Abstract
The alimentary tract in chickens plays a crucial role in immune cell formation and immune challenges, which regulate intestinal flora and sustain extra-intestinal immunity. The interaction between pathogenic microorganisms and the host commensal microbiota as well as the variety and integrity of gut microbiota play a vital role in health and disease conditions. Thus, several studies have highlighted the importance of gut microbiota in developing immunity against viral infections in chickens. The gut microbiota (such as different species of Lactobacillus, Blautia Bifidobacterium, Faecalibacterium, Clostridium XlVa, and members of firmicutes) encounters different pathogens through different mechanisms. The digestive tract is a highly reactive environment, and infectious microorganisms can disturb its homeostasis, resulting in dysbiosis and mucosal infections. Avian influenza viruses (AIV) are highly infectious zoonotic viruses that lead to severe economic losses and pose a threat to the poultry industry worldwide. AIV is a challenging virus that affects gut integrity, disrupts microbial homeostasis and induces inflammatory damage in the intestinal mucosa. H9N2 AIV infection elevates the expression of proinflammatory cytokines, such as interferon (IFN-γ and IFNα) and interleukins (IL-17A and IL-22), and increases the proliferation of members of proteobacteria, particularly Escherichia coli. On the contrary, it decreases the proliferation of certain beneficial bacteria, such as Enterococcus, Lactobacillus and other probiotic microorganisms. In addition, H9N2 AIV decreases the expression of primary gel-forming mucin, endogenous trefoil factor family peptides and tight junction proteins (ZO-1, claudin 3, and occludin), resulting in severe intestinal damage. This review highlights the relationship among AIV, gut microbiota and immunity in chicken.
Key words: avian influenza, chicken, dysbiosis, gut health, immunity, microbiome, probiotics
INTRODUCTION
The microbiome is a vast collection of various microorganisms that are attached to host mucosal surfaces and it plays an essential role in host homeostasis (Das and Nair, 2019; Meijerink et al., 2021). Similar to the mammalian gut microbiome, the chicken gut microbiome is structurally diverse, with communities comprising different bacteria, methanogenic archaea, fungi and viruses (Meijerink et al., 2021). The normal gastrointestinal (GIT) microbiome is essential for the growth and development of an organism, resulting in energy-rich short-chain fatty acid generation, intestinal villus and crypt shape development, nutrient uptake, host metabolism, immune system modulation and development, and infection resistance (Hooper et al., 2012; Yeoman et al., 2012; Swelum et al., 2020; Meijerink et al., 2021). Microbiota can be found throughout the alimentary tract with great diversity and abundance in the ceca (1010–1011 cells/g of cecal content), where a longer transit period of the digestive fluid allows more extensive microbial fermentation (Yeoman et al., 2012).
Compared to mammalian GIT, the chicken GIT is proportionally shorter in length and exhibits a shorter transit time. Yet, chickens are highly efficient in converting consumed feed into meat (Tolkamp et al., 2010). The extraction of energy and nutrients from food does not solely depend on host physiology but is also attributed to the symbiotic interactions with microorganisms that reside in the GIT. The chicken GIT is a complex ecological niche colonized by millions of microorganisms (O'Hara and Shanahan, 2006). This complex microbial community, known as the gut microbiota, comprises commensal, symbiotic, and pathogenic microorganisms that co-exist in close association with the host, and its importance in vital functions is such that it can be considered an additional organ (O'Hara and Shanahan, 2006).
In the gut microbiota, the three domains of life, including archaea, eubacteria, and eukarya, as well as viruses co-exist in close interaction with the host, with bacteria being a predominant member. At an estimated density of 107 to 1011 bacteria/g of digesta, the gut bacterial density is considered one of the highest cell densities for any ecosystem (Apajalahti et al., 2004). Furthermore, the collective gut microbial genome encodes proteins involved in several metabolic pathways, with a potential that surpasses the capacity of the host genome. Thus far, extensive studies have been conducted on chicken microbiota focusing primarily on the bacterial population in the gut (Apajalahti et al., 2004).
Egg-laying chickens (layers) exhibit age-dependent growth of the alimentary microbiome. In the first week of life in these chickens, approximately 20% to 50% of the gut microbiome is predominated by the phylum proteobacteria, primarily including the family enterobacteriaceae and genus Escherichia, whereas the remainder of the gut microbiome comprises the family lachnospiraceae of the phylum firmicutes (Videnska et al., 2014b). From the second to the fourth week of life, the abundance of proteobacteria decreases to <10%, while firmicutes, mainly the families lachnospiraceae and ruminococcaceae, comprise the remaining 90% of the overall gut microbiome (Videnska et al., 2014b).
At the age of 2 to 6 mo, the phylum bacteroidetes replaces firmicutes, where the families rikenellaceae, porphyromonadaceae and bacteroidaceae are predominantly found, whereas at the age of >7 mo, the phyla firmicutes and bacteroidetes exhibit equal abundance. By the age of 34 mo, bacteria belonging to the phylum proteobacteria return and constitutes 5% of the total microbiome, with Desulfovibrio and Succinivibrio represents the predominant genera (Videnska et al., 2014b).
The structure of the broiler chicken microbiome varies from that of the layer microbiome. The GIT microbiome of broilers is mainly dominated by the phyla firmicutes and proteobacteria, representing 76% and 14% of the microbiome, respectively. In contrast, bacteroidetes and actinobacteria together represent only 6.5% of the microbiome (Videnska et al., 2014a). In addition to their nutritional role mediated via the production of enzymes and catabolism of feed polysaccharides (Beckmann et al., 2006), species complexity and microbiome diversity are closely linked to infection tolerance (Yeoman et al., 2012). Various ecological factors, including age, breed, hygiene, feeding, temperature and housing, may affect the microbiome of chickens (Clavijo and Florez, 2018; Ocejo et al., 2019).
Dysbacteriosis; avian gut microbiome is dynamic but plastic, meaning that it can recover its community structure despite rapid changes in the alimentary tract environment (Abd El‐Hack et al., 2021a). Nevertheless, prolonged disturbance of microbial diversity, also known as dysbiosis or dysbacteriosis, can increase an organism's susceptibility to various diseases due to the overgrowth of potentially harmful microorganisms and subsequent infections (Abd El-Hack et al., 2021b). This condition leads to significant loss of overall host health, productive performance, and animal well-being as well as subsequent economic loss. It also poses a threat to food safety in case of zoonotic pathogens (El-Saadony et al., 2021a, 2021b, 2021c).
The gut microbiota plays a crucial role in the regulation and stimulation of host reactions to various respiratory infections (Schuijt et al., 2016; El-Naggar et al., 2022) caused by viruses (Oh et al., 2014; Setta et al., 2018), bacteria (Abd El Hamid et al., 2019; Marouf et al., 2022), parasites (Salem et al., 2022a, 2022b), and fungi (McAleer et al., 2016). The role of the alimentary tract microbiome in chickens in combating bacterial pathogens, such as Clostridium perfringens and Salmonella spp., has been discussed extensively (Videnska et al., 2013; Varmuzova et al., 2016). In a previous study where chickens were injected with Campylobacter jejuni, both antibiotic-treated and specific pathogen-free (SPF) chickens exhibited a considerably higher colony count (colony-forming units of C. jejuni) in their cecal load associated with alimentary tract injuries than conventional birds supplied with the exact count (Han et al., 2017).
The alimentary microbiome can control the antiviral immune response (Budden et al., 2017). However, the current knowledge about alimentary microbiome function in the tolerance or susceptibility to viral pathogens in chickens remains insufficient. Recent studies have reported that commensal microbiota may have a protective influence on the influenza virus infection (Abt et al., 2012). In chickens, many viruses can disrupt the gut microbiota, such as Marek's disease virus (Perumbakkam et al., 2014, 2016), infectious bursal disease virus (Li et al., 2018) and avian influenza viruses (AIV, Li et al., 2018). Thus, viruses possibly influence the homeostatic relationship between the alimentary tract microbiome and the host. Furthermore, the GIT microbiome has also been noted to play a critical role in the immune response to vaccination (Abt et al., 2012; Oh et al., 2014).
In this current review, we shed light on the interaction between the GIT microbiome and AIV and their effect on immune modulation in chickens.
Functions of Gut Microbiota
Within the GIT, continuous interactions occur among host cells, gut microbiota and digesta, and these interactions highlight the exceptionally essential role of gut microbiota in the host health (Waite and Taylor, 2014). For example, gut microbiota has been proven to confer protection against pathogenic microbes, promote beneficial effects in the development of the intestinal morphology and immunology, facilitate feed digestion and absorption of otherwise indigestible nutrients by the host, breaks down toxic compounds and provide beneficial metabolic products (Waite and Taylor, 2014).
A balanced gut microbial community provides a protective mechanism that might prevent the overgrowth of pathogenic bacteria by competitive exclusion, meaning, gut microbiota that settle first in the epithelium compete for space and nutrients with potential pathogens, which might contribute to create an unsuitable environmental atmosphere for potential pathogens or even inhibit their growth through the production of antimicrobial compounds such as bacteriocins (Garcia Gutierrez et al., 2019). Several studies have demonstrated the efficacy of competitive exclusion in conferring protection against enteric pathogens in young chicks as previously reported by Mead (2000).
The gut microbiota influences the maturation and functioning of the intestinal epithelium by promoting cell proliferation and differentiation, triggering enzyme and hormone secretions, and stimulating the maintenance of the intestinal barrier integrity. For example, Bacteroides thetaiotaomicron stimulates the secretion of a protein essential for the maintenance of desmosomes in the epithelial villus, and the bacterial peptidoglycan favors the reinforcement of the tight junctions of the epithelial barrier by activating Toll-like receptor 2 signaling (Lutgendorff et al., 2008). Studies on germ-free chicks showed reduced intestinal movement and body temperature and poor immunity compared with normal chicken; however, their overall health status improved after the administration of normal microbiome (Niba et al., 2009).
The gut microbiota is thought to stimulate immunological responses by activating base levels of inflammation and influence the development of the cellular and humoral immune systems during early life (Cebra, 1999). Experimental organisms lacking gut microbiota are more prone to disease development and generally exhibit deficiencies in their immunological mechanisms, such as slower development of lymphoid cells or poorer development of secondary lymphoid tissues, compared with their wild-type counterparts (Hooper, 2004).
The gut microbiota exhibits remarkable metabolic activity, playing an important role in the host metabolism. By metabolizing nutrient substrates, the gut microbiota produces large amounts of metabolites that aid the host in fulfilling its energy requirements (Józefiak et al., 2008). For example, in carbohydrate fermentation, certain groups of bacteria are responsible for breaking down complex substrates such as nonstarch polysaccharides, which requires specialized hydrolytic enzymes (Józefiak et al., 2008). The resulting metabolites then become available to other members of the microbiota to produce amino acids and short-chain fatty acids (SCFA; primarily acetate, propionate, and butyrate) through subsequent fermentation. These compounds then become readily accessible to the host. These SCFAs are an essential energy source for epithelial cells and have anti-inflammatory and anti-oxidative effects (Józefiak et al., 2008).
Certain vitamins that cannot be synthesized by the host, such as vitamins K and B, can be provided by members of the gut microbiota, and vitamin K and B deficiencies have been reported in animal models lacking gut microbiota (Montalto et al., 2009). By contributing to the breakdown of nutrients that are not digestible by the host and thereby providing the available nutrients, the GIT microbiome influences the growth rate and performance of the host (Montalto et al., 2009). The gut microbiota can also exert certain negative effects, such as competition for substrates or the production of potentially harmful metabolites. For example, proteolytic fermentation by gut microbiota may result in the formation of potentially harmful substances, such as ammonia, indoles and phenols. Moreover, the ability of certain groups of microorganisms to metabolize drugs can compromise the efficacy of these drugs (Swanson, 2015).
ENTERO-TROPISM OF AIV
Avian influenza is a worldwide zoonotic disease and is usually accompanied by significant disruption of the poultry industry as well as epidemics and pandemics (Adlhoch et al., 2021; Hu et al., 2021). It is well known that the poultry entero-tropism of low pathogenic avian influenza (LPAI) is higher than that of highly pathogenic avian influenza (HPAI), which is more linked to the respiratory epithelium (Post et al., 2012). However, effects of the infection caused by HPAI H5N1 on the gut microbiota or fecal bacterial communities of migrating Whooper swans resulted in the disruption of the GIT microbiome structure mediated by a modification in the dominance of bacterial genera such as Lactobacillus and Aeromonas (Zhao et al., 2018). This compositional and characteristic shift of the fecal microbiome may result from the hazardous effect of gut-linked infection aggravating disease transmission (Zhao et al., 2018). In addition, the HPAI 2016 H5N8 virus exhibited a degree of attachment to the wild duck gut epithelia comparable to that of LPAI H4N5 virus. More than that of 2005 H5N1 virus for two of the four duck spp., and chicken tested, indicating that 2016 H5N8 may have gained a parallel entero-tropism to LPAI viruses without losing the respirotropism of older HPAI viruses of the Goose/Guangdong lineage. The elevated entero-tropism of 2016 H5N8 implies that this virus remained in the wild waterfowl hosts for a prolonged period (Caliendo et al., 2020).
Moreover, H9N2 is a significant menace to public health because it may multiply in mammalian cells with no previous acclimatization (Lin et al., 2000; Wan et al., 2008; Zhang et al., 2013), and in humans, HPAI viruses were shown to possess endogenous genes from avian H9N2 viruses (Guan et al., 2015). The LPAI H9N2 subtype has attained a panzootic proportion, causing infections in turkeys, chickens, domestic ducks, ostriches and pheasants (Umar et al., 2016; Adlhoch et al., 2021). Thus, to enhance avian productivity and health, the reduction and control of H9N2 outbreaks can have key advantages in decreasing the exposition of mammals to the virus.
CLASSIFICATION, MORPHOLOGY, AND COMPOSITION OF AIV
AIVs belong to the family orthomyxoviridae, which are single-stranded eight-segmented negative-sense RNA (−ssRNA) viruses that encode at least ten viral proteins (Capua and Alexander, 2004). The family is classified into three main genera of influenza types A, B, and C and two other unknown genera, isavirus, and thogotovirus (Suarez, 2008). Among other influenza viruses, AIVs belonging to the genus influenza A spread most widely, with members infecting avian and mammalian species (Capua and Alexander, 2004). AIVs are morphologically variable, and this variability is controlled by the matrix protein M1 (Suarez, 2008).
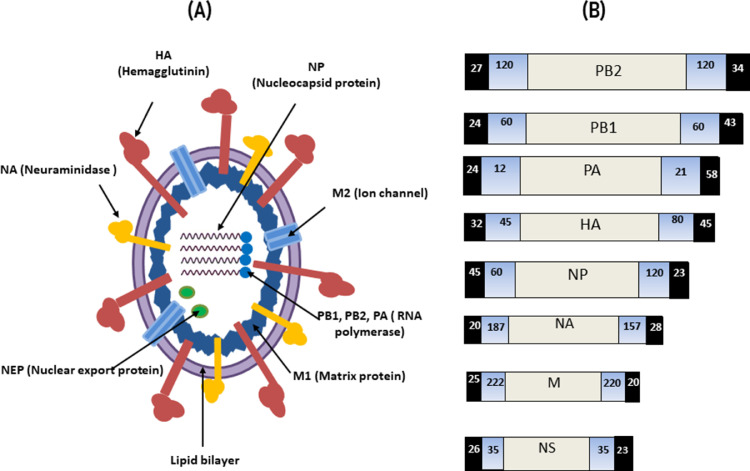
The viruses are pleomorphic, roughly filamentous and several microns in length or spherical with a size of 80 to 120 nm (Figure 1) (Causey and Edwards, 2008). The virion in any morphological form is encapsulated with a lipid envelope derived from the host cell with globular spikes or projections of membrane proteins (Figure 1) (Suarez, 2008). Despite its small genomic size, the AIV has evolved various molecular methods to express several viral proteins from a single gene segment. Until 2001, the eight RNA segments of AIV were supposed to encode ten proteins (Kang et al., 2021).
Figure 1.
(A) The virion structure and (B) genome organization of IAV.
Nevertheless, in 2001, an eleventh protein called PB1-F2, which is translated using an alternative open reading frame within PB1, was identified, and six additional viral proteins were discovered after 2009 (Vasin et al., 2014). Influenza A viruses are further categorized based on strains or subtypes according to their envelope proteins, neuraminidase (NA) and hemagglutinin (HA). Currently, nine (N1–N9) and 16 subtypes (H1–H16) of NA and HA, respectively, are known (Capua and Alexander, 2004). Both HA and NA can elicit subtype-specific immune responses that protect against infections caused by the same subtype and partially protect against infection caused by different subtypes (Swayne, 2008).
PATHOGENICITY AND VIRULENCE OF AIV
The HA surface protein is the major antigenic determinant resulting in clinical signs and immune responses (Perdue, 2008; Hu et al., 2021). HA is a polyprotein with a rounded head cleaved into two subunits (HA-2 and HA-1). The virus attaches to the host cell through the globular head of HA. Hence, antibodies are directed against the globular head, thus preventing the binding of the viral receptor site to the host cell. The globular head also changes rapidly because of mutation, known as antigenic drift, to evade host cell antibodies (Perdue, 2008).
The fusion of the host cell with the virus particle to release the virion to begin viral replication in the host cell is also mediated by the fusion peptide on the HA. Uncleaved HA polyprotein is termed HAO, which must be cleaved into HA-1 and HA-2 at the point of their disulfide linkage (the proteolytic cleavage site) before the virus can infect a cell. The basic amino acid sequence of the proteolytic cleavage site on the HAO determines the pathogenicity of AIVs (Perdue, 2008; Kang et al., 2021). Based on pathogenicity in chickens and turkeys, these viruses are categorized into LPAI, which causes mild respiratory disease or a drop in egg production, and HPAI, which causes severe systemic disease and exhibits a mortality rate of up to 100% (Figure 2) (OIE, 2006).
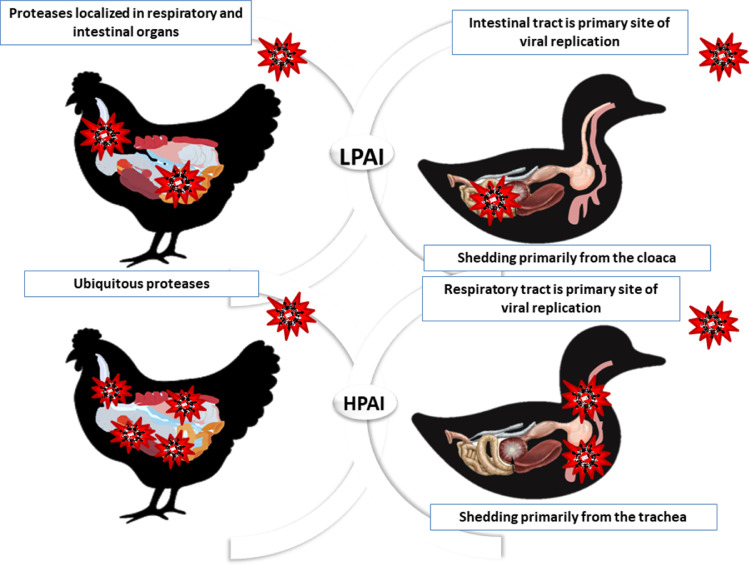
Figure 2.
Localization of systemic highly pathogenic avian influenza (HPAI) infection versus low pathogenic avian influenza (LPAI) infection in chicken and duck. In chickens, proteases in gut and respiratory systems enable LPAI and HPAI replication while in ducks, their gut is the primary organ for LPAI replication and shedding and respiratory tract is the main site for HPAI replication and shedding.
At the clinical level, the virulence of AIV varies significantly with host species and age, environmental factors, other pathogens’ presence, and immunity (Kang et al., 2021). In wildfowl, these viruses are completely accommodated and produce no symptoms of infection. Thus, these birds are considered the natural host of AIV (Webster et al., 1992). Therefore, wild birds are considered to be the primary source or maintenance host for all influenza viruses that infect mammals (Webster et al., 1992). Conversely, domestic birds such as turkeys, chickens, ostriches, and quail are sensitive to AIV outbreaks (Perez et al., 2003). Clinical manifestations of AIV infection in poultry range from asymptomatic to a broad spectrum of symptoms such as severe respiratory illness, production loss, and acute fatal infection with elevated morbidity and mortality (Suarez and Schultz-Cherry, 2000). According to the World Organisation for Animal Health, formerly known as the Office International des Epizooties (OIE), AIV is classified into two pathotypes, LPAI and HPAI.
At least 75% of the total cases of mortality in 4 to 8-wk-old chickens are mainly due to HPAI viruses, whereas LPAI viruses are generally considerably less virulent (Lee and Saif, 2009). Nevertheless, significant symptoms occur in chickens as a result of LPAI infection, which usually causes infection when the birds are subjected to environmental stressors or coinfected with other pathogens (Nili and Asasi, 2003). Although LPAI viruses can exhibit any HA antigen type (H1–H18), all HPAI were detected with only H5 or H7 antigens (Swayne, 2008). The segmented nature of the AIV genome elevates the possibility of reasserting viral gene segments from a mixed illness with two or more virus subtypes, leading to the formation of pandemic viruses (de Silva et al., 2012). Globally, LPAI viruses such as the H9N2 subtype have become more dominant since the early 1990s and are associated with elevated morbidity and mortality and a significant decrease in egg curve (Alexander, 2007). However, data regarding the disease symptoms in birds experimentally challenged with LPAI viruses remain limited.
Earlier, LPAI viruses were discovered in the secretions from the respiratory tract and droppings of chickens inoculated intranasally, orally, or intratracheally. Some viruses were isolated from the droppings as early as on postinfection day 2 (Swayne and Beck, 2005). The LPAI subtype H5N2 was primarily observed in oropharyngeal samples compared with cloacal swabs during the clinical phase, but more viruses were observed in the droppings than in respiratory secretions after host recovery. Earlier studies have revealed that LPAI viruses are primarily limited to the respiratory and intestinal tracts as these sites contain host proteases, such as trypsin-like enzymes, required for cleavage and HA glycoprotein production (Alexander, 2000). Nevertheless, recent studies have reported a much broader tissue tropism of LPAI viruses in the brain, spleen, cardiac, hepatic, and renal tissues (Post et al., 2012, 2013). Stimulating a GIT immune reaction by the rapid replication of LPAI viruses is crucial in protecting birds from the lethal impact of HPAI viruses (Hu et al., 2021).
PATHOGENESIS OF AIV
Infection through the respiratory tract with aerosolized droplets or the GIT via the orofecal route, AIV initiates infection and replication in the host cell. HPAI induces systemic disease in humans and animals through the bloodstream and lymphatics and may attack the nervous system (Perkins and Swayne, 2003). The HPAI H5N1 virus isolated from the vascular endothelium, heart, pancreas, brain, and adrenal glands of chickens and other gallinaceous birds leads to cardiovascular damage due to endothelial activation and disruption, leukocyte activation with systemic cytokine release, cardiopulmonary failure, and multiorgan damage (Perkins and Swayne, 2003).
Pathological and clinical findings vary based on several determinants, with LPAI infection in chickens and turkeys often being accompanied by mild respiratory distress and reduced egg production. Tracheitis, sinusitis, air saculitis, nephritis, ovaritis, and oviduct lesions with egg peritonitis in layers can be observed sometimes (Suarez, 2008; Wakawa et al., 2008; Chrzastek et al., 2021). Clinical signs of HPAI infection may be absent in the peracute disease form. Respiratory distress, coughing, sneezing, cyanosis of combs and wattle, hemorrhages on the shank, bleeding from the nares, diarrhea, circling, incoordination, and death may also be noted (Wakawa et al., 2008). Pathological lesions may include hydropericardium, hemorrhages in the mesentery and pericardial serosa, pulmonary edema, mucus in the trachea, pinpoint hemorrhages in the proventriculus, serous exudates in the body cavities, pancreatic necrosis, and nonsuppurative brain lesions (Perkins and Swayne, 2003; Li and Chen, 2021).
GUT MICROBIOTA AND CHICKEN HEALTH
The host and microbiome are in a stable relationship where their communication pattern depends on microbial ecology and host immune status (El-Saadony et al., 2022). Microbiota composition can be affected by various dietary factors such as the components of diet, concentrations of nutrients (carbohydrates, fats, proteins, water, minerals, vitamins, and fibers), physical characteristics/structure of feed (grain type and particle size), or supplementation of feed (Abou-Kassem et al., 2021a).
Various methods have been suggested to maintain the resistance of the gut mucosa and microbiome to avoid excessive inflammatory reactions (Meijerink et al., 2021). Members of particular microbial groups, such as species of lactobacilli, can stimulate the habituation of dendritic cells by intraepithelial lymphocytes toward a tolerogenic phenotype, thus stimulating the differentiation of T cells into regulatory T (Treg) and T helper (Th) 2 subsets (Zeuthen et al., 2008). Another approach of initiating tolerance is to decrease the toxic effect of lipopolysaccharides (LPS), which is available in the GIT and can cause systemic toxic shock (Sekirov et al., 2010). LPS reduction can be performed by dephosphorylation of the LPS endotoxin via the activity of intestinal alkaline phosphatase, which was reported to lower MyD88- and TNF-α-mediated recruitment of neutrophils at the gut epithelium, thereby enhancing tolerance to the gut microbiome and decreasing inflammatory reaction (Sekirov et al., 2010). Another protective mechanism is the gut mucosal barrier, which provides both physical and chemical protection and helps maintain host–microbial homeostasis (Li and Chen, 2021).
Chemical barriers, which comprise the regenerating islet-derived 3 family of proteins, antimicrobial peptides, lysozyme, and secretory phospholipase A2, help maintain the separation of GIT microorganisms from intraepithelial cells (IEC) by killing pathogens that invade the gut mucosa (Okumura and Takeda, 2018). The glycocalyx provides a physical barrier on the microvilli of absorptive IEC, and cell junctions connecting the IEC help in the spatial separation of the GIT microbiome, thus preventing the penetration of pathogens into the gut mucosa (Srinivasan, 2010).
The host prevents undesirable responses toward the beneficial members of the GIT microbiome while exhibiting immune response against pathogens; this interspecies balance is known as “Eubiosis.” The normal microbiome of the GIT stimulates the maturation and upkeep of gut immunity and homeostasis (Rakoff-Nahoum and Medzhitov, 2007). The gut microbiome also controls the adaptive immune response, with particular groups boosting the population of regulatory and effector T-cell such as Th17 and Treg cells (Hooper et al., 2012). For instance, segmented filamentous bacteria stimulate robust specific and nonspecific Th17 reactions, exerting detrimental and positive effects on the host (Hooper et al., 2012).
The gut microbiome also plays a role in preventing intestinal diseases and protecting against the growth and colonization of important pathogens through competitive exclusion, including repressive element generation and nutrient exhaustion (Hooper et al., 2012). A shift in the relative profusion of the GIT microbiome, which is called dysbiosis, results in the disturbance of commensal-mediated propagation tolerance to gut diseases, which is accompanied by different infections (Spor et al., 2011). Additionally, a change in the composition of the bacterial community may ease the proliferation of harmful subgroups of endogenous bacteria, also known as pathobionts, within the gut (Egan et al., 2012).
The pathogens’ primary ports of entry are the mucosal surfaces with abundant microorganisms, implying that the beneficial interaction between commensal microbiota and mucosal surfaces plays a unique role in host protection. Various interactions among pathogens and the commensal microbiome have been reported in the literature. For instance, the gut microbiome prevents the development of bacterial infection by generating biosurfactants, competing for attachment and nutrients, secreting metabolites with antimicrobial activities, and promoting the action of secondary intestinal lymphoid organs, which are the first line of defense in the gut mucosa (Hooper et al., 2012; Meijerink et al., 2021; Shi et al., 2021).
Although the lack of knowledge regarding the interrelationship between commensal microbiota and viral pathogens indicates strong evidence assuming that the normal microbiome can directly or indirectly affect viral infections to protect the host or control viral multiplication and transmission. In addition to exerting local effects, the gut microbiome affects host resistance/infection at other mucosal surfaces.
THE BENEFICIAL ROLE OF MICROBIOTA AGAINST VIRAL INFECTIONS
Normal microbiome plays a particular role in the protection against viruses. The gut flora promotes the progress of immune cells and boosts the innate antiviral immunity (Alqazlan et al., 2020; Shi et al., 2021). Commensal microorganisms release soluble factors that protect the intestinal epithelial cells from rotavirus infection by glycan modification in the epithelial cell membrane, thereby inhibiting pathogen attachment (Varyukhina et al., 2012).
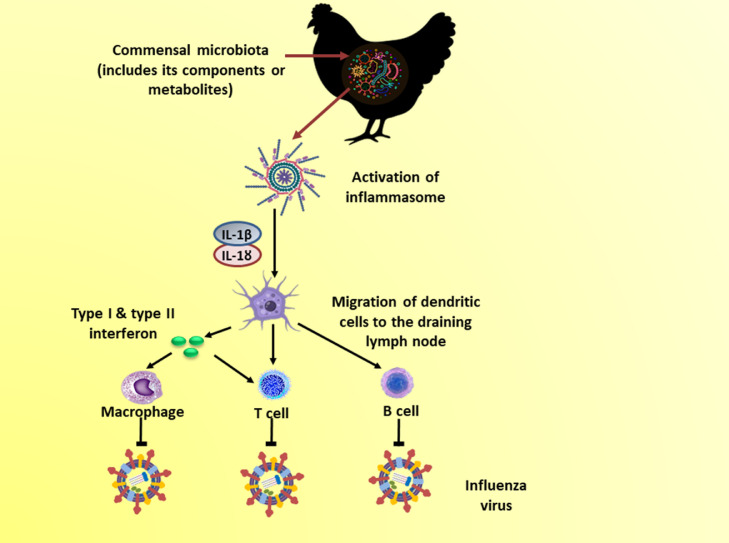
These studies highlight the crucial role of the commensal microbiota in protecting the host against AIV infection. Additionally, these studies suggest the significance of the gut microbiome in protection against pathogens at other mucosal surfaces, including the respiratory system (Samy and Naguib, 2018). Mechanisms underlying the control of AIV infection by the gut microbiome are summarized in Figure 3.
Figure 3.
Mechanisms underlying the suppression of influenza virus infection by the commensal microbiota.
AIV, GUT MICROBIOTA AND CHICKEN IMMUNE RESPONSE
Successful invasion of the mucous layer, AIV primarily targets the respiratory and intestinal epithelia, resulting in the rapid killing of infected cells (Kaufmann et al., 2001; Huang et al., 2021). The viral RNA is identified by different pattern recognition receptors (PRR) of the innate immune system, leading to the production of proinflammatory cytokines such as type-I interferons (IFN), chemokines, and eicosanoids (Iwasaki and Pillai, 2014). If the innate immune system fails to detect AIV, resulting in infection, the adaptive immune response combats the infection.
At least three categories of PRR recognize pathogen-associated molecular patterns (PAMP) of AIV that are either found on the virus or generated during infection. These receptors are retinoic acid-inducible gene I (RIG-I), TLR, and nucleotide oligomerization domain (NOD)-like receptors (NLR) (Pulendran and Maddur, 2012). In the case of ssRNA recognition by TLR7, viral replication is unnecessary because the receptor can recognize genomic ssRNA, resulting in myeloid differentiation primary response-88 (MyD88)-mediated activation of the transcription factor nuclear factor-κB (NF-κB) or interferon regulatory factor 7; this activation leads to the expression of proinflammatory cytokines and type-I IFN (Lund et al., 2004). In addition, TLR7 promotes B-cell-mediated antibody responses against AIV infection (Iwasaki and Pillai, 2014). Currently, the significance of TLR8 in AIV infection is unknown (Iwasaki and Pillai, 2014; Huang et al., 2021).
Influenza infection stimulates RIG-I in macrophages, respiratory epithelial cells, and mast cells. Hosts with a nonfunctional variant of RIG-I exhibit a significantly weak antiviral response, indicating that RIG-I is one of the main pathways through which the host combats AIV infection (Pulendran and Maddur, 2012). Chickens lack RIG-I receptors; nonetheless, MDA5 in chickens may recognize the same ligands as RIG-I (Barber et al., 2010). The NLR are mainly expressed in the cytosol and react with different PAMP, leading to inflammatory reactions in mitogen-activated protein kinases, NF-κB, and MAVS–IRF3-dependent pathways (Pulendran and Maddur, 2012). Pyrin domain-containing protein 3 or cryopyrin (NLRP3), NLR family CARD-containing protein 2 (NLRC2 or NOD2) on NLR responding to influenza virus NLRX1 are the major NLR included. NLRP3 stimulates the production of pro-IL-1β and pro-IL-18, and both NLRC2 and NLRX1 stimulate the synthesis of type-I IFN (Huang et al., 2021).
Microbial ligands, such as LPS, released by selective members of the microbiota stimulate translational and transcriptional activation of pro-IL-18 and pro-IL-1β (signal 1), often via TLR, IL-1R, or TNF receptor. In influenza-infected cells, the virus produces signal 2, necessary to form the NLRP3 inflammasome and stimulate caspase-1, which cleaves pro-IL-18 and pro-IL-1β into their mature forms (Bauernfeind et al., 2011). Although, to the best of our knowledge, no specific PAMP that interact with NLRP3 have been identified yet, ligands of influenza viruses, such as PB1- F2, M2, and viral RNA, and those of normal microbiome initiate the activation of NLRP3 (Pulendran and Maddur, 2012; Chrzasteka et al., 2021).
Identification of the virus by innate receptors is followed by the rapid stimulation of antiviral effector responses such as the secretion of IL-6, IL-1β, and TNF-α from macrophages and monocytes (Herold et al., 2006). The innate antiviral response triggers the secretion of type-I IFN from virus-infected cells (Hiscott, 2007). Type-I IFN are antiviral effectors essential for suppressing viral replication and stimulating cells involved in innate immunity, particularly dendritic cells, thereby facilitating the stimulation of adaptive immunity (Pulendran and Maddur, 2012). Receptor–IFN binding leads to the stimulation of the Janus kinase and signal transducer and activator of transcription pathways, resulting in the stimulation of hundreds of interferon-stimulated genes (ISG) (Yan and Chen, 2012). ISG, which include myxovirus resistance protein, IFN-inducible transmembrane protein, ribonuclease L, 2′–5′-oligoadenylate synthase, and RNA-activated protein kinase, viperin, and thethrin, play essential roles in the immune response against AIV (Iwasaki and Pillai, 2014).
IMPLEMENTING CHICKEN MODEL FOR THE INTERACTION AMONG AIV, GUT MICROBIOTA AND CHICKEN IMMUNE RESPONSES
Models have been used to investigate virus–microbiome interactions; of them, SPF or antibiotic-treated organisms have been used most widely. However, SPF models can be colonized with one or more bacterial strains to study the interaction between microbiota and viruses as they provide a microorganism-free controlled environment (Robinson and Pfeiffer, 2014). Furthermore, SPF chickens experimentally infected with H9N2 AIV exhibited the disruption and damage of intestinal microbiota as well as the inflammatory destruction of the intestinal mucosa-associated marked increase in the genera Escherichia, particularly E. coli, (P < 0.01) 5 d after infection and a significant decrease in obligate anaerobic lactic acid-producing bacteria Lactobacillus, Enterococcus, Streptococcus, and another probiotic bacteria (P < 0.01) (Li et al., 2018; Alqazlan et al., 2020; Shi et al., 2021).
H9N2 AIV infection leads to a significant reduction in the mRNA expression of tight junction proteins (MUC, ZO-1, claudin 3, and occludin), endogenous trefoil (TFF2), and primary gel-forming mucin (MUC2). The mRNA expression of proinflammatory cytokines such as IFN-γ, IL-22, IFN-α, IL-1β, IL-6, and IL-17A were elevated (Li et al., 2018; Samy and Naguib, 2018; Abaidullah et al., 2019; Alqazlan et al., 2020; Chrzasteka et al., 2021). Simultaneously, the expression of TFF2 and MUC2, claudin, occludin, and ZO was significantly decreased, resulting in substantial damage of the gut epithelial tight junctions and the destruction of mucin layer covering the intestinal villi, enabling secondary infection by various bacteria, particularly pathogenic E. coli, which results in severe diarrhea that commonly follow H9N2 infection (Barbour et al., 2009; Li et al., 2018). Intestinal mucosal damage may encourage pathogens to induce systemic infection (Chappell et al., 2009). Similarly, TFF2 can suppress inducible nitric oxide synthase (in monocytes and inflammatory components and control monocyte nitric oxide -mediated inflammation-inducing colitis (Giraud et al., 2004).
H9N2 infection results in a marked increase in ileal butyrate production, which may be considered a self-regulating mechanism to tolerate this infection (Li et al., 2018). In a previous study, AIV-infected broiler chickens showed increased proliferation of pathobionts such as Vampirovibrio, Clostridium cluster-XIVb, and Ruminococcus sp. (Barjesteh et al., 2015). The proliferation of these pathogens induces the liberation of proinflammatory cytokines such as IL-6 and IL-1B (Oakley and Kogut, 2016). Mice infected with HPAI viruses showed similar outcomes with the elevated synthesis of IFN-γ and IL-17A, leading to gut dysbiosis (Wang et al., 2014). The interaction among AIV, GIT microbiome, and chicken immune responses is presented in Figure 4.
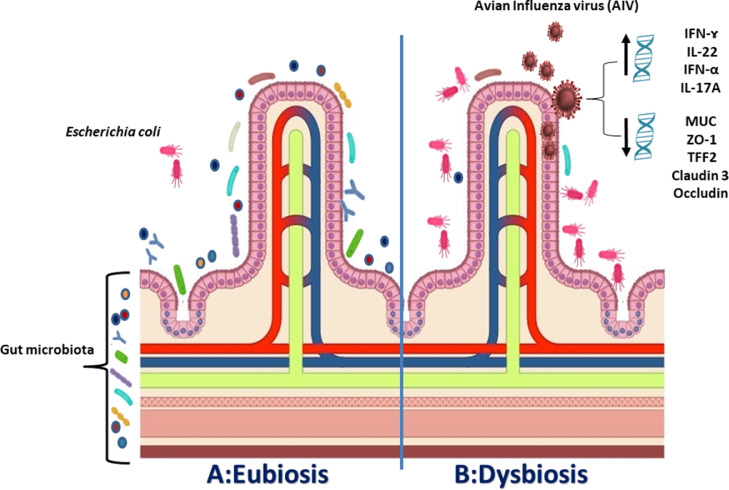
Figure 4.
(A) Eubiosis; a sort of balanced environment “Eco-system” express the presence of different types of gut microbiota together providing intestinal integrity and limiting the attachment of pathogenic pathogens as Escherichia coli. (B) Dysbiosis; explain that during the infection with AIV, the number of gut microbiota is significantly decreased while the number of secondary pathogenic bacteria increased. Furthermore, genes expression for proinflammatory cytokines IFN-ɤ, IFN-α, IL-1β, IL-6, IL-22, and IL-17A were significantly increased while, genes expression for MUC, ZO-1, Claudin 3, Occludin, TFF2, and Muc2 (responsible for intestinal mucin layer and intestinal mucosa healthiness) were significantly decreased resulting in an eruption of intestinal mucosa enabling secondary infection with E. coli which may lead to systemic infection.
ENHANCEMENT OF AVIAN GUT MICROBIOTA
The chicken meat industry has long relied upon the use of antibiotic growth promoters (AGP) administered in chickens through diet to enhance feed conversion ratios and ensure animal weight gain as well as low morbidity and mortality due to clinical and subclinical infections (El-Shall et al., 2021; Arif et al., 2022). The benefits of AGP are, in part, due to the modulation of the host immune reaction after the reduction of the total bacterial load in the GIT and the suppression of potential pathogens (Salem et al., 2021). However, the increasing spread of antimicrobial resistance (multidrug resistance against antibiotics) and the associated threats to public health have prompted governments to limit the use of AGP on farms (El-Tarabily et al., 2021).
There are many safe, natural substances with proven efficacy that have been used to promote intestinal health and boost gut microbiome activities by competing with various pathogens in chickens; the examples include herbal extracts, essential oils, amino acids, prebiotics, probiotics, synbiotics, exogenous enzymes, organic acids, and nanoparticles synthesized via green synthesis (Alagawany et al., 2018; Abd El-Ghany et al., 2021; Abd El-Hack et al., 2022a). These natural substances stimulate the growth of birds, enhance their productivity and immunity, facilitate pathogen resistance, and ensure the production of organic avian products with high nutritive value; these substances are safe for human consumption (El-Saadony et al., 2020; Saad et al., 2021a,b; Abd El-Hack et al., 2022b,c).
Prebiotics are compounds that pass undigested through the proximal parts of the GIT and can induce the growth or activity of beneficial microorganisms that colonize the hindgut, thus improving host physiology. Most prebiotics belong to the groups of galactooligosaccharides, fructooligosaccharides, raffinose family oligosaccharides, and mannanoligosaccharides and are indigestible by the host. However, the gut microbiota can break them down to produce SCFA such as propionate, acetate, and butyrate (Yaqoob et al., 2021). Probiotics are viable microorganisms used as a feed additive, which exert beneficial effects on health when administered in sufficient quantities to promote a good balance among the microbial members present in the GIT (Abd El-Hack et al., 2020).
Organic acids and their salts have gained popularity because of their nutritional value and antimicrobial properties that can elicit positive effects on growth performance; they can be present as simple monocarboxylic acids (formic, acetic, propionic, and butyric acids), carboxylic acids with a hydroxyl group (lactic, malic, tartaric, and citric acids) or short-chain carboxylic acids containing double bonds (fumaric and sorbic acids); they are widely distributed in nature as the normal components of plants or animal cells (Huyghebaert et al., 2011). Organic acids are sometimes found as their sodium, potassium, or calcium salts, which are the forms of choice in the feed manufacturing process because they are more stable, odorless, and easier to handle than the more volatile acid forms (Huyghebaert et al., 2011).
Herbal extracts, nanopreparations, and nanoparticles synthesized via green synthesis are usually incorporated into the diet or feed of poultry birds to improve their performance (Reda et al., 2020, 2021a,b; Abd El-Hack et al., 2021c). The effects of these compounds on enteric diseases, GIT integrity, nutrient digestibility, immunity, and productivity have been comprehensively reviewed in chickens (Alagawany et al., 2015, 2021; Abou-Kassem et al., 2021b).
CONCLUSIONS
Gut microbiota, including different species of Lactobacillus, Blautia Bifidobacterium, Faecalibacterium, Clostridium XlVa, and members of firmicutes, play a significant role in the prevention and control of AIV and other infections. During AIV infection, the expression of proinflammatory cytokines such as IFN-γ, IFNα, IL-17A, and IL-22 is increased significantly. The expression of TFF2, MUC, ZO-1, claudin 3, and occludin is significantly decreased, resulting in decreased mucosal integrity and increased inflammatory response, leading to severe intestinal damage and dysbiosis. Furthermore, the pathogens can invade the intestine, causing a systemic reaction. Consequently, using feed supplements containing probiotics, prebiotics, synbiotics, herbal extracts, essential oils, essential amino acids, organic acids, and exogenous enzymes in chicken diet enhances the local immunity in the intestines, ensuring healthy intestinal mucosa and reducing the chance of infection with pathogens such as H9N2 AIV.
AUTHOR CONTRIBUTIONS
All authors equally contributed to writing this review article. All authors reviewed and approved the final version of the manuscript.
Acknowledgments
Prof. Khaled A. El-Tarabily thanks the library at Murdoch University, Australia, for the valuable online resources and comprehensive databases.
Disclosures
The authors declare no conflict of interest.
References
- Abaidullah M., Peng S., Kamran M., Song X., Yin Z. Current findings on gut microbiota mediated immune modulation against viral diseases in chicken. Viruses. 2019;11:681. doi: 10.3390/v11080681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Ghany W.A., Shaalan M., Salem H.M. Nanoparticles applications in poultry production: an updated review. Worlds Poult. Sci. J. 2021;77:1001–1025. [Google Scholar]
- Abd El-Hack M.E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Shafi M.E., Albaqami N.M., Taha A.E., Ashour E.A. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals. 2021;11:1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., Gado A.R., Nader M.M., Saad A.M., El-Tahan A.M., Taha A.E., Salem H.M., El-Tarabily K.A. Hot red pepper powder as a safe alternative to antibiotics in organic poultry feed: an updated overview. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Saad A.M., Salem H.M., Ashry N.M., Abo Ghanima M.M., Shukry M., Swelum A.A., Taha A.E., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: a comprehensive review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Salem H.M., El-Tahan A.M., Soliman M.M., Youssef G.B.A., Taha A.E., Soliman S.M., Ahmed A.E., El-Kott A.F., Al Syaad K.M., Swelum A.A. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird's health and production. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El -Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A., Al-Wajeeh A.S., Al-Shargi O., Taha A.E., Mesalam N.M., Abdel-Moneim A.E. Prebiotics can restrict Salmonella populations in poultry: a review. Anim. Biotech. 2021;19:1–10. doi: 10.1080/10495398.2021.1883637. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Qattan S.Y., Batiha G.E., Khafaga A.F., Abdel-Moneim A.M.E., Alagawany M. Probiotics in poultry feed: a comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020;104:1835–1850. doi: 10.1111/jpn.13454. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.E., Khafaga A.F., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. Int. 2021;28:4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abd El Hamid M.I., Abd El-Moaty D.A.M., El-Sergany E.F., Salem H.M., El-Sawy H., Abbas A.M. Utility of molecular biology tools for identification and characterization of Egyptian Riemerella anatipestifer duck isolates. Inter. J. Vet. Sci. 2019;8:335–341. [Google Scholar]
- Abou-Kassem D.E., El-Abasy M.M., Al-Harbi M.S., Abol-Ela S., Salem H.M., El-Tahan A.M., El-Saadony M.T., Abd El-Hack M.E., Ashour E.A. Influences of total sulfur amino acids and photoperiod on growth, carcass traits, blood parameters, meat quality and cecal microbial load of broilers. Saudi J. Biol. Sci. 2021;29:1683–1693. doi: 10.1016/j.sjbs.2021.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Kassem D.E., Mahrose K.M., El-Samahy R.A., Shafi M.E., El-Saadony M.T., Abd El-Hack M.E., El-Sharnouby M.E., Taha A.E., Ashour E.A. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. Ital. J. Anim. Sci. 2021;20:896–910. [Google Scholar]
- Abt M.C., Osborne L.C., Monticelli L.A., Doering T.A., Alenghat T., Sonnenberg G.F., Paley M.A., Antenus M., Williams K.L., Erikson J., Wherry E.J., Artis D. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlhoch C., Fusaro A., Gonzales J.L., Kuiken T., Marangon S., Niqueux E., Staubach C., Terregino C., Aznar I., Guajardo I.Munoz, Lima E., Baldinelli F. Avian influenza overview February–May 2021. EFSA J. 2021;19:e06951. doi: 10.2903/j.efsa.2021.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Abd El-Hack M.E., Farag M.R., Elnesr S.S., El-Kholy M.S., Saadeldin I.M., Swelum A.A. Dietary supplementation of Yucca schidigera extract enhances productive and reproductive performances, blood profile, immune function, and antioxidant status in laying Japanese quails exposed to lead in the diet. Poult. Sci. 2018;97:3126–3137. doi: 10.3382/ps/pey186. [DOI] [PubMed] [Google Scholar]
- Alagawany M., El-Hindawy M., Attia A., Farag M., Abd El-Hack M. Influence of dietary choline levels on growth performance and carcass characteristics of growing Japanese quail. Adv. Anim. Vet. Sci. 2015;3:109–115. [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Madkour M., Reda F.M. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander D.J. A review of avian influenza in different bird species. Vet. Microbiol. 2000;74:3–13. doi: 10.1016/s0378-1135(00)00160-7. [DOI] [PubMed] [Google Scholar]
- Alexander D.J. An overview of the epidemiology of avian influenza. Vaccine. 2007;25:5637–5644. doi: 10.1016/j.vaccine.2006.10.051. [DOI] [PubMed] [Google Scholar]
- Alqazlan N., Alizadeh M., Boodhoo N., Taha-Abdelaziz K., Nagy E., Bridle B., Sharif S. Probiotic lactobacilli limit avian influenza virus subtype H9N2 replication in chicken cecal tonsil mononuclear cells. Vaccines. 2020;8:605. doi: 10.3390/vaccines8040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apajalahti J., Kettunen A., Graham H. Characteristics of the gastrointestinal microbial communities, with special reference to the chicken. World's Poult. Sci. J. 2004;60:223–232. [Google Scholar]
- Arif M., Baty R.S., Althubaiti E.H., Ijaz M.T., Fayyaz M., Shafi M.E., Albaqami N.M., Alagawany M., Abd El-Hack M.E., Taha A.E., Salem H.M., El-Tahan A.M., Elnesr S.S. The impact of betaine supplementation in quail diet on growth performance, blood chemistry, and carcass traits. Saudi J. Biol. Sci. 2022;29:1604–1610. doi: 10.1016/j.sjbs.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber M.R.W., Aldridge Jr J.R., Webster R.G., Magor K.E. Association of RIG-I with innate immunity of ducks to influenza. Proc. Natl. Acad. Sci. USA. 2010;107:5913–5918. doi: 10.1073/pnas.1001755107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour E.K., Mastori F.A., Nour A.M.A., Shaib H.A., Jaber L.S., Yaghi R.H., Sabra A., Sleiman F.T., Sawaya R.K., Niedzwieck A., Tayeb I.T., Kassaify Z.G., Rath M., Harakeh S., Barbour K.E. Standardization of a new model of H9N2/Escherichia coli challenge in broilers in Lebanon. Vet. Ital. 2009;45:317–322. [PubMed] [Google Scholar]
- Barjesteh N., Shojadoost B., Brisbin J.T., Emam M., Hodgins D.C., Nagy É., Sharif S. Reduction of avian influenza virus shedding by administration of Toll-like receptor ligands to chickens. Vaccine. 2015;33:4843–4849. doi: 10.1016/j.vaccine.2015.07.070. [DOI] [PubMed] [Google Scholar]
- Bauernfeind F., Ablasser A., Bartok E., Kim S., Schmid-Burgk J., Cavlar T., Hornung V. Inflammasomes: current understanding and open questions. Cell. Mol. Life Sci. 2011;68:765–783. doi: 10.1007/s00018-010-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann L., Simon O., Vahjen W. Isolation and identification of mixed linked β-glucan degrading bacteria in the intestine of broiler chickens and partial characterization of respective 1,3-1,4-beta-glucanase activities. J. Basic Microbiol. 2006;46:175–185. doi: 10.1002/jobm.200510107. [DOI] [PubMed] [Google Scholar]
- Budden K.F., Gellatly S.L., Wood D.L., Cooper M.A., Morrison M., Hugenholtz P., Hansbro P.M. Emerging pathogenic links between microbiota and the gut–lung axis. Nat. Rev. Microbiol. 2017;15:55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- Caliendo V., Leijten L., Begeman L., Poen M.J., Fouchier R.A.M., Beerens N., Kuiken T. Enterotropism of highly pathogenic avian influenza virus H5N8 from the 2016/2017 epidemic in some wild bird species. Vet. Res. 2020;51:117. doi: 10.1186/s13567-020-00841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capua I., Alexander D.J. Avian influenza: recent developments. Avian Pathol. 2004;33:393–404. doi: 10.1080/03079450410001724085. [DOI] [PubMed] [Google Scholar]
- Causey D., Edwards S.V. Ecology of avian influenza in birds. J. Infect. Dis. 2008;197:S29–S33. doi: 10.1086/524991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra J.J. Influences of microbiota on intestinal immune system development. Am. J. Clin. Nutr. 1999;69:1046s–1051s. doi: 10.1093/ajcn/69.5.1046s. [DOI] [PubMed] [Google Scholar]
- Chappell L., Kaiser P., Barrow P., Jones M.A., Johnston C., Wigley P. The immunobiology of avian systemic salmonellosis. Vet. Immunol. Immunopathol. 2009;128:53–59. doi: 10.1016/j.vetimm.2008.10.295. [DOI] [PubMed] [Google Scholar]
- Chrzastek K., Leng J., Zakaria M.K., Bialy D., Ragione R.La, Shelton H. Low pathogenic avian influenza virus infection retards colon microbiome diversification in two different chicken lines. Anim. Microbiome. 2021;3:64. doi: 10.1186/s42523-021-00128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavijo V., Florez M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult. Sci. 2018;97:1006–1021. doi: 10.3382/ps/pex359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B., Nair G.B. Homeostasis and dysbiosis of the gut microbiome in health and disease. J. Biosci. 2019;44:117. [PubMed] [Google Scholar]
- de Silva U.C., Tanaka H., Nakamura S., Goto N., Yasunaga T. A comprehensive analysis of reassortment in influenza A virus. Biol. Open. 2012;1:385–390. doi: 10.1242/bio.2012281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan C.E., Cohen S.B., Denkers E.Y. Insights into inflammatory bowel disease using Toxoplasma gondii as an infectious trigger. Immunol. Cell Biol. 2012;90:668–675. doi: 10.1038/icb.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Naggar M.S., Ibrahim H.M., Salem H.M., Marouf S. A novel locally prepared inactivated bivalent mycoplasma vaccine for chicken flocks in Egypt. Adv. Anim. Vet. Sci. 2022;10:55–61. [Google Scholar]
- El-Saadony M.T., Abd El-Hack M.E., Swelum A.A., Al-Sultan S.I., El-Ghareeb W.R., Hussein E.O., Ba-Awadh H.A., Akl B.A., Nader M.M. Enhancing quality and safety of raw buffalo meat using the bioactive peptides of pea and red kidney bean under refrigeration conditions. Ital. J. Anim. Sci. 2021;20:762–776. [Google Scholar]
- El-Saadony M.T., Elsadek M.F., Mohamed A.S., Taha A.E., Ahmed B.M., Saad A.M. Effects of chemical and natural additives on cucumber juice's quality, shelf life, and safety. Foods. 2020;9:639. doi: 10.3390/foods9050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Taha T.F., Najjar A.A., Zabermawi N.M., Nader M.M., AbuQamar S.F., El-Tarabily K.A., Salama A. Selenium nanoparticles from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi as a new source from human breast milk. Saudi J. Biol. Sci. 2021;28:6782–6794. doi: 10.1016/j.sjbs.2021.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Salem H.M., El-Tahan A.M., Abd El-Mageed T.A., Soliman S.M., Khafaga A.F., Swelum A.A., Ahmed A.E., Alshammari F.A., Abd El-Hack M.E. The control of poultry salmonellosis using organic agents: an updated overview. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Zabermawi N.M., Zabermawi N.M., Burollus M.A., Shafi M.E., Alagawany M., Abd El-Hack M.E. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: a review. Food Rev. Int. 2021;37:1–23. [Google Scholar]
- El-Shall N.A., Abd El-Hack M.E., Albaqami N.M., Khafaga A.F., Taha A.E., Swelum A.A., El-Saadony M.T., Salem H.M., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A., Elbestawy A.R. Phytochemical control of poultry coccidiosis: a review. Poult. Sci. 2021;101 doi: 10.1016/j.psj.2021.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F., Elwan H.A.M., Elnesr S.S., Abd El-Hack M.E. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021;28:5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gutierrez E., Mayer M.J., Cotter P.D., Narbad A. Gut microbiota as a source of novel antimicrobials. Gut Microbes. 2019;10:1–21. doi: 10.1080/19490976.2018.1455790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud A.S., Pereira P.M., Thim L., Parker L.M., Judd L.M. TFF-2 inhibits iNOS/NO in monocytes and nitrated protein in healing colon after colitis. Peptides. 2004;25:803–809. doi: 10.1016/j.peptides.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Guan J., Fu Q., Sharif S. Replication of an H9N2 avian influenza virus and cytokine gene expression in chickens exposed by aerosol or intranasal routes. Avian Dis. 2015;59:263–268. doi: 10.1637/10972-110714-Reg. [DOI] [PubMed] [Google Scholar]
- Han Z., Willer T., Li L., Pielsticker C., Rychlik I., Velge P., Kaspers B., Rautenschlein S. Influence of the gut microbiota composition on Campylobacter jejuni colonization in chickens. Infect. Immun. 2017;85:e00380–17. doi: 10.1128/IAI.00380-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold S., von Wulffen W., Steinmueller M., Pleschka S., Kuziel W.A., Mack M., Srivastava M., Seeger W., Maus U.A., Lohmeyer J. Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. J. Immunol. 2006;177:1817–1824. doi: 10.4049/jimmunol.177.3.1817. [DOI] [PubMed] [Google Scholar]
- Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J. Biol. Chem. 2007;282:15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- Hooper L.V. Bacterial contributions to mammalian gut development. Trends Microbiol. 2004;12:129–134. doi: 10.1016/j.tim.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Hooper L.V, Littman D.R., Macpherson A.J. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Peng P., Li J., Zhang Q., Li R., Wang X., Gu M., Hu Z., Hu S., Liu X., Jiao X., Peng D., Liu X. Single dose of bivalent H5 and H7 influenza virus-like particle protects chickens against highly pathogenic H5N1 and H7N9 avian influenza viruses. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.774630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D., Chao Y.C., Lv Z., Jan J.T., Yang Y.C., Hsiao P.W., Wu C.Y., Liao C.H., Wu T.H., Wang L.C. Comparison of chicken immune responses after inoculation with H5 avian influenza virus like particles produced by insect cells or pupae. J. Vet. Res. 2021;65:139–145. doi: 10.2478/jvetres-2021-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghebaert G., Ducatelle R., Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011;187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Iwasaki A., Pillai P.S. Innate immunity to influenza virus infection. Nat. Rev. Immunol. 2014;14:315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Józefiak D., Kaczmarek S., Rutkowski A. A note on the effects of selected prebiotics on the performance and ileal microbiota of broiler chickens. J. Anim. Feed Sci. 2008;17:392–397. [Google Scholar]
- Kang Y.M., Cho H.K., Kim J.H., Lee S.J., Park S.J., Kim D.Y., Kim S.Y., Park J.W., Lee M.H., Kim M.C., Kang H.M. Single dose of multi-clade virus-like particle vaccine protects chickens against clade 2.3.2.1 and clade 2.3.4.4 highly pathogenic avian influenza viruses. Sci. Rep. 2021;11:13786. doi: 10.1038/s41598-021-93060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann A., Salentin R., Meyer R.G., Bussfeld D., Pauligk C., Fesq H., Hofmann P., Nain M., Gemsa D., Sprenger H. Defense against influenza A virus infection: essential role of the chemokine system. Immunobiology. 2001;204:603–613. doi: 10.1078/0171-2985-00099. [DOI] [PubMed] [Google Scholar]
- Lee C.W., Saif Y.M. Avian influenza virus. Comp. Immunol. Microbiol. Infect. Dis. 2009;32:301–310. doi: 10.1016/j.cimid.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Li C., Chen H. H7N9 influenza virus in China. Cold Spring Harb. Perspect. Med. 2021;11 doi: 10.1101/cshperspect.a038349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Liu X., Chen F., Zuo K., Wu C., Yan Y., Chen W., Lin W., Xie Q. Avian influenza virus subtype H9N2 affects intestinal microbiota, barrier structure injury, and inflammatory intestinal disease in the chicken ileum. Viruses. 2018;10:270. doi: 10.3390/v10050270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.P., Shaw M., Gregory V., Cameron K., Lim W., Klimov A., Subbarao K., Guan Y., Krauss S., Shortridge K., Webster R., Cox N., Hay A. Avian-to-human transmission of H9N2 subtype influenza A viruses: relationship between H9N2 and H5N1 human isolates. Proc. Natl. Acad. Sci. USA. 2000;97:9654–9658. doi: 10.1073/pnas.160270697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J.M., Alexopoulou L., Sato A., Karow M., Adams N.C., Gale N.W., Iwasaki A., Flavell R.A. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorff F., Akkermans L., Soderholm J.D. The role of microbiota and probiotics in stress-induced gastrointestinal damage. Curr. Mol. Med. 2008;8:282–298. doi: 10.2174/156652408784533779. [DOI] [PubMed] [Google Scholar]
- Marouf S., Khalf M.A., Alorabi M., El-Shehawi A.M., El-Tahan A.M., AbdEl-Hack M.E., El-Saadony M.T., Salem H.M. Mycoplasma gallisepticum: a devastating organism for the poultry industry in Egypt. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAleer J.P., Nguyen N.L.H., Chen K., Kumar P., Ricks D.M., Binnie M., Armentrout R.A., Pociask D.A., Hein A., Yu A., Vikram A., Bibby K., Umesaki Y., Rivera A., Sheppard D., Ouyang W., Hooper L.V., Kolls J.K. Pulmonary Th17 antifungal immunity is regulated by the gut microbiome. J. Immunol. 2016;197:97–107. doi: 10.4049/jimmunol.1502566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead G.C. Prospects for 'competitive exclusion' treatment to control salmonellas and other foodborne pathogens in poultry. Vet. J. 2000;159:111–123. doi: 10.1053/tvjl.1999.0423. [DOI] [PubMed] [Google Scholar]
- Meijerink N., de Oliveira J.E., van Haarlem D.A., Hosotani G., Lamot D.M., Stegeman J.A., Rutten V.P.M.G., Jansen C.A. Glucose oligosaccharide and long-chain glucomannan feed additives induce enhanced activation of intraepithelial NK Cells and relative abundance of commensal lactic acid bacteria in broiler chickens. Vet. Sci. 2021;8:110. doi: 10.3390/vetsci8060110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalto M., D'onofrio F., Gallo A., Cazzato A., Gasbarrini G. Intestinal microbiota and its functions. Dig. Liver. Dis. 2009;3:30–34. [Google Scholar]
- Niba A.T., Beal J.D., Kudi A.C., Brooks P.H. Bacterial fermentation in the gastrointestinal tract of non-ruminants: influence of fermented feeds and fermentable carbohydrates. Trop. Anim. Health Prod. 2009;41:1393–1407. doi: 10.1007/s11250-009-9327-6. [DOI] [PubMed] [Google Scholar]
- Nili H., Asasi K. Avian influenza (H9N2) outbreak in Iran. Avian Dis. 2003;47:828–831. doi: 10.1637/0005-2086-47.s3.828. [DOI] [PubMed] [Google Scholar]
- Oakley B.B., Kogut M.H. Spatial and temporal changes in the broiler chicken cecal and fecal microbiomes and correlations of bacterial taxa with cytokine gene expression. Front. Vet. Sci. 2016;3:11. doi: 10.3389/fvets.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocejo M., Oporto B., Hurtado A. 16S rRNA amplicon sequencing characterization of caecal microbiome composition of broilers and free-range slow-growing chickens throughout their productive lifespan. Sci. Rep. 2019;9:2506. doi: 10.1038/s41598-019-39323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J.Z., Ravindran R., Chassaing B., Carvalho F.A., Maddur M.S., Bower M., Hakimpour P., Gill K.P., Nakaya H.I., Yarovinsky F., Sartor R.B., Gewirtz A.T., Pulendran B. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity. 2014;41:478–492. doi: 10.1016/j.immuni.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara A.M., Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE. 2006. The World Organization for Animal Health. Accessed May 2022. https://www.oie.int/en/home
- Okumura R., Takeda K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018;38:5. doi: 10.1186/s41232-018-0063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue M.L. In: Pages 23–41 in Avian Influenza. Swayne D.E., editor. Blackwell Publishing; Iowa: 2008. Molecular determinants of pathogenicity for AI viruses. [Google Scholar]
- Perez D.R., Webby R.J., Hoffmann E., Webster R.G. Land-based birds as potential disseminators of avian mammalian reassortant influenza A viruses. Avian Dis. 2003;47:1114–1117. doi: 10.1637/0005-2086-47.s3.1114. [DOI] [PubMed] [Google Scholar]
- Perkins L.E.L., Swayne D.E. Comparative susceptibility of selected avian and mammalian species to a Hong Kong-origin H5N1 high-pathogenicity avian influenza virus. Avian Dis. 2003;47:956–967. doi: 10.1637/0005-2086-47.s3.956. [DOI] [PubMed] [Google Scholar]
- Perumbakkam S., Hunt H.D., Cheng H.H. Marek's disease virus influences the core gut microbiome of the chicken during the early and late phases of viral replication. FEMS Microbiol. Ecol. 2014;90:300–312. doi: 10.1111/1574-6941.12392. [DOI] [PubMed] [Google Scholar]
- Perumbakkam S., Hunt H.D., Cheng H.H. Differences in CD8αα and cecal microbiome community during proliferation and late cytolytic phases of Marek's disease virus infection are associated with genetic resistance to Marek's disease. FEMS Microbiol. Ecol. 2016;92:fiw188. doi: 10.1093/femsec/fiw188. [DOI] [PubMed] [Google Scholar]
- Post J., Burt D.W., Cornelissen J.B., Broks V., van Zoelen D., Peeters B., Rebel J.M. Systemic virus distribution and host responses in brain and intestine of chickens infected with low pathogenic or high pathogenic avian influenza virus. Virol. J. 2012;9:61. doi: 10.1186/1743-422X-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post J., Geus E.D., Vervelde L., Cornelissen J.B.W.J., Rebel J.M.J. Systemic distribution of different low pathogenic avian influenza (LPAI) viruses in chicken. Virol. J. 2013;10:23. doi: 10.1186/1743-422X-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B., Maddur M.S. In: Influenza Pathogenesis and Control-Volume II. Oldstone M.B.A., Compans R.W., editors. Springer International Publishing; Switzerland: 2012. Innate immune sensing and response to influenza; pp. 139–157. [Google Scholar]
- Rakoff-Nahoum S., Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Attia A.I., El-Sayed S.A., Ahmed S.Y., Madkour M., Alagawany M. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilization, blood metabolites and intestinal microbiota. Ital. J. Anim. Sci. 2021;20:324–335. [Google Scholar]
- Reda F., El-Saadony M.T., El-Rayes T., Farahat M., Attia G., Alagawany M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10:754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C.M., Pfeiffer J.K. Viruses and the microbiota. Annu. Rev. Virol. 2014;1:55–69. doi: 10.1146/annurev-virology-031413-085550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A.M., El-Saadony M.T., Mohamed A.S., Ahmed A.I., Sitohy M.Z. Impact of cucumber pomace fortification on the nutritional, sensorial and technological quality of soft wheat flour-based noodles. Int. J. Food Sci. Technol. 2021;56:3255–3268. [Google Scholar]
- Saad A.M., Sitohy M.Z., Ahmed A.I., Rabie N.A., Amin S.A., Aboelenin S.M., Soliman M.M., El-Saadony M.T. Biochemical and functional characterization of kidney bean protein alcalase-hydrolysates and their preservative action on stored chicken meat. Molecules. 2021;26:4690. doi: 10.3390/molecules26154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Ismael E., Shaalan M. Evaluation of the effects of silver nanoparticles against experimentally induced necrotic enteritis in broiler chickens. Int. J. Nanomed. 2021;16:6783–6796. doi: 10.2147/IJN.S319708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Khattab M.S., Yehia N., Abd El-Hack M.E., El-Saadony M.T., Alhimaidi A.R., Swelum A.A., Attia M.M. Morphological and molecular characterization of Ascaridia columbae in the domestic pigeon (Columba livia domestica) and the assessment of its immunological responses. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Salem M.A., Soliman M.M., Althobaiti S.A., Khafaga A.K., El-Tahan A.M., El-Saadony M.T., Attia M.M. Parasitological and histopathological examination of Cocktail love birds infected with Eimeria aratinga (Apicomplexa: Eimeriidae) Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samy A., Naguib M.M. Avian respiratory coinfection and impact on avian influenza pathogenicity in domestic poultry: field and experimental findings. Vet. Sci. 2018;5:23. doi: 10.3390/vetsci5010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuijt T.J., Lankelma J.M., Scicluna B.P., e Melo F.de S., Roelofs J.J.T.H., de Boer J.D., Hoogendijk A.J., de Beer R., de Vos A., Belzer C., de Vos W.M., van der Poll T., Wiersinga W.J. The gut microbiota plays a protective role in the host defense against pneumococcal pneumonia. Gut. 2016;65:575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirov I., Russell S.L., Antunes L.C., Finlay B.B. Gut microbiota in health and disease. Physiol. Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Setta A., Salem H.M., Elhady M., El-Hussieny A., Arafa A. Molecular and genetic characterization of infectious bronchitis viruses isolated from commercial chicken flocks in Egypt between 2014 and 2016. J. World Poult. Res. 2018;8:01–08. [Google Scholar]
- Shi H.Y., Zhu X., Li W.L., Mak J.W.Y., Wong S.H., Zhu S.T., Guo S.L., Chan F.K.L., Zhang S.T., Siew C.N. Modulation of gut microbiota protects against viral respiratory tract infections: a systematic review of animal and clinical studies. Eur. J. Nutr. 2021;60:4151–4174. doi: 10.1007/s00394-021-02519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spor A., Koren O., Ley R. Unraveling the effects of the environment and host genotype on the gut microbiome. Nat. Rev. Microbiol. 2011;9:279–290. doi: 10.1038/nrmicro2540. [DOI] [PubMed] [Google Scholar]
- Srinivasan N. Telling apart friend from foe: discriminating between commensals and pathogens at mucosal sites. Innate Immun. 2010;16:391–404. doi: 10.1177/1753425909357577. [DOI] [PubMed] [Google Scholar]
- Suarez D.L. In: Pages 3–22 in Avian Influenza. Swayne D.E., editor. Blackwell Publishing; Ames: 2008. Influenza A virus. [Google Scholar]
- Suarez D.L., Schultz-Cherry S. Immunology of avian influenza virus: a review. Dev. Comp. Immunol. 2000;24:269–283. doi: 10.1016/s0145-305x(99)00078-6. [DOI] [PubMed] [Google Scholar]
- Swanson H.I. Drug metabolism by the host and gut microbiota: a partnership or rivalry? Drug. Metab. Dispos. 2015;43:1499–1504. doi: 10.1124/dmd.115.065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayne D.E. In: Pages 287–297 in Avian Influenza. Swayne D.E., editor. Blackwell Publishing; Ames: 2008. Avian influenza control strategies. [Google Scholar]
- Swayne D.E., Beck J.R. Experimental study to determine if low-pathogenicity and high-pathogenicity avian influenza viruses can be present in chicken breast and thigh meat following intranasal virus inoculation. Avian Dis. 2005;49:81–85. doi: 10.1637/7260-081104R. [DOI] [PubMed] [Google Scholar]
- Swelum A.A., Shafi M.E., Albaqami N.M., El-Saadony M.T., Elsify A., Abdo M., Taha A.E., Abdel-Moneim A.E., Al-Gabri N.A., Almaiman A.A. COVID-19 in human, animal, and environment: a review. Front. Vet. Sci. 2020;7:578. doi: 10.3389/fvets.2020.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolkamp, B., E. Wall, R. Roehe, J. Newbold, and K. Zaralis. 2010. Review of nutrient efficiency in different breeds of farm livestock. Report to DEFRA (IF0183).
- Umar S., Guerin J.L., Ducatez M.F. Low pathogenic avian influenza and coinfecting pathogens: a review of experimental infections in avian models. Avian Dis. 2016;61:3–15. doi: 10.1637/11514-101316-Review. [DOI] [PubMed] [Google Scholar]
- Varmuzova K., Kubasova T., Davidova-Gerzova L., Sisak F., Havlickova H., Sebkova A., Faldynova M., Rychlik I. Composition of gut microbiota influences resistance of newly hatched chickens to Salmonella enteritidis infection. Front. Microbiol. 2016;7:957. doi: 10.3389/fmicb.2016.00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varyukhina S., Freitas M., Bardin S., Robillard E., Tavan E., Sapin C., Grill J.P., Trugnan G. Glycan-modifying bacteria-derived soluble factors from Bacteroides thetaiotaomicron and Lactobacillus casei inhibit rotavirus infection in human intestinal cells. Microbes Infect. 2012;14:273–278. doi: 10.1016/j.micinf.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Vasin A.V., Temkina O.A., Egorov V.V., Klotchenko S.A., Plotnikova M.A., Kiselev O.I. Molecular mechanisms enhancing the proteome of influenza A viruses: an overview of recently discovered proteins. Virus Res. 2014;185:53–63. doi: 10.1016/j.virusres.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Videnska P., Rahman M.M., Faldynova M., Babak V., Matulova M.E., Prukner-Radovcic E., Krizek I., Smole-Mozina S., Kovac J., Szmolka A., Nagy B., Sedlar K., Cejkova D., Rychlik I. Characterization of egg-laying hen and broiler fecal microbiota in poultry farms in Croatia, Czech Republic, Hungary and Slovenia. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnska P., Sedlar K., Lukac M., Faldynova M., Gerzova L., Cejkova D., Sisak F., Rychlik I. Succession and replacement of bacterial populations in the caecum of egg-laying hens over their whole life. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnska P., Sisak F., Havlickova H., Faldynova M., Rychlik I. Influence of Salmonella enterica serovar enteritidis infection on the composition of chicken cecal microbiota. BMC Vet. Res. 2013;9:140. doi: 10.1186/1746-6148-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite D.W., Taylor M.W. Characterizing the avian gut microbiota: membership, driving influences, and potential function. Front. Microbiol. 2014;5:223. doi: 10.3389/fmicb.2014.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakawa A.M., Sa'idu L., Kazeem H.M., Fatihu M.Y., Admau J., Mamman P.H., Abdu P.A., Bello M.B., Kwanashie C. Highly pathogenic avian influenza in waterfowls in Zaria, Nigeria. Niger. Vet. J. 2008;29:55–58. [Google Scholar]
- Wan H., Sorrell E.M., Song H., Hossain M.J., Ramirez-Nieto G., Monne I., Stevens J., Cattoli G., Capua I., Chen L.M., Donis R.O., Busch J., Paulson J.C., Brockwell C., Webby R., Blanco J., Al-Natour M.Q., Perez D.R. Replication and transmission of H9N2 influenza viruses in ferrets: evaluation of pandemic potential. PLoS One. 2008;3:e2923. doi: 10.1371/journal.pone.0002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Li F., Wei H., Lian Z.X., Sun R., Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell–dependent inflammation. J. Exp. Med. 2014;211:2397–2410. doi: 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R., Bean W.J., Gorman O.T., Chambers T.M., Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N., Chen Z.J. Intrinsic antiviral immunity. Nat. Immunol. 2012;13:214–222. doi: 10.1038/ni.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqoob M., Abd El-Hack M.E., Hassan F., El-Saadony M.T., Khafaga A., Batiha G., Yehia N., Elnesr S., Alagawany M., El-Tarabily K.A., Wang M. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoman C.J., Chia N., Jeraldo P., Sipos M., Goldenfeld N.D., White B.A. The microbiome of the chicken gastrointestinal tract. Anim. Health Res. Rev. 2012;13:89–99. doi: 10.1017/S1466252312000138. [DOI] [PubMed] [Google Scholar]
- Zeuthen L.H., Fink L.N., Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-β. Immunology. 2008;123:197–208. doi: 10.1111/j.1365-2567.2007.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Zhang Z., Yu Z., Li L., Cheng K., Wang T., Huang G., Yang S., Zhao Y., Feng N., Fu J., Qin C., Gao Y., Xia X. Domestic cats and dogs are susceptible to H9N2 avian influenza virus. Virus Res. 2013;175:52–57. doi: 10.1016/j.virusres.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Zhao N., Wang S., Li H., Liu S., Li M., Luo J., Su W., He H. Influence of novel highly pathogenic avian influenza A (H5N1) virus infection on migrating whooper swans fecal microbiota. Front. Cell Infect. Microbiol. 2018;8:46. doi: 10.3389/fcimb.2018.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]