Abstract
Objective:
To present the framework for Stanford Fertility and Reproductive Health’s comprehensive reproductive biobanking initiatives and results of first year of recruitment.
Design:
Technical description paper
Setting:
Academic fertility center
Patients:
Fertility patients >18 years of age
Intervention:
Offer and enroll patients interested in research in biobanking protocols
Main Outcome Measures:
Patient recruitment and sample inventory from 9/2020 to 9/2021
Results:
A total of 253 patients have enrolled in Stanford Fertility and Reproductive Health biobanking initiatives since 9/2020. Current inventory is 1,176 samples including serum, plasma, buffy coat, endometrium, maternal decidua, miscarriage chorionic villi, and human embryos (zygote, cleavage, and blastocyst stage).
Conclusion:
This biobanking initiative addresses a critical unmet need in reproductive health research to make it possible for patients to donate excess embryos and gametes and preserves valuable somatic and reproductive tissues that would otherwise be discarded for future research. We present the framework of this biobanking initiative in an effort to support future efforts to establish similar biorepositories.
Keywords: biobank, embryo research, reproductive biobank, fertility biobank, biorepository
Introduction
The field of Biorepository and Biospecimen Science was officially introduced in 2006, and the popularity of biobanks has significantly increased over the last two decades (1, 2). Although the majority of patients report little to no prior knowledge of biobanking, they support and recognize its value once introduced to the concept (3, 4). Most patients who are willing to donate are driven by their own altruism or a desire to find cures for their and their loved ones’ conditions (2, 3). Participating in biobanking initiatives empowers patients to help society and the scientific community without having to participate beyond their clinical care (3).
From the perspective of researchers, the growth of biobanks improves efficiency for population-based research and precision medicine (5, 6). Biobanks have standard operating procedures for the collection, processing, storage, and distribution of specimens and data that allow them to provide quality-controlled samples with important phenotypic information (1, 2, 6). The demand for high quality specimens and associated data has increased over the last few years, making biobanks necessary for a wide variety of scientific research (1, 6).
Although biobanks in general have rapidly grown in popularity, there are still very few fertility and reproductive health-based biobanks (7–12). Among those, the types and varieties of reproductive tissue stored and the potential for future research using banked specimens varies. Schon et al. reported recently on a reproductive biorepository at the University of Michigan for a variety of reproductive specimens including semen, sperm, follicular fluid, granulosa cells, immature oocytes, ovarian and uterine tissue, and blood samples (11). A unique consideration among patients receiving fertility treatment is the disposition of excess embryos, oocytes, and sperm (6, 13–15). It has become a standard practice to generate excess embryos or gametes during fertility treatment, and patients overwhelmingly support having donation to research as an option in their consideration of final disposition (13–15). In a 2019 position statement, the ASRM (American Society of Reproductive Medicine) Ethics in Embryo Research Task Force and the ASRM Ethics Committee stated that “scientific research using human embryos advances human health and offspring well-being, and provides vital insights into the mechanisms for reproduction and disease” (16). Furthermore, the task force stated that “embryo research, with either existing embryos or those produced specifically for research purposes, is ethically acceptable as a means of obtaining new knowledge that may benefit human health, offspring well-being, or reproduction provided certain guidelines and safeguards are followed” (16). Despite donation of embryos to research being highly desired by both fertility patients and the scientific community, it is not commonly offered in the US.
Herein, we report biobanking efforts in our institution for excess embryos, oocytes, and sperm (the RENEW Biobank: Regenerative medicine through the Ethical procurement of Nonviable or Excess cellular Waste), as well as excess somatic tissues including endometrium and pregnancy tissue generated during routine fertility evaluation and treatment (the ROSE Biobank: Reproductive research On Specimens in Excess). This biobanking initiative addresses a critical unmet need to make it possible for patients to donate excess embryos and gametes to research and preserves valuable somatic tissue that would otherwise be discarded for future research (6, 13–15). We present the framework of this biobanking initiative and results of the first year of recruitment in an effort to support future efforts to establish similar biorepositories.
Materials and Methods
Dual Biobanking Initiatives
We have two separate biobanking initiatives that function synergistically. The purpose of the ROSE Biobank is to collect and store somatic specimens that are generated in excess of clinical need during the course of fertility evaluation and treatment, with the option of an additional blood draw for banking during routine venipuncture. The accepted specimen types for the ROSE Biobank are blood, urine, endometrium, and pregnancy tissue (decidua and villi). The purpose of the RENEW Biobank is to collect and store reproductive specimens that patients decide they no longer need once they complete their fertility treatment, including embryos, oocytes, sperm, ovarian tissue, testicular tissue, and follicular fluid. The ROSE Biobank was established in 2020 and the RENEW Biobank was established in 2008. However, the RENEW Biobank halted enrollment in 2014 and only resumed in 2020 with the ROSE Biobank.
We established separate biobanks because each curates different specimen types at different timepoints in a patient’s fertility journey and embryo/gamete donation requires more regulatory oversight given the samples donated and the legal requirements for using these samples in research; examples of such legal requirements are California’s laws on who must consent to donate embryos and how long an embryo can be used for research before it must be discarded (17). The RENEW Biobank also has specific IRB approval to accept donations from patients outside of Stanford given the national demand for embryo/gamete donation. Furthermore, since the biobanks have separate protocols, patients can choose to enroll in both, one, or neither. The ROSE and RENEW biobank protocols have been approved by the Stanford Institutional Review Board (IRB-55252, IRB-10466, respectively) and by the Stem Cells Research Oversight panel (SCRO-792, SCRO-795, respectively).
Infrastructure
Daily operations of the ROSE and RENEW Biobanks are managed by 1.0 full time equivalent of a clinical research coordinator and overseen by a faculty member with effort as needed. In addition, the ROSE and RENEW Biobanks have advisory boards comprised of content experts in the fields of reproductive endocrinology and infertility, embryology, andrology, perinatal stem cell biology, and bioethics. Equipment for sample processing is provided by the Stanford Department of OB/GYN and the Stanford Fertility and Reproductive Health Clinic. Freezer space is allocated by the Stanford Department of OB/GYN. Our embryo biobanking initiative, including required equipment and freezers, is financially supported entirely by our department.
Patient Eligibility
All adult patients (>18 years of age) are eligible to participate in biobanking regardless of sex, gender, race, ethnicity, or fertility diagnosis. Donation to the ROSE Biobank is currently limited to patients seen in the Stanford Fertility and Reproductive Health Clinic. Of note, oocyte donors and gestational carriers are eligible to participate. All patients who created and stored their embryos in states that allow human embryo research using autologous gametes are eligible to donate their reproductive tissues to the RENEW Biobank (Supplementary Table 1). All embryos are eligible for donation to the RENEW Biobank regardless of ploidy (euploid, aneuploid, mosaic, untested) and infectious disease carrier status. Patients who created and stored their embryos in states that allow human embryo research using donor gametes are eligible to donate only if the oocyte or sperm donor’s consent forms do not specifically prohibit research.
Patient Recruitment for ROSE Biobank
Patients are recruited for the ROSE Biobank through encounters that occur during routine clinical care. All patients complete an intake form before their new patient visit that includes the following question: “As a patient at Stanford Fertility and Reproductive Health, you will have the opportunity to participate in our research. Would you like to learn more about studies you may be eligible for?” If a patient answers “yes” to this question, patients are contacted for recruitment after the new patient visit.
Patients are also enrolled prior to a clinic procedure (i.e. endometrial biopsy), or prior to a surgical procedure (oocyte retrieval, hysteroscopy, uterine dilation and curettage, microscopic testicular sperm extraction, and electroejaculation). The research team receives permission from the patient’s care team prior to contacting patients. The research team presents the ROSE Biobank and patients have the option of enrolling prior to their planned procedure, or at a later time.
Patient Recruitment for RENEW Biobank
At Stanford Fertility and Reproductive Health, patients are educated on the option to donate embryos to research prior to the creation of embryos or cryopreservation of oocytes or sperm. As part of the informed consent process, patients are asked to make the following decision regarding excess embryos: I/we consent to have excess embryos frozen OR I/we do not wish to freeze excess embryos and choose 1) donate to research; 2) donate to quality improvement techniques; or 3) discard. In the case of oocytes or embryos that are unsuitable for clinical use or otherwise not cryopreserved, patients are given the same disposition options. As part of their family-building journey, patients later meet with a clinical coordinator to review their disposition options. Patients who continue to be interested in donating embryos or gametes to research are then referred to the RENEW Biobank research team. Clinical consent forms indicating interest in donation to research are insufficient to enroll in the RENEW Biobank, which requires an independent consent process pioneered at Stanford (18). In addition, patients who maintain embryos and gametes in cryostorage receive an annual bill. This bill includes information about the RENEW biobank for donated reproductive tissues including embryos, with contact information for the research team.
The RENEW Biobank also has partnerships with many clinics and storage facilities across the country. When patients express that they are interested in considering final embryo disposition options, these institutes offer donation to the RENEW biobank as an option along with the other typical options (discard, donation to another couple, etc.) (13–15). Patients from clinics not directly affiliated with the RENEW Biobank can access the website (https://med.stanford.edu/hesc/donations/tissue.html) and are encouraged to contact the research team directly to express interest and receive more information. A description of all of our biobanking initiatives is also on our clinic website (https://www.stanfordchildrens.org/en/service/fertility-and-reproductive-health/biobanking).
Informed Consent Process
For both biobanks, patients complete an online electronic consent form (eConsent) that is administered through REDCap software. This electronic platform allows patients to complete the eConsent at their convenience and save a digital copy for their reference, and also simplifies book-keeping and data organization processes for the research team. In addition to the online eConsent, patients enrolling in the RENEW Biobank also complete an electronic donor health questionnaire (Supplementary Table 2).
Both biobanks utilize a one-time consent. Patients do not consent to a particular research project, but instead consent for donation of specific tissue types to be used for a variety of research. This authorization for the use of these tissues expires per IRB requirements in 2060 for the RENEW Biobank and 2120 for the ROSE Biobank.
When a patient consents to enroll in the ROSE Biobank, they consent to donate blood, urine, endometrium, decidua, and villi that are generated in excess through clinical standard of care procedures and would otherwise be discarded. They have the option of consenting to have their specimens possibly used for somatic cell nuclear transfer and/or genetic reprogramming, but they can enroll without agreeing to this kind of research. In addition, ROSE patients have the option to donate two tubes of blood while they are already undergoing a clinical blood draw or IV placement. Patients are informed that they are able to enroll in ROSE without consenting to donate additional blood.
The ethical framework for donation of human embryos to research was pioneered at Stanford and described by Kalista et al. (6, 18). Patients enrolling in the RENEW Biobank have the option of consenting to both stem cell and non-stem cell research or only non-stem cell research. Embryos created with autologous gametes can be donated to the RENEW Biobank with the consent of both parents. Patients who utilized donor gametes in the creation of their embryos may only donate to non-stem cell research due to the legal requirements in the state of California that both intended parents and gamete donors must consent for stem cell research (17). RENEW patients also have the ability to choose whether they would like to be re-contacted in the case of a researcher needing more health information, or in the case of a researcher discovering relevant information for themselves and/or their family based on results from genetic testing done on the donated tissue. The consent form states that the RENEW Biobank does not support embryo research with reproductive intent and that donated tissue will never be transferred to a uterus or result in pregnancy. The consent form also states that all embryos are discarded according to ASRM guidelines and California law by 12 days of age (17, 19).
Protection of Patient Privacy
All donated specimens are de-identified and each enrolled patient is assigned an anonymous and random number ID. No identifying information is listed on the vials or distributed to the researchers. Patients are informed that despite all of the safety measures that we will use, we cannot guarantee that their identity will never become known. If a patient consents to be re-contacted and researchers decide that re-contact is necessary, biobank personnel can re-identify distributed specimens based on the patient ID and will contact patients directly; PHI is not distributed to researchers. Both our biobanks have received a Certificate of Confidentiality from the NIH.
Sample Collection, Processing, and Storage
ROSE Biobank
Blood
The research team saves serum that is present in excess after clinical testing and, among patients who opt in, serum, plasma and buffy coat from two extra tubes of blood when patients undergo venipuncture. For clinical testing, our lab freezes and stores serum up to one week after completion of analyses. Among patients who have consented, this excess serum is transferred to the ROSE Biobank. For patients who additionally consent to donate blood at the time of clinical venipuncture, sample processing is detailed in Supplementary Table 3. Serum and plasma can be utilized for numerous metabolomic, proteomic, immunologic and epigenetic assays and the buffy coat can be used for DNA analysis.
Endometrium
Endometrium is collected during biopsies that are required for clinical care. Clinicians are asked to save any excess tissue that they do not need to submit for testing. Sample processing is detailed in Supplementary Table 3. These snap frozen tissues can then be used for a variety of downstream analyses including transcriptome, proteome, epigenome and metabolomic analyses.
Decidua and Villi
Decidua and villi are collected during dilation and curettage (D&C) procedures that patients elect for in the event of a miscarriage. Clinicians are asked to save any excess tissue that they do not need to submit for pathology or cytogenetic testing. Sample processing including visual dissection of decidua and villi as described by Murugappan et al. is detailed in Supplementary Table 3 (20). These snap frozen samples can be used for molecular and gene expression assays, histopathology and immunohistochemistry.
RENEW Biobank
Tissues are received in batch shipments using dry nitrogen shippers and in the majority of cases, the cost of shipping from other locations is absorbed by the RENEW biobank. Tissues are stored in a large liquid nitrogen dewar that is regularly monitored and refilled by the research team.
Data collection
Relevant de-identified information is distributed to researchers who request samples from our biobanks. For samples from the ROSE Biobank, this information includes sex, race, ethnicity, BMI, smoking history, and infertility diagnoses associated with the distributed samples. The specific sample information includes patient age at collection, clinical purpose of collection, and patient’s obstetric history, pregnancy status, and medications at the time of sample collection. For samples from the RENEW biobank, information is reported for both individuals in a patient couple and includes sex, gender, race, ethnicity, sexual orientation, religious affiliation, education level, infertility diagnoses, prior fertility treatments, gravidity/parity, surgical history, allergies, chronic conditions, and family history of chronic conditions. The specific sample information includes patient age at retrieval, freezing protocol, stage when frozen, grade when frozen, and PGT results (if applicable).
Results
Patient Demographics
A total of 115 female and 11 male patients have donated samples to the ROSE Biobank in the first year of recruitment. Patient demographics are shown in Table 1. The most common infertility diagnosis was male infertility (24.6%) followed by diminished ovarian reserve (17.5%).
Table 1 –
ROSE Biobank Demographics
| Female (n=115) | Male (n=11) | |
|---|---|---|
|
| ||
| Average Age at consent | 36.31 years old | 37.45 years old |
|
| ||
| Average BMI at consent | 25.36 kg/m2 | 27.00 kg/m2 |
|
| ||
| Race | ||
|
|
||
| American Indian/Alaska Native | ||
| Asian | 54 (47%) | 3 (27.3%) |
| Native Hawaiian/Pacific Islander | 1 (0.9%) | |
| Black/African American | 4 (3.5%) | |
| White | 44 (38.3%) | 5 (45.4%) |
| More Than One Race | 2 (1.7%) | 1 (9.1%) |
| Other | 8 (7%) | 2 (18.2%) |
| Unknown/Not Reported | 2 (1.7%) | |
|
| ||
| Ethnicity | ||
|
|
||
| Not Hispanic/Latinx | 102 (88.7%) | 8 (72.7%) |
| Hispanic/Latinx | 8 (7%) | 1 (9.1%) |
| Unknown/Not Reported | 5 (4.3%) | 2 (18.2%) |
|
| ||
| Infertility Diagnosis | ||
|
|
||
| Diminished Ovarian Reserve | 22 (19.1%) | |
| Endometriosis | 7 (6.1%) | |
| Fertility Preservation | 8 (7%) | |
| Hypothalamic Amenorrhea | 1 (0.9%) | |
| Indication for use of Gestational Carrier | 1 (0.9%) | |
| Male Infertility | 26 (22.6%) | 5 (45.5%) |
| Other Ovulation Disorders | 3 (2.6%) | |
| Polycystic Ovarian Syndrome | 13 (11.3%) | |
| Recurrent Pregnancy Loss | 36 (31.3%) | 6 (54.5%) |
| Tubal Factor | 4 (3.5%) | 1 (9.1%) |
| Unexplained Infertility | 17 (14.8%) | |
| Uterine Factor | 6 (5.2%) | |
| Other | 7 (6.1%) | |
| Not Applicable* | 8 (7%) | |
|
| ||
| Gravidity/Parity at 1st collection | ||
|
|
||
| Average # of pregnancies | 2.00 (Range: 0 – 11) | |
| Average # of live births | 0.45 (Range: 0 – 2) | |
| Average # of miscarriages | 1.46 (Range: 0 – 9) | |
Encompasses patients without infertility, including third party reproduction, same sex patients, and single gene disorders.
A total of 109 male/female couples and 9 females have donated to the RENEW Biobank. Patient demographics are shown in Table 2. The most common infertility diagnosis was unexplained infertility (25%). Patients enrolling in RENEW also answered questions regarding their attitudes towards final embryo disposition (Table 2). One such question was to rate the difficulty of their decision to donate to our biobank on a scale of 1 to 5 with 5 being the most difficult. 119 patients (52.4%) rated their decision to donate as a 1 or 2, 44 patients (19.4%) rated it as a 3, and 63 patients (27.8%) rated it as a 4 or 5.
Table 2 –
RENEW Biobank Demographicsγ
| Female (n=118)* | Male (n=109) | |
|---|---|---|
|
| ||
| Race | ||
|
|
||
| Asian | 26 (22%) | 20 (18.3%) |
| East Asian | 12 (10.2%) | 7 (6.4%) |
| South Asian | 12 (10.2%) | 12 (11%) |
| Southeast Asian | 2 (1.7%) | 1 (0.9%) |
| Black or African American | 1 (0.8%) | |
| White | 78 (66.1%) | 85 (78%) |
| Middle Eastern/North African | 1 (0.9%) | |
| More than one race | 5 (4.2%) | 2 (1.8%) |
| East Asian, White | 4 (3.4%) | |
| East Asian, White, Native Hawaiian/Other | ||
| Pacific Islander | 1 (0.9%) | |
| South Asian, White | 1 (0.8%) | 1 (0.9%) |
| Other | 1 (0.9%) | |
| Unknown/Not Reported | 8 (6.8%) | |
|
| ||
| Ethnicity | ||
|
|
||
| Not Hispanic/Latinx | 106 (89.8%) | 104 (95.4%) |
| Hispanic/Latinx | 2 (1.7%) | 4 (3.7%) |
| Unknown/Not Reported | 10 (8.5%) | 1 (0.9%) |
|
| ||
| Gender ** | ||
|
|
||
| Female (cisgender) | 110 (93.2%) | |
| Male (cisgender) | 109 (100%) | |
| Unknown/Not Reported | 8 (6.8%) | |
|
| ||
| Sexual Orientation | ||
|
|
||
| Heterosexual/straight | 102 (86.4%) | 105 (96.3%) |
| Homosexual/gay or lesbian | 5 (4.2%) | 1 (0.9%) |
| Bisexual | 2 (1.7%) | 1 (0.9%) |
| Pansexual | 1 (0.8%) | |
| Choose not to disclose | 2 (1.7%) | 2 (1.8%) |
| Unknown/Not Reported | 6 (5.1%) | |
|
| ||
| Religious Affiliation | ||
|
|
||
| Buddhist | 2 (1.7%) | 2 (1.8%) |
| Christian | 57 (48.3%) | 50 (45.9%) |
| Catholic | 20 (16.9%) | 21 (19.3%) |
| Protestant | 21 (17.8%) | 18 (16.5%) |
| Orthodox | 6 (5.1%) | 5 (4.6%) |
| Undisclosed sect | 10 (8.5%) | 6 (5.5%) |
| Hindu | 8 (6.8%) | 8 (7.3%) |
| Jewish | 8 (6.8%) | 5 (4.6%) |
| Muslim | 1 (0.9%) | |
| Not religious/atheist | 22 (18.6%) | 28 (25.7%) |
| Other | 3 (2.5%) | 5 (4.6%) |
| Choose not to disclose | 10 (8.5%) | 10 (9.2%) |
| Unknown/Not Reported | 8 (6.8%) | |
|
| ||
| Highest Education Level | ||
|
|
||
| High School | 5 (4.6%) | |
| Some College | 4 (3.4%) | 15 (13.8%) |
| Bachelor’s Degree | 39 (33.1%) | 40 (36.7%) |
| Master’s Degree | 49 (41.5%) | 30 (27.5%) |
| Doctoral/Professional Degree (PhD, MD, JD) | 15 (12.7%) | 17 (15.6%) |
| Other | 1 (0.8%) | |
| Choose not to disclose | 2 (1.7%) | 2 (1.8%) |
|
| ||
| Infertility Diagnosis (n=116 patients/patient couples) | ||
|
|
||
| Diminished Ovarian Reserve | 3 (2.6%) | |
| Endometriosis | 8 (6.9%) | |
| Hypothalamic Amenorrhea | 1 (0.9%) | |
| Indication for use of Gestational Carrier | 1 (0.9%) | |
| Male Infertility | 18 (15.5%) | |
| Polycystic Ovarian Syndrome | 20 (17.2%) | |
| Recurrent Pregnancy Loss | 2 (1.7%) | |
| Tubal Factor | 6 (5.2%) | |
| Unexplained Infertility | 29 (25%) | |
| Uterine Factor | 2 (1.7%) | |
| Other | 13 (11.2%) | |
| Not Applicable | 11 (9.5%) | |
| Unknown/Not Reported | 22 (19%) | |
|
| ||
| On a scale of 1-5 (5 being the most difficult), how difficult was your decision to donate? | ||
|
|
||
| 1 | 33 (28%) | 40 (36.7%) |
| 2 | 19 (16.1%) | 27 (24.8%) |
| 3 | 25 (21.2%) | 19 (17.4%) |
| 4 | 24 (20.3%) | 13 (11.9%) |
| 5 | 16 (13.6%) | 10 (9.2%) |
| Unknown/Not Reported | 1 (0.8%) | |
| Median Rating | 3 (IQR = 3: Q1 = 1, Q3 = 4) | 2 (IQR = 2: Q1 = 1, Q3 = 3) |
|
| ||
| If you did not have the option to donate your embryos to research what would be your second choice? | ||
|
|
||
| Continue to store them | 17 (14.4%) | 13 (11.9%) |
| Discard them | 77 (65.3%) | 78 (71.6%) |
| Donate them to another couple | 18 (15.3%) | 15 (13.8%) |
| Other | 5 (4.2%) | 3 (2.8%) |
| Unknown/Not Reported | 1 (0.8%) | |
|
| ||
| Please rate the quality of your counseling regarding what to do with embryos still in storage upon completion of your fertility treatment on a scale of 1-5 (5 being the best). | ||
|
|
||
| 1 | 19 (16.1%) | 13 (11.9%) |
| 2 | 16 (13.6%) | 15 (13.8%) |
| 3 | 21 (17.8%) | 28 (25.7%) |
| 4 | 20 (16.9%) | 16 (14.7%) |
| 5 | 41 (34.7%) | 37 (33.9%) |
| Unknown/Not Reported | 1 (0.8%) | |
| Median Rating | 4 (IQR = 3: Q1 = 2, Q3 = 5) | 3 (IQR = 3: Q1 = 2, Q3 = 5) |
|
| ||
| Do you think that you would have benefitted from additional counseling throughout your treatment regarding what to do with embryos in storage upon completion of your fertility treatment? Please rate the degree to which you believe you would have benefitted from additional counseling on a scale of 1-5 (5 being the largest benefit). | ||
|
|
||
| 1 | 38 (32.2%) | 43 (39.4%) |
| 2 | 18 (15.3%) | 14 (12.8%) |
| 3 | 21 (17.8%) | 21 (19.3%) |
| 4 | 18 (15.3%) | 16 (14.7%) |
| 5 | 22 (18.6%) | 15 (13.8%) |
| Unknown/Not Reported | 1 (0.8%) | |
| Median Rating | 3 (IQR = 3: Q1 = 1, Q3 = 4) | 2 (IQR = 3: Q1 = 1, Q3 = 4) |
Includes single mothers and lesbian couples using donor sperm
No patients indicated that they identify as transgender or genderqueer/non-binary/gender non-conforming
This questionnaire is voluntary and patients can choose to leave questions unanswered. Responses are condensed and do not reflect all options given in the questionnaire; please refer to Supplementary Table 2 for a copy of the questionnaire with all answer choices.
Enrollment
An average of 9.7 patients enrolled per month in the ROSE Biobank and an average of 9.8 patients enrolled per month in the RENEW Biobank over the past year (Figure 1). From an average of 35 new patient consults per week, our goal was to recruit 2 patients per week in each biobanking initiative and we exceeded this goal in our first year of recruitment.
Figure 1 –
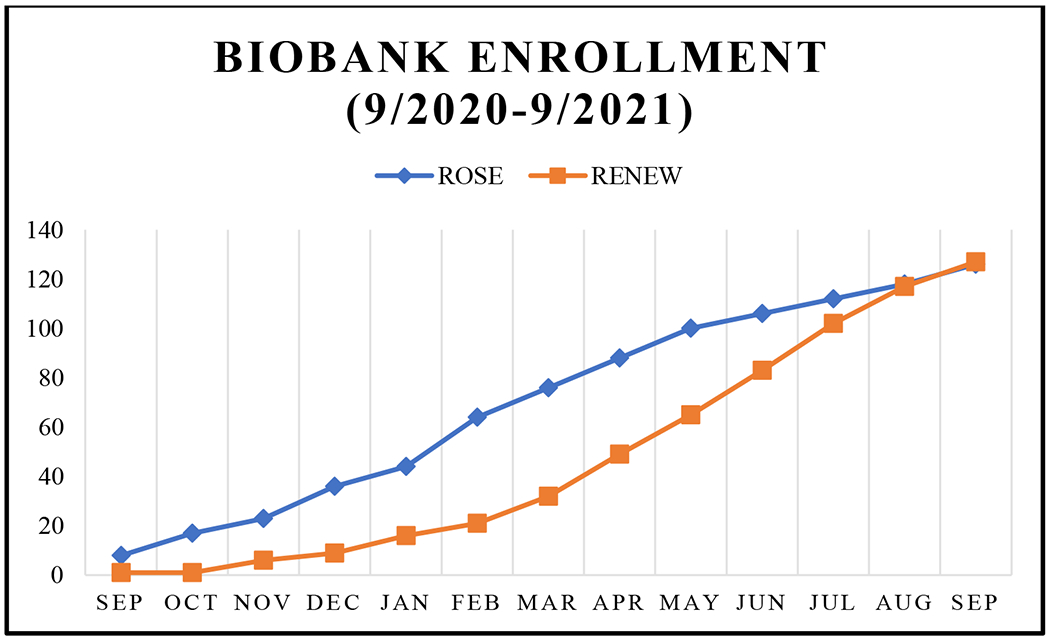
Cumulative Enrollment Per Month
* Although the RENEW Biobank was established in 2008, we are only showing cumulative enrollment from when we resumed enrolling again in 2020 with the ROSE Biobank
Sample Inventory
From September 2020 to September 2021, we have acquired 129 serum samples (267 aliquots), 65 plasma samples (317 aliquots), and 65 buffy coat samples (124 aliquots) in the ROSE Biobank (Table 3). 34 patients have donated blood at multiple timepoints. Of the 117 patients who were given the option to donate excess blood, 100 opted in (85.47%). In addition to blood, there are 35 endometrium samples (48 aliquots) and 28 pregnancy tissue samples (157 aliquots of decidua and 71 aliquots of villi) in the ROSE Biobank.
Table 3 –
Sample Inventory
| ROSE Biobank Sample Types | Number of Samples | Number of Aliquots |
|---|---|---|
|
| ||
| Blood | ||
|
|
||
| Serum | 129 | 267 |
| Plasma | 65 | 317 |
| Buffy coat | 65 | 124 |
| Endometrium | 35 | 48 |
|
|
||
| Pregnancy Tissue | ||
|
|
||
| Decidua | 28 | 157 |
| Villi | 26 | 71 |
|
| ||
| Patients with multiple sample donations | 52 | |
| Average number of donations for those who have multiple | 2.7 | |
| Patients with multiple blood samples | 34 | |
| Patients with paired blood and endometrium | 17 | |
| Patients with paired blood and pregnancy tissue | 19 | |
|
| ||
| RENEW Biobank Sample Types | Number of Samples (total specimens)* | |
|
| ||
| Embryos | ||
|
|
||
| 2PN | 74 | |
| Cleavage Stage | 287 | |
| Blastocyst | 467 | |
The RENEW Biobank has 7 pts who have consented to donate oocytes and 62 pts who have consented to donate embryos, but these tissues have not been received yet and are not counted in this inventory.
There is currently a total of 828 embryos in the RENEW Biobank, including zygote, cleavage and blastocyst stage. Additionally, there are 7 patients who have consented to donate oocytes and 62 couples who have consented to donate embryos with planned shipments.
Discussion
We report a framework for banking diverse reproductive tissue types that are routinely generated as part of fertility evaluation and treatment and are typically present in excess upon completion of clinical care. We present a streamlined workflow for patient recruitment, consent process, and details of sample processing and storage that maximize future research applications of the cryopreserved tissue. Furthermore, we describe the results of our first year of recruitment. Specimens from the ROSE Biobank have already enabled advanced, untargeted proteomic and metabolomic analysis of serum to explore novel markers of infertility and ovarian aging (unpublished data). Embryos from the RENEW Biobank have already been used in a variety of research including studies focused improving IVF outcomes (21–24).
There are several unique aspects of our biobanking initiative. The ROSE Biobank is a curated biorepository for blood (serum, plasma, buffy coat), endometrium, and pregnancy tissue (decidua and villi). Notably, we have collected paired samples of blood and endometrium and blood and pregnancy tissue as well as longitudinal samples of blood to enable complementary evaluations of reproductive tissues. For all patients, comprehensive clinical data is collected to further support translational and clinical research. The one-time consent utilized for the ROSE and RENEW Biobanks streamlines the recruitment process for the research team because patients do not need to be re-contacted to donate individual tissue types. In addition, prior research has shown that the majority of patients prefer a one-time informed consent for biobanking with a broad scope of permission for research (4).
Current limitations of our biobanking initiative include lack of ethnic diversity and limited number of oocytes. While patients from multiples races are represented in the ROSE and RENEW Biobanks, less than 10% of participants are of Hispanic ethnicity. As our biobanking initiatives primarily recruit from our fertility and reproductive health clinic, this ethnic distribution is reflective of our patient population. As our clinical services expand to satellite centers, we anticipate recruiting from increasingly diverse patient populations. An additional limitation of our biobanks is that while we have the capacity to store mature, unfertilized oocytes for research, we have not yet received this tissue type. This could be due to the fact that since oocyte cryopreservation is a relatively recent option widely available for patients, many are still anticipating use of their oocytes for their own procreative purposes. Seven patients from other locations, however, have consented to donating oocytes and shipments are planned. A limitation of biobanking initiatives in general is the lack of a registration requirement to better delineate efforts being made to support utilization of tissues for research. An international registry of biobanks would provide documentation of the various biobanks in the world and the kinds of tissues they procure so that researchers can easily find specimens.
Our biobanking initiative addresses a critical unmet need to allow patients the option of donating excess reproductive tissue to research (6, 13–15). Unlike many other medical treatments, patients undergoing IVF treatment usually create and freeze embryos that they do not ever use either because the embryos are aneuploid or because the patients have completed their family-building (6, 13, 15). If they decide that they do not want to continue storing the embryos for their own reproductive use, they generally have the following options: discard the embryos, donation to another patient for reproductive use, or donation to research and/or clinical training (13–15). Several studies have described patients’ attitudes toward embryo donation. A fertility clinic in Missouri recently reviewed their patients’ embryo disposition choices from 2000 to 2020 and found that roughly 45% of their patients expressed interest in donation to research (13). The RENEW Biobank donor health questionnaire responses also suggest that donation to research is a desired option for patients who are considering final embryo disposition, with over half of patients rating their decision to donate as a 1 or 2 on a scale of 5 with 5 being the most difficult. Additional studies are needed to better understand the counseling patients receive around embryo disposition and to further characterize their understanding of donation to research. Despite many patients preferring the option to donate to research and the unequivocal value of human embryo research for advancing human health, it is not commonly offered within the US. Through the RENEW Biobank we are able to give patients from any state that allows human embryo research the choice to donate, even if their clinics do not have their own research programs.
There are several key lessons learned from our experience. A streamlined, one-time consent administered electronically is well-received by patients and greatly reduces the administrative burden on the research team. A downside to the one-time consent process is that adding tissue types to either biobank requires re-consenting all enrolled patients. Inclusion of gestational carriers and oocyte donors provides a valuable source of healthy control patients but are a minority of our patient population. We plan to expand collection to the broader Department of OB/GYN to further recruit healthy control patients.
In summary, we report a framework for Stanford Fertility and Reproductive Health’s comprehensive biobanking initiatives in an effort to guide the establishment of future biobanks. Future directions for our biobanking initiative include the development of methods for sample distribution to internal and external collaborators and procurement of intramural and extramural funding to support this resource-intensive but valuable initiative.
Supplementary Material
Funding:
This work was supported by the Stanford Department of Obstetrics & Gynecology. Dr. Murugappan’s work related to non-embryo biobank (ROSE) research was supported by the National Institutes of Health Grant 1K12HD103084.
Footnotes
Disclosures: Drs. Alvero, Eisenberg and Murugappan are Scientific Advisors to Hannah Life Technologies. Drs. Alvero, Aghajanova and Behr are Scientific Advisors to Orchid Bioscience. Dr. Eisenberg is a Scientific Advisor to Roman, Dadi, and Underdog.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.De Souza YG, Greenspan JS. Biobanking past, present and future: responsibilities and benefits. AIDS 2013;27:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaught JB. Biorepository and biospecimen science: a new focus for CEBP. Cancer Epidemiol Biomarkers Prev 2006;15:1572–3. [DOI] [PubMed] [Google Scholar]
- 3.Domaradzki J, Pawlikowski J. Public Attitudes toward Biobanking of Human Biological Material for Research Purposes: A Literature Review. Int J Environ Res Public Health 2019;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon CM, L’Heureux J, Murray JC, Winokur P, Weiner G, Newbury E et al. Active choice but not too active: public perspectives on biobank consent models. Genet Med 2011;13:821–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrick DM, Mette E, Hoyle B, Rogers SD, Gillanders EM, Schully SD et al. The use of biospecimens in population-based research: a review of the National Cancer Institute’s Division of Cancer Control and Population Sciences grant portfolio. Biopreserv Biobank 2014;12:240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurlbut JB. Promising waste: biobanking, embryo research, and infrastructures of ethical efficiency. Monash Bioeth Rev 2015;33:301–24. [DOI] [PubMed] [Google Scholar]
- 7.Krawetz SA, Casson PR, Diamond MP, Zhang H, Legro RS, Schlaff WD et al. Establishing a biologic specimens repository for reproductive clinical trials: technical aspects. Syst Biol Reprod Med 2011;57:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruth KS, Perry JR, Henley WE, Melzer D, Weedon MN, Murray A. Events in Early Life are Associated with Female Reproductive Ageing: A UK Biobank Study. Sci Rep 2016;6:24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santillan MK, Leslie KK, Hamilton WS, Boese BJ, Ahuja M, Hunter SK et al. “Collection of a lifetime: a practical approach to developing a longitudinal collection of women’s healthcare biological samples”. Eur J Obstet Gynecol Reprod Biol 2014;179:94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenk M, Huppertz B, Obermayer-Pietsch B, Kastelic D, Hormann-Kropfl M, Weiss G. Biobanking of different body fluids within the frame of IVF-a standard operating procedure to improve reproductive biology research. J Assist Reprod Genet 2017;34:283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schon SB, Raja N, Xu M, Cameron H, Yang K, Reynolds J et al. Establishing a reproductive biorepository for basic and translational research: experience developing the reproductive subjects registry and sample repository. J Assist Reprod Genet 2021;38:2097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheldon E, Vo KC, McIntire RA, Aghajanova L, Zelenko Z, Irwin JC et al. Biobanking human endometrial tissue and blood specimens: standard operating procedure and importance to reproductive biology research and diagnostic development. Fertil Steril 2011;95:2120–2, 2 e1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander VM, Riley JK, Jungheim ES. Recent trends in embryo disposition choices made by patients following in vitro fertilization. J Assist Reprod Genet 2020;37:2797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyerly AD, Steinhauser K, Voils C, Namey E, Alexander C, Bankowski B et al. Fertility patients’ views about frozen embryo disposition: results of a multi-institutional U.S. survey. Fertil Steril 2010;93:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rinehart LA. Storage, transport, and disposition of gametes and embryos: legal issues and practical considerations. Fertil Steril 2021;115:274–81. [DOI] [PubMed] [Google Scholar]
- 16.Ethics in Embryo Research Task F, Ethics Committee of the American Society for Reproductive Medicine. Electronic address aao, Ethics Committee of the American Society for Reproductive M. Ethics in embryo research: a position statement by the ASRM Ethics in Embryo Research Task Force and the ASRM Ethics Committee. Fertil Steril 2020;113:270–94. [DOI] [PubMed] [Google Scholar]
- 17.California Code of Regulations. In: California So, ed. 17, 2021. [Google Scholar]
- 18.Kalista T, Freeman HA, Behr B, Pera RR, Scott CT. Donation of embryos for human development and stem cell research. Cell Stem Cell 2011;8:360–2. [DOI] [PubMed] [Google Scholar]
- 19.Ethics Committee of the American Society for Reproductive Medicine. Electronic address aao. Disposition of unclaimed embryos: an Ethics Committee opinion. Fertil Steril 2021;116:48–53. [DOI] [PubMed] [Google Scholar]
- 20.Murugappan G, Gustin S, Lathi RB. Separation of miscarriage tissue from maternal decidua for chromosome analysis. Fertil Steril 2014;102:e9–e10. [DOI] [PubMed] [Google Scholar]
- 21.Arand J, Reijo Pera RA, Wossidlo M. Reprogramming of DNA methylation is linked to successful human preimplantation development. Histochem Cell Biol 2021;156:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chavez SL, Loewke KE, Han J, Moussavi F, Colls P, Munne S et al. Dynamic blastomere behaviour reflects human embryo ploidy by the four-cell stage. Nat Commun 2012;3:1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol 2010;28:1115–21. [DOI] [PubMed] [Google Scholar]
- 24.Yanez LZ, Han J, Behr BB, Pera RAR, Camarillo DB. Human oocyte developmental potential is predicted by mechanical properties within hours after fertilization. Nat Commun 2016;7:10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


