Abstract
Systemic toxicity due to chemotherapy contributes to poor prognosis in patients receiving chemotherapy. The present study, therefore, explores the role of Ellagic acid, a phytochemical, in modulating cisplatin (CP) toxicity in dimethylhydrazine-induced colorectal cancer. Colons excised from DMH administered animals showed abnormal crypts and bulges over the mucosal surface. SEM revealed significant alterations and dysplastic lesions in DMH administered mice. Animals receiving combined treatment showed improvement in colonic epithelium with lesser irregularities. DMH and CP administration disturbed the membrane dynamics and integrity as observed with the fluorescent probes DPH and pyrene. However, EA co-supplementation with CP proved to be beneficial in normalizing the altered membrane. Ellagic acid co-supplementation along with CP; therefore, showed great promise and helped restore the membrane alterations in the colon caused due to CP-induced toxicity and DMH insult. These observations could pave way towards developing a combination therapy targeting colon carcinogenesis in future.
Keywords: Ellagic acid, Cisplatin, DMH, Colorectal cancer, Membrane
Highlights
-
•
Colorectal tumor initiation as evident by the presence of ACFs (pre-neoplastic lesions).
-
•
DMH and Cisplatin altered the membrane dynamics.
-
•
Ellagic acid helped restore the membrane structure as evidenced through SEM.
-
•
Membrane dynamics were also improved, as evidenced through lipid packing and lateral diffusion.
-
•
Structural alterations monitored through FT-IR were also improved upon Ellagic acid co-supplementation.
1. Introduction
Colorectal cancer (CRC) accounts for the third most commonly diagnosed cancer in men and second in women worldwide [1]. The incidence rates for colon and rectal cancers may increase by 90.0% and 124.2%, respectively, for patients between the ages of 20–34 years by 2030 [2]. When deciding on the treatment regimen, chemotherapy is one of the preferred choices. In this regard, the fundamental reason patients discontinue chemotherapy is when treatment-induced toxicity outweighs the benefits derived.
The present communication is in continuation with previous reports from our laboratory, wherein chemotherapy induced toxicity to various organs, viz; Liver [3], Testis, and Kidney [4], and its amelioration with ellagic acid were reported. To further highlight the importance of the membrane system and its modulation by chemotherapy, the present study undertook to evaluate the membrane alterations induced by cancer and chemotherapy and its modulation by ellagic acid.
Different cancer-causing antigens, metabolites, etc, have been reported to alter the histoarchitecture of normal cells to their malignant phenotypes [5]. Additionally, membrane lipids regulate a variety of cellular functions [6]. Numerous alterations occur in the lipid composition of cell membranes, altering the membrane fluidity, during their transformation into malignant phenotypes [7,8].
The plasma membrane constitutes the first cellular barrier to antineoplastic agents, and its importance in the diffusion of drugs has been well established [9]. Cisplatin; in particular, reduces the activity of certain ion channels, transport protein [10], and various plasma membrane enzymes [11].
Secondary metabolites from plants are important sources of molecules with great potential for chemotherapy [12,13]. These nutraceuticals have been accounted to increase the anticancer activities as well as reduce the severe side effects of antitumor drugs [14,15] Among them, dietary polyphenols have gained remarkable attention worldwide due to their incredible antioxidant properties, and their enormous abundance in our diet [16,17].
The present study, therefore, explores the role of Ellagic acid in modulating the topography and lipid dynamics of the colonic membranes after CP exposure in DMH-induced colorectal cancer [18].
2. Materials and methods
2.1. Animal procurement and experimental conditions
Male Laca mice (25–30g) were procured from the Central Animal House, Panjab University, Chandigarh (India). All experimental procedures were done by the ethical guidelines (PU/IAEC/S/16/30), approved by the Institutional Animal Ethics Committee (IAEC), and conducted according to the Indian National Science Academy guidelines.
Group I: Control (No special treatment), Group II: DMH (30 mg/kg b.w s.c in normal saline; once a week from 1st to 20th week), Group III: DMH (same as group II) + EA (10 mg/kg b.w p.o daily in corn oil from 21st to 26th week) [4,19], Group IV: DMH + CP (5 mg/kg b.w i.p), Group V: DMH + CP + EA.
2.2. Aberrant crypt foci (ACFs)
At the 12th and 20th weeks of the treatment period, formaldehyde-fixed colons were stained and visualized using a light microscope [20].
2.3. Scanning electron microscopy
Glutaraldehyde and paraformaldehyde-fixed specimens were transferred to professional processing and imaging facility for SEM.
2.4. Lipid packing analysis
The fluorescent probe 1, 6-diphenyl-1, 3, 5- hexatriene (DPH) was used to investigate the fluidity studies (rotational diffusion) [21].
2.5. Lateral diffusion
Pyrene fluorescence excimer (dimer) formation was used to study the lateral diffusion in the membrane [22].
2.6. Fourier-transform infrared spectroscopy (FTIR)
Colon tissues were flash-frozen and processed for FT-IR. Spectral records of 64 interferograms at a spectral resolution of 2 cm−1 and a sampling interval of 1 cm−1 were averaged for each spectrum. The whole data was then corrected with background energy reading from a blank KBr pellet. sample.
2.7. Statistical analysis
All values were expressed as mean ± S.D. Statistical significance of values were determined using paired sample T test and analysis of variance (ANOVA), followed by least significant differnence (LSD) post hoc test. Result were considered significant at p≤0.05.
3. Results
3.1. Aberrant crypt foci
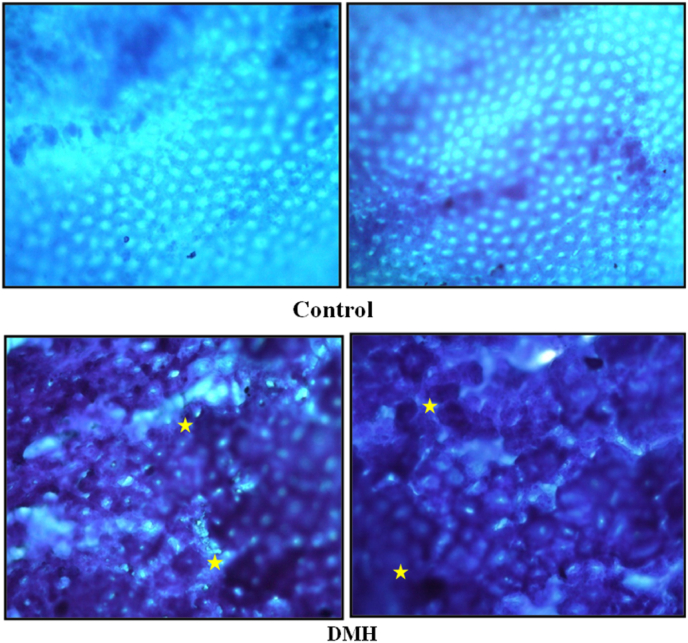
Normal crypts were evident in the control group whereas DMH administered animals showed darkly stained sections as well as abnormal crypts and bulges over the mucosal surface (Fig. 1).
Fig. 1.
Aberrant crypt foci observed after 20 weeks of DMH treatment (Irregular luminal opening (Stars), thicker epithelial lining) [100x].
3.2. Topographical studies
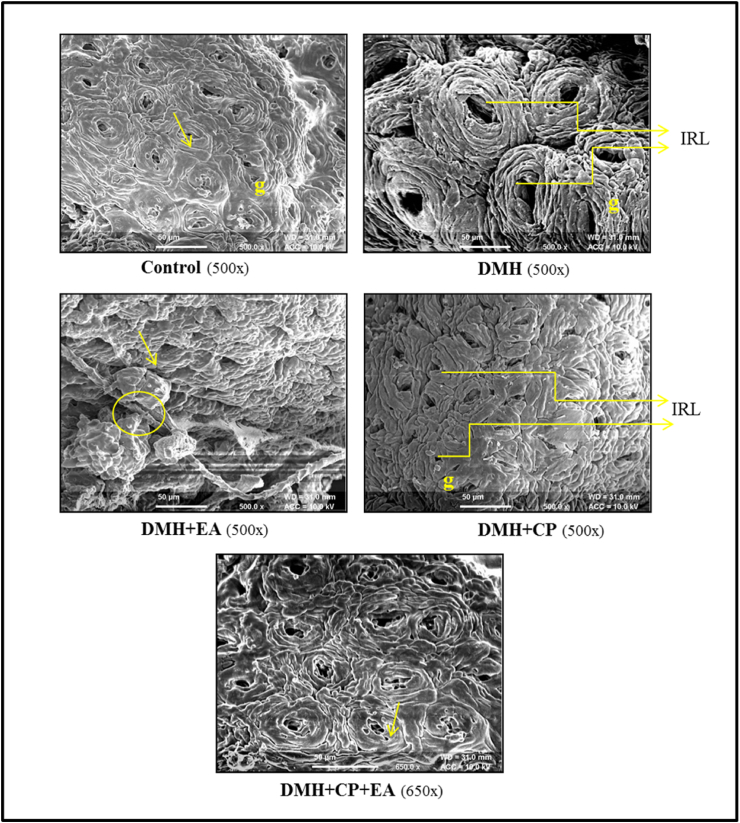
Topographical observation of intestinal surface from control animals revealed a flat and relatively even contoured surface; the margins of the cells were well defined. DMH and DMH + EA treated animals showed enlarged cells causing the surface to appear irregular. Additionally, dysplastic lesions were observed with small areas of epithelial sloughing. DMH + CP and DMH + CP + EA groups showed a flat lesion recognized as an area with small crypt openings and loss of goblet cells (Fig. 2).
Fig. 2.
Topographical changes in colon tissue of animals subjected to DMH, Cisplatin, Ellagic acid, and their co-treatment through SEM [Crypts (arrows), goblet cells (g), dysplastic lesions (circle), irregular luminal openings (IRL)].
3.3. Membrane dynamics
3.3.1. Pyrene fluorescence
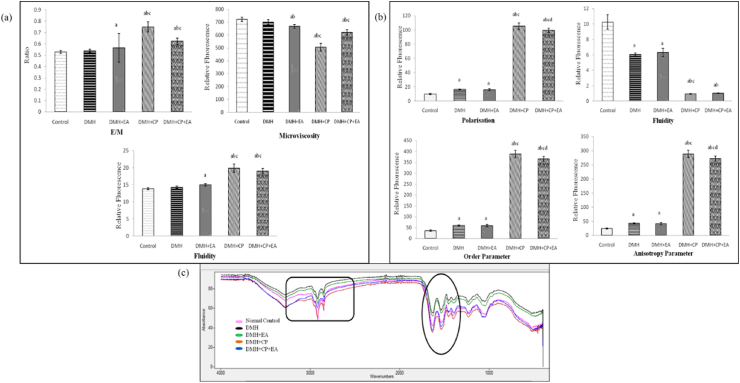
An appreciable (p≤0.05) raise in E/M ratio was observed in DMH + EA, DMH + CP, and DMH + CP + EA groups when compared to control. E/M ratio hiked significantly (p≤0.05) in both the CP treated groups when compared with DMH and DMH + EA groups. However, EA treatment in DMH + CP animals led to a non-significant decrease in the E/M ratio when compared to DMH + CP treated animals (Fig. 3a).
Fig. 3.
(a) Effect of DMH, Ellagic Acid, Cisplatin and their co-treatment on the Excimer/Monomer ratio (E/M), Microviscosity, and membrane fluidity as measured by pyrene fluorescence studies in the colon tissue. (b) Effect of DMH, Cisplatin, and Ellagic acid and their co-treatment on the polarization, fluidity, order parameter, and anisotropy parameter as measured by the DPH fluorescence studies in the colon tissue. Data are expressed as Mean ± SD (n = 6). Data is analyzed using one-way ANOVA followed by a post hoc test. “a” p≤0.05 significant concerning control group. “b” p≤0.05 significant concerning DMH group. “c” p≤0.05 significant concerning DMH + EA group. “d” p≤0.05 significant concerning DMH + CP group. (c) Representative FT-IR spectrum of colon tissue of mice subjected to DMH, Cisplatin, and Ellagic acid treatment showing regions of variations.
A noteworthy (p≤0.05) decline in microviscosity was observed in DMH + EA, DMH + CP, and DMH + CP + EA groups than in control and DMH groups. Microviscosity diminished significantly (p≤0.05) in both the CP treated groups when compared with DMH + EA treated animals. EA treatment in DMH + CP animals led to an increase in microviscosity when compared to DMH + CP treated animals; however, the increase was insignificant (Fig. 3a).
A substantial rise in fluidity was observed in DMH + EA, DMH + CP, and DMH + CP + EA groups than in the control. Fluidity significantly (p≤0.05) increased in both the CP treated groups when compared with DMH and DMH + EA groups (Fig. 3a).
3.3.2. DPH fluorescence
A substantial (p≤0.05) raise in polarization, order parameter and anisotropy parameter was found in all the DMH treated groups when compared to control. A significant (p≤0.05) increase was also observed in both the cisplatin-treated groups when compared with DMH as well as DMH + EA groups; however, a notable (p≤0.05) decrease was observed in the DMH + CP + EA group than DMH + CP treated animals.
A considerable (p≤0.05) decrease in fluidity was found in all the DMH treated groups than in the control. Significant (p≤0.05) decrease in DMH + CP and DMH + CP + EA was observed in DMH and DMH + EA treated mice (Fig. 3b).
3.4. FT-IR
Peak 1660: Alpha structure: A significant (p≤0.05) decrease was observed in the area of the DMH + EA group when compared with control and DMH. Administration of CP to DMH injected mice caused a (p≤0.05) decrease in the area when compared with control, DMH, and DMH + EA groups. EA administration to CP treated tumor-bearing mice further decreased the area significantly (p≤0.05) when compared to the DMH + CP group (Fig. 3c, Table 1)
Table 1.
The FT-IR band area values of various functional groups from the colon of mice treated with DMH, Ellagic Acid, Cisplatin, and their co-treatment after 26 weeks.
| Functional groups | Peak/Group | Control | DMH | DMH + EA | DMH + CP | DMH + CP + EA |
|---|---|---|---|---|---|---|
| Alpha structure | 1660 | 1.32 ± 0.04 | 0.97 ± 0.04 | 0.73 ± 0.047 ab | 0.21 ± 0.05 abc | 0.14 ± 0.01 abcd |
| Beta sheets | 1670 | 0.17 ± 0.01 | 0.18 ± 0.00 | 0.20 ± 0.04 ab | 0.24 ± 0.03 abc | 0.24 ± 0.00 abc |
| Beta turns | 1667 | 0.88 ± 0.02 | 0.12 ± 0.05a | 0.63 ± 0.08 ab | 0.10 ± 0.02 ac | 0.80 ± 0.03 bcd |
| Amide I | 1650 | 0.61 ± 0.01 | 0.69 ± 0.02 | 0.72 ± 0.07 | 0.24 ± 0.05 abc | 0.14 ± 0.02 abcd |
| Amide II | 1534 | 0.33 ± 0.02 | 0.39 ± 0.01 | 0.30 ± 0.03 | 0.61 ± 0.06 abc | 0.37 ± 0.10d |
| Amide III | 1306 | 0.25 ± 0.04 | 0.25 ± 0.00 | 0.12 ± 0.03 ab | 0.10 ± 0.01 ab | 0.24 ± 0.08 cd |
| Fatty Acids/Nucleic Acids | 1744/1082 | 4.10 ± 0.77 | 7.02 ± 0.32 | 6.67 ± 0.97 ab | 5.86 ± 0.55 ab | 5.66 ± 0.18 ab |
| Phosphate group of nucleic acids | 1081 | 0.54 ± 0.09 | 0.61 ± 0.07 | 0.26 ± 0.02 ab | 0.40 ± 0.03 abc | 0.39 ± 0.26 abc |
| RNA/DNA | 1121/1020 | 0.72 ± 0.05 | 0.56 ± 0.06 | 0.56 ± 0.02 | 0.18 ± 0.03 abc | 0.28 ± 0.02 abcd |
| C–O (H) stretching | 1170 | 0.17 ± 0.01 | 0.18 ± 0.01 | 0.13 ± 0.03 b | 0.16 ± 0.02 | 0.21 ± 0.13 abcd |
Units: Arbitrary units.
Data are expressed as Mean ± SD (n = 5). Data is analyzed using one-way ANOVA followed by a post hoc test. “a” p≤0.05 significant concerning control group. “b” p≤0.05 significant concerning DMH group. “c” p≤0.05 significant concerning DMH + EA group. “d” p≤0.05 significant concerning DMH + CP group.
Peak 1667: Beta turns: All DMH-treated animals showed a significant (p≤0.05) decline in beta turns when compared with control. EA treatment in tumor-bearing mice led to a notable (p≤0.05) increase in beta turns when compared to DMH-treated animals. DMH + CP treated animals showed a substantial (p≤0.05) decrease from the DMH + EA group. EA + CP combined treatment to tumor-bearing mice substantially (p≤0.05) elevated the area in comparison with DMH, DMH + EA, and DMH + CP groups.
Peak 1306: Amide III: Peak area in the DMH + EA group showed a (p≤0.05) decline from the control and DMH group. The area of DMH + CP treated animals also showed a significant (p≤0.05) reduction in control and DMH groups. The area in the DMH + CP + EA group improved significantly (p≤0.05) when compared to, DMH + EA and DMH + CP groups.
Peak 1744/Peak1082: An (p≤0.05) increase was observed in the FA/NA ratio of the DMH group when compared to the control. Peak ratio in DMH + EA, DMH + CP, and DMH + CP + EA showed a significant (p≤0.05) increase from the control group; however, these groups showed an appreciable (p≤0.05) decrease from the DMH group.
Peak 1081: A significant (p≤0.05) decrease in peak area was observed in DMH + EA, DMH + CP, and DMH + CP + EA groups in comparison with control and DMH groups. However, a notable (p≤0.05) increment in the area was observed in both the CP injected groups when compared to DMH + EA treated animals.
Peak 1121/Peak 1020: No significant difference was observed in the RNA/DNA ratio of DMH and DMH + EA group when compared to control. The peak ratio in DMH + CP and DMH + CP + EA groups showed a considerable (p≤0.05) decrease from the control, DMH and DMH + EA groups. However, an appreciable (p≤0.05) increment in the ratio was observed in CP and EA co-treated animals than in DMH + CP animals.
Peak 1170: C–O (H) stretching: EAtreat tumor-bearing bearing mice lowered the area significantly (p≤0.05) when compared to DMH-treated animals. However, EA and CP combined treatment to tumor-bearing mice significantly (p≤0.05) raise the peak area when observed in comparison with all other groups.
4. Discussion
Cisplatin is one of the most commonly used chemotherapeutic drugs used for a wide spectrum of human malignancies. Its use; however, is warranted because of an array of associated side effects ranging from nephrotoxicity [4]to ototoxicity, damage to the peripheral nervous system, liver [3], and testes [4].
Toxicity resulting from chemical exposure can manifest itself in various ways such as changes in membrane structure and dynamics. The generation of reactive oxygen species after exposure to any toxicant makes lipids in the cell membrane highly susceptible to peroxidation.
Intestinal surfaces from DMH-treated animals showed irregular crypt surface and dysplastic lesions. The cells were enlarged, making the surface appear irregular (elevated surfaces), with small areas indicating epithelial sloughing. These findings are in concordance with the ACF observations which also revealed an increase in tumor incidence on the administration of DMH. Similar results were reported earlier on alterations occurring in colon tissue with DMH administration [23,24].
Free radicals are well recognized for their “dual role” in the living system [25,26]. ROS readily attacks the polyunsaturated fatty acids, initiating a self-propagating chain reaction [27]. Increased oxidative stress due to amplified ROS resulting in enhanced LPO production by carcinogens and chemotherapy leads to disorganization and disruption of membranes [28,29].
Pyrene forms intermolecular excimers upon its embedding in the phospholipid vesicles and biological membranes [30]. This property of pyrene has been exploited in exploring the dynamic properties of membrane lipids by determining the lateral diffusion and thus depicts the changes in microviscosity under pathological conditions. Increased E/M ratio in the DMH group leads to a decrease in the microviscosity which in turn is inversely related to the membrane fluidity. Following a similar pattern, an increase in membrane fluidity was observed in the DMH + CP treated group. LPO has been shown to perturb the bilayer structure of biomembranes and modify membrane fluidity [28,29]. Since the membrane is considered two-dimensional, any change in membrane order may not be uniform and restricted to a unique location in the membrane. The increased lateral diffusion of the pyrene probe thus understandably might have resulted due to partial lipid removal and more motional freedom of the probe in the hydrocarbon phase. The increased excimer formation has been reported in the literature to be evidential of enhanced lateral mobility of the probe in the bilayer, thereby, increasing the fluidity of the membrane.
However, simultaneous supplementation of CP and EA increased microviscosity leading to a decrease in the membrane fluidity which may be due to the decreased diffusion of pyrene in the membrane. Oxidative stress has also been correlated with membrane fluidity [31,32]. Numerous studies have revealed that CP and EA have pronounced effects on the physical state of the membrane [33]. CP causes DNA-adduct formation and induces apoptosis through plasma membrane disruption [34]. Supplementation of EA + CP to the DMH treated mice attenuated the alterations induced in the anisotropy, order parameter, and fluidity of the membrane.
The steady-state polarization of 1, 6-diphenyl-1,3,5-hexatriene (DPH) was employed to examine the rotational diffusion and order parameter of the membrane (short-range order). The polarization of the fluorescence of a molecule relies on the rate of rotation where the binding of the fluorophore to the biological membrane can be analyzed by a rise in the polarization of the fluorescence probe [35]. DPH being hydrophobic readily accommodates the hydrocarbon part of the phospholipid bilayer. Emission anisotropy too correlates well with the order of phospholipid chains [36]. As a consequence of the resistance offered by the microenvironment to the motion of the probe, rotational motion affects the fluorescence polarization that provides an estimate of the environmental resistance and is inversely correlated as a measure of fluidity.
Results from DPH fluorescence depicted an increased order parameter in DMH treated group indicating a dense lipid packing in the membrane bilayer. Fluorescence polarization was also found to be elevated in DMH treated animals which indicated a reduction in the membrane fluidity implying the more rigid membrane that offers a hindered channel for lipophilic solutes to move across the membrane barrier [37]. Treatment with CP and combined treatment of CP + EA resulted in increased polarization which could be due to increased ROS and LPO, thereby increasing the number of free lipids; which also led to a significant rise in the anisotropy and membrane lipid order, thereby suggesting lesser rigidity of the membrane, reflecting the breakdown of lipid packing in cancerous tissue [38].
FTIR spectroscopy was used presently to determine the protein secondary structure. A large amount of CP is reported to bind to proteins (forms platinum-protein adducts), both extracellularly and intracellularly for its anti-cancer activity [39]. Oxidative stress as well as metabolic modifications due to DMH and CP might have altered the protein structures which can be inferred from irregularities in protein secondary structure as well as the disordered state of plasma membrane caused by peroxidative damage. Protein interface with cisplatin-DNA complex induces DNA bending and structural changes [40]. Structural changes caused due to ROS generation [41] after DMH and CP treatment thereby disrupted the membranes and hence altered the amide bands. A significant increase was also observed in the RNA/DNA ratio of the DMH and DMH + CP treated group.
DMH is known to cause DNA mutations thereby initiating the process of carcinogenesis [42]. The biological activity of CP is strictly associated with the plastination of nuclear DNA [43], the formation of intrastrand cross-links, and thus DNA bending. RNA/DNA ratio gives an idea of the transcriptional status of the cell [44] indicating the transformation of cells from normal to malignant ones. Carcinogenesis is directly proportional to lipidogenesis [45]. In the present study, a notable increase in fatty acid content was evident in DMH-treated animals. CP causes significant fatty acid oxidation which can be well correlated to the decline in fatty acid to nucleic acid ratio. EA treatment to CP administered mice further decreased the fatty acid content which may be attributed to its pro-oxidant nature in tumorous tissue [46].
Ellagic acid supplementation, on the contrary, shielded the membrane structure by DMH and Cisplatin insult. Thus, ellagic acid supplementation shows great promise in reducing the DMH and cisplatin-induced membrane alterations. However, further studies in this direction are a need of the hour wherein alternative medicine can be explored in carving a holistic approach towards patient health as well as managing chemotherapy-induced toxic insult.
Author contributions
Conception: Dr. Pavitra Ranawat Data curation: Yasmeen Goyal Analysis of Data: Dr. Pavitra Ranawat, Yasmeen Goyal Preparation of the manuscript: Dr. Pavitra Ranawat, Yasmeen Goyal Revision for important Intellectual content: Dr. Pavitra Ranawat, Yasmeen Goyal, Prof. Ashwani Koul Supervision: Dr. Pavitra Ranawat, Prof. Ashwani Koul.
Conflicts of interest
The authors have no conflict of interest to report.
Declaration of competing interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Acknowledgment
UGC- BSR fellowship, New Delhi, India (Award No: F.25-1/2013- 14(BSR)/7–209/2009), DST-PURSE Grant, New Delhi, India (Award No: 49/RPC-29/04/16).
References
- 1.Ferlay J., Rosso S., Comber H., et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur. J. Cancer. 2013;49(6):1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Bailey C.E., Yuan Hu C., Nancy You Y., et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surgery. 2015;150(1):17–22. doi: 10.1001/jamasurg.2014.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal Y., Koul A., Ranawat P. Ellagic acid ameliorates cisplatin induced hepatotoxicity in colon carcinogenesis. Environ. Toxicol. 2019;34(7):804–813. doi: 10.1002/tox.22747. [DOI] [PubMed] [Google Scholar]
- 4.Goyal Y., Koul A., Ranawat P. Ellagic acid ameliorates cisplatin toxicity in chemically induced colon carcinogenesis. Mol. Cell. Biochem. 2019;453(1):205–215. doi: 10.1007/s11010-018-3446-1. [DOI] [PubMed] [Google Scholar]
- 5.Skrzydlewska E., Sulkowski S., Koda M., et al. Lipid peroxidation and antioxidant status in colorectal cancer. World J. Gastroenterol.: WJG. 2005;11(3):403. doi: 10.3748/wjg.v11.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLaughlin S., Wang J., Gambhir A., et al. PIP2 and proteins: interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct. 2002;31(1):151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 7.Cascio M. Connexins and their environment: effects of lipids composition on ion channels. Biochim. Biophys. Acta (BBA) Biomembr. 2005;171(2):142–153. doi: 10.1016/j.bbamem.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Carradoriad D., dos Santos A.G., Masquelier J., et al. The origin of neural stem cells impacts their interactions with targeted-lipid nanocapsules: potential role of plasma membrane lipid composition and fluidity. J. Contr. Release. 2018;292:248–255. doi: 10.1016/j.jconrel.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Merry S., Kaye S. Tumour cell resistance to anthracyclines—a review. Cancer Chemother. Pharmacol. 1985;14(2):96–103. doi: 10.1007/BF00434344. [DOI] [PubMed] [Google Scholar]
- 10.Grunicke H., Hofmann J. Cytotoxic and cytostatic effects of antitumor agents induced at the plasma membrane level. Pharmacol. Ther. 1992;55(1):1–30. doi: 10.1016/0163-7258(92)90027-w. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal S.K., Rade I.N. Effect of cisplatin on the plasma membrane phosphatase activities in ascites sarcoma-180 cells: a cytochemical study. J. Histochem. Cytochem. 1983;31(2):307–317. doi: 10.1177/31.2.6300219. [DOI] [PubMed] [Google Scholar]
- 12.R Craig C., E Stitzel R. Lippincott Williams & Wilkins; 2004. Modern Pharmacology with Clinical Application. [Google Scholar]
- 13.Butler L., Bacon M., Carey M., et al. Determining the relationship between toxicity and quality of life in an ovarian cancer chemotherapy clinical trial. J. Clin. Oncol. 2004;22(12):2461–2468. doi: 10.1200/JCO.2004.01.106. [DOI] [PubMed] [Google Scholar]
- 14.Carrillo-Navas H., Cruz-Olivares J., Varela-Guerrero V., et al. Rheological properties of a double emulsion nutraceutical system incorporating chia essential oil and ascorbic acid stabilized by carbohydrate polymer–protein blends. Carbohydr. Polym. 2012;87(2):1231–1235. doi: 10.1016/j.carbpol.2011.09.005. [DOI] [Google Scholar]
- 15.Ahmad A., Li Y., Bao B., et al. Epigenetic regulation of miRNA-cancer stem cells nexus by nutraceuticals. Mol. Nutr. Food Res. 2014;58(1):79–86. doi: 10.1002/mnfr.201300528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao M., Zhao Z., Lv P., et al. Quantitative combination of natural anti-oxidants prevents metabolic syndrome by reducing oxidative stress. Redox Biol. 2015;6:206–217. doi: 10.1016/j.redox.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moilanen J., Karonen M., Tähtinen P., et al. Biological activity of ellagitannins: effects as anti-oxidants, pro-oxidants, and metal chelators. Phytochemistry. 2016;125:65–72. doi: 10.1016/j.phytochem.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Cecil C., Lacal P.M., Tentori L., et al. Experimental evidence of the antitumor, antimetastaic & antiangiogenic activity of ellagic acid. Nutrients. 2018;10(11):1756. doi: 10.3390/nu10111756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs S., Harmon B.E., Ollberding N.J., Wilkens L.R., Monroe K.R., Kolonel L.N., Le Marchand L., Boushey C.J., Maskarinec G. Among 4 diet quality indexes, only the alternate Mediterranean diet score is associated with better colorectal cancer survival and only in African American women in the multiethnic cohort. J. Nutr. 2016;146:1746–1755. doi: 10.3945/jn.116.234237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whiteley L.O., Hudson L., JR., Pretlow T.P. Aberrant crypt foci in the colonic mucosa of rats treated with a genotoxic and nongenotoxic colon carcinogen. Toxicol. Pathol. 1996;24(6):681–689. doi: 10.1177/019262339602400602. [DOI] [PubMed] [Google Scholar]
- 21.Swapna I., Sathya Sai Kumar K.V., Murthy Ch R.K., et al. Membrane alterations and fluidity changes in cerebral cortex during acute ammonia intoxication. Neurotoxicology. 2006;27(3):402–408. doi: 10.1016/j.neuro.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 22.B Massey J., She H.S., Gotto A.M., et al. Lateral distribution of phospholipid and cholesterol in apolipoprotein A-I recombinants. Biochem. 1985;24(25):7110–7116. doi: 10.1021/bi00346a014. [DOI] [PubMed] [Google Scholar]
- 23.Paulsen J.E., Steffensen I.L., Namork E., et al. Scanning electron microscopy of aberrant crypt foci in rat colon. Carcinogenesis. 1994;15(10):2371–2373. doi: 10.1093/carcin/15.10.2371. [DOI] [PubMed] [Google Scholar]
- 24.K Rawat J., Roy S., Singh M., et al. Transcutaneous vagus nerve stimulation regulates the cholinergic anti-inflammatory pathway to counteract 1, 2-dimethylhydrazine induced colon carcinogenesis in albino wistar rats. Front. Pharmacol. 2019;10:1–13. doi: 10.3389/fphar.2019.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bragado P., Armesilla A., Silva A., et al. Apoptosis by cisplatin requires P53 mediated P38α MAPK activation through ROS generation. Apoptosis. 2007;12(9):1733–1742. doi: 10.1007/s10495-007-0082-8. [DOI] [PubMed] [Google Scholar]
- 26.Dasari S., Cisplatin P.B Tchounwou. Cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka A., Yamamoto A., Murota K., et al. Polyunsaturated fatty acids induce ovarian cancer cell death through ROS-dependent MAP kinase activation. Biochem. Biophys. Res. Commun. 2017;493(1):468–473. doi: 10.1016/j.bbrc.2017.08.168. [DOI] [PubMed] [Google Scholar]
- 28.N Chatterjee S., Agarwal S. Liposomes as membrane model for study of lipid peroxidation. Free Radic. Biol. Med. 1988;4(1):51–72. doi: 10.1016/0891-5849(88)90011-1. [DOI] [PubMed] [Google Scholar]
- 29.Spengler M.I., Bertoluzzo S.M., Catalani G., et al. Study on membrane fluidity and erythrocyte aggregation in equine, bovine, and human species. Clin. Hemorheol. Microcirc. 2008;38(1):171–176. [PubMed] [Google Scholar]
- 30.Yu T., Afanas’ev S.A., Putrova O.D., et al. Age-related characteristics of erythrocyte membrane microviscosity in experimental cardiosclerosis. Adv. Gerontol. 2013;3(3):211–214. doi: 10.1134/S2079057013030119. [DOI] [PubMed] [Google Scholar]
- 31.Ibrahim P.Singh S. Curcumin activates erythrocyte membrane acetylcholinesterase. Lett. Drug Des. Discov. 2013;10(6):550–556. doi: 10.2174/1570180811310060012. [DOI] [Google Scholar]
- 32.Guţu M., Rusu V., Ştefănescu C. Membrane fluidity--biophysical parameter in relation to membrane transport processes. Revista Medico-Chirurgicala a Societatii de Medici Si Naturalisti Din Iasi. 2011;115(1):153–162. [PubMed] [Google Scholar]
- 33.Rebillard A., Tekpli X., Meurette O., et al. Cisplatin-induced apoptosis involves membrane fluidification via inhibition of NHE1 in human colon cancer cells. Cancer Res. 2007;67(16):7865–7874. doi: 10.1158/0008-5472.CAN-07-0353. [DOI] [PubMed] [Google Scholar]
- 34.Rebillard A., Lagadic-Gossmann D., T Dimanche-Boitrel M. Cisplatin cytotoxicity: DNA and plasma membrane targets. Curr. Med. Chem. 2008;152(6):2656–2663. doi: 10.2174/092986708786242903. [DOI] [PubMed] [Google Scholar]
- 35.Bhardwaj P., Kumar M., Dhatwalia S.K., et al. Acetyl-11-Keto-β-Boswellic acid modulates membrane dynamics in benzo(a)Pyrene-induced lung carcinogenesis. Mol. Cell. Biochem. 2019;460(1–2):17–27. doi: 10.1007/s11010-019-03566-z. [DOI] [PubMed] [Google Scholar]
- 36.Halder A., Maity B.Saha P., et al. Lipid chain saturation and the cholesterol in the phospholipid membrane affect the spectroscopic properties of lipophilic dye nile red. Spectrochim. Acta Mol. Biomol. Spectrosc. 2018;191:104–110. doi: 10.1016/j.saa.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 37.R Naylor M., Ly A.M., Handford M.J., et al. Lipophilic permeability efficiency reconciles the opposing roles of lipophilicity in membrane permeability and aqueous solubility. J. Med. Chem. 2018;61(24):11169–11182. doi: 10.1021/acs.jmedchem.8b01259. [DOI] [PubMed] [Google Scholar]
- 38.Abdelrazzak A.B., El-Bahy G.S. FT-IR spectroscopic investigation of ionizing radiation-induced damage in the small intestine of whole-body irradiated rats. Vib. Spectrosc. 2018;99:146–150. doi: 10.1016/j.vibspec.2018.09.007. [DOI] [Google Scholar]
- 39.Calderone V., Casini A., Mangani S., et al. Structural investigation of cisplatin-protein interactions: selective platination of His19 in a cuprozinc superoxide dismutase. Angew. Chem. Int. Ed. 2006;45(8):1267–1269. doi: 10.1002/anie.200502599. [DOI] [PubMed] [Google Scholar]
- 40.Neault J.F., Tajmir-Riahi H.A. Interaction of cisplatin with human serum albumin. Drug binding mode and protein secondary structure. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1998;1384(1):153–159. doi: 10.1016/S0167-4838(98)00011-9. [DOI] [PubMed] [Google Scholar]
- 41.Engelke L.H., Hamacher A., Proksch P., et al. Ellagic acid and resveratrol prevent the development of cisplatin resistance in the epithelial ovarian cancer cell line A2780. J. Cancer. 2016;7(4):353–363. doi: 10.7150/jca.13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pamplona-Silva M.T., Morandi W.V., Bernardi L., et al. Brown flaxseed prevents DNA damage induced by 1,2-dimethylhydrazine in a pre-clinical model. Braz. Arch. Biol. Technol. 2018;61:1–13. doi: 10.1590/1678-4324-2018180303. [DOI] [Google Scholar]
- 43.Benkafadar N., Menardo J., Bourien J., et al. Reversible P53 inhibition prevents cisplatin ototoxicity without blocking chemotherapeutic efficacy. EMBO Mol. Med. 2017;9(1):7–26. doi: 10.15252/emmm.201606230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang H., Wang J., Li W., et al. Serum HBV DNA plus RNA shows superiority in reflecting the activity of intrahepatic CccDNA in treatment-naïve HBV-infected individuals. J. Clin. Virol. 2018;99–10:71–78. doi: 10.1016/j.jcv.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 45.Murata S. vol. 319. 2016. New chemotherapeutic agents: monoterpenes and fatty acid synthase inhibitors. (Colorectal Cancer - from Pathogenesis to Treatment). [DOI] [Google Scholar]
- 46.Fernando W., Vasantha Rupasinghe H.P., Hoskin D.W. Dietary phytochemicals with anti-oxidant and pro-oxidant activities: a double-edged sword in relation to adjuvant chemotherapy and radiotherapy? Cancer Lett. 2019;452:168–177. doi: 10.1016/j.canlet.2019.03.022. [DOI] [PubMed] [Google Scholar]