Abstract
Although xanthorrhizol, a sesquiterpenoid oil obtained from the rhizome of Curcuma xanthorrhiza Roxb., known as Java turmeric, has many pharmacological effects, its pharmacokinetics remain unclear. Therefore, we investigated the pharmacokinetics of xanthorrhizol in mice and rats. Xanthorrhizol was administered intravenously and orally to mice, while xanthorrhizol and a Java turmeric supercritical extract were administered orally to rats. The terminal half-life (t1/2), clearance, and absolute bioavailability (BA) of xanthorrhizol in mice were almost 8 h, 6.5 L/h/kg, and 10.2%, respectively. In comparison, the clearance of xanthorrhizol was 3-fold higher in rats than mice. The absolute BAs of xanthorrhizol in rats were 12.9% and 13.4% after oral administration of xanthorrhizol and a supercritical extract, respectively. Our results regarding the pharmacokinetics of xanthorrhizol could guide the conversion of intravenous and oral doses, and help identify the optimal maintenance doses of xanthorrhizol and the extract for desirable pharmacodynamic effects.
Keywords: Xanthorrhizol, Java turmeric extract, Pharmacokinetics, Mice, Rats
Introduction
Xanthorrhizol is a sesquiterpenoid oil isolated from the rhizome of Curcuma xanthorrhiza Roxb., also known as Java turmeric (Rimpler et al., 1970). We have investigated the pharmacological activities of the active substance xanthorrhizol, including ethanol and supercritical extracts, over 20 years. Xanthorrhizol was shown to have remarkable anticariogenic activity against Streptococcus mutans, with a minimum inhibitory concentration of 9 µM (Hwang et al., 2000). In addition, xanthorrhizol had anti-metastatic activity in a lung cancer model through multiple signaling pathways, including extracellular signal-regulated kinase (ERK), cyclooxygenase-2 (COX-2), and matrix metalloproteinase-9 (MMP-9) (Choi et al., 2004). In neuronal and microglial cells, xanthorrhizol had anti-oxidant and anti-inflammatory effects, suggesting the possible use of oil for the treatment of neurological disorders related to reactive oxygen species and inflammation (Lim et al., 2005). The ethanol extracts of Java turmeric decreased the postprandial glucose and triglyceride levels in high-fat diet-induced obese mice (Kim et al., 2014). Recently, a standardized supercritical extract of the root was shown to ameliorate periodontitis by inhibiting gum inflammation and improving bone remodeling in a lipopolysaccharide-induced periodontitis rat model (Kook et al., 2018).
In addition to pharmacodynamic information, pharmacokinetic information of xanthorrhizol is also important to determine the relationship between xanthorrhizol activity and systemic and/or tissue exposure during administration as a food supplement or candidate drug. Therefore, we investigated the pharmacokinetics of xanthorrhizol after intravenous and oral administration of xanthorrhizol to mice, and oral administration of xanthorrhizol and a Java turmeric supercritical extract to rats. The plasma concentrations were measured using a validated analytical method based on high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) (Choi et al., 2017).
Materials and methods
Materials
Xanthorrhizol was extracted and purified to achieve a chemical purity of over 95% according to the standard operating procedure in our lab (Kim et al., 2014). The chemical structure of xanthorrhizol was confirmed by nuclear magnetic resonance spectroscopy and mass spectrometry. The rhizomes of Curcuma xanthorrhiza (Nutribiotech Co., Ltd, Seoul, Korea) were extracted using a supercritical CO2 fluid extraction system (SCFE-P400; Ilshin Autoclave Co., Ltd., Daejeon, Korea) (Kook et al., 2018). The chemical content of xanthorrhizol in the extract was 30%. All other chemicals and solvents were of the highest analytical grade available.
Animal study
After a 7-day acclimatization, 10 young adult Sprague-Dawley rats (300–320 g) were randomly divided into two groups (n = 5 rats in both) for oral administration of xanthorrhizol (15 mg/kg) or the extract (200 mg/kg). Thirteen mice (25–28 g) were divided into two groups for intravenous (5 mg/kg) or oral (50 mg/kg) administration of xanthorrhizol. The mice were further allocated to two sampling times, and the plasma concentration was measured at each time point for three mice.
Xanthorrhizol was dissolved in 20% polyethylene glycol 400 aqueous solution and corn oil for intravenous and oral administration, respectively. The Java turmeric supercritical extract was suspended in corn oil. The administration volume was 1 mL/kg for rats and 10 mL/kg for mice. Heparinized blood samples were serially obtained from rat subclavian veins at 2, 5, 15 min, and 0.5, 1, 1.5, 2, 3, 4, 6, 8 and 10 h for intravenous administration; at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, and 12 h for oral administration, and mouse orbital sinus at 2, 5, 15 min, and 0.5, 1, 1.5, 2, 4, 8, 12 h for intravenous administration; at 0.25, 0.5, 0.75, 1, 2, 4, 8, 12, and 24 h for oral administration, and spun at 13,200 rpm for 10 min. Plasma was separated and frozen until analysis. This study was approved by the Institutional Animal Care and Use Committee (IACUC) of Chung-Ang University, South Korea (Permit No.: 2017-00024).
Measurement of plasma xanthorrhizol concentration by HPLC-MS/MS
The plasma xanthorrhizol concentration was measured using a validated analytical method based on HPLC-MS/MS (Choi et al., 2017). Briefly, acetonitrile (90 µL), including 50 ng/mL of diclofenac (internal standard, IS), was added to plasma (30 µL). After centrifugation, the supernatant (5 µL) was injected into a reversed-phase column (Atlantis T3, 50 × 2.1 mm internal diameter, 3 μm particle size; Agilent, Wilmington, DE, USA) at 30 °C. The mobile phase consisted of 20 mM ammonium acetate aqueous solution and acetonitrile (20:80, v/v), delivered at a rate of 0.2 mL/min using an HP 1260 series pump (Agilent).
Xanthorrhizol and the IS were quantified using an API 4000 LC/MS/MS system (AB SCIEX, Framingham, MA, USA) equipped with an electrospray ionization interface. The turbo ion spray interface was operated at 4500 V and 450 °C. The ion transitions, from the precursor to the product, mainly involved deprotonated ions [M − H]− at m/z 216.9→132.8 (declustering potential, − 105 eV; collision energy, − 44 eV) for xanthorrhizol and 296.1→251.7 (declustering potential, − 65 eV; collision energy, − 18 eV) for the IS. Quantification was performed by selective reaction monitoring of deprotonated precursor ions and related product ions based on the ratio of the area under the peak for each solution.
Pharmacokinetic data
Average plasma xanthorrhizol concentrations from the three mice at each time point were used to determine the systemic exposure. The plasma xanthorrhizol concentration in individual rats was analyzed independently.
The time course of the plasma xanthorrhizol concentration was used to determine the pharmacokinetic parameters: the maximum concentration (Cmax) and time-to-Cmax (Tmax) were determined from the plasma concentration time curve; the elimination rate constant (k) was calculated by linear regression of the log-transformed plasma drug concentration at time points near the terminal phase; the area under the plasma concentration time curve (AUCt) was calculated using the trapezoidal rule, and the concentration at the last sampling time (Clast)/k was added to obtain the AUC to infinite (AUCinf); and clearance was calculated as dose/AUCinf, indicating the distribution volume of xanthorrhizol completely removed per unit time.
The absolute bioavailability (BA, F (%)) of xanthorrhizol in mice and rats was estimated using the equation AUCinf,p.o. × Dosei.v./(AUCinf,i.v. × Dosep.o.). Data are presented as mean ± standard deviation. Previously reported pharmacokinetic parameters (Choi et al., 2017; Noh et al., 2020), measured after intravenous administration of xanthorrhizol in rats, were used to determine the absolute BA of xanthorrhizol in rats and compare it with that after oral administration.
Statistical analysis
The pharmacokinetic parameters were statistically analyzed using Student’s t-test. A p-value < 0.05 was considered statistically significant. The data were analyzed using the Statistical Package for the Social Sciences (SPSS) 26 (IBM Corp., Armonk, NY, USA).
Results and discussion
Pharmacokinetics of xanthorrhizol in mice
The time courses of plasma xanthorrhizol concentration following oral (50 mg/kg) and intravenous (5 mg/kg) administration in mice are shown in Fig. 1, and the pharmacokinetic parameters are listed in Table 1. The time courses were obtained from the average value at each time point, i.e., by the naïve-pooled method, since serial sampling is not appropriate in small animals like mice. The relative standard deviation (standard deviation × 100/mean) at each time point, representing interindividual variation, was 8–29%, which was not excessively large considering that the data were obtained from different subjects. Two blood samples were collected from each eye using a heparinized capillary at different time points, with the total amount of blood collected being less than 10% of the total body weight to minimize hemodynamic changes due to blood loss.
Fig. 1.

Time courses of plasma xanthorrhizol concentrations following intravenous (●, 5 mg/kg) and oral (○, 50 mg/kg) administrations in mice
Table 1.
Pharmacokinetic parameters of xanthorrhizol after intravenous (5 mg/kg) and oral (50 mg/kg) administrations of xanthorrhizol in mice
| Parameter | Intravenous | Oral |
|---|---|---|
| AUCinf (ng h/mL) | 754 | 767 |
| Cmax (ng/mL) | – | 85 |
| Tmax (h) | – | 1.0 |
| t1/2 (h) | 7.9 | 8.3 |
| CL (L/h/kg) | 6.63 | 6.52a |
| F (%) | – | 10.2 |
aBioavailability was taken into account
AUCinf, area under the plasma concentration time to infinite; Cmax, maximum concentration; Tmax, time-to-Cmax; t1/2, half-life; CL, clearance; F(%), bioavailability
The plasma xanthorrhizol concentration reached its peak (Cmax, 85 ng/mL) almost 1 h (Tmax) after oral administration of 50 mg/kg of xanthorrhizol. After relatively rapid decay for up to 4 h after administration, xanthorrhizol disappeared from the circulation with a half-life of 8.3 h equivalent to 0.09 h− 1 of the elimination rate constant in the terminal phase. The AUC and total clearance were 767 ng h/mL and 6.52 L/h/kg, respectively.
Xanthorrhizol (5 mg/kg) demonstrated bimodal decay after intravenous administration: the plasma concentration rapidly decreased for up to 2 h after administration, followed by a slow decay for 7.9 h, thereby demonstrating a half-life equivalent to 0.09 h− 1 of the elimination rate constant in the terminal phase. The AUC and total clearance were 754 ng h/mL and 6.63 L/h/kg, respectively. The absolute BA of xanthorrhizol was estimated to be 10.2%.
Pharmacokinetics of xanthorrhizol in rats
The time courses of plasma xanthorrhizol concentration following oral (15 mg/kg) and intravenous (5 mg/kg) administration in rats are shown in Fig. 2, and the pharmacokinetic parameters are listed in Table 2. The parameters were obtained using the standard two-stage method: in the first step, the parameters were individually calculated from the time course of the plasma xanthorrhizol concentration in each subject. Then, in the second step, the mean and standard deviation of each parameter were estimated.
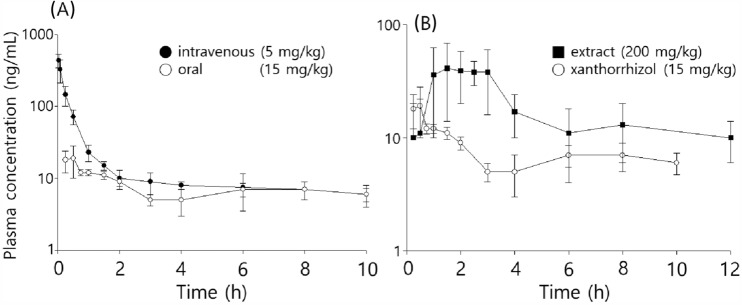
Fig. 2.
A time courses of plasma xanthorrhizol concentrations following oral (○, 15 mg/kg) and intravenous (●, 5 mg/kg, Choi et al., 2017) administrations of xanthorrhizol in rats; B Plasma xanthorrhizol concentrations versus time after oral administration of xanthorrhizol (○, 15 mg/kg) and a Java turmeric supercritical extract (■, 200 mg/kg) in rats
Table 2.
Pharmacokinetic parameters of xanthorrhizol after oral administration of xanthorrhizol (15 mg/kg) and a Java turmeric supercritical extract (200 mg/kg; 60 mg/kg as xanthorrhizol) in rats (mean ± SD, n = 5)
| Parameter | Intravenousa | Oral | |
|---|---|---|---|
| Xanthorrhizol (5 mg/kg) | Xanthorrhizol (15 mg/kg) | Supercritical extract (200 mg/kg) | |
| AUCinf (ng h/mL) | 229 ± 28 | 92 ± 5 | 353 ± 165 |
| Cmax (ng/mL) | – | 18 ± 8 | 55 ± 22 |
| Tmax (h) | – | 0.4 ± 0.2 | 2.2 ± 0.5 |
| t1/2 (h) | 5.6 ± 0.5 | 7.5 ± 0.3 | 5.6 ± 4.1 |
| CL (L/h/kg) | 19.1 ± 2.3 | 21.0 ± 1.1b | 22.8 ± 10.5b |
| F (%) | – | 12.9 | 13.4 |
aThe data were retrieved from our previous work (Choi et al., 2017)
bBioavailability was taken into account
AUCinf, area under the plasma concentration time to infinite; Cmax, maximum concentration; Tmax, time-to-Cmax; t1/2, half-life; CL, clearance; F(%), bioavailability
As shown in Fig. 2 A, after oral administration of xanthorrhizol, the maximum concentration (Cmax, 19 ng/mL) was observed at 0.5 h (Tmax). After reaching the peak, a bi-exponential decay was observed: a relatively rapid decrease for up to 3 h, followed by a slow decay for 7.0 h (t1/2), representing the terminal phase. The mean clearance and AUCinf were 21.0 L/h/kg and 92 ng h/mL, respectively. The total clearance and half-life were similar to those previously obtained after intravenous administration (19.1 L/h/kg and 5.6 h, respectively). The absolute BA was 13%.
The mean Cmax of xanthorrhizol following oral administration of the extract (200 mg/kg, 60 mg/kg as xanthorrhizol) was 55 ng/mL at 2.2 h (Fig. 2B). However, the mean plasma concentrations at 1–3 h after administration plateaued, which might be attributable to interindividual variation in the time to peak concentration. The relative standard deviations of the other parameters after xanthorrhizol extract intake were much higher than those following the oral administration of xanthorrhizol. The second peak was observed at 8 h, which likely reflected involvement of the enterohepatic circulation and/or delayed absorption from the gastrointestinal tract. Because this phenomenon was not observed after xanthorrhizol administration, it is possible that the ingredients in the extract affect the absorption of xanthorrhizol. However, there was no difference in the clearance of xanthorrhizol after the administration of xanthorrhizol and the extract (21.0 ± 1.1 vs. 22.8 ± 10.5 L/h/kg, respectively); therefore, the effects of the ingredients may be negligible (Table 2). Further studies are needed to explain the double-peak phenomenon (e.g., by using a bile duct-cannulated rat and in situ intestine perfusion experiment).
After oral administration of the extract, the absolute BA of xanthorrhizol was 13.4%, which was similar to that following oral administration of xanthorrhizol alone and almost 3% higher than that in mice. However, these differences were negligible considering that the value was obtained using the naïve-pooled method in mice.
The absolute oral BA is widely used to convert an intravenous dose to an oral dose and vice versa. The clearance can be used to determine the maintenance dose or dosing rate (k0) required to reach an effective mean plasma concentration at steady state (Css, Css = k0/Cl). Therefore, our results could inform the design of further studies on pharmacokinetics and pharmacodynamics after administration of xanthorrhizol and the extract.
In summary, the absolute oral BA of xanthorrhizol was 10.2% in mice and 12.9% in rats. The BA of the Java turmeric extract, including 30% xanthorrhizol, was 13.4% after oral absorption. The clearance rates of xanthorrhizol in mice and rats were 6.6 and 22.8 L/h/kg, respectively. Our results provide important information regarding the conversion of an intravenous dose to an oral dose, and will aid the selection of appropriate maintenance doses of xanthorrhizol and the extract.
Acknowledgements
This research was supported by the Chung-Ang University Research Scholarship Grants in 2020 and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2022R1A5A6000760).
Declarations
Conflict of interest
The authors declare no conflict of interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Juhyung Kang and Jihyun Won have contributed equally to this work.
Contributor Information
Jae-Kwan Hwang, Email: jkhwang@yonsei.ac.kr.
Wonku Kang, Email: wkang@cau.ac.kr.
References
- Choi MA, Kim SH, Chung WY, Hwang JK, Park KK. Xanthorrhizol a natural sesquiterpenoid from Curcuma xanthorrhiza, has an anti-metastatic potential in experimental mouse lung metastasis model. Biochemical and Biophysical Research Communications. 2004;326:210–217. doi: 10.1016/j.bbrc.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Choi S, Kim M, Kim C, Hwang JK, Kang W. Quantitative determination of xanthorrhizol in rat plasma by HPLC-MS/MS and its application to a pharmacokinetic study. Journal of Pharmaceutical and Biomedical Analysis. 2017;132:56–59. doi: 10.1016/j.jpba.2016.09.043. [DOI] [PubMed] [Google Scholar]
- Hwang JK, Shim JS, Baek NI, Pyun YR. Xanthorrhizol: a potential antibacterial agent from Curcuma xanthorrhiza against Streptococcus mutans. Planta Medica. 2000;66:196–197. doi: 10.1055/s-0029-1243135. [DOI] [PubMed] [Google Scholar]
- Kim MB, Kim C, Song Y, Hwang JK. Antihyperglycemic and anti-inflammatory effects of standardized Curcuma xanthorrhiza Roxb extract and its active compound xanthorrhizol in high-fat diet-induced obese mice. Evidence-Based Complementary and Alternative Medicine. 2014;2014:205915. doi: 10.1155/2014/205915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook KE, Kim C, Kang W, Hwang JK. Inhibitory Effect of Standardized Curcuma xanthorrhiza Supercritical Extract on LPS-Induced Periodontitis in Rats. Journal of Microbiology and Biotechnology. 2018;28:1614–1625. doi: 10.4014/jmb.1808.08052. [DOI] [PubMed] [Google Scholar]
- Lim CS, Jin DQ, Mok H, Oh SJ, Lee JU, Hwang JK, Ha I, Han JS. Antioxidant and antiinflammatory activities of xanthorrhizol in hippocampal neurons and primary cultured microglia. Journal of Neuroscience Research. 2005;82:831–838. doi: 10.1002/jnr.20692. [DOI] [PubMed] [Google Scholar]
- Noh K, Back HM, Shin BS, Kang W. Pharmacokinetics of shikimic acid following intragastric and intravenous administrations in rats. Pharmaceutics. 2020;12:824. doi: 10.3390/pharmaceutics12090824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimpler H, Hänsel R, Kochendoerfer L. Xanthorrhizol: a new sesquiterpene from Curcuma xanthorrhiza. Zeitschrift für Naturforschung B. 1970;25:995–998. doi: 10.1515/znb-1970-0917. [DOI] [PubMed] [Google Scholar]