNA of the deletion strains (Fig. 5B). The amounts of tRNA relative to rRNA were similar in the rrn+ and Δ1 (TA566) strains, and a substantial increase in the relative amount of tRNA occurred as the number of deletions increased from two to six. This overproduction of tRNA contributes significantly to the maintenance of a relatively constant level of total RNA in the rrn deletion strains and supports a model in which tRNA derepression occurs similarly to that of rRNA (11, 21). This result also shows that the reduction in the amount of rRNA in the rrn deletion strains is surprisingly moderate. The total rRNA/total protein ratio in the Δ6 strain, in which all of the rRNA is provided by one operon, rrnC, is approximately 56% of that measured in the rrn+ strain (Fig. 5A). This represents an approximately sixfold increase in the amount of rRNA produced by rrnC if one takes into account gene dosage effects contributed by the distance from the origin of replication. (Gene dosage was calculated as described in reference 7, assuming that DNA replication and cell division are not disturbed in the Δ6 strain. The number of rrn operons per cell in the Δ6 strain at a doubling time of 61 min is approximately 2.4, versus 27.1 in rrn+ cells with a doubling time of 30 min. Since the amount of rRNA in Δ6 strains is 56% of that in rrn+ cells, the amount of rRNA produced per operon is increased about sixfold in strains containing only rrnC.)
The total RNA/total protein and tRNA/rRNA ratios in the Δ7 prrn strain (TA527) carrying pHK-rrnC+ were similar to those in the rrn+ strain (Fig. 5B), indicating that the single rRNA operon (rrnC) on this pSC101-based plasmid was able to supply sufficient rRNA to restore these balances.
Ribosome efficiency.
The rRNA/protein ratio is proportional to the number of ribosomes per protein, i.e., the ribosome concentration (5). In the Δ2 strain, the percent decrease in the growth rate was similar to the decrease in the rRNA/protein ratio (Fig. 5A), suggesting that the reduced growth rate of this strain was caused by a reduced ribosome concentration. When the number of rrn deletions was extended from three to six, however, the decrease in the growth rate was greater than that of the rRNA/protein ratio. This difference between the decreases in the growth rate and the rRNA/protein ratio was most pronounced in the Δ7 prrn strain, in which the rRNA/protein ratio was completely restored to wild-type levels but the growth rate was not. These results suggest that the reduced growth rates of the multiply deleted strains are not simply due to reduced ribosome concentrations. It has been proposed that ribosome concentration and efficiency are growth limiting in any living cell whose protein turnover is negligible and that the growth rate of an exponential-phase culture is proportional to the multiplication product of these two values (5). According to this theory, the reduced growth rates of the Δ3 to Δ7 strains can be attributed, at least in part, to reduced ribosome efficiencies. We have calculated the theoretical ribosome efficiency in each of the rrn deletion strains by using the equation presented in reference 5. By these calculations, the ribosome efficiency of Δ7 prrn strains falls to below 80% of wild-type levels (Fig. 5A).
There are several ways in which the ribosome efficiency could be affected by rrn inactivation. Ribosome efficiency is determined by the fraction of active ribosomes (i.e., the fraction of ribosomes that are actively engaged in peptide chain elongation) and the rate of peptide chain elongation per ribosome (5). The fraction of active ribosomes is thought to be constant (∼80%) at a wide range of growth rates in wild-type E. coli (13). This value may not apply to rrn deletion strains, however. We have previously demonstrated, for example, that the number of molecules of translation initiation factor IF3 per ribosome is significantly decreased in rrn deletion strains (8). Since IF3 stimulates the dissociation of vacant 70S ribosomes and thereby promotes the recycling of the ribosomal subunits for new initiation events (17), the reduced ribosome efficiency that we observe in Δ3 to Δ6 strains may be due to a decrease in the active ribosome fraction.
The other determinant of ribosome efficiency, the rate of peptide chain elongation, is also likely to be affected by the rrn deletions. The chain elongation rate is influenced by the concentrations of several factors, such as tRNA, GTP, and elongation factors. We suspect that the concentrations of at least some tRNA molecules may be inadequate in rrn deletion strains. In Δ6 and Δ7 prrn strains, for example, the spacer tRNAs for Ile-1 and Ala-1B are expressed from a plasmid and their quantities may differ from those present in the rrn+ strain. Alternatively, since derepression of rrn expression leads to the overexpression of distal tRNAs and other tRNAs encoded outside of the rRNA operons (Fig. 5B) (11), titration of the tRNA modification machinery by these tRNAs could lead to a reduced chain elongation rate.
In addition to the quantitative differences discussed above, ribosomes in rrn deletion strains may exhibit qualitative differences. Since IF3 ensures the accuracy of translation initiation by preventing initiation at codons other than AUG, GUG, or UUG (32), a reduced IF3/ribosome ratio in rrn deletion strains could result in the overproduction of certain proteins that are normally poorly expressed. Such an unbalanced expression of proteins might also be predicted to have consequences for the growth rate.
Applications of rrn deletion strains.
Using the Δ7 prrn strain, we have successfully constructed strains containing an rrn operon from a foreign microorganism, such as Salmonella typhimurium and P. vulgaris (1). Characterization of these strains with hybrid ribosomes emphasizes the usefulness of this system for evolutionary studies of the translation machinery. The deletion strains can also be used to examine current models of rrn regulation and to answer questions about the evolution of bacteria with multiple rrn operons. The Δ7 prrn strain should be especially useful for in vitro analysis of ribosome functions, since pure mutant ribosome populations from this strain are available (1). In addition, Δ7 prrn provides a powerful method for the isolation of new rRNA mutations, including conditionally lethal mutations for examining essential functions of rRNA. Questions concerning specific rRNA domains or sequences, modified bases, particular structures, long-range interactions, changes leading to drug resistance, and interaction with other components of the translation apparatus should all be more readily addressed by using the deletion strains described here.
ACKNOWLEDGMENTS
We thank Al Dahlberg and members of his laboratory for enthusiastic discussions of and constant interest in this work and Pat Dennis for advice and discussions. T.A. is especially thankful to Tokio Kogoma for many suggestions on strain construction.
National Institutes of Health grant GM24751 to C.L.S. supported these studies.
REFERENCES
- 1.Asai T, Zaporojets D, Squires C, Squires C L. An Escherichia colistrain with all chromosomal rRNA operons inactivated: complete exchange of rRNA genes between bacteria. Proc Natl Acad Sci USA. 1999;96:1971–1976. doi: 10.1073/pnas.96.5.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beresford T, Condon S. Cloning and partial characterization of genes for ribonucleic acid in Lactococcus lactis subsp. lactis. FEMS Microbiol Lett. 1991;62:319–323. doi: 10.1016/0378-1097(91)90178-d. [DOI] [PubMed] [Google Scholar]
- 3.Blomfield I C, Vaughn V, Rest R F, Eisenstein B I. Allelic exchange in Escherichia coli using the Bacillus subtilis sacBgene and a temperature-sensitive pSC101 replicon. Mol Microbiol. 1991;5:1447–1457. doi: 10.1111/j.1365-2958.1991.tb00791.x. [DOI] [PubMed] [Google Scholar]
- 4.Bott K, Stewart G C, Anderson A G. Genetic mapping of cloned ribosomal RNA genes. In: Hoch J A, Ganesan A T, editors. Syntro Conference on Genetics and Biotechnology of Bacilli. New York, N.Y: Academic Press; 1984. pp. 19–34. [Google Scholar]
- 5.Bremer H, Dennis P P. Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C: ASM Press; 1996. pp. 1553–1569. [Google Scholar]
- 6.Clarke L, Carbon J. A colony bank containing synthetic Col E1 hybrid plasmids representative of the entire E. coligenome. Cell. 1976;9:91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- 7.Condon C, French S, Squires C, Squires C L. Deletion of functional ribosomal RNA operons in Escherichia colicauses increased expression of the remaining intact copies. EMBO J. 1993;12:4305–4315. doi: 10.1002/j.1460-2075.1993.tb06115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condon C, Liveris D, Squires C, Schwartz I, Squires C L. rRNA operon multiplicity in Escherichia coli and the physiological implications of rrninactivation. J Bacteriol. 1995;177:4152–4156. doi: 10.1128/jb.177.14.4152-4156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Condon C, Philips J, Fu Z-Y, Squires C, Squires C L. Comparison of the expression of the seven ribosomal RNA operons in Escherichia coli. EMBO J. 1992;11:4175–4185. doi: 10.1002/j.1460-2075.1992.tb05511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Condon C, Squires C, Squires C L. Control of rRNA transcription in Escherichia coli. Microbiol Rev. 1995;59:623–645. doi: 10.1128/mr.59.4.623-645.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong H, Nilsson L, Kurland C G. Co-variation of tRNA abundance and codon usage in Escherichia coliat different growth rates. J Mol Biol. 1996;260:649–663. doi: 10.1006/jmbi.1996.0428. [DOI] [PubMed] [Google Scholar]
- 12.Ellwood M, Nomura M. Deletion of a ribosomal ribonucleic acid operon in Escherichia coli. J Bacteriol. 1980;143:1077–1080. doi: 10.1128/jb.143.2.1077-1080.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forchhammer J, Lindahl L. Growth rate of polypeptide chains as a function of the cell growth rate in a mutant of Escherichia coli15. J Mol Biol. 1971;55:563–568. doi: 10.1016/0022-2836(71)90337-8. [DOI] [PubMed] [Google Scholar]
- 14.Gaal T, Bartlett M S, Ross W, Turnbough C L, Gourse R L. Transcription regulation by initiating NTP concentration: rRNA synthesis in bacteria. Science. 1997;278:2092–2097. doi: 10.1126/science.278.5346.2092. [DOI] [PubMed] [Google Scholar]
- 15.Garnier T, Canard B, Cole S T. Cloning, mapping, and molecular characterization of the rRNA operons of Clostridium perfringens. J Bacteriol. 1991;173:5431–5438. doi: 10.1128/jb.173.17.5431-5438.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gay P, Le Coq D, Steinmetz M, Berkelman T, Kado C I. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J Bacteriol. 1985;164:918–921. doi: 10.1128/jb.164.2.918-921.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godefroy-Colburn T, Wolfe A D, Dondon J, Grunberg-Manago M, Dessen P, Pantaloni P. Light-scattering studies showing the effect of initiation factors on the reversible dissociation of Escherichia coliribosomes. J Mol Biol. 1975;94:461–478. doi: 10.1016/0022-2836(75)90215-6. [DOI] [PubMed] [Google Scholar]
- 18.Gourse R L, de Boer H A, Nomura M. DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, and antitermination. Cell. 1986;44:197–205. doi: 10.1016/0092-8674(86)90498-8. [DOI] [PubMed] [Google Scholar]
- 19.Gourse R L, Gaal T, Bartlett M S, Appleman J A, Ross W. rRNA transcription and growth rate-dependent regulation of ribosome synthesis in Escherichia coli. Annu Rev Microbiol. 1996;50:645–677. doi: 10.1146/annurev.micro.50.1.645. [DOI] [PubMed] [Google Scholar]
- 20.Gutterson N I, Koshland D E. Replacement and amplification of bacterial genes with sequences altered in vitro. Proc Natl Acad Sci USA. 1983;80:4894–4898. doi: 10.1073/pnas.80.16.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jinks-Robertson S, Gourse R, Nomura M. Expression of rRNA and tRNA genes in Escherichia coli: evidence for feedback regulation by products of rRNA operons. Cell. 1983;33:865–876. doi: 10.1016/0092-8674(83)90029-6. [DOI] [PubMed] [Google Scholar]
- 22.Jinks-Robertson S, Nomura M. Ribosomes and tRNA. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 2. Washington, D.C: American Society for Microbiology; 1987. pp. 1358–1385. [Google Scholar]
- 23.Keener J, Nomura M. Regulation of ribosome synthesis. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 1417–1431. [Google Scholar]
- 24.Kiss A, Sain B, Venetianer P. The number of rRNA genes in Escherichia coli. FEBS Lett. 1977;79:77–79. doi: 10.1016/0014-5793(77)80354-2. [DOI] [PubMed] [Google Scholar]
- 25.Komine Y, Adachi T, Inokuchi H, Ozeki H. Genomic organization and physical mapping of the transfer RNA genes in Escherichia coliK12. J Mol Biol. 1990;212:579–598. doi: 10.1016/0022-2836(90)90224-A. [DOI] [PubMed] [Google Scholar]
- 26.LaFauci G, Widom R L, Eisner R L, Jarvis E D, Rudner R. Mapping of rRNA genes with integrable plasmids in Bacillus subtilis. J Bacteriol. 1986;165:204–214. doi: 10.1128/jb.165.1.204-214.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maaløe O, Kjeldgaard N O. Control of macromolecular synthesis. W. A. New York, N.Y: Benjamin; 1966. [Google Scholar]
- 28.Nomura M, Morgan E A, Jaskunas S R. Genetics of bacterial ribosomes. Annu Rev Genet. 1977;11:297–347. doi: 10.1146/annurev.ge.11.120177.001501. [DOI] [PubMed] [Google Scholar]
- 29.Sarmientos P, Sylvester J E, Contente S, Cashel M. Differential stringent control of the tandem E. coli ribosomal RNA promoters from the rrnA operon expressed in vivoin multicopy plasmids. Cell. 1983;32:1337–1346. doi: 10.1016/0092-8674(83)90314-8. [DOI] [PubMed] [Google Scholar]
- 30.Singer M, Baker T A, Schnitzler G, Deischel S M, Goel M, Dove W, Jaacks K J, Grossman A D, Erickson J W, Gross C A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989;53:1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slater S, Maurer R. Simple phagemid-based system for generating allele replacements in Escherichia coli. J Bacteriol. 1993;175:4260–4262. doi: 10.1128/jb.175.13.4260-4262.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sussman J K, Simons E L, Simons R W. Escherichia coli translation initiation factor 3 discriminates the initiation codon in vivo. Mol Microbiol. 1996;21:347–360. doi: 10.1046/j.1365-2958.1996.6371354.x. [DOI] [PubMed] [Google Scholar]
- 33.Triman K, Becker E, Dammel C, Katz J, Mori H, Douthwaite S, Yapijakis C, Yoast S, Noller H F. Isolation of temperature-sensitive mutants of 16S rRNA in Escherichia coli. J Mol Biol. 1989;209:645–653. doi: 10.1016/0022-2836(89)92000-7. [DOI] [PubMed] [Google Scholar]
- 34.Tulloch D L, Finch L R, Hillier A J, Davidson B E. Physical map of the chromosome of Lactococcus lactis subsp. lactisDL11 and localization of six putative rRNA operons. J Bacteriol. 1991;173:2768–2775. doi: 10.1128/jb.173.9.2768-2775.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uhlin B E, Volkert M R, Clark A J, Sancar A, Rupp W D. Nucleotide sequence of a recAoperator mutation. LexA/operator-repressor binding/inducible repair. Mol Gen Genet. 1982;185:251–254. doi: 10.1007/BF00330794. [DOI] [PubMed] [Google Scholar]