Abstract
Objective
Sickle cell disease (SCD) is a highly morbid condition notable for recurrent hospitalisations due to vaso-occlusive crises and complications of end organ damage. Little is known about the use of inpatient palliative care services in adult patients with SCD. This study aims to evaluate inpatient palliative care use during SCD-related hospitalisations overall and during terminal hospitalisations. We hypothesise that use of palliative care is low in SCD hospitalisations.
Design
A retrospective cross-sectional study using data from the National Inpatient Sample from 2008 to 2017 was conducted.
Setting
US hospitals from 47 states and the District of Columbia.
Participants
Patients >18 years old hospitalised with a primary or secondary International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) or ICD-10-CM diagnosis of SCD were included.
Primary and secondary outcome measures
Palliative care service use (documented by ICD-9-CM and ICD-10-CM diagnosis codes V66.7 and Z51.5).
Results
987 555 SCD-related hospitalisations were identified, of which 4442 (0.45%) received palliative care service. Palliative care service use increased at a rate of 9.2% per year (95% CI 5.6 to 12.9). NH-black and Hispanic patients were 33% and 53% less likely to have palliative care services compared with NH-white patients (OR 0.67; 95% CI 0.45 to 0.99 and OR 0.47; 95% CI 0.26 to 0.84). Female patients (OR 0.40; 95% CI 0.21 to 0.76), Medicaid use (OR 0.40; 95% CI 0.21 to 0.78), rural (OR 0.47; 95% CI 0.28 to 0.79) and urban non-teaching hospitals (OR 0.61; 95% CI 0.47 to 0.80) each had a lower likelihood of palliative care services use.
Conclusion
Use of palliative care during SCD-related hospitalisations is increasing but remains low. Disparities associated with race and gender exist for use of palliative care services during SCD-related hospitalisation. Further studies are needed to guide evidence-based palliative care interventions for more comprehensive and equitable care of adult patients with SCD.
Keywords: adult palliative care, haematology, anaemia
Strengths and limitations of this study.
Our study is the first of its kind to investigate palliative care use among patients with sickle cell disease (SCD) using a national database.
International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and ICD-10-CM codes for palliative care are not specific to palliative care provided by a palliative care specialist as these codes also encompass patients who are in comfort care status.
Our study investigated inpatient palliative care use and, hence our findings cannot be extrapolated to outpatient setting where patients with SCD receive most of their care.
Introduction
Sickle cell disease (SCD) is a group of inherited red blood cell disorders in which abnormality in haemoglobin beta gene results in abnormal shape and sickling of red blood cells under stress. Physiological manifestations of the illness arise from end organ ischaemia, including acute pain from vaso-occlusive crises, cerebrovascular accident, acute chest syndrome, pulmonary hypertension, heart failure, renal failure, severe anaemia, and recurrent infections.1 Approximately 100 000 Americans predominantly of African (90%), Middle Eastern and Mediterranean descent are affected by SCD.2 Historically, SCD was a childhood disease—life expectancy for those with the disease was 20 years in 1970. Currently, life expectancy with SCD extends into adulthood (42–48 years) but remains significantly lower than the general population, especially for sickle cell anaemia—the most severe form of SCD.3 As an increasing number of people with SCD survive into adulthood, there is a rising need for effective transitions to adult medicine involving multidisciplinary care teams.4
SCD is a highly morbid condition. Recurrent hospitalisation is common in SCD due to vaso-occlusive crises and complications of end organ damage.5 6 During their last year of life, people with SCD were hospitalised for an average of 42 days over five admissions.7 Hospital admissions and ED visits increase sharply a month before death and most patients with SCD die in the hospital (63%) or emergency department (15%).7 Patients with SCD experience increased rates of depression and anxiety,8–10 chronic pain11 and decreased health-related quality of life.12 Increased healthcare use13 and symptom burden are salient opportunities for palliative care intervention in this patient population. Palliative care provides holistic care for patients with serious chronic illnesses using a multidisciplinary team approach.14 Palliative care has been shown to increase patient’s quality of life and decrease healthcare costs15 16 but the impact of palliative care on SCD is not well characterised.17
Furthermore, little is known about the use of inpatient palliative care services in adult patients with SCD. To address this gap, we used national data to evaluate inpatient palliative care use during SCD-related hospitalisations overall and during terminal hospitalisations (hospitalisations that resulted in patients’ death in-hospital regardless of cause of death). We identified patient and hospital characteristics associated with use of hospital-based palliative care services in SCD. We hypothesise that use of palliative care is low in SCD hospitalisations.
Methods
Data source
Data from 2008 to 2017 National Inpatient Sample (NIS), the largest all-payer, nationally representative inpatient database in the USA was used. The NIS is part of a group of databases developed by the Healthcare Utilisation Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality.18 This public database contains longitudinal data from a stratified sample of all discharges from US hospitals from 47 states and the District of Columbia, excluding rehabilitation and long-term acute care hospitals, thereby estimating about a 20% sample of all hospital discharges. When weighted, each year, NIS contains data from about 35 million hospitalisations.
Study population and variables
The analysis was restricted to hospitalisations in adults aged 18 years and above. Diagnoses and procedures are coded using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes till the third-quarter of 2015, after which HCUP transitioned to ICD-10-CM format. To identify the hospitalisations of interest, we first scanned the diagnosis codes (the principal diagnosis and up to 29 secondary diagnoses) in each patient’s discharge record for an indication of SCD (ICD-9-CM—282.41, 282.42, 282.61, 282.62, 282.63, 282.64, 282.60, 282.68, 282.69; ICD-10-CM - D57.4x, D57.0x, D57.2x, D57.1x, D57.8x). The study’s primary outcome was palliative care use which was identified using ICD-9-CM diagnosis code V66.7 and ICD-10-CM diagnosis code Z51.5. The ICD-9-CM and ICD-10-CM palliative care codes capture hospital encounters by a palliative care specialist or service, patients admitted for palliative care, and patients on comfort measures only status. There were no changes in the criteria for palliative care code with the transition from ICD-9-CM to ICD-10-CM. Covariates included in the study were sociodemographics for the patient which consisted of patients’ age, race/ethnicity—categorised as non-Hispanic (NH)-white, NH-black, Hispanic and NH-others, sex, insurance type—Medicare, Medicaid, private insurance, self-pay and others, median household income based on zipcodes; and hospital characteristics such as hospital location, hospital type (urban vs rural and teaching vs non-teaching) and hospital bed size.
Statistical analysis
All statistical tests were two tailed with the type-I error of 5% and were conducted by using R V.3.5.1 (University of Auckland, Auckland, New Zealand) and R Studio V.1.1.423 (Boston, Massachusetts, USA) and Joinpoint Regression Programme, V.4.7.0.0 (National Cancer Institute).
Temporal trends analyses were performed using Joinpoint regression technique, which enables examination of the trend of an outcome (in this case, palliative care use) over the study period.19 The results are represented in the form of average annual percentage change and its 95% CI. We scrutinised the temporal trends in the rates of palliative care use among patients with SCD overall, and among those who died in hospital. Next, we examined the sociodemographic and hospitalisation characteristics of patients with SCD and those with SCD who received palliative care during their hospitalisation. Prevalence rates of SCD and palliative care use among SCD hospitalisations were calculated, stratified by patients’ race/ethnicity. Furthermore, after excluding records with missing values in model variables, we conducted adjusted survey logistic regression models to examine the patient sociodemographic and hospitalisation characteristics (exposure variables) associated with palliative care use (outcome variable) among all SCD hospitalisations and among those which resulted in patients’ death in hospital. All the patient demographic and hospital characteristics were loaded into the association models to obtain adjusted OR and 95% CIs.
Patient and public involvement
Patients were not involved in this retrospective analysis of an anonymised national inpatient database.
Results
Trends in the rate of palliative care use in SCD-related hospitalisations
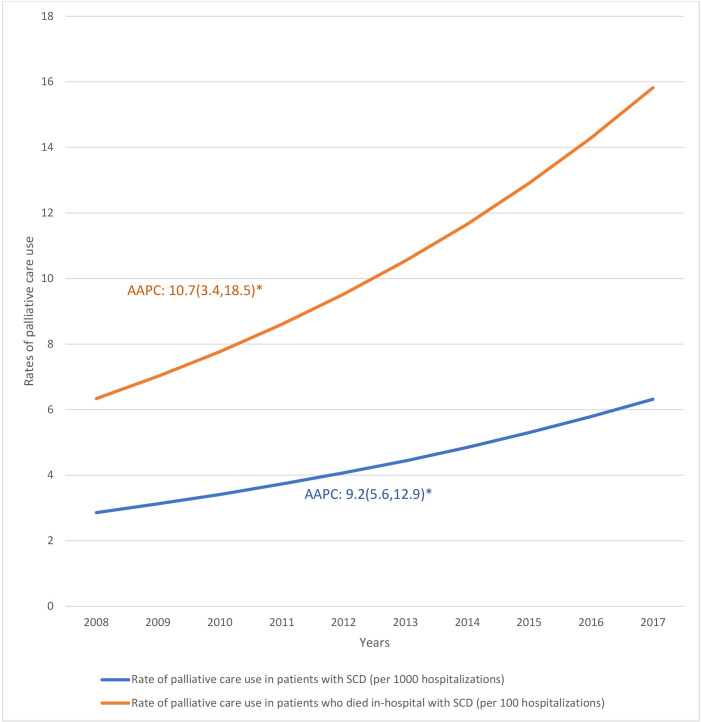
During the study period of 1 January 2008 to 31 December 2017, the NIS contained 987 555 SCD-related hospitalisations of which 6327 (0.64%) were terminal admissions. There were 4442 (0.45%) SCD-related hospitalisations that received palliative care service. Figure 1 shows temporal trends in palliative care use. The rate of inpatient palliative care service use increased at an average of 9.2% per year (95% CI 5.6% to 12.9%) for all SCD-related hospitalisations—from 2.86 per 1000 hospitalisations in 2008 to 6.32 per 1000 hospitalisations in 2017; and an average of 10.7% (95% CI 3.4% to 18.5%) for terminal hospitalisations—6.34 per 1000 hospitalisations in 2008 to 15.82 per 1000 hospitalisations in 2017.
Figure 1.
Temporal trends in the rates of palliative care use 2008–2017. AAPC, average annual percentage change; SCD, sickle cell disease. ‘*’ indicates statistically significant findings.
Patient characteristics: SCD hospitalisations with palliative care use
Table 1 shows patient characteristics for SCD-related hospitalisations and patients who received palliative care services. The prevalence of SCD-related hospitalisation was highest among the 18–39 years age group (9.7 per 1000 SCD hospitalisations) and decreased with increasing age. Conversely, the prevalence of inpatient palliative care use was highest in the oldest age group (34.8 per 1000 SCD hospitalisation) and lowest in the 18–39 years age group (3.8 per 1000 SCD hospitalisations). The prevalence of SCD-related hospitalisation was highest among NH-blacks (20.7 per 1000 SCD hospitalisations) and lowest in NH-whites (0.1 per 1000 SCD hospitalisations) while the prevalence of inpatient palliative care services was highest in NH-whites (12.6 per 1000 SCD hospitalisations) and lowest in Hispanics (3.6 per 1000 SCD hospitalisations).
Table 1.
Patient characteristics among SCD-related hospitalisations and among those with SCD who received palliative care during their hospitalisation
| Total N=307 709 982 |
SCD-related hospitalisations N=987 555 |
Prevalence of SCD-related hospitalisations (per 1000 hospitalisations) | SCD-related hospitalisations receiving palliative care N=4442 |
Prevalence of palliative care use (per 1000 SCD hospitalisations) | |
| Age group | |||||
| 18–39 years | 75 567 499 | 733 837 | 9.7 | 2824 | 3.8 |
| 40–59 years | 78 526 326 | 214 939 | 2.7 | 1160 | 5.4 |
| 60–79 years | 101 611 492 | 35 362 | 0.3 | 339 | 9.6 |
| 80+ years | 52 004 665 | 3417 | 0.1 | 119 | 34.8 |
| Race/ethnicity | |||||
| NH-white | 192 567 014 | 16 962 | 0.1 | 214 | 12.6 |
| NH-black | 41 490 748 | 857 006 | 20.7 | 3773 | 4.4 |
| Hispanic | 29 897 432 | 32 676 | 1.1 | 116 | 3.6 |
| Others | 17 422 072 | 23 026 | 1.3 | 157 | 6.8 |
| Missing | 26 332 714 | 57 883 | 2.2 | 181 | 3.1 |
| Sex | |||||
| Male | 125 745 784 | 427 285 | 3.4 | 1921 | 4.5 |
| Female | 181 778 482 | 559 845 | 3.1 | 2521 | 4.5 |
| Missing | 185 716 | 424 | 2.3 | – | – |
| Discharge status | |||||
| Routine | 202 797 074 | 853 218 | 4.2 | 2619 | 3.1 |
| Transfer | 55 186 460 | 37 409 | 0.7 | 447 | 11.9 |
| Died | 6 740 712 | 6327 | 0.9 | 685 | 108.3 |
| DAMA | 3 847 845 | 44 520 | 11.6 | 69 | 1.5 |
| Other | 38 933 666 | 45 507 | 1.2 | 622 | 13.7 |
| Missing | 204 225 | 573 | 2.8 | – | – |
| Zip code income quartile | |||||
| Lowest quartile | 89 460 128 | 478 674 | 5.4 | 2053 | 4.3 |
| Second quartile | 78 497 949 | 219 619 | 2.8 | 801 | 3.6 |
| Third quartile | 71 474 969 | 163 233 | 2.3 | 836 | 5.1 |
| Highest quartile | 61 340 502 | 100 769 | 1.6 | 703 | 7.0 |
| Missing | 6 936 433 | 25 259 | 3.6 | 48 | 1.9 |
| Primary payer | |||||
| Medicare | 141 357 438 | 315 777 | 2.2 | 1736 | 5.5 |
| Medicaid | 50 563 783 | 428 967 | 8.5 | 1725 | 4.0 |
| Private insurance | 88 933 878 | 170 459 | 1.9 | 671 | 3.9 |
| Self-pay | 26 247 689 | 70 526 | 2.7 | 304 | 4.3 |
| Missing | 607 194 | 1825 | 3.0 | ─ | ─ |
| Hospital characteristics | |||||
| Hospital region | |||||
| Northeast | 33 888 883 | 46 282 | 1.4 | 1226 | 6.1 |
| Midwest | 107 572 349 | 234 180 | 2.2 | 1014 | 5.4 |
| South | 164 884 575 | 701 680 | 4.3 | 1820 | 3.6 |
| West | 1 364 173 | 5411 | 4.0 | 382 | 4.4 |
| Hospital bed size | |||||
| Small | 46 455 596 | 114 366 | 2.5 | 331 | 2.9 |
| Medium | 81 563 430 | 258 481 | 3.2 | 998 | 3.9 |
| Large | 178 326 782 | 609 296 | 3.4 | 3108 | 5.1 |
| Missing | 1 364 173 | 5411 | 4.0 | ─ | ─ |
| Hospital location and teaching status | |||||
| Rural | 33 888 883 | 46 282 | 1.4 | 96 | 2.1 |
| Urban non-teaching | 107 572 349 | 234 180 | 2.2 | 749 | 3.2 |
| Urban teaching | 164 884 575 | 701 680 | 4.3 | 3591 | 5.1 |
| Missing | 1 364 173 | 5411 | 4.0 | ─ | ─ |
Prevalence is the rate of the outcome (SCD or palliative care use) among each patient characteristics.
‘–’ represents cell values <10, which have been suppressed to preserve patient confidentiality, as per HCUP guidelines.
DAMA, Discharged Against Medical Advice; HUCP, Healthcare Utilisation Project; NH, non-Hispanic; SCD, sickle cell disease.
Regional and hospital characteristics: SCD hospitalisations with palliative care use
SCD hospitalisation was lowest in the Northeast (1.4 per 1000 SCD hospitalisations) but the prevalence of inpatient palliative care service was highest in this region (6.1 per 1000 SCD hospitalisations). Conversely, the South region had the highest prevalence of SCD hospitalisation (4.3 per 1000 SCD hospitalisations) but the lowest use of inpatient palliative care services (3.6 per 1000 SCD hospitalisations). Large and urban teaching hospitals had the highest prevalence of SCD hospitalisation (3.4 and 4.3 per 1000 hospitalisations, respectively) and highest level of inpatient palliative care service use (5.1 and 5.1 per 1000 SCD hospitalisations, respectively).
Factors associated with inpatient palliative care use in SCD
Table 2 shows factors associated with inpatient palliative care use in SCD hospitalisations. During terminal hospitalisations patients between 40 and 59 years were more likely to receive palliative care services than patients 18–39 years old (OR 1.81, 95% CI 1.19 to 2.75). NH-black and Hispanic patients were less likely to receive inpatient palliative care services compared with NH-white (OR 0.67; 95% CI 0.45 to 0.99 and OR 0.47; 95% CI 0.26 to 0.84, respectively). Likewise, during terminal admissions use of palliative care services was less likely among NH-black and Hispanic patients, respectively, when compared with NH-white (OR 0.33; 95% CI 0.16 to 0.68 and OR 0.28; 95% CI 0.09 to 0.91, respectively). Female patients were less likely to receive palliative care services than male patients (OR 0.40; 95% CI 0.21 to 0.76). Compared with routine discharges, hospitalisations resulting in death and transfer to another healthcare facility were associated with increased odds of use of inpatient palliative care services (OR 39.36; 95% CI 30.83 to 49.98, and OR 3.71; 95% CI, 2.73 to 5.03, respectively) while discharge against medical advice was associated with decreased odds of use of inpatient palliative care services (OR 0.48; 95% CI 0.28 to 0.84).
Table 2.
Factors associated with palliative care use in patients with SCD—overall and among those who died in hospital
| Palliative care use in SCD hospitalisations | Palliative care use among patients with SCD who died in hospital | |
| OR (95% CI) | OR (95% CI) | |
| Age group | ||
| 18–39 years | Reference | Reference |
| 40–59 years | 0.98 (0.81 to 1.18) | 1.81 (1.19 to 2.75)* |
| 60–79 years | 0.95 (0.70 to 1.30) | 1.13 (0.60 to 2.12) |
| 80+ years | 2.34 (1.41 to 3.88)* | 2.29 (0.79 to 6.64) |
| Race/ethnicity | ||
| NH-white | Reference | Reference |
| NH-black | 0.67 (0.45 to 0.99)* | 0.33 (0.16 to 0.68)* |
| Hispanic | 0.47 (0.26 to 0.84)* | 0.28 (0.09 to 0.91)* |
| Others | 0.83 (0.49 to 1.39) | 0.41 (0.12 to 1.42) |
| Sex | ||
| Male | Reference | Reference |
| Female | 0.40 (0.21 to 0.76)* | 1.45 (1.00 to 2.11) |
| Discharge status | ||
| Routine | Reference | Reference |
| Transfer | 3.71 (2.73 to 5.03)* | |
| Died | 39.26 (30.83 to 49.98)* | – |
| DAMA | 0.48 (0.28 to 0.84)* | – |
| Other | 4.14 (3.20 to 5.35)* | – |
| Zip code income quartile | ||
| Lowest quartile | Reference | Reference |
| Second quartile | 0.89 (0.71 to 1.11) | 0.69 (0.42 to 1.14) |
| Third quartile | 1.24 (0.85 to 1.80) | 0.48 (0.25 to 0.91)* |
| Highest quartile | 1.58 (1.02 to 2.45)* | 1.46 (0.79 to 2.71) |
| Primary payer | ||
| Medicare | Reference | Reference |
| Medicaid | 0.40 (0.21 to 0.78)* | 0.72 (0.21 to 2.48) |
| Private insurance | 1.30 (1.02 to 1.65)* | 1.03 (0.59 to 1.79) |
| Self-pay | 1.22 (0.93 to 1.59) | 1.12 (0.64 to 1.99) |
| Hospital characteristics | ||
| Hospital region | ||
| Northeast | Reference | Reference |
| Midwest | 1.00 (0.57 to 1.75) | 1.18 (0.57 to 2.46) |
| South | 0.69 (0.42 to 1.11) | 1.47 (0.78 to 2.78) |
| West | 0.72 (0.40 to 1.31) | 1.63 (0.77 to 3.41) |
| Hospital bed size | ||
| Small | Reference | Reference |
| Medium | 1.47 (1.02 to 2.12)* | 1.18 (0.57 to 2.44) |
| Large | 1.91 (1.30 to 2.82)* | 1.52 (0.78 to 2.96) |
| Hospital location and teaching status | ||
| Urban teaching | Reference | Reference |
| Rural | 0.47 (0.28 to 0.79)* | 0.75 (0.33 to 1.73) |
| Urban non-teaching | 0.61 (0.47 to 0.80)* | 0.63 (0.40 to 0.99) |
’–’ represents the absence of variable in the model.
‘*’ indicates statistically significant findings.
DAMA, Discharged Against Medical Advice; NH, non-Hispanic; SCD, sickle cell disease.
Patients living in zip codes in the highest income quartile were more likely to receive inpatient palliative care services (OR 1.58; 95% CI 1.02 to 2.45) than those in the lowest quartile. Compared with Medicare patients, those with Medicaid had lesser odds (OR 0.40; 95% CI 0.21 to 0.78), while patients with private insurance had higher odds (OR 1.30; 95% CI 0.93 to 1.59) of receiving palliative care services. During terminal admissions, there were no significant differences in palliative care use across different payors.
Patients admitted to medium or large hospitals were more likely (OR 1.47; 95% CI 1.02 to 2.12 and OR 1.91; 95% CI 1.30 to 2.82, respectively) to receive palliative care services than patients in small hospitals while patients admitted to rural or urban non-teaching hospitals were less likely to receive palliative care services than those in urban teaching hospitals (OR 0.47; 95% CI 0.28 to 0.79 and OR 0.61; 95% CI 0.47 to 0.80, respectively).
Discussion
In this study of national trends, we found overall low use of palliative care services during SCD-related hospitalisations. Palliative care use was more likely among patients who were NH-white male, in the highest income quartile, with private insurance, and who received care in large or urban academic hospitals. NH-black and Hispanic patients were less likely to receive palliative care services especially during terminal hospitalisations.
While early palliative care has gained acceptance in cancer care,20 its adoption in other serious life-limiting conditions such as congestive heart failure, chronic kidney disease and SCD lags. SCD has traditionally been viewed as a childhood disease as most patients did not survive to adulthood in the past. Currently, most patients with SCD survive to adulthood.3 Furthermore, some patients with SCD experience complications and end-organ damage from their disease resulting in a shortened life expectancy than the general population. Our study found a higher prevalence of palliative care service use in terminal admissions when compared with admissions with routine discharge or transfer to another level of care. This highlights an opportunity for palliative care involvement earlier in the disease course in SCD, facilitating more holistic care of patients. Earlier initiation of palliative care grants patients psychosocial support and symptom management from an interdisciplinary team,21 as well as honest discussions regarding goals of care and patient’s preference for end of life.
Our study showed most SCD-related hospitalisations and palliative care service use were in the NH-black group. However, further analysis accounting for covariates revealed that NH-black patients were significantly less likely to receive palliative care during hospitalisation compared with NH-white patients. Notably, race/ethnicity was the only factor associated with palliative care use in both SCD and terminal hospitalisations. Other studies have shown racial disparities in the use of palliative care services in other chronic diseases.22 23 Furthermore, studies have shown that black patients are less likely to use hospice services and more likely to receive intensive life-sustaining procedures at end of life compared with white patients.24 Factors identified to drive this racial disparity in hospice use include knowledge deficits,25 mistrust of the healthcare system,26 miscommunication and misunderstanding of treatment options,27 and preference for more aggressive care.28 Similar studies are lacking to elucidate factors implicated in the racial difference in non-hospice palliative care use across all life-limiting diseases.29 Palliative care has a strong perceived association with hospice with many patients and families unable to differentiate between the two. Therefore, the factors identified above relating to hospice use are likely to also play a role in racial differences observed in palliative care use in patients with chronic conditions such as SCD. Further studies are needed to investigate patient and system factors associated with racial differences in palliative care use in SCD.
Gender differences in the use of inpatient palliative care services were found. Although male and female patients had a similar prevalence of SCD-related hospitalisation, female patients were 60% less likely to receive palliative care services. This finding is contrary to that of other studies which show that women are more receptive to palliative care than men.30 Pain is the most prominent feature of SCD and hence it is plausible that inpatient palliative care services in SCD hospitalisations primarily involve pain management. Studies have shown that although most chronic pain conditions are more prevalent in women than men, and women report greater pain than men after invasive procedures, women receive less analgesia and are more likely to have their pain attributed to psychological causes.31 It is possible that palliative care consultants are more frequently involved for specialist-level pain management for male than female patients accounting for the gender difference observed in our study. This finding requires further investigation as the study by McClish et al demonstrates that men and women with SCD report similar pain experiences.32
We also found socioeconomic differences in the use of palliative care in SCD similar to other diseases.23 33 34 Patients with zip codes in the highest income quartile and those with private insurance were more likely to receive palliative care, while patients with Medicaid were less likely to receive palliative care services. Insurance dictates patient’s access to specialty care with patients on Medicaid reporting more challenge than those with private insurance accessing certain specialty care particularly in the outpatient setting.35 36
Regional differences were detected in our study. The South region had the highest number of SCD hospitalisations but the lowest prevalence of palliative care use. This finding is notable as two of the three states (Florida, Texas and New York, USA) with the highest population of patients with SCD are in the South. Hospital characteristics were also predictive of palliative care use with medium, large and urban teaching hospitals associated with greater use of palliative care in SCD similar to findings from other studies.22 33 34 While many patients with SCD use larger, urban hospitals for their care,37 this study highlights the need for improvement in access to palliative care services across all settings in the USA, particularly in small and rural hospitals.
To our knowledge, our study is the first of its kind to investigate palliative care use among patients with SCD using a national data base. Limitations exist in our study one of which is the validity of the ICD-9 and ICD-10 codes for identification of palliative care consultations. Investigators have sought to validate these codes, however, these studies use data from a single institution38 or from the VA system.39 Furthermore, the ICD-9 and ICD-10 codes for palliative care are not specific to palliative care provided by a palliative care specialist as these codes also encompass patients who are in comfort care status.40 Not all patients in comfort care are managed by a palliative care specialist and as such it is difficult to delineate which hospitalisations were indeed associated with the involvement of a palliative care specialist. In addition, our dataset does not allow us to elucidate the nature of palliative care interventions provided during admissions hence it is unclear if these interventions were for symptom management, goals of care discussion, counselling and support, or transition to comfort care. Given utilisation of aggregate data in which a hospitalisation is considered a single event, we are unable to identify the number of patients who benefited from palliative care services as multiple admissions could be associated with one patient. As such, we suspect that our study overestimates the prevalence of palliative care use in SCD. Lastly, our study investigated inpatient palliative care use and hence, our findings cannot be extrapolated to outpatient settings where patients with SCD receive most of their care.
SCD-related morbidity and mortality have a significant impact on patient self-reported quality-of-life outcomes and healthcare utilisation and cost. Early palliative care might guide patients’ treatment decisions and mitigate initiation of interventions that are not in line with a patients’ wishes at the end of life. Early palliative care also has the potential to improve symptom management and consequently improve quality of life for individuals with SCD. Studies exploring barriers and facilitators to early palliative care at the provider (SCD providers, palliative care specialists), patient and system levels do not exist and are needed for future recommendation for PC interventions in this population. Racial disparity in the use of palliative care by patients with SCD should be investigated with studies assessing knowledge of palliative care and barriers to palliative care use by patients with SCD. Furthermore, studies addressing system barriers to palliative care use among minority patients are needed as the implication spans across other life-limiting illnesses.
Supplementary Material
Footnotes
Contributors: EN-O is the guarantor and was involved in the conception of the study, interpretation of data, drafting and revision of the manuscript. DD was involved in the conception of the study, analysis of the data, drafting, and revision of the manuscript. HMS was involved in the conception of the study and revision of the manuscript. LA was involved in the interpretation of data and drafting of the manuscript. MM was involved in the interpretation of data, drafting and revision of the manuscript. MA was involved in the interpretation of data and drafting of the manuscript. ADN was involved in the conception of the study and revision of the manuscript.
Funding: Research funding support was provided by the US Department of Health and Human Services and Health Resources and Services Administration for Baylor College of Medicine Center of Excellence in Health Equity, Training, and Research (Grant No: D34HP31024). Additional support is provided to Dr. Naik from the Houston VA HSR&D Center for Innovations in Quality, Effectiveness, and Safety (CIN 13-413).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. We used data from the 2008 to 2017 National Inpatient Sample (NIS) which is provided by the Healthcare Utilisation Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ). Data can be accessed through the HCUP website.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved as exempt by the Institutional Review Board (IRB) of Baylor College of Medicine.
References
- 1.Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med 2017;377:1561–73. 10.1056/NEJMra1510865 [DOI] [PubMed] [Google Scholar]
- 2.Brousseau DC, Panepinto JA, Nimmer M, et al. The number of people with sickle-cell disease in the United States: national and state estimates. Am J Hematol 2010;85:77–8. 10.1002/ajh.21570 [DOI] [PubMed] [Google Scholar]
- 3.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. life expectancy and risk factors for early death. N Engl J Med 1994;330:1639–44. 10.1056/NEJM199406093302303 [DOI] [PubMed] [Google Scholar]
- 4.Saulsberry AC, Porter JS, Hankins JS. A program of transition to adult care for sickle cell disease. Hematology Am Soc Hematol Educ Program 2019;2019:496–504. 10.1182/hematology.2019000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the multicenter study of hydroxyurea in sickle cell anemia. N Engl J Med 1995;332:1317–22. 10.1056/NEJM199505183322001 [DOI] [PubMed] [Google Scholar]
- 6.Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA 2003;289:1645–51. 10.1001/jama.289.13.1645 [DOI] [PubMed] [Google Scholar]
- 7.Johnston EE, Adesina OO, Alvarez E, et al. Acute care utilization at end of life in sickle cell disease: highlighting the need for a palliative approach. J Palliat Med 2020;23:24–32. 10.1089/jpm.2018.0649 [DOI] [PubMed] [Google Scholar]
- 8.Levenson JL, McClish DK, Dahman BA, et al. Depression and anxiety in adults with sickle cell disease: the PiSCES project. Psychosom Med 2008;70:192–6. 10.1097/PSY.0b013e31815ff5c5 [DOI] [PubMed] [Google Scholar]
- 9.Adam SS, Flahiff CM, Kamble S, et al. Depression, quality of life, and medical resource utilization in sickle cell disease. Blood Adv 2017;1:1983–92. 10.1182/bloodadvances.2017006940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurence B, George D, Woods D. Association between elevated depressive symptoms and clinical disease severity in African-American adults with sickle cell disease. J Natl Med Assoc 2006;98:365–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Brandow AM, Carroll CP, Creary S, et al. American Society of hematology 2020 guidelines for sickle cell disease: management of acute and chronic pain. Blood Adv 2020;4:2656–701. 10.1182/bloodadvances.2020001851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S, Vania DK, Bhor M, et al. Patient-Reported outcomes and economic burden of adults with sickle cell disease in the United States: a systematic review. Int J Gen Med 2020;13:361–77. 10.2147/IJGM.S257340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kauf TL, Coates TD, Huazhi L, et al. The cost of health care for children and adults with sickle cell disease. Am J Hematol 2009;84:323–7. 10.1002/ajh.21408 [DOI] [PubMed] [Google Scholar]
- 14.Sepúlveda C, Marlin A, Yoshida T, et al. Palliative care: the world Health organization's global perspective. J Pain Symptom Manage 2002;24:91–6. 10.1016/s0885-3924(02)00440-2 [DOI] [PubMed] [Google Scholar]
- 15.Casarett D, Pickard A, Bailey FA, et al. Do palliative consultations improve patient outcomes? J Am Geriatr Soc 2008;56:593–9. 10.1111/j.1532-5415.2007.01610.x [DOI] [PubMed] [Google Scholar]
- 16.Gade G, Venohr I, Conner D, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med 2008;11:180–90. 10.1089/jpm.2007.0055 [DOI] [PubMed] [Google Scholar]
- 17.Ajayi TA, Edmonds KP, Thornberry K, et al. Palliative care teams as advocates for adults with sickle cell disease. J Palliat Med 2016;19:195–201. 10.1089/jpm.2015.0268 [DOI] [PubMed] [Google Scholar]
- 18.NIS database documentation. Available: https://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp [Accessed 1 Jun 2021].
- 19.National Cancer Institute . Joinpoint trend analysis software. Available: https://surveillance.cancer.gov/joinpoint/ [Accessed 1 Jun 2021].
- 20.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733–42. 10.1056/NEJMoa1000678 [DOI] [PubMed] [Google Scholar]
- 21.Osunkwo I, Andemariam B, Minniti CP, et al. Impact of sickle cell disease on patients' daily lives, symptoms reported, and disease management strategies: results from the International sickle cell world assessment survey (sway). Am J Hematol 2021;96:404–17. 10.1002/ajh.26063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen Y, Jiang C, Koncicki HM, et al. Trends and racial disparities of palliative care use among hospitalized patients with ESKD on dialysis. J Am Soc Nephrol 2019;30:1687–96. 10.1681/ASN.2018121256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K, Gani F, Canner JK, et al. Racial disparities in utilization of palliative care among patients admitted with advanced solid organ malignancies. Am J Hosp Palliat Care 2021;38:539–46. 10.1177/1049909120922779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ornstein KA, Roth DL, Huang J, et al. Evaluation of racial disparities in hospice use and end-of-life treatment intensity in the REGARDS cohort. JAMA Netw Open 2020;3:e2014639. 10.1001/jamanetworkopen.2020.14639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson KS, Kuchibhatla M, Tulsky JA. Racial differences in self-reported exposure to information about hospice care. J Palliat Med 2009;12:921–7. 10.1089/jpm.2009.0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cort MA. Cultural mistrust and use of hospice care: challenges and remedies. J Palliat Med 2004;7:63–71. 10.1089/109662104322737269 [DOI] [PubMed] [Google Scholar]
- 27.Mack JW, Paulk ME, Viswanath K, et al. Racial disparities in the outcomes of communication on medical care received near death. Arch Intern Med 2010;170:1533–40. 10.1001/archinternmed.2010.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnato AE, Anthony DL, Skinner J, et al. Racial and ethnic differences in preferences for end-of-life treatment. J Gen Intern Med 2009;24:695–701. 10.1007/s11606-009-0952-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson KS. Racial and ethnic disparities in palliative care. J Palliat Med 2013;16:1329–34. 10.1089/jpm.2013.9468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saeed F, Hoerger M, Norton SA, et al. Preference for palliative care in cancer patients: are men and women alike? J Pain Symptom Manage 2018;56:1–6. 10.1016/j.jpainsymman.2018.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gott M, Morgan T, Williams L. Gender and palliative care: a call to arms. Palliat Care Soc Pract 2020;14:263235242095799. 10.1177/2632352420957997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClish DK, Levenson JL, Penberthy LT, et al. Gender differences in pain and healthcare utilization for adult sickle cell patients: the PiSCES project. J Womens Health 2006;15:146–54. 10.1089/jwh.2006.15.146 [DOI] [PubMed] [Google Scholar]
- 33.Patel AA, Walling AM, Ricks-Oddie J, et al. Palliative care and health care utilization for patients with end-stage liver disease at the end of life. Clin Gastroenterol Hepatol 2017;15:1612–9. 10.1016/j.cgh.2017.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu KG, Shen JJ, Kim PC, et al. Trends of hospital palliative care utilization and its associated factors among patients with systemic lupus erythematosus in the United States from 2005 to 2014. Am J Hosp Palliat Care 2020;37:164–71. 10.1177/1049909119891999 [DOI] [PubMed] [Google Scholar]
- 35.Lee L, Smith-Whitley K, Banks S, et al. Reducing health care disparities in sickle cell disease: a review. Public Health Rep 2019;134:599–607. 10.1177/0033354919881438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Timbie JW, Kranz AM, Mahmud A, et al. Specialty care access for Medicaid enrollees in expansion states. Am J Manag Care 2019;25:e83–7. [PMC free article] [PubMed] [Google Scholar]
- 37.Fingar KR, Owens PL, Reid LD. Characteristics of inpatient hospital stays involving sickle cell disease, 2000-2016: statistical brief #251. Healthcare Cost and Utilization Project (HCUP) statistical briefs 2006. [PubMed]
- 38.Hua M, Li G, Clancy C, et al. Validation of the V66.7 code for palliative care consultation in a single academic medical center. J Palliat Med 2017;20:372–7. 10.1089/jpm.2016.0363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feder SL, Redeker NS, Jeon S, et al. Validation of the ICD-9 diagnostic code for palliative care in patients hospitalized with heart failure within the Veterans health administration. Am J Hosp Palliat Care 2018;35:959–65. 10.1177/1049909117747519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stubbs JM, Assareh H, Achat HM, et al. Specialist palliative care activity at an acute care tertiary hospital and its representation in administrative data. Am J Hosp Palliat Care 2021;38:216–22. 10.1177/1049909120939861 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. We used data from the 2008 to 2017 National Inpatient Sample (NIS) which is provided by the Healthcare Utilisation Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ). Data can be accessed through the HCUP website.