Abstract
Objectives
Based on available data, the histological predictors of long-term outcome of lupus nephritis (LN) are not clearly defined. Aims of this retrospective study were: (i) to evaluate the change of chronicity index from the first to second kidney biopsy and to find the predictors of chronicity index increase and (ii) to detect the clinical/histological features at first and at second kidney biopsy associated with long-term kidney function impairment.
Methods
Among 203 biopsy proven LN subjects, 61 repeated kidney biopsy 49 months after the first biopsy. The reasons for repeated biopsy were: nephritic flares in 25 (41%), proteinuric flares in 21 (36%) of patients and protocol biopsy in 14 (23%) of cases.
Results
During 23-year follow-up, 25 patients presented a decrease in glomerular filtration rate (eGFR) ≥30%. At repeat biopsy, chronicity index increased in 44 participants (72%) and did not increase in 17 (28%). Nephritic syndrome and serum creatinine >1.6 mg/dL at presentation correlated with chronicity index increase (p=0.031, 0.027, respectively), cyclophosphamide therapy tended to protect against chronicity index increase (p=0.059). Kidney flares occurred in 53.6% of patients with vs 23.5% of those without chronicity index increase (p=0.035). Chronicity index increases of 3.5 points in patients with kidney flares vs 2 in those without flares (p=0.001). At second, but not at first kidney biopsy, two different models predicted eGFR decrease at multivariate analysis. The first included activity index >3 (OR: 3.230; p=0.013) and chronicity index >4 (OR: 2.905; p=0.010), and the second model included moderate/severe cellular/fibrocellular crescents (OR: 4.207; p=0.010) and interstitial fibrosis (OR: 2.525; p=0.025).
Conclusion
At second biopsy, chronicity index increased in 3/4 of participants. Its increase was predicted by kidney dysfunction at presentation and occurrence of LN flares. Kidney function impairment was predicted by both activity and chronicity index and by some of their components at repeated biopsy, but not at first biopsy.
Keywords: Lupus Nephritis; Lupus Erythematosus, Systemic; Cyclophosphamide
WHAT IS ALREADY KNOWN ON THIS TOPIC
A second kidney biopsy may be of help in predicting the long-term renal survival in lupus nephritis (LN).
WHAT THIS STUDY ADDS
This study evaluated, for the first time, the changes in chronicity index from first to second kidney biopsy and looked for the predictors of chronicity index increase in LN. The histological features at first and at second biopsy were tested as predictors of kidney failure.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study underlines the important role of baseline serum creatinine, of initial immunosuppressive therapy and of LN flares in predicting irreversible increase in chronic kidney lesions and emphasises the role of repeated kidney biopsy to provide useful information on management and long-term prognosis of LN.
Introduction
Kidney biopsy (KB) is recommended in presence of clinical signs of active lupus nephritis (LN) to confirm the diagnosis, to assess the prognosis and initiate the treatment.1 Years ago, it was suggested to add to the histological classification of LN activity and chronicity indices to better predict the kidney outcome of LN.2 3 Further studies found that activity index may indicate whether a treatment should be aggressive or not while chronicity index seemed to be associated with poor prognosis.4–11 Kojo et al5 found that extracapillary proliferation, glomerular sclerosis and fibrous crescents were independent predictors of poor kidney outcome, while Hsieh et al6 reported that tubulointerstitial inflammation and fibrosis, but not glomerular injury, were correlated with the risk of end stage kidney disease. In a retrospective study on 203 patients with LN followed for a median period of 14 years, interstitial inflammation was the only component of activity index associated with decrease in creatinine clearance. Such a decrease was observed only when interstitial inflammation was associated with tubular atrophy/interstitial fibrosis. Among the components of chronicity index, glomerular sclerosis, fibrous crescents and interstitial fibrosis were independent predictors of kidney function impairment (KFI) at multivariate analysis.11
Studies on repeated KB are few and report contrasting results. Some studies were based on protocol biopsies,12–16 while other reports included biopsies done for clinical reasons.17–19 A study that included protocol biopsy concluded that no histological variables at repeat biopsy were predictive of long-term kidney function.13 Other reports found that chronicity index predicted poor kidney outcome15 16 19, while some authors found that activity index at repeat biopsy but not chronicity index correlated with long-term kidney function.12 14 Few reports investigated if single components of the activity index at repeat biopsy were associated with doubling serum creatinine. Endocapillary proliferation and interstitial inflammation predicted doubling serum creatinine in a study,14 while subendothelial immune deposits, cellular crescents and fibrinoid necrosis/karyorrhexis12 or mesangial, subendothelial and subepithelial deposits were associated with the same endpoint in other studies.17 In our experience, cellular crescents in more than 30% of glomeruli predicted poor kidney function at multivariate analysis together with chronicity index ≥5.18
When comparing first and repeat KB, available data suggest that activity index can increase, reduce or remain unchanged; on the other hand, chronicity index generally increases.20 However, the prognostic value of the single components of chronicity index at repeat biopsy was never analysed. First aim of the study was assessing how chronicity index changes from the first to second biopsy and to find the clinical predictors of chronicity index variation in a single-centre cohort of patients with LN.
A second aim of our study was to detect the clinical/histological features at first and at second KB associated with long-term KFI.
Patients and methods
Out of 203 adults with biopsy-proven LN described elsewhere,11 we selected for this study the subjects who received a second KB. The reasons for repeated biopsy were: proteinuric flare, nephritic flare and protocol biopsy. The definition of nephritic and proteinuric biopsy are reported in table 1.21
Table 1.
Definition of kidney outcome, of indications to second biopsy and of histological score
| Definition of kidney events | ||||
| KFI | Decrease in eGFR ≥30% confirmed by at least three determinations for at least 3 months | |||
| Proteinuric flare21 | Increase in proteinuria without modification of serum creatinine of at least 2 g/24 hours if the previous proteinuria was <3.5 g/24 hours or doubling if previous proteinuria was ≥3.5 g/24 hours | |||
| Nephritic flare21 | Increase in serum creatinine of at least 30% over the last value, associated with nephritic urinary sediment, with or without increased proteinuria | |||
| Arterial hypertension | Systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg in sitting position (mean of three consecutive measurements) | |||
| Complete kidney response | Proteinuria<0.5 g/24 hours, normal or near normal eGFR (within 10% of normal eGFR of previously abnormal) | |||
| No complete kidney response | All the other cases | |||
| Histological assessment | ||||
| Light microscopy | Specimen fixed in 5% formalin Stains used: H&E, periodic acid-Schiff, silver methenamine and Masson’s trichrome/AFOG |
|||
| 0 | 1+ | 2+ | 3+ | |
| Neutrophil infiltration/karyorrhexis Endocapillary hypercellularity Hyaline deposits Cellular/fibrocellular crescents Fibrous crescents Fibrinoid necrosis Glomerular sclerosis |
Absent | Mild (<25% of glomeruli) |
Moderate (25%–50% of glomeruli) |
Severe (>50% of glomeruli) |
| Interstitial inflammation Interstitial fibrosis Tubular atrophy |
Absent | Mild (<25% of the cortex) |
Moderate (25%–50% of the cortex) |
Severe (>50% of the cortex) |
AFOG, Acid Fuchsin Orange G; eGFR, estimated glomerular filtration rate; KFI, kidney function impairment.
All the participants had first and repeated kidney biopsies classified according to the ISN/RPS criteria.22 All biopsies had at least 10 glomeruli each, evaluated by light microscopy and immunofluorescence.23 Patients requiring kidney replacement therapy at presentation or with inadequate biopsy were excluded from the study.
As previously reported,9 11 in 2003, all renal biopsies performed before 2002 in our centre were reclassified according to the ISN/RPS criteria22 by two nephrologists expert in kidney pathology (GB, GM) on the basis of light microscopy, immunofluorescence23 or re-evaluation of slides when necessary. Disagreements were adjudicated by consensus. The activity and chronicity indices were estimated by a semiquantitative scoring system according to Austin et al3 4 24 and by the recent revision of SLE classification.25
The starting point of the study was the date of the first biopsy and the end of the study was the date of the last check-up or death. For the aims of the study, KFI was defined by a decrease in estimated glomerular filtration rate (eGFR) of ≥30% over the baseline, confirmed by at least three determinations for at least 3 months. At second biopsy, no patient had KFI. The eGFR was assessed using CKD-EPI formula.26 The histological score to classify the kidney lesions is reported in table 1.
All patients received ‘specific therapy’ based on histological and clinical data and were regularly followed by a dedicated team. Induction therapy consisted of three intravenous methylprednisolone pulses (500–1000 mg/die) followed by oral prednisone 0.5 mg/kg/day in a single morning administration for 4 weeks, then progressively tapered to a maintenance of 5–7.5 mg per day. Glucocorticoids were associated with oral cyclophosphamide (1.5–2 mg/kg/day for 3 months) in severe cases or with azathioprine in milder cases.27 Maintenance with an immunosuppressive drug has become pivotal since the 1990s.28 More recently, mycophenolate mofetil was frequently used both in the induction and in the maintenance phase.
At each follow-up visit, clinical, laboratory and therapeutic data were regularly recorded until the last check-up in December 2021.
At the time of KB, all participants signed an informed consent for the scientific use of their anonymised records. This study adheres to the tenets of the Declaration of Helsinki.
Patient and public involvement
It was not possible to involve patients in the design, or conduct, or reporting, or dissemination plans of our research.
Statistical analysis
Descriptive statistics were calculated as median and IQR, since the distribution of most variables was not normal according to the Shapiro normality test. For the same reason, the difference of continuous variables between groups was tested with t-test or non-parametric Wilcoxon test for independent samples. χ² test was used to test associations between qualitative or dichotomised variables. Fisher’s exact test was used instead of χ² when expected cell counts were ≤5. For linear regression, we used Pearson’s correlation. Kaplan-Meier estimate was used for survival curves and Mantel-Cox log-rank test was used to test their difference. The Cox proportional hazards model, both univariate and multivariate, was used to find the predictors of KFI development over time. The ISN/RPS histological classes, activity and chronicity indices and all their components, and clinical features at first and at second biopsy have been tested as predictors of KFI development.
Different dichotomisations of ordinal variables ranging 0–3 were tested (eg, 0 vs 1–3, 0–1 vs 2–3) and the best one, according to its p value in the statistical models, was retained. Activity and chronicity indexes were dichotomised according to their median values.
The SPSS statistical package has been used for all the analyses (V.25, IBM, Armonk, New York, USA).29
Results
Sixty-one subjects who respected the inclusion criteria were included in this retrospective study. Of them, 55 were females (90%) and 60 Caucasians (98%). Their median age at first biopsy was 28 years (IQR 22–36). The second KB was performed at 49 (27-96) months median time after the first biopsy.
Events leading to second biopsy were: proteinuric flares in 21 (36%) cases, nephritic flares in 25 (41%), protocol biopsy or clinical decisions in 14 (23%). Ten protocol biopsies were performed after 2–3 years of maintenance therapy as part of a previous randomised pilot study.30 The last four biopsies were performed to decide about treatment reduction/change in patients with severe adverse events (two biopsies) or with severe histological lesions (two patients). Repeat and protocol biopsies were quite distributed over the years covered by the study, in particular 23 biopsies were performed between 1984 and 1994, 22 between 1995 to 2004 and the remaining 16 between 2005 and 2018.
The median time between first and second biopsy was 61 (36–102) months for proteinuric flares, 79 (28–112) months for nephritic flares and 27 (26.5–28) months for protocol biopsies. The clinical/histological and therapeutical characteristics of the whole group and of the three subgroups at first and second biopsy are reported in table 2.
Table 2.
Clinical/histological features, therapy at first and second kidney biopsy and patient/kidney outcomes in all patients and in those who received a first and a second kidney biopsy for proteinuric flare, nephritic flare or protocol biopsy
| Variables | All pts (n=61) | Proteinuric flare (n=22) | Nephritic flare (n=25) | Protocol biopsy (n=14) | ||||
| Data at 1st KB |
Data at 2nd KB |
Data at 1st KB |
Data at 2nd KB |
Data at 1st KB |
Data at 2nd KB |
Data at 1st KB |
Data at 2nd KB |
|
| Male/Female, n* of pts (%) | 6 (10)/55 (90) | 2 (9)/20 (91) | 4 (16)/21 (84) | 0/14 (100) | ||||
| Age at kidney biopsy, years | 28 (22–36) | 34 (28–45) | 29 (22–32) | 34 (28–44) | 27 (21–35) | 34 (28–47) | 28 (27–48) | 33 (29–47) |
| Serum creatinine, mg/dL | 1.1 (0.8–1.6) | 1.1 (0.9–1.6) | 1 (0.7–1.3) | 0.9 (0.7–1) | 1.2 (1–1.6) | 1.6 (1.5–2.4) | 1.3 (1–1.6) | 1 (0.9–1.1) |
| eGFR, mL/min/1.73 m2 | 66 (44–99.5) | 66.5 (42–87) | 79 (59–115) | 106 (75–112) | 65 (43–89) | 37 (28–49) | 57 (42–66) | 68 (58–86) |
| Proteinuria, g/die | 3.3 (1.8–5.6) | 2.4(1-5) | 3.4 (1.4–5.7) | 2.7 (2–4.5) | 4.4(3-6) | 4.1 (1.9–6.8) | 2.9 (1.8–3.9) | 0.2 (0.2–0.4) |
| Urinary erythrocytes, n*/HPF | 20 (7–40) | 5 (0–16) | 13 (5–21) | 5 (0–13) | 34 (12–52) | 17.5 (1–40) | 40 (14–40) | 3 (1–5) |
| Serum C3, mg/dL† | 50 (40–67) | 80 (50–95) | 54 (42–66) | 69 (56–97) | 50 (36–73) | 61 (42–90) | 50 (45–59) | 97 (85–112) |
| Serum C4, mg/dL‡ | 7 (5–13.5) | 13.5(10-18) | 11.5(5-15) | 11(9-15) | 5.5 (5–9) | 13 (9.5–17) | 7 (5–11) | 17 (16–19) |
| Arterial hypertension, n*(%) | 32 (52) | 31 (51) | 11 (50) | 10 (45.5) | 11(44) | 17 (68) | 10 (71) | 4 (28) |
| Months from 1* to 2* KB | 49 (27–96) | 61 (36–102) | 79 (28–112) | 27 (26.5–28) | ||||
| Induction therapy, n* pts(%) | ||||||||
| Methylprednisolone pulses IST induction Cyclophosphamide na |
38 (70) 39 (72) 26 (48) 7 |
28 (49) 23 (40) 13 (23) 4 |
11 (55) 14 (70) 10 (50) 2 |
11 (52) 9 (43) 5 (24) 1 |
17 (81) 13 (62) 8 (38)¶ 4 |
16 (69.5) 13 (56.5) 8 (35) 2 |
10 (77) 12 (92)§ 8 (61) 1 |
1 (7.7) 1 (7.7) 0 1 |
| Maintenance therapy, n* (%) MMF/AZA/CsA, % na |
28 (51) 9/34.5/11 6 |
33 (58) 30/26/14 4 |
5 (26) –/26/– 3 |
11 (52) 38/9/14 1 |
10 (45) 14/27/4.5 3 |
12 (53) 22/35/4 2 |
13 (93) 14/57/36 – |
10 (77) 31/38/31 1 |
| Follow-up, years | 23 (17.5–32) | 30 (20.8–34.5) | 21.6 (13–30) | 22 (21–28) | ||||
| KFI, n* pts (%) | 25 (41%)** | 6 (27%) | 16 (64%)†† | 3 (21%) | ||||
| ESRD, n* pts (%) | 12 (20%) | 4 (18%) | 7 (28%) | 1 (7%) | ||||
| Death, n* pts (%) | 14 (23%) | 6 (27%) | 7 (28%) | 1 (7%) | ||||
| Histological class, n* | ||||||||
| I/II/III/IV/V Mixed classes |
–/–/7/47/7 7 |
1/6/12/36/6 13 |
–/–/2/15/5‡‡ 2 |
–/1/6/12/3 8 |
–/–/3/21/1 2 |
–/1/3/20/1 2 |
–/–/2/11/1 3 |
1/4/3/4/2 3 |
| Activity index | 7 (5–10) | 3 (0–6) | 7.5 (6–10) | 2.5 (0.25–5) | 7.5 (5–10) | 5 (3–9) | 6 (4–10) | 1 (0–2) |
| Chronicity index | 1 (0–3) | 4 (2–6) | 1 (0–2) | 4 (2–4) | 1 (0–2) | 5 (2–7) | 3 (2–4)§§/¶¶ | 3 (2–6) |
| Increase in CI from 1st and 2nd KB | 2 (0–4) | 2 (0–2.75) | 3 (2–6)*** | 0.5 (0–2) | ||||
| N* pts (%) with no increase of CI between first and second KB | 17 (28%)††† | 7 (32%) | 3 (12%)‡‡‡ | 7 (50%) | ||||
*1 data missing.
†Normal values 90–180 mg/dL.
‡Normal values 10–40 mg/dL.
§P=0.053; refers to the comparison of the frequencies of induction therapy in protocol biopsy group vs nephritic flare and proteinuric flare groups.
¶P=0.028; refers to the comparison of the frequencies of cyclophosphamide therapy in nephritic flare group vs proteinuric flare and protocol biopsy.
**P=0.009; refers to comparison of the frequencies of KFI between the three different groups.
††P=0.001; refers to the comparison of the frequencies of KFI in nephritic flare group vs proteinuric flare and protocol biopsy groups.
‡‡P=0.038; refers to the comparison of incidences of class V NL in proteinuric flare group vs nephritic flare and protocol biopsy groups.
§§P=0.002; refers to the comparison between proteinuric flare and protocol biopsy group.
¶¶P=0.001; refers to the comparison between proteinuric flare and nephitic biopsy group.
***P=0.001; refers to the comparison of nephritic flare group vs proteinuric flare and protocol biopsy groups.
†††P=0.035; refers to comparison between the three different groups.
‡‡‡P=0.021; refers to the comparison of nephritic flare group vs proteinuric flare and protocol biopsy groups.
AZA, Azathioprine; CsA, Ciclosporin; eGFR, estimated glomerular filtration rate; ESRD, End Stage Renal Disease; HPF, High-power field; IST, Immunosuppressive Therapy; KB, kidney biopsy; KFI, kidney function impairment; MMF, mycophenolate mofetil.
At first KB 11.5% of patients had class III, 77% class IV and 11.5% class V LN. Class V was more frequent in biopsies performed for proteinuric flares. It was diagnosed in 23% of participants who received a repeat biopsy for proteinuric flares in comparison to 4% of subjects with nephritic flares, and of 7% in protocol group (p=0.038). There were no other significant differences between the three groups in the clinical characteristics at first KB.
At first KB, induction therapy with cyclophosphamide was more frequent in the protocol group. Cyclophosphamide was employed in 61% of patients in protocol group, in 50% of those in proteinuric group and 38% in the nephritic group (p=0.028).
Table 2 (and online supplemental table 1) reported changes in histological classes from the first to the second biopsy. Altogether, class transformation occurred in 47% of patients, 71.5% of the patients in class V (3 patients switched to class III and two to class IV), 57% in class III (one patient changed to class II and three to class IV) and 34% in class IV (online supplemental table 2). Activity index, from first to the second biopsy reduced in 44 patients, increased in 13 and was unchanged in the last four patients.
lupus-2022-000721supp001.pdf (71KB, pdf)
The median follow-up from the first KB and from the second to last observation were respectively 23 (17.5–32) and 15 (8.4–21) years. Twenty-five patients (41%) developed KFI in 3 years median time (2–13.3) after the second biopsy.
At the end of observation, 64% of participants who received a second biopsy for nephritic flares, developed KFI vs 27% of those who had proteinuric flares and 21% in the protocol group (p=0.001). Patients with nephritic flares had also a higher increase of chronicity index in comparison to the first biopsy (median increase 3 (2-6)) than those with proteinuric flares (2 (0–2.75)) and those who received biopsy for protocol or clinical events 0.5 (0–2) p=0.001). Altogether, 88% of patients with nephritic flares had chronicity index increase from the first to the second biopsy versus 68% in proteinuric group and 50% in the other groups (p=0.021).
Changes in chronicity index from first to second biopsy and predictors of increase in chronicity index
In 17 patients (28%), there was no increase in the chronicity index between the first and the second biopsy (2 (IQR 1–3)). In the other 44 participants, the chronicity index increased from a median of 1 (IQR 0–2) to 2 (IQR 2–5) (table 3).
Table 3.
Comparison of clinical data, histological characteristics and therapy at first kidney biopsy between patients who had and those who did not have increase in chronicity index between first and second kidney biopsy
| No increase in Chronicity Index (n=17) |
Increase in Chronicity Index (n=44) |
P value | |
| Male/Female, n* of pts (%) | 2 (12)/ 15 (88) | 4 (9)/ 40 (91) | 0.753 |
| Age at kidney biopsy, years | 27 (24.3–33) | 29 (21.8–36.7) | 0.750 |
| Months between LN diagnosis and biopsy | 2.8 (1–16) | 5 (0.9–37) | 0.280 |
| Months between SLE and biopsy | 24.4 (7–87.6) | 27.7 (4.8–96) | 0.960 |
| Months between first and second biopsy | 29.2 (26.8–64.2) | 54 (27–99.5) | 0.580 |
| Clinical data at first kidney biopsy | |||
| Nephritic syndrome, n* of pts (%) | 3 (17.6) | 21 (47.7) | 0.031 |
| Serum creatinine, mg/dL | 1.1 (0.8–1.3) | 1.1 (0.9–1.6) | 0.169 |
| Serum creatinine ≥1.65 mg/dL, n* of pts (%) | 0 | 10/42 (45)* | 0.027 |
| eGFR, mL/min/1.73 m2 | 67 (57.2–97.6) | 65.7 (42.3–97.6) | 0.369 |
| Proteinuria, g/die | 3.3 (1.5–5.6) | 3.4 (2–5.5) | 0.442 |
| Urinary erythrocytes (number/HPF) | 21.5 (7.8–40) | 17(6-40) | 0.369 |
| Serum C3, mg/dL† | 50 (46-77) | 50 (37-64) | 0.272 |
| Serum C4, mg/dL‡ | 9.5 (5.3–12) | 6 (5-14) | 0.999 |
| Arterial hypertension, n* of pts (%) | 9 (53%) | 23 (52.3%) | 0.963 |
| Therapy | |||
| Methylprednisolone pulses, n* of pts (%) | 11 (68.75%) | 27 (73%) | 0.754 |
| IST Induction, n* of pts (%) Cyclophosphamide |
14 (87.5%) 11 (68.8%) |
25 (65.7%) 15 (40.5%) |
0.130
0.059 |
| Maintenance, (%) MMF/AZA/CSA Na |
9 (53%) 6% / 41% / 6% 0 |
19 (43%) 4.5% / 27% / 11.5% 6 |
0.840 |
| Complete response at 1 year, n* of pts (%) | 8/16 (50%) | 17/40 (42.5%) | 0.610 |
| Kidney flares, n* of pts (%)§ Creatinine flares, n* of pts (%) |
4/17 (23.5%) 0 |
22/41 (53.6%) 9/41 (22%) |
0.035
0.035 |
| Histological characteristic | |||
| Histological classes (%) | |||
| III/IV/V Mixed classes |
17.5/65/17.5 12 |
6.8/82/9 11 |
0.3/0.15/0.3 0.9 |
| Activity index | 6 (3–10) | 7 (6–10) | 0.692 |
| Endocapillary hypercellularity¶>1, pts (%) | 8 (47) | 25 (57.8) | 0.492 |
| Neutrophils infiltration/karyorrhexis¶>1, pts(%) | 6 (35) | 23 (53.3) | 0.233 |
| Cellular/fibrocellular crescents¶>1, pts (%) | 1 (6) | 6 (13.3) | 0.394 |
| Hyaline deposits/wire loops¶>1, pts (%) | 9 (56)** | 25 (57.8) | 0.968 |
| Fibrinoid necrosis††>0, pts (%) | 6 (35) | 23 (51) | 0.234 |
| Interstitial inflammation††>0, pts (%) | 8 (47) | 19 (42.2) | 0.784 |
| Chronicity Index | 2 (1–3) | 1 (0–2) | 0.209 |
| Glomerular sclerosis††>0, pts (%) | 9 (53%) | 16 (35.5%) | 0.238 |
| Fibrous crescents††>0, pts (%) | 10 (59%) | 17 (37.8%) | 0.154 |
| Tubular atrophy††>0, pts (%) | 3 (17.6%) | 7 (17.7%) | 0.869 |
| Interstitial fibrosis††>0, pts (%) | 8 (47%) | 13 (31%) | 0.197 |
P values are evaluated with t-test for independent samples and with χ² test between qualitative or dichotomised variables.
Bold values indicates the significant results.
*10 missing data.
†Normal values 90–180 mg/dL.
‡Normal values 10–40 mg/dL.
§10 missing data (2 data in the group of patients who did not increase Chronicity Index and 8 data in the other group).
¶These variables were categorised as: 0+1 vs 2+3.
** 1 missing data.
††These variables were categorised as: 0 vs 1+2+3, being: 0 if absent; 1+ if mild (in less than 25% of glomeruli and/or in tubulointerstitial cortex); 2+ if moderate (in between 25% and less than 50% of glomeruli and/or in tubulointerstitial cortex) and, 3+ if severe (in more than 50% of glomeruli and/or in tubulointerstitial cortex).
eGFR, estimated glomerular filtration rate; LN, lupus nephritis; MMF, mycophenolate mofetil.
The time between the first and the second biopsy did not correlate with the increase in chronicity index (Spearman’s rho: 0.104; p=0.427), although patients with chronicity index increase received the second KB 54 (27–99.5) months after the first biopsy in comparison to 29.2 (26.8–64.2) months in those without increase in chronicity index (p=0.580).
Presentation with nephritic syndrome (47.7% in participants with increase in chronicity index vs 17.6% in those with no increase; p=0.031) and serum creatinine ≥1.6 mg/dL at first biopsy (45% in patients with chronicity index increase vs in no participants without increase; p=0.027), predicted increase in chronicity index. Instead, induction treatment with any immunosuppressive therapy (87.5% in those without chronicity index increase vs 65.7% in those with increase; p=0.139) and with cyclophosphamide (68.8% in those without increase of chronicity index vs 40.5% in those with increase; p=0.059), tended to protect from chronicity index increase.
There were no differences in chronicity index increase in patients who received or did not receive maintenance immunosuppressive therapy. Forty-three per cent of patients with a chronicity index increase between the two biopsies received maintenance therapy in comparison to 53% of those who did not increase the chronicity index (p=0.804).
Increase in chronicity index was more frequent in class IV than in the other classes but the difference was not significative (82% vs 65%; p=0.154). Neither the baseline value of activity and chronicity indices nor any of their components were associated with the changes in chronicity index between the first and second biopsy.
Among the time dependent factors, the achievement of complete kidney remission 1 year after the start of therapy did not protect from chronicity index increase (complete remission in 50% of participants without chronicity index increase versus 42.5% in those with no remission; p=0.610).
Chronicity index increased in 53.6% of participants who had one or more kidney flares before the second biopsy versus 23.5% of those without flares (p=0.035). The median chronicity index increase was 3.5 (1.75–6) in subjects who developed kidney flares versus an increase of 2 (0–2) in those who did not (p=0.001). Nephritic flares occurred in 22% of subjects with chronicity index increase but in none of participants without increase (p=0.035).
The difference in renal flares between patients who did or did not receive a maintenance immunosuppressive therapy before the second KB was not significant, but it was numerically higher in patients who did not receive maintenance therapy (55.2% vs 32%; p=0.08).
Clinical and histological predictors of kidney function impairment
KFI occurred in 25 patients (41%). In online supplemental table 3, the characteristics of patients who developed KFI are reported and compared with those of patients who did not develop it.
Because the study covers a period of several decades, we tested at univariate analysis the year of first and second KB: these variables were not predictive of KFI, (year of first KB: OR 1.015; p=0.448; CI 0.977 to 1.054; year of second KB: OR 1.010; p=0.572; 0.975–1.046). Moreover, Pearson’s correlation between KFI and years of KB were not statistically significative (first KB: R=−0.192, p=0.153; second KB: R=−0.132, p=0.324).
At univariate analysis (table 4), cellular/fibrocellular crescents in more than 25% of glomeruli (OR 5.805; p=0.023; CI 1.275 to 26.441) was the only histological parameter at first biopsy associated with KFI. No clinical feature at the first biopsy was predictive of KFI.
Table 4.
Clinical and histological predictors of KFI (univariate and multivariate analysis)
| Univariate analysis | Multivariate analysis 1 | Multivariate analysis 2 | |||||||
| OR | CI | P | OR | CI | P | OR | CI | P value | |
| Histological features at first kidney biopsy | |||||||||
| Cellular/fibrocellular crescents>1* | 5.805 | 1.275 to 26.441 | 0.023 | ||||||
| Clinical features at second kidney biopsy | |||||||||
| Serum creatinine | 1.329 | 1.123 to 1.572 | 0.001 | ||||||
| Proteinuria>3.5 g/die | 3.239 | 1.457 to 7.197 | 0.004 | ||||||
| Arterial hypertension | 3.384 | 1.374 to 8.337 | 0.008 | ||||||
| Histological features at second kidney biopsy | |||||||||
| Activity Index >3 | 3.808 | 1.516 to 9.564 | 0.004 | 3.230 | 1.275 to 8.183 | 0.013 | |||
| Cell/fibrocell crescents>1* | 4.141 | 1.406 to 12.199 | 0.010 | 4.207 | 1.416 to 12.500 | 0.010 | |||
| Hyaline deposits>1* | 2.836 | 1.216 to 6.614 | 0.016 | ||||||
| Chronicity Index>4 | 3.476 | 1.544 to 7.824 | 0.003 | 2.905 | 1.285 to 6.566 | 0.010 | |||
| Fibrous crescents>1* | 2.902 | 1.148 to 7.335 | 0.024 | ||||||
| Interstitial fibrosis >1* | 2.498 | 1.114 to 5.598 | 0.026 | 2.525 | 1.120 to 5.691 | 0.025 | |||
*These variables were categorised as: 0+1 vs 2+3, being: 0 if absent; 1+ if mild (in less than 25% of glomeruli and/or in tubulointerstitial cortex); 2+ if moderate (in between 25% and less than 50% of glomeruli and/or in tubulointerstitial cortex) and 3+ if severe (in more than 50% of glomeruli and/or in tubulointerstitial cortex).
KFI, kidney function impairment.
At second biopsy, among the clinical features, serum creatinine (OR 1.329 for any increase in 1 mg/dL; p=0.001; CI 1.123 to 1.572) nephrotic proteinuria (OR 3.239; p=0.004; CI 1.457 to 7.197) and arterial hypertension (OR 3.384; p=0.008; CI 1.374 to 8.337) were significantly related to the development of kidney function deterioration at univariate analysis. Among the histological characteristics, activity index ≥3 (OR 3.808; p=0.004; CI 1.516 to 9.564), moderate/severe cellular/fibrocellular crescents (OR 4.141; p=0.010; CI 1.406 to 12.199) and hyaline deposits (OR 2.836; p=0.016; CI 1.216 to 6.614) were significantly associated with long-term KFI at univariate analysis. In addition, chronicity index >4 (OR 3.476; p=0.003; CI 1.544 to 7.824), moderate/severe fibrous crescents (OR 2.902; p=0.024; CI 1.148 to 7.335) and interstitial fibrosis (OR 2.498; p=0.026; CI 1.114 to 5.598) predicted decline of eGFR ≥30% at univariate analysis.
At multivariate analysis, two histological models with the same power were associated with the development of KFI (likelihood ratio p=5e−10 in both). Model 1 included activity index ≥3 (OR:3.230; p=0.013; CI 1.275 to 8.183) and chronicity index >4 (OR:2.905; p=0.010; CI 1.285 to 6.566) (figure 1A, B). Model 2 included moderate/severe cellular/fibrocellular crescents (OR:4.207; p=0.010; CI 1.416 to 12.500) and of interstitial fibrosis (OR:2.525; p=0.025; CI 1.120 to 5.691) (figure 1C, D).
Figure 1.
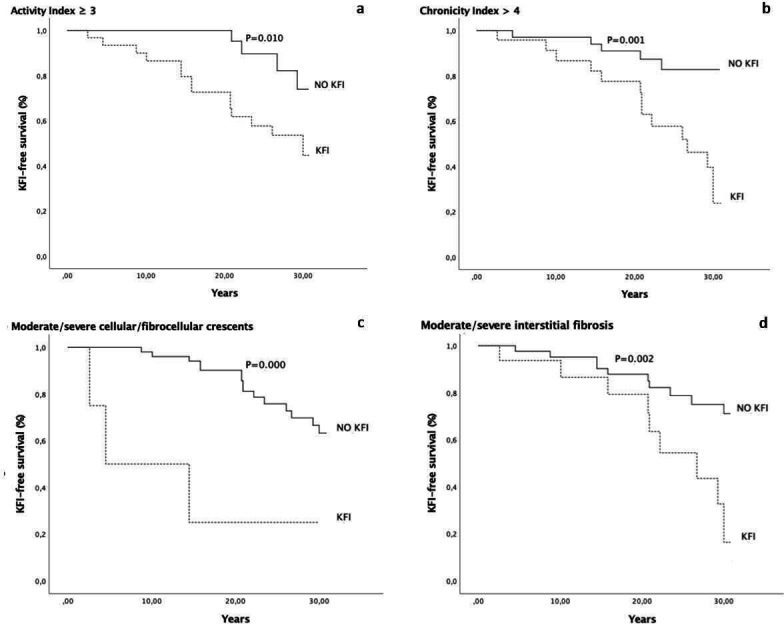
Kidney function impairment-free survival curve in patients with activity index ≥or <3 (A), chronicity index >or ≤4 (B), with or without moderate/severe cellular/fibrocellular crescents (C) and with or without moderate/severe interstitial fibrosis (D) at the second kidney biopsy. KFI, kidney function impairment.
Clinical and histological predictors of kidney function impairment occurring within 5 years after the second kidney biopsy
Thirteen patients developed KFI within 5 years after the second biopsy (online supplemental table 4).
At univariate analysis, among clinical and histological features at first KB, proteinuria >3.5 g/day (OR=3.677; p=0.000, CI 2.707 to 24.909) and cellular/fibrocellular crescents in more than 25% of glomeruli (OR=8.212; p=0.000, CI 2.707 to 24.909) were the only parameters predictive of KFI. At the second KB, among clinical features, serum creatinine (OR=2.387; p=0.000; CI 1.719 to 3.316) and nephrotic proteinuria (OR=3.070; p=0.049; CI 1.004 to 9.390) predicted KFI al univariate analysis. Among the histological features moderate/severe cellular/fibrocellular crescents (OR=10.577; p=0.000; CI 2.836 to 39.444), chronicity index >4 (OR=23.698; p=0.001; CI 3.075 to 182.613), moderate/severe fibrous crescents (OR=5.602; p=0.002; CI 1.873 to 16.754) and interstitial fibrosis (OR=6.545; p=0.026; CI 2.129 to 20.118) were associated with KFI within 5 years from the second biopsy.
At multivariate analysis, moderate/severe cellular/fibrocellular crescents (OR 31.955, p=0.000; CI 5.160 to 197.910) and chronicity index >4 (OR: 39.078; p=0.001; CI 4.375 to 349.071) were independent predictors of KFI within 5 years.
Online supplemental table 5 summarises the combination of the different risk factors that contributed to the study end-points: (a) chronicity index increase from first to second KB and (b) long-term KFI.
Discussion
The first goal of the study was to evaluate changes in chronicity index between first and second KB and to detect the clinical factors associated with chronicity index increase. Chronicity index increased from the first to the second biopsy in 72% of participants, and it remained unchanged in the other 28% of patients.
Three quarter of patients who underwent repeat biopsy for nephritic or proteinuric flares had chronicity index increase, instead chronicity index remained unchanged in half of protocol biopsies.
Nephritic syndrome and an elevated serum creatinine at the time of first biopsy correlated with increase in chronicity index, while the use of cyclophosphamide in induction therapy tended to protect against this increase. Nephritic syndrome is often associated with or followed by an increase in serum creatinine, that may recover incompletely. Previous studies already reported that impaired kidney function at onset of LN is correlated with poor kidney prognosis.4 9 31 32 The efficacy of cyclophosphamide therapy in inducing LN remission is well known, and even the long-term data demonstrate its efficacy.9 33 34 In a cohort of 39 patients with LN who underwent repeat biopsy after 2 years of induction therapy, the group treated with cyclophosphamide had a significantly lower increase in chronicity index compared the group treated with azathioprine.13 One may speculate that cyclophosphamide might effectively protect from development of chronic lesions by healing active histological lesions and preventing their transformation into chronic scars compared with other immunosuppressors. Since our paper encompasses many decades, some patients did not receive maintenance immunosuppressive therapy, which started to become indispensable in 90s.28 However, the absence of immunosuppressive drugs in maintenance therapy was not associated with an increase in chronicity index.
The clinical response at 1 year did not protect from worsening of chronicity index. This is not strikingly surprising, since clinical and histological remissions are often discordant.15 16 35 36 Furthermore, it is still unknown how long it is needed by an active injury to heal or progress into a chronic lesion.37 Among the time dependent factors, kidney flares, nephritic flares, were strongly correlated with chronicity index increase. This result confirms a previous report outlining that nephritic flare was associated with a 27-fold risk of doubling creatinine value, compared with patients without flares or with proteinuric flare.21 Parikh et al38 added that also the relative duration of kidney exacerbation is an independent predictor of incident and progressive chronic kidney disease. The deleterious influence of kidney exacerbation may possibly be due to the incomplete reversal of the lesions caused by flares despite therapy. The rate of renal flares was not different between patients who received maintenance immunosuppressive therapy and those who did not.
The other purpose of this study was to detect the clinical-histological features associated with KFI in the long term. Patients who received a second biopsy for nephritic flares developed more frequently KFI than those with proteinuric flares and those of protocol group.
We found that features at second biopsy were more predictive than those at first biopsy. At first KB, among clinical and histological features, only the presence of cellular/fibrocellular crescents predicted KFI at univariate analysis. At second biopsy, high serum creatinine, nephrotic syndrome and arterial hypertension predicted KFI. Among the histological features, activity index ≥3, moderate/severe cellular/fibrocellular crescents and hyaline deposits, chronicity index >4, moderate/severe fibrous crescents and interstitial fibrosis were all associated to KFI at univariate analysis. At multivariate analysis, two different histological models with the same power were able to predict KFI. The first model included the association of activity index ≥3 with chronicity index >4, and the second model included the association between moderate-severe cellular/fibrocellular crescents and interstitial fibrosis. When we looked at patients who developed KFI within 5 years of the second KB, we found that both active and chronic lesions at the second biopsy confirmed to be predictors of KFI. In fact, at multivariate analysis, chronicity index >4 and moderate/severe cellular/fibrocellular crescents at second biopsy were the independent histological predictors of KFI at multivariate analysis.
These data confirm in a larger monocentric series with a longer follow-up the results of a small previous multicentric report that outlined the prognostic significance of activity and chronicity index at second biopsy, but not at baseline.18 In another cohort of 77 subjects with LN who underwent a second protocol KB, Alsuwaida et al14 reported that the doubling of serum creatinine was predicted by an activity index >0, by endocapillary proliferation and interstitial inflammation at second KB. There was a non-significant trend towards a better outcome in those with chronicity index <3. Hill et al12 also found that the activity index at the second biopsy was associated with the doubling serum creatinine while the chronicity index at the second biopsy did not predict the outcome. Other studies found that only the chronicity index at second biopsy predicted renal kidney outcome,15 16 19 but the individual components of the index were not investigated. Some discrepancies with our study may be due to the fact that only 23% of our cases were protocol biopsies, while most other studies were based on systematic protocol biopsies.12–16 However, there is general agreement that the histological data at repeated biopsy are more predictive than those at first KB.12 14–20
Altogether, our results showed a strict interplay between renal flare, chronicity index increase and KFI. However, not all renal and nephritic flares cause an increase in chronicity index. If renal flares are timely diagnosed and aggressively treated the clinical manifestations reverse and renal function is preserved.27 A repeat KB is an important tool to assess the sequelae of nephritic flares on kidney tissue and to obtain information for the long-term outcome.
In conclusion, this study underlines the central role of serum creatinine, LN flares and immunosuppressive therapy in predicting and preventing irreversible chronic kidney lesions and emphasises the role of repeated KB to provide useful information on management and long-term prognosis of LN.
However, this report has some limitations; it has a retrospective nature and all, but one participant were Caucasians, so these results cannot be extended to other ethnicities. The indications for repeated KB were not homogeneous. Finally, since most data came from a real-world LN cohort, treatment and duration of follow-up were not standardised.
Footnotes
Contributors: GM and CP conceptualised the study. GP wrote the original draft. GM, GP, GF and FR were responsible for investigation, data curation and formal analysis. GB, GM, GP and CP reviewed and edited the manuscript. GB, MC, CP, GP and FR were responsible for visualisation and validation. GM is the guarantor of this study.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Ethics Committee of Fondazione IRCCS Policlinico di Milano, Italy (protocol number 505_2019bis). Participants gave informed consent to participate in the study before taking part.
References
- 1.Fanouriakis A, Kostopoulou M, Cheema K, et al. 2019 update of the joint European League against rheumatism and European renal Association–European dialysis and transplant association (EULAR/ERA–EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis 2020;79:713–23. 10.1136/annrheumdis-2020-216924 [DOI] [PubMed] [Google Scholar]
- 2.Carette S, et al. Controlled studies of oral immunosuppressive drugs in lupus nephritis. Ann Intern Med 1983;99:1–8. 10.7326/0003-4819-99-1-1 [DOI] [PubMed] [Google Scholar]
- 3.Austin HA, Muenz LR, Joyce KM, et al. Prognostic factors in lupus nephritis. contribution of renal histologic data. Am J Med 1983;75:382–91. 10.1016/0002-9343(83)90338-8 [DOI] [PubMed] [Google Scholar]
- 4.Austin HA, Boumpas DT, Vaughan EM, et al. Predicting renal outcomes in severe lupus nephritis: contributions of clinical and histologic data. Kidney Int 1994;45:544–50. 10.1038/ki.1994.70 [DOI] [PubMed] [Google Scholar]
- 5.Kojo S, SADA KEN-EI, Kobayashi M, et al. Clinical usefulness of a prognostic score in histological analysis of renal biopsy in patients with lupus nephritis. J Rheumatol 2009;36:2218–23. 10.3899/jrheum.080793 [DOI] [PubMed] [Google Scholar]
- 6.Hsieh C, Chang A, Brandt D, et al. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res 2011;63:865–74. 10.1002/acr.20441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alsuwaida AO. Interstitial inflammation and long-term renal outcomes in lupus nephritis. Lupus 2013;22:1446–54. 10.1177/0961203313507986 [DOI] [PubMed] [Google Scholar]
- 8.Obrișcă B, Jurubiță R, Andronesi A, et al. Histological predictors of renal outcome in lupus nephritis: the importance of tubulointerstitial lesions and scoring of glomerular lesions. Lupus 2018;27:1455–63. 10.1177/0961203318776109 [DOI] [PubMed] [Google Scholar]
- 9.Moroni G, Vercelloni PG, Quaglini S, et al. Changing patterns in clinical–histological presentation and renal outcome over the last five decades in a cohort of 499 patients with lupus nephritis. Ann Rheum Dis 2018;77:1318–25. 10.1136/annrheumdis-2017-212732 [DOI] [PubMed] [Google Scholar]
- 10.Tao J, Wang H, Yu X-J, et al. A validation of the 2018 revision of international Society of Nephrology/Renal pathology Society classification for lupus nephritis: a cohort study from China. Am J Nephrol 2020;51:483–92. 10.1159/000507213 [DOI] [PubMed] [Google Scholar]
- 11.Moroni G, Porata G, Raffiotta F, et al. Beyond ISN/RPS lupus nephritis classification: adding chronicity index to clinical variables predicts kidney survival. Kidney360 2022;3:122–32. 10.34067/KID.0005512021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill GS, Delahousse M, Nochy D, et al. Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages. Kidney Int 2001;59:304–16. 10.1046/j.1523-1755.2001.00492.x [DOI] [PubMed] [Google Scholar]
- 13.Grootscholten C, Bajema IM, Florquin S, et al. Treatment with cyclophosphamide delays the progression of chronic lesions more effectively than does treatment with azathioprine plus methylprednisolone in patients with proliferative lupus nephritis. Arthritis Rheum 2007;56:924–37. 10.1002/art.22449 [DOI] [PubMed] [Google Scholar]
- 14.Alsuwaida A, Husain S, Alghonaim M, et al. Strategy for second kidney biopsy in patients with lupus nephritis. Nephrology Dialysis Transplantation 2012;27:1472–8. 10.1093/ndt/gfr517 [DOI] [PubMed] [Google Scholar]
- 15.Zickert A, Sundelin B, Svenungsson E, et al. Role of early repeated renal biopsies in lupus nephritis. Lupus Sci Med 2014;1:e000018. 10.1136/lupus-2014-000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malvar A, Pirruccio P, Alberton V, et al. Histologic versus clinical remission in proliferative lupus nephritis. Nephrol Dial Transplant 2017;32:1338–44. 10.1093/ndt/gfv296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esdaile J, Joseph L, MacKenzie T, et al. The pathogenesis and prognosis of lupus nephritis: information from repeat renal biopsy. Semin Arthritis Rheum 1993;23:135–48. 10.1016/S0049-0172(05)80019-8 [DOI] [PubMed] [Google Scholar]
- 18.Moroni G, Pasquali S, Quaglini S, et al. Clinical and prognostic value of serial renal biopsies in lupus nephritis. Am J Kidney Dis 1999;34:530–9. 10.1016/S0272-6386(99)70082-X [DOI] [PubMed] [Google Scholar]
- 19.Greloni G, Scolnik M, Marin J, et al. Value of repeat biopsy in lupus nephritis flares. Lupus Sci Med 2014;1:e000004. 10.1136/lupus-2013-000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moroni G, Depetri F, Ponticelli C. Lupus nephritis: when and how often to biopsy and what does it mean? J Autoimmun 2016;74:27–40. 10.1016/j.jaut.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 21.Moroni G, Quaglini S, Maccario M, et al. “Nephritic flares” are predictors of bad long-term renal outcome in lupus nephritis. Kidney Int 1996;50:2047–53. 10.1038/ki.1996.528 [DOI] [PubMed] [Google Scholar]
- 22.Weening JJ, D'Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 2004;65:521–30. 10.1111/j.1523-1755.2004.00443.x [DOI] [PubMed] [Google Scholar]
- 23.Fogazzi GB, Bajetta M, Banfi G, et al. Comparison of immunofluorescent findings in kidney after snap-freezing and formalin fixation. Pathol Res Pract 1989;185:225–30. 10.1016/S0344-0338(89)80256-0 [DOI] [PubMed] [Google Scholar]
- 24.Austin HA, Muenz LR, Joyce KM, et al. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int 1984;25:689–95. 10.1038/ki.1984.75 [DOI] [PubMed] [Google Scholar]
- 25.Bajema IM, Wilhelmus S, Alpers CE, et al. Revision of the International Society of Nephrology/Renal pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of health activity and chronicity indices. Kidney Int 2018;93:789–96. 10.1016/j.kint.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis 2014;63:820–34. 10.1053/j.ajkd.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moroni G, Quaglini S, Gallelli B, et al. The long-term outcome of 93 patients with proliferative lupus nephritis. Nephrol Dial Transplant 2007;22:2531–9. 10.1093/ndt/gfm245 [DOI] [PubMed] [Google Scholar]
- 28.Boumpas DT, Austin HA, Vaughn EM, et al. Controlled trial of pulse methylprednisolone versus two regimens of pulse cyclophosphamide in severe lupus nephritis. Lancet 1992;340:741–5. 10.1016/0140-6736(92)92292-N [DOI] [PubMed] [Google Scholar]
- 29.. Available: https://www.ibm.com/analytics/spss-statistics-software
- 30.Moroni G, Doria A, Mosca M, et al. A randomized pilot trial comparing cyclosporine and azathioprine for maintenance therapy in diffuse lupus nephritis over four years. CJASN 2006;1:925–32. 10.2215/CJN.02271205 [DOI] [PubMed] [Google Scholar]
- 31.Contreras G, Pardo V, Cely C, et al. Factors associated with poor outcomes in patients with lupus nephritis. Lupus 2005;14:890–5. 10.1191/0961203305lu2238oa [DOI] [PubMed] [Google Scholar]
- 32.Chan TM, Tse KC, Tang CSO, et al. Long-term outcome of patients with diffuse proliferative lupus nephritis treated with prednisolone and oral cyclophosphamide followed by azathioprine. Lupus 2005;14:265–72. 10.1191/0961203305lu2081oa [DOI] [PubMed] [Google Scholar]
- 33.Houssiau FA, Vasconcelos C, D'Cruz D, et al. The 10-year follow-up data of the Euro-Lupus nephritis trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis 2010;69:61–4. 10.1136/ard.2008.102533 [DOI] [PubMed] [Google Scholar]
- 34.Ponticelli C, Escoli R, Moroni G. Does cyclophosphamide still play a role in glomerular diseases? Autoimmun Rev 2018;17:1022–7. 10.1016/j.autrev.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 35.Malvar A, Alberton V, Lococo B, et al. Kidney biopsy–based management of maintenance immunosuppression is safe and may ameliorate flare rate in lupus nephritis. Kidney Int 2020;97:156–62. 10.1016/j.kint.2019.07.018 [DOI] [PubMed] [Google Scholar]
- 36.Parodis I, Tamirou F, Houssiau FA. Prediction of prognosis and renal outcome in lupus nephritis. Lupus Sci Med 2020;7:e000389. 10.1136/lupus-2020-000389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nachman PH. Repeat kidney biopsy for lupus nephritis: an important step forward. Kidney Int 2018;94:659–61. 10.1016/j.kint.2018.06.026 [DOI] [PubMed] [Google Scholar]
- 38.Parikh SV, Nagaraja HN, Hebert L, et al. Renal flare as a predictor of incident and progressive CKD in patients with lupus nephritis. Clin J Am Soc Nephrol 2014;9:279–84. 10.2215/CJN.05040513 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lupus-2022-000721supp001.pdf (71KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.


