Summary
Radium is refocused from the viewpoint of an environmental pollutant and cancer therapy using alpha particles, where it mainly exists as a hydrated ion. We investigated the radium hydration structure and the dynamics of water molecules by extended X-ray absorption fine structure (EXAFS) spectroscopy and ab initio molecular dynamics (AIMD) simulation. The EXAFS experiment showed that the coordination number and average distance between radium ion and the oxygen atoms in the first hydration shell are 9.2 ± 1.9 and 2.87 ± 0.06 Å, respectively. They are consistent with those obtained from the AIMD simulations, 8.4 and 2.88 Å. The AIMD simulations also revealed that the water molecules in the first hydration shell of radium are less structured and more mobile than those of barium, which is an analogous element of radium. Our results indicate that radium can be more labile than barium in terms of interactions with water.
Subject areas: Nuclear structure, Nuclear spectroscopy, Molecular dynamics
Graphical abstract

Highlights
-
•
Extended X-ray absorption fine structure (EXAFS) measurement revealed the hydration structure of radium ion
-
•
Ab initio molecular dynamics (AIMD) simulation brought consistent results
-
•
AIMD revealed the structural and dynamic properties of the water molecules
-
•
The hydration structure of radium ion is more labile than that of barium ion
Nuclear structure; Nuclear spectroscopy; Molecular dynamics
Introduction
Radium (Ra) is a popular radioactive element, having no stable isotopes and adverse health effects. Ra has not been in the focus of environmental science for a long time owing to its rarity. Since the 1980s, the concentration of Ra in drinking and groundwater gained the attention of the scientific community (Hess et al., 1985; Zhuo et al., 2001; Szabo et al., 2012; Guo et al., 2018). In addition, the environmental transport of Ra is crucial for geological disposal of high-level radioactive waste, such as spent fuel because 226Ra is a descendant nuclide of 238U, which has a long half-life (1,600 years). The concentration of Ra in the environment depends on various factors, such as pH, redox state, ionic strength, and mineral component. The amount of Ra tends to be higher in an anoxic environment (Szabo et al., 2012), causing a higher concentration in deeper groundwater than in surface water. Taking advantage of this trend, Ra can be used as a natural tracer for detecting submarine groundwater discharge (Burnett et al., 2006). Ra is also used for dating volcanic ejecta (Tanguy et al., 2007) and buried sediment (Olley et al., 1996), using the uranium and thorium decay chains. However, shale gas extraction using new drilling techniques, such as horizontal drilling and hydraulic fracturing, has become active recently, raising concerns about the contamination of Ra in surface and drinking water (Vengosh et al., 2014). As described above, the long-lived isotope of Ra such as 226Ra is basically toxic. However, 223Ra with a half-life of 11.43 days has recently been used to treat bone metastasis in castration-resistant prostate cancer (CRPC) (Deshayes et al., 2017; Delgado Bolton and Giammarile, 2018; Morris et al., 2019).
Ra mainly exists as a hydrated divalent cation in the environment and does not form any pure Ra minerals because of its low concentration (Langmuir et al., 1985). Therefore, the environmental behavior of Ra2+ is governed by coprecipitation, recrystallization, and adsorption reactions with other alkaline earth elements (Kraemer and Reid, 1984; Langmuir and Melchior, 1985; Beaucaire and Toulhoat, 1987; Vinson et al., 2013). The adsorption of Ra2+ on clay minerals and iron (hydr)oxides has also been reported (Martin et al., 2003; Hidaka et al., 2007). This behavior has also been studied by numerous laboratory experiments (Doerner and Hoskins, 1925; Curti et al., 2010; Hedstrom et al., 2013; Chen and Kocar, 2018). For example, the early uptake of Ra2+ during the recrystallization and coprecipitation of barite (barium sulfate), has been observed (Hedstrom et al., 2013). Similar chemical reactions were applied to bone metastasis in CRPC in the field of nuclear medicine, substituting 223Ra for calcium in hydroxyapatite complexes. Atomic-level investigation of hydration and dehydration of Ra2+ is essential to understand these reactions such as adsorption and coprecipitation which occur at the solid-water interface with hydration and/or dehydration processes. However, the hydration properties of Ra2+ have not been well studied.
Numerical simulations are effective in studying the hydration structure of Ra2+ safely. The formation of Ra2+ hydrated clusters was studied via static quantum mechanical calculations. Namely, the fragment molecular orbital–molecular dynamics simulation approach was applied to study the hydration structures in an aqueous solution (Matsuda and Mori, 2014b). Classical molecular dynamics, ab initio quantum chemical calculations with the relativistic model core potential (MCP) method, and fragment molecular orbital–molecular dynamics were conducted to study the hydration of Ra2+ and compare it with other hydrated divalent alkaline earth metal ions (Matsuda and Mori, 2014a). Recently, classical molecular dynamics simulation with the force field fitted to the results of quantum mechanical calculations was conducted (Pappalardo et al., 2021). In the hydrated barium ion (Ba2+), an analog element of Ra cases, it has been shown that full-ab initio molecular dynamics (AIMD) simulation is one of the best simulation methods compared with other methods, including QM/MM, a hybrid method of quantum and classical calculations, in the accuracy for reproducing experimental results (Yamaguchi et al., 2021). The full-AIMD simulations have not been applied to hydrated Ra2+ systems mainly owing to the higher computational cost of AIMD than those of other methods. However, the recent development of supercomputers enables large-scale full-AIMD simulations even for large systems such as the hydrated Ra2+ systems with a large number of water molecules.
There are some experimental methods to directly investigate the hydration structure at the atomic level, such as extended X-ray absorption fine structure (EXAFS) measurement, X-ray and neutron diffraction measurements, vibration spectroscopic measurement, and nuclear magnetic resonance (Marcus, 2010). EXAFS is suitable for studying the hydration structures of Ra2+ owing to its high selectivity of elements and high sensitivity, which are crucial for measuring a dilute solution such as hydrated Ra2+. As mentioned above, no spectroscopic experiment has been performed on hydrated Ra2+ because of the radiation toxicity of Ra. However, some facilities have beamlines to measure radioactive materials. SPring–8, a synchrotron radiation facility in Japan, has two beamlines that can be applied to radioactive materials.
Therefore, in this study, EXAFS was applied for the first time to determine the hydration structure of Ra2+ using the BL22XU beamline of SPring–8. Additionally, the AIMD simulations in this study were conducted to evaluate the average hydration structure and the dynamic properties of the hydration structure. These methods are complementary, and their combination revealed the microscopic hydration structure of Ra2+ and its dynamics. Our study clarifies the differences between the hydration properties of Ra2+ and Ba2+, which is often used as a non-radioactive analog of Ra2+.
Results and discussion
Hydration structure in comparison between extended X-ray absorption fine structure and ab initio molecular dynamics results
The EXAFS analysis showed that the average distance between the Ra2+ and the oxygen atoms of the water molecules in the first hydration shell () and coordination number (CN) were 2.87 ± 0.06 Å and 9.2 ± 1.9, respectively (Figure 1 and Table 1). As this is the first atomic-level structural information obtained for hydrated Ra2+, these values could not be validated by direct comparison with other spectroscopic methods. Hence, we considered the consistency with the experimental results of other alkaline earth metal ions. Table 2 shows the monotonic increase in the distance between the cation and oxygen atoms in the hydration water molecules, obtained using various methods, including EXAFS, with the atomic number. The effective ionic radii shown in previous studies (Shannon, 1976) have the same tendency (Table 2), and the value of Ra2+ is consistent with the trend. We also evaluated the effective radius of a water molecule, defined by the difference between the interatomic distance and the effective ionic radius (Marcus, 1988). The radius of the water molecule hydrated to Ra2+ was determined to be 1.39 Å. This value is almost the same as other alkaline earth metal elements (Table 2) and as the radius of O2− used for calculating the effective ionic radius (1.40 Å) (Shannon, 1976). These results mean that the interatomic distance between Ra2+ and oxygen atoms determined by the experimental EXAFS method in this study is consistent with the results of other alkaline earth metal elements, confirming the adequacy of the obtained values. The CN is directly related to the cation–oxygen distance because a longer cation–oxygen distance implies a larger shell volume for water molecules. Therefore, the observed CN is consistent with other alkaline earth metal elements (Table 2).
Figure 1.
EXAFS spectrum
EXAFS spectrum of hydrated Ra2+ in (A) -space and (B) R-space. The horizontal and vertical axes represent (Å−1) and in (A) and interatomic distance and the Fourier transformation magnitude in (B), where is EXAFS oscillation. The dotted line represents the fitted result.
Table 1.
Extended X-ray absorption fine structure spectroscopy parameters for fitting
| range (Å−1) | Shell | CN | (Å) | (eV) | (× 10−3 Å2) | (× 10−4 Å3) | (%) |
|---|---|---|---|---|---|---|---|
| 2.3–9.0 | Ra–O | 9.2 ± 1.9 | 2.87 ± 0.06 | 6.3 ± 2.1 | 26.5 ± 0.9 | 7.3 ± 38.2 | 1.29 |
CN, , , , , and represent the coordination number, the averaged interatomic distance between Ra2+ and nearest oxygen atoms of water molecules, threshold shift, DW factor, the third-order cumulant, and residual factor.
Table 2.
The averaged distance between ion () and the oxygen atoms of water molecules in the first hydration shell () and coordination number (CN) observed via experiments, effective ionic radii (EIR) and CN, and the differences between and EIR for alkali earth metal ions
| Ion ( | (Å) | CN | EIR (Å) (Shannon, 1976) | CN (Shannon, 1976) | (Å) | Method |
|---|---|---|---|---|---|---|
| Ra2+ | 2.87 ± 0.06 (This study) | 9.2 ± 1.9 (This study) | 1.48 | 8 | 1.39 | EXAFS |
| Ba2+ | 2.79 ± 0.02 (Yamaguchi et al., 2021) | 8.0 ± 1.9 (Yamaguchi et al., 2021) | 1.42 | 8 | 1.37 | EXAFS |
| Sr2+ | 2.64 ± 0.01 (Yokoyama, 1995) | 8.1 ± 0.1 (Yokoyama, 1995) | 1.26 | 8 | 1.38 | XRD and ND |
| Ca2+ | 2.40 ± 0.04 (Yokoyama, 1995) | 6.3 ± 0.7 (Yokoyama, 1995) | 1.00 | 6 | 1.40 | XRD and ND |
| Mg2+ | 2.09 ± 0.04 (Yokoyama, 1995) | 6.0 ± 0.1 (Yokoyama, 1995) | 0.72 | 6 | 1.37 | XRD |
XRD and ND means X-ray diffraction and neutron diffraction, respectively. The values of Sr2+, Ca2+, and Mg2+ reported in the literature (Yokoyama, 1995) were average of the reported values in literature (Ohtaki and Radnai, 1993; Persson et al., 1995).
AIMD simulation is a reliable method for comparison with experimental results. In the Ba2+ case, the results obtained by EXAFS and AIMD simulations are consistent (Yamaguchi et al., 2021). We note that the results of AIMD simulations depend on the exchange-correlation functionals and the strongly constrained and appropriately normed (SCAN) XC functional showed good agreement with EXAFS experiment results. Therefore, the results using the SCAN XC functional are mainly discussed in this study. The Supplementary Information shows the comparison of the results with several other XC functionals (Figures S3–S8 and Table S1).
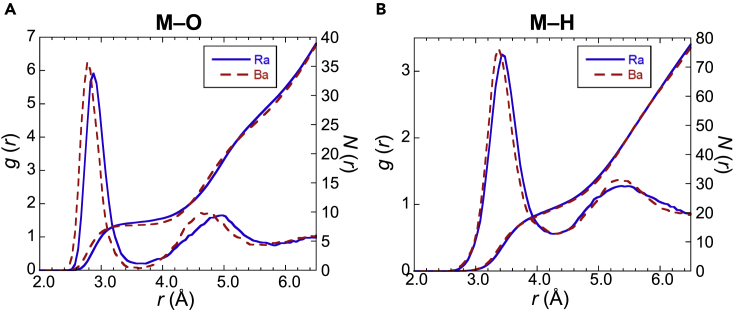
The radial distribution functions (RDFs), , of Ra–O and radium–hydrogen (Ra–H) were evaluated by AIMD simulations (Figure 2). The running integration numbers, , of Ra–O and Ra–H were obtained by the RDFs. The and CN can be obtained from the and , and compared with the experimental EXAFS results. The of Ra–O has two peaks corresponding to the first and second hydration shells (Figure 2A). The first peak position, i.e., 2.88 Å of for Ra–O, is consistent with as determined by the EXAFS experiment (2.87 ± 0.06 Å). This result is consistent with the results predicted by other simulation methods (Table 3). The CN is defined by the value of at the first minimum of , i.e., 8.4. This value is consistent with the experimental value (9.2 ± 1.9). All of the and CN values obtained with several XC functionals are consistent with the experimental results (Figure S3).
Figure 2.
Radial distribution functions and running integration numbers
Radial distribution functions and running integration numbers of (A) M−O and (B) M−H evaluated using the AIMD simulation; M represents the metal ion, Ba2+ or Ra2+.
Table 3.
Comparison of hydration structures of Ba2+ and Ra2+. , CN, tilt angle (), Debye–Waller factor (), and for Ra2+ and Ba2+
| M | Method | (Å) | CN | (Å2) | (Å) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Ra2+ | Exp. | EXAFS | 2.87 ± 0.06 | 9.2 ± 1.9 | – | 0.027 | – | This study |
| Ra2+ | Sim. | AIMD SCAN | 2.88 | 8.4 | 132 | 0.041 | 0.31 | This study |
| Ra2+ | Sim. | FMO-MD | 2.85 | 8.1 | – | – | – | Previous study (Matsuda and Mori, 2014b) |
| Ra2+ | Sim. | MCP | 2.80–2.95 | 7–9 | – | – | – | Previous study (Matsuda and Mori, 2014a) |
| Ra2+ | Sim. | MD | 2.93 | 9.8 | 135 | 0.034 | 0.24 | Previous study (Pappalardo et al., 2021) |
| Ba2+ | Exp. | EXAFS | 2.79 ± 0.02 | 8.0 ± 1.9 | – | 0.020 | – | Previous study (Yamaguchi et al., 2021) |
| Ba2+ | Exp. | EXAFS | 2.81 ± 0.03 | 8.1 ± 0.3 | – | – | – | Previous study (Persson et al., 1995) |
| Ba2+ | Exp. | EXAFS | 2.85 ± 0.02 | 8.0 ± 0.2 | – | – | – | Previous study (Migliorati, Caruso and D’Angelo, 2019) |
| Ba2+ | Sim. | AIMD SCAN | 2.78 | 7.8 | 138 | 0.031 | 0.26 | This study and previous study (Yamaguchi et al., 2021) |
| Ba2+ | Sim. | AIMD PW91 | 2.80 | 8.0 | – | – | – | Previous study (Chaudhari et al., 2015) |
| Ba2+ | Sim. | MD | 2.81 | 9.4 | 138 | 0.034 | 0.22 | Previous study (Pappalardo et al., 2021) |
| Ba2+ | Sim. | MD | 2.85 | 8.1 | – | – | – | Previous study (Migliorati, Caruso and D’Angelo, 2019) |
| Ba2+ | Sim. | QM/MM | 2.86 | 9.3 | – | – | – | Previous study (Hofer et al., 2005) |
The RDF of Ra–H also has two peaks corresponding to the first and second shells (Figure 2B). The value of for Ra–H at the first minimum (0.6) is much larger than that of Ra–O (0.2). In addition, the CN of hydrogen is 21.5, which deviates from the number of protons expected by the CN of oxygen (). This deviation can be explained by the rotation of water molecules in the first and second shells, which increases the of Ra–H between the first and second shells. This trend is also shown in the results of Ba2+.
Structural properties of water molecules around radium ion
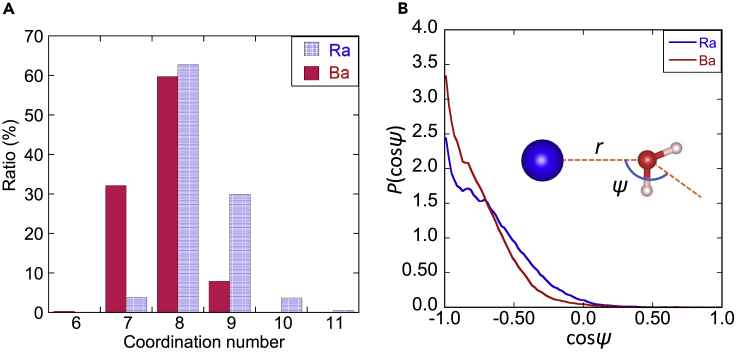
The AIMD simulation results were analyzed in more detail to obtain the structural properties of water molecules hydrating Ra2+ and Ba2+. The AIMD simulation showed that the histogram of the number of water molecules in the first hydration shell fluctuates (Figure 3A). In Ra2+, the eight- and nine-coordinate structures are primary and secondary, respectively, and the seven- and ten-coordinate structures appear rarely. The probability distribution for Ba2+ differs from Ra2+, i.e., the primary structure is the same, but the secondary and tertiary structures are seven- and nine-coordinate, respectively. The difference between the CNs of Ra2+ and Ba2+ reflects these differences between the ancillary structures. The larger secondary structure of Ra2+ can be explained by the difference in ionic radii of Ra2+ and Ba2+, i.e., a larger ionic radius provides a larger first hydration shell volume that can contain a greater number of water molecules.
Figure 3.
Histograms of coordination numbers and probability distribution of tilt angle
(A) Histograms of coordination numbers and (B) the probability distribution of tilt angle () of water molecules hydrating of Ba2+ and Ra2+ evaluated using the AIMD simulation. As for Figure 3B, the horizontal and vertical axes represent the cosine of the tilt angle and the probability distribution , respectively. The inset shows the simple model with a cation and a water molecule discussed in the text. The probability distribution of the tilt angle is a monotonically decreasing function of the distance between the cation and oxygen in the water molecule . See the text for details.
The tilt angle between the dipole vector of a water molecule in the first shell and the vector pointing from the water oxygen to the metal ion is a good indicator of the structural property of the water molecule. Figure 3B shows the flatter probability distribution of the tilt angles for Ra2+ than Ba2+. The flatter distribution implies that the collision between water molecules easily changes the direction of the dipole vector. To understand the difference, we considered a simple model that consists of a cation (M) and a water molecule shown in the inset of Figure 3B. Obviously, the energy to tilt the dipole vector is a monotonic decrease function of the . The is shorter than the (Table 3). Therefore, the direction of the dipole vector of a water molecule in the first shell of Ra2+ can be changed more readily than that of Ba2+. This explains the origin of the flatter distribution of for Ra2+.
The AIMD simulations were also used to evaluate the second cumulants (SCs) of the and (Table 3). SC is defined as follows:
| (Equation 1) |
where , , and represent the distance between an ion and the oxygen atoms of the water molecules in the first hydration shell, its average, and the average of , respectively.
SC is associated with the Debye–Waller (DW) factor in spectroscopic experiments. The value of the SC for Ra2+ (0.041 Å2) is slightly larger than that for Ba2+ (0.031 Å2). Their relationship is consistent with the higher DW factor of Ra2+ in the EXAFS experiments (Table 3). Comparison between these results implies that the hydration structure of Ra2+ is more disordered and flexible than that of Ba2+. This tendency has been confirmed by the simulation results with other XC functionals.
Furthermore, the eccentricity was evaluated for Ra2+ and Ba2+ via AIMD simulations (Table 3). is the average distance between the cation and center of mass of the water molecules in the first hydration shell, defined in a previous study (Caralampio et al., 2017) as follows:
| (Equation 2) |
where represents the center of mass of the water molecules in the first hydration shell. The obtained values were 0.31 Å (Ra2+) and 0.26 Å (Ba2+), which were compared with the classical MD results (Pappalardo et al., 2021) of 0.24 Å (Ra2+) and 0.22 Å (Ba2+). Consistent result of the value of Ra2+ being higher than that of Ba2+ was obtained via the simulation method. These results imply that the hydration shell of Ba2+ is structured to a greater extent than that of Ra2+.
Dynamic properties of water molecules around radium ion
The hydration structure is not static but dynamic. To evaluate the dynamical property, the mean residence time (MRT) of water molecules in the first hydration shell was evaluated by the “direct” method (Hofer et al., 2004), which is defined as
| (Equation 3) |
where , , and are the sampling time (50 ps), average CN, and number of the exchange events that lasted longer ps, respectively. The MRTs for Ra2+ with and 0.5 ps are shorter than those of Ba2+, which is consistent with the previous study (Pappalardo et al., 2021) (Table 4). The MRTs were also calculated for other functionals, suggesting that the dispersion interaction critically affects them (Tabls S1). The lifetime of CN was also evaluated (Figure 4), which depends on . The CN with the longest lifetime of CN of Ra2+ is eight-coordinate both for and 0.5 ps, and those of Ba2+ is seven- and eight-coordinates for and 0.5 ps, respectively. These results are consistent with the structural properties, i.e., the CN (Table 3) and histogram of the CNs (Figure 3). The longest lifetime of the CN of Ra2+ is shorter than that of Ba2+ for and 0.5 ps.
Table 4.
Dynamic properties of Ba2+ and Ra2+
| Property | (ps) | Ba2+ |
Previous study (Pappalardo et al., 2021) | Ra2+ |
Previous study (Pappalardo et al., 2021) |
|---|---|---|---|---|---|
| This study | This study | ||||
| 0 | 28 | – | 102 | – | |
| 0.5 | 4 | – | 11 | – | |
| MRT (ps) | 0 | 14 | 38 | 4 | 20 |
| MRT (ps) | 0.5 | 98 | – | 38 | – |
Figure 4.
Lifetimes of the CN
Lifetimes of the CN of Ba2+ and Ra2+ for (A) ps and (B) . The horizontal and vertical axes represent CN and lifetime (ps), respectively. Red square and blue circle represent the lifetimes of the CN of Ba2+ and Ra2+, respectively.
These results suggest that the water molecules in the first hydration shell of Ra2+ are more labile than those of Ba2+. Some previous results support the interpretation, e.g., Ra2+ has the poorest structured water molecules in the hydration shell among the alkaline earth ions (Matsuda and Mori, 2014a), and Ra2+ has the largest self-diffusion coefficient at infinite dilution in water among the alkaline earth ions owing to the smallest ionic potential (Yuan-Hui and Gregory, 1974).
Comparison between radium and other alkaline earth metal ions
Barium(II) has often been used as an analog of Ra2+ because Ba2+ has an ionic radius close to Ra2+ and often shows similar physical and chemical properties to Ra2+. However, the quantitative differences between them have been scarcely discussed owing to the lack of spectroscopic and theoretical results regarding Ra2+. Our EXAFS and AIMD results in this study clarify the differences. As shown by the simulations and confirmed via the experiments, the CN of Ra2+ is larger than that of Ba2+. Our AIMD simulations showed that the difference in the CNs between Ra2+ and Ba2+ is owing to the difference in the dynamic motion of water molecules. Both hydrated Ra2+ and Ba2+ primarily have an eight-coordinate structure. However, the secondary structures determine the CNs of the ions: the dominant structure is nine-coordinate for Ra2+, while that for Ba2+ is seven-coordinate. In addition, structural properties, such as the tilt angle, SC, and , imply that the bonds between the water molecules in the first hydration shell of Ra2+ are weaker than those in the first hydration shell of Ba2+. These differences are also found in the dynamical properties: the residence time and the longest lifetime of CN of Ra2+ are shorter than Ba2+, suggesting the water molecules hydrating Ra2+ are more mobile than those of Ba2+.
From these results, it appears that Ra2+ is possibly more labile than Ba2+. Therefore, for example, the difference between Ra2+ and Ba2+ should be considered when the reaction processes between these cations and solids are considered. It has been reported that Ba2+ promotes the dissolution of quartz owing to the short residence time of water molecules hydrating Ba2+ (Dove and Nix, 1997; Dove, 1999). Our results indicate that the water molecules hydrating Ra2+ are more mobile than Ba2+ as confirmed by the shorter residence time of those hydrating Ra2+. Therefore, Ra2+ can promote dissolution to a greater extent than Ba2+. This chemical property can be closely related to the vitrification of radioactive waste (Gin et al., 2017) for long-term storage because radioactive wastes contain uranium and its descendant nuclides, such as Ra, and fission products (Swedish, 2011). For the removal of Ra2+ from groundwater, coprecipitation with barite is effective (Kraemer and Reid, 1984; Langmuir and Melchior, 1985; Beaucaire and Toulhoat, 1987; Martin et al., 2003; Vinson et al., 2013). The kinetics of coprecipitation of Ra2+ with barite is fast, and the activation energy is lower than Ba2+ and Sr2+ (Hedstrom et al., 2013). This behavior of Ra2+ can be explained by the higher mobility of water molecules hydrating Ra2+. The present result has an implication for incorporating Ra2+ into apatite (calcium phosphate), the primary step of 223Ra therapy for CRPC. Owing to the ionic radius of Ra2+ being much larger than that of Ca2+, the incorporation of Ra2+ into the bone is not favorable for its stability. However, the high mobility of water molecules hydrating Ra2+ shown by this study has a possibility to promote the incorporation reaction in some way, such as kinetic way.
Conclusions
The average structure of hydration water molecules around Ra2+ was observed by EXAFS spectroscopy, and the values of CN and were determined to be 9.2 ± 1.9 Å and 2.87 ± 0.06 Å, respectively. These values were consistent with the values of the other divalent alkaline earth ions. The results of our AIMD study provided values of 8.4 and 2.88 Å for CN and , respectively, and these values agree with the experimentally obtained ones. AIMD simulations were also performed to investigate the structural properties of water molecules by calculating the tilt angle between the dipole vector of a water molecule in the first shell and the vector pointing from the water oxygen to the metal ion, the SC of the distance between the first shell ion and oxygen, and the . The results imply that the water molecules in the first shell of Ra2+ are structured to a less extent than those of Ba2+. The dynamical properties were investigated via MRT and the lifetime of CN. The results showed shorter MRT and the shorter longest lifetime of CN for Ra2+ than those for Ba2+, suggesting the more labile hydration structure of Ra2+. The mobile water molecules in the first hydration shell of Ra2+ can explain the fast coprecipitation of Ra2+ with barite, and they predict that Ra2+ can promote the hydrolysis of quartz or other oxides to a greater extent than Ba2+. This study marks the beginning of a systematic scientific investigation of its transport in the environment and living organisms. These investigations contribute to improving environmental pollution owing to Ra and to the development of cancer therapy.
Limitations of the study
A cell containing 100 H2O molecules and a Ra2+ was simulated for 60 ps by AIMD simulation in this study. This calculated cell and time can be small and short. Errors in the fitted parameters of EXAFS were estimated to be generally ±0.02 A˚ for R, ± 20% for CN, and 20% for (O’Day et al., 1994).
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Sr-resin | Eichrom Technologies, Inc., USA | https://www.eichrom.com/eichrom/products/sr-resin/ |
| Thallium oxide | FUJIFILM Wako Pure Chemical Corporation | 203-00812 |
| Software and algorithms | ||
| The Vienna Ab initio Simulation Package (VASP) | Universität Wien | https://www.vasp.at/ |
| Python version 3.9 | Python Software Foundation | https://www.python.org |
| VESTA | Koichi Momma and Fujio Izumi | https://jp-minerals.org/vesta/en/ |
| FEFF | University of Washington | http://monalisa.phys.washington.edu/feffproject-feff.html |
| REX2000 | Rigaku Co., Tokyo, Japan | Version 2.5.9 |
| Other | ||
| 19-element solid-state detector (SSD) | CANBERRA | GL0110S |
| Bag made with polyethylene and nylon | FUKUSUKE KOGYO CO., LTD. | 0704997 |
| Bag made with polypropylene | AS ONE Corporation | 1-1471-01 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Akiko Yamaguchi (yamaguchi.akiko@jaea.go.jp).
Materials availability
This study did not generate new unique reagents.
Method details
EXAFS measurement
The hydration structure of Ra2+ was investigated at the atomic-level by EXAFS spectroscopy. The EXAFS measurements were conducted by detecting the fluorescence X-ray of Ra. The energy of Ra Lα1 (12.339 keV) is close to that of lead (Pb) Lβ4 12.307 (keV). Thus, Pb, a descendant element of Ra, should be removed from the samples. Therefore, Ra2+ solution was refined with 0.001 M nitric acid using Sr-resin (Eichrom Technologies, Inc., USA) within 3 weeks before the EXAFS measurement. A solution of 50 μL containing 2 MBq Ra, corresponding to approximately 4 mM, was interfused to a sponge made by polyvinyl alcohol and packed into bags made with polyethylene and nylon thrice and a bag made with polypropylene, following the Japanese Act (Nuclear Regulation Authority, 1957) for treating radio isotope.
The EXAFS spectra were collected at BL22XU in SPring–8 (Harima, Japan). The energy of incident X-ray was monochromatized with the monochromator of two parallel Si(111) crystals and calibrated by assigning the LI-edge of thallium to 15.353 keV. For the calibration, a pellet of thallium oxide (Tl2O3) diluted by boron nitride was placed downstream of the ion chamber for transmission mode (I1) and upstream of the ion chamber for second transmission mode (I2). The X-ray absorption near edge structure of Tl was measured using I1 and I2 simultaneously with the Ra EXAFS measurement. The LIII-edge EXAFS spectra of Ra were collected in fluorescence mode. The fluorescence X-ray of Ra Lα1 was observed by a 19-element solid-state detector (SSD) positioned at 45° to the incident X-ray. The EXAFS measurements of Ra were repeated 11 times (one scan: 60 min.) and the obtained spectra were summated.
The Ra EXAFS spectra analysis was conducted using REX2000 (Rigaku Co., Tokyo, Japan). Backscattering amplitude and phase shift were obtained by FEFF 7.0 (Ankudinov and Rehr, 1997), using a radium sulfate structure (Matyskin et al., 2017). They were plotted in Figures S1A and S1B. The curve obtained by the inverse Fourier transformation of the normalized EXAFS oscillation with a limited range from 1.84 to 2.85 Å was used for the fitting by REX2000 (Figure S1C).
AIMD simulation
The hydration structure of Ra2+ was investigated in more detail by ab initio molecular dynamics (AIMD) simulations based on density functional theory (DFT), using the Vienna ab initio simulation package (Kresse and Hafner, 1993; Kresse and Furthmüller, 1996). The dynamics of a Ra2+ and 100 water molecules in a cubic cell of side length 14.457 Å with periodic boundary conditions were calculated using Γ-only k-point DFT. For the calculation of the hydrated Ra2+ system, the total valence state of the cell was adjusted to +2, and a jellium background was introduced for charge neutralization. The Nosé–Hoover thermostat was used for the NVT simulation with a time step of 0.25 fs at 330 K (Nosé, 1984; Hoover, 1985). This temperature is suitable for the simulation of bulk water using the strongly constrained and appropriately normed (SCAN) meta-GGA exchange-correlation (XC) functional (Chen et al., 2017; Zheng et al., 2018; LaCount and Gygi, 2019; Xu et al., 2019; Dellostritto et al., 2020; Duignan et al., 2020a, 2020b).
This simulation in the main text was conducted with the SCAN meta-GGA XC functional (Sun et al., 2015). In general, the results of DFT calculations depend on the XC functionals. Therefore, the AIMD simulations with the Perdew–Burke–Ernzerhof (PBE) (Perdew et al., 1996), Becke–Lee–Yang–Parr (BLYP) (Becke, 1988; Lee et al., 2011), PBE-D3, and BLYP-D3 (Grimme, 2006; Grimme et al., 2010; Grimme et al., 2011) GGA XC functionals were performed for comparison. The PBE-D3 and BLYP-D3 GGA XC functionals include the zero damping DFT-D3 dispersion interaction correction (Grimme et al., 2010). For all calculations, snaps from 10 to 60 ps were analyzed after 10 ps equilibration. The time evolutions of the total energies of the hydrated Ra2+ and Ba2+ systems with these XC functionals were evaluated to confirm their thermal equilibriums (Figure S2).
Quantification and statistical analysis
EXAFS fitting was performed using FEFF 7.0 (Ankudinov and Rehr, 1997) and REX2000 (Rigaku Co., Tokyo, Japan). Analysis of AIMD simulation was conducted based on Equations 1, 2, and 3 described in the main text.
Acknowledgments
The AIMD simulation was performed on the supercomputing system HPE SGI8600 at Japan Atomic Energy Agency. XAFS analysis was conducted with the approval of KEK-PF (Proposal Nos. 2018G089, 2018G575, and 2019S1001) and SPring-8 (Proposal Nos. 2019A1523, 2019B1498, 2020A0174, 2021A3712, and 2021B3720). The crystal structures were drawn with VESTA (Momma and Izumi, 2011). This study was partially supported by JSPS KAKENHI Grant Number 19K23432 and by the JAEA Fund for Exploratory Researches (Houga fund).
Author contributions
The refining of radium was conducted by K. Nagata and T. Yoshimura. EXAFS measurement was carried out by A. Yamaguchi, K. Tanaka, K. Shimojo, Y. Kaneta, S. Matsuda, and T. Sekiguchi under the supervision of T. Kobayashi and H. Tanida. AIMD simulation was carried and analyzed by A. Yamaguchi and K. Kobayashi under the supervision of M. Okumura. Experiments were planned and designed by A. Yamaguchi under the supervision of K. Yokoyama, T. Yaita, and Y. Takahashi. The article was drafted by A. Yamaguchi and critically revised by K. Kobayashi, Y. Takahashi, and M. Okumura.
Declaration of interests
The authors declare no competing interests.
Published: August 19, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.104763.
Supplemental information
Data and code availability
-
•
Data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original codes.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Ankudinov A.L., Rehr J.J. Relativistic calculations of spin-dependent x-ray-absorption spectra. Phys. Rev. B. 1997;56:R1712–R1716. doi: 10.1103/PhysRevB.56.R1712. [DOI] [Google Scholar]
- Beaucaire C., Toulhoat P. Redox chemistry of uranium and iron, radium geochemistry, and uranium isotopes in the groundwaters of the Lodeve Basin, Massif Central, France. Appl. Geochem. 1987;2:417–426. doi: 10.1016/0883-2927(87)90025-4. [DOI] [Google Scholar]
- Becke A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A. Gen. Phys. 1988;38:3098–3100. doi: 10.1103/PhysRevA.38.3098. [DOI] [PubMed] [Google Scholar]
- Burnett W.C., Aggarwal P.K., Aureli A., Bokuniewicz H., Cable J.E., Charette M.A., Kontar E., Krupa S., Kulkarni K.M., Loveless A., et al. Quantifying submarine groundwater discharge in the coastal zone via multiple methods. Sci. Total. Environ. 2006;367:498–543. doi: 10.1016/j.scitotenv.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Caralampio D.Z., Martínez J.M., Pappalardo R.R., Marcos E.S. The hydration structure of the heavy-alkalines Rb+ and Cs+ through molecular dynamics and X-ray absorption spectroscopy: surface clusters and eccentricity. Phys. Chem. Chem. Phys. 2017;19:28993–29004. doi: 10.1039/c7cp05346k. [DOI] [PubMed] [Google Scholar]
- Chaudhari M.I., Soniat M., Rempe S.B. Octa-coordination and the aqueous Ba2+ ion. J. Phys. Chem. B. 2015;119:8746–8753. doi: 10.1021/acs.jpcb.5b03050. [DOI] [PubMed] [Google Scholar]
- Chen M., Ko H.Y., Remsing R.C., Calegari Andrade M.F., Santra B., Sun Z., Selloni A., Car R., Klein M.L., Perdew J.P., Wu X. Ab initio theory and modeling of water. Proc. Natl. Acad. Sci. USA. 2017;114:10846–10851. doi: 10.1073/pnas.1712499114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.A., Kocar B.D. Radium sorption to iron (hydr)oxides, pyrite, and montmorillonite: implications for mobility. Environ. Sci. Technol. 2018;52:4023–4030. doi: 10.1021/acs.est.7b05443. [DOI] [PubMed] [Google Scholar]
- Curti E., Fujiwara K., Iijima K., Tits J., Cuesta C., Kitamura A., Glaus M.A., Müller W. Radium uptake during barite recrystallization at 23±2°C as a function of solution composition: an experimental 133Ba and 226Ra tracer study. Geochem. Cosmochim. Acta. 2010;74:3553–3570. doi: 10.1016/j.gca.2010.03.018. [DOI] [Google Scholar]
- Delgado Bolton R.C., Giammarile F. Bone radionuclide therapy and increased survival with radium-223 is the way to go for nuclear medicine: the offer that oncologists cannot refuse. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:822–823. doi: 10.1007/s00259-017-3913-z. [DOI] [PubMed] [Google Scholar]
- Dellostritto M., Xu J., Wu X., Klein M.L. Aqueous solvation of the chloride ion revisited with density functional theory: impact of correlation and exchange approximations. Phys. Chem. Chem. Phys. 2020;22:10666–10675. doi: 10.1039/c9cp06821j. [DOI] [PubMed] [Google Scholar]
- Deshayes E., Roumiguie M., Thibault C., Beuzeboc P., Cachin F., Hennequin C., Huglo D., Rozet F., Kassab-Chahmi D., Rebillard X., Houédé N. Radium 223 dichloride for prostate cancer treatment. Drug Des. Dev. Ther. 2017;11:2643–2651. doi: 10.2147/DDDT.S122417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerner H.A., Hoskins W.M. Co-precipitation of radium and barium sulfates. J. Am. Chem. Soc. 1925;47:662–675. doi: 10.1021/ja01680a010. [DOI] [Google Scholar]
- Dove P.M. The dissolution kinetics of quartz in aqueous mixed cation solutions. Geochem. Cosmochim. Acta. 1999;63:3715–3727. doi: 10.1016/S0016-7037(99)00218-5. [DOI] [Google Scholar]
- Dove P.M., Nix C.J. The influence of the alkaline earth cations, magnesium, calcium and barium on the dissolution kinetics of quartz. Geochem. Cosmochim. Acta. 1997;61:3329–3340. doi: 10.1016/S0016-7037(97)00217-2. [DOI] [Google Scholar]
- Duignan T.T., Mundy C.J., Schenter G.K., Zhao X.S. Method for accurately predicting solvation structure. J. Chem. Theor. Comput. 2020;16:5401–5409. doi: 10.1021/acs.jctc.0c00300. [DOI] [PubMed] [Google Scholar]
- Duignan T.T., Schenter G.K., Fulton J.L., Huthwelker T., Balasubramanian M., Galib M., Baer M.D., Wilhelm J., Hutter J., Del Ben M., et al. Quantifying the hydration structure of sodium and potassium ions: taking additional steps on Jacob’s Ladder. Phys. Chem. Chem. Phys. 2020;22:10641–10652. doi: 10.1039/c9cp06161d. [DOI] [PubMed] [Google Scholar]
- Gin S., Jollivet P., Tribet M., Peuget S., Schuller S. Radionuclides containment in nuclear glasses: an overview. Radiochim. Acta. 2017;105:927–959. doi: 10.1515/ract-2016-2658. [DOI] [Google Scholar]
- Grimme S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006;27:1787–1799. doi: 10.1002/jcc.20495. [DOI] [PubMed] [Google Scholar]
- Grimme S., Antony J., Ehrlich S., Krieg H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010;132 doi: 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
- Grimme S., Ehrlich S., Goerigk L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011;32:1456–1465. doi: 10.1002/jcc.21759. [DOI] [PubMed] [Google Scholar]
- Guo H., Zhao W., Li H., Xiu W., Shen J. High radionuclides in groundwater of an inland basin from Northwest China: origin and fate. ACS Earth Space Chem. 2018;2:1137–1144. doi: 10.1021/acsearthspacechem.8b00108. [DOI] [Google Scholar]
- Hedström H., Ramebäck H., Ekberg C. A study of the Arrhenius behavior of the co-precipitation of radium, barium and strontium sulfate. J. Radioanal. Nucl. Chem. 2013;298:847–852. doi: 10.1007/s10967-013-2431-0. [DOI] [Google Scholar]
- Hess C.T., Michel J., Horton T.R., Prichard H.M., Coniglio W.A. The occurrence of radioactivity in public water supplies in the United States. Health. Phys. 1985;48:553–586. doi: 10.1097/00004032-198505000-00002. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Horie K., Gauthier-Lafaye F. Transport and selective uptake of radium into natural clay minerals. Earth. Planet. Sci. Lett. 2007;264:167–176. doi: 10.1016/j.epsl.2007.09.027. [DOI] [Google Scholar]
- Hofer T.S., Tran H.T., Schwenk C.F., Rode B.M. Characterization of dynamics and reactivities of solvated ions by ab initio simulations. J. Comput. Chem. 2004;25:211–217. doi: 10.1002/jcc.10374. [DOI] [PubMed] [Google Scholar]
- Hofer T.S., Rode B.M., Randolf B.R. Structure and dynamics of solvated Ba(II) in dilute aqueous solution - an ab initio QM/MM MD approach. Chem. Phys. 2005;312:81–88. doi: 10.1016/j.chemphys.2004.11.023. [DOI] [Google Scholar]
- Hoover W.G. Canonical dynamics: equilibrium phase-space distributions. Physiol. Rev. 1985;31:1695–1697. doi: 10.1007/BF00419952. [DOI] [PubMed] [Google Scholar]
- Kraemer T.F., Reid D.F. The occurrence and behavior of radium in saline formation water of the U.S. Gulf Coast region. Chem. Geol. 1984;46:153–174. doi: 10.1016/0009-2541(84)90186-4. [DOI] [Google Scholar]
- Kresse G., Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. Condens. Matter. 1996;54:11169–11186. doi: 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- Kresse G., Hafner J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B Condens. Matter. 1993;47:558–561. doi: 10.1103/PhysRevB.47.558. [DOI] [PubMed] [Google Scholar]
- LaCount M.D., Gygi F. Ensemble first-principles molecular dynamics simulations of water using the SCAN meta-GGA density functional. J. Chem. Phys. 2019;151:164101. doi: 10.1063/1.5124957. [DOI] [PubMed] [Google Scholar]
- Langmuir D., Riese A.C., Langmuir D R.A., Langmuir D., Riese A.C. The thermodynamic properties of radium. Geochem. Cosmochim. Acta. 1985;49:1593–1601. doi: 10.1016/0016-7037(85)90264-9. [DOI] [Google Scholar]
- Langmuir D., Melchior D. The geochemistry of Ca, Sr, Ba and Ra sulfates in some deep brines from the Palo Duro Basin, Texas. Geochem. Cosmochim. Acta. 1985;49:2423–2432. doi: 10.1016/0016-7037(85)90242-X. [DOI] [Google Scholar]
- Lee C., Yang W., Parr R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- Marcus Y. Ionic radii in aqueous solutions. Chem. Rev. 1988;88:1475–1498. doi: 10.1021/cr00090a003. [DOI] [Google Scholar]
- Marcus Y. Effect of ions on the structure of water. Pure. Appl. Chem. 2010;82:1889–1899. doi: 10.1351/PAC-CON-09-07-02. [DOI] [Google Scholar]
- Martin A.J., Crusius J., Jay McNee J., Yanful E.K. The mobility of radium-226 and trace metals in pre-oxidized subaqueous uranium mill tailings. Appl. Geochem. 2003;18:1095–1110. doi: 10.1016/S0883-2927(02)00243-3. [DOI] [Google Scholar]
- Matsuda A., Mori H. A quantum chemical study on hydration of Ra (II): comparison with the other hydrated divalent alkaline earth metal ions. J. Comput. Chem. Jpn. 2014;13:105–113. doi: 10.2477/jccj.2013-0011. [DOI] [Google Scholar]
- Matsuda A., Mori H. Theoretical study on the hydration structure of divalent radium ion using fragment molecular orbital-molecular dynamics (FMO-MD) simulation. J. Solut. Chem. 2014;43:1669–1675. doi: 10.1007/s10953-014-0235-7. [DOI] [Google Scholar]
- Matyskin A.V., Ylmen R., Lagerkvist P., Ramebäck H., Ekberg C. Crystal structure of radium sulfate: an X-ray powder diffraction and density functional theory study. J. Solid State Chem. 2017;253:15–20. doi: 10.1016/j.jssc.2017.05.024. [DOI] [Google Scholar]
- Migliorati V., Caruso A., D’Angelo P. Unraveling the hydration properties of the Ba2+ aqua ion: the interplay of quantum mechanics, molecular dynamics, and EXAFS spectroscopy. Inorg. Chem. 2019;58:14551–14559. doi: 10.1021/acs.inorgchem.9b02204. [DOI] [PubMed] [Google Scholar]
- Momma K., Izumi F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011;44:1272–1276. doi: 10.1107/S0021889811038970. [DOI] [Google Scholar]
- Morris M.J., Corey E., Guise T.A., Gulley J.L., Kevin Kelly W., Quinn D.I., Scholz A., Sgouros G. Radium-223 mechanism of action: implications for use in treatment combinations. Nat. Rev. Urol. 2019;16:745–756. doi: 10.1038/s41585-019-0251-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosé S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984;81:511–519. doi: 10.1063/1.447334. [DOI] [Google Scholar]
- Nuclear Regulation Authority . Japanese Law Translation; 1957. Act on prevention of radiation hazards due to radioisotopes etc., Law No. 167.https://www.japaneselawtranslation.go.jp/en/laws/view/3962 Latest Revision in September 11, 2019. [Google Scholar]
- O’Day P.A., Rehr J.J., Zabinsky S.I., Brown G.E.J. Extended X-ray absorption fine structure (EXAFS) analysis of disorder and multiple scattering in complex crystalline solids. J. Am. Chem. Soc. 1994;116:2938–2949. [Google Scholar]
- Ohtaki H., Radnai T. Structure and dynamics of hydrated ions. Chem. Rev. 1993;93:1157–1204. doi: 10.1021/cr00019a014. [DOI] [Google Scholar]
- Olley J.M., Murray A., Roberts R.G. The effects of disequilibria in the uranium and thorium decay chains on burial dose rates in fluvial sediments. Quat. Sci. Rev. 1996;15:751–760. doi: 10.1016/0277-3791(96)00026-1. [DOI] [Google Scholar]
- Pappalardo R.R., Caralampio D.Z., Martínez J.M., Sánchez Marcos E. Hydration of heavy alkaline-earth cations studied by molecular dynamics simulations and X-ray absorption spectroscopy. Inorg. Chem. 2021;60:13578–13587. doi: 10.1021/acs.inorgchem.1c01888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdew J.P., Burke K., Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- Persson I., Sandstrom M., Yokoyama H., Monika C. Structure of the solvated strontium and barium ions in aqueous, dimethl sulfoxide and pyridine solution, and crystal structure of strontium and barium hydroxide octahydrate. Z. Naturforsch. 1995;50:21–37. doi: 10.1515/zna-1995-0105. [DOI] [Google Scholar]
- Shannon R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta. Crystallogr. A.,Found. Crystallogr. 1976;32:751–767. doi: 10.1107/S0567739476001551. [DOI] [Google Scholar]
- Sun J., Ruzsinszky A., Perdew J.P. Strongly constrained and appropriately normed semilocal density functional. Phys. Rev. Lett. 2015;115 doi: 10.1103/PhysRevLett.115.036402. [DOI] [PubMed] [Google Scholar]
- Swedish N.F. 2011. Long-term Safety for the Final Repository for Spent Nuclear Fuel at Forsmark Main Report of the SR-Site Project Volume III. [Google Scholar]
- Szabo Z., dePaul V.T., Fischer J.M., Kraemer T.F., Jacobsen E. Occurrence and geochemistry of radium in water from principal drinking-water aquifer systems of the United States. Appl. Geochem. 2012;27:729–752. doi: 10.1016/j.apgeochem.2011.11.002. [DOI] [Google Scholar]
- Tanguy J.C., Condomines M., Le Goff M., Chillemi V., La Delfa S., Patanè G. Mount Etna eruptions of the last 2, 750 years: revised chronology and location through archeomagnetic and 226Ra-230Th dating. Bull. Volcanol. 2007;70:55–83. doi: 10.1007/s00445-007-0121-x. [DOI] [Google Scholar]
- Vengosh A., Jackson R.B., Warner N., Darrah T.H., Kondash A. A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ. Sci. Technol. 2014;48:8334–8348. doi: 10.1021/es405118y. [DOI] [PubMed] [Google Scholar]
- Vinson D.S., Tagma T., Bouchaou L., Dwyer G.S., Warner N.R., Vengosh A. Occurrence and Mobilization of Radium in Fresh to Saline Coastal Groundwater Inferred from Geochemical and Isotopic Tracers (Sr, S, O, H, R., Rn) Appl. Geochem. 2013;38:161–175. doi: 10.1016/j.apgeochem.2013.09.004. [DOI] [Google Scholar]
- Xu J., Chen M., Zhang C., Wu X. First-principles study of the infrared spectrum in liquid water from a systematically improved description of H-bond network. Phys. Rev. B. 2019;99 doi: 10.1103/PhysRevB.99.205123. [DOI] [Google Scholar]
- Yamaguchi A., Kobayashi K., Takahashi Y., Machida M., Okumura M. Hydration structures of barium ions: Ab initio molecular dynamics simulations using the SCAN meta-GGA density functional and EXAFS spectroscopy studies. Chem. Phys. Lett. 2021;780 doi: 10.1016/j.cplett.2021.138945. [DOI] [Google Scholar]
- Yokoyama H. Japanese Society of Chemistry. Center for Academic Publications Japan; 1995. Molecular pictures of solution, ‘structure of hydrated ions’. [Google Scholar]
- Yuan-Hui L., Gregory S. Diffusion of ions in sea water and in deep-sea sediments. Geochem. Cosmochim. Acta. 1974;38:703–714. doi: 10.1016/0016-7037(74)90145-8. [DOI] [Google Scholar]
- Zheng L., Chen M., Sun Z., Ko H.Y., Santra B., Dhuvad P., Wu X. Structural, electronic, and dynamical properties of liquid water by ab initio molecular dynamics based on SCAN functional within the canonical ensemble. J. Chem. Phys. 2018;148:164505. doi: 10.1063/1.5023611. [DOI] [PubMed] [Google Scholar]
- Zhuo W., Iida T., Yang X. Occurrence of 222Rn, 226Ra, 228Ra and U in groundwater in fujian province, China. J. Environ. Radioact. 2001;53:111–120. doi: 10.1016/S0265-931X(00)00108-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original codes.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.