Abstract
Drug resistance is a major concern nowadays, and finding alternatives of the well-known antibiotic is necessary. Green nanoparticles are emerging as a tenable alternative to this with a large spectrum of activity. The present manuscript describes an eco-friendly approach for green synthesis of silver nanoparticles from both in vitro and in vivo leaf extract of Coleus forskohlii. Leaf extracts were used in synthesis of nanoparticles which were further analyzed through UV–Vis, dynamic light scattering, energy-dispersive spectroscopy, and transmission electron microscopy. Antimicrobial activity of silver nanoparticles alone, as well as crude extract of the plant itself, was carried out against eight multidrug-resistant respiratory tract infecting pathogenic strains. Satisfactory antimicrobial activities were found with nanoparticles, in vitro and in vivo leaf extracts. However, gradually higher to lower inhibition potential against pathogenic bacterial strains was found in silver nanoparticles, in vitro and in vivo leaf extracts. Seven bioactive compounds were detected in the crude extract through gas chromatography–mass spectroscopy analysis. Results revealed that nanoparticle formation occurred in a wide range of sizes (10–50 nm) and shapes (trigonal, hexagonal, spherical, rod). The diversity in size and shape of the nanoparticles makes them biologically active. Silver nanoparticle exhibits significantly better antimicrobial activities as compared to the plant extract in case of nearly all pathogens with a maximum zone of inhibition of 15.33 ± 0.94 mm where more than 12 well-known antibiotics failed to respond. Because of this broad-spectrum activity of nanoparticles as well as the leaf extracts against life-threatening microbes, it can be used as future generation drugs.
Keywords: Antimicrobial activity, Multidrug resistant bacteria, Nanoparticle, Respiratory tract infection
Introduction
Green synthesis of nanoparticles is an eco-friendly bioreduction process. Nanoparticles are presently gaining interest due to their extended biological and chemical properties and less toxic potential than chemically synthesized nanoparticles (Verma et al. 2019). Silver has been used in water purification and food preservation (Silver 2003). Silver is an active antimicrobial agent and is more active on gram-negative than gram-positive bacteria due to their peptidoglycan layer (Jung et al. 2008). Nanosilver being less reactive has an advantage over bulk silver; henceforth, nanosilver is well suited for clinical applications (Manikandan et al. 2017).
Silver nanoparticles have wide applications in drug delivery, water treatment, food industries, agriculture, textile industries, as antifungal, antibacterial, and anti-cancer agents (Deshmukh et al. 2019; Salem et al. 2020; Kale et al. 2021). Nanoparticles also have a positive impact on the growth and development of plants (Verma et al. 2018).
Chemical-based synthesis of the metal nanoparticle is commonly used worldwide. However, most chemicals used to synthesize nanoparticles are toxic in nature and cause environmental pollution; therefore, chemically synthesized nanoparticles are avoided in the medical field (Mousavi et al. 2018). In addition to size, the shape of nanoparticles is also an important criterion for medical purposes. Chemically synthesized nanoparticles are mainly uniform and restricted in shape like spherical, whereas the varied range of shapes of nanoparticles was synthesized from biological material which is much more effective against microorganisms (Dahl et al. 2007; Murphy et al. 2008).
The multidrug-resistant (MDR) strains were a great concern in the medical field. Due to excessive exposure of antibiotics, bacteria become drug-resistant by altering the target side, enzymatic degradation, decreasing membrane permeability, and efflux pumping (Baptista et al. 2018; Gupta et al. 2019). In this context, nano-sized metal particles should provide a better alternative to conventional antibiotics for those MDR strains and also to be regarded as next-generation antibiotics (Rai et al. 2012).
Medicinal plants are always a good source of natural therapy and are useful in treating bacteria and fungus (Haque et al. 2018; Chakraborty et al. 2019; Gantait et al. 2021). Coleus forskohlii of the Lamiaceae family is an aromatic medicinal plant of Indian origin. The fresh leaves and roots are used to treat hypertension, rheumatism, whooping cough, etc. (Mitra et al. 2020; Reddymalla et al. 2021). In addition, this aromatic plant contains several bioactive chemicals such as carvacrol (monoterpenoids), caryophyllene (bicyclic sesquiterpene), and many flavonoids (Ram and Mehrotra 1970).
Multidrug resistance is a major concerning phenomenon for bacteria today and finding an alternative drug is the primary criteria to fight against those disease-causing microbes. Traditional antibiotics are unable to kill them due to several mechanisms developed in them to resist the drugs that were discussed earlier. Natural resources are the major stuff to combat those pathogens till now (Chakraborty et al. 2022). In the present study, various bioactive compound enriched leaf extract and green-synthesized nanoparticles were used to treat them and found to be very much effective. The present manuscript's results must help to find an alternative drug to fight against those multidrug-resistant pathogens in a broad range. In-vitro propagation of the plant also help to obtain those compounds as well as nanoparticles over the years (Haque and Ghosh 2020).
In this study, a new approach has been used in the synthesis of the nanoparticle by Coleus forskohlii leaf. This study includes (1) in vitro propagation of the plant Coleus forskohlii to yield huge biomass in limited space throughout the year, (2) efficiency of in vivo and in vitro plants on the synthesis of nanoparticles, (3) nanoparticle synthesis and characterization via UV–Vis spectroscopy, dynamic light scattering (DLS), energy-dispersive spectroscopy (EDS), and transmission electron microscopy (TEM), (4) effect of both bio-nanoparticle and the plant on MDR respiratory tract infecting (RTI) human pathogenic bacteria, and (5). Gas chromatography–mass spectroscopy (GC–MS) profiling of the plant crude extract to identify the compounds responsible for the inhibition of microbes.
Materials and method
Micropropagation of Coleus forskohlii
Healthy shoot tips of about 1.0–1.2 cm in length were collected from mother plants grown inside the shade-net house and used for culture initiation (Fig. 1A). The surface of the explants was disinfected following the method described by Haque and Ghosh (2019) with minor modification. Explants were washed with 1.0% liquid detergent (Tween®-20) for 2 min, then 2.0% fungicide (Bavistine®) for 10 min, and finally disinfected with 0.1% mercuric chloride (HgCl2) for 6 min and rinsed thrice with sterile distilled water to remove trace of HgCl2. The shoot tips were implanted in Murashige and Skoog medium (Murashige and Skoog 1962) fortified with 6.98-µM kinetin (KIN) and 0.54 µM α-naphthaleneacetic acid (NAA). Cultures were then incubated inside the tissue culture laboratory at 25 °C under 16 h/8 h photoperiod and 65% relative humidity. After 30 days, all cultures were subcultured in the same medium composition for elongation of multiplied shoots. Each elongated shoot of about 2.0–2.5 cm (after 2–3 weeks) was separated from a cluster of multiplied shoots, and individual shoots were implanted in MS medium for growth and biomass production.
Fig. 1.
Micropropagation of Coleus forskohlii A Field grown plants, B, C in vitro multiplication, D in vitro grown complete plant with the well-developed root system
Preparation of plant extract
The fresh leaves of in vivo grown plants and in vitro regenerated plants were washed several times with running tap water and then with distilled water twice and finally with Milli-Q water. Dried leaves of 5.0 g were subjected to Soxhlet extraction ethanol for 16 h and the resulting extract yield was 0.967 g. After extraction, the crude extract of 100 mg was mixed in 100 ml Milli-Q water and filtered through Whatman No. 1 filter paper. The filtered extract was used as a reducing agent for silver nanoparticle synthesis. The rest of the crude material was used to study the activity against microbes.
Gas chromatography–mass spectroscopy (GC–MS) profiling
Ethanol extracts of the leaves of in vitro grown C. forskohlii plants were investigated for the identification of different bioactive compounds by gas chromatography–mass spectroscopy (GC–MS). The operation was performed using column: TRWAXMS 30 × 0.25 mm × 0.25 µm df, equipment: Trace GC ultra, Thermo fisher Scientific India Pvt. Ltd. Gas flow: 1.0 ml/min split 20:1 detector: mass detector Polaris Q mass software data collection XCALIBUR. 1.0 µl of the extract was injected into the injection port of the GC column. The oven temperature program was no hold up to 50 ºC, for 2 min at the rate of 10 ºC/min, and 5 min hold up to 270 ºC. Injector temperature was 250 ºC and total GC–MS running time was 28 min. Helium gas was used as carrier gas at a constant flow rate of 1.0 ml/min. Bioactive compounds were identified based on their mass spectral pattern compared to spectral databases of NIST Library (USA). Inlet line temperature was 250 ºC and source temperature was 230 ºC. Mass scan (m/z) was 50−650, the solvent delay was 0 − 4 min, and the total MS running time was 27 min.
Synthesis of nanoparticle
In vivo and in vitro leaf extract of C. forskohlii in four different concentrations (2.0, 2.5, 3.0, and 3.5 mg/ml) were separately added to 30 ml of 1 mM silver nitrate solution and incubated for 12 h at 26 °C temperature in a shaking incubator. As a result, the solution started to turn transparent to yellow–red and finally dark brown, indicating silver nanoparticle formation (Nayak et al. 2020).
Characterization of nanoparticles
UV–vis spectra analysis
Primary identification of silver nanoparticles by determining the bioreduction of silver ions into silver nanoparticles was carried out in a UV–vis spectrophotometer. Dilutions were made by dissolving into Milli-Q water for better peak detection, and subsequent scans were done between 300 and 700 nm wavelengths in a spectrophotometer (SHIMADZU, UV – 1800, UV SPECTROPHOTOMETER, JAPAN) (Anbu et al. 2019).
TEM, EDS, and DLS analysis
Silver nanoparticles were visualized under a high-resolution transmission electron microscope (HRTEM: JEM-2100, JEOL, Japan) to study the morphology of nanoparticles. TEM grids were prepared by placing 5 μL of the silver nanoparticle solutions on carbon-coated copper grids and drying them under the lamp. HRTEM operating at an accelerating voltage of 200 kV is equipped with energy-dispersive spectroscopy (EDS) analysis (Oxford INCA instruments). The EDS spectrum of nanoparticles was recorded to confirm the elemental composition of the nanosilver particle. A homogeneous solution was prepared by sonicating the silver nanoparticles. Dynamic light scattering (DLS) analysis was done with a Zetasizer Nano ZS90 instrument. To obtain the average size, the experiment was repeated thrice (Anbu et al. 2019).
Estimation of antibacterial activity by agar cup method
The comparative antibacterial activities of the leaf extracts (both in vivo and in vitro plant) and silver nanoparticles were effectively accessed against human pathogenic bacteria. The agar cup method was assayed to determine the antibacterial activity, and the plates of Muller–Hinton agar media were prepared for the agar cup method as described by Haque et al. (2017). DMSO was used as a negative control to evaluate the antimicrobial activity with no zone of inhibition. Fifteen well-known antibiotics were used as a positive control with high resistance properties of the bacteria (Table 4). All the experiments were designed in triplicate to obtain the resulting data. The plates were incubated at 37 °C for 24 to 48 h, and the maximum zone of inhibition was observed and measured to conclude the effectivity.
Table 4.
Drug resistance of eight pathogenic bacteria against 15 well-known antibiotics
| Pathogen | Bacterial strain | Antibiotics | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ciprofloxacin | Amoxicillin | Erythromycin | Aztreonam | Teicoplanin | Clindamycin | Vancomycin | Rifampicin | Tetracycline | Ertapenum | Ampicillin | Tiacarcillin | Piperacillin | Cefalotin | Cefoxitin | |||
| Strain type | Strain name | ||||||||||||||||
| ISOLATE NO 396617 | RTI | Staphylococcus aureus | R | R | R | R | S | R | S | S | R | R | S | S | S | − | S |
| ISOLATE NO 156081 | RTI | Klebsiella pneumoniae | S | S | R | R | R | − | S | − | R | R | R | R | R | R | S |
| ISOLATE NO 156822 | RTI | Klebsiella sp | S | S | R | R | R | I | S | S | R | R | − | R | R | S | S |
| ISOLATE NO 156482 | RTI | Klebsiella pneumoniae | R | R | R | − | R | I | R | R | R | I | − | − | S | I | I |
| ISOLATE NO 154467 | RTI | Klebsiella sp | − | S | R | − | I | I | S | − | R | R | R | R | S | − | S |
| ISOLATE NO 16486 | RTI | Staphylococcus aureus | S | S | S | S | R | S | S | S | S | S | I | R | R | I | S |
| BT- 122,606 | RTI | Klebsiella pneumoniae | S | − | S | S | S | − | S | S | S | − | − | − | − | I | − |
| BT- 122,967 | RTI | Staphylococcus saprophyticus | S | I | R | − | R | R | R | R | − | I | − | R | R | − | R |
R resistant, S sensitive, I intermediate, ‘–’ not tested, RTI respiratory tract infection
Determination of minimum inhibitory and cidal concentration
To determine the minimum inhibitory concentration (MIC) of both extracts and nanoparticles against the bacterial strain, “two-fold broth dilution analysis” was done by following the method of Wiegand et al (2008) with minor modification. Dimethyl sulfoxide (DMSO) was used as a solvent for dissolving of the crude extract and the nanoparticles to make 100 mg/ml concentration as well as used as a negative control with no inhibition potential. Then, the 100 µl inoculum was added to each tube and incubated at 37 °C for 24 h. The minimum concentration that is effective in inhibition is further plated to determine the minimum bactericidal concentration (MBC).
Statistical data analyses
Each experiment, for determination of antimicrobial activity by agar well diffusion method, was repeated thrice with 5 concentrations per replicate. All data were subjected to SPSS software for Windows (IBM® SPSS, version 19.0, Chicago, IL) to determine the statistical data analysis.
Results
Micropropagation
Profuse shoot multiplication with 18 ± 0.62 shoots per explants occurred within 4 weeks of implantation in Murashige and Skoog (MS) medium fortified with 6.98 µM kinetin (KIN) and 0.54 µM α-naphthaleneacetic acid (NAA). Multiplied shoots were further elongated in the same medium composition (Fig. 1B, C). Further growth of individual shoots and in vitro plant biomass production occurred in plant growth regulator-free medium (Fig. 1D).
Gas chromatography–mass spectroscopy (GC–MS) profiling
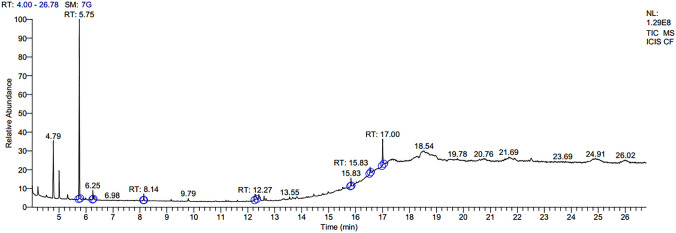
Seven major compounds were found in the gas chromatography–mass spectroscopy (GC–MS) spectrum of ethanolic leaf extract of C. forskohlii (Fig. 2). The major area in the spectrum was occupied by the terpene molecule camphor (Table 1). In addition, two alkane hydrocarbons were found viz. Dodecane and 7-methyl pentadecane. Another steroidal compound 1-monolinoleoylglycerol trimethylsilyl ether and ester compound tetracosyl trifluroacetate were found in the leaf extract. Two more important compounds trans-13-docosenamide and lidocaine were also present in the crude extract. As in vitro plants have significantly higher potential in the inhibition of microbes as compared to in vivo plants, GC–MS profiling was done only on in vitro plant extract to identify the compounds.
Fig. 2.
GC–MS chromatography of ethanolic leaf extracts of Coleus forskohlii
Table 1.
GC–MS profile of in vitro leaves of Coleus forskohlii
| Sl.no | Chemical constituents | Molecular formula | Mol wt | Retention time | % Area | Nature | Biological activities |
|---|---|---|---|---|---|---|---|
| 1 | Camphor | C10H16O | 152.24 | 5.75 | 70.36 | Terpene | Antifungal activity, Antibacterial activity, Anti carcinogenic property, small amount is used in aromatherapy and skin therapy, High amount is toxic in nature (Rahman et al. 2016) |
| 2 | Dodecane | C12H26 | 170.34 | 6.25 | 3.04 | Alkane hydrocarbon | Antibacterial activity (Togashi et al. 2007) |
| 3 | 7-methyl Pentadecane | C16H34 | 226.45 | 8.14 | 2.13 | Alkane hydrocarbon | Antimicrobial activity (Begum et al. 2016) |
| 4 | Lidocaine | C14H22N2O | 234.34 | 12.27 | 5.33 | – | Used in treatment of cardiac arrhythmias (Spracklen et al. 1968). Antimicrobial activity (Parr et al. 1999) |
| 5 | Trans-13-docosenamide | C22H43NO | 337.58 | 15.83 | 3.29 | – | Antimicrobial activity (Rukshana et al. 2017) |
| 6 | Tetracosyl trifluroacetate | C26H49F3O2 | 450.66 | 16.54 | 2.62 | Ester | Antibacterial and antifungal activity (Du et al. 2009) |
| 7 | 1-Monolinoleoylglycerol trimethylsilyl ether | C27H54O4SI2 | 498 | 17.00 | 13.23 | Steroid | Antimicrobial, anti-inflammatory, anti-androgenic, anti-cancer, anti-arthritic, anti-asthma, diuretic (Parthipan et al. 2015) |
Characterization of silver nanoparticles
UV–Vis spectra analysis
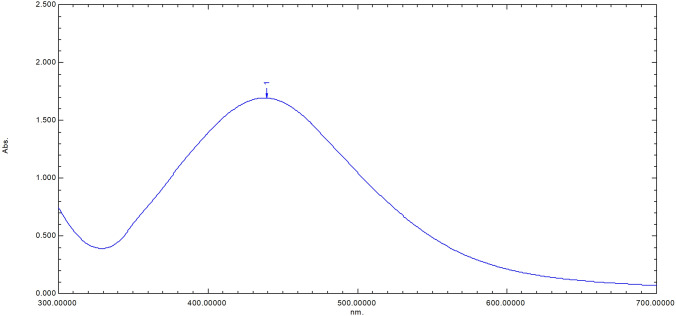
The presence of nanoparticles was confirmed with the color change to pale brown after 12 h of incubation at 26 ˚C temperature. Due to the excitation of SPR, the color change of silver nanoparticles occurred (Xiaoming et al. 2009). The UV–visible spectrum of the nanoparticle synthesized from plant extract resulted in maximum absorption at 420 nm (Fig. 3).
Fig. 3.
UV- visible spectrum of silver bio-nanoparticle synthesized from leaves of Coleus forskohlii
TEM and EDS analysis
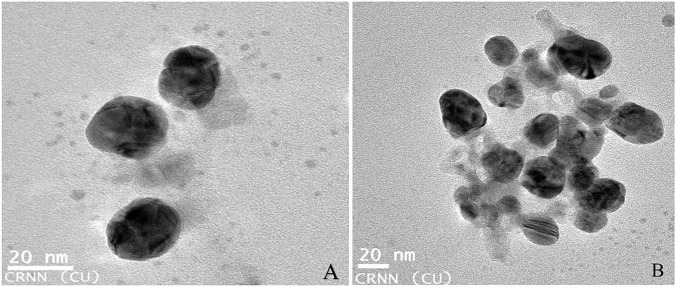
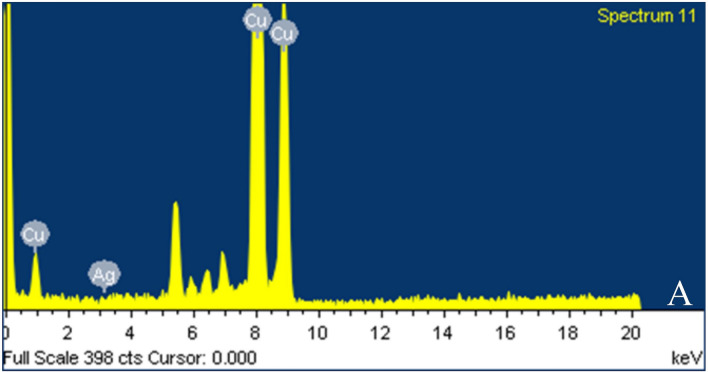
High-resolution transmission electron microscope (HRTEM) was used to analyze the actual size distribution and shape of the nanoparticle. The size distribution of leaf extract-mediated nanoparticles ranges from 5 to 30 nm (Fig. 4A, B). Nanoparticles with various shapes i.e., trigonal, hexagonal, spherical, rod-shaped, etc.were obtained. The percentage of weight and atomics of the silver nanoparticles determined by the energy-dispersive spectroscopy (EDS) spectrum (Fig. 5) were 0.83 and 0.49, respectively.
Fig. 4.
HRTEM image of silver bio-nanoparticle synthesized from Coleus forskohlii A Single distribution of nanoparticles, B Aggregative distribution of nanoparticles
Fig. 5.
EDS spectrum of silver bio-nanoparticles synthesized from Coleus forskohlii show the presence of silver
DLS analysis
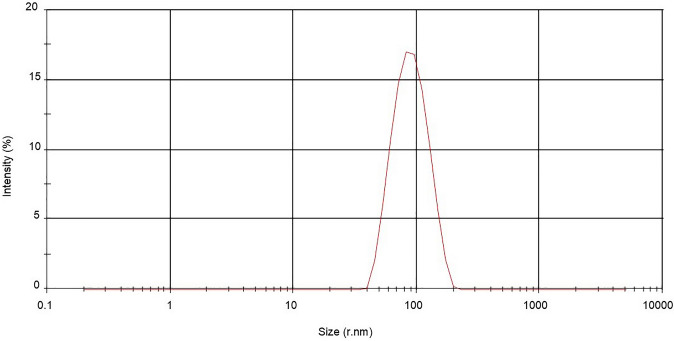
The Dynamic light scattering (DLS) instrument was used to measure the coated thickness with a covering agent enveloping the silver nanoparticle along with the actual size in this case. The average size distribution of green-synthesized nanoparticles was 86.20 nm with 1 peak at 92.43 nm and the intensity was 100% (Fig. 6).
Fig. 6.
DLS spectrum of silver bio-nanoparticles synthesized from Coleus forskohlii
Isolation and identification of RTI bacterial pathogens
Pure isolated colonies were selected by plating the respiratory specimens (e.g., sputum, pleural fluid, bronchial aspirate etc.) on MacConkey agar and Nutrient agar plates (Himedia®, India) at a temperature of 37 ± 2 °C for 24 h. VITEK 2 COMPACT SYSTEM BIOMERIUEX machine was used to identify the pathogenic strains and 3.0 ml of half-normal saline containing Vitek ID tubes were incubated with 3‒4 colonies of the sample. McFarland’s reading of ID tubes was taken with the instrument provided adjusting the MCF to 0.5 to 0.63. The suspensions from ID tubes were then transferred to AST tubes with the help of a pipette, and ID and AST cards were inserted into the tubes within 30 min of transfer. Data were collected after completion. Other tests like oxidase activity, oxidation fermentation, catalase production, indole test, Voges‒Proskauer test, and hydrogen sulfide production were done for initial identification. After identification, the strains were subjected to screening for antibiotic sensitivity assay.
Antibacterial activity
The antibacterial activity of the silver nanoparticles synthesized from the plant extract was higher than the plant crude extract. Both in vivo and in vitro plant extracts along with synthesized nanoparticles were experimented upon eight pathogenic strains of human respiratory tract infecting (RTI) bacteria (Fig. 7). Ethanolic extract of in vitro leaves exhibits better inhibition potential than in vivo plant leaves of C. forskohlii. In-vitro leaf extract exhibited maximum inhibition against Staphylococcus aureus (ISOLATE NO 396617) with ZI = 14.66 ± 0.47 mm, whereas ZI = 10.66 ± 0.47 mm was observed in the case of in vivo plant extract. As compared to plant extract (both in vitro and in vivo), the nanoparticles showed better inhibition with ZI = 15.33 ± 0.94 mm against the same pathogenic strain of RTI (Table 2). Nanoparticles showed higher inhibition potential against seven out of eight strains of pathogenic bacteria, whereas ethanolic extract of the plant has higher effectivity against only one strain (BT-122967) compared to the silver nanoparticle. Effective antimicrobial activity is seen against all the pathogens by both plant extract and silver nanoparticles, but maximum effectiveness was perceived by the nanoparticles against a maximum number of bacteria than the plant extract where conventional antibiotics ailed to respond. The lowest MIC and bactericidal concentrations are seen in the case of nanoparticles than the extracts used (Table 3).
Fig. 7.
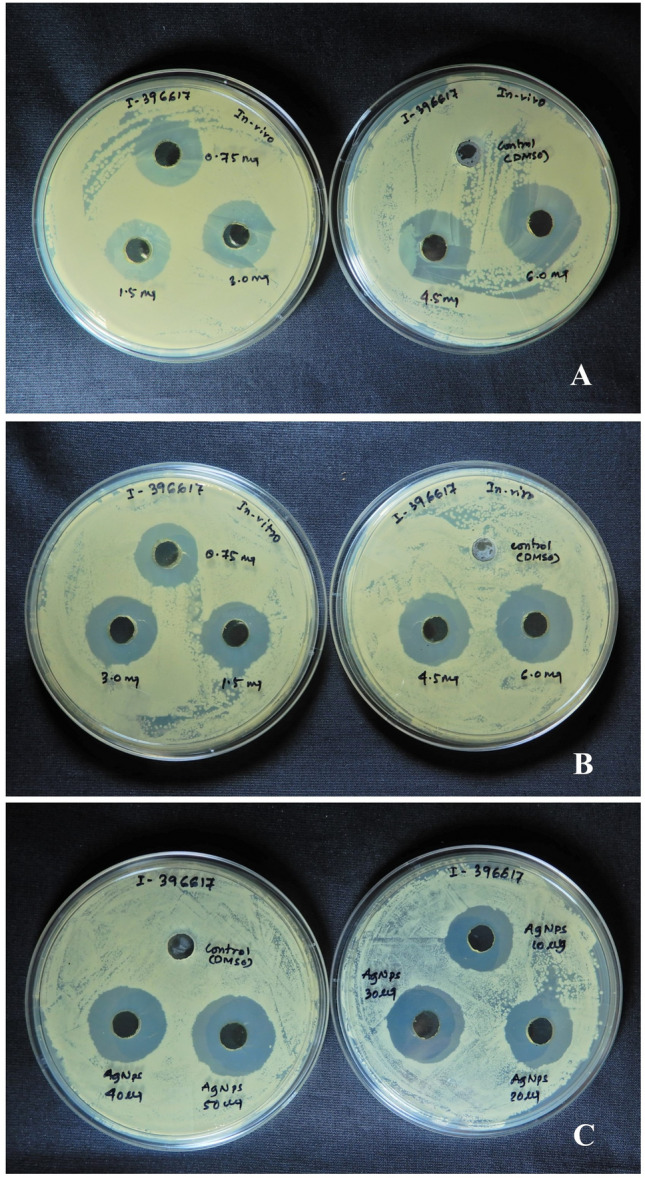
Antimicrobial activity study of A in vivo plant extract against the pathogen ISOLATE NO 396617. B in vitro plant extract against the same pathogen. C Synthesized bio-nanoparticle against the same pathogen
Table 2.
Comparative antimicrobial activity of Coleus forskohlii (ethanol extract) and silver bio-nanoparticle against RTI clinical pathogens
| Antibacterial materials | Extract/cup (mg) | Zone of inhibition | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pathogenic bacteria | |||||||||
| ISOLATE NO 396617 | ISOLATE NO 156081 | ISOLATE NO 156822 | ISOLATE NO 156482 | ISOLATE NO 154467 | ISOLATE NO 16486 | BT-122606 | BT-122967 | ||
| Plant extract (In vivo) | 0.75 | 8.33 ± 0.47 | 0 | 0 | 0 | 7.33 ± 0.47 | 0 | 0 | 6.66 ± 0.47 |
| 1.5 | 9.33 ± 0.47 | 6.66 ± 0.47 | 0 | 7.33 ± 0.47 | 8.00 ± 0.81 | 7.66 ± 0.47 | 0 | 8.66 ± 0.47 | |
| 3.0 | 9.66 ± 0.47 | 7.66 ± 0.47 | 7.00 ± 0 | 8.00 ± 0.81 | 9.33 ± 0.94 | 8.33 ± 0.47 | 8.00 ± 0.81 | 10.00 ± 0 | |
| 4.5 | 10.33 ± 0.47 | 9.00 ± 0.81 | 8.00 ± 0.81 | 8.33 ± 0.47 | 10.00 ± 0.81 | 10.00 ± 0.81 | 8.33 ± 0.47 | 10.33 ± 0.47 | |
| 6.0 | 10.66 ± 0.47 | 9.33 ± 0.47 | 9.33 ± 0.47 | 9.33 ± 0.47 | 10.33 ± 0.94 | 10.33 ± 0.94 | 8.66 ± 0.94 | 11.00 ± 0 | |
| Plant extract (In vitro) | 0.75 | 10.66 ± 0.47 | 7.33 ± 0.94 | 0 | 0 | 8.66 ± 0.47 | 0 | 0 | 7.66 ± 1.24 |
| 1.5 | 11.33 ± 1.24 | 9.33 ± 1.69 | 7.33 ± 1.24 | 7.33 ± 0.47 | 10.33 ± 0.94 | 6.66 ± 0.94 | 7.33 ± 1.24 | 9.33 ± 0.94 | |
| 3.0 | 12.33 ± 1.24 | 9.66 ± 1.24 | 7.00 ± 0 | 8.00 ± 0.81 | 11.00 ± 0.81 | 8.33 ± 0.94 | 9.00 ± 1.63 | 11.66 ± 1.24 | |
| 4.5 | 14.00 ± 0.81 | 11.66 ± 0.94 | 9.66 ± 0.47 | 8.33 ± 0.47 | 11.33 ± 0.94 | 10.33 ± 1.69 | 11.00 ± 1.63 | 12.00 ± 0.81 | |
| 6.0 | 14.66 ± 0.47 | 12.00 ± 0.81 | 10.33 ± 0.47 | 9.33 ± 0.47 | 12.33 ± 0.94 | 11.33 ± 1.24 | 12.66 ± 0.47 | 13.33 ± 0.47 | |
| Nanoparticle | 50 µg | 15.33 ± 0.94 | 14.66 ± 0.94 | 11.33 ± 0.94 | 13 ± 0.81 | 14.33 ± 0.94 | 12.33 ± 0.47 | 12.66 ± 0.47 | 10.66 ± 1.24 |
| DMSO | 50 µl | – | – | – | – | – | – | – | – |
ISOLATE NO 396617—Staphylococcus aureus; ISOLATE NO 156081—Klebsiella pneumoniae; ISOLATE NO 156822—Klebsiella sp.; ISOLATE NO 156482—Klebsiella pneumoniae; ISOLATE NO 154467—Klebsiella sp.; ISOLATE NO 16486—Staphylococcus aureus; BT-122606—Klebsiella pneumoniae; BT-122967—Staphylococcus saprophyticus
Table 3.
MIC and MBC of in vivo field grown and in vitro tissue culture-raised plant extract and nanoparticle
| Type | Strain | Inhibition type | Inhibition and cidal concentration | ||
|---|---|---|---|---|---|
| In-vivo plant (mg/mL) | In-vitro plant (mg/mL) | Nanoparticle (µg/ml) | |||
| Respiratory tract infecting pathogenic bacteria | ISOLATE NO-42571 | MIC | 5.00 | 3.75 | 2.50 |
| MBC | 7.50 | 6.25 | 5.00 | ||
| ISOLATE NO-42423 | MIC | 5.50 | 4.25 | 2.50 | |
| MBC | 6.25 | 5.75 | 5.00 | ||
| ISOLATE NO-43181 | MIC | 7.00 | 6.00 | 3.00 | |
| MBC | 8.25 | 7.50 | 6.50 | ||
| ISOLATE NO-42269 | MIC | 9.00 | 8.75 | 4.00 | |
| MBC | 10.50 | 10.00 | 6.75 | ||
| ISOLATE NO-42230 | MIC | 5.50 | 4.75 | 3.50 | |
| MBC | 6.75 | 6.25 | 6.50 | ||
| ISOLATE NO-43546 | MIC | 8.75 | 7.00 | 2.75 | |
| MBC | 9.25 | 8.50 | 5.25 | ||
| ISOLATE NO-43571 | MIC | 10.00 | 7.25 | 5.50 | |
| MBC | 12.75 | 10.00 | 7.75 | ||
| ISOLATE NO-43627 | MIC | 6.75 | 4.00 | 3.50 | |
| MBC | 8.25 | 6.00 | 5.50 | ||
Discussion
The plant C. forskohlii is an important medicinal plant with a strong antimicrobial activity (Chakraborty et al. 2022). Most of the studies related to the antimicrobial potential of this plant focused on root because of the presence of the active compound named forskolin, having a great medicinal value (Illiano et al. 2018). In addition to root, the antimicrobial activity of leaf and stem was also examined by a few researchers, and they reported root extract is more potent than the rest (Malleswari et al. 2013). Although they examined the activity of leaf extract on standard microbes, no such work has yet been done on the pathogenic strains. In the present study, we found that the leaf extract of C. forskohlii has displayed antimicrobial potential against bacteria resistant to commercially available antibiotics (Table 4). The tested isolates were maintained by weekly subculture in a fresh media. The standard MTCC strains were used to maintain the overall quality of the microbial assay, similar findings were previously reported by us (Haque et al. 2017). Plant extract contains major bioactive components viz., alkaloids, terpene, saponins, and flavonoids which can cause inhibition of bacteria which is very significant. Similar findings were previously reported by Haque et al. (2017) in the case of Bacopa monnieri, where tissue-cultured raised plants prove effective against MDR urinary tract infecting bacteria. According to the findings, plant-derived metabolites and bioactive compounds can be used in biomedical sciences due to their versatility in the inhibition potential against multidrug-resistant bacterial pathogens.
Gas chromatography–mass spectroscopy (GC–MS) profiling of the crude leaf extract revealed the presence of seven major compounds and all of which have great antimicrobial activity (Table 1). Camphor is the major active compound present in the leaf extract. This terpene molecule, camphor, has antifungal activity, antibacterial activity, and anti-carcinogenic properties and is used in a small amount in aromatherapy and skin therapy (Rahman et al. 2016). Dodecane and 7-methyl pentadecane are alkane hydrocarbons with an elevated higher inhibition potency against microbes (Togashi et al, 2007; Begum et al, 2016). Another compound lidocaine is found which is used in the treatment of cardiac arrhythmias and also has some antibacterial activity (Spracklen et al. 1968; Parr et al. 1999). 1-monolinoleoylglycerol trimethylsilyl ether is a steroid having antimicrobial, anti-inflammatory, anti-androgenic, anti-cancer, anti-arthritic, and anti-asthma activity (Parthipan et al. 2015). Two more important compounds tetracosyl trifluroacetate and trans-13-docosenamide both have antimicrobial activity (Du et al. 2009; Rukshana et al. 2017). The presence of these biologically active compounds in crude leaf extract may be responsible for significant antimicrobial activity.
Nanoparticles itself have an extensive account of antimicrobial activity. Biologically synthesized nanoparticles have a greater advantage in inhibition potential over chemically synthesized nanoparticles. As the presently described protocol is followed by green synthesis, it may be used in medical science due to its nontoxicity (Zarei et al. 2021). The shape and size of the metal nanoparticles are the most important factors for antimicrobial activity. Nanoparticles exhibit enhanced antimicrobial activity when their size is less than 50 nm, whereas above 50 nm their effectivity becomes lesser (Farouk et al. 2020). Corresponding to the result, the nanoparticle is significantly more effective on all pathogens used in the present study compared in vivo and in vitro leaf extract of the plant. Nanoparticles cause damage to the bacterial cell membrane by causing pores and damage by disintegrating the membrane. Smaller nanoparticles can cause this type of damage rather than large-size nanoparticles (Sharmila et al. 2018; Pirtarighat et al. 2019). Corroborating the literature, the size of the nanoparticles synthesized in this study is under 30 nm in size and may cause a large extent of inhibition against the bacteria. The shape of the nanoparticles is also an important parameter to treat the microorganisms. Silver nanoparticle synthesized from the leaf of C. forskohlii shows the maximum number of triangular-shaped nanoparticles as well as rod, spherical and oval-shaped nanoparticle. The previous study shows that the triangular nanoparticles are more effective than spherical and rod-shaped nanoparticles (Pal et al. 2007). In the present study, green-synthesized silver nanoparticle shows colloidal nature under a high-resolution transmission electron micrograph, and this nature of nanoparticles has a catalytic activity which is very much effective in the destabilization of enzymes of the microbes (Suganya et al. 2015). Silver nanoparticles are also able to increase the level of H2O2 in the treated bacterial cell which can cause cell damage due to increased toxicity in the bacterial cell (Kim and Lee 2021). Because of these mechanisms, silver nanoparticles cause maximum inhibition against all pathogens in the present study. Similarly, nanosilver particle was efficiently synthesized by tissue culture-raised leaves and callus of Costus speciosus and Sesuvium portulacastrum, respectively (Nabikhan et al. 2010; Parthipan et al. 2015).
In the previous study, Ramaswamy and Krishnamoorthy (2015) reported the synthesis of silver nanoperticles only from the root extract of C. forskohlii. The present study describes an efficient and alternative strategy of using profusely growing leaves of this medicinal plant for the synthesis of nanoparticles with promising antimicrobial potency. Furthermore, as the leaves of this plant can be propagated in a shorter period of time using the tissue culture technique in our laboratory, compared to the roots, the possibility of using leaf culture for commercial production is more. Accordingly, the availability of rapidly and clonally propagated disease-free plants C. forskohlii is ensured throughout the year.
Conclusion
In the present study, the leaf extract of Coleus forskohlii has been used for the synthesis of silver nanoparticles of distinct shapes and sizes, thereby proposing a greener, safe, and environment-friendly protocol for the synthesis of nanoparticles. The coating of biological molecules on the surface of nanoparticles makes them biocompatible in comparison with those prepared by chemical methods. These nanoparticles exhibit excellent antimicrobial activity against pathogenic RTI strains of bacteria compared to that of in vivo and in vitro leaf extracts. Gas chromatography–mass spectroscopy studies of in vitro leaf extract have confirmed the presence of seven biologically active compounds with high antimicrobial potency levels. May these compounds help effective binding with the cell wall which is required for better antimicrobial activity of the nanoparticles. Thus, the large spectrum of antimicrobial activity of these bioactive nanoparticles can be proved to be an important tool in many potential biomedical applications and also a ray of hope to treat health-threatening bacterial infections. The nanoparticles, as well as extracts, can be used in future medicine which can help to treat RTI infection and other infectious pathogens.
Acknowledgements
AC, SMH and BG thankful to Swami Kamalasthananda, Principal, Ramakrishna Mission Vivekananda Centenary College, Rahara, Kolkata (India), for the facilities provided for the present study and Centre for research in nanoscience and nanotechnology, University of Calcutta for the use of transmission electron microscope. DD acknowledges technical and management support provided by Ashok Laboratory Clinical Testing Centre Private Limited, Kolkata.
Author contribution
AC screened the antimicrobial activities and GC–MS of plant extract and EDS, TEM study of nanoparticle and write up a part of manuscript. SMH performed all the plant tissue culture related experiments and write up a part of manuscript. DD collected the clinical isolates and checked their response against multidrug. DG performed DLS under the guidance of DM. SM and BG was involved in result interpretation and made necessary correction in the write up. Final approval of the article was done by BG. All authors read and approved the final manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Avijit Chakraborty, Email: avijit.microbio@gmail.com.
Sk Moquammel Haque, Email: moquammel@ecgcollege.org.
Debasish Ghosh, Email: debasishghosh2609@gmail.com.
Diganta Dey, Email: diganta_dey2006@yahoo.com.
Swapna Mukherjee, Email: swamuk15@gmail.com.
Dilip K. Maity, Email: maitydkcu@gmail.com
Biswajit Ghosh, Email: ghosh_b2000@yahoo.co.in.
References
- Anbu P, Gopinath SC, Yun HS, Lee CG. Temperature-dependent green biosynthesis and characterization of silver nanoparticles using balloon flower plants and their antibacterial potential. J Mol Struct. 2019;1177:302–309. [Google Scholar]
- Baptista PV, McCusker MP, Carvalho A, Ferreira DA, Mohan NM, Martins M, Fernandes AR. Nano-strategies to fight multidrug-resistant bacteria: a Battle of the titans. Front Microbiol. 2018;9:1441. doi: 10.3389/fmicb.2018.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum IF, Mohankumar R, Jeevan M, Ramani K. GC–MS analysis of bioactive molecules derived from Paracoccus pantotrophus FMR19 and the antimicrobial activity against bacterial pathogens and MDROs. Indian J Microbiol. 2016;56:426–432. doi: 10.1007/s12088-016-0609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A, Kundu S, Ghosh B. Endophytism in Zingiberaceae: Elucidation of beneficial impact. Endophytes and Second Metab. 2019;10(1007):978–983. [Google Scholar]
- Chakraborty A, Haque SM, Dey D, Mukherjee S, Ghosh B. Detection of UTI pathogen-killing properties of Coleus forskohlii from tissue cultured in vitro and ex vitro plants. Proc Natl Acad Sci India Sect B Biol Sci. 2022;92:157–169. [Google Scholar]
- Dahl JA, Maddux BLS, Hutchison JE. Toward greener nano-synthesis. Chem Rev. 2007;107:2228–2269. doi: 10.1021/cr050943k. [DOI] [PubMed] [Google Scholar]
- Deshmukh SP, Patil SM, Mullani SB, Delekar SD. Silver nanoparticles as an effective disinfectant: a review. Mater Sci Eng C. 2019;97:954–965. doi: 10.1016/j.msec.2018.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Wang Y, Hao X, Li C, Peng Y, Wang J, Zhou L. Antimicrobial phenolic compounds from Anabasis aphylla L. Nat Prod Commun. 2009;4:385–388. [PubMed] [Google Scholar]
- Farouk F, Abdelmageed M, Azam Ansari M, Azzazy HM. Synthesis of magnetic iron oxide nanoparticles using pulp and seed aqueous extract of Citrullus colocynth and evaluation of their antimicrobial activity. Biotechnol Lett. 2020;42:231–240. doi: 10.1007/s10529-019-02762-7. [DOI] [PubMed] [Google Scholar]
- Gantait S, Mahanta M, Bera S, Verma SK. Advances in biotechnology of Emblica officinalis Gaertn. Syn. Phyllanthus emblica L.: a nutraceuticals-rich fruit tree with multifaceted ethnomedicinal uses. 3 Biotech. 2021;11(2):1–25. doi: 10.1007/s13205-020-02615-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Mumtaz S, Li CH, Hussain I, Rotello VM. Combatting antibiotic-resistant bacteria using nanomaterials. Chem Soc Rev. 2019;48:415–427. doi: 10.1039/c7cs00748e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque SM, Ghosh B. A submerged culture system for rapid micropropagation of the commercially important aquarium plant, ‘Amazon sword’ (Echinodorus ‘Indian Red’) In Vitro Cell Dev Biol Plant. 2019;55:81–87. [Google Scholar]
- Haque SM, Chakraborty A, Dey D, Mukherjee S, Nayak S, Ghosh B. Improved micropropagation of Bacopa monnieri (L.) Wettst. (Plantaginaceae) and antimicrobial activity of in vitro and ex vitro raised plants against multidrug-resistant clinical isolates of urinary tract infecting (UTI) and respiratory tract infecting (RTI) bacteria. Clin Phytosci. 2017;3:17. [Google Scholar]
- Haque SM, Chakraborty A, Ghosh B. Callus mediated shoot organogenesis and regeneration of cytologically stable plants of Ledebouria revoluta: an ethnomedicinal plant with promising antimicrobial potency. J Genet Eng Biotech. 2018;16:645–651. doi: 10.1016/j.jgeb.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque SM, Ghosh B. Productive method for commercial propagation through direct embryogenesis and organogenesis in Ledebouria revoluta—an underutilized medicinal plant with cardioprotective properties. Ind Crop Prod. 2020;157:112941. [Google Scholar]
- Illiano M, Conte M, Sapio L, Nebbioso A, Spina A, Naviglio S. Forskolin sensitizes human acute myeloid leukemia cells to H3K27me2/3 demethylases GSKJ4 inhibitor via Protein Kinase A. Front Pharmacol. 2018;9:792. doi: 10.3389/fphar.2018.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung WK, Koo HC, Kim KW, Shin S, Kim SH, Park YH. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl Environ Microbiol. 2008;74:2171–2178. doi: 10.1128/AEM.02001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale SK, Parishwad GV, Patil ASHAS. Emerging agriculture applications of silver nanoparticles. ES Food Agrofor. 2021;3:17–22. [Google Scholar]
- Kim S, Lee DG. Silver nanoparticles-induced H2O2 triggers apoptosis-like death and is associated with dinF in Escherichia coli. Free Rad Res. 2021;55:107–118. doi: 10.1080/10715762.2020.1866178. [DOI] [PubMed] [Google Scholar]
- Malleswari D, Bagyanarayana G, Hindumathi A. Anti-bacterial activity of Coleus forskohlii extracts against some pathogenic bacteria. J Nat Prod Plant Resour. 2013;3:75–78. [Google Scholar]
- Manikandan V, Velmurugan P, Park JH, Chang WS, Park YJ, Jayanthi P, Oh BT. Green synthesis of silver oxide nanoparticles and its antibacterial activity against dental pathogens. 3 Biotech. 2017;7:1–9. doi: 10.1007/s13205-017-0670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra M, Gantait S, Mandal N. Coleus forskohlii: advancements and prospects of in vitro biotechnology. Appl Microbiol Biotechnol. 2020;104:2359–2371. doi: 10.1007/s00253-020-10377-6. [DOI] [PubMed] [Google Scholar]
- Mousavi SM, Hashemi SA, Ghasemi Y, Atapour A, Amani AM, SavarDashtaki A, Arjmand O. Green synthesis of silver nanoparticles toward bio and medical applications: review study. Artif Cells Nanomed Biot. 2018;46:S855–S872. doi: 10.1080/21691401.2018.1517769. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:495–497. [Google Scholar]
- Murphy CJ, Gole AM, Stone JW, Sisco PN, Alkilany AM, Goldsmith EC, Baxter SC. Gold nanoparticles in biology: beyond toxicity to cellular imaging. Acc Chem Res. 2008;41:1721–1730. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- Nabikhan A, Kandasamy K, Raj A, Alikunhi NM. Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L. Colloids Surf B. 2010;79:488–493. doi: 10.1016/j.colsurfb.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Nayak S, Bhat MP, Udayashankar AC, Lakshmeesha TR, Geetha N, Jogaiah S. Biosynthesis and characterization of Dillenia indica-mediated silver nanoparticles and their biological activity. Appl Organomet Chem. 2020;34:5567. [Google Scholar]
- Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr AM, Zoutman DE, Davidson JS. Antimicrobial activity of lidocaine against bacteria associated with nosocomial wound infection. Ann Plas Surg. 1999;43:239–245. doi: 10.1097/00000637-199909000-00003. [DOI] [PubMed] [Google Scholar]
- Parthipan B, Suky MGT, Mohan VR. GC-MS Analysis of Phytocomponents in Pleiospermiumalatum (Wall. ex Wight & Arn.) Swingle, (Rutaceae) J Pharmacogn Phytochem. 2015;4:216–222. [Google Scholar]
- Pirtarighat S, Ghannadnia M, Baghshahi S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J Nanostruct Chem. 2019;9:1–9. [Google Scholar]
- Rahman FA, Priya V, Gayathri R, Geetha RV. In vitro antibacterial activity of camphor oil against oral microbes. Int J Pharm Sci Rev Res. 2016;39:119–121. [Google Scholar]
- Rai MK, Deshmukh SD, Ingle AP, Gade AK. Silver nanoparticles: the powerful nano-weapon against multidrug-resistant bacteria. J Appl Microbiol. 2012;112:841–852. doi: 10.1111/j.1365-2672.2012.05253.x. [DOI] [PubMed] [Google Scholar]
- Ram PR, Mehrotra BN. Compendium of Indian medicinal plants, CDRI Lucknow and publication and information directorate. New Delhi. 1970;2:79–201. [Google Scholar]
- Ramaswamy M, Krishnamoorthy R. synthesis of silver nanoparticles and its antimicrobial activity of Coleus forskohlii. World J Pharm Sci. 2015;4:673–678. [Google Scholar]
- Reddymalla NR, Pureti S, Kolluru VC. Medicinal plants. Cham: Springer; 2021. Cultivation and utilization of Coleus species; pp. 229–251. [Google Scholar]
- Rukshana MS, Doss A, Kumari PR. Phytochemical screening and GC-MS analysis of leaf extract of Pergulariadaemia (Forssk) Chiov. Asian J Plant Sci Res. 2017;7:9–15. [Google Scholar]
- Salem SS, El-Belely EF, Niedbała G, Alnoman MM, Hassan SED, Eid AM, Fouda A. Bactericidal and in-vitro cytotoxic efficacy of silver nanoparticles (Ag-NPs) fabricated by endophytic actinomycetes and their use as coating for the textile fabrics. Nanomaterials. 2020;10:2082. doi: 10.3390/nano10102082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmila G, Muthukumaran C, Sandiya K, Santhiya S, Pradeep RS, Kumar NM, Thirumarimurugan SN, M, Biosynthesis, characterization, and antibacterial activity of zinc oxide nanoparticles derived from Bauhinia tomentosa leaf extract. J Nanostruct Chem. 2018;8:293–299. [Google Scholar]
- Silver S. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev. 2003;27:341–353. doi: 10.1016/S0168-6445(03)00047-0. [DOI] [PubMed] [Google Scholar]
- Spracklen FH, Kimerling JJ, Besterman EM, Litchfield JW. Use of lignocaine in treatment of cardiac arrhythmias. Br Med J. 1968;1:89. doi: 10.1136/bmj.1.5584.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganya KU, Govindaraju K, Kumar VG, Dhas TS, Karthick V, Singaravelu G, Elanchezhiyan M. Blue-green alga mediated synthesis of gold nanoparticles and its antibacterial efficacy against Gram-positive organisms. Mater Sci Eng C. 2015;47:351–356. doi: 10.1016/j.msec.2014.11.043. [DOI] [PubMed] [Google Scholar]
- Togashi N, Shiraishi A, Nishizaka M, Matsuoka K, Endo K, Hamashima H, Inoue Y. Antibacterial activity of long-chain fatty alcohols against Staphylococcus aureus. Molecules. 2007;12:139–148. doi: 10.3390/12020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SK, Das AK, Patel MK, Shah A, Kumar V, Gantait S. Engineered nanomaterials for plant growth and development: a perspective analysis. Sci Total Environ. 2018;630:1413–1435. doi: 10.1016/j.scitotenv.2018.02.313. [DOI] [PubMed] [Google Scholar]
- Verma SK, Das AK, Gantait S, Kumar V, Gurel E. Applications of carbon nanomaterials in the plant system: a perspective view on the pros and cons. Sci Total Environ. 2019;667:485–499. doi: 10.1016/j.scitotenv.2019.02.409. [DOI] [PubMed] [Google Scholar]
- Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3:163. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- Xiaoming S, Liming Z, Songhua H. Amplified immune response by ginsenoside-based nanoparticles (ginsomes) Vaccine. 2009;27:2306–2311. doi: 10.1016/j.vaccine.2009.02.040. [DOI] [PubMed] [Google Scholar]
- Zarei M, Karimi E, Oskoueian E, Es-Haghi A, Yazdi MET. Comparative study on the biological effects of sodium citrate-based and apigenin-based synthesized silver nanoparticles. Nutr Cancer. 2021;73:1511–1519. doi: 10.1080/01635581.2020.1801780. [DOI] [PubMed] [Google Scholar]