Abstract
Heat stress (HS) compromises the yield and quality of poultry products and endangers the sustainability of the poultry industry. Despite being homeothermic, chickens, especially fast-growing broiler lines, are particularly sensitive to HS due to the phylogenetic absence of sweat glands, along with the artificial selection-caused increase in metabolic rates and limited development of cardiovascular and respiratory systems. Clinical signs and consequences of HS are multifaceted and include alterations in behavior (e.g., lethargy, decreased feed intake, and panting), metabolism (e.g., catabolic state, fat accumulation, and reduced skeletal muscle accretion), general homeostasis (e.g., alkalosis, hormonal imbalance, immunodeficiency, inflammation, and oxidative stress), and gastrointestinal tract function (e.g., digestive and absorptive disorders, enteritis, paracellular barrier failure, and dysbiosis). Poultry scientists and companies have made great efforts to develop effective solutions to counteract the detrimental effects of HS on health and performance of chickens. Feeding and nutrition have been shown to play a key role in combating HS in chicken husbandry. Nutritional strategies that enhance protein and energy utilization as well as dietary interventions intended to restore intestinal eubiosis are of increasing interest because of the marked effects of HS on feed intake, nutrient metabolism, and gut health. Hence, the present review series, divided into Part I and Part II, seeks to synthesize information on the effects of HS on physiology, gut health, and performance of chickens, with emphasis on potential solutions adopted in broiler chicken nutrition to alleviate these effects. Part I provides introductory knowledge on HS physiology to make good use of the nutritional themes covered by Part II.
Keywords: chicken, heat stress, physiology, metabolism, gut health
Introduction
Heat stress (HS) affects performance, health, and welfare of commercially-reared birds (Renaudeau et al., 2012; Rostagno, 2020) and alters their meat (Song and King, 2015; Wang et al., 2017; Zaboli et al., 2019) and egg quality (Mack et al., 2013; Barrett et al., 2019), thereby endangering the sustainability of the poultry industry. This environmental stressor has commonly impacted poultry flocks raised in tropical and subtropical regions of the world. However, as a result of global warming, high environmental temperatures have become a large-scale issue that severely threatens poultry producers located in temperate areas as well (Renaudeau et al., 2012). Extreme heatwaves have already caused devastating events for the poultry industry, such as the sudden death of more than 700,000 birds in California (Nienaber and Hahn, 2007). St-Pierre et al. (2003) reported that the financial burden of HS amounts to $128–165 million per year for the US poultry industry alone. Although this figure still represents a general reference, recent estimates cannot be easily found in the literature.
In addition to causing evident changes in chicken behavior (Wang W. C. et al., 2018), HS negatively acts upon metabolism and general homeostasis (Rhoads et al., 2013) and impairs the functionality of the digestive system (Rostagno, 2020). Interestingly, several physiological and pathophysiological responses to HS are evolutionary conserved across different animal species (Lambert et al., 2002; Pearce et al., 2012; Koch et al., 2019; Kaufman et al., 2021), including humans (Snipe et al., 2018). For instance, the reduction in feed intake is one of the most common HS reactions because it is an effective way to limit the generation of metabolic heat due to digestion, absorption, and nutrient metabolism (Baumgard and Rhoads, 2013).
Given its tremendous relevance to the whole sector, poultry scientists and companies have been committed to developing reliable tools against HS. Engineering solutions and equipment intended to optimize the environmental control of poultry houses, along with management measures and genetic selection, can undoubtedly aid poultry producers in counteracting hot conditions (Lin et al., 2006b; Naga Raja Kumari and Narendra Nath, 2018; Saeed et al., 2019; Liang et al., 2020; Wasti et al., 2020; Goel, 2021; Nawaz et al., 2021; Vandana et al., 2021). Feeding strategies and dietary interventions can also help relieve HS effects on poultry (Gous and Morris, 2005; Lin et al., 2006b; Wasti et al., 2020; Vandana et al., 2021). For instance, increasing energy and nutrient density of the diet can counterbalance the decreased feed consumption of birds exposed to HS (Wasti et al., 2020). Moreover, researchers dealing with HS have been testing feed supplements aimed at promoting gastrointestinal (GI) health (Lian et al., 2020), which is a mainstay for the modern animal science and livestock industry (Kogut and Arsenault, 2016). Gut health is multifaceted and simultaneously influenced by composition and properties of the diet, digestion and absorption processes, integrity of the GI epithelium, plasticity and resilience of the GI immune system, and dynamics of the GI microbiota (Brugaletta et al., 2020). Pioneering studies conducted in the 1980s revealed that the GI microbiota is a main target of HS and that dietary supplementation of probiotic blends can attenuate HS in chickens (Suzuki et al., 1983, 1989). Probiotics have been shown to drive the formation of a desirable and protective GI microbiota (Baldwin et al., 2018), while properly reestablishing eubiosis following environmental stress, such as the exposure to elevated temperatures (Sugiharto et al., 2017). Along with probiotics, other GI microbiota modulators and potentially gut health-enhancing additives have been tested in poultry nutrition to promote eubiosis under HS conditions, such as prebiotics, synbiotics, postbiotics, phytochemicals, and amino acids, to name but a few (Lian et al., 2020; He et al., 2021).
Over the last years, HS-mediated alterations of physiology and gut health of chickens have received considerable attention. Therefore, this review series composed of Part I and Part II, was conceived to summarize relevant knowledge about these topics and examine some feeding and nutritional interventions that have been proposed to mitigate HS in broiler chickens. The present Part I discusses the effects of HS on physiology and gut health of chickens, while Part II (Teyssier et al., 2022a) provides an overview of potential solutions employed in broiler chicken nutrition to minimize the detrimental effects of HS.
Heat stress effects on physiology of chickens
Chickens are homeotherms that can keep their body temperature tightly regulated across a wide range of external temperatures. However, high environmental temperatures can overwhelm the thermoregulatory mechanisms, causing an imbalance between the amount of metabolic heat produced by chickens and their own capacity to dissipate body heat to the environment. This alteration results in an abnormal increase in body temperature (hyperthermia) and triggers HS (Renaudeau et al., 2012; Rostagno, 2020). In addition to being potentially lethal, HS has a broad-spectrum effect on behavior, physiology, gut health, welfare, and productive performance of chickens. It is worth pointing out that fast-growing and highly efficient broiler chickens (Havenstein et al., 1994, 2003b; Zuidhof et al., 2014; Tallentire et al., 2018), the outcome of about 70 years of genetic progress, are even less thermotolerant and more susceptible to HS than slow-growing lines due to extremely high metabolic rates and poorly developed cardiovascular and respiratory systems (Cahaner and Leenstra, 1992; Yunis and Cahaner, 1999; Havenstein et al., 2003a; Gous and Morris, 2005; Lu et al., 2007; Yahav, 2009; Xu et al., 2018). In this regard, Gogoi et al. (2021) recently proved that the physiological response to HS is more severe and heat tolerance is lower in heavy broilers than in lighter birds of the same line and age.
Heat stress and behavior
“Cooling behaviors” (Wang W. C. et al., 2018) are the most prominent HS-caused modifications in chicken behavior. As their name suggests, these abnormal behaviors are intended to cool the body down to restore normothermia. Chickens lack sweat glands, which would facilitate latent heat loss by evaporation of the perspiration, and have relatively limited unfeathered body surface areas to provide an effective loss of sensible heat through conduction, radiation, and convection (Nichelmann et al., 1986; Yunis and Cahaner, 1999; Renaudeau et al., 2012; Rostagno, 2020). As the ambient temperature rises, the thermal gradient between the body surface and the surrounding environment lessens while the dissipation of sensible heat becomes decreasingly effective. Therefore, chickens suffering from environment-induced hyperthermia increase their respiratory rate (thermal tachypnea/polypnea or panting) to maximize the loss of latent heat via evaporation of water from the respiratory tract (Jukes, 1971; Teeter et al., 1985). While sensible heat loss is restricted by the body-to-environment thermal gradient, relative humidity imposes a ceiling on water evaporation and, therefore, on latent heat dissipation (Renaudeau et al., 2012). Thus, elevated ambient temperature associated with high relative humidity considerably limit heat removal from the body and magnify the injurious effects of HS on chickens (Rajaei-Sharifabadi et al., 2017; Goel, 2021). Under persistent HS conditions, thermal polypnea turns into a slower and deeper panting phase, also called thermal hyperpnea (Hales, 1973; IUPS Thermal Commission, 1987; Renaudeau et al., 2012). Even though panting improves evaporative cooling through latent heat dissipation, it has some drawbacks for chickens (Marder and Arad, 1989). Dehydration, the most intuitive panting-related disadvantage, usually results in higher water requirement and consumption (Wang W. C. et al., 2018). Panting also increases CO2 exhalation leading to hypocapnia and, eventually, to respiratory alkalosis, a disorder of the acid-base balance (Richards, 1970; Marder and Arad, 1989; Renaudeau et al., 2012; Beckford et al., 2020; Wasti et al., 2020). Alkalosis poses a risk to the egg industry because it reduces blood ionized calcium and therefore negatively affects eggshell mineralization (Odom et al., 1986). However, HS-induced respiratory alkalosis is a great threat to broiler growers as well (Teeter et al., 1985; Borges et al., 2007). Chickens subjected to HS frequently lift their wings (Wang W. C. et al., 2018) to expose body areas uncovered by feathers in an attempt to enhance the sensible heat flow toward the environment. Despite being fundamental to preserving or reestablishing euthermia, panting and raising wings are energy-intensive activities (Brackenbury and Avery, 1980; Dale and Fuller, 1980) which deplete the amount of calories that would be allocated to productive purposes (Yahav et al., 2004; Baumgard and Rhoads, 2013).
Chickens kept at high temperatures become lethargic, spending more time resting (e.g., squatting close to the ground) and less time feeding and walking. This unfavorably affects feed intake (Wang W. C. et al., 2018) and skeletal health (Hester et al., 2013). Limiting feed consumption is a highly conserved survival mechanism employed by animals to reduce the thermogenesis from digestive, absorptive, and nutrient utilization processes (Baumgard and Rhoads, 2013). Reduced performance of heat-stressed chickens have traditionally been attributed to reduced feed intake (Dale and Fuller, 1980; Teeter et al., 1985). However, pair-feeding models—adopted to minimize the confounding effects of dissimilar feed consumption between birds under thermoneutral conditions and their heat-stressed counterparts—revealed that up to 40% of body weight gain loss of broilers subjected to HS are unrelated to anorexia (Dale and Fuller, 1980; Geraert et al., 1996a; Ain Baziz et al., 1996; Lu et al., 2007; Zuo et al., 2015; Lu et al., 2018; Teyssier et al., 2022b). Readers are referred to Part II of this review series for more information on the effects of HS on feed intake (Teyssier et al., 2022a). Table 1 provides an overview of heat stress effects on chicken behavior.
TABLE 1.
Overview of heat stress effects on chicken behavior.
| Class | Heat stress effect | Pros | Cons | References a |
|---|---|---|---|---|
| Behavior | ↑ respiratory rate (thermal polypnea or panting) → thermal hyperpnea | ↑ latent heat dissipation (evaporative heat loss through the respiratory tract) | Dehydration → higher water requirement and consumption | Richards (1970) , Jukes (1971) , Brackenbury and Avery (1980) , Dale and Fuller (1980) , Teeter et al. (1985) , Odom et al. (1986) , Marder and Arad (1989) , Yahav et al. (2004) , Borges et al. (2007) , Renaudeau et al. (2012), Rhoads et al. (2013), Wang W. C. et al. (2018) , Beckford et al. (2020) , Wasti et al. (2020) |
| ↑ CO2 loss → hypocapnia → respiratory alkalosis (acid-base imbalance) → ↓ blood calcium for eggshell mineralization and ↓ growth performance | ||||
| ↑ energy expenditure to maintain euthermia → ↓ performance | ||||
| Wing lifting | ↑ exposition of unfeathered body surfaces → ↑ sensible heat loss | ↑ energy expenditure to maintain euthermia → ↓ performance | Dale and Fuller (1980) , Baumgard and Rhoads (2013), Wang W. C. et al. (2018) | |
| Lethargy → ↓ feeding and walking | ↓ metabolic heat from digestion, absorption, and nutrient utilization | ↓ performance | Dale and Fuller (1980) , Teeter et al. (1985) , Geraert et al. (1996a) , Baumgard and Rhoads (2013), Hester et al. (2013) , Wang W. C. et al. (2018) | |
| ↓ bone/skeletal health |
Upward arrow (↑), increase; downward arrow (↓), decrease; rightward arrow (→), consequence/degeneration.
Include studies on non-avian species that have exhibited comparable heat stress effects and responses to those observed in chickens. Studies with the focus on chickens or poultry are highlighted in bold.
Heat stress and lipid metabolism
Direct effects of HS upon physiology, other than reduced feed intake, remarkably contribute to impair chicken performance (Dale and Fuller, 1980; Geraert et al., 1996a; Renaudeau et al., 2012). Heat-stressed animals paradoxically show a restricted fat mobilization notwithstanding their negative energy balance and catabolic state (Baumgard and Rhoads, 2013). Indeed, not only chickens (Bobek et al., 1997; Lu et al., 2018), but also pigs (Pearce et al., 2013a; Victoria Sanz Fernandez et al., 2015), and dairy cattle (Rhoads et al., 2009) kept in warm environments show a progressive reduction in circulating non-esterified (free) fatty acids (NEFA)—a reliable indicator of lipid metabolism—suggesting a limited use of fat energy stores. Extensive research has also revealed that heat-stressed chickens deposit more visceral (abdominal), subcutaneous, and intramuscular fat (Kleiber and Dougherty, 1934; Kubena et al., 1972; Ain Baziz et al., 1996; Yunianto et al., 1997; He et al., 2015; Lu et al., 2018, 2019). A greater lipid retention at the peripheral body sites can further hinder the dissipation of sensible heat (Renaudeau et al., 2012), increasing the risk of severe hyperthermia. The hampered fat mobilization is a metabolic adaptation likely due to hyperinsulinemia triggered by HS, at least in pigs and cattle (Baumgard and Rhoads, 2013). In contrast to mammals, however, heat-stressed chickens do not usually show a spike in blood insulin levels (Geraert et al., 1996b; Tang et al., 2013; Belhadj Slimen et al., 2016), although Lu et al. (2019) reported an increase and a decrease in circulating insulin at 7 and 14 days of HS, respectively. Moreover, avian insulin lacks a powerful antilipolytic effect, while the importance of its signaling cascades in the adipose tissue of chickens is still unclear and a matter of debate (Dupont et al., 2012, 2015). Therefore, several questions about the role of insulin in fat metabolism of chickens undergoing HS remain unanswered at present. It is worth noting, however, that the altered lipid metabolism is not limited to a reduced utilization of fat storages because heat-challenged chickens also show an overexpression of proteins involved in the hepatic de novo lipogenesis, along with fat accumulation in the liver (Flees et al., 2017; Lu et al., 2019). HS effects on chicken lipid metabolism are summarized in Table 2.
TABLE 2.
Overview of heat stress effects on chicken lipid metabolism.
| Class | Heat stress effect | Pros | Cons | References a |
|---|---|---|---|---|
| Lipid metabolism | ↓ fat mobilization and ↑ hepatic lipogenesis → ↑ fat retention and deposition | — | ↑ carcass adiposity | Kleiber and Dougherty (1934) , Kubena et al. (1972) , Ain Baziz et al. (1996) , Geraert et al. (1996a) , Yunianto et al. (1997) , Bobek et al. (1997) , Rhoads et al. (2009), Renaudeau et al. (2012), Baumgard and Rhoads (2013), Pearce et al. (2013a), Victoria Sanz Fernandez et al. (2015), He et al. (2015) , Flees et al. (2017) , Lu et al. (2018) , Lu et al. (2019) |
| ↓ sensible heat dissipation |
Upward arrow (↑), increase; downward arrow (↓), decrease; rightward arrow (→), consequence/degeneration.
Include studies on non-avian species that have exhibited comparable heat stress effects and responses to those observed in chickens. Studies with the focus on chickens or poultry are highlighted in bold.
Heat stress and skeletal muscle protein metabolism
In addition to an increase in fat content, HS has been demonstrated to alter the carcass composition of broiler chickens by lowering the lean tissue proportion, especially the breast yield (Howlider and Rose, 1989; Geraert et al., 1996a; Ain Baziz et al., 1996; Temim et al., 2000; Zuo et al., 2015; Lu et al., 2018; Qaid and Al-Garadi, 2021; Zampiga et al., 2021). First molecular insights suggested that HS-mediated depression in muscle protein deposition is mostly attributable to a reduced protein synthesis rather than a more pronounced protein breakdown (Temim et al., 2000). Zuo et al. (2015) showed, however, that the cause for the diminished lean mass accretion can be muscle-specific, with the breast showing a decreased protein synthesis while the thigh an augmented protein degradation. They also associated the impaired protein synthesis with a lower expression of insulin-like growth factor 1 (IGF-1), phosphatidylinositol 3-kinase (PI3K), and p70S6 kinase (S6K) and the higher protein degradation with an upregulation of muscle atrophy F-box (MAFbx or atrogin-1). Ma et al. (2021) recently confirmed the modifications in S6K and MAFbx expression caused by HS. S6K is indispensable in controlling protein synthesis and muscle development in chickens (Bigot et al., 2003; Duchêne et al., 2008; Everaert et al., 2010). Interestingly, Boussaid-Om Ezzine et al. (2010) detected a limited response of the S6K signaling pathway to anabolic stimuli in heat-stressed broiler chickens. Lu et al. (2018) measured an increase in blood uric acid, urea, and proteinogenic amino acids (AA)—in spite of a marked decrease in feed intake and breast yield—along with a reduction in glucose and NEFA. Consequently, they postulated that heat-exposed chickens mobilize protein reservoirs of skeletal muscles, particularly the breast, to compensate for the inability to extract energy from stored fat. In this regard, plasmatic levels of creatine, 3-methylhistidine, and urea have been used as biomarkers to assess muscle protein breakdown induced by HS (Rhoads et al., 2013). The hypothesis formulated by Lu et al. (2018) has been supported by Ma et al. (2021) who found that HS reduces plasmatic glucogenic AA, increases AA uptake of the liver and its glucogenic potential, and enhances the activity of hepatic transaminases that deaminize AA to make them precursors for gluconeogenesis. Furthermore, Zampiga et al. (2021) observed that heat-stressed broilers exhibit a drop in blood glucogenic precursors and breast muscle free AA, despite a rise in circulating protein-building AA concomitant with a substantial feed intake reduction. Table 3 presents a summary of heat stress effects on skeletal muscle protein metabolism of chickens.
TABLE 3.
Overview of heat stress effects on skeletal muscle protein metabolism of chickens.
| Class | Heat stress effect | Pros | Cons | References a |
|---|---|---|---|---|
| Protein metabolism | ↓ protein synthesis and ↑ protein breakdown in skeletal muscles | Supply of glucogenic precursors to the liver | ↓ lean tissue yield (especially breast yield) | Howlider and Rose (1989) , Ain Baziz et al. (1996) , Geraert et al. (1996a) , Temim et al. (2000) , Boussaid-Om Ezzine et al. (2010) , Rhoads et al. (2013), Zuo et al. (2015) , Lu et al. (2018) , Ma et al. (2021) , Qaid and Al-Garadi (2021) , Zampiga et al. (2021) |
Upward arrow (↑), increase; downward arrow (↓), decrease; rightward arrow (→), consequence/degeneration.
Include studies on non-avian species that have exhibited comparable heat stress effects and responses to those observed in chickens. Studies with the focus on chickens or poultry are highlighted in bold.
Heat stress and hormonal levels
Chickens subjected to HS share numerous hormonal variations with mammalian species. HS activates the hypothalamic-pituitary-adrenal axis, leading to a marked increase in circulating glucocorticoids, particularly corticosterone (Geraert et al., 1996b; Yunianto et al., 1997; Quinteiro-Filho et al., 2010, 2012; Rajaei-Sharifabadi et al., 2017; Lu et al., 2019; Beckford et al., 2020; Ma et al., 2021). In chickens, high levels of corticosterone have been reported to decrease growth potential, induce proteolysis and suppress protein synthesis in skeletal muscles, and increase fat deposition (Decuypere and Buyse, 1988; Dong et al., 2007; Yuan et al., 2008), all of which are typical HS consequences (Rhoads et al., 2013). It has been proposed that corticosterone impairs muscle protein metabolism by inducing the abovementioned changes in S6K and MAFbx expression (Ma et al., 2021), while also exerting a lipogenic effect by promoting the expression of fatty acid synthase (FASN) in hepatocytes and adipocytes (Gonzalez-Rivas et al., 2020). However, a recent investigation demonstrated that treating heat-stressed chicken myotubes with corticosterone does not intensify proteolysis and does not increase the expression of MAFbx compared to the HS treatment alone (Furukawa et al., 2021). The latter interesting results have been obtained in vitro, and therefore further research may be needed to elucidate the role of corticosterone in the altered protein metabolism of heat-stressed chickens.
Additionally, since hypercorticosteronemia is immunosuppressive (Quinteiro-Filho et al., 2010), heat-challenged chickens have a compromised immunocompetence and are more prone to infectious diseases (Renaudeau et al., 2012; Farag and Alagawany, 2018; Chauhan et al., 2021). In this regard, Hirakawa et al. (2020) detected serious immunological disorders in heat-stressed broilers, such as a decrease in immunoglobulin production against a prototype antigen as well as atrophy and dysfunction of primary and secondary lymphoid tissues accompanied by lymphocyte depression. The authors mentioned hypercorticosteronemia among the plausible reasons for these anomalies in the immune system. Corticosterone-related immune dysfunctions of chickens have thoroughly been described by Shini et al. (2010).
Reductions in hematic triiodothyronine (T3) and thyroxine (T4) have also been observed in laying hens (de Andrade et al., 1977; Bobek et al., 1997) and broiler chickens (Geraert et al., 1996b; Yunianto et al., 1997; Sohail et al., 2010; Rajaei-Sharifabadi et al., 2017; Beckford et al., 2020) undergoing HS. These variations, which might be caused by decreased size and activity of the thyroid (Huston and Carmon, 1962; Dale and Fuller, 1980; Yunianto et al., 1997), have also been measured in heat-stressed dairy cattle (Chen et al., 2018). It has commonly been assumed that the thyroid response to high environmental temperatures is an adaptive mechanism that allows animals to lower their basal metabolism and thermogenesis in order to prevent overheating (Renaudeau et al., 2012; Chen et al., 2018; Gonzalez-Rivas et al., 2020). The hypothyroid-like condition can partly justify growth depression (McNabb and Darras, 2015), increased carcass adiposity (Decuypere and Buyse, 1988; Geraert et al., 1996b), and decreased egg production and shell quality (de Andrade et al., 1977) observed during HS. Table 4 briefly illustrates the effects of heat stress on chicken hormonal levels.
TABLE 4.
Overview of heat stress effects on chicken hormonal levels.
Upward arrow (↑), increase; downward arrow (↓), decrease; rightward arrow (→), consequence/degeneration.
Include studies on non-avian species that have exhibited comparable heat stress effects and responses to those observed in chickens. Studies with the focus on chickens or poultry are highlighted in bold.
Heat stress effects on gut health of chickens
Gut health should be addressed in a holistic way (Oviedo-Rondón, 2019) by taking into consideration the major elements that synergistically affect it, namely the GI epithelium, the GI immune system, and the GI microbiota (Kogut et al., 2017). Being the largest body surface exposed to the environment, the gastrointestinal tract (GIT) is repeatedly threatened by a wide variety of harmful factors (Yegani and Korver, 2008), like noxious feed-derived compounds and pathogenic microorganisms. Applegate and Troche (2014) emphasized that the GIT accomplishes conflicting tasks, that is maximizing nutrient uptake while recognizing multiple antigenic stimuli and tolerating the resident microbiota. Hence, integrity and proper morpho-functionality of the GIT are of utmost importance in ensuring optimal health and productivity for chickens.
Heat stress and gastrointestinal epithelium
The GI epithelium, arranged in a single-cell layer, takes an active part in the integrated gut immune system, forming a barrier reinforced by tight junction (TJ) proteins, secreting mucus and antimicrobial/host defense peptides (AMP/HDP), and expressing pattern recognition receptors (PRR) that orchestrate the enteral immune response (Smith et al., 2014; Chen et al., 2015; Broom and Kogut, 2018a).
TJs, the uppermost component of the apical junctional complex, seal the interstice between adjoining columnar epithelial cells (Farquhar and Palade, 1963) and encompass transmembrane (claudins and occludin) and scaffolding/peripheral/plaque proteins (zonula occludens—ZO). Through their binding domains, ZO anchor to claudins and occludin on one side and to the perijunctional actomyosin ring on the other side, thereby making a bridge between transmembrane TJs and the cytoskeleton (Ulluwishewa et al., 2011). TJs are primarily responsible for controlling the paracellular pathway that, unlike the pump- and channel-dependent transcellular transports, allows a passive transepithelial diffusion via two main routes, known as the pore pathway and the leak pathway (Dokladny et al., 2016). The pore pathway relies on claudins and limits the passage of charged and large molecules (greater than 4 Å), while the leak pathway, governed by occludin and ZO, can be crossed by big solutes, including bacterial lipopolysaccharides (LPS) (Anderson and Van Itallie, 2009; Dokladny et al., 2016; France and Turner, 2017). Under HS, the cardiovascular system responds in another evolutionary-preserved adaptation whereby a large volume of blood is shunted from the splanchnic tissues to peripheral areas of the body to maximize the dissipation of sensible heat (Hales, 1973; Lambert, 2009). This occurs to the detriment of the GIT because the altered blood pressure is mostly compensated by a sympathetically driven vasoconstriction of viscera (Table 5). The resulting hypoperfusion implicates a reduced supply of nutrients and oxygen to the GIT, which prompts deleterious effects on the intestinal mucosa (Lambert, 2009; Baumgard and Rhoads, 2013; Rostagno, 2020). In light of the remarkable energy and protein demands of the digestive system, a sub-optimal trophism of the GI epithelium negatively affects cell turnover and the maintenance of a robust intestinal barrier (Koutsos and Arias, 2006). On the other hand, the inadequate oxygenation leads to hypoxia, a condition that profoundly alters the cellular bioenergetic pathways and promotes the generation of reactive oxygen and nitrogen species (ROS and RNS, respectively) (Hall et al., 1999). Moreover, hyperthermia triggers ROS and RNS production per se (Hall et al., 2001) and impairs the enzymatic antioxidant systems (Farag and Alagawany, 2018), directly contributing to the establishment of a pro-oxidative scenario. Lin et al. (2006a) proved that elevated ambient temperatures provoke oxidative stress in chickens, while Tan et al. (2010) suggested that HS can depress the mitochondrial respiratory chain activity with consequent overproduction of ROS and oxidative injury. Worthy of mention is also the research on oxidative damage affecting the skeletal muscles, particularly the Pectoralis major, of heat-stressed broilers. Several authors demonstrated a rise in mitochondrial membrane potential, a high production of mitochondrial superoxide and ROS, and a considerable increase in malondialdehyde level (marker of lipid peroxidation) in breast muscles of broilers exposed to HS (Mujahid et al., 2006; Wang et al., 2009; Azad et al., 2010; Kikusato and Toyomizu, 2013). On the other hand, studies focused on the GIT have reported that oxidative stress destabilizes the TJ-regulated paracellular barrier and increases intestinal permeability (Rao, 2008; Bischoff et al., 2014). Myosin light-chain kinase (MLCK) is involved in this cascade of events because it regulates the circumferential contractions of the actomyosin ring and, indirectly, the TJ-controlled paracellular pathway (France and Turner, 2017). The actomyosin ring contractions can be triggered by several physiological and pathological stimuli. Oxidative stress has been shown to cause such contractions and, consequently, to affect the MLCK-regulated localization of ZO proteins and downregulate their expression, contributing to the deterioration of the paracellular barrier (González-Mariscal et al., 2011).
TABLE 5.
Overview of heat stress effects on the cardiovascular system of chickens.
| Class | Heat stress effect | Pros | Cons | References a |
|---|---|---|---|---|
| Cardiovascular system | Peripheral vasodilatation and GIT vasoconstriction | ↑ sensible heat dissipation | GIT hypoperfusion → | Hales (1973), Hall et al. (1999), Koutsos and Arias (2006) , Rao (2008), Lambert (2009), Baumgard and Rhoads (2013), Bischoff et al. (2014), Rostagno (2020) |
| ↓ nutrient supply to the GIT → ↓ GI barrier and functionality | ||||
| GIT hypoxia → oxidative stress → ↓ GI barrier and functionality |
Upward arrow (↑), increase; downward arrow (↓), decrease; rightward arrow (→), consequence/degeneration.
Include studies on non-avian species that have exhibited comparable heat stress effects and responses to those observed in chickens. Studies with the focus on chickens or poultry are highlighted in bold.
Along with oxidative stress, the aforementioned increment in corticosterone levels further weakens the intestinal barrier (Quinteiro-Filho et al., 2012). Transepithelial electrical resistance (TEER)—i.e., the epithelium resistance to ion passage—and mucosa-to-serosa flux of marker probes (e.g., fluorescein isothiocyanate-dextran) have commonly been used to evaluate the paracellular barrier stability and integrity (Shen et al., 2011; Bischoff et al., 2014; Awad et al., 2017; Ma et al., 2018; Gilani et al., 2021). A steady paracellular pathway shows high values of TEER and effectively obstructs the flux of markers, whereas low TEER and high marker passage indicate poor barrier functions. HS has been shown to considerably reduce TEER and significantly increase the migration of marker tracers across the intestinal epithelium in numerous animal models (Dokladny et al., 2016), pigs (Pearce et al., 2013b), and broiler chickens (Song et al., 2014; Tabler et al., 2020). These variations are indicators of a “leaky gut” that barely holds noxious luminal compounds (Shen et al., 2011; Awad et al., 2017; Ma et al., 2018; Ruff et al., 2020). Translocation of pathogen-associated molecular patterns (PAMP) from the intestinal lumen to the underlying lamina propria is a major consequence of an increased paracellular permeability. The gut contains a massive amount of PAMPs, mainly LPS of Gram-negative bacteria (Wassenaar and Zimmermann, 2018), which can bind to a class of PRRs known as Toll-like receptors (TLR). Intestinal TLRs are particularly differentiated in chickens (Keestra et al., 2013) and have been shown to play a pivotal role in maintaining gut homeostasis and evoking inflammatory responses in the case of infections or other insults, such as hypoxia and tissue injury (Gribar et al., 2008). These receptors are also involved in epithelial cell proliferation, wound healing, stability of TJs, and modulation of immunoglobulin A (IgA) production and AMP expression (Abreu, 2010; Iizuka and Konno, 2011). Furthermore, TLRs are rather non-responsive to the multitude of commensal microorganisms inhabiting the GIT, yet are constantly responsive to PAMPs and host indicators of cell damage (Harris et al., 2006; Kogut et al., 2017; Madsen and Park, 2017). The ability to distinguish between useful microbes and those undesirable—or that can become such, like pathobionts (Round and Mazmanian, 2009)—is one of the most fascinating properties of the GI immune system (Mowat, 2018). At the basolateral membrane of the intestinal epithelium, LPS are recognized by the TLR4–MD-2 receptor complex (Shimazu et al., 1999; Abreu, 2010; Keestra et al., 2013) whose activation initiates an intracellular signaling cascade upregulating the expression of several pro-inflammatory cytokines (Vaure and Liu, 2014). The latter signaling molecules, also released by LPS-stimulated innate immune cells, foster a vicious cycle that deteriorates the intestinal barrier (Lambert, 2009). Tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and interferon gamma (IFN-γ) have been reported to ruin the paracellular barrier, thereby increasing LPS leakage (Turner et al., 2014; Dokladny et al., 2016; Awad et al., 2017; Ma et al., 2018). Specifically, TNF-α has been shown to initiate the actomyosin ring contractions and, subsequently, to cause occludin internalization and TJ disassociation (Turner et al., 2014). Pro-inflammatory cytokines evoke a local inflammatory response aggravating the damages to the enteric mucosa. Quinteiro-Filho et al. (2010, 2012) reported that heat-stressed broilers manifest mild multifocal enteritis. Enteral inflammation has been shown to shorten the lifespan of enterocytes and cause crypt hyperplasia and villus atrophy (Smith et al., 2014). These alterations in intestinal epithelium morphology (microarchitecture), along with increased cell apoptosis and reduced cell proliferation, have recently been observed in broiler chickens exposed to HS (He et al., 2018a, 2018b; Liu et al., 2020, 2022; Nanto-Hara et al., 2020). In their pair-feeding study with broilers, Nanto-Hara et al. (2020) evidenced that intestinal morphological damage and increased intestinal permeability are direct consequences of HS rather than of feed intake reduction induced by HS itself. The resultant nutrient malabsorption and energy expenditure to sustain the GI immune response severely impact chicken performance and can be a predisposing factor for additional health problems (Broom and Kogut, 2018b).
In addition to initiating a local inflammation, luminal LPS can permeate the portal circulation whereby they reach and compromise the liver (Wang et al., 2015). Once exceeding the hepatic detoxification potential, LPS can diffuse throughout the bloodstream causing endotoxemia (Baumgard and Rhoads, 2013; Alhenaky et al., 2017; Epstein and Yanovich, 2019; Nanto-Hara et al., 2020). The resulting systemic inflammatory reactions force the organism to adjust nutrient partition and divert energy to support the immune system; this substantially depresses chicken performance (Broom and Kogut, 2018b; Ruff et al., 2020). At the worst, endotoxemia can lead to multi-organ failure and lethal septic shock (Wassenaar and Zimmermann, 2018).
According to Rostagno (2020), anomalies in the transcellular transport are another reason for intestinal permeability problems of chickens under HS. Indeed, a loss of epithelial integrity can degenerate into cell damage and consequent opening of TJ-independent pathways (France and Turner, 2017). Enteric bacteria can cross the altered and more permeable intestinal epithelium and, eventually, reach the liver or even migrate to other organs or tissues. For example, heat-stressed broilers showed a greater hepatic Salmonella invasion due to increased intestinal permeability (Alhenaky et al., 2017). This event, commonly called “bacterial translocation”, can be prelude to extraintestinal issues, such as deteriorations of liver functionality and health (Ilan, 2012; Ducatelle et al., 2018) as well as bacterial chondronecrosis with osteomyelitis (BCO) (Wideman, 2016). HS effects on the GI epithelium of chickens are summed up in Table 6.
TABLE 6.
Overview of heat stress effects on the GI epithelium of chickens.
| Class | Heat stress effect | Pros | Cons | References a |
|---|---|---|---|---|
| GI epithelium | Altered GI epithelium morphology (microarchitecture) and enterocyte lifecycle | — | Digestive and absorptive dysfunctions → ↓ performance | Lambert (2009), Song et al. (2014) , Vaure and Liu (2014), Wang et al. (2015), Wideman (2016) , Dokladny et al. (2016) , Alhenaky et al. (2017) , Awad et al. (2017), France and Turner (2017), Ma et al. (2018), Wassenaar and Zimmermann (2018), Ducatelle et al. (2018) , He et al. (2018a) , He et al. (2018b) , Epstein and Yanovich (2019), Nanto-Hara et al. (2020) , Ruff et al. (2020 ), Tabler et al. (2020) , Liu et al. (2020) , Liu et al. (2022) |
| ↑ paracellular permeability (↓ transepithelial electrical resistance and ↑ mucosa-to-serosa flux of markers) → “leaky gut” | ||||
| ↓ GI epithelium integrity | LPS/endotoxins leakage →↑ pro-inflammatory cytokines → GI inflammation and ↓ GI barrier | |||
| ↓ liver health and functionalityEndotoxemia → systemic inflammation, multi-organ failure, and septic shock | ||||
| “Bacterial translocation” →↓ liver health and functionalitybacterial chondronecrosis with osteomyelitis |
Upward arrow (↑), increase; downward arrow (↓), decrease; rightward arrow (→), consequence/degeneration.
Include studies on non-avian species that have exhibited comparable heat stress effects and responses to those observed in chickens. Studies with the focus on chickens or poultry are highlighted in bold.
Heat stress and gastrointestinal microbiota
Microbiota and microbiome are similar-sounding words that are often used interchangeably. However, the microbiota represents a cluster of microorganisms residing in a specific environment (Marchesi and Ravel, 2015), such as an area of human or animal bodies (Clavijo and Flórez, 2018), while the microbiome unifies the metagenome of a microbiota (i.e., the collection of microbial genomes) and its surrounding environment (Marchesi and Ravel, 2015). The alimentary canal of chickens harbors an extremely complex microbial population that consists of bacteria, archea, protozoa, fungi, and viruses (Yeoman et al., 2012). The GI microbiota extends the genome of the host and substantially influences its physiology (Koutsos and Arias, 2006), almost acting as a supplementary—or “neglected” (Bocci, 1992)—organ. It is a widely held view that the GI microbiota is instrumental in programming and modulating both the gastroenteric (Iyer and Blumberg, 2018; Cheng et al., 2019) and systemic (Belkaid and Hand, 2014; Zheng et al., 2020) immune system of humans and animals, including poultry (Broom and Kogut, 2018c). This notion is supported by gnotobiotic models in which germ-free mice (Round and Mazmanian, 2009; Belkaid and Hand, 2014; Iyer and Blumberg, 2018) and chickens (Dibner et al., 2008) have been reported to suffer from severe developmental deficiencies and dysfunctions of the GI immunity. In addition to its immunogenic and immunoregulatory roles, the GI microbiota considerably influences growth, morphology, and function of the intestine in chickens (Dibner et al., 2008; Pan and Yu, 2014).
A myriad of host- and environment-related variables affects the GI microbiota (Kers et al., 2018). For instance, data from several studies indicate that high ambient temperatures can dramatically shape the GI microbiota. Specifically, it has been demonstrated that HS perturbs the GI microbiota in rats (Suzuki et al., 1983, 1989), poultry (Suzuki et al., 1983, 1989; Lan et al., 2004; Burkholder et al., 2008; Song et al., 2014; Wang X. J. et al., 2018; He et al., 2019b; Shi et al., 2019; Xing et al., 2019; Zhu et al., 2019; Liu et al., 2020; Wang et al., 2020; He et al., 2021; Liu et al., 2022), dairy cattle (Chen et al., 2018), and pigs (He et al., 2019a; Le Sciellour et al., 2019; Xiong et al., 2020), pushing it to dysbiosis. Dysbiosis (dysbacteriosis) is an alteration in the gut microbiota with an overgrowth of harmful microorganisms, or a depletion of beneficial bacteria, which can weaken the fragile equilibrium between the host and its GI microbiota (Walker, 2017; Ducatelle et al., 2018). A dysbiotic state is often associated with depression in nutrient digestion, loss of intestinal barrier function, and GI inflammation (Chen et al., 2015; Ducatelle et al., 2018), whereas eubiosis, referred to as a balanced microbial ecosystem (Iebba et al., 2016), can enhance health, productivity, and ability of chickens to withstand environmental stressors (Kogut, 2019). Although cutting-edge analytical techniques are currently available to study the GI microbiota (Borda-Molina et al., 2018), the modifications in structure, composition, and functions of the GI microbiota of heat-stressed chickens are still to be fully understood (He et al., 2021; Liu et al., 2022). However, changes in GI morphology, mucus quantity and composition, and attachment sites, coupled with an accumulation of poorly digested or even undigested feed components, are all plausible reasons for HS-caused dysbiosis.
The commensal microbiota is able to hinder the colonization and proliferation of allochthonous and pathogenic microorganisms in the GI ecosystem (Schneitz, 2005). This protective mechanism, conventionally termed competitive exclusion (CE) or “Nurmi concept”, was firstly observed in newly hatched chicks acquiring resistance to Salmonella challenges if previously inoculated per os with a suspension of crop and intestinal contents collected from healthy adult chickens (Nurmi and Rantala, 1973). Chichlowski et al. (2007) specified that CE is a physical blockage of intestinal niches carried out by beneficial bacteria to the detriment of opportunistic pathogens. Desirable bacteria can also compete with pathogens for nutrients, and produce microbiostatic and microbicidal substances, such as organic acids and bacteriocins (Pan and Yu, 2014; Clavijo and Flórez, 2018). However, aberrant microbiotas of chickens subjected to HS have been related to an increased susceptibility to intestinal colonization of Salmonella Enteritidis (Burkholder et al., 2008; Soliman et al., 2009). Tsiouris et al. (2018) also demonstrated that HS can promote the expansion of Clostridium perfringens in the chicken intestine, becoming a predisposing factor for necrotic enteritis outbreak in flocks reared under hot conditions. C. perfringens can also release enterotoxins that, together with other harmful bacterial effectors, may impair TJs and gut barrier functions (Awad et al., 2017). Taken together, dysbiosis, intestinal barrier disorders, and mucosa inflammation are interconnected and fuel each other (Ducatelle et al., 2018), exacerbating the negative effects of HS on gut health, physiology, and performance of chickens (Tables 7, 8).
TABLE 7.
Overview of heat stress effects on the GI microbiota of chickens.
| Class | Heat stress effect | Pros | Cons | References a |
|---|---|---|---|---|
| GI microbiota | Perturbation of the GI ecosystem and microbial community stability → dysbiosis | — | Positive feedback loop among dysbiosis, GI barrier dysfunction, and GI inflammation → ↓ health and performance | Suzuki et al. (1983) , Suzuki et al. (1989) , Lan et al. (2004) , Burkholder et al. (2008) , Soliman et al. (2009) , Song et al. (2014) , Chen et al. (2018), Tsiouris et al. (2018) , Wang X. J. et al. (2018) , Wang et al. (2020) , Ducatelle et al. (2018) , Shi et al. (2019) , Xing et al. (2019) , Zhu et al. (2019) , He et al. (2019a), He et al. (2019b) , He et al. (2021), Le Sciellour et al. (2019), Xiong et al. (2020) , Liu et al. (2020) , Liu et al. (2022) |
| ↑ susceptibility to GI pathogen colonization → ↑ GI disorders (e.g., necrotic enteritis) → ↓ health and performance |
Upward arrow (↑), increase; downward arrow (↓), decrease; rightward arrow (→), consequence/degeneration.
Include studies on non-avian species that have exhibited comparable heat stress effects and responses to those observed in chickens. Studies with the focus on chickens or poultry are highlighted in bold.
TABLE 8.
Overview of heat stress effects on the inflammatory state of chickens.
| Class | Heat stress effect | Pros | Cons | References a |
|---|---|---|---|---|
| Inflammatory state | Enteritis and systemic inflammation | Response to endotoxemia, microbial infection, and GI tissue injury | ↑ energy expenditure to sustain the immune system → ↓ performance | Quinteiro-Filho et al. (2010) , Quinteiro-Filho et al. (2012) , Broom and Kogut (2018b), Ruff et al. (2020) |
Upward arrow (↑), increase; downward arrow (↓), decrease; rightward arrow (→), consequence/degeneration.
Include studies on non-avian species that have exhibited comparable heat stress effects and responses to those observed in chickens. Studies with the focus on chickens or poultry are highlighted in bold.
Conclusion
Nowadays poultry farmers must deal with HS at almost every latitude because climate change has made high temperatures a pressing issue no longer limited to hot countries. Consequently, it would be advisable to update the estimate of costs and economic losses caused by HS to realize its actual impact on the global poultry industry.
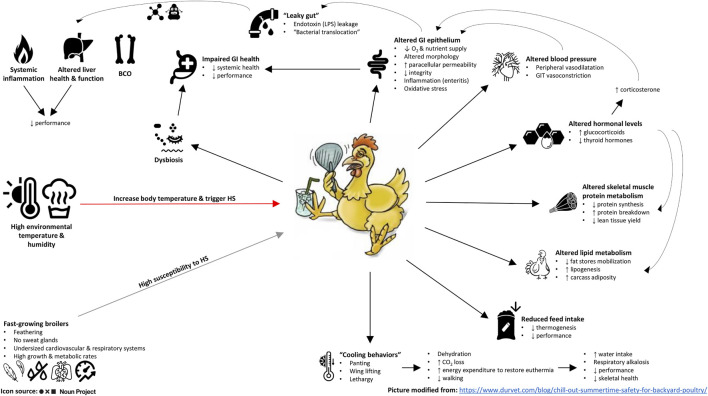
According to the literature reviewed here, HS provokes a wide range of deleterious effects on chickens, especially those belonging to modern high-performing lines (Figure 1). Firstly, HS negatively affects immunohomeostasis, hormonal equilibrium, and inflammatory and oxidative status. More studies on these physiologic alterations and their interconnections can help develop multitargeted solutions to help chickens combat HS more effectively. Secondly, HS promotes tissue catabolism and a substantial modification in protein and lipid metabolism. While there is evidence to assert that HS affects skeletal muscle accretion of chickens via both protein synthesis inhibition and protein degradation stimulation, further investigations are needed to clarify the underlying causes of the blunted fat mobilization in heat-stressed chickens. Lastly, high temperatures can be deemed to be a “dysbiogenic stressor” that undermines gut functionality and disrupts the host-microbiota interrelationship. Reinforcing the intestinal barrier, restoring digestive and absorptive processes, rebalancing the GI microbiota, and lowering the GI inflammation and oxidative stress seem therefore essential to increase HS tolerance and resilience for chickens.
FIGURE 1.
Summary chart of the main effects of heat stress on modern (broiler) chicken lines. LPS, lipopolysaccharides; BCO, bacterial chondronecrosis with osteomyelitis.
In conclusion, reversing the homeostatic and metabolic perturbations induced by HS and conferring enteral protection appear to be promising approaches to fight against this growing threat to the poultry industry sustainability.
Author contributions
GB and FS conceived this review series. GB wrote Part I of the series under the supervision of FS. J-RT, SR, SD, and FS revised this manuscript. All authors read and approved the submitted version of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AA, amino acids; AMP/HDP, antimicrobial/host defense peptide; BCO, bacterial chondronecrosis with osteomyelitis; CE, competitive exclusion; FASN, fatty acid synthase; GI(T), gastrointestinal (tract); HS, heat stress; IFN-γ interferon gamma; IGF-1, insulin-like growth factor 1; IL-1β, interleukin 1 beta; LPS, lipopolysaccharides; MAFbx, muscle atrophy F-box; MLCK, myosin light-chain kinase; NEFA, non-esterified fatty acids; PAMP, pathogen-associated molecular pattern; PI3K, phosphatidylinositol 3-kinase; RNS, reactive nitrogen species; ROS, reactive oxygen species; S6K, p70S6 kinase; IgA, immunoglobulin A; T3, triiodothyronine; T4, thyroxine; TEER, transepithelial electrical resistance; TJ, tight junction; TLR, Toll-like receptor; TNF-α, tumor necrosis factor alpha; ZO, zonula occludens.
References
- Abreu M. T. (2010). Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 10, 131–144. 10.1038/nri2707 [DOI] [PubMed] [Google Scholar]
- Ain Baziz H., Geraert P. A., Padilha J. C. F., Guillaumin S. (1996). Chronic heat exposure enhances fat deposition and modifies muscle and fat partition in broiler carcasses. Poult. Sci. 75, 505–513. 10.3382/ps.0750505 [DOI] [PubMed] [Google Scholar]
- Alhenaky A., Abdelqader A., Abuajamieh M., Al-Fataftah A.-R. (2017). The effect of heat stress on intestinal integrity and Salmonella invasion in broiler birds. J. Therm. Biol. 70, 9–14. 10.1016/j.jtherbio.2017.10.015 [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Van Itallie C. M. (2009). Physiology and function of the tight junction. Cold Spring Harb. Perspect. Biol. 1, a002584. 10.1101/cshperspect.a002584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate T. J., Troche C. (2014). “Influence of probiotics on intestinal structure and barrier functionality of poultry,” in Probiotics in poultry production: concept and applications. Editors Abdelrahman W. H. A., Mohnl M. (5M Publishing Ltd; ), 51–70. [Google Scholar]
- Awad W., Hess C., Hess M. (2017). Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins 9, 60. 10.3390/toxins9020060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad M. A. K., Kikusato M., Maekawa T., Shirakawa H., Toyomizu M. (2010). Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 155, 401–406. 10.1016/j.cbpa.2009.12.011 [DOI] [PubMed] [Google Scholar]
- Baldwin S., Hughes R. J., Van T. T. H., Moore R. J., Stanley D. (2018). At-hatch administration of probiotic to chickens can introduce beneficial changes in gut microbiota. PLoS One 13, e0194825. 10.1371/journal.pone.0194825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett N. W., Rowland K., Schmidt C. J., Lamont S. J., Rothschild M. F., Ashwell C. M., et al. (2019). Effects of acute and chronic heat stress on the performance, egg quality, body temperature, and blood gas parameters of laying hens. Poult. Sci. 98, 6684–6692. 10.3382/ps/pez541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgard L. H., Rhoads R. P. (2013). Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 1, 311–337. 10.1146/annurev-animal-031412-103644 [DOI] [PubMed] [Google Scholar]
- Beckford R. C., Ellestad L. E., Proszkowiec-Weglarz M., Farley L., Brady K., Angel R., et al. (2020). Effects of heat stress on performance, blood chemistry, and hypothalamic and pituitary mRNA expression in broiler chickens. Poult. Sci. 99, 6317–6325. 10.1016/j.psj.2020.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhadj Slimen I., Najar T., Ghram A., Abdrrabba M. (2016). Heat stress effects on livestock: molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 100, 401–412. 10.1111/jpn.12379 [DOI] [PubMed] [Google Scholar]
- Belkaid Y., Hand T. W. (2014). Role of the microbiota in immunity and inflammation. Cell 157, 121–141. 10.1016/j.cell.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigot K., Taouis M., Tesseraud S. (2003). Refeeding and insulin regulate S6K1 activity in chicken skeletal muscles. J. Nutr. 133, 369–373. 10.1093/jn/133.2.369 [DOI] [PubMed] [Google Scholar]
- Bischoff S. C., Barbara G., Buurman W., Ockhuizen T., Schulzke J.-D., Serino M., et al. (2014). Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol. 14, 189. 10.1186/s12876-014-0189-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobek S., Sechman A., Wieczorek E., Wronska-Fortuna D., Koziec K., Niezgoda J. (1997). Reverse 3, 3′, 5′-triiodothyronine (rT 3 ) enhances hyperglycemic and lipemic effects of heat-stress in chickens. Horm. Metab. Res. 29, 252–254. 10.1055/s-2007-979031 [DOI] [PubMed] [Google Scholar]
- Bocci V. (1992). The neglected organ: bacterial flora has a crucial immunostimulatory role. Perspect. Biol. Med. 35, 251–260. 10.1353/pbm.1992.0004 [DOI] [PubMed] [Google Scholar]
- Borda-Molina D., Seifert J., Camarinha-Silva A. (2018). Current perspectives of the chicken gastrointestinal tract and its microbiome. Comput. Struct. Biotechnol. J. 16, 131–139. 10.1016/j.csbj.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges S. A., Da Silva A. V. F., Maiorka A. (2007). Acid-base balance in broilers. World's. Poult. Sci. J. 63, 73–81. 10.1017/S0043933907001286 [DOI] [Google Scholar]
- Boussaid-Om Ezzine S., Everaert N., Métayer-Coustard S., Rideau N., Berri C., Joubert R., et al. (2010). Effects of heat exposure on Akt/S6K1 signaling and expression of genes related to protein and energy metabolism in chicken (Gallus gallus) pectoralis major muscle. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 157, 281–287. 10.1016/j.cbpb.2010.07.001 [DOI] [PubMed] [Google Scholar]
- Brackenbury J, Avery P. (1980). Energy consumption and ventilatory mechanisms in the exercising fowl. Comp. Biochem. Physiol. Part A Physiol. 66, 439–445. 10.1016/0300-9629(80)90189-9 [DOI] [Google Scholar]
- Broom L. J., Kogut M. H. (2018a). Gut immunity: Its development and reasons and opportunities for modulation in monogastric production animals. Anim. Health Res. Rev. 19, 46–52. 10.1017/S1466252318000026 [DOI] [PubMed] [Google Scholar]
- Broom L. J., Kogut M. H. (2018b). Inflammation: Friend or foe for animal production? Poult. Sci. 97, 510–514. 10.3382/ps/pex314 [DOI] [PubMed] [Google Scholar]
- Broom L. J., Kogut M. H. (2018c). The role of the gut microbiome in shaping the immune system of chickens. Vet. Immunol. Immunopathol. 204, 44–51. 10.1016/j.vetimm.2018.10.002 [DOI] [PubMed] [Google Scholar]
- Brugaletta G., De Cesare A., Zampiga M., Laghi L., Oliveri C., Zhu C., et al. (2020). Effects of alternative administration programs of a synbiotic supplement on broiler performance, foot pad dermatitis, caecal microbiota, and blood metabolites. Animals. 10, 522. 10.3390/ani10030522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder K. M., Thompson K. L., Einstein M. E., Applegate T. J., Patterson J. A. (2008). Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella Enteritidis colonization in broilers. Poult. Sci. 87, 1734–1741. 10.3382/ps.2008-00107 [DOI] [PubMed] [Google Scholar]
- Cahaner A., Leenstra F. (1992). Effects of high temperature on growth and efficiency of male and female broilers from lines selected for high weight gain, favorable feed conversion, and high or low fat content. Poult. Sci. 71, 1237–1250. 10.3382/ps.0711237 [DOI] [PubMed] [Google Scholar]
- Chauhan S. S., Rashamol V. P., Bagath M., Sejian V., Dunshea F. R. (2021). Impacts of heat stress on immune responses and oxidative stress in farm animals and nutritional strategies for amelioration. Int. J. Biometeorol. 65, 1231–1244. 10.1007/s00484-021-02083-3 [DOI] [PubMed] [Google Scholar]
- Chen J., Tellez G., Richards J. D., Escobar J. (2015). Identification of potential biomarkers for gut barrier failure in broiler chickens. Front. Vet. Sci. 2, 14. 10.3389/fvets.2015.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Wang J., Peng D., Li G., Chen J., Gu X., et al. (2018). Exposure to heat-stress environment affects the physiology, circulation levels of cytokines, and microbiome in dairy cows. Sci. Rep. 8, 14606. 10.1038/s41598-018-32886-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H.-Y., Ning M.-X., Chen D.-K., Ma W.-T. (2019). Interactions between the gut microbiota and the host innate immune response against pathogens. Front. Immunol. 10, 607. 10.3389/fimmu.2019.00607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichlowski M., Croom J., McBride B. W., Havenstein G. B., Koci M. D. (2007). Metabolic and physiological impact of probiotics or direct-fed-microbials on poultry: a brief review of current knowledge. Int. J. Poult. Sci. 6, 694–704. 10.3923/ijps.2007.694.704 [DOI] [Google Scholar]
- Clavijo V., Flórez M. J. V. (2018). The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult. Sci. 97, 1006–1021. 10.3382/ps/pex359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N. M., Fuller H. L. (1980). Effect of diet composition on feed intake and growth of chicks under heat stress: II. Constant vs. Cycling temperatures. Poult. Sci. 59, 1434–1441. 10.3382/ps.0591434 [DOI] [PubMed] [Google Scholar]
- de Andrade A. N., Rogler J. C., Featherston W. R., Alliston C. W. (1977). Interrelationships between diet and elevated temperatures (cyclic and constant) on egg production and shell quality. Poult. Sci. 56, 1178–1188. 10.3382/ps.0561178 [DOI] [Google Scholar]
- Decuypere E., Buyse J. (1988). “Thyroid hormones, corticosterone, growth hormone and somatomedins in avian species: general effects and possible implications in fattening,” in Leanness in domestic birds. Editors Leclercq B., Whitehead C. C. (Butterworth-Heinemann; ), 295–312. 10.1016/b978-0-408-01036-8.50030-0 [DOI] [Google Scholar]
- Dibner J. J., Richards J. D., Knight C. D. (2008). Microbial imprinting in gut development and health. J. Appl. Poult. Res. 17, 174–188. 10.3382/japr.2007-00100 [DOI] [Google Scholar]
- Dokladny K., Zuhl M. N., Moseley P. L. (2016). Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J. Appl. Physiol. 120, 692–701. 10.1152/japplphysiol.00536.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Lin H., Jiao H. C., Song Z. G., Zhao J. P., Jiang K., et al. (2007). Altered development and protein metabolism in skeletal muscles of broiler chickens (Gallus gallus domesticus) by corticosterone. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 147, 189–195. 10.1016/j.cbpa.2006.12.034 [DOI] [PubMed] [Google Scholar]
- Ducatelle R., Goossens E., De Meyer F., Eeckhaut V., Antonissen G., Haesebrouck F., et al. (2018). Biomarkers for monitoring intestinal health in poultry: present status and future perspectives. Vet. Res. 49, 43. 10.1186/s13567-018-0538-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchêne S., Audouin E., Berri C., Dupont J., Tesseraud S. (2008). Tissue-specific regulation of S6K1 by insulin in chickens divergently selected for growth. Gen. Comp. Endocrinol. 156, 190–198. 10.1016/j.ygcen.2007.12.008 [DOI] [PubMed] [Google Scholar]
- Dupont J., Métayer-Coustard S., Ji B., Ramé C., Gespach C., Voy B., et al. (2012). Characterization of major elements of insulin signaling cascade in chicken adipose tissue: apparent insulin refractoriness. Gen. Comp. Endocrinol. 176, 86–93. 10.1016/j.ygcen.2011.12.030 [DOI] [PubMed] [Google Scholar]
- Dupont J., Rideau N., Simon J. (2015). “Endocrine pancreas,” in Sturkie’s avian physiology. Editor Scanes C. G. (London, UK: Academic Press; ), 613–631. [Google Scholar]
- Epstein Y., Yanovich R. (2019). Heatstroke. N. Engl. J. Med. 380, 2449–2459. 10.1056/NEJMra1810762 [DOI] [PubMed] [Google Scholar]
- Everaert N., Swennen Q., Coustard S. M., Willemsen H., Careghi C., Buyse J., et al. (2010). The effect of the protein level in a pre-starter diet on the post-hatch performance and activation of ribosomal protein S6 kinase in muscle of neonatal broilers. Br. J. Nutr. 103, 206–211. 10.1017/S0007114509991735 [DOI] [PubMed] [Google Scholar]
- Farag M. R., Alagawany M. (2018). Physiological alterations of poultry to the high environmental temperature. J. Therm. Biol. 76, 101–106. 10.1016/j.jtherbio.2018.07.012 [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. (1963). Junctional complexes in various epithelia. J. Cell Biol. 17, 375–412. 10.1083/jcb.17.2.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flees J., Rajaei-Sharifabadi H., Greene E., Beer L., Hargis B. M., Ellestad L., et al. (2017). Effect of Morinda citrifolia (Noni)-Enriched diet on hepatic heat shock protein and lipid metabolism-related genes in heat stressed broiler chickens. Front. Physiol. 8, 919. 10.3389/fphys.2017.00919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- France M. M., Turner J. R. (2017). The mucosal barrier at a glance. J. Cell Sci. 130, 307–314. 10.1242/jcs.193482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Toyomizu M., Kikusato M. (2021). Possible role of corticosterone in proteolysis, glycolytic, and amino acid metabolism in primary cultured avian myotubes incubated at high-temperature conditions. Domest. Anim. Endocrinol. 76, 106608. 10.1016/j.domaniend.2021.106608 [DOI] [PubMed] [Google Scholar]
- Geraert P. A., Padilha J. C. F., Guillaumin S. (1996a). Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: growth performance, body composition and energy retention. Br. J. Nutr. 75, 195–204. 10.1079/bjn19960124 [DOI] [PubMed] [Google Scholar]
- Geraert P. A., Padilha J. C. F., Guillaumin S. (1996b). Metabolic and endocrine changes induced by chronic heate xposure in broiler chickens: biological and endocrinological variables. Br. J. Nutr. 75, 205–216. 10.1079/bjn19960125 [DOI] [PubMed] [Google Scholar]
- Gilani S., Chrystal P. V., Barekatain R. (2021). Current experimental models, assessment and dietary modulations of intestinal permeability in broiler chickens. Anim. Nutr. 7, 801–811. 10.1016/j.aninu.2021.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A. (2021). Heat stress management in poultry. J. Anim. Physiol. Anim. Nutr. 105, 1136–1145. 10.1111/jpn.13496 [DOI] [PubMed] [Google Scholar]
- Gogoi S., Kolluri G., Tyagi J. S., Marappan G., Manickam K., Narayan R., et al. (2021). Impact of heat stress on broilers with varying body weights: Elucidating their interactive role through physiological signatures. J. Therm. Biol. 97, 102840. 10.1016/j.jtherbio.2021.102840 [DOI] [PubMed] [Google Scholar]
- González-Mariscal L., Quirós M., Díaz-Coránguez M. (2011). ZO proteins and redox-dependent processes. Antioxid. Redox Signal. 15, 1235–1253. 10.1089/ars.2011.3913 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rivas P. A., Chauhan S. S., Ha M., Fegan N., Dunshea F. R., Warner R. D., et al. (2020). Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 162, 108025. 10.1016/j.meatsci.2019.108025 [DOI] [PubMed] [Google Scholar]
- Gous R. M., Morris T. R. (2005). Nutritional interventions in alleviating the effects of high temperatures in broiler production. World's. Poult. Sci. J. 61, 463–475. 10.1079/WPS200568 [DOI] [Google Scholar]
- Gribar S. C., Richardson W. M., Sodhi C. P., Hackam D. J. (2008). No longer an innocent bystander: epithelial toll-like receptor signaling in the development of mucosal inflammation. Mol. Med. 14, 645–659. 10.2119/2008-00035.Gribar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales J. R. S. (1973). Effects of exposure to hot environments on the regional distribution of blood flow and on cardiorespiratory function in sheep. Pflugers Arch. 344, 133–148. 10.1007/BF00586547 [DOI] [PubMed] [Google Scholar]
- Hall D. M., Baumgardner K. R., Oberley T. D., Gisolfi C. V. (1999). Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am. J. Physiol. 276, G1195–G1203. 10.1152/ajpgi.1999.276.5.g1195 [DOI] [PubMed] [Google Scholar]
- Hall D. M., Buettner G. R., Oberley L. W., Xu L., Matthes R. D., Gisolfi C. V., et al. (2001). Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. Heart Circ. Physiol. 280, H509–H521. 10.1152/ajpheart.2001.280.2.h509 [DOI] [PubMed] [Google Scholar]
- Harris G., KuoLee R., Chen W. (2006). Role of toll-like receptors in health and diseases of gastrointestinal tract. World J. Gastroenterol. 12, 2149–2160. 10.3748/wjg.v12.i14.2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenstein G. B., Ferket P. R., Scheideler S. E., Larson B. T. (1994). Growth, livability, and feed conversion of 1957 vs 1991 broilers when fed “typical” 1957 and 1991 broiler diets. Poult. Sci. 73, 1785–1794. 10.3382/ps.0731785 [DOI] [PubMed] [Google Scholar]
- Havenstein G. B., Ferket P. R., Qureshi M. A. (2003a). Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 82, 1509–1518. 10.1093/PS/82.10.1509 [DOI] [PubMed] [Google Scholar]
- Havenstein G. B., Ferket P. R., Qureshi M. A. (2003b). Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 82, 1500–1508. 10.1093/PS/82.10.1500 [DOI] [PubMed] [Google Scholar]
- He S., Zhao S., Dai S., Liu D., Bokhari S. G. (2015). Effects of dietary betaine on growth performance, fat deposition and serum lipids in broilers subjected to chronic heat stress. Anim. Sci. J. 86, 897–903. 10.1111/asj.12372 [DOI] [PubMed] [Google Scholar]
- He X., Lu Z., Ma B., Zhang L., Li J., Jiang Y., et al. (2018a). Chronic heat stress damages small intestinal epithelium cells associated with the adenosine 5′-monophosphate-activated protein kinase pathway in broilers. J. Agric. Food Chem. 66, 7301–7309. 10.1021/acs.jafc.8b02145 [DOI] [PubMed] [Google Scholar]
- He X., Lu Z., Ma B., Zhang L., Li J., Jiang Y., et al. (2018b). Effects of chronic heat exposure on growth performance, intestinal epithelial histology, appetite-related hormones and genes expression in broilers. J. Sci. Food Agric. 98, 4471–4478. 10.1002/jsfa.8971 [DOI] [PubMed] [Google Scholar]
- He J., Guo H., Zheng W., Xue Y., Zhao R., Yao W., et al. (2019a). Heat stress affects fecal microbial and metabolic alterations of primiparous sows during late gestation. J. Anim. Sci. Biotechnol. 10, 84. 10.1186/s40104-019-0391-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., He Y., Pan D., Cao J., Sun Y., Zeng X., et al. (2019b). Associations of gut microbiota with heat stress-induced changes of growth, fat deposition, intestinal morphology, and antioxidant capacity in ducks. Front. Microbiol. 10, 903. 10.3389/fmicb.2019.00903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Maltecca C., Tiezzi F. (2021). Potential use of gut microbiota composition as a biomarker of heat stress in monogastric species: A review. Animals. 11, 1833. 10.3390/ani11061833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester P. Y., Enneking S. A., Haley B. K., Cheng H. W., Einstein M. E., Rubin D. A., et al. (2013). The effect of perch availability during pullet rearing and egg laying on musculoskeletal health of caged White Leghorn hens. Poult. Sci. 92, 1972–1980. 10.3382/ps.2013-03008 [DOI] [PubMed] [Google Scholar]
- Hirakawa R., Nurjanah S., Furukawa K., Murai A., Kikusato M., Nochi T., et al. (2020). Heat stress causes immune abnormalities via massive damage to effect proliferation and differentiation of lymphocytes in broiler chickens. Front. Vet. Sci. 7, 46. 10.3389/fvets.2020.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlider M. A. R., Rose S. P. (1989). Rearing temperature and the meat yield of broilers. Br. Poult. Sci. 30, 61–67. 10.1080/00071668908417125 [DOI] [Google Scholar]
- Huston T. M., Carmon J. L. (1962). The influence of high environmental temperature on thyroid size of domestic fowl. Poult. Sci. 41, 175–179. 10.3382/ps.0410175 [DOI] [Google Scholar]
- Iebba V., Totino V., Gagliardi A., Santangelo F., Cacciotti F., Trancassini M., et al. (2016). Eubiosis and dysbiosis: the two sides of the microbiota. New Microbiol. 39, 1–12. [PubMed] [Google Scholar]
- Iizuka M., Konno S. (2011). Wound healing of intestinal epithelial cells. World J. Gastroenterol. 17, 2161–2171. 10.3748/wjg.v17.i17.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan Y. (2012). Leaky gut and the liver: A role for bacterial translocation in nonalcoholic steatohepatitis. World J. Gastroenterol. 18, 2609–2618. 10.3748/wjg.v18.i21.2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUPS Thermal Commission (1987). Glossary of terms for thermal physiology. Pflugers Arch. 57, 567–587. 10.1007/bf00586542 [DOI] [PubMed] [Google Scholar]
- Iyer S. S., Blumberg R. S. (2018). “Influence of the gut microbiome on immune development during early life,” in Physiology of the gastrointestinal tract. Editor Said H. M. (London, UK: Academic Press; ), 767–774. 10.1016/B978-0-12-809954-4.00034-7 [DOI] [Google Scholar]
- Jukes M. M. (1971). “Transport of blood gases,” in Physiology and biochemistry of the domestic fowl. Editors Bell D. J., Freeman B. M. (New York, NY: Academic Press; ), Vol. 1. [Google Scholar]
- Kaufman J. D., Seidler Y., Bailey H. R., Whitacre L., Bargo F., Lüersen K., et al. (2021). A postbiotic from Aspergillus oryzae attenuates the impact of heat stress in ectothermic and endothermic organisms. Sci. Rep. 11, 6407. 10.1038/s41598-021-85707-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keestra A. M., de Zoete M. R., Bouwman L. I., Vaezirad M. M., van Putten J. P. M. (2013). Unique features of chicken Toll-like receptors. Dev. Comp. Immunol. 41, 316–323. 10.1016/j.dci.2013.04.009 [DOI] [PubMed] [Google Scholar]
- Kers J. G., Velkers F. C., Fischer E. A. J., Hermes G. D. A., Stegeman J. A., Smidt H., et al. (2018). Host and environmental factors affecting the intestinal microbiota in chickens. Front. Microbiol. 9, 235. 10.3389/fmicb.2018.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusato M., Toyomizu M. (2013). Crucial role of membrane potential in heat stress-induced overproduction of reactive oxygen species in avian skeletal muscle mitochondria. PLoS One 8, e64412. 10.1371/journal.pone.0064412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiber M., Dougherty J. E. (1934). The influence of environmental temperature on the utilization of food energy in baby chicks. J. Gen. Physiol. 17, 701–726. 10.1085/jgp.17.5.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F., Thom U., Albrecht E., Weikard R., Nolte W., Kuhla B., et al. (2019). Heat stress directly impairs gut integrity and recruits distinct immune cell populations into the bovine intestine. Proc. Natl. Acad. Sci. U. S. A. 116, 10333–10338. 10.1073/pnas.1820130116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M. H., Arsenault R. J. (2016). Editorial: Gut health: The new paradigm in food animal production. Front. Vet. Sci. 3, 71. 10.3389/fvets.2016.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M., Yin X., Yuan J., Broom L. (2017). Gut health in poultry. CAB. Rev. 12 (031), 1–7. 10.1079/PAVSNNR201712031 [DOI] [Google Scholar]
- Kogut M. H. (2019). The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed Sci. Technol. 250, 32–40. 10.1016/j.anifeedsci.2018.10.008 [DOI] [Google Scholar]
- Koutsos E. A., Arias V. J. (2006). Intestinal ecology: interactions among the gastrointestinal tract, nutrition, and the microflora. J. Appl. Poult. Res. 15, 161–173. 10.1093/japr/15.1.161 [DOI] [Google Scholar]
- Kubena L. F., Lott B. D., Deaton J. W., Reece F. N., May J. D. (1972). Body composition of chicks as influenced by environmental temperature and selected dietary factors. Poult. Sci. 51, 517–522. 10.3382/ps.0510517 [DOI] [Google Scholar]
- Lambert G. P., Gisolfi C. V., Berg D. J., Moseley P. L., Oberley L. W., Kregel K. C., et al. (2002). Selected contribution: hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J. Appl. Physiol. 92, 1750–1761. 10.1152/japplphysiol.00787.2001 [DOI] [PubMed] [Google Scholar]
- Lambert G. P. (2009). Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J. Anim. Sci. 87, E101–E108. 10.2527/jas.2008-1339 [DOI] [PubMed] [Google Scholar]
- Lan P. T. N., Sakamoto M., Benno Y. (2004). Effects of two probiotic Lactobacillus strains on jejunal and cecal microbiota of broiler chicken under acute heat stress condition as revealed by molecular analysis of 16S rRNA genes. Microbiol. Immunol. 48, 917–929. 10.1111/j.1348-0421.2004.tb03620.x [DOI] [PubMed] [Google Scholar]
- Le Sciellour M., Zemb O., Hochu I., Riquet J., Gilbert H., Giorgi M., et al. (2019). Effect of chronic and acute heat challenges on fecal microbiota composition, production, and thermoregulation traits in growing pigs1, 2. J. Anim. Sci. 97, 3845–3858. 10.1093/jas/skz222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian P., Braber S., Garssen J., Wichers H. J., Folkerts G., Fink-Gremmels J., et al. (2020). Beyond heat stress: Intestinal integrity disruption and mechanism-based intervention strategies. Nutrients 12, 734. 10.3390/nu12030734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Tabler G. T., Dridi S. (2020). Sprinkler technology improves broiler production sustainability: From stress alleviation to water usage conservation: a mini review. Front. Vet. Sci. 7, 544814. 10.3389/fvets.2020.544814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Decuypere E., Buyse J. (2006a). Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 144, 11–17. 10.1016/j.cbpa.2006.01.032 [DOI] [PubMed] [Google Scholar]
- Lin H., Jiao H. C., Buyse J., Decuypere E. (2006b). Strategies for preventing heat stress in poultry. World's. Poult. Sci. J. 62, 71–86. 10.1079/WPS200585 [DOI] [Google Scholar]
- Liu G., Zhu H., Ma T., Yan Z., Zhang Y., Geng Y., et al. (2020). Effect of chronic cyclic heat stress on the intestinal morphology, oxidative status and cecal bacterial communities in broilers. J. Therm. Biol. 91, 102619. 10.1016/j.jtherbio.2020.102619 [DOI] [PubMed] [Google Scholar]
- Liu W.-C., Pan Z.-Y., Zhao Y., Guo Y., Qiu S.-J., Balasubramanian B., et al. (2022). Effects of heat stress on production performance, redox status, intestinal morphology and barrier-related gene expression, cecal microbiome, and metabolome in indigenous broiler chickens. Front. Physiol. 13, 890520. 10.3389/fphys.2022.890520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q., Wen J., Zhang H. (2007). Effect of chronic heat exposure on fat deposition and meat quality in two genetic types of chicken. Poult. Sci. 86, 1059–1064. 10.1093/ps/86.6.1059 [DOI] [PubMed] [Google Scholar]
- Lu Z., He X., Ma B., Zhang L., Li J., Jiang Y., et al. (2018). Serum metabolomics study of nutrient metabolic variations in chronic heat-stressed broilers. Br. J. Nutr. 119, 771–781. 10.1017/S0007114518000247 [DOI] [PubMed] [Google Scholar]
- Lu Z., He X. F., Ma B. B., Zhang L., Li J. L., Jiang Y., et al. (2019). Increased fat synthesis and limited apolipoprotein B cause lipid accumulation in the liver of broiler chickens exposed to chronic heat stress. Poult. Sci. 98, 3695–3704. 10.3382/ps/pez056 [DOI] [PubMed] [Google Scholar]
- Ma T. Y., Nighot P., Al-Sadi R. (2018). “Tight junctions and the intestinal barrier,” in Physiology of the gastrointestinal tract. Editor Said H. M. (London, UK: Academic Press; ), 587–639. 10.1016/B978-0-12-809954-4.00025-6 [DOI] [Google Scholar]
- Ma B., Zhang L., Li J., Xing T., Jiang Y., Gao F., et al. (2021). Heat stress alters muscle protein and amino acid metabolism and accelerates liver gluconeogenesis for energy supply in broilers. Poult. Sci. 100, 215–223. 10.1016/j.psj.2020.09.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack L. A., Felver-Gant J. N., Dennis R. L., Cheng H. W. (2013). Genetic variations alter production and behavioral responses following heat stress in 2 strains of laying hens. Poult. Sci. 92, 285–294. 10.3382/ps.2012-02589 [DOI] [PubMed] [Google Scholar]
- Madsen K., Park H. (2017). “Immunologic response in the host,” in The microbiota in gastrointestinal pathophysiology. Editors Floch M. H., Ringel Y., Walker W. A. (London, UK: Academic Press; ), 233–241. 10.1016/B978-0-12-804024-9.00026-4 [DOI] [Google Scholar]
- Marchesi J. R., Ravel J. (2015). The vocabulary of microbiome research: a proposal. Microbiome 3, 31. 10.1186/s40168-015-0094-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder J., Arad Z. (1989). Panting and acid-base regulation in heat stressed birds. Comp. Biochem. Physiol. A Comp. Physiol. 94, 395–400. 10.1016/0300-9629(89)90112-6 [DOI] [PubMed] [Google Scholar]
- McNabb F. M. A., Darras V. M. (2015). “Thyroids,” in Sturkie’s avian physiology. Editor Scanes C. G. (London, UK: Academic Press; ), 535–547. [Google Scholar]
- Mowat A. M. (2018). To respond or not to respond — a personal perspective of intestinal tolerance. Nat. Rev. Immunol. 18, 405–415. 10.1038/s41577-018-0002-x [DOI] [PubMed] [Google Scholar]
- Mujahid A., Sato K., Akiba Y., Toyomizu M. (2006). Acute heat stress stimulates mitochondrial superoxide production in broiler skeletal muscle, possibly via downregulation of uncoupling protein content. Poult. Sci. 85, 1259–1265. 10.1093/ps/85.7.1259 [DOI] [PubMed] [Google Scholar]
- Naga Raja Kumari K., Narendra Nath D. (2018). Ameliorative measures to counter heat stress in poultry. World's. Poult. Sci. J. 74, 117–130. 10.1017/S0043933917001003 [DOI] [Google Scholar]
- Nanto-Hara F., Kikusato M., Ohwada S., Toyomizu M. (2020). Heat stress directly affects intestinal integrity in broiler chickens. J. Poult. Sci. 57, 284–290. 10.2141/jpsa.0190004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz A. H., Amoah K., Leng Q. Y., Zheng J. H., Zhang W. L., Zhang L., et al. (2021). Poultry response to heat stress: Its physiological, metabolic, and genetic implications on meat production and quality including strategies to improve broiler production in a warming world. Front. Vet. Sci. 8, 699081. 10.3389/fvets.2021.699081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichelmann M., Baranyiová E., Goll; B., Tzschentke R. (1986). Influence of feather cover on heat balance in laying hens (Gallus domesticus). J. Therm. Biol. 11, 121–126. 10.1016/0306-4565(86)90032-X [DOI] [Google Scholar]
- Nienaber J. A., Hahn G. L. (2007). Livestock production system management responses to thermal challenges. Int. J. Biometeorol. 52, 149–157. 10.1007/s00484-007-0103-x [DOI] [PubMed] [Google Scholar]
- Nurmi E., Rantala M. (1973). New aspects of Salmonella infection in broiler production. Nature 241, 210–211. 10.1038/241210a0 [DOI] [PubMed] [Google Scholar]
- Odom T. W., Harrison P. C., Bottje W. G. (1986). Effects of thermal-induced respiratory alkalosis on blood ionized calcium levels in the domestic hen. Poult. Sci. 65, 570–573. 10.3382/ps.0650570 [DOI] [PubMed] [Google Scholar]
- Oviedo-Rondón E. O. (2019). Holistic view of intestinal health in poultry. Anim. Feed Sci. Technol. 250, 1–8. 10.1016/j.anifeedsci.2019.01.009 [DOI] [Google Scholar]
- Pan D., Yu Z. (2014). Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 5, 108–119. 10.4161/gmic.26945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S. C., Mani V., Boddicker R. L., Johnson J. S., Weber T. E., Ross J. W., et al. (2012). Heat stress reduces barrier function and alters intestinal metabolism in growing pigs. J. Anim. Sci. 90, 257–259. 10.2527/jas.52339 [DOI] [PubMed] [Google Scholar]
- Pearce S. C., Gabler N. K., Ross J. W., Escobar J., Patience J. F., Rhoads R. P., et al. (2013a). The effects of heat stress and plane of nutrition on metabolism in growing pigs. J. Anim. Sci. 91, 2108–2118. 10.2527/jas.2012-5738 [DOI] [PubMed] [Google Scholar]
- Pearce S. C., Mani V., Boddicker R. L., Johnson J. S., Weber T. E., Ross J. W., et al. (2013b). Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS One 8, e70215. 10.1371/journal.pone.0070215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaid M. M., Al-Garadi M. A. (2021). Protein and amino acid metabolism in poultry during and after heat stress: a review. Animals. 11, 1167. 10.3390/ani11041167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinteiro-Filho W. M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M. L., Sakai M., Sá L. R. M., et al. (2010). Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 89, 1905–1914. 10.3382/ps.2010-00812 [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W. M., Rodrigues M. V., Ribeiro A., Ferraz-de-Paula V., Pinheiro M. L., Sá L. R. M., et al. (2012). Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: role of acute hypothalamic-pituitary-adrenal axis activation. J. Anim. Sci. 90, 1986–1994. 10.2527/jas.2011-3949 [DOI] [PubMed] [Google Scholar]
- Rajaei-Sharifabadi H., Greene E., Piekarski A., Falcon D., Ellestad L., Donoghue A., et al. (2017). Surface wetting strategy prevents acute heat exposure–induced alterations of hypothalamic stress– and metabolic-related genes in broiler chickens. J. Anim. Sci. 95, 1132–1143. 10.2527/jas2016.1290 [DOI] [PubMed] [Google Scholar]
- Rao R. (2008). Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front. Biosci. 13, 7210–7226. 10.2741/3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudeau D., Collin A., Yahav S., de Basilio V., Gourdine J. L., Collier R. J. (2012). Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 6, 707–728. 10.1017/S1751731111002448 [DOI] [PubMed] [Google Scholar]
- Rhoads M. L., Rhoads R. P., VanBaale M. J., Collier R. J., Sanders S. R., Weber W. J., et al. (2009). Effects of heat stress and plane of nutrition on lactating holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 92, 1986–1997. 10.3168/jds.2008-1641 [DOI] [PubMed] [Google Scholar]
- Rhoads R. P., Baumgard L. H., Suagee J. K. (2013). 2011 and 2012 early careers achievement awards: metabolic priorities during heat stress with an emphasis on skeletal muscle. J. Anim. Sci. 91, 2492–2503. 10.2527/jas.2012-6120 [DOI] [PubMed] [Google Scholar]
- Richards S. A. (1970). Physiology of thermal panting in birds. Ann. Biol. Anim. Biochim. Biophys. 10, 151–168. 10.1051/rnd:19700614 [DOI] [Google Scholar]
- Rostagno M. H. (2020). Effects of heat stress on the gut health of poultry. J. Anim. Sci. 98, skaa090. 10.1093/jas/skaa090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round J. L., Mazmanian S. K. (2009). The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9, 313–323. 10.1038/nri2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff J., Barros T. L., Tellez G., Blankenship J., Lester H., Graham B. D., et al. (2020). Research Note: evaluation of a heat stress model to induce gastrointestinal leakage in broiler chickens. Poult. Sci. 99, 1687–1692. 10.1016/j.psj.2019.10.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed M., Abbas G., Alagawany M., Kamboh A. A., Abd El-Hack M. E., Khafaga A. F., et al. (2019). Heat stress management in poultry farms: a comprehensive overview. J. Therm. Biol. 84, 414–425. 10.1016/j.jtherbio.2019.07.025 [DOI] [PubMed] [Google Scholar]
- Schneitz C. (2005). Competitive exclusion in poultry––30 years of research. Food control. 16, 657–667. 10.1016/j.foodcont.2004.06.002 [DOI] [Google Scholar]
- Shen L., Weber C. R., Raleigh D. R., Yu D., Turner J. R. (2011). Tight junction pore and leak pathways: A dynamic duo. Annu. Rev. Physiol. 73, 283–309. 10.1146/annurev-physiol-012110-142150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D., Bai L., Qu Q., Zhou S., Yang M., Guo S., et al. (2019). Impact of gut microbiota structure in heat-stressed broilers. Poult. Sci. 98, 2405–2413. 10.3382/ps/pez026 [DOI] [PubMed] [Google Scholar]
- Shimazu R., Akashi S., Ogata H., Nagai Y., Fukudome K., Miyake K., et al. (1999). MD-2, a molecule that confers lipopolysaccharide responsiveness on toll-like receptor 4. J. Exp. Med. 189, 1777–1782. 10.1084/jem.189.11.1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shini S., Huff G. R., Shini A., Kaiser P. (2010). Understanding stress-induced immunosuppression: exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poult. Sci. 89, 841–851. 10.3382/ps.2009-00483 [DOI] [PubMed] [Google Scholar]
- Smith A. L., Powers C., Beal R. K. (2014). “The avian enteric immune system in health and disease,” in Avian immunology. Editors Shat K. A., Kaspers B., Kaiser P. (Academic Press; ), 227–250. [Google Scholar]
- Snipe R. M. J., Khoo A., Kitic C. M., Gibson P. R., Costa R. J. S. (2018). The impact of exertional-heat stress on gastrointestinal integrity, gastrointestinal symptoms, systemic endotoxin and cytokine profile. Eur. J. Appl. Physiol. 118, 389–400. 10.1007/s00421-017-3781-z [DOI] [PubMed] [Google Scholar]
- Sohail M. U., Ijaz A., Yousaf M. S., Ashraf K., Zaneb H., Aleem M., et al. (2010). Alleviation of cyclic heat stress in broilers by dietary supplementation of mannan-oligosaccharide and lactobacillus-based probiotic: dynamics of cortisol, thyroid hormones, cholesterol, C-reactive protein, and humoral immunity. Poult. Sci. 89, 1934–1938. 10.3382/ps.2010-00751 [DOI] [PubMed] [Google Scholar]
- Soliman E. S., Taha E., Infante K. D., Laboy K., Sobieh M. A., Reddy P. G., et al. (2009). Stressors influence on Salmonella enterica serovar Enteritidis colonization in broilers. Am. J. Anim. Vet. Sci. 4, 42–48. 10.3844/ajavsp.2009.42.48 [DOI] [Google Scholar]
- Song D. J., King A. J. (2015). Effects of heat stress on broiler meat quality. World's. Poult. Sci. J. 71, 701–709. 10.1017/S0043933915002421 [DOI] [Google Scholar]
- Song J., Xiao K., Ke Y. L., Jiao L. F., Hu C. H., Diao Q. Y., et al. (2014). Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 93, 581–588. 10.3382/ps.2013-03455 [DOI] [PubMed] [Google Scholar]
- St-Pierre N. R., Cobanov B., Schnitkey G. (2003). Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86, E52–E77. 10.3168/jds.S0022-0302(03)74040-5 [DOI] [Google Scholar]
- Sugiharto S., Yudiarti T., Isroli I., Widiastuti E., Kusumanti E. (2017). Dietary supplementation of probiotics in poultry exposed to heat stress – a review. Ann. Anim. Sci. 17, 591–604. 10.1515/aoas-2016-0062 [DOI] [Google Scholar]
- Suzuki K., Harasawa R., Yoshitake Y., Mitsuoka T. (1983). Effects of crowding and heat stress on intestinal flora, body weight gain, and feed efficiency of growing rats and chicks. Nihon Juigaku Zasshi. 45, 331–338. 10.1292/jvms1939.45.331 [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kodama Y., Mitsuoka T. (1989). Stress and intestinal flora. Bifidobact. Microflora 8, 23–38. 10.12938/bifidus1982.8.1_23 [DOI] [Google Scholar]
- Tabler T. W., Greene E. S., Orlowski S. K., Hiltz J. Z., Anthony N. B., Dridi S., et al. (2020). Intestinal barrier integrity in heat-stressed modern broilers and their ancestor wild jungle fowl. Front. Vet. Sci. 7, 249. 10.3389/fvets.2020.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallentire C. W., Leinonen I., Kyriazakis I. (2018). Artificial selection for improved energy efficiency is reaching its limits in broiler chickens. Sci. Rep. 8, 1168. 10.1038/s41598-018-19231-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G.-Y., Yang L., Fu Y.-Q., Feng J.-H., Zhang M.-H. (2010). Effects of different acute high ambient temperatures on function of hepatic mitochondrial respiration, antioxidative enzymes, and oxidative injury in broiler chickens. Poult. Sci. 89, 115–122. 10.3382/ps.2009-00318 [DOI] [PubMed] [Google Scholar]
- Tang S., Yu J., Zhang M., Bao E. (2013). Effects of different heat stress periods on various blood and meat quality parameters in young arbor Acer broiler chickens. Can. J. Anim. Sci. 93, 453–460. 10.4141/CJAS2013-041 [DOI] [Google Scholar]
- Teeter R. G., Smith M. O., Owens F. N., Arp S. C., Sangiah S., Breazile J. E., et al. (1985). Chronic heat stress and respiratory alkalosis: occurrence and treatment in broiler chicks. Poult. Sci. 64, 1060–1064. 10.3382/ps.0641060 [DOI] [PubMed] [Google Scholar]
- Temim S., Chagneau A.-M., Peresson R., Tesseraud S. (2000). Chronic heat exposure alters protein turnover of three different skeletal muscles in finishing broiler chickens fed 20 or 25% protein diets. J. Nutr. 130, 813–819. 10.1093/jn/130.4.813 [DOI] [PubMed] [Google Scholar]
- Teyssier J. R., Brugaletta G., Dridi S., Sirri F., Rochell S. J. (2022a). A review of heat stress in broiler chickens. Part II: Insights into protein and energy utilization and feeding. Front. Physiol. 13, 943612. 10.3389/fphys.2022.943612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssier J. R., Preynat A., Cozannet P., Briens M., Mauromoustakos A., Greene E. S., et al. (2022b). Constant and cyclic chronic heat stress models differentially influence growth performance, carcass traits and meat quality of broilers. Poult. Sci. 101, 101963. 10.1016/j.psj.2022.101963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiouris V., Georgopoulou I., Batzios C., Pappaioannou N., Ducatelle R., Fortomaris P., et al. (2018). Heat stress as a predisposing factor for necrotic enteritis in broiler chicks. Avian Pathol. 47, 616–624. 10.1080/03079457.2018.1524574 [DOI] [PubMed] [Google Scholar]
- Turner J. R., Buschmann M. M., Romero-Calvo I., Sailer A., Shen L. (2014). The role of molecular remodeling in differential regulation of tight junction permeability. Semin. Cell Dev. Biol. 36, 204–212. 10.1016/j.semcdb.2014.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulluwishewa D., Anderson R. C., McNabb W. C., Moughan P. J., Wells J. M., Roy N. C., et al. (2011). Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 141, 769–776. 10.3945/jn.110.135657 [DOI] [PubMed] [Google Scholar]
- Vandana G. D., Sejian V., Lees A. M., Pragna P., Silpa M. V., Maloney S. K., et al. (2021). Heat stress and poultry production: Impact and amelioration. Int. J. Biometeorol. 65, 163–179. 10.1007/s00484-020-02023-7 [DOI] [PubMed] [Google Scholar]
- Vaure C., Liu Y. (2014). A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 5, 316. 10.3389/fimmu.2014.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria Sanz Fernandez M., Johnson J. S., Abuajamieh M., Stoakes S. K., Seibert J. T., Cox L., et al. (2015). Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol. Rep. 3, e12315. 10.14814/phy2.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. A. (2017). “Dysbiosis,” in The microbiota in gastrointestinal pathophysiology. Editors Floch M. H., Ringel Y., Walker W. A. (London, UK: Academic Press; ), 227–232. [Google Scholar]
- Wang R. R., Pan X. J., Peng Z. Q. (2009). Effects of heat exposure on muscle oxidation and protein functionalities of pectoralis majors in broilers. Poult. Sci. 88, 1078–1084. 10.3382/ps.2008-00094 [DOI] [PubMed] [Google Scholar]
- Wang L., Llorente C., Hartmann P., Yang A.-M., Chen P., Schnabl B., et al. (2015). Methods to determine intestinal permeability and bacterial translocation during liver disease. J. Immunol. Methods 421, 44–53. 10.1016/j.jim.2014.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. H., Liang R. R., Lin H., Zhu L. X., Zhang Y. M., Mao Y. W., et al. (2017). Effect of acute heat stress and slaughter processing on poultry meat quality and postmortem carbohydrate metabolism. Poult. Sci. 96, 738–746. 10.3382/ps/pew329 [DOI] [PubMed] [Google Scholar]
- Wang M., Lin X., Jiao H., Uyanga V., Zhao J., Wang X., et al. (2020). Mild heat stress changes the microbiota diversity in the respiratory tract and the cecum of layer-type pullets. Poult. Sci. 99, 7015–7026. 10.1016/j.psj.2020.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. C., Yan F. F., Hu J. Y., Amen O. A., Cheng H. W. (2018a). Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J. Anim. Sci. 96, 1654–1666. 10.1093/jas/sky092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. J., Feng J. H., Zhang M. H., Li X. M., Ma D. D., Chang S. S., et al. (2018b). Effects of high ambient temperature on the community structure and composition of ileal microbiome of broilers. Poult. Sci. 97, 2153–2158. 10.3382/ps/pey032 [DOI] [PubMed] [Google Scholar]
- Wassenaar T. M., Zimmermann K. (2018). Lipopolysaccharides in food, food supplements, and probiotics: should we be worried? Eur. J. Microbiol. Immunol. 8, 63–69. 10.1556/1886.2018.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasti S., Sah N., Mishra B. (2020). Impact of heat stress on poultry health and performances, and potential mitigation strategies. Animals. 10, 1266. 10.3390/ani10081266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wideman R. F. (2016). Bacterial chondronecrosis with osteomyelitis and lameness in broilers: A review. Poult. Sci. 95, 325–344. 10.3382/ps/pev320 [DOI] [PubMed] [Google Scholar]
- Xing S., Wang X., Diao H., Zhang M., Zhou Y., Feng J., et al. (2019). Changes in the cecal microbiota of laying hens during heat stress is mainly associated with reduced feed intake. Poult. Sci. 98, 5257–5264. 10.3382/ps/pez440 [DOI] [PubMed] [Google Scholar]
- Xiong Y., Yi H., Wu Q., Jiang Z., Wang L. (2020). Effects of acute heat stress on intestinal microbiota in grow-finishing pigs, and associations with feed intake and serum profile. J. Appl. Microbiol. 128, 840–852. 10.1111/jam.14504 [DOI] [PubMed] [Google Scholar]
- Xu Y., Lai X., Li Z., Zhang X., Luo Q. (2018). Effect of chronic heat stress on some physiological and immunological parameters in different breed of broilers. Poult. Sci. 97, 4073–4082. 10.3382/ps/pey256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahav S., Straschnow A., Luger D., Shinder D., Tanny J., Cohen S. (2004). Ventilation, sensible heat loss, broiler energy, and water balance under harsh environmental conditions. Poult. Sci. 83, 253–258. 10.1093/ps/83.2.253 [DOI] [PubMed] [Google Scholar]
- Yahav S. (2009). Alleviating heat stress in domestic fowl: different strategies. World's. Poult. Sci. J. 65, 719–732. 10.1017/S004393390900049X [DOI] [Google Scholar]
- Yegani M., Korver D. R. (2008). Factors affecting intestinal health in poultry. Poult. Sci. 87, 2052–2063. 10.3382/ps.2008-00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoman C. J., Chia N., Jeraldo P., Sipos M., Goldenfeld N. D., White B. A., et al. (2012). The microbiome of the chicken gastrointestinal tract. Anim. Health Res. Rev. 13, 89–99. 10.1017/S1466252312000138 [DOI] [PubMed] [Google Scholar]
- Yuan L., Lin H., Jiang K. J., Jiao H. C., Song Z. G. (2008). Corticosterone administration and high-energy feed results in enhanced fat accumulation and insulin resistance in broiler chickens. Br. Poult. Sci. 49, 487–495. 10.1080/00071660802251731 [DOI] [PubMed] [Google Scholar]
- Yunianto V. D., Hayashit K., Kaiwda S., Ohtsuka A., Tomita Y. (1997). Effect of environmental temperature on muscle protein turnover and heat production in tube-fed broiler chickens. Br. J. Nutr. 77, 897–909. 10.1079/bjn19970088 [DOI] [PubMed] [Google Scholar]
- Yunis R., Cahaner A. (1999). The effects of the naked neck (Na) and frizzle (F) genes on growth and meat yield of broilers and their interactions with ambient temperatures and potential growth rate. Poult. Sci. 78, 1347–1352. 10.1093/ps/78.10.1347 [DOI] [PubMed] [Google Scholar]
- Zaboli G., Huang X., Feng X., Ahn D. U. (2019). How can heat stress affect chicken meat quality? – a review. Poult. Sci. 98, 1551–1556. 10.3382/ps/pey399 [DOI] [PubMed] [Google Scholar]
- Zampiga M., Laghi L., Zhu C., Cartoni Mancinelli A., Mattioli S., Sirri F., et al. (2021). Breast muscle and plasma metabolomics profile of broiler chickens exposed to chronic heat stress conditions. Animal 15, 100275. 10.1016/J.ANIMAL.2021.100275 [DOI] [PubMed] [Google Scholar]
- Zheng D., Liwinski T., Elinav E. (2020). Interaction between microbiota and immunity in health and disease. Cell Res. 30, 492–506. 10.1038/s41422-020-0332-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Liao R., Wu N., Zhu G., Yang C. (2019). Heat stress mediates changes in fecal microbiome and functional pathways of laying hens. Appl. Microbiol. Biotechnol. 103, 461–472. 10.1007/s00253-018-9465-8 [DOI] [PubMed] [Google Scholar]
- Zuidhof M. J., Schneider B. L., Carney V. L., Korver D. R., Robinson F. E. (2014). Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 93, 2970–2982. 10.3382/ps.2014-04291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J., Xu M., Abdullahi Y. A. uwal., Ma L., Zhang Z., Feng D., et al. (2015). Constant heat stress reduces skeletal muscle protein deposition in broilers. J. Sci. Food Agric. 95, 429–436. 10.1002/JSFA.6749 [DOI] [PubMed] [Google Scholar]