Abstract
Objective
In order to identify areas of unmet need in patients with primary biliary cholangitis (PBC), this study sought to use real-world observational healthcare data to characterise the burden in patients with PBC and in PBC patients with a recorded diagnosis of pruritus.
Design
This retrospective, cross-sectional database study compared prevalence of prespecified comorbidities and medications in the PBC population and PBC-pruritus subpopulation with non-cases using an indirect standardisation approach. The PBC population was identified from the US IBM MarketScan Commercial Claims and Medicare Supplemental Database during 2016 using International Classification of Diseases 10th Revision, Clinical Modification codes (≥2 claims for PBC); the PBC-pruritus subpopulation additionally had ≥1 claim for pruritus during this period. Non-cases had no claims for PBC. Indirect age-sex standardised prevalence ratios (iSPR) and 95% confidence intervals (CIs) were calculated for prespecified comorbidities and medications recorded during 2017.
Results
The PBC population (N=1963) and PBC-pruritus subpopulation (N=139) had significantly higher prevalence of fatigue (19.9%, iSPR (95% CI): 1.51 (1.36 to 1.66); 26.6%, 2.10 (1.48 to 2.90)), depression/anxiety (21.3%, 1.09 (0.99 to 1.20); 28.1%, 1.46 (1.04 to 2.00)) and sleep-related issues (6.9%, 1.18 (0.99 to 1.40); 14.4%, 2.58 (1.58 to 3.99)) compared with non-cases. Bile acid sequestrants were prescribed in 5.8% and 18.0% of the PBC and PBC-pruritus populations, respectively. In general, a higher prevalence of comorbidities and medication use was observed in the PBC-pruritus subpopulation compared with the PBC population and non-cases.
Conclusion
Despite availability of treatments for PBC, the PBC population had a higher burden of comorbidities than non-cases. This burden was even greater among the PBC-pruritus subpopulation, with a particularly high prevalence of sleep disorders and depression/anxiety. Despite this, pruritus remains undertreated highlighting a need for treatments specifically indicated for cholestatic pruritus.
Keywords: PRIMARY BILIARY CIRRHOSIS, CHOLESTATIC LIVER DISEASES, EPIDEMIOLOGY
WHAT IS ALREADY KNOWN ON THIS TOPIC
Pruritus is one of the most common conditions associated with primary biliary cholangitis (PBC) affecting up to 75% of individuals at some point during their disease course. It has a negative impact on health-related quality of life with limited treatment options.
WHAT THIS STUDY ADDS
This study provides broad real-world insights relating to the holistic burden among patients with PBC and PBC-associated pruritus in a relatively large sample.
Prevalence of comorbidities, particularly fatigue, depression or anxiety, and sleep-related issues, and medication use were generally higher in the PBC-pruritus subpopulation compared with non-cases, as well as in the PBC population relative to non-cases.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study highlights the impact of pruritus on symptoms and comorbidities related to PBC such as fatigue, depression and anxiety, and sleep disorders, which ultimately have a profoundly negative impact on health-related quality of life.
Existing literature indicates that currently available treatments for pruritus associated with PBC have significant limitations in terms of efficacy and tolerability, which is reinforced by our findings that show a relatively low use of pruritus treatments among the PBC-pruritus population.
This suggests there might be a need for more effective treatments that may effectively reduce the overall disease burden in patients with PBC.
Introduction
Primary biliary cholangitis (PBC) is a rare chronic autoimmune cholestatic liver disease characterised by ductopenia, cholestasis and biliary fibrosis resulting in end-stage liver disease and associated complications.1 The global prevalence of PBC is estimated to range from 1.9 to 40.2 per 100 000 individuals.2 3 Diagnosis is typically made between 55 and 65 years of age, and women are disproportionately affected with a female-to-male ratio of up to 9:1.4 5
Pruritus is one of the most common conditions associated with PBC, affecting approximately 50% of patients at any given point after diagnosis and up to 75% of patients over the course of the disease.6–9 Other conditions associated with PBC include extrahepatic autoimmune diseases, as well as symptoms such as abdominal discomfort, bone and joint pain, restless legs syndrome and cognitive dysfunction.1 The burden associated with PBC may be further compounded by pruritus, which has a profoundly negative impact on the health-related quality of life (HRQoL) of affected patients.1 10–13 Pruritus is also associated with sleep disturbance, fatigue and depression, and suicidal ideation.10 11 13 14
The recommended first-line therapy for patients with PBC, for which treatment is usually life-long, is ursodeoxycholic acid (UDCA).1 15 Treatment can improve prognosis1 16 and reduce mortality,17 18 but does not improve fatigue or alleviate pruritus.9 19 Obeticholic acid (OCA) is approved and recommended as second-line therapy for PBC either alone (for those who are unable to tolerate UDCA) or in combination with UDCA (for those without a sufficient response to UDCA).1 20 However, pruritus is the most commonly reported adverse effect with OCA,21 and has been included as a warning and precaution in the prescribing information by the US Food and Drug Administration.20
The only treatment for cholestatic pruritus with a specific indication is the bile-acid sequestrant cholestyramine.1 22 23 However, cholestyramine interferes with the absorption of other medications, including UDCA or OCA.1 22 It also has several gastrointestinal side effects and tolerability issues.1 22 23 Treatment options for pruritus that are used off-label include rifampicin, fibrates, selective serotonin reuptake inhibitors (eg, sertraline), opiate antagonists (eg, naltrexone) and antihistamines (primarily for their sedative properties).1 22 However, these treatments have limited efficacy.8 22 24
Large insurance claims databases are particularly invaluable for assessing the real-world management and burden in patients with rare diseases, such as PBC. Insights can be gained into comorbidities, medication usage and measures of disease-related burden among patients with PBC, regardless of physician specialty. The aim of this study was to assess the prevalence of prespecified comorbidities and medication use in patients with PBC, particularly in those with pruritus, and to compare with age-matched and sex-matched individuals without PBC, using claims data from a US health insurance database. Thus, the overarching objective of this work is to better understand and describe the overall holistic burden in the PBC population and those with a recorded diagnosis of pruritus using real-world data in order to identify areas of unmet need with the aim of improving patient care in the future. To the best of our knowledge, this is the first such assessment reported in the literature.
Materials and methods
Study design and data source
This retrospective, cross-sectional study used de-identified US administrative data from the IBM MarketScan Commercial Claims and Medicare Supplemental Database. This database provides a nationally representative sample of insured individuals living in the USA and includes information on inpatient and outpatient medical data and outpatient prescription drug data.
bmjgast-2021-000857supp001.pdf (220.8KB, pdf)
Claims and prescriptions were evaluated during a 12-month observation period (1 January to 31 December 2017) to record the occurrence of events of interest. The reference date was 1 January 2017. The 12-month period immediately prior to this was defined as the lead-in period, during which cases and baseline characteristics were identified.
Eligibility criteria
Eligible patients were required to be at least 18 years of age at reference date with uninterrupted enrolment, including pharmacy and medical benefits, during the lead-in period and throughout the entire observation period, and at least one record of any type during the lead-in period, both for cases and non-cases, to ascertain patients were active in the healthcare system. No exclusion criteria were employed.
Analysis populations
Prevalent PBC cases were identified using 2020 International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes for PBC (K74.3). These cases formed the PBC population, defined as individuals with at least two claims for PBC during the full study period, of which at least one had to occur during the lead-in period. This approach was informed by an earlier validation study, which reported that the optimal algorithm to identify PBC cases in administrative data included the use of at least two PBC-related codes, with a sensitivity of 94% and a positive predictive value of 73%.25 A subset of the PBC population, hereafter referred to as the PBC-pruritus subpopulation, was identified using the same criteria for PBC; however, individuals were additionally required to have at least one claim for pruritus during the lead-in period. As no ICD-10-CM code exists for PBC-specific pruritus, these patients were identified using a general pruritus code (ICD-10 L29 (pruritus, unspecified)). The non-case populations (ie, comparator populations) were identified as those not meeting the case definition (ie, with no ICD-10-CM diagnosis code for PBC or no PBC-pruritus at any time prior to 31 December 2016).
Study outcome variables and data analysis
Data on prespecified comorbidities and medications of interest were extracted from the data source based on the presence of at least one claim or prescription fill, respectively, during the observation period. The prevalence of comorbidities and medication use in the PBC population, PBC-pruritus subpopulation and non-case population was assessed.
Prespecified comorbidities of interest were categorised as either autoimmune conditions (known to be more common in patients with PBC), other liver conditions, or potential symptoms and/or comorbidities associated with PBC such as fatigue, depression or anxiety, and sleep-related issues. Prespecified comorbidities were identified using their respective ICD-10-CM diagnosis codes (see online supplemental file 1). Prespecified concomitant medications (including treatments for PBC, pruritus and other comorbidities) were identified using national drug codes and the Healthcare Common Procedure Coding System. The 30 most common comorbidities and medications in the PBC and PBC-pruritus populations identified from the data are presented in online supplemental tables 1 and 2, respectively.
bmjgast-2021-000857supp002.pdf (790.2KB, pdf)
Age, as on reference date, was determined and categorised into broad groups. Indirect age-sex standardised prevalence ratios (iSPRs) – a ratio of the prevalence of a given comorbidity or medication in the PBC population relative to the prevalence in the non-case population – were calculated. iSPR values greater or less than 1 indicate that the event is either more or less common in the PBC population relative to the non-case population, respectively. This approach improves estimate precision by leveraging the size of the non-case population while accounting for differences in age and sex distributions between the case and non-case populations. Further details of the mathematical approaches used to calculate iSPRs are provided in the online supplemental methods.
Statistical analyses
No formal power calculations were performed. Analyses included complete data available from all eligible cases. To determine potential differences between the case and non-case populations, 95% CIs of iSPRs were calculated.26 Analyses were conducted using the Instant Health Data Platform (Panalgo, Boston, MA; R V3.2.1).
Results
Patient characteristics
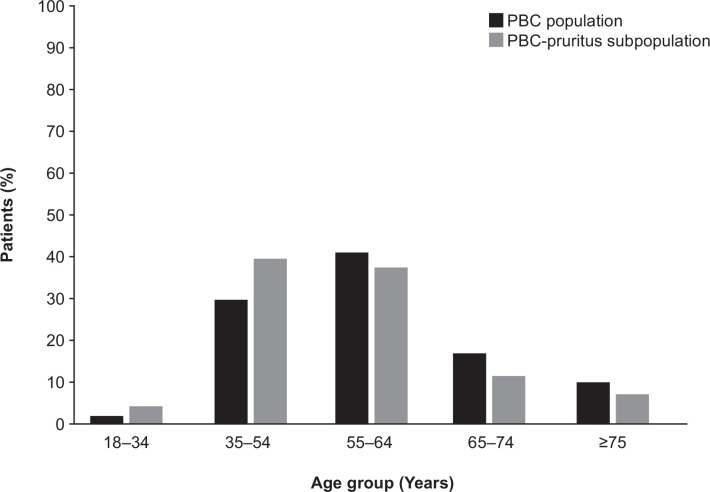
Of 10 247 555 individuals in the data source, 1963 were included in the PBC population, of whom 139 had a pruritus code during the lead-in period and were included in the PBC-pruritus subpopulation. In both populations, women comprised the majority of cases (PBC population: 90%; PBC-pruritus subpopulation: 91%). Over 40% of all PBC cases were in the 55–64 year age group; fewer than 5% were in the 18–34 year age group, and fewer than 10% were ≥75 years. By contrast, the PBC-pruritus subpopulation was skewed towards the younger (35–54 years) age group compared with the overall PBC population (~40% vs~30%, respectively; figure 1).
Figure 1.
Age distribution in the PBC population (N=1963) and the PBC-pruritus subpopulation (N=139). PBC, primary biliary cholangitis.
Comorbidities during the observation period
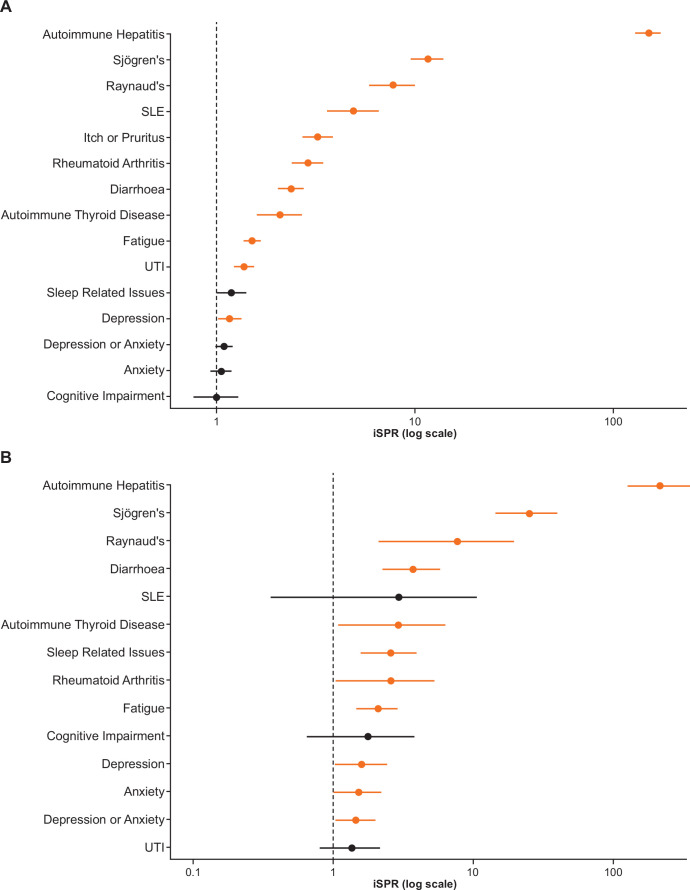
With respect to PBC symptoms of interest, pruritus was observed in 6.8% of the PBC population (based on claims during the observation period), a three-fold higher prevalence relative to non-cases (1.5%) (iSPR (95% CI): 3.24 (2.71 to 3.84)) (table 1), and was more frequently observed in the younger population (18–34 years) compared with other age groups (online supplemental table 3).
Table 1.
Observed crude point prevalence and iSPR of prespecified comorbidities among patients in the PBC population or the PBC-pruritus subpopulation relative to the respective non-case population
| Comorbidity | PBC population (N=1963) |
PBC-pruritus subpopulation (N=139) |
Non-case population (N=10 245 592)* | ||
| % | iSPR (95% CI)† | % | iSPR (95% CI)† | % | |
| PBC symptoms of interest | |||||
| Diarrhoea | 9.3 | 2.38 (2.05 to 2.75) | 13.7 | 3.73 (2.25 to 5.83) | 3.1 |
| Pruritus | 6.8 | 3.24 (2.71 to 3.84) | N/A | N/A | 1.5 |
| UTI | 13.8 | 1.37 (1.21 to 1.54) | 13.0 | 1.36 (0.81 to 2.15) | 6.6 |
| Prevalent psychiatric and other conditions affecting QoL | |||||
| Cognitive impairment | 3.1 | 1.00 (0.77 to 1.29) | 4.3 | 1.77 (0.65 to 3.85) | 1.7 |
| Depression or anxiety | 21.3 | 1.09 (0.99 to 1.20) | 28.1 | 1.46 (1.04 to 2.00) | 15.9 |
| Fatigue | 19.9 | 1.51 (1.36 to 1.66) | 26.6 | 2.10 (1.48 to 2.90) | 10.4 |
| Sleep-related issues | 6.9 | 1.18 (0.99 to 1.40) | 14.4 | 2.58 (1.58 to 3.99) | 4.4 |
| Liver and autoimmune conditions | |||||
| Autoimmune hepatitis | 9.5 | 151.31 (130.40 to 174.62) | 12.2 | 218.07 (127.03 to 349.15) | 0.03 |
| Autoimmune thyroid disease | 3.0 | 2.09 (1.59 to 2.70) | 4.3 | 2.92 (1.07 to 6.36) | 1.0 |
| Raynaud’s syndrome | 2.9 | 7.75 (5.87 to 10.04) | 2.9 | 7.72 (2.10 to 19.75) | 0.2 |
| Rheumatoid arthritis | 6.3 | 2.89 (2.40 to 3.45) | 5.0 | 2.58 (1.04 to 5.31) | 1.2 |
| Sjögren’s syndrome | 5.8 | 11.64 (9.60 to 13.98) | 11.5 | 25.20 (14.40 to 40.93) | 0.2 |
| SLE | 2.4 | 4.91 (3.60 to 6.52) | 1.4 | 2.95 (0.36 to 10.67) | 0.3 |
*N for the non-case population (N=10 245 592), relates to the comparison versus the PBC population not vs the PBC-pruritus population (N=9 984 009).
†Compared with non-case population.
CI, confidence interval; iSPRs, indirect age-sex standardised prevalence ratios; N/A, not applicable; PBC, primary biliary cholangitis; QoL, quality of life; SLE, systemic lupus erythematosus; UTI, urinary tract infection.
Diarrhoea was the most common symptom of interest in the PBC population (9.3%) and the PBC-pruritus subpopulation (13.7%) (table 1, online supplemental figure 1); in both cases, the prevalence of diarrhoea was at least triple that observed in the non-case population (3.1%) (iSPR (95% CI): 2.38 (2.05 to 2.75) and 3.73 (2.25 to 5.83), respectively) (table 1, figure 2). Compared with non-cases (6.6%), the prevalence of urinary tract infection was also higher in the PBC population (13.8%; iSPR (95% CI): 1.37 (1.21 to 1.54)) and in the PBC-pruritus subpopulation (13.0%) (table 1, online supplemental figure 1); however, the iSPR was not significant for the PBC-pruritus subpopulation relative to non-cases as the CI crossed unity (iSPR (95% CI): 1.36 (0.81 to 2.15)) (table 1, figure 2).
Figure 2.
Prevalence of prespecified comorbidities among (A) patients in the PBC population (N=1963) or (B) the PBC-pruritus subpopulation (N=139) relative to the respective non-case populations*. *Ns for the non-case population differs between analysis A and B: non-cases for analysis A (PBC population) N=10 245 592; non-cases for analysis B (PBC-pruritus subpopulation) N=9 984 009. Prevalence of prespecified comorbidities in the PBC population or the PBC-pruritus subpopulation, using prevalence in the non-case population as reference. iSPRs ˃1 imply greater prevalence in either of the PBC case populations versus the non-case population. Crude point prevalence (%) and iSPRs (95% CIs) for each of the comorbidities shown are also reported in table 1. iSPRs, indirect age-sex standardised prevalence ratios; PBC, primary biliary cholangitis; SLE, systemic lupus erythematosus; UTI, urinary tract infection.
Prevalent fatigue, psychiatric conditions and sleep disturbance
Among the prespecified comorbidities, fatigue was most frequently reported in both the PBC population and PBC-pruritus subpopulation compared with non-cases: 19.9% (iSPR (95% CI): 1.51 (1.36 to 1.66)) and 26.6% (iSPR (95% CI): 2.10 (1.48 to 2.90)), respectively (table 1). A higher prevalence of depression or anxiety was also observed in both PBC populations compared with non-cases: 21.3% (iSPR (95% CI): 1.09 (0.99 to 1.20) and 28.1% (iSPR (95% CI): 1.46 (1.04 to 2.00)) (table 1). The prevalence of all prespecified psychiatric comorbidities (except cognitive impairment) was also significantly higher in the PBC-pruritus subpopulation, and the magnitude of the difference relative to non-cases was greater than in the PBC population (figure 2). The greatest difference was observed for sleep-related issues, which was 2.6-fold higher in the PBC-pruritus subpopulation compared with non-cases (14.4%; iSPR (95% CI): 2.58 (1.58 to 3.99)) compared with 1.2-fold higher in the PBC population (6.9%; iSPR (95% CI: 1.18 (0.99 to 1.40)) (table 1).
Liver and autoimmune conditions
Of all the prespecified comorbidities assessed, autoimmune hepatitis showed the greatest difference in prevalence between both the PBC population (9.5%; iSPR (95% CI): 151.31 (130.40 to 174.62)) and the PBC-pruritus subpopulation (12.2%; 218.07 (127.03 to 349.15)) compared with non-cases (table 1, figure 2). This was followed (by descending prevalence in the PBC-pruritus subset) by Sjögren’s syndrome (5.8%, 11.64 (9.60 to 13.98); 11.5%, 25.20 (14.40 to 40.93)), rheumatoid arthritis (6.3%, 2.89 (2.40 to 3.45); 5.0%, 2.58 (1.04 to 5.31)), autoimmune thyroid disease (3.0%, 2.09 (1.59 to 2.70); 4.3%, 2.92 (1.07 to 6.36)), Raynaud’s syndrome (2.9%, 7.75 (5.87 to 10.04); 2.9%, 7.72 (2.10 to 19.75)) and systemic lupus erythematosus (2.4%, 4.91 (3.60 to 6.52); 1.4%, 2.95 (0.36 to 10.67)) (table 1, figure 2).
Treatments during the observation period
Approved and off-label treatments for PBC
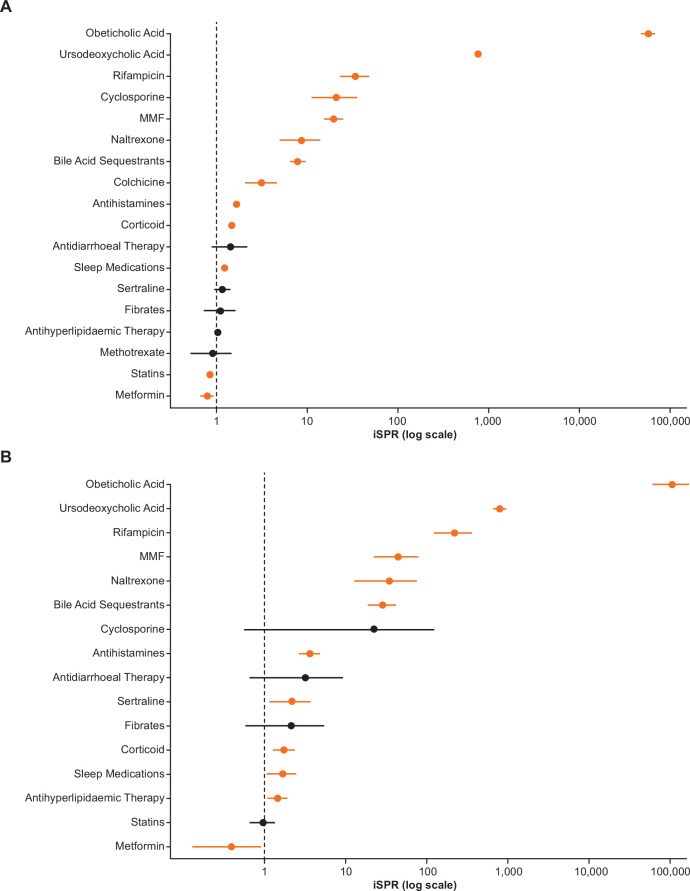
Over 80% of the PBC population and PBC-pruritus subpopulation received UDCA therapy in the 12-month observation period (87.8% and 83.5%, respectively, vs 0.1% for non-cases). The corresponding data for OCA use was 6.9% and 11.5% versus 0%, respectively, indicating greater use of OCA in the PBC-pruritus subpopulation (table 2, figure 3). The use of fibrates, which are sometimes used off-label as a treatment for PBC and are thought to have a benefit on pruritus, was more prevalent in the PBC-pruritus subpopulation compared with the PBC population and non-cases (1.3% and 2.9% vs 1.4%, respectively). Most of the other prespecified medications were also more frequently prescribed in both PBC populations compared with non-cases (table 2, figure 3).
Table 2.
Observed crude point prevalence and iSPR of prespecified medications among patients in the PBC population or the PBC-pruritus subpopulation relative to the respective non-case population
| Medication | PBC population (N=1963) | PBC-pruritus subpopulation (N=139) | Non-case population (N=10 245 592)* | ||
| % | iSPR (95% CI)† | % | iSPR (95% CI)† | % | |
| Antidiarrhoeal therapy | 1.1 | 1.42 (0.88 to 2.18) | 2.2 | 3.18 (0.66 to 9.30) | 0.5 |
| Antihistamines | 15.7 | 1.66 (1.48 to 1.86) | 34.5 | 3.62 (2.67 to 4.80) | 8.5 |
| Antihyperlipidaemic therapy | 31.6 | 1.02 (0.94 to 1.11) | 38.1 | 1.45 (1.08 to 1.89) | 20.9 |
| Bile acid sequestrants‡ | 5.8 | 7.86 (6.48 to 9.44) | 18.0 | 28.54 (18.47 to 42.12) | 0.4 |
| Colchicine | 1.4 | 3.14 (2.07 to 4.57) | 0 | 0 | 0.6 |
| Corticoid | 29.4 | 1.47 (1.35 to 1.59) | 33.8 | 1.73 (1.27 to 2.30) | 17.1 |
| Cyclosporine | 0.7 | 20.94 (11.15 to 35.81) | 0.7 | 22.29 (0.56 to 124.20) | 0.03 |
| Fibrates | 1.3 | 1.10 (0.72 to 1.61) | 2.9 | 2.14 (0.58 to 5.48) | 1.4 |
| MMF | 3.5 | 19.64 (15.25 to 24.90) | 7.9 | 44.51 (22.22 to 79.65) | 0.2 |
| Metformin | 8.0 | 0.79 (0.67 to 0.92) | 3.6 | 0.39 (0.13 to 0.91) | 7.6 |
| Methotrexate | 0.8 | 0.90 (0.51 to 1.46) | 0 | 0 | 0.5 |
| Naltrexone | 0.8 | 8.63 (4.93 to 14.02) | 4.3 | 34.54 (12.67 to 75.17) | 0.1 |
| OCA‡ | 6.9 | 57 510.56 (48 218.86 to 68 070.71) | 11.5 | 105,298.43 (60 187.15 to 170,998.05) | 0 |
| Penicillamine | 0.1 | 163.02 (4.13 to 908.31) | 0 | 0 | 0 |
| Rifampicin‡ | 1.5 | 33.87 (22.68 to 48.65) | 10.8 | 218.08 (122.06 to 359.69) | 0.1 |
| Sertraline‡ | 5.0 | 1.16 (0.94 to 1.41) | 9.4 | 2.16 (1.15 to 3.70) | 3.6 |
| Sleep medications | 12.9 | 1.22 (1.07 to 1.38) | 16.6 | 1.65 (1.05 to 2.47) | 7.7 |
| Statins | 24.5 | 0.84 (0.77 to 0.92) | 23.7 | 0.96 (0.66 to 1.34) | 19.6 |
| UDCA§ | 87.8 | 766.60 (730.84 to 803.66) | 83.5 | 796.69 (658.32 to 955.55) | 0.1 |
*N for the non-case population (N=10 245 592), relates to the comparison versus the PBC population not versus the PBC-pruritus population (N=9 984 009).
†Compared with non-cases (comparator) population.
‡Guideline-recommended pruritus treatment (AASLD and EASL).
§PBC treatment.
AASLD, American Association for the Study of Liver Diseases; CI, confidence interval; EASL, European Association for the Study of the Liver; iSPRs, indirect age-sex standardised prevalence ratios; MMF, mycophenolate mofetil; OCA, obeticholic acid; PBC, primary biliary cholangitis; UDCA, ursodeoxycholic acid.
Figure 3.
Prevalence of prespecified medications among (A) patients in the PBC population (N=1963) or (B) the PBC-pruritus subpopulation (N=139) relative to the respective non-case populations*. *Ns for the non-case population differs between analysis A and B: non-cases for analysis A (PBC population) N=10 245 592; non-cases for analysis B (PBC-pruritus subpopulation) N=9 984 009. Prevalence of prespecified medications in the PBC population or the PBC-pruritus subpopulation, using prevalence in the non-case population as reference. iSPRs ˃1 imply greater medication prevalence in either of the PBC case populations versus the PBC non-case population. Crude point prevalence (%) and iSPRs (95% CIs) for each of the medications shown are also reported in table 2.iSPRs, indirect age-sex standardised prevalence ratios; PBC, primary biliary cholangitis; MMF, mycophenolate mofetil.
Recommended treatments for pruritus in PBC according to global guidelines
Bile acid sequestrants were the most frequently prescribed pruritus therapies in the PBC-population and PBC-pruritus subpopulation compared with non-cases (5.8% and 18.0% vs 0.4%, respectively) (table 2). A similar trend was also observed for rifampicin (1.5% and 10.8% vs 0.1%) (table 2). Similarly, naltrexone was prescribed to 0.8% and 4.3% of the PBC and PBC-pruritus subpopulation versus 0.1% for non-cases (table 2). A near two-fold higher prevalence of sertraline use was observed in the PBC-pruritus subpopulation relative to the PBC population and non-cases (5.0% and 9.4% vs 3.6%) (table 2). Similarly, antihistamine prescriptions were more than two-fold higher in the PBC-pruritus subpopulation than in the PBC population (15.7% and 34.5% vs 8.5%) (table 2).
Immunosuppressants
The use of immunosuppressants among PBC cases was also significantly higher than in non-cases. The immunosuppressive drugs that were more commonly prescribed in the PBC-pruritus subpopulation relative to the PBC population included corticoids (29.4% and 33.8% vs 17.1%), mycophenolate mofetil (3.5% and 7.9% vs 0.2%), and cyclosporine (0.7% and 0.7% vs 0.03%) (table 2).
Other treatments
Additional medications that were prescribed for the treatment of comorbidities that were higher in PBC-pruritus subpopulation than in PBC cases and non-cases included sleep medications (12.9% and 16.6% vs 7.7%), antidiarrhoeal treatment (1.1% and 2.2% vs 0.5%) and antihyperlipidaemic agents (including statins) (31.6% and 38.1% vs 20.9%) (table 2).
Discussion
This retrospective cross-sectional study indicates that patients with PBC experience a significant burden from their disease, including a high prevalence of comorbidities, particularly psychiatric disorders, fatigue and sleep disturbance. Furthermore, these comorbidities were more common in the presence of pruritus.
Consistent with the literature, a female-to-male ratio of PBC cases of 9:1 was observed, with most cases between 55 and 64 years of age.2 27 28 By contrast, pruritus was more frequent (15%) in the youngest age group (18–34 years), and a considerable proportion of patients (~40%) in the PBC-pruritus subpopulation was in the 35–54 year age group, suggesting that patients can suffer with pruritus and its consequential impact on well-being and productivity for many years. An association between younger age and persistent high levels of pruritus has also been reported previously based on patient data in the UK.6
We observed a low (~7%) prevalence of pruritus in the overall PBC population. This is inconsistent with the published literature that reports approximately 50% of patients are affected at any given point after a PBC diagnosis and up to 75% are affected by it over the course of their disease.6–9 The lower prevalence of pruritus we observed may indicate that pruritus is under-reported in observational electronic healthcare databases. It is also possible that physicians may have prescribed a treatment for pruritus, without recording a pruritus diagnosis (discussed further below). The low prevalence of pruritus also highlights the possibility that pruritus remains under-recorded, and diagnosis may be limited to patients with more severe presentations. Nevertheless, the prevalence of pruritus was still significantly higher in the PBC population compared with non-cases.
A key finding of this study is the increased comorbid medical burden for patients with PBC and pruritus relative to non-cases, particularly for comorbidities with a major impact on HRQoL such as psychiatric conditions (depression or anxiety) as well as fatigue and sleep-related issues. The magnitude of difference in the prevalence of these conditions was greater in the PBC-pruritus subpopulation compared with broader PBC group; for example, fatigue was up to two-fold higher in the PBC-pruritus subpopulation and 1.5-fold higher in the broader PBC population versus non-cases. These findings are in agreement with a recently published review of the literature, which concluded that pruritus, fatigue, depression, anxiety and sleep disorders are the most frequently reported symptoms that patients with PBC experience, and these symptoms have a considerable negative impact on their HRQoL.12 However, it should be emphasised here that comparisons between the PBC population and the PBC-pruritus subpopulation are reported only for illustrative purposes, as any apparent differences between iSPRs may be attributable to differences in population structure of the two samples; however, the age-sex structure of the PBC and PBC-pruritus groups was broadly similar. Nevertheless, we acknowledge that our conclusion that patients with PBC and pruritus have an increased comorbid medical burden may be influenced by the fact that patients with more severe presentations of pruritus may have received the L29 code for pruritus (as noted above).
Diarrhoea is a commonly observed symptom in patients with PBC,29 which is reflected in our analysis wherein diarrhoea was the most common prespecified symptom of interest in both the PBC population and the PBC-pruritus subpopulation. The occurrence of diarrhoea in these populations may also be secondary to the use of UDCA and (to a lesser extent) bile acid resins.
Consistent with the literature, comorbidities with the greatest difference between PBC cases and non-cases were autoimmune disorders such as autoimmune hepatitis, followed by Sjögren’s syndrome, rheumatoid arthritis and autoimmune thyroid disease. The prevalence of autoimmune hepatitis observed in our PBC case population (9.5%) is consistent with that reported elsewhere (8%–10%).1 However, it should be noted that autoimmune hepatitis/PBC overlap syndrome is often over diagnosed in clinical practice,30 and it is possible that this may also be the case in our PBC case population; however, the available data source does not include additional clinical information, details of diagnostic tests or the diagnostic criteria used to further classify these patients. Use of immunosuppressants (corticoids and mycophenolate mofetil) among PBC cases was also significantly higher than in non-cases. Although the indication cannot be confirmed based on the available data source, the higher use of these medications is likely related to the higher rate of autoimmune comorbidities, which further adds to the already substantial burden of disease in these patients.
Regarding treatment of PBC, UDCA was prescribed in close to 90% of the PBC population with only 7% of this population receiving OCA. The relatively low OCA usage is perhaps expected in this study given that data from this analysis are from 2017 and OCA was only approved in the USA in 2016.20 It is also interesting to note that UDCA usage was lower and OCA use higher in the PBC-pruritus subpopulation (84% and 12%, respectively). As UDCA failures have previously been reported to have a higher rate of pruritus31 and pruritus is a known side effect of OCA,21 this difference in PBC management in this dataset is likely to arise from a combination of these factors. However, it should be noted that it is not possible to deduce any causal associations from this analysis.
Medications recommended for the treatment of pruritus in PBC were expectedly more prevalent in the PBC population and PBC-pruritus subpopulation compared with non-cases. Bile acid sequestrants were among the most commonly used medications in the PBC-pruritus subpopulation; however, the rate of use was still relatively low (18%) despite cholestyramine being the only approved treatment for cholestatic pruritus.1 22 23 This observation is consistent with other studies, which also reported low rates of bile acid sequestrant use. In a UK cohort, only 24% of patients with PBC who had experienced pruritus during their illness reported receiving treatment with cholestyramine.6 Similarly, a US study of the TARGET PBC database reported that only 16% of patients with PBC with pruritus had received bile acid sequestrants.32
Use of off-label therapies, including rifampicin, naltrexone and sertraline, was significantly higher in the PBC pruritus subpopulation compared with non-cases; however, as for bile acid sequestrants, the overall frequency of use was low (11%, 4% and 9%, respectively). Although not considered effective for cholestatic pruritus according to current guidelines,1 22 antihistamines were prescribed to 35% of the PBC-pruritus subpopulation, which is three-fold higher compared with non-cases.
Given that on-label and off-label use of bile acid sequestrants, rifampicin and naltrexone is unlikely to be substantially different between the PBC and non-case populations (with the exception of treating pruritus), the fact that their use in the PBC population in this dataset (5.8%, 1.5% and 0.8%, respectively) was higher compared with the very low rates in non-cases (0.4%, 0.1% and 0.1%, respectively) is likely attributable, at least partly, to their use for treating pruritus. This is also the case, although to a lesser extent, for both sertraline and antihistamines which have higher use in the PBC population relative to non-cases. Together this strongly indicates that there is a large population of patients being treated for pruritus who are not coded for as such in clinical practice and thus are difficult to identify in claims databases, and further supports the point noted above, that the prevalence of pruritus in the PBC sample observed in this analysis is likely a significant underestimate of the true rate.
Reflecting the higher prevalence of sleep disorders, depression and anxiety among the PBC pruritus subpopulation, sleep medications and antidepressants (ie, sertraline) were prescribed more often in this group compared with non-cases. It is important to note that information of indications for each medicine prescribed was not available in the database. Therefore, the relationship between comorbidity and treatment is based merely on clinical assumptions.
Although administrative claims data provide valuable real-world information, there are inherent challenges and limitations with this type of data. Identification of cases was based on ICD codes alone with no available information regarding laboratory parameters and diagnostic criteria, which may vary among physicians. However, to minimise error rates and increase robustness of the results, an eligibility criterion of at least two diagnostic codes for PBC was included. This approach is based on a previous validation study which showed that the optimal algorithm to identify confirmed PBC cases in administrative data included two or more uses of a PBC code, with sensitivity of 94% and positive predictive value of 73%.25 Nevertheless, the use of ICD codes alone to specify comorbidities may result in underestimation of some comorbidities, as this method assumes that the absence of a code for a given condition implies absence of the condition itself. Moreover, this study could not account for missing data, such as duration, adherence or continuity of treatment, exclusion of over-the-counter medications and the quality of available data that were solely collected for billing purposes. Individuals with chronic conditions typically use healthcare resources to a greater extent than those without such illnesses, potentially introducing artefacts in our analysis and inflating differences between populations. To account for such errors, a healthcare encounter during the lead-in period was specified as an inclusion criterion, which was also applicable to the non-case population. Caution should be exercised when generalising our findings to the wider PBC population, especially in different countries and varying healthcare systems. Additionally, potential differences in clinical practice and socioeconomic discrepancies, such as access to care, should also be considered.33 34 Collectively, these limitations highlight that there is a clear need for high quality data, including from both structured and unstructured databases as well as patient-reported outcome data to fully address the burden of PBC and associated pruritus in real-world practice.
Despite these limitations this study is unique in that it provides a comprehensive, real-world perspective of the overall burden of PBC and PBC-associated pruritus in a relatively large population, especially given the rarity of the disease. Our analyses confirm current knowledge regarding the burden of pruritus and highlight the substantial impact of pruritus on psychiatric and sleep disorders in patients with PBC. The results also emphasise that pruritus might be often underdiagnosed and, consequently, undertreated in clinical practice, reflecting a clear unmet need for newer effective and safe treatments for cholestatic pruritus.
Acknowledgments
The authors would like to thank Jolyon Fairburn-Beech for analytic support and quality assurance. Editorial support (in the form of writing assistance, including development of the initial draft based on author direction, assembling tables and figures, collating authors’ comments, grammatical editing, and referencing) was provided by Katalin Bartus, PhD, of Fishawack Indicia Ltd., part of Fishawack Health, and was funded by GSK.
Footnotes
UG and DCG contributed equally.
Presented at: Some elements of these data were presented at The Liver Meeting – 71st Annual Meeting of the American Association for the Study of Liver Diseases (Gungabissoon U, et al. Hepatology 2020;72:741A).
Contributors: UG, DCG and GR contributed to the conception and design of the study. UG, DCG, ARdS and HS contributed to data analysis or interpretation. UG is acting as the guarantor for the overall content of the manuscript. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors, take responsibility for the integrity of the work as a whole, contributed to the writing and reviewing of the manuscript and have given final approval for the version to be published. All authors had full access to the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Funding: This study was funded by GSK (GSK212487/209984).
Competing interests: All authors are employees and stock/shareholders of GSK.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This was a claims database study exclusively using pre-existing, de-identified claims data and patient recruitment was not carried out specifically for this study. Thus, no patient involvement existed in this study and all applicable laws regarding patient privacy were followed. Informed consent, ethics committee or institutional review board approval were not required as patient identification was not included as part of the analysis and omitted from publication.
References
- 1.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver . EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol 2017;67:145–72. 10.1016/j.jhep.2017.03.022 [DOI] [PubMed] [Google Scholar]
- 2.Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol 2012;56:1181–8. 10.1016/j.jhep.2011.10.025 [DOI] [PubMed] [Google Scholar]
- 3. Orphanet. Prevalence and incidence of rare diseases: Bibliographic data. Available: https://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf
- 4.Hirschfield GM, Dyson JK, Alexander GJM, et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut 2018;67:1568–94. 10.1136/gutjnl-2017-315259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones DE. Primary biliary cholangitis. BMJ best practice. Available: https://bestpractice.bmj.com/topics/en-gb/344
- 6.Hegade VS, Mells GF, Fisher H, et al. Pruritus is common and undertreated in patients with primary biliary cholangitis in the United Kingdom. Clin Gastroenterol Hepatol 2019;17:1379–87. 10.1016/j.cgh.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 7.Oeda S, Takahashi H, Yoshida H, et al. Prevalence of pruritus in patients with chronic liver disease: a multicenter study. Hepatol Res 2018;48:E252–62. 10.1111/hepr.12978 [DOI] [PubMed] [Google Scholar]
- 8.Talwalkar JA, Lindor KD. Primary biliary cirrhosis. Lancet 2003;362:53–61. 10.1016/S0140-6736(03)13808-1 [DOI] [PubMed] [Google Scholar]
- 9.Talwalkar JA, Souto E, Jorgensen RA, et al. Natural history of pruritus in primary biliary cirrhosis. Clin Gastroenterol Hepatol 2003;1:297–302. 10.1016/S1542-3565(03)00134-4 [DOI] [PubMed] [Google Scholar]
- 10.Bergasa NV. Pruritus and fatigue in primary biliary cirrhosis. Clin Liver Dis 2003;7:879–900. 10.1016/S1089-3261(03)00105-3 [DOI] [PubMed] [Google Scholar]
- 11.Levy C. Management of pruritus in patients with cholestatic liver disease. Gastroenterol Hepatol 2011;7:615–7. [PMC free article] [PubMed] [Google Scholar]
- 12.Sivakumar T, Kowdley KV. Anxiety and depression in patients with primary biliary cholangitis: current insights and impact on quality of life. Hepat Med 2021;13:83–92. 10.2147/HMER.S256692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trivedi HD, Lizaola B, Tapper EB, et al. Management of pruritus in primary biliary cholangitis: a narrative review. Am J Med 2017;130:744.e1–744.e7. 10.1016/j.amjmed.2017.01.037 [DOI] [PubMed] [Google Scholar]
- 14.Beuers U, Kremer AE, Bolier R, et al. Pruritus in cholestasis: facts and fiction. Hepatology 2014;60:399–407. 10.1002/hep.26909 [DOI] [PubMed] [Google Scholar]
- 15.Lu M, Li J, Haller IV, et al. Factors associated with prevalence and treatment of primary biliary cholangitis in United States health systems. Clin Gastroenterol Hepatol 2018;16:1333–41. 10.1016/j.cgh.2017.10.018 [DOI] [PubMed] [Google Scholar]
- 16.Wong KA, Bahar R, Liu CH, et al. Current treatment options for primary biliary cholangitis. Clin Liver Dis 2018;22:481–500. 10.1016/j.cld.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 17.Beuers U, Gershwin ME, Gish RG, et al. Changing nomenclature for PBC: from 'cirrhosis' to 'cholangitis'. J Hepatol 2015;63:1285–7. 10.1016/j.jhep.2015.06.031 [DOI] [PubMed] [Google Scholar]
- 18.Lu M, Zhou Y, Haller IV, et al. Increasing prevalence of primary biliary cholangitis and reduced mortality with treatment. Clin Gastroenterol Hepatol 2018;16:1342–50. 10.1016/j.cgh.2017.12.033 [DOI] [PubMed] [Google Scholar]
- 19.Lindor KD, Dickson ER, Baldus WP, et al. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology 1994;106:1284–90. 10.1016/0016-5085(94)90021-3 [DOI] [PubMed] [Google Scholar]
- 20.Food and Drug Administration . OCALIVA (obeticholic acid) prescribing information, 2016. [Google Scholar]
- 21.Nevens F, Andreone P, Mazzella G, et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med 2016;375:631–43. 10.1056/NEJMoa1509840 [DOI] [PubMed] [Google Scholar]
- 22.Lindor KD, Bowlus CL, Boyer J, et al. Primary biliary cholangitis: 2018 practice guidance from the American association for the study of liver diseases. Hepatology 2019;69:394–419. 10.1002/hep.30145 [DOI] [PubMed] [Google Scholar]
- 23.Lindor KD, Gershwin ME, Poupon R, et al. Primary biliary cirrhosis. Hepatology 2009;50:291–308. 10.1002/hep.22906 [DOI] [PubMed] [Google Scholar]
- 24.Yosipovitch G, Bernhard JD. Clinical practice. chronic pruritus. N Engl J Med 2013;368:1625–34. 10.1056/NEJMcp1208814 [DOI] [PubMed] [Google Scholar]
- 25.Myers RP, Shaheen AAM, Fong A, et al. Validation of coding algorithms for the identification of patients with primary biliary cirrhosis using administrative data. Can J Gastroenterol 2010;24:175–82. 10.1155/2010/237860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am J Epidemiol 1990;131:373–5. 10.1093/oxfordjournals.aje.a115507 [DOI] [PubMed] [Google Scholar]
- 27.Kim WR, Lindor KD, Locke GR, et al. Epidemiology and natural history of primary biliary cirrhosis in a US community. Gastroenterology 2000;119:1631–6. 10.1053/gast.2000.20197 [DOI] [PubMed] [Google Scholar]
- 28.Lleo A, Wang G-Q, Gershwin ME, et al. Primary biliary cholangitis. Lancet 2020;396:1915–26. 10.1016/S0140-6736(20)31607-X [DOI] [PubMed] [Google Scholar]
- 29.Liu Chen Kiow J, Vincent C, Sidani S, et al. High occurrence of small intestinal bacterial overgrowth in primary biliary cholangitis. Neurogastroenterol Motil 2019;31:e13691. 10.1111/nmo.13691 [DOI] [PubMed] [Google Scholar]
- 30.Trivedi PJ, Hirschfield GM. Review article: overlap syndromes and autoimmune liver disease. Aliment Pharmacol Ther 2012;36:517–33. 10.1111/j.1365-2036.2012.05223.x [DOI] [PubMed] [Google Scholar]
- 31.Carbone M, Mells GF, Pells G, et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology 2013;144:560–9. 10.1053/j.gastro.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 32.Carey E, Smith H, McLaughlin M. The pervasive impact of pruritus on quality of life in patients with primary biliary cholangitis (PBC): real world experience in TARGET-PBC. Hepatology 2020;72:766A–7. 10.1002/hep.31579 [DOI] [Google Scholar]
- 33.Peters MG, Di Bisceglie AM, Kowdley KV, et al. Differences between Caucasian, African American, and Hispanic patients with primary biliary cirrhosis in the United States. Hepatology 2007;46:769–75. 10.1002/hep.21759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabiee A, Polanco NAP, Vara AFDL, et al. Hispanic patients with primary biliary cholangitis have decreased access to care compared to non-Hispanics. J Clin Transl Hepatol 2020;8:1–6. 10.14218/JCTH.2020.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2021-000857supp001.pdf (220.8KB, pdf)
bmjgast-2021-000857supp002.pdf (790.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.