Abstract
In response to a growing population and rising food demand, the food industry has come up with a wide array of alterations, innovations, and possibilities for making meat in vitro. In addition to revolutionizing the meat industry, this advancement also has profound effects on the environment, health, and welfare of animals. Thus, rather than using slaughtered animals, animal cells are employed to generate cell-based meat, with the cells' proliferation and differentiation taking place in the culture environment. The primary goal of this paper is to examine the overall mechanism and numerous approaches involved in the creation of cell-based meat. It also covers upcoming issues like technical, consumer, and regulatory issues, environmental concerns, the economy, cost of the product, health and safety concerns, and ethical, religious, and societal taboos. Finally, it assesses the future prospects of cell-based meat production.
Graphical abstract
Keywords: In vitro, Animal cell, Cell-based meat, Proliferation, Differentiation
Introduction
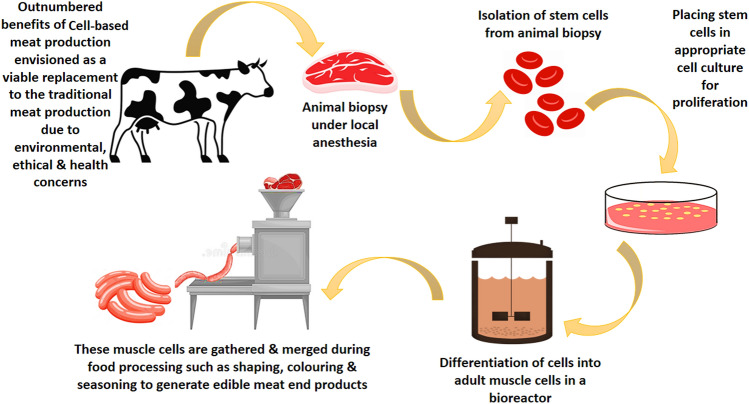
Feeding 10 billion people by 2050 is humanity's top problem today (UNPD, 2019). Meat is one of the most important dietary resources and nutrients for the majority of people, with over 300 million tonnes consumed in 2014 and predicted growth of 76% by 2050 (Alexandratos and Bruinsma, 2012). To accommodate expanding meat demand, about 70 billion animals are grown and slaughtered each year around the world (Dopelt et al., 2019). Animal farming and slaughter on a large scale pose serious environmental, ethical, and health concerns. The main disadvantages of traditional meat production are greenhouse gas emissions from biogas and manure. Since it is unclean and violent, animal slaughtering pollutes the environment. Cattle genetic diversity has been lost as a result of crossbreeding to produce just high-yielding animals. The proliferation of weeds by animals has resulted in the loss of crops and natural wildlife variety, Soil erosion is caused by the grazing of meat-producing animals. Consumption of red meat raises ethical concerns and human health issues, Superbugs, and antibiotic resistance genes are spreading, as the drugs are leftover from animal feed. Farm animals provide a risk of zoonotic illnesses (viruses, bacteria, fungus, and other pathogens). Due to outbreaks of African swine fever, avian flu, and other animal ailments, traditional livestock production has become increasingly precarious (Dixon et al., 2020; Normile, 2008; Singh et al., 2020; Taylor et al., 2020). In light of these issues, an alternative meat production technology that is extremely efficient, environmentally friendly, and long-lasting is required. Cell-based meat has been named one of the World Economic Forum’s “Top 10 Emerging Technologies of 2018,” and has been lauded as a viable solution to animal-production challenges (Cann, 2016; Jiang et al., 2020; Lee et al., 2020). Cell-based meat (also known as in vitro meat, lab-grown meat, clean meat, or synthetic meat) is edible muscle tissue created by culturing stem cells in a controlled culture and physiological environment in a laboratory utilizing tissue engineering and computational simulation technologies (Mengistie, 2020). In other words, cell-based meat is produced by growing stem cells outside the animal for human consumption from which it originates. In most cases, self-renewing and differentiating stem cells are isolated from an animal biopsy and placed in an appropriate medium containing nutrients, energy sources, growth hormones, and other variables necessary for the stem cells to develop and differentiate into adult muscle cells in a bioreactor, resulting in muscle fibers, fat, and other cell types that make up muscle tissue. Cell (or tissue) culturing can be used to make edible animal muscle, generally known as meat, by multiplying a small number of muscle cells into a large amount of tissue. These cells are gathered and merged after food processing such as shape, coloring, and seasoning to generate edible meat end-products (Arshad et al., 2017; Bhat et al., 2015; Datar and Betti, 2010; Kadim et al., 2015). In vitro meat is envisioned as a viable replacement for traditional meat due to significant benefits such as animal-free production, up to a 50% decrease in energy use, 75–95% lower greenhouse gas emissions, 99% less land use, and 80–95% less water usage (Tuomisto and Teixeira de Mattos, 2011). Furthermore, as compared to actual animal meat, cell-based meat would have a far lower carbon impact. Unlike traditional animal meat, where 75–95% of the feed consumed by the animal is utilized for metabolism and the creation of unappealing body features like exoskeleton (hair, horns, and hooves) and neurological systems, the energy requirements will be reduced (Bhat and Fayaz, 2011). To improve output and profit, various medications, such as tranquillizers, additives, and steroids, are given to the animals before they are killed under the traditional system. The animal’s body is frequently not properly cleaned, and the animal becomes ill as a result. All of these problems are avoided when lab meat is used. The risk of disease outbreaks is lowered because cell-based meat is not generated from animals grown in confined spaces. As a result, costly immunizations against dangerous diseases are no longer required (Srutee et al., 2021). Cell-based meat will become less expensive to produce in the long run, and it may be more cost-effective than regular meat if it is produced more effectively (Bryant, 2020). Thus, the outnumbered benefits of cell-based meat techniques compared to traditional meat production opened the field of scope for this innovative technique.
As cell-based meat is still in its infancy due to its immature manufacturing technology and high production costs, researchers are working feverishly to enhance technical aspects to speed up the industrialization and commercialization process (Guan et al., 2021).
Methodology used for review
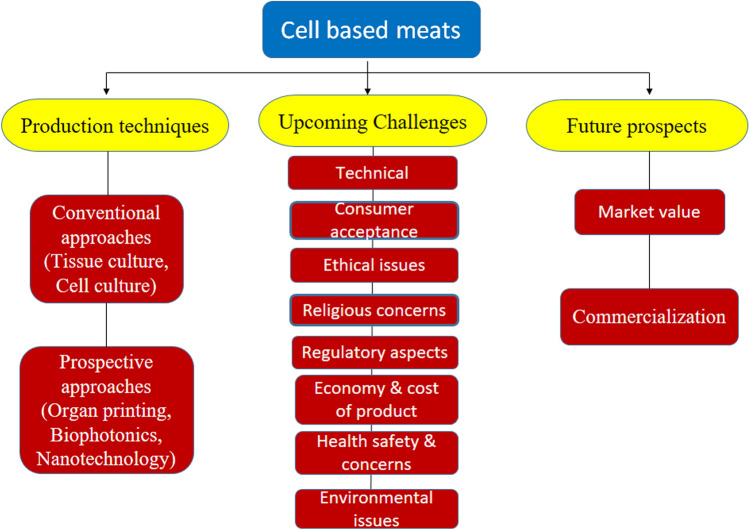
The authors have tried to present an overview of existing cell-based meat production processes and analyze the technological hurdles for cell-based meat. Meanwhile, further research is being focused on the marketing and commercialization of cell-based meat (Fig. 1).
Fig. 1.
Overview of the paper, detailing production techniques, upcoming challenges, and the prospects of cell-based meat
First, Scopus and Google Scholar were used to perform a literature search. Data was gathered and discussed with appropriate references. Although the majority of the articles were published during the last decade, earlier references were included in some cases to emphasize conclusions and facts that had not before been reported in a comparable context. Recent advancements have been given more attention.
Mechanism of production
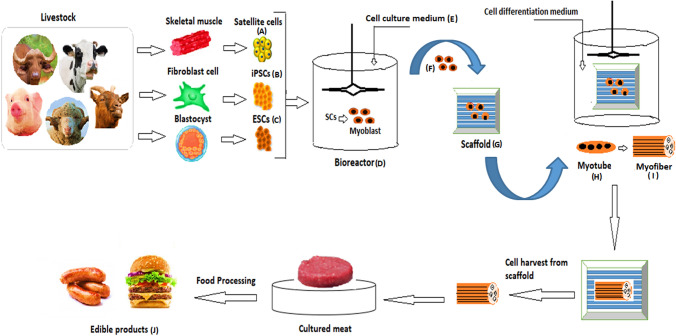
The cell-based meat production method is a combination of tissue and food engineering techniques where the production process can be divided into four major steps as shown in Fig. 2.
Fig. 2.
Stem cell technique for producing cell-based meat
Stem cell collection
Stem cells are a kind of progenitor cell that can proliferate and differentiate into different types of cells with specialized functions (Dodson et al., 2015). Muscle stem cells, or myosatellite stem cells (Fig. 2. Number A), induced pluripotent stem cells (Fig. 2. Number B), and embryonic stem cells (Fig. 2. Number C) are all possible sources of stem cells. Muscle cells are produced from embryonic stem cells (ES cells), myosatellite cells, or specialized cells in muscle tissue (Pandurangan and Kim, 2015). These cells can be readily obtained from a biopsy of living animal tissue after enzymatic digestion and mechanical disruption, as well as purification utilizing flow sorting with specific surface markers (Ding et al., 2017). ES cell lines have endless potential for regeneration, but mutations can accumulate over generations, and muscle cells require particular stimulation. Myosatellite cells have a limited potential to regenerate, although they can more closely mimic myogenesis. These satellite cells mature into myotubes and mature myofibrils quickly, making them the ideal cell source for skeletal muscle tissue formation (Bach et al., 2003). Breakthroughs in cell culture techniques and stem cell engineering, particularly the creation of induced pluripotent stem cells (iPSCs) from somatic cells via genome reprogramming, are propelling in vitro meat research (Singh et al., 2020). Although a variety of cell sources can be used to manufacture cell-based meat, each cell type requires a separate ex vivo expansion and differentiation procedure due to its growth and development properties (Fish et al., 2020; Stephens et al., 2018; Zhang et al., 2020).
Proliferation of cell
After stem cells have been extracted, they must be expanded in order to attain massive cell numbers. The laboratory culture scale of flasks or dishes is insufficient to fulfill market demand, necessitating the development of a large-scale bioreactor system (Fig. 2. Number D) (Post et al., 2020). Tissue development should be promoted by the design of a bioreactor. The effectiveness of scaled-up cell-based meat production is dependent on adequate oxygen perfusion during cell seeding and development on the scaffold. Bioreactors provide for better mass transfer between the culture media and the cells, resulting in optimal oxygen perfusion. A rotating wall vessel is a form of bioreactor that circles at a speed that balances centrifugal, drag, and gravitational forces, allowing the three-dimensional culture to be submerged in the medium and assisting in the creation of tissue with a structure similar to that found in vivo. This bioreactor design provides for maximal mass transfer while minimizing shear stress. Another type of bioreactor is the direct perfusion bioreactor, which is better suited for growing on scaffolds. A porous scaffold circulates the medium, with gas exchange taking place in an external fluid loop. This bioreactor has a high mass transfer rate as well as a lot of shear stress (Kurt et al., 2021). For stem cell development and differentiation into muscle cells, a cell culture medium (Fig. 2. Number E) is used. Dulbecco’s Modified Eagle Medium (DMEM), Fetal Bovine Serum (20%), and Horse Serum (10%) were employed by Danoviz and Yablonka-Reuveni to successfully develop the proliferation and differentiation of myosatellite cells. The DMEM is a four-fold concentration of vitamins and amino acids that is a modification of Basal Medium Eagle (BME), according to the study. There’s also 4.5 g/L glucose, 0.11 g/L sodium pyruvate, 100 U/mL penicillin, 0.0000001 g/L streptomycins, and 0.004 M l-glutamine in it. Fetal bovine serum (20%) includes fibroblast and insulin-like growth factors, is heat-inactive, and encourages myoblast differentiation and proliferation. Because of its ability to enhance myoblast differentiation and proliferation, horse serum (10%) was used (Danoviz and Yablonka- Reuveni, 2012). After two weeks in culture, 81% of the cultures demonstrated tissue adherence to the culture vessel, 63% showed self-healing, and 74% showed cell proliferation using a self-organized approach. Explanted tissue expanded by over 14% when fetal bovine serum was used as the nutritional medium, and by over 13% when the mushroom extract was used. It was revolutionary to discover goldfish explant tissue growth in a serum-free medium. The most challenging obstacle for cell-based meat is substituting natural extracts for serum-free media, which scientists and companies are still working on (Benjaminson et al., 2002). Growth factors are essential for cell proliferation and growth at the same time. To make recombinant proteins, purified growth factors or hormones from plants, animals, or transgenic bacterial species can be added to the culture medium (Houdebine, 2009). Furthermore, the approach should use a low-cost serum-free medium and online monitoring of various parameters such as pH, dissolved oxygen and carbon dioxide, essential nutrient concentrations, and metabolic waste (Allan et al., 2019). At the same time, medium recycling with automated removal of hazardous wastes and nutrient replacement based on monitoring feedback is crucial to maximize resource use and keep production costs low (Guan et al., 2021).
Differentiation of cell
When it comes to structuring differentiated muscle cells, proliferated cells (Fig. 2. Number F) must be placed into scaffolds (Fig. 2. Number G), which are heterogeneous structures that affect attributes such as texture. Collagen-based meshwork and microcarrier beads are often utilized as scaffolds since they are biocompatible and biodegradable. The scaffold-cultured cells were plated in a nutrient-filled bioreactor that could be stationary or rotated. Myotubes (Fig. 2, number H) are generated when cells unite and develop into myofibers with the help of a differentiation medium (Fig. 2. Number I). This process produces meat that is soft and boneless, which may be used to make hamburgers and sausages, among other things. The main drawback of this procedure is that it cannot produce highly structured or three-dimensional meat, such as steaks. The co-culture of myoblasts and fibroblasts is a potential approach for in vitro meat production (Ding et al., 2017). Once the required number of cells has been attained, cells are stimulated to develop into myotubes, adipocytes, or other mature cell types in muscular tissues. Because cell maturity has a substantial impact on characteristics, structure, and nutrient content, such as proteins, fatty acids, and vitamins, the maturity degree of the final cells produced is an important assessment criterion at this stage (Wuyi, 2019). Despite the fact that muscle stem cells are assumed to have a high potential for myogenic differentiation, the diameter, length, and protein content of ex vivo produced myofibers varies greatly depending on the culture conditions and may be significantly lower than actual muscle fibers (Braga et al., 2017; Lamarche et al., 2021; Park et al., 2016). As a result, it is vital to maximize the differentiation state and improve the maturity of differentiated cells based on the process of in vivo muscle tissue growth (Wuyi, 2019).
Cell harvest, assembly, and food processing
In the final stage of the cell-based meat manufacturing process, the mature cells are collected and processed, including molding, coloring, and seasoning, to produce the cell-based meat end-product (XinRui et al., 2019). Since typical cell culture can only create a two-dimensional (2D) thin cell layer, a piece of marbleized and structured meat requires the assembly of myofibers, adipocytes, and maybe connective tissue cells (Zhang et al., 2020). Furthermore, the molding technique could be used throughout the cell differentiation step, which involves co-culturing distinct cell types in a biomimetic three-dimensional (3D) environment provided by the scaffold or hydrogel (Tuomisto and Teixeira de Mattos, 2011). Finally, food processing such as the inclusion of heme protein and flavoring agents may result in the final product (Wuyi, 2019). Cell-based meat can be utilized to make burgers, sausages, and other edible products (Fig. 2. Number J) using a variety of food processing procedures.
Production techniques
The techniques used for the production of cell-based meats are grouped as conventional and prospective approaches.
Conventional techniques
The two methodologies used in the traditional way of cell-based meat production are self-organizing and scaffolding approaches. Muscle tissue is employed for in vitro culturing in the self-organizing approach, whereas stem cells are used for proliferation and differentiation into myofibrils in the scaffolding technique.
Self-organizing/tissue culture techniques
Muscle stem cells were separated and assembled in an organized manner to produce cell-based meat. The growth of existing muscle tissue can also be aided by coculturing the cells in a suitable medium. This technology was initially used to create cell-based meat when Benjaminson and colleagues (2002) used it to make fish muscle explants from Carassius auratus (goldfish). They cultured the skeletal muscle explant for seven days in a variety of media and found that the explant with dissociated goldfish skeletal muscle grew rapidly and resembled a fresh fish fillet due to the culture media, which mimicked in vivo conditions. It also covers the testing of fetal bovine serum, fish meal extract, and mushroom extracts, among other growth mediums. He also evaluated how each growth medium affected the muscle tissue growth of the explants, which led him to explore alternatives to fetal bovine serum (Benjaminson et al., 2002). This approach can produce highly structured meat identical to in vivo meat without the need for scaffolds because the explant comprises all of the tissues. The main disadvantage of this approach is that the formed cells become necrotic due to a lack of blood supply, nutrient supply, and cell separation from the surface. This is because culture media can only resemble in vitro meat under specified conditions. By cultivating the tissue explant onto a network of edible porous polymer scaffolds through which nutrients can be perfused to the cells by functioning as an artificial capillary, tissue culture and tissue engineering can overcome this limitation (Zandonalla, 2003). Additionally, customized bioreactors with low shear force and homogenous perfusion for huge quantities have been developed.
Cell culture/scaffold-based technique
The utilization of scaffolds allows for the production of soft consistency or boneless meat, which is the second type of cell-based meat production technique. Scaffolds play an essential role in tissue engineering by providing temporary structural support, assisting in the transmission of important nutrients, eliminating waste materials, and fostering the growth of functioning tissues and organs. By arranging differentiated myotubes in a ring on a scaffold and allowing them to grow in size and protein content, a single piece of muscle tissue can be replicated to more than a trillion strands (Bhat et al., 2017; Chriki and Hocquette, 2020; Mengistie, 2020). Stem cells, such as satellite cells, ESCs, and iPSCs, are extracted and cultivated, and/or co-cultured with adipocytes, in this procedure. When stem cells are embedded in edible scaffolds or carriers, such as collagen meshwork or microcarrier beads, differentiation from myotubes to myofibers occurs. Finally, the myofibers obtained can be collected, processed, cooked, and consumed as an emulsion or ground meat product (Bhat et al., 2015; Drury and Mooney, 2003). Even though the most important processes are the differentiation of myotubes from stem cells and myofibers from myotubes, this can be accomplished in vitro by developing developmental pathways for muscle fibers. Various growth factors and signaling molecules are used for this, including insulin growth factor (IGF), platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), Wnt3a, Wnt7a, Notch, and forskolin (Chargé and Rudnicki, 2004; Will et al., 2015). One of the key challenges is the difficulty in constructing suitable scaffolds with the appropriate thickness and mechanical properties to facilitate cell attachment and development. Scaffold biomaterials must be edible and/or biodegradable while yet preserving structural integrity and sustaining cells in bioreactors. The scaffolds must allow for the right arrangement of lipids, muscles, and connective elements to mimic the feel of traditional meat (Choudhury et al., 2020).
Prospective techniques
With recent advancements in technologies and scientific innovations in the field of cell-based meat, various emerging techniques like organ printing, nanotechnology and biophotonics fall under the prospective approaches for cell-based meat production.
Organ printing
For small-scale production of cell-based meat, cell culture and tissue culture can be utilized but they lack the fundamental features and aspects of usable and appropriately tasting meat, such as vascularization, consistency, and fat marbling. Other hypothetical alternatives, such as organ printing, could, in the future, provide a viable option for producing fully structured flesh. Three- dimensional (3D) or four-dimensional (4D) bioprinting technologies can be used to manufacture biological tissue constructs that replicate the anatomical, structural, and functional characteristics of original organs or tissues when used in conjunction with tissue engineering concepts. Organs made with 3D printing technology would have appropriate vascularization to allow blood flow to the entire product, as well as the fundamental cellularity of the individual organ (Bhat et al., 2017; Gillispie et al., 2019). 3D printing is a new, advanced, and sophisticated tissue engineering process that involves fusing sprayed cell encapsulated hydrogels over a printed scaffold to create the desired 3D shape and structure (Jung et al., 2016). 3D bioprinting is one of the most powerful and appealing tools for providing functionally and anatomically similar organs or tissues for regenerative tissue and organ clinical applications because it deposits biomaterials and multiple cell types into a single 3D tissue architecture with high precision. This technology is used for tissue regeneration such as muscle, bone, and cardiovascular tissues, and it uses similar technology. 4D printing is an extension of 3D printing that adds one more dimension of transformation over time where the target organs or tissues are sensitive to parameters like humidity and temperature (Javaid and Haleem, 2019). There are a variety of 3D printers available, including laser-assisted, inkjet, and microextrusion bioprinters, with different printing precision and specifications.
Microextrusion printers are slower and less expensive and work with the continuous release of materials. The most expensive and highest-resolution printers are laser-assisted printers. The cheapest printers are ink-jet printers, which have small droplet sizes and low viscosity (Bhat et al., 2019). The only drawback of this approach is that it is expensive and still in development (Gaydhane et al., 2018). Spraying a suspension of myoblast cell encapsulated hydrogel over a gel made from biological sources could reduce in vitro meat production costs (Singh et al., 2020). Using diverse hydrogel compositions and cell types, researchers from all around the world have attempted to produce tissue-like biological structures. Despite its potential in regenerative medicine to generate several transplantable tissues, such as cartilage, skin, and bone, organ printing is still in its infancy, and existing bioprinting options face greater technical challenges in terms of controlled cell distributions, high-resolution cell deposition, innervation, and vascularization within complex 3D tissues (Mandrycky et al., 2016). Due to the presence of vasculature and intramuscular fat, cell-based meat mimics of specific components like beef steak, pig shoulders, and other meats typically require spatial resolution and redefinition, affecting the taste, flavor, and texture to match the originals. Improving scaffolds (preferably hydrogel scaffolds), introducing varied muscle types, and even constructing functional organs in ex vivo, can be handled by utilizing 3D printing (Lee et al., 2019). During contraction, 3D printing of muscle is defined by cell distribution, alignment, and synchronization; nevertheless, biochemical compatibility, resolution, and throughput are important considerations (Beauchamp et al., 2017). The mechanical qualities of meat-ink are also a determining aspect of 3D-printable meat items, according to a recent study. The choice of bioprinter nozzle, pressure, and shear stress all influence cell development and differentiation in mouse myoblast cell culture (C2C12) (Handral et al., 2022). Overall, 3D printing has advantages such as speed of production, shape adjustment, uniform distribution of nutritional content (protein, fat), and easy preparation, even in space stations. A 3D bioprinted rib-eye steak was manufactured for the first time in 2021 by an Israeli business called “Aleph farms” (Liu et al., 2017; Newswire, 2021). Nonetheless, 3D printing may offer novel ways to manage the nutritious profile of cultured tissues, particularly fat and proteins, as well as the major hurdles of cell-based meat production, like protein, fat, and other nutritional content, along with realistic texture (Handral et al., 2022). In the future, 3D printing will be used in the food sector to customize 3D foods with specific nutrients and personalized textures and shapes. However, the appearance of 3D-printed foods is one of the most crucial components of their adoption. According to some research, these produced meals should be viewed as unique in the future to encourage entrepreneurship and growth for a more sustainable food chain. Surprisingly, experts believe that the future will be a confluence of 3D printing and cooking on a single device, which is a critical possibility for the development of 4D printing equipment to assist with food supply and management (Baiano, 2020). In vitro meat paired with 3D printing technology offers an uncompromising solution for averting future food crises, minimizing animal cruelty, and significantly lowering greenhouse gas emissions and water waste. Nutrigenomics allows for the creation of tailored food based on a person’s genetic information, lifestyle difficulties, and nutritional inadequacies (Prakash et al., 2019).
Nanotechnology-based technique
Nanotechnology is a rapidly evolving technology that involves the testing, manufacture, and modification of nano-sized materials with unique properties that are less than 100 nm in diameter (Tabassum et al., 2018). Nanotechnology is the atomic and molecular level fabrication and alteration of materials. With a speculative technology in mind, such as an assembler or a robot the size of a molecule capable of moving matter at the atomic and molecular level, this quickly growing science has enormous promise. With the aid of these molecular-scale small robots, nanotechnologists are investigating all of the potential and helpful technological interventions that they would want to make. Knowing that everything is made up of the same fundamental atoms that are just organized in different ways allows us to build almost any material we desire from the ground up by putting together exactly the molecules we want (Bhat et al., 2017). Nanotechnology can play a crucial role in the creation and packaging of novel meat products since nanofibers occur naturally in meat and have an impact on the texture and color of meat after cooking. Nanotechnology is also frequently used in the packaging of meat products. A packaging film comprised of nanoclays dispersed on a polyamide-6 (PA6) matrix is used to package beef products. This nanoclays packaging film improves the stiffness of meat products while also improving O2 barrier properties (Picouet et al., 2014). Nanotechnology’s function in meat packaging has several benefits, including mechanical tolerance, heat resistance, increased biodegradability, and improved barrier qualities. They can also be used with antimicrobial boosters and spoilage detectors as packaging materials. While layer-by-layer (LbL) deposition techniques can be used to wrap meat and other food goods in nanolaminates or edible coatings, which can increase the shelf-life of the products. These cutting-edge packaging solutions assure the quality of meat during transport, enabling storage and potential dissemination, as well as an open line of communication with customers (Sharma et al., 2017). Furthermore, greater nutritional bioavailability, enhanced sensory acceptance, focused distribution of bioactive chemicals, and improved antimicrobial effects of preservatives are all promising areas for nanoscience involvement in meat science and technology (Singh et al., 2020). To detect the presence of microbial pathogens, pollution, and poisons, food-based nanosensors such as e-nose, e-tongue, and lab-on-chip nanosensors for pathogen detection, surface-enhanced Raman scattering-based sensors, and aptamer-based sensors are utilized exclusively. They have the ability to give advantages such as rapid sensitivity, accuracy, and functional detection to assist in the removal of contaminants and the provision of high-quality meat to stakeholders (Kulshreshtha et al., 2017).
Bio-photonics
Biophotonics, a technology that uses light to hold matter particles together, is another speculative approach for cell-based meat (Bhat et al., 2019). Biophotonics, a new prospective technology for the generation of in vitro meat that relies on the effects of lasers to transport matter particles into specific organizational structures, is a novel approach to using light to bind together particles of matter. Even though the principles behind this sector are unknown, it produces "optical matter" in the form of three-dimensional chess boards or hexagonal arrays in which crystalline materials, such as polystyrene beads, can be bonded together by infrared light nets. The substance will disintegrate when the light is turned off (Bhat et al., 2017). By holding cells together using laser light, it can eliminate the requirement for scaffolding in tissue creation (Gaydhane et al., 2018).
Upcoming challenges
Various challenges affecting cell-based meat production are discussed below, and it is summarized in Table 1.
Table 1.
Various challenges affecting cell-based meat production (technical, consumer acceptance, ethical issues, religious concerns, regulatory aspects, economy and cost of production, health safety and concerns, environmental issues)
| Technical |
● Cell source collection ● Culture medium preparation ● Scaffold selection ● Up scaling process and need of bioreactor ● Mimicry and sensorial resembles |
| Consumer acceptance |
● Unnaturalness ● Unappealing ● Unaccepted price |
| Ethical issues |
● Tampering with God’s creatures ● Promoting cell-based meat is one form of advertising red meat as a must diet ● Why not plant based meat? ● Risk of cannibalism |
| Religious concern | ● Questioning whether dietary requirements of Islamic, Jewish, Christian, Hindu and Buddhism is done |
| Regulatory aspects |
● To create guidelines for cell-based meat acceptability and commercialization ● Implementation of safety evaluation and regulatory policies |
| Economy and cost of product |
● Affects nations relying on conventional meat production ● Unemployment in agricultural sectors ● This is a risky innovation that requires skilled labors and investment ● Cost of culture medium |
| Health safety and concerns |
● Use of plastic wares create endocrine disrupting chemicals (EDCs) ● Processed meats create a chance of cancer and cardiovascular diseases ● Safety aspects of used culture medium is unclear |
| Environmental issues |
● Greenhouse gas emission is moderate compared to plant/ insect based proteins ● Energy consumption is high compared to conventional meat |
Technical
The critical step in obtaining the best cell source for cell-based meat manufacturing is difficult. Regardless of whether cells are derived from cell lines or primary cells, both have disadvantages. Cell lines can be formed genetically and chemically, or they can develop naturally by mutations (Ramboer et al., 2014). These immortalized cells proliferate and differentiate more quickly, potentially minimizing the need for additional animal biopsies. Cell lines, on the other hand, have several disadvantages, such as sub-culturing, misidentification, and continued evolution (Stephens et al., 2018). Another option is to extract the primary cells found in the original tissues, which would entail keeping a small herd of animals and harvesting cells for culture as needed. Muscle stem cells are the most commonly researched stem cells (satellite cells). However, because of their ability to expand in the absence of animal serum and their increased proliferation capacity, mesenchymal stem cells, and other multipotent cells are increasingly being researched. Embryonic stem cells, which have an infinite proliferative capacity, are also a possibility, however guiding them into a muscle cell lineage seems to be more challenging (CookMyosite, 2016). Human primary cells are also available for scientific purposes. Harvesting the proper cell type from the source tissues, in both terms of cell numbers and uniformity, can be expensive and technically challenging, and the amount of cells recovered is sometimes insufficient for useful interpretation. The responsiveness of cells to the culture conditions and their growth behavior will also be affected by sample variability. It’s still unclear which animal species, breed, or tissue will produce the best cell supply, which is a huge concern (Bhat et al., 2019).
The development of an adequate culture medium is one of the top priorities for cell-based meat as a cell culture-based product. The culture medium, in particular, must enable efficient cell proliferation and differentiation while also taking into account cost, availability, and food safety considerations (Guan et al., 2021). Cultured media components are currently from animal origins, such as chicken embryo extract, fetal calf serum, or horse serum, as compared to plant-based and microorganisms. To comply with the purpose of cell-based meat, which is to avoid animal husbandry and slaughter, all animal components should be removed from the manufacturing process. Incorporating serum into the production system will not only raise issues about acceptance, but will also result in a plethora of additional serious difficulties. Apart from being a potential source of contamination and infections, and a cause of significant variable between batches, the large supply of serum-based medium required for commercial production would not be logistically or ethically desirable. Furthermore, creating serum-free and animal component-free media is crucial for ensuring optimal safety, sustainability, controllability, and accuracy of the cell culture process. It is possible and feasible to develop serum-free media for a single cell type, allowing for precise nutrition delivery during the cell’s proliferation and differentiation phase. Myoblasts, on the other hand, lack such a medium, necessitating close scientific scrutiny (Bhat et al., 2019; Guan et al., 2021). The most important cost driver in the cell-based meat manufacturing process is the culture medium, which accounts for 55% to 95% of the entire production cost. As a result, cost reduction is still a major priority in the development and improvement of the cell-based meat production process (Kolkmann et al., 2020).
The scaffold’s composition is similar to the problem with cell-based meat production. Ideal scaffolds for cell adhesion and growth have a large specific surface area, are flexible in contraction and relaxation, and have strong cell affinity and compatibility. Simultaneously, investigate the digestibility, edibility, safety, economics, and scalability of cell-based meat (Browe and Freeman, 2019). Depending on their degradability and edibility, bio-based scaffold materials are usually classed as non-edible, non-edible but degradable, or edible scaffolds (Bodiou et al., 2020). If degradable scaffolds are used, they must remain stable during the cell growth procedure and be degraded either naturally or artificially before cell-based meat products can be obtained, thus the deterioration procedure and duration must be properly monitored to avoid cell damage or gene and protein expression interferon (Guan et al., 2021). Extruded gelatin microfibre scaffolds have been utilized to improve cow and rabbit muscle cells’ adherence, development, and maturity (MacQueen et al., 2019). Furthermore, since these natural scaffolds are derived from animals, their use will fall short of the cell-based meat’s main goals. The texture, taste, thermal stability, cooking properties, and nutritional content of scaffolds, as well as the cost and availability of scalable production, must all be considered while developing cell-based meat products (Guan et al., 2021). In scaffold-based production, the disassembly of the scaffolding structure is also a hurdle. Traditionally, sheets of cells have been physically or enzymatically removed from the scaffolding system, causing damage to both the cells and the extracellular matrix they produce, but new approaches are increasingly being employed to eliminate this problem (Canavan et al., 2005).
Designing and developing intelligent bioreactors for cell-based meat is a technical challenge, with the potential to be the largest in the field of mammalian cell culture. For example, if one ton of cell-based meat is produced, the needed cell number is in the range of 1014, and the maximum accessible cell density in bioreactors is 1–3 × 107 × 0.001 L, the basic configuration is one 10 m3 or ten 1 m3 bioreactors (Post et al., 2020). Publications show bioreactor expansion up to 5 L, however, with today’s commercially accessible technologies, bioreactors up to 2000 L are possible (Schnitzler et al., 2016). To put the scale of cell-based meat production into perspective, 8 × 1012 cells are required to extract one kilogram of protein from muscle cells, which would necessitate a 5000 L traditional stirred tank bioreactor. While this amount is common in established bioprocessing, tissue engineering and mesenchymal stem cell growth have yet to be established. Different bioreactor designs, like fluidized bed bioreactors and hollow fiber membrane bioreactors, can theoretically achieve higher cell densities, although they are currently less well-established for cell expansion. Scale-up (in a few large bioreactors) and scale-out (in a large number of smaller bioreactors) are both significant challenges (Stephens et al., 2018). Consumers expect cell-based meat to be as good as, if not better than meat produced by farm animals in terms of color, flavor, texture, and nutrition, so it’s structural and biochemical composition must be comparable to regular meat (Bhat et al., 2019).
It’s difficult to replicate the flavor of meat in vitro because it comprises over a thousand fat-derived and water-soluble components (Sharma et al., 2015). Due to reduced myoglobin expression in cell-based cells under ambient oxygen conditions, the hue of cell-based meat generated in laboratories today tends to be more yellowish than reddish or pinkish (Post and Hocquette, 2017). Several studies have found that by exposing cultured bovine muscle fibers to low oxygen levels, myoglobin expression is increased, leading the color to resemble that of actual meat (Kanatous and Mammen, 2010). Many recently released imitation meat products contain plant-based heme to mimic the color and flavor of real meat, therefore heme and iron fortification throughout the processing step should be addressed. Adipose tissue is required for meat texture, juiciness, and flavor, hence co-culturing muscle cells with adipose tissue cells is critical. Increasing the number of cell types during co-culturing, on the other hand, considerably expands the number of test circumstances, making cell co-culturing experimentally difficult. Furthermore, different cell types have distinct nutritional requirements, and muscle cells mature considerably faster than adipose tissue, which takes two to three months to mature into whole adipose tissue. Extensive experimentation and effort will be required to optimize the experimental circumstances. Aside from these factors, there are a few more that influence the development of sensory qualities and meat quality, including biochemical and physical changes that occur during the aging process (Bhat et al., 2019).
Consumer acceptance and perception
The public’s perception and acceptance of new technologies and goods is always a challenge, and cell-based meat is no exception (Bryant, 2020). After conducting a thorough study based on 14 studies on consumer acceptance of cell-based meat (summarized in Table 2), researchers determined that people have differing attitudes about cell-based meat (Bryant and Barnett, 2018). Many customers believe that cell-based meat has a poor flavor, texture, or color than traditional meat. Tucker emphasizes this argument, arguing that the rejection of cell-based meat was mostly due to a lack of sensory appeal (Tucker, 2014). Some participants wanted to compare the aesthetic appeal of cell-based and regular meat. In another research, it was revealed that participants in all three nations of their studies reported issues with flavor and texture (some of them believed it will be soft or boring texture). Cell-based meat was expected to have a lesser flavor and texture, as well as be less expensive than conventional meat, according to the participants (Bekker et al., 2017a, 2017b; O’Keefe et al., 2016). Those who were critical of the flavor and texture frequently emphasized the lack of fat content in the cell-based meats as well (Laestadius and Caldwell, 2015). Aside from sensory factors (primarily color, tenderness, and flavor), psychological factors (including cultural factors and lifestyle), assurances of hygiene and safety, and marketing factors such as price, brand, and labels based on origin, safety, local production, and ethical production are the main factors that currently influence cell-based meat purchases and consumption. In a study it claimed that 65.3% of consumers would be willing to try cell-based meat, with 32.6% wanting to eat it on a regular basis, 47.7% more eager to eat it than soy-based meat alternatives, and 31.5% willing to eat it as a replacement for conventional meat. Meanwhile, in another study only between 5 and 11% would consume cell-based meat (Hocquette et al., 2015; Slade, 2018; Wilks and Phillips, 2017).
Table 2.
Summary of 14 studies related to consumer acceptance and perceptions of cell-based meat
| Authors | The most important findings |
|---|---|
| Tucker (2014) | Although most people had an unfavorable opinion of cell-based meat, some people (especially men, younger people, middle-income people, and city dwellers) had a favorable opinion. Animal ethics and higher protein productivity were the main perceived benefits, while sensory qualities, unnaturalness, and perceived unhealthiness were the key reported downsides |
| Verbeke et al. (2015a; 2015b) | Disgust and weirdness were among the first reactions. Participants saw few personal benefits, but recognized social benefits such as food security and the environment. Health, safety, and negative social and economic consequences are all personal and society risks. Further issues included the necessity for regulation and unambiguous labeling, as well as the inevitable scientific progress, governance, and risk control |
| Marcu et al. (2015) |
Anchoring on more familiar technologies, utilizing metaphors and ordinary arguments to block off debate, and establishing polarities were among the tactics used by participants to make sense of the situation Others, on the other hand, posed questions and participated in realistic cost–benefit analysis |
| Hocquette et al. (2015) | The majority of respondents thought the meat industry had significant environmental and animal welfare issues, and that cell-based meat was viable and realistic. However, only a small percentage of people choose cell-based meat as their first option for reducing meat-related issues. The majority believed it would not be healthy or tasty, and that customers would reject it. Despite this, many people were in favor of funding more research on cell-based meat |
| Verbeke et al. (2015a; 2015b) | When compared to only providing basic information, providing additional information about the advantages of cell-based meat improved acceptance. Acceptance is hampered by both financial and sensory expectations |
| Laestadius and Caldwell (2015) | The majority of the remarks were unfavorable. Positive feedback focused on animal welfare, the environment, and public health benefits, while negative feedback focused on cell-based meat’s artificial and unpleasant appearance |
| Laestadius (2015) | Both favorable and negative comments had comparable principles (animal welfare, sustainability, equality, naturalness, and maximizing limited resources), but participants viewed cell-based meat differently. Themes that are comparable to the ones mentioned previously |
| O’Keefe et al. (2016) | A positive discussion about cell-based meat was largely fueled by a sense of scientific advancement. Although the main apparent benefit was animal welfare, much of the discussion focused on sustainability. Many people had concerns about the product’s safety and nutritional value, and most felt that it would have to be less expensive than traditional meat to win acceptability |
| Siegrist and Sütterlin (2017) | The health risks associated with cell-based meat were considered to be less acceptable than those associated with regular meat. Perceived naturalness was the sole mediator of this impact |
| Bekker et al. (2017a; 2017b) | Positive and negative information regarding cell-based meat (or a comparable product) altered explicit, but not implicit, attitudes about it. For more familiar participants, there was less of an effect |
| Wilks and Phillips (2017) | The majority of respondents were willing to test cell-based meat, but just one third were willing to consume it on a regular basis or as a substitute for conventional meat. Men were more receptive than women, and liberals were more susceptible than conservatives. Price, taste, and unnaturalness were the main concerns |
| Bekker et al. (2017a; 2017b) | The majority of the connections were about the future and societal consequences. In terms of physical qualities and composition, cell-based meat was thought to be equivalent to normal meat, while some participants thought it wasn’t’ real’ meat. Depending on how liberal their definition of meat was, this differed between participants from different nations |
| Siegrist et al. (2018) | As cell-based meat is perceived as unnatural, it has a lower acceptance rate than normal meat. The discussion about cell-based meat increased people’s acceptance of traditional meat. Non-technical descriptions of cell-based meat are accepted more readily than technical statements, owing to a sense of unnaturalness and unpleasantness |
| Slade (2018) | A small percentage of participants (11%) preferred cell-based meat to conventional meat or plant-based meat. Men, younger people, more educated people, those who eat meat alternatives, and those who care about the environment had a higher preference for cell-based meat |
People who have been informed about the environmental and health benefits of cell-based meat are more likely to accept it. Food safety and pricing are the most common consumer concerns, as are uncertainties such as unnaturalness, unhealthy, unattractive flavor, and an unexpected cost. Following that, researchers examined customer acceptance of cell-based meat after tasting it, emphasizing the role of positive information in enhancing acceptance and willingness to try it. Surprisingly, 58% said they would pay a 37% premium for cell-based meat over regular meat (Rolland et al., 2020).
Existing studies on public views of cell-based meat use a number of approaches, but they all reveal that people have a wide range of emotions about it, ranging from extremely positive to extremely negative, with a lot of grey areas in between. Although cell-based meat is primarily aimed at meat-eaters, vegetarians who abstain from eating meat out of compassion for animals may find it appealing. As a result, the benefits of cell-based meat to the environment and animal welfare should be quantified and shared effectively (Milburn, 2016). According to social media surveys and comments on media articles on cell-based meat, the perceived unnaturalness and unattractive features of cell-based meat can be a worry (Laestadius and Caldwell, 2015). In a poll of 1890 scientists and students on cell-based meat, multiple correspondence analysis identified three distinct groups of respondents: those in favor, those against, and those who had no viewpoint (Hocquette et al., 2015). A survey of 673 people in the United States found that while more than two-thirds said they would try cell-based meat, just one-third said they would eat it on a daily basis (Wilks and Phillips, 2017). As a result, public science popularization of cell-based meat should be carried out through a range of channels in addition to seeking scientific advancements. The objective of creation, the method of manufacturing, the benefits, and drawbacks of cell-based meat, as well as the nutritional content, food safety, and health consequences of consumables, should all be made freely available to the general public. Once consumers have formed an accurate perception, they can select whether or not to eat cell-based meat (Guan et al., 2021).
Although some people consider cell-based meat to be unnatural and reject it, others welcome it since it is animal-friendly, healthful, and safe. Meat may be advised by doctors and used as a medical diet in the future since the contents of meat, such as lipids, proteins, and iron can be controlled. People of varied religious and cultural backgrounds have different viewpoints. Some people regard cell-based meat as a form of disrespect toward God and the environment, while others support it since it eliminates animal slaughter. Public perceptions of technology have a significant impact on its acceptability. Only by delivering the correct message through the right channels, such as media coverage, public product debuts, and scientists raising public knowledge through debates and conversations about new technology, public perceptions can be changed. Governments must work on regulatory guidelines to aid the acceptability of cell-based meat and to keep a check on quality and purpose before these items can become commercially successful (Gaydhane et al., 2018).
Ethical considerations
People assume that anything natural is wholesome and safe in comparison to manmade materials. The ethical society opposed and despised the practice of growing animal parts in the lab since it appeared to be messing with God’s creation and humiliating the creatures. Some individuals are also worried that cell-based meat may eventually overtake animal farming, resulting in fewer healthy farm animals (Bhat et al., 2015; Milburn, 2016; Schaefer and Savulescu, 2014). Animal suffering and death is one of the most serious ethical concerns surrounding modern cell-based meat processing methods.
Biopsies from donor animals are harvested for stem cells, and a medium based on fetal calf serum (blood from fetuses recovered from slaughtered pregnant cows) is used in the current production procedure. Despite the fact that such animal biopsies are thought to be painless (or utilize tissues such as feathers) and research towards an animal-free growing medium is ongoing, meat produced in labs and by start-up enterprises has yet to be devoid of all the animal’s peculiarities.
Another issue with marketing cell-based meat is that it is unethical to advertise unhealthy food, even if we believe it will be farmed appropriately in the future. Experts are strongly opposed to advertising products that are harmful to people’s health, such as fast food for children or smoking. There is growing evidence that consuming meat, especially processed red meats, is linked to some malignancies and cardiovascular disorders. Because current cell-based meat technology can only produce processed meats, promoting cell-based meat would not only put meat at the center of the human diet but would also encourage people to eat more processed meats.
It would be unethical to redirect resources into a system that is less efficient than plants if cell-based meat was stated to be a necessity for responsibly feeding the world’s expanding population in the future. Furthermore, redirecting nutrients and plant products for human consumption instead of using them to grow cell-based meat might be more successful in preventing future global famine.
Other concerns with cell-based meat production include its apparent unnaturalness, the potential for cannibalism, and the fact that it will increase reliance on multinational food corporations while decreasing local self-sufficiency (Bhat et al., 2019).
Religious restrictions and social taboos
Religious leaders are still debating whether cell-based meat is kosher (kosher for Jewish dietary requirements), halal (halal for Muslim consumers who follow Islamic norms), and what to do if no animals are available for religious rites (Hindu consumers). The rabbinical perspective on Judaism is ambiguous. Some believe that cell-based meat can only be kosher if the cells were generated from a slaughtered kosher animal. Others say that regardless of where the cells used to make cell-based meat come from, they will lose their original identity. As a result, the finished product cannot be deemed unsafe for human consumption (Krautwirth, 2018). The Lord directs the Christian community to identify the names of edible animal meat and non-touchable animals, as well as the source of stem cells, in Deuteronomy 14:3–21 of the Holy Bible. “Thou shalt not consume any unclean thing, but ox, sheep, and goat are the best.” However, you are not allowed to consume camels or pigs. It also has a Jewish connection (Mengistie, 2020).
The most important question for the Islamic community is whether the cell-based meat is halal, or whether it fits Islamic guidelines. Because meat culturing is a relatively new discovery, traditional Islamic jurists, whom Muslims frequently consult, have never questioned its halal status. As a result, current Islamic jurists have assumed this responsibility. The halal status of cell-based meat can be determined by looking at the source of the cells and the serum medium used in its production. Only halal in vitro meat can be labeled if the stem cells were derived from a halal slaughtered animal and no blood or serum was utilized in the operation. Serum should be avoided unless it can be demonstrated that the meat will not be affected as a result of its interaction with the serum (due to the danger of contamination) (Hamdan et al., 2018). However, peaceful ideals urge a vegan diet in Hinduism and Buddhism, and only a small number of Hindus choose cell-based meat because most Hindus accept the humane death of animals for food. Beef, on the other hand, is prohibited due to the reverence with which cows are held (Mattick et al., 2015a, 2015b).
Regulatory challenges
Food regulatory agencies must focus on creating guidelines to promote the acceptability of cell-based meat and channel research and development efforts for speedy commercialization. Consumers would feel more at ease with reference criteria, and entrepreneurs pursuing the commercialization of cell-based meat would have less mistrust (Gaydhane et al., 2018).
In March 2019, the US Department of Agriculture (USDA) and the Food and Drug Administration (FDA) agreed on each agency’s regulatory responsibilities for cell-based meat. In summary, the FDA will oversee cell collection and harvesting progress. The USDA will be in charge of regulating the manufacturing and labeling of food products derived from these cells (Food and Drug Administration, 2019). The European Union (EU) was the first to suggest it, the United States was the most active booster, and Israel and Singapore were enthusiastic participants in terms of the global cell-based meat industry’s regulatory stance (Listek, 2020; Post et al., 2020). The EU Novel Foods Regulation ((EU) 2015/2283), which took effect on January 1, 2018, specifically classifies goods made by animal cell or tissue culture as new foods, reducing legal stumbling blocks to the selling of cell-based meat (EFSA, 2018). FDA and the United States Department of Agriculture Food Safety and Inspection Service (USDAFSIS) collaborated to establish a regulatory framework to monitor cell-based meat in the US (FDA, 2019). Food Standards Australia New Zealand (FSANZ), a legislative authority responsible for developing food standards for Australia and New Zealand, has announced that cell-based meat may be included in their current Food Standards Code, but that specialist premarket certification is required (FSANZ, 2017). In China, the cell-based meat industry’s supervisory structure and necessary legislation are being established and upgraded. According to Chinese researchers, cell-based meat should be treated as a novel food raw material, and handled using the National Management Method for Safety Review of New Food Raw Materials (Bonny et al., 2015). The regulatory agency in charge of developing food standards in Australia and New Zealand, Food Standards Australia New Zealand (FSANZ), has announced that cell-based meat may be included in the current Food Standards Code, but that expert premarket certification is required (TingWei et al., 2019).
The safety evaluation and regulatory policy of cell-based meat, as a type of brand new meat product, must be carefully investigated and formulated. The safety of donor animals and the entire cell-based meat production process should be improved, and an independent standard system and an objective regulatory system should be developed to assess food safety risks and nutrient content. Singapore officials claimed to have approved the application of Eat Just to publicly sell a cell-based chicken product in December 2020. It is the first regulatory issue for cell-based meat in the globe (Poinski, 2020). The food safety risk of cell-based meat during the production process involves chemical safety, bio-safety, and nutrition security, and no detailed information regarding the texture, flavor, or nutrients of this product, such as the amino acid composition, protein, fat, or mineral content, can be found (TingWei et al., 2019). Antibiotics and hormones, as well as medium components and extra additions for cell proliferation and differentiation, should all be used in accordance with the applicable regulations (Chriki and Hocquette, 2020; Fujita et al., 2010; Schnitzler et al., 2016). Several additives necessitate the establishment of food product usage criteria due to their initial application in the food sector. Before premarket approval for cell-based meat, the possibility for gene and protein variation owing to long-term in vitro cultivation should be assessed. If gene modification is employed in the manufacturing process, the entire product should be tested repeatedly for sensitization, toxicity, and tumorigenicity (Edelman et al., 2005; Mohorich and Reese, 2019). In a nutshell, food safety evaluation standards and policy restrictions for all products and materials in the cell-based meat industrial chain should be developed as soon as possible to lead cell-based meat research.
Cost of production and economy
One of the major challenges of cell-based meat is its cost of production. Some research groups have recently produced cell-based chicken, beef, and marine items on a pilot and larger scale, with costs ranging from $66.4/kg to $2200.5/kg (Scipioni, 2020; Starostinetskaya, 2021). Broiler chicken costs $2.1–3.9/kg, beef cattle costs $5.6–10.2/kg, and pig costs $2.7–7.1/kg, all of which are much less than the present cost of cell-based meat (USDA, 2021). As a result, cost reduction remains a primary priority in the development and improvement of the cell-based meat manufacturing process. Because of the need for a chemically defined and animal-free medium, which necessitates the use of somewhat expensive recombinant growth factors in place of bovine sera, the culture medium is the major cost driver in the cell-based meat production process, accounting for 55–95% production cost (Kolkmann et al., 2020; Ng and Kurisawa, 2021).
In vitro meat production will undoubtedly have an impact on the economies of nations that engage in large-scale conventional meat production and rely on meat exports to other countries. In places where cell-based meat production is being introduced on a large scale, this technology will have an impact on agricultural employment. These production centers will cut pollution by being close to cities to reduce transportation costs, but this may not be good for the countryside (Bhat et al., 2015). The initial investment is higher and requires skilled laborers. As this is a risky innovation, traditional banks will be reluctant to give financial support to these developments. Furthermore, the production of cell-based meat is not going to benefit the poor in developing countries soon.
Health safety
The cell culture technique would be a first step toward determining the health and environmental safety of in vitro meat production. The culture medium is provided with nutrients, hormones, and growth factors to maintain cell growth, proliferation, and differentiation in cell culture. However, researchers are unaware of whether these compounds have any short-term or long-term harmful effects on human health (Chriki and Hocquette, 2020). Furthermore, plastic wares used for cell culture, such as culture flasks, culture plates, Petri dishes, and so on, could be a significant source of endocrine-disrupting chemicals (EDCs), as EDCs such as bisphenol A (BPA) and dibutyl phthalate (DBP) are used to provide texture and flexibility to plastic items. As a result of the widespread use of plastic goods in cell culture, EDCs may accumulate in culturing cells that can be in stem cells or matured muscle cells. As a result, doing a toxicological assessment of cell-based meat before commercialization has become feasible (Manikkam et al., 2013; Singh et al., 2020). Eating meat, particularly processed red meat, is linked to some cancers and cardiovascular diseases too.
Environmental
In a survey, cell-based meat was compared against a variety of animal protein alternatives (plant, mycoprotein, dairy, and chicken) by using a different comparison field. The researchers discovered that lab-grown meat had a lower environmental impact than conventional beef and perhaps pork, but a larger impact than chicken and plant protein production, owing to high energy demands, except land use and terrestrial soil and freshwater ecotoxicity (Smetana et al., 2015). According to another study, cell-based meat uses fewer agricultural inputs and occupies less area than livestock, but these advantages may come at the expense of more intensive energy use (Mattick et al., 2015a, 2015b). Cell-based meat may generate more externalities than several meat alternatives, such as gluten, soymeal, or insect-based substitutes, and some components, such as glucose and amino acids, must be present in high concentrations for cell-based meat production, and these components have a significant impact on the environmental footprint (Post et al., 2020; Smetana et al., 2015). Greenhouse gas emissions by the cell-based meat products are compared to three distinct beef production systems. Even though cell-based meat produces far less pollution than cattle, they find that cell-based meat is not always more climate-friendly in one scenario. This is because carbon dioxide (CO2) from energy generation accounts for almost all of the CO2 emitted by cell-based meat, and CO2 lasts longer in the atmosphere than methane or nitrous oxide emitted by conventional meat production. As a result, they come to the conclusion that cell-based meat, in the long run, may pose more climate difficulties than cattle. However, because the simulations only provide a result after hundreds of years, this conclusion is highly speculative. Further investigation reveals that the scholarly literature on cell-based meat that has been predicted is unreliable. Because the technology is still in its infancy, future emissions will be dictated by how manufacturing is finished and scaled, as well as the ability to reduce emissions in other parts of the life cycle. It also underlines the significance of combining impact analyses with more coherent renewable energy supply scenarios in the future (Lynch and Pierrehumbert, 2019).
According to another study, although greenhouse gas emissions will be reduced by 78–96%, land use will be reduced by 99%, water consumption will be reduced by 82–96%, and energy consumption will be reduced by 7–45% when compared to conventional farming, except for conventional poultry meat, which requires less energy. According to a comparable study, cell-based beef has a lower heating potential than normal beef. However, cell-based pork and chicken meat may require a large amount of energy, resulting in a higher heating potential than conventional products (Tuomisto and Teixeira de Mattos, 2011).
Switching from meat to alternative proteins can result in massive decreases in greenhouse gas emissions, particularly when using plant or insect-based proteins. While current estimates of cell-based meat emissions imply relatively moderate savings only, that also depends on how the production of cell-based meat is scaled up, because depending on that, large reductions in emissions are possible (Godfray, 2019).
Future prospects and concluding remarks
Cell-based meat technology has progressed from a simple concept to a growing number of businesses all aiming to produce cell-based meat for human consumption. Since this idea is still in the works, we believe that a massive shift in animal farming is unavoidable. Furthermore, with the introduction of this technology, a big number of non-meat eaters may be persuaded to eat cell-based meat because it is safe and devoid of animal killing and suffering. Consumers who prefer vegetarianism for ethical reasons will be drawn to cell-based meat. Because in vitro meat production is a controlled and manipulatable technology, it is feasible to adjust the quality of meat to produce “designer meat” on a long-term basis. Fundamental challenges such as cell sources, mimicking the in vivo environment of myogenesis, cost of production, nutritional characteristics, texture and taste, and consumer perceptions, all of which should be properly addressed through interdisciplinary scientific interventions (Bhat et al., 2019; Singh et al., 2020).
The first “Cell-based Meat Symposium,” held in Norway in 2008, predicted that within the next five to ten years, the first commercial cell-based meat products would be available at prices comparable to European beef ($5200–$5500 per tonne or $3300–$3500) and readily available in supermarkets for each person (Penn, 2018). Moreover, cell-based meat production at a business level even now requires substantial in-depth studies because, in the coming years, cell-based meat will be an integral part of the human diet. However, in the near term, the exceptionally high cost of biologically synthesized meat is the main obstacle to its commercialization, so scientists are trying to dig hard and devote their expertise and time to optimize the overall cost and commercialization (Bhat et al., 2019). To put it into perspective past few years, Mark Post’s first burger cost $330,000 to prepare, and Memphis Meats was producing meat for less than a fifth of that price within a few years. Mosa Meat’s burgers will cost roughly $10 per patty by 2020, and around the same as the least expensive meat on the market five years later, according to Mark Post (Grass, 2019). Cell-based meat could be produced in countries with little or no agricultural land. It’s not surprising, then, that Singapore was the first country to market cell-based meat. Changes in the sites of local and global meat production should restructure economic and social networks in such a way that forecasts are difficult to make now because the location of cell-based meat production and the exact inputs necessary are unknown (Treich, 2021).
External stress factors (management and environment) will also be excluded from meat culturing in bioreactors, resulting in the most consistent production of high-quality meat. This will enable the meat to be raised in regions where the climate and land are unsuitable. Furthermore, compared to traditional meat production, cell-based meat will produce less waste (Bhat et al., 2019). It is thought that if meat substitutes such as Quorn and tofu gain acceptance and people shift to semi-vegetarianism, it will be easier to accept cell-based meat in the future. Practically cell-based meat may not be a quick solution to food shortages. However, since the nutrient composition can be tweaked, its acceptance as “medicinal” meat may increase. Taste, scalability, and even cost become less of an issue in this instance, just like people don’t mind taking vitamin pills instead of eating fruits and vegetables. Traditional animal farming will continue to play a significant role in meeting protein dietary demand. Another ray of hope is growing public awareness and consciousness about the environment, which may entice some people to try cell-based meat (Gaydhane et al., 2018).
Likewise, the meat industry of the future will definitely be more complex than that of today, with a greater variety of meat products or meat replacements available on the market from a variety of sources or techniques (Bonny et al., 2015; Bonny et al., 2017). All protein sources have disadvantages and advantages that affect business feasibility and customer expectation (Bonny et al., 2017). To be successful, new products must be commercially viable alternatives to existing meat production. Because customers are likely to refer to items with comparable market positioning, the success of cell-based meat as an alternative, substitute, or supplement to conventional meat will be critical (van der Weele et al., 2019; Verbeke et al., 2015a, 2015b). The global cell-based meat industry is anticipated to reach USD 214 million in 2025. In a typical scenario, it is expected to reach USD 593 million by 2032, suggesting a 15.7% CAGR from 2025 to 2032. New firms are being launched by entrepreneurs in an attempt to break into the market (Gertenbach et al., 2021). By the year 2025, India is expected to have clean meat marketing. Two institutions, CCMB in Hyderabad and ICT in Mumbai, have recently inked MoUs with the Good Food Institute and the Humane Society of India. IIT Guwahati researchers developed lab-grown meat and applied for a patent on it (Srutee et al., 2021).
Even though cell-based meat is still in its early stages, it is a promising technology that provides a safe and disease-free way to meet rising meat demand without sacrificing animals while also reducing environmental impact and human disease burden. Furthermore, it has the potential to combine a low environmental impact with nutritional and taste attributes that are comparable to conventional meat. With a rapidly rising global population, cell-based meat would supply healthful, nutritious, and sustainable nourishment for future generations. Food shortages would be alleviated, food-borne infections would be reduced, pollution would be reduced, and the output of cell-based meat would increase. Cell-based meat would reduce the reliance on natural resources and land resources, allowing the area to be used for other productive and recreational purposes. However, there are technical, ethical, religious, regulatory, and public neophobia challenges with in vitro meat that must be addressed before it can be included in the human food chain. In the event of future pandemics, the demand for animal protein sources will increase. In such a time of crisis, in vitro meat would be the best approach to alleviate the food crisis and the best option for improving the human population’s nutritious profile through protein-integrated manufacturing protocols.
Acknowledgements
The authors would like to express their gratitude to the people who have been instrumental in the successful completion of this manuscript. This work was not supported by any funding.
Author contributions
AB: investigation, writing-original draft, writing-review and editing, visualization, graphical representation. KP: writing-review, technical editing, supervision. RU: conceptualization, flow of the manuscript, writing-review and editing, supervision, management.
Declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alexandratos N, Bruinsma J. World agriculture towards 2030/2050: the 2012 revision. ESA Working paper No. 12-03. Rome, Agricultural Development Economics Division. Food and Agriculture Organization of the United Nations (FAO). https://www.fao.org/economic/esa (2012)
- Allan SJ, De Bank PA, Ellis MJ. Bioprocess design considerations for cultured meat production with a focus on the expansion bioreactor. Frontiers in Sustainable Food Systems. 2019;3:44. doi: 10.3389/fsufs.2019.00044. [DOI] [Google Scholar]
- Arshad MS, Javed M, Sohaib M, Saeed F, Imran A, Amjad Z. Tissue engineering approaches to develop cultured meat from cells: a mini review. Cogent Food & Agriculture. 2017;3:1320814. doi: 10.1080/23311932.2017.1320814. [DOI] [Google Scholar]
- Bach AD, Stern-Straeter J, Beier JP, Bannasch H, Stark GB. Engineering of muscle tissue. Clinics in Plastic Surgery. 2003;30:589–599. doi: 10.1016/S0094-1298(03)00077-4. [DOI] [PubMed] [Google Scholar]
- Baiano A. 3D printed foods: a comprehensive review on technologies, nutritional value, safety, consumer attitude, regulatory framework, and economic and sustainability issues. Food Reviews International. 2020;38:1–31. [Google Scholar]
- Beauchamp MJ, Nordin GP, Woolley AT. Moving from millifluidic to truly microfluidic sub-100-μm cross-section 3D printed devices. Analytical and Bioanalytical Chemistry. 2017;409:4311–4319. doi: 10.1007/s00216-017-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker GA, Fischer AR, Tobi H, van Trijp HC. Explicit and implicit attitude toward an emerging food technology: the case of cultured meat. Appetite. 2017;108:245–254. doi: 10.1016/j.appet.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Bekker GA, Tobi H, Fischer AR. Meet meat: an explorative study on meat and cultured meat as seen by Chinese, Ethiopians and Dutch. Appetite. 2017;114:82–92. doi: 10.1016/j.appet.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Benjaminson MA, Gilchriest JA, Lorenz M. In vitro edible muscle protein production system (MPPS): stage 1, fish. Acta Astronautica. 2002;51:879–889. doi: 10.1016/S0094-5765(02)00033-4. [DOI] [PubMed] [Google Scholar]
- Bhat ZF, Fayaz H. Prospectus of cultured meat—advancing meat alternatives. Journal of Food Science and Technology. 2011;48:125–140. doi: 10.1007/s13197-010-0198-7. [DOI] [Google Scholar]
- Bhat ZF, Kumar S, Fayaz H. In vitro meat production: challenges and benefits over conventional meat production. Journal of Integrative Agriculture. 2015;14:241–248. doi: 10.1016/S2095-3119(14)60887-X. [DOI] [Google Scholar]
- Bhat ZF, Kumar S, Bhat HF. In vitro meat: a future animal-free harvest. Critical Reviews in Food Science and Nutrition. 2017;57:782–789. doi: 10.1080/10408398.2014.924899. [DOI] [PubMed] [Google Scholar]
- Bhat ZF, Morton JD, Mason SL, Bekhit AE, Bhat HF. Technological, regulatory, and ethical aspects of in vitro meat: a future slaughter-free harvest. Comprehensive Reviews in Food Science and Food Safety. 2019;18:1192–1208. doi: 10.1111/1541-4337.12473. [DOI] [PubMed] [Google Scholar]
- Bodiou V, Moutsatsou P, Post MJ. Microcarriers for upscaling cultured meat production. Frontiers in Nutrition. 2020;7:10. doi: 10.3389/fnut.2020.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonny SP, Gardner GE, Pethick DW, Hocquette JF. What is artificial meat and what does it mean for the future of the meat industry? Journal of Integrative Agriculture. 2015;14:255–263. doi: 10.1016/S2095-3119(14)60888-1. [DOI] [Google Scholar]
- Bonny SP, Gardner GE, Pethick DW, Hocquette JF. Artificial meat and the future of the meat industry. Animal Production Science. 2017;57:2216–2223. doi: 10.1071/AN17307. [DOI] [Google Scholar]
- Braga M, Simmons Z, Norris KC, Ferrini MG, Artaza JN. Vitamin D induces myogenic differentiation in skeletal muscle derived stem cells. Endocrine Connections. 2017;6:139–150. doi: 10.1530/EC-17-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browe D, Freeman J. Optimizing C2C12 myoblast differentiation using polycaprolactone–polypyrrole copolymer scaffolds. Journal of Biomedical Materials Research Part A. 2019;107:220–231. doi: 10.1002/jbm.a.36556. [DOI] [PubMed] [Google Scholar]
- Bryant CJ. Culture, meat, and cultured meat. Journal of Animal Science. 2020;98:skaa172. doi: 10.1093/jas/skaa172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant C, Barnett J. Consumer acceptance of cultured meat: a systematic review. Meat Science. 2018;143:8–17. doi: 10.1016/j.meatsci.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Canavan HE, Cheng X, Graham DJ, Ratner BD, Castner DG. Cell sheet detachment affects the extracellular matrix: a surface science study comparing thermal liftoff, enzymatic, and mechanical methods. Journal of Biomedical Materials Research Part a: an Official Journal of the Society for Biomaterials, the Japanese Society for Biomaterials, and the Australian Society for Biomaterials and the Korean Society for Biomaterials. 2005;75:1–3. doi: 10.1002/jbm.a.30297. [DOI] [PubMed] [Google Scholar]
- Cann O. These are the top 10 emerging technologies of 2016. In World Economic Forum. Available from: https://www.ospi.es/export/sites/ospi/documents/documentos/WEF_Top-10-emerging-technologies-of-2017.pdf. Accessed 28 Nov 2017
- Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiological Reviews. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Choudhury D, Tseng TW, Swartz E. The business of cultured meat. Trends in Biotechnology. 2020;38:573–577. doi: 10.1016/j.tibtech.2020.02.012. [DOI] [PubMed] [Google Scholar]
- Chriki S, Hocquette JF. The myth of cultured meat: a review. Frontiers in Nutrition. 2020;7:7. doi: 10.3389/fnut.2020.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CookMyoSite. skMDC Skeletal Muscle-Derived Cells. Available from: https://www.cookmyosite.com/skmdc. Accessed 2016
- Danoviz ME, Yablonka-Reuveni Z. Myogenesis. Totowa: Humana Press; 2012. Skeletal muscle satellite cells: background and methods for isolation and analysis in a primary culture system; pp. 21–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datar I, Betti M. Possibilities for an in vitro meat production system. Innovative Food Science & Emerging Technologies. 2010;11:13–22. doi: 10.1016/j.ifset.2009.10.007. [DOI] [Google Scholar]
- Ding S, Wang F, Liu Y, Li S, Zhou G, Hu P. Characterization and isolation of highly purified porcine satellite cells. Cell Death Discovery. 2017;3:1–11. doi: 10.1038/cddiscovery.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon LK, Stahl K, Jori F, Vial L, Pfeiffer DU. African swine fever epidemiology and control. Annual Review of Animal Biosciences. 2020;8:221–246. doi: 10.1146/annurev-animal-021419-083741. [DOI] [PubMed] [Google Scholar]
- Dodson MV, Allen RE, Du M, Bergen WG, Velleman SG, Poulos SP, Fernyhough-Culver M, Wheeler MB, Duckett SK, Young MR, Voy BH. Invited review: evolution of meat animal growth research during the past 50 years: adipose and muscle stem cells. Journal of Animal Science. 2015;93:457–481. doi: 10.2527/jas.2014-8221. [DOI] [PubMed] [Google Scholar]
- Dopelt K, Radon P, Davidovitch N. Environmental effects of the livestock industry: the relationship between knowledge, attitudes, and behavior among students in Israel. International Journal of Environmental Research and Public Health. 2019;16:1359. doi: 10.3390/ijerph16081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/S0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- Edelman PD, McFarland DC, Mironov VA, Matheny JG. Commentary: in vitro-cultured meat production. Tissue Engineering. 2005;11:659–662. doi: 10.1089/ten.2005.11.659. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority. Novel food. Available from: https://www.efsa.europa.eu/en/topics/topic/novel-food. Accessed 2018
- Fish KD, Rubio NR, Stout AJ, Yuen JS, Kaplan DL. Prospects and challenges for cell-cultured fat as a novel food ingredient. Trends in Food Science & Technology. 2020;98:53–67. doi: 10.1016/j.tifs.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration. Formal agreement between FDA and USDA regarding oversight of human produced using animal cell technology derived from cell lines of USDA-amendable species. Available from: https://www.fda.gov/food/domestic-interagency-agreements-food/formal-agreement-between-fda-and-usda-regarding-oversight-human-food-produced-using-animal-cell. Accessed 3 July 2019
- Food and Drug Administration. USDA and FDA announce a formal agreement to regulate cell-cultured food products from cell lines of livestock and poultry. Available from: https://www.usda.gov/media/press-releases/2019/03/07/usda-and-fda-announce-formal-agreement-regulate-cell-cultured-food. Accessed 7 Mar 2019
- Food Standards Australia New Zealand. Australia New Zealand food standards code—standard 1.5.1—novel foods. Available from: https://www.legislation.gov.au/Details/F2015L00403. Accessed 2017
- Fujita H, Endo A, Shimizu K, Nagamori E. Evaluation of serum-free differentiation conditions for C2C12 myoblast cells assessed as to active tension generation capability. Biotechnology and Bioengineering. 2010;107:894–901. doi: 10.1002/bit.22865. [DOI] [PubMed] [Google Scholar]
- Gaydhane MK, Mahanta U, Sharma CS, Khandelwal M, Ramakrishna S. Cultured meat: state of the art and future. Biomanufacturing Reviews. 2018;3:1–10. doi: 10.1007/s40898-018-0005-1. [DOI] [Google Scholar]
- Gertenbach L, Lamla J, Laser S. Eating ourselves out of industrial excess? Degrowth, multi-species conviviality and the micro-politics of cultured meat. Anthropological Theory. 2021;21:386–408. doi: 10.1177/1463499620981544. [DOI] [Google Scholar]
- Gillispie GJ, Park J, Copus JS, Asari AK, Yoo JJ, Atala A, Lee SJ. Principles of regenerative medicine. Cambridge: Academic Press; 2019. Three-dimensional tissue and organ printing in regenerative medicine; pp. 831–852. [Google Scholar]
- Godfray HCJ. Meat: The future series-alternative proteins. World Economic Forum. (2019)
- Grass S. Negative impacts of the beef industry: lab-grown meat. WRIT Journal of First-Year Writing. 2019;2:7. doi: 10.25035/writ.02.02.07. [DOI] [Google Scholar]
- Guan X, Lei Q, Yan Q, Li X, Zhou J, Du G, Chen J. Trends and ideas in technology, regulation and public acceptance of cultured meat. Future Foods. 2021;3:100032. doi: 10.1016/j.fufo.2021.100032. [DOI] [Google Scholar]
- Hamdan MN, Post MJ, Ramli MA, Mustafa AR. Cultured meat in Islamic perspective. Journal of Religion and Health. 2018;57:2193–2206. doi: 10.1007/s10943-017-0403-3. [DOI] [PubMed] [Google Scholar]
- Handral H K, Hua Tay S, Wan Chan W, Choudhury D. 3D printing of cultured meat products. Critical Reviews in Food Science and Nutrition. 2022;62:272–281. doi: 10.1080/10408398.2020.1815172. [DOI] [PubMed] [Google Scholar]
- Hocquette A, Lambert C, Sinquin C, Peterolff L, Wagner Z, Bonny SP, Lebert A, Hocquette JF. Educated consumers don’t believe artificial meat is the solution to the problems with the meat industry. Journal of Integrative Agriculture. 2015;14:273–284. doi: 10.1016/S2095-3119(14)60886-8. [DOI] [Google Scholar]
- Houdebine LM. Production of pharmaceutical proteins by transgenic animals. Comparative Immunology, Microbiology and Infectious Diseases. 2009;32:107–121. doi: 10.1016/j.cimid.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaid M, Haleem A. 4D printing applications in medical field: a brief review. Clinical Epidemiology and Global Health. 2019;7:317–321. doi: 10.1016/j.cegh.2018.09.007. [DOI] [Google Scholar]
- Jiang G, Ameer K, Kim H, Lee EJ, Ramachandraiah K, Hong GP. Strategies for sustainable substitution of livestock meat. Foods. 2020;9:1227. doi: 10.3390/foods9091227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JW, Lee JS, Cho DW. Computer-aided multiple-head 3D printing system for printing of heterogeneous organ/tissue constructs. Scientific Reports. 2016;6:1–9. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadim IT, Mahgoub O, Baqir S, Faye B, Purchas R. Cultured meat from muscle stem cells: a review of challenges and prospects. Journal of Integrative Agriculture. 2015;14:222–233. doi: 10.1016/S2095-3119(14)60881-9. [DOI] [Google Scholar]
- Kanatous SB, Mammen PP. Regulation of myoglobin expression. Journal of Experimental Biology. 2010;213:2741–2747. doi: 10.1242/jeb.041442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolkmann AM, Post MJ, Rutjens MA, Van Essen AL, Moutsatsou P. Serum-free media for the growth of primary bovine myoblasts. Cytotechnology. 2020;72:111–120. doi: 10.1007/s10616-019-00361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krautwirth R. Will lab-grown meat find its way to your table? Available from: https://yuobserver.org/2018/05/will-lab-grown-meat-find-way-table/. Accessed 10 May 2018
- Kulshreshtha NM, Shrivastava D, Bisen PS. Nanobiosensors. Amsterdam: Elsevier; 2017. Contaminant sensors: nanotechnology-based contaminant sensors; pp. 573–628. [Google Scholar]
- Kurt E, Klont E, Ergun O, Klont R. White paper cell cultured meat. Austin Food Sciences. 2021;6:1041. [Google Scholar]
- Laestadius LI. Public perceptions of the ethics of in-vitro meat: determining an appropriate course of action. Journal of Agricultural and Environmental Ethics. 2015;28:991–1009. doi: 10.1007/s10806-015-9573-8. [DOI] [Google Scholar]
- Laestadius LI, Caldwell MA. Is the future of meat palatable? Perceptions of in vitro meat as evidenced by online news comments. Public Health Nutrition. 2015;18:2457–2467. doi: 10.1017/S1368980015000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche É, AlSudais H, Rajgara R, Fu D, Omaiche S, Wiper-Bergeron N. SMAD2 promotes myogenin expression and terminal myogenic differentiation. Development. 2021;148:dev195495. doi: 10.1242/dev.195495. [DOI] [PubMed] [Google Scholar]
- Lee AR, Hudson AR, Shiwarski DJ, Tashman JW, Hinton TJ, Yerneni S, Bliley JM, Campbell PG, Feinberg AW. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365:482–487. doi: 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Yong HI, Kim M, Choi YS, Jo C. Status of meat alternatives and their potential role in the future meat market—a review. Asian-Australasian Journal of Animal Sciences. 2020;33:1533. doi: 10.5713/ajas.20.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listek V. The cultured meat revolution: Singapore and Israel one step closer to commercializing lab grown chicken. Available from: https://3dprint.com/276467/the-cultured-meat-revolution-singapore-and-israel-one-step-closer-to-commercializing-lab-grown-chicken/. Accessed 9 Dec 2020
- Liu Z, Zhang M, Bhandari B, Wang Y. 3D printing: printing precision and application in food sector. Trends in Food Science & Technology. 2017;69:83–94. doi: 10.1016/j.tifs.2017.08.018. [DOI] [Google Scholar]
- Lynch J, Pierrehumbert R. Climate impacts of cultured meat and beef cattle. Frontiers in Sustainable Food Systems. 2019 doi: 10.3389/fsufs.2019.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen LA, Alver CG, Chantre CO, Ahn S, Cera L, Gonzalez GM, O’Connor BB, Drennan DJ, Peters MM, Motta SE, Zimmerman JF. Muscle tissue engineering in fibrous gelatin: implications for meat analogs. NPJ Science of Food. 2019;3:1–12. doi: 10.1038/s41538-019-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrycky C, Wang Z, Kim K, Kim DH. 3D bioprinting for engineering complex tissues. Biotechnology Advances. 2016;34:422–434. doi: 10.1016/j.biotechadv.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PloS One. 2013;8:e55387. doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu A, Gaspar R, Rutsaert P, Seibt B, Fletcher D, Verbeke W, Barnett J. Analogies, metaphors, and wondering about the future: lay sense-making around synthetic meat. Public Understanding of Science. 2015;24:547–562. doi: 10.1177/0963662514521106. [DOI] [PubMed] [Google Scholar]
- Mattick CS, Landis AE, Allenby BR, Genovese NJ. Anticipatory life cycle analysis of in vitro biomass cultivation for cultured meat production in the United States. Environmental Science & Technology. 2015;49:11941–11949. doi: 10.1021/acs.est.5b01614. [DOI] [PubMed] [Google Scholar]
- Mattick CS, Wetmore JM, Allenby BR. An anticipatory social assessment of factory-grown meat. IEEE Technology and Society Magazine. 2015;34:56–64. doi: 10.1109/MTS.2015.2395967. [DOI] [Google Scholar]
- Mengistie D. Lab-growing meat production from stem cell. Journal of Nutrition & Food Sciences. 2020;3:100015. [Google Scholar]
- Milburn J. Chewing over in vitro meat: animal ethics, cannibalism and social progress. Res Publica. 2016;22:249–265. doi: 10.1007/s11158-016-9331-4. [DOI] [Google Scholar]
- Mohorčich J, Reese J. Cell-cultured meat: lessons from GMO adoption and resistance. Appetite. 2019;143:104408. doi: 10.1016/j.appet.2019.104408. [DOI] [PubMed] [Google Scholar]
- Newswire PR. Aleph farms and the technion reveal world’s first cultivated Ribeye steak. Available from: https://www.prnewswire.com/il/news-releases/aleph-farms-and-the-technion-reveal-worlds-first-cultivated-ribeye-steak-301224800.html. Accessed 9 Feb 2021
- Ng S, Kurisawa M. Integrating biomaterials and food biopolymers for cultured meat production. Acta Biomaterialia. 2021;124:108–129. doi: 10.1016/j.actbio.2021.01.017. [DOI] [PubMed] [Google Scholar]
- Normile D. Driven to extinction. 319: 1606-1609 (2008) [DOI] [PubMed]
- O'Keefe L, McLachlan C, Gough C, Mander S, Bows-Larkin A. Consumer responses to a future UK food system. British Food Journal. 2016;118:412–428. doi: 10.1108/BFJ-01-2015-0047. [DOI] [Google Scholar]
- Pandurangan M, Kim DH. A novel approach for in vitro meat production. Applied Microbiology and Biotechnology. 2015;99:5391–5395. doi: 10.1007/s00253-015-6671-5. [DOI] [PubMed] [Google Scholar]
- Park SY, Yun Y, Lim JS, Kim MJ, Kim SY, Kim JE, Kim IS. Stabilin-2 modulates the efficiency of myoblast fusion during myogenic differentiation and muscle regeneration. Nature Communications. 2016;7:1–15. doi: 10.1038/ncomms10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn J. Cultured meat: lab-grown beef and regulating the future meat market. UCLA Journal of Environmental Law and Policy. 2018;36:104. doi: 10.5070/L5361039902. [DOI] [Google Scholar]
- Picouet PA, Fernandez A, Realini CE, Lloret E. Influence of PA6 nanocomposite films on the stability of vacuum-aged beef loins during storage in modified atmospheres. Meat Science. 2014;96:574–580. doi: 10.1016/j.meatsci.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Poinski M. Eat just lands first regulatory approval for cell-based meat. Available from: https://www.fooddive.com/news/eat-just-lands-first-regulatory-approval-for-cell-based-meat/589907/. Accessed 2 Dec2020
- Post MJ, Hocquette JF. New aspects of meat quality. Amsterdam: Elsevier; 2017. New sources of animal proteins: cultured meat; pp. 425–441. [Google Scholar]
- Post MJ, Levenberg S, Kaplan DL, Genovese N, Fu J, Bryant CJ, Negowetti N, Verzijden K, Moutsatsou P. Scientific, sustainability and regulatory challenges of cultured meat. Nature Food. 2020;1:403–415. doi: 10.1038/s43016-020-0112-z. [DOI] [Google Scholar]
- Prakash S, Bhandari BR, Godoi FC, Zhang M. Fundamentals of 3D food printing and applications. Amsterdam: Elsevier; 2019. Future outlook of 3D food printing; pp. 373–381. [Google Scholar]
- Ramboer E, De Craene B, De Kock J, Vanhaecke T, Berx G, Rogiers V, Vinken M. Strategies for immortalization of primary hepatocytes. Journal of Hepatology. 2014;61:925–943. doi: 10.1016/j.jhep.2014.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland NC, Markus CR, Post MJ. The effect of information content on acceptance of cultured meat in a tasting context. PLoS One. 2020;15:e0231176. doi: 10.1371/journal.pone.0231176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer GO, Savulescu J. The ethics of producing in vitro meat. Journal of Applied Philosophy. 2014;31:188–202. doi: 10.1111/japp.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler AC, Verma A, Kehoe DE, Jing D, Murrell JR, Der KA, Aysola M, Rapiejko PJ, Punreddy S, Rook MS. Bioprocessing of human mesenchymal stem/stromal cells for therapeutic use: current technologies and challenges. Biochemical Engineering Journal. 2016;108:3–13. doi: 10.1016/j.bej.2015.08.014. [DOI] [Google Scholar]
- Scipioni J. This restaurant will be the first ever to serve labgrown chicken (for $23). Available from: https://www.cnbc.com/2020/12/18/singapore-restaurant-first-ever-to-serve-eat-just-lab-grown-chicken.html. Accessed 18 Dec 2020
- Sharma S, Thind SS, Kaur A. In vitro meat production system: why and how? Journal of Food Science and Technology. 2015;52:7599–7607. doi: 10.1007/s13197-015-1972-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C, Dhiman R, Rokana N, Panwar H. Nanotechnology: an untapped resource for food packaging. Frontiers in Microbiology. 2017;8:1735. doi: 10.3389/fmicb.2017.01735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist M, Sütterlin B. Importance of perceived naturalness for acceptance of food additives and cultured meat. Appetite. 2017;113:320–326. doi: 10.1016/j.appet.2017.03.019. [DOI] [PubMed] [Google Scholar]
- Siegrist M, Sütterlin B, Hartmann C. Perceived naturalness and evoked disgust influence acceptance of cultured meat. Meat Science. 2018;139:213–219. doi: 10.1016/j.meatsci.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Singh A, Verma V, Kumar M, Kumar A, Sarma DK, Singh B, Jha R. Stem cells-derived in vitro meat: from petri dish to dinner plate. Critical Reviews in Food Science and Nutrition. 2020;30:1–4. doi: 10.1080/10408398.2020.1856036. [DOI] [PubMed] [Google Scholar]
- Slade P. If you build it, will they eat it? Consumer preferences for plant-based and cultured meat burgers. Appetite. 2018;125:428–437. doi: 10.1016/j.appet.2018.02.030. [DOI] [PubMed] [Google Scholar]
- Smetana S, Mathys A, Knoch A, Heinz V. Meat alternatives: life cycle assessment of most known meat substitutes. The International Journal of Life Cycle Assessment. 2015;20:1254–1267. doi: 10.1007/s11367-015-0931-6. [DOI] [Google Scholar]
- Srutee R, R. S S, Uday SA. Clean meat: techniques for meat production and its upcoming challenges. Animal Biotechnology. 2021;3:1–9. doi: 10.1080/10495398.2021.1911810. [DOI] [PubMed] [Google Scholar]
- Starostinetskaya A. This startup makes lab-grown chicken for less than $10 per serving. Available from: https://vegnews.com/2021/2/lab-grown-chicken-less-than-10-per-serving. Accessed 2 Feb 2021
- Stephens N, Di Silvio L, Dunsford I, Ellis M, Glencross A, Sexton A. Bringing cultured meat to market: technical, socio-political, and regulatory challenges in cellular agriculture. Trends in Food Science & Technology. 2018;78:155–166. doi: 10.1016/j.tifs.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabassum N, Verma V, Kumar M, Kumar A, Singh B. Nanomedicine in cancer stem cell therapy: from fringe to forefront. Cell and Tissue Research. 2018;374:427–438. doi: 10.1007/s00441-018-2928-5. [DOI] [PubMed] [Google Scholar]
- Taylor RA, Condoleo R, Simons RR, Gale P, Kelly LA, Snary EL. The risk of infection by African swine fever virus in European swine through boar movement and legal trade of pigs and pig meat. Frontiers in Veterinary Science. 2020;6:486. doi: 10.3389/fvets.2019.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TingWei W, JingWen Z, XinRui Z, GuoQiang Z, XueLiang L, GuoCheng D, Jian C, XiuLan S. Research progress on lab-grown meat risk prevention and safety management norms. Food and Fermentation Industries. 2019;45:254–258. [Google Scholar]
- Treich N. Cultured meat: promises and challenges. Environmental and Resource Economics. 2021;79:33–61. doi: 10.1007/s10640-021-00551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker CA. The significance of sensory appeal for reduced meat consumption. Appetite. 2014;81:168–179. doi: 10.1016/j.appet.2014.06.022. [DOI] [PubMed] [Google Scholar]
- Tuomisto HL, Teixeira de Mattos MJ. Environmental impacts of cultured meat production. Environmental Science & Technology. 2011;45:6117–6123. doi: 10.1021/es200130u. [DOI] [PubMed] [Google Scholar]
- United Nations Development Programme. World population prospects 2019: highlights. Available from: https://population.un.org/wpp/Publications/Files/WPP2019_10KeyFindings.pdf. Accessed June 2019
- United States Department of Agriculture. Meat price spreads. Available from: https://www.ers.usda.gov/data-products/meat-price-spreads/. Accessed 2021
- Van der Weele C, Feindt P, van der Goot AJ, van Mierlo B, van Boekel M. Meat alternatives: an integrative comparison. Trends in Food Science & Technology. 2019;88:505–512. doi: 10.1016/j.tifs.2019.04.018. [DOI] [Google Scholar]
- Verbeke W, Marcu A, Rutsaert P, Gaspar R, Seibt B, Fletcher D, Barnett J. ‘Would you eat cultured meat?’: Consumers' reactions and attitude formation in Belgium, Portugal and the United Kingdom. Meat Science. 2015;102:49–58. doi: 10.1016/j.meatsci.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Verbeke W, Sans P, Van Loo EJ. Challenges and prospects for consumer acceptance of cultured meat. Journal of Integrative Agriculture. 2015;14:285–294. doi: 10.1016/S2095-3119(14)60884-4. [DOI] [Google Scholar]
- Wilks M, Phillips CJ. Attitudes to in vitro meat: a survey of potential consumers in the United States. PloS One. 2017;12:e0171904. doi: 10.1371/journal.pone.0171904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will K, Schering L, Albrecht E, Kalbe C, Maak S. Differentiation of bovine satellite cell-derived myoblasts under different culture conditions. In Vitro Cellular & Developmental Biology-Animal. 2015;51:885–889. doi: 10.1007/s11626-015-9916-9. [DOI] [PubMed] [Google Scholar]
- Wuyi LA. Review on the Genetic regulation of myogenesis and muscle development. American Journal of Biochemistry and Biotechnology. 2019;15:1–12. doi: 10.3844/ajbbsp.2019.1.12. [DOI] [Google Scholar]
- XinRui Z, GuoQiang Z, XueLiang L, XiuLan S, JingWen Z, GuoCheng D, Jian C. Commercial production of artificial meat. Food and Fermentation Industries. 2019;45:248–253. [Google Scholar]
- Zandonella C. Tissue engineering: the beat goes on. Nature. 2003;421:884–887. doi: 10.1038/421884a. [DOI] [PubMed] [Google Scholar]
- Zhang G, Zhao X, Li X, Du G, Zhou J, Chen J. Challenges and possibilities for bio-manufacturing cultured meat. Trends in Food Science & Technology. 2020;97:443–450. doi: 10.1016/j.tifs.2020.01.026. [DOI] [Google Scholar]