Abstract
Objective
The objective of this study was the visualization of hot spots and evolving trends in research on the association between vitamin D and infections through the use of bibliometric analysis.
Methods
Based on 3046 relevant articles collected in the Web of Science Core Collection for the period of 2001–2021, the data were processed using CiteSpace software. GraphPad software was used for some of the graphics.
Results
A total of 3,046 literature were retrieved, with an average citation frequency of 27.89 times. The number of published papers in the direction of “Immunology” (453 articles, 14.9%) and “Infectious diseases” (312 articles, 10.2%) is much higher. The United States presents the highest publication count (890, 29.2%) and shows a strong leadership in this field. Country burst shows that since 2015, many developing countries and low-income countries have carried out enthusiastic research in this regard, including China, Pakistan, and Iran. As for institutions, the League of European Research Universities produces a larger proportion of articles (220, 7.2%). In terms of authors, Martineau AR and Camargo CA have the highest number of published articles, contributing 30 (0.99%) and 28 articles (0.92%), respectively. Major studies are supported by the United States Department of Health Human Services funding (394, 12.9%). According to the keyword co-occurrence diagram, the 10 most frequent keywords from 2001 to 2021 are “vitamin D”, “infection”, “d deficiency”, “risk”, “association”, “expression”, “disease”, “d supplementation”, “vitamin d deficiency”, and “children”. The top 10 cited articles in 2021 are all related to COVID-19, suggesting it is a hotspot in recent times.
Conclusion
Research on the association between vitamin D and infection has grown rapidly since 2012 and is generally developing well. While developed Western countries continue to be leading roles in this field, research trends in developing countries are also very promising. It is demonstrated that the relationship between vitamin D and respiratory infections, especially respiratory viruses and the more recently COVID-19, has received a lot of attention in the last two decades, suggesting that this is the hotspot and frontier of research issue.
Keywords: vitamin D, infectious disease, hot spots, trends, visualization analysis
Introduction
As a fat-soluble secosteroid, the two main forms of vitamin D in nature are vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Vitamin D2 is mainly of plant origin, while vitamin D3 accounts for about 80%−90% of the total in higher animals (1). Vitamin D3 could be synthesized through exposure to ultraviolet B (UV B) radiation from 7-dehydrocholesterol in the skin (1), which is the major source for most people. If the endogenous synthesis is deficient, usually due to limited skin exposure to sunlight, then dietary supply becomes critical. Both vitamin D2 and vitamin D3 are inactive and need two consecutive hydroxylation steps to develop fully active vitamin D. Vitamin D is first transported to the liver via vitamin D binding protein (DBP). In the liver, vitamin D2 and vitamin D3 undergo hydroxylation to 25(OH)D, which is then re-hydroxylated in the kidneys to 1,25(OH)2D (calcitriol) (2).
25(OH)D is the main circulating metabolite of vitamin D and the most recognized indicator of vitamin D status currently due to its longer half-life (about 2–3 weeks) (3). Circulating 25(OH)D is tightly bound to DBP (85–90%) or albumin (10–15%), and only a very small fraction is present in free form in the circulation (4). The free hormone hypothesis states that only unbound hormone can be biologically active (5). This hypothesis is supported by observations in DBP-deficient mice. These DBP null mice, although with largely undetectable 25(OH)D levels, did not show signs of vitamin D deficiency unless given a vitamin D-deficient diet (6). It is suggested that DBP is a key reservoir of vitamin D metabolites and may reduce the risk of vitamin D deficiency when ingestion or epidermal production is restricted. Polymorphisms in DBP are associated with disease susceptibility (7).
Similar to other steroid hormones, the active form of vitamin D, 1,25(OH)2D, functions by binding to the vitamin D receptor (VDR) to a specific DNA sequence, thereby transcriptionally regulating gene expression and mediating cellular responses (8). VDRs are present in a wide range of cells along with organs, such as the brain, heart, small intestine, colon, osteoblasts, activated T and B lymphocytes, and monocytes (1). Studies also showed that vitamin D can directly or indirectly interact with a wide range of genes (9). The latest study suggested that a dose-dependent alteration in the expression of genes was observed after 25(OH)D supplementation, with 162, 320, and 1,289 genes up- or downregulated, respectively (10). The effects of vitamin D involve anti-proliferation, pro-differentiation, anti-angiogenesis, inhibition of metastasis, and induction of apoptosis in cancer cells (1). Other effects include the increase in insulin secretion, modulation of renin–angiotensin–aldosterone effect, and various immunomodulatory effects, including control of immune activation on the one hand and enhancement of anti-infection defense on the other hand (11–13).
These suggest that vitamin D may play a broad role in human health besides bone health, especially in cancer, cardiovascular disease, diabetes mellitus, and autoimmune diseases (14–16). The latest observational analysis published in Lancet Diabetes & Endocrinol suggests a non-linear dose–response relationship between 25(OH)D concentration and cardiovascular disease, stroke, and mortality outcomes (17). Meanwhile, the further genetic analysis for individuals with low concentrations of 25(OH)D provides strong proof supporting a causal relationship between 25(OH)D concentrations and the risk of all-cause mortality at a threshold of approximately 40 nmol/L (17), which is consistent with the previous Mendelian randomization analysis (18). Recent studies also highlight that vitamin D plays an important role in infectious diseases (19, 20). The overall effect of vitamin D deficiency in infections is associated with alteration of the critical immune response such as genetic expression related to antioxidants, cytokine storm, metabolism, and cellular function (21). However, there are arguments that vitamin D does not do much for infections. The coronavirus disease 2019 (COVID-19) epidemic raging around the world has pushed the debate to its climax. Of note, in stark contrast to the importance of vitamin D in health, vitamin D deficiency is prevalent worldwide, regardless of age, ethnicity, latitude, and economic development (22–25). Since the issue has been recognized and taken seriously, the volume of research literature in this direction grows rapidly in recent years. Bibliometric techniques can be used to explore the dynamics of a specialty, mapping from a research frontier to its knowledge base in a time-varying manner (26). With the use of this technique, our studies analyze the relevant data and try to present a realistic and intuitive picture of the evolving trends of research hotspots on the association between vitamin D and infections, to assist a better understanding of the research dynamics in this area.
Materials and Methods
Data collection
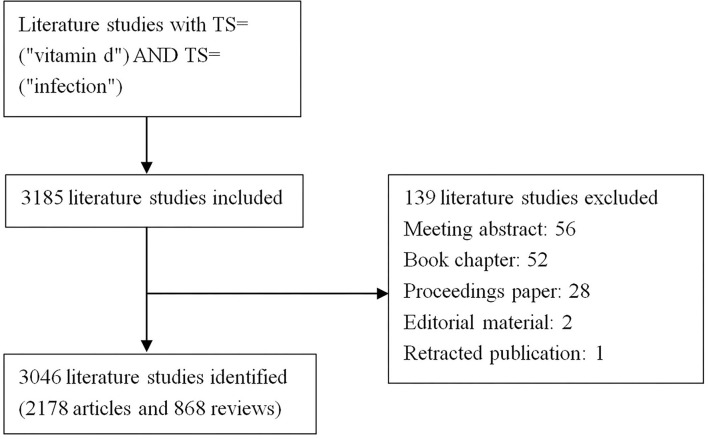
We performed a systematic search of the literature within the Web of Science Core Collection (WoSCC) database using the strategy described below: TS = (“vitamin d”) AND TS = (“infection”) AND Articles OR Review Articles (Document Types) AND Language = English, with a period limited from 2001 to 2021. To avoid the impact of frequent database updates, all literature searching and data collecting were conducted within 1 day on 17 April 2022. A total of 3,185 records were accessed. Then, we excluded 139 data about meeting abstract, book chapter, proceedings paper, editorial material, and retracted publication. The final search yielded 3,046 papers, including 2,178 articles and 868 reviews. The procedure of searching was presented in Figure 1.
Figure 1.
Flowchart of literature screening. Data collection was done on a single day 17 April 2022. A total of 3,185 records from the Web of Science Core Collection (WoSCC) database were retrieved. Then, 139 data were excluded including meeting abstracts, book chapters, proceeding papers, editorial materials, and retracted publications. Finally, 3,046 pieces of data were obtained, including 2,178 articles and 868 reviews. Data were imported into the CiteSpace software (version 5.8.R3) for further analysis.
Data analysis
Retrieved literature data were exported in TXT format and then imported to CiteSpace software (version 5.8.R3) for further analysis and processing. The specific parameters in CiteSpace were set as follows: method (LLR), time slicing (January 2001–December 2020), years per slice (1), term source (title, abstract, author keywords, and keyword plus), node type (select one of the following options at a time: keyword, country), and selection criteria: Top N = 50.
The number of publications, major research institutions, leading countries and authors, keywords, and other indicators in the research field of the association between vitamin D and infections was analyzed. By adjusting the relevant parameters, co-occurrence analysis, cluster analysis, and visualization graphs were performed for keywords. In the generated map, centrality was used to reflect the importance of the node in the network. Centrality value > 0.1 was generally considered a comparatively important node. The higher frequency of co-occurrence and higher centrality indicated that the node was more important in this field.
The results of keyword co-occurrence and keyword cluster represented the evolution of research themes in the field over a defined time interval. The result of keyword burst indicated a sharp increase in the intensity of a research direction over different periods, which was used to identify research hotspots. Highly cited articles were summarized and served the same purpose. Country burst showed rapid growth in the number of citations to literature published by that country over this time frame, which was used to indicate the research fervor in a country.
Microsoft Excel (version 2016) and GraphPad software (version 9.3.1) were also applied in data drawing.
Results
The global growth trend of publication outputs
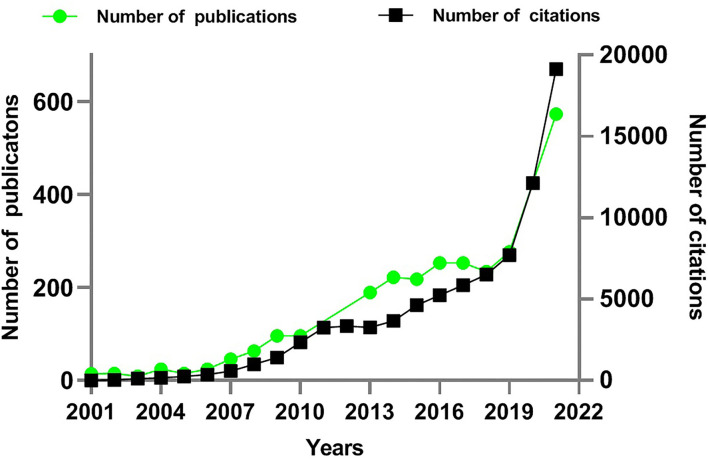
The number of publications is an important index to visualize the trend of the research field. As presented in Figure 2, from 2001 to 2012, there were <100 relevant articles per year on vitamin D and infection. From 2012 onwards, the field has welcomed a rapid growth in the number of literature, reaching 573 articles by 2021. Of note, more than twice as many articles were published in 2021 than in 2019, most possibly due to the sudden outbreak of COVID-19 epidemic. The mean citation frequency is 27.89 times each, and the H-index is 128 times. The cited literature has increased from 3,335 in 2012 to 19,140 in 2021. All these papers cover 82 research directions, with more articles published in the field of “Immunology” (453 articles, 14.9%) and “Infectious Diseases” (312 articles, 10.2%). Other popular areas of research include general internal medicine, nutrition dietetics, and endocrinology metabolism (Table 1).
Figure 2.
Times cited and publications over time (2001–2021). The green line represents the number of published articles. The black line represents the number of cited articles.
Table 1.
Top five based on the number of documents (2001–2021).
| Field | Record count | % of 3,046 | |
|---|---|---|---|
| Research Areas | Immunology | 453 | 14.9 |
| Infectious Diseases | 312 | 10.2 | |
| General Internal Medicine | 311 | 10.2 | |
| Nutrition Dietetics | 274 | 9.0 | |
| Endocrinology Metabolism | 227 | 7.5 | |
| Countries | USA | 890 | 29.2 |
| England | 316 | 10.4 | |
| Italy | 242 | 7.9 | |
| China | 229 | 7.5 | |
| India | 163 | 5.4 | |
| Affiliations | League of European Research Universities | 220 | 7.2 |
| University of London | 133 | 4.4 | |
| Harvard University | 120 | 3.9 | |
| University of California System | 101 | 3.3 | |
| Egyptian Knowledge Bank | 78 | 2.6 | |
| Authors | Martineau AR | 30 | 0.99 |
| Camargo CA | 28 | 0.92 | |
| Hewison M | 18 | 0.59 | |
| Sun J | 17 | 0.56 | |
| Griffiths CJ | 15 | 0.49 | |
| Funding Agencies | United States Department of Health Human Services | 394 | 12.9 |
| National Institutes of Health | 393 | 12.9 | |
| European Commission | 161 | 5.3 | |
| NIH National Institute of Allergy Infectious Diseases | 141 | 4.6 | |
| UK Research Innovation | 96 | 3.2 |
Analysis of country contribution and country burst
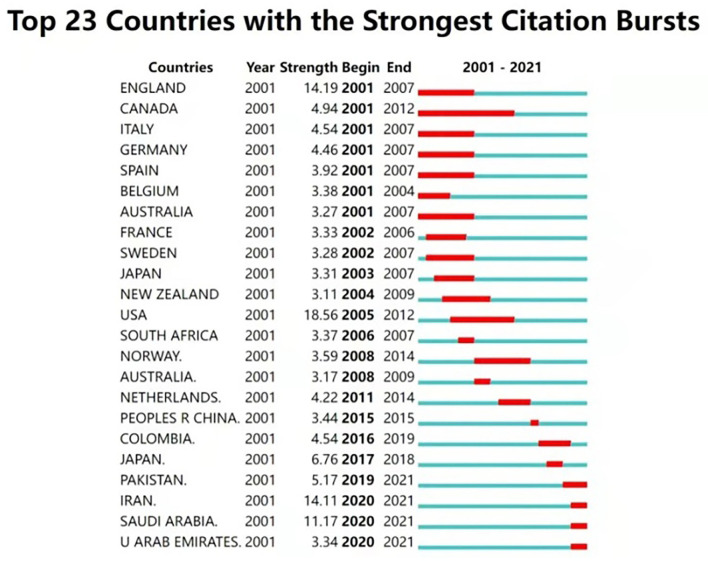
A total of 122 countries or regions have contributed to the research on the association between vitamin D and infections. The United States (US) ranks first and leads the way in the number of publications (890, 29.2%), followed by England (316, 10.4%), Italy (242, 7.9%), China (229, 7.5%), and India (163, 5.4%). Table 1 also shows the top five prominent sources of funding. The major funding agencies include the United States Department of Health Human Services and the National Institutes of Health (NIH), all of which are US organizations with approximately the same number of grants. Meanwhile, we further analyzed the strongest citation bursts of publications by country/region from 2001 to 2021 (Figure 3). The result shows that until 2011, the dominant nations were Western developed countries. Since 2015, many developing countries and low-income countries have carried out enthusiastic research in this regard, including China, Pakistan, and Iran. Recently, several Middle Eastern countries have also shown a high enthusiasm for research, such as Saudi Arabia and the United Arab Emirates.
Figure 3.
Top 23 countries with the strongest citation bursts (2001–2021). The blue line represents the period, and the red line represents the duration of the citation burst.
Analysis of institutions and authors
As shown in Table 1, among the 3,918 institutions contributing to the study of this area, the League of European Research Universities (LERU) published the largest number of papers (220, 7.2%). The following institutions include the University of London (133, 4.4%), Harvard University (120, 3.9%), University of California System (101, 3.3%), and Egyptian Knowledge Bank (78, 2.6%). As for authors, Martineau AR and Camargo CA are the two with the highest number of published articles, contributing 30 (0.99%) and 28 articles (0.92%), respectively. Other authors include Hewison M, Sun J, and Griffiths CJ (Table 1). Their research topics encompass the pathophysiology of vitamin D-related diseases and clinical studies. Interestingly, all these five authors are from universities either in the US or the United Kingdom (United Kingdom). Martineau AR and Griffiths CJ are both colleagues serving at the Queen Mary University of London. Hewison M is at another university in the United Kingdom, namely the Institute of Metabolism and Systems Research, University of Birmingham. Camargo CA works at Massachusetts General Hospital, Harvard Medical School, while Sun J is at the University of Illinois at Chicago.
Analysis of research topic and frontiers
Top ten highly cited articles
Highly cited articles refer to publications with a high citation frequency and a high impact, which could reflect hotspots and depth of research in this field. Table 2 shows the most cited 10 articles in terms of the association between vitamin D and infections. The article published in Science by Liu and colleagues in 2006 was the most cited article, with an impressive frequency of 2,599 citations. This article, along with the tenth-ranked literature, discussed the specific mechanisms of vitamin D in the treatment of tuberculosis. Their findings highlighted the critical role of vitamin D in the antimicrobial response in innate immunity. The fifth- and sixth-ranked articles showed the correlation between vitamin D and virus infections and upper respiratory tract infections, respectively. The second-, seventh-, and eighth-ranked articles summarized evidence from randomized controlled studies to investigate whether vitamin D supplementation could prevent viral infections. These highly cited articles illustrate the continued interest in the association of vitamin D with infections over the past two decades.
Table 2.
Top 10 high-cited references related to vitamin D and infections.
| Ranking | Title | References | Journal | Year | Cited by |
|---|---|---|---|---|---|
| 1 | Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response | Liu, PT, et al. | Science | 2006 | 2,599 |
| 2 | Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data | Martineau, AR, et al. | BMJ-British medical journal | 2017 | 833 |
| 3 | Genetic dissection of immunity to mycobacteria: The human model | Casanova, JL; Abel, L | Annual review of immunology | 2002 | 736 |
| 4 | Environmental risk factors for multiple sclerosis. Part I: The role of infection | Ascherio, A; Munger, KL. | Annals of neurology | 2004 | 694 |
| 5 | Epidemic influenza and vitamin D | Cannell, JJ, et al. | Epidemiology and infection | 2006 | 660 |
| 6 | Association Between Serum 25-Hydroxyvitamin D Level and Upper Respiratory Tract Infection in the Third National Health and Nutrition Examination Survey | Ginde, AA; Mansbach, JM and Camargo, CA | Archives of internal medicine | 2009 | 615 |
| 7 | Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren | Urashima, et al. | American journal of clinical nutrition | 2010 | 563 |
| 8 | Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths | Grant, WB, et al. | Nutrients | 2020 | 552 |
| 9 | Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity | Adams, JS and Hewison, M | Nature clinical practice endocrinology & metabolism | 2008 | 544 |
| 10 | Vitamin D3 Induces Autophagy in Human Monocytes/ Macrophages via Cathelicidin | Yuk, JM, et al. | Cell host & microbe | 2009 | 542 |
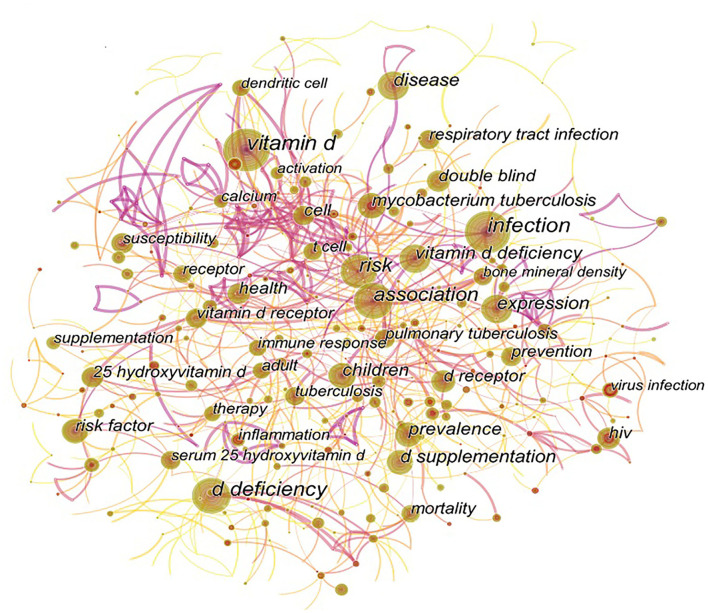
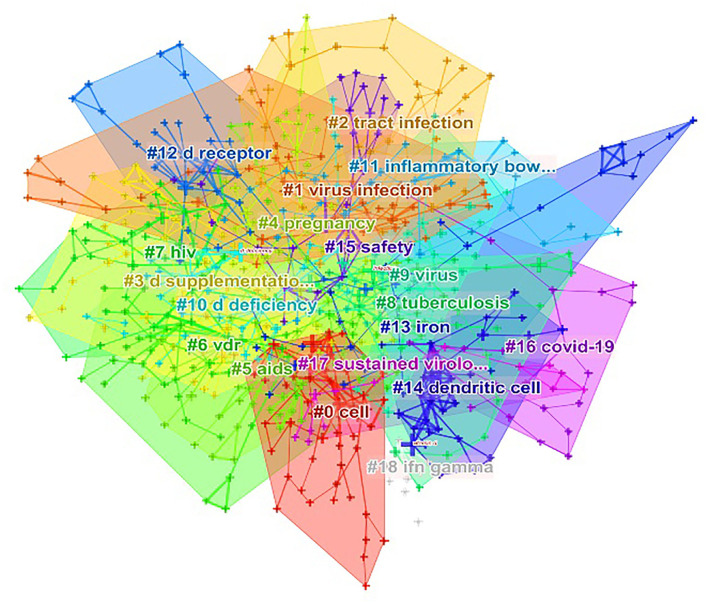
Keyword co-occurrence and cluster
Two or more keywords appearing in the same literature are considered as one co-occurrence. Keyword co-occurrence map is based on the frequency of keyword co-occurrence in the cited literature. The keyword co-occurrence analysis helps to identify research hotspots and predict research trends in certain fields. The keyword co-occurrence diagram is presented in Figure 4. The top 35 keywords based on the co-occurrence frequency are displayed in Table 3. As shown in Figure 4, besides vitamin D and infection, the 10 most frequent keywords from 2001 to 2021 are “d deficiency”, “risk”, “association”, “expression”, “disease”, “d supplementation”, “vitamin d deficiency”, and “children”. Centrality is a measurement of the importance of a node in network analysis. Based on the centrality ranking, the top five keywords are “cell” (centrality 0.22), “epstein barr virus” (centrality 0.2), “allele” (centrality 0.18), “susceptibility” (centrality 0.15), and “calcitriol” (centrality 0.15).
Figure 4.
Keyword co-occurrence network (2001–2021). The nodes represent keywords, and the larger nodes indicate more research articles in that direction. The lines linking the nodes indicate the research connection between keywords, and the thicker the line, the stronger the connection. The top 10 co-occurrence keywords are “vitamin D”, “infection”, “d deficiency”, “risk”, “association”, “expression”, “disease”, “d supplementation”, “vitamin d deficiency”, and “children”.
Table 3.
Keyword co-occurrence frequency (Top 35 in count order, 2001–2021).
| Keywords | Count | Centrality | First appearance year |
|---|---|---|---|
| vitamin d | 594 | 0.01 | 2001 |
| infection | 502 | 0.01 | 2001 |
| d deficiency | 424 | 0.07 | 2006 |
| risk | 364 | 0.04 | 2004 |
| association | 356 | 0.09 | 2001 |
| expression | 226 | 0.09 | 2001 |
| disease | 223 | 0.01 | 2001 |
| d supplementation | 211 | 0.01 | 2009 |
| vitamin d deficiency | 192 | 0.07 | 2001 |
| children | 181 | 0.11 | 2003 |
| prevalence | 181 | 0.02 | 2007 |
| mycobacterium tuberculosis | 158 | 0.1 | 2001 |
| risk factor | 147 | 0.01 | 2002 |
| d receptor | 146 | 0.01 | 2007 |
| double blind | 140 | 0.03 | 2010 |
| hiv | 130 | 0 | 2005 |
| cell | 129 | 0.22 | 2004 |
| health | 126 | 0 | 2009 |
| prevention | 124 | 0 | 2009 |
| respiratory tract infection | 121 | 0.01 | 2010 |
| vitamin d receptor | 121 | 0.06 | 2007 |
| 25 hydroxyvitamin d | 117 | 0.09 | 2006 |
| mortality | 114 | 0.05 | 2006 |
| receptor | 94 | 0.02 | 2008 |
| adult | 93 | 0.03 | 2002 |
| pulmonary tuberculosis | 91 | 0.08 | 2002 |
| immune response | 89 | 0.03 | 2007 |
| susceptibility | 88 | 0.15 | 2002 |
| tuberculosis | 87 | 0.03 | 2004 |
| t cell | 87 | 0.01 | 2007 |
| supplementation | 86 | 0.01 | 2008 |
| serum 25 hydroxyvitamin d | 85 | 0.06 | 2008 |
| inflammation | 80 | 0.04 | 2010 |
| therapy | 79 | 0.04 | 2007 |
| calcium | 78 | 0.1 | 2001 |
Keyword cluster is a network of clusters formed by keywords with similar research topics to reveal the main themes. Generally, clusters are efficient and credible when silhouette > 0.7. A total of 19 distinct clusters are obtained. Within each cluster, the title word used with high frequency in the article serves as an identifier for the cluster connotation. Clusters are numbered from 0 in CiteSpace, namely, cluster #0 is the largest cluster, while cluster #1 is the next largest, and so on. According to the keyword cluster analysis, the top three clusters are “cell”, “virus infection” and “tract infection” (Figure 5 and Table 4). In cluster #0, the related keywords include “risk”, “vitamin d receptor polymorphisms” and “covid-19”. In cluster#1 “virus infection”, the most appeared keywords include “epstein barr virus”, “interferon”, “diet” and “polymorphism”. In cluster #2 “tract infection”, the major keywords mentioned are “influenza a”, “lung infection” and “rsv bronchiolitis”. “tuberculosis” ranked as cluster #8, and the newly emerged “covid-19” ranked as cluster #16.
Figure 5.
Keyword cluster analysis (2001–2021). There are 19 clusters in total and are distinguished by different colors. Cluster #0 is the largest one, followed by cluster #1, and onwards. The top three clusters are “cell”, “virus infection,” and “tract infection”.
Table 4.
Cluster summary (19 clusters by size, 2001–2021).
| ClusterID | Label (LLR)* | Size | mean(Year) |
|---|---|---|---|
| 0 | cell | 49 | 2011 |
| 1 | virus infection | 44 | 2013 |
| 2 | tract infection | 43 | 2016 |
| 3 | d supplementation | 41 | 2011 |
| 4 | pregnancy | 41 | 2013 |
| 5 | aids | 40 | 2010 |
| 6 | vdr | 40 | 2011 |
| 7 | hiv | 40 | 2010 |
| 8 | tuberculosis | 37 | 2008 |
| 9 | virus | 36 | 2011 |
| 10 | d deficiency | 36 | 2012 |
| 11 | inflammatory bowel disease | 35 | 2013 |
| 12 | d receptor | 32 | 2006 |
| 13 | iron | 30 | 2012 |
| 14 | dendritic cell | 30 | 2005 |
| 15 | safety | 30 | 2010 |
| 16 | covid-19 | 23 | 2016 |
| 17 | sustained virological response | 14 | 2013 |
| 18 | ifn gamma | 9 | 2004 |
Only the first keyword of per cluster is listed.
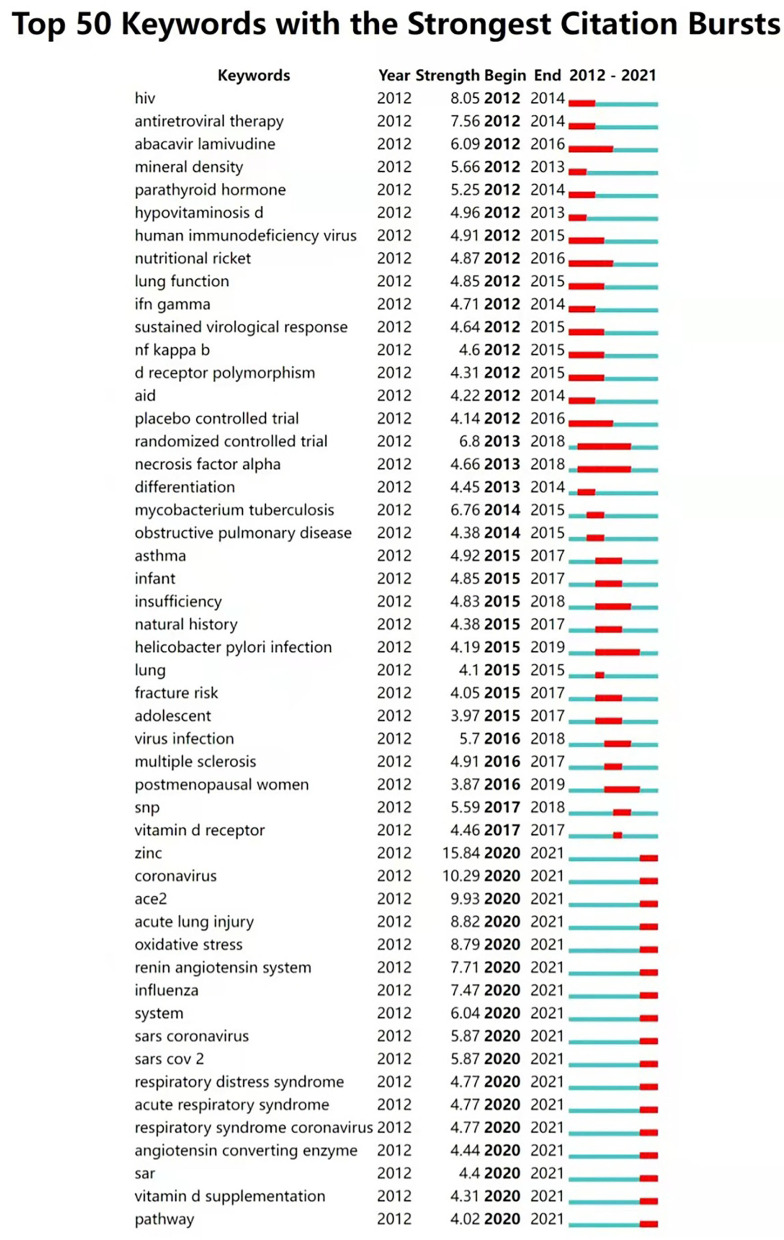
Keyword bursts and the most recent publications
Keyword bursts refer to the sudden increase of keywords in a specific research area at a certain time. Combined with keyword co-occurrence and cluster analysis, it can present a more comprehensive picture of the evolution of research trends and hotspots in related fields. Figure 6 shows the list of top 50 keywords bursts during the last decade. Keywords with higher strength include “zinc” (15.84), “coronavirus” (10.29), “ace2” (9.93), “acute lung injury” (8.82), “oxidative stress” (8.79), “hiv” (8.05), “renin angiotensin system” (7.71), and “influenza” (7.47). “mycobacterium tuberculosis” also has a high strength of 6.76. Keywords with a long duration of citation burst include “randomized controlled trial” (2013-2018), “necrosis factor alpha” (2013-2018), “placebo controlled trial” (2012-2016), “abacavir lamivudine” (2012-2016). “nutritional ricket” (2012-2016), “sustained virological response” (2012-2015), “nf kappa b” (2012-2015), and “d receptor polymorphism” (2012-2015). The latest burst keywords include “coronavirus” (2020-2021), “acute lung injury” (2020-2021), “oxidative stress” (2020-2021), “renin angiotensin system” (2020-2021), and so on.
Figure 6.
Top 50 keywords with the strongest citation burst (2012–2021). The blue line indicates the time-lapse, and the red line indicates the duration of the quote burst, which shows the progression of cutting-edge hot topics.
The most highly cited articles in 2021 are presented in Table 5, which are all related to COVID-19. These articles focus on the role of vitamin D in the pathogenesis of COVID-19 and the relevance to the disease, such as outcomes, severity, etc.
Table 5.
Top cited articles in 2021 related to vitamin D and infection.
| Title | Corresponding Authors | Journal | Cited by |
Impact Factor (2021) |
|---|---|---|---|---|
| Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections | Zimmermann, P | ARCHIVES OF DISEASE IN CHILDHOOD | 121 | 4.973 |
| Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: could they help against COVID-19? | Stojanovska, L | MATURITAS | 78 | 5.110 |
| Vitamin D Status in Hospitalized Patients with SARS-CoV-2 Infection | Hernandez, Jose L. | JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM | 66 | 6.134 |
| Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis | Li, H | INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES | 52 | 12.074 |
| The impact of outdoor air pollution on COVID-19: a review of evidence from in vitro, animal, and human studies | Bourdrel, T | EUROPEAN RESPIRATORY REVIEW | 46 | 9.553 |
| The link between COVID-19 and VItamin D (VIVID): a systematic review and meta-analysis | Fuleihan, GEH | METABOLISM-CLINICAL AND EXPERIMENTAL | 43 | 13.934 |
| Vitamin D Deficiency Is Inversely Associated with COVID-19 Incidence and Disease Severity in Chinese | Cheng, LM | JOURNAL OF NUTRITION | 43 | 4.687 |
| A systematic review of COVID-19 and obstructive sleep apnoea | Miller, MA | SLEEP MEDICINE REVIEWS | 39 | 11.401 |
| Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID-19 | Sharma, A | VIRUS RESEARCH | 35 | 6.286 |
| Association of Vitamin D Status with SARS-CoV-2 Infection or COVID-19 Severity: a systematic review and Meta-analysis | Kazemi, A; Mohammadi, V | ADVANCES IN NUTRITION | 34 | 11.576 |
Discussion
Research trends
According to the qualitative and quantitative investigations conducted by CiteSpace, the scientific production in the field of vitamin D and infections as well as the researchers devoted to it have been growing over the last 20 years. As presented in Figure 2, articles published since 2012 account for 78.2% of the total produced articles in 20 years. Our results are in agreement with previous studies carried out by Shi and colleagues in 2019. Their findings revealed that in recent years, especially from 2015 to 2018, the hot research topic on vitamin D-related diseases has shifted significantly from musculoskeletal-related to the non-musculoskeletal-related area, such as neuropsychological, cardiovascular disease, cancer, and infectious diseases (27). Of note, the 998 articles published during 2020 and 2021 account for nearly one-third of all published 3,046 articles in the last two decades (Figure 2), which is linked to the global outbreak of the COVID-19 epidemic in late 2019.
As illustrated in Table 1, in this field, the US, the UK, Italy, China, and India ranked the top five countries in the total number of publications. Of the top five contributing institutions, namely the League of European Research Universities, University of London, Harvard University, University of California System, and Egyptian Knowledge Bank, two belong to the United States and two in Europe. In terms of the number of individual publications, Martineau AR and Camargo CA ranked as the top two authors. Of the five authors with the highest number of published articles, two professors, Camargo CA and Sun J, work in US universities, while the rest three authors Martineau AR, Griffiths CJ, and Hewison M come from UK institutions. What is even more remarkable is that these authors have remained focused on the field for more than one decade and continue to present high-quality papers up to now. As for the top five funding agencies, three are affiliated with the United States and the rest are from Europe. Hence, our results reveal the consistency in the leading authors, institutions, and countries. Meanwhile, our study also shows the absolute leadership of the Western countries in this field, which was consistent with previous studies (28). In the early twentieth century, researchers in the Western world identified the structure of vitamin D (29) and have consistently attached importance to the study of vitamin D. Owing to the emphasis of governments, outstanding research institutions, intense academic atmosphere, and sufficient research funding, Western developed countries have made outstanding contributions in this area.
Interestingly, further analysis of the countries burst (Figure 3) shows that, in addition to the traditional academic giants, research in developing countries and Middle Eastern countries has been increasing in recent years. Compared to Western developed countries, medical facilities and healthcare framework of developing countries are still relatively lagging behind. However, vitamin D deficiency is more prevalent in low- and middle-income countries, including India and Iran (24). Meanwhile, based on studies in the Middle East, clothing style is also an essential factor in determining vitamin D levels (30). The entire skin-covered dressing style, limited outdoor activities due to the summer heat, limited vitamin D fortification, and dietary habits might explain the extremely low vitamin D concentrations in Middle East countries (31–34). In addition, although serum or plasma 25(OH)D concentrations are considered to be the most reliable biomarkers for determining vitamin D status (3), it is often difficult to monitor or obtain data of 25(OH)D in populations in low- and middle-income countries, which would hamper the effort to evaluate vitamin D status. It is expected that these studies on vitamin D will draw the attention of the relevant authorities in these countries and lead to the adoption of necessary actions.
Research focuses
According to the results of co-occurring keywords (Figure 4) and keyword cluster (Figure 5), it is illustrated that the relationship between vitamin D and respiratory infections, especially respiratory viruses and the more recently coronaviruses, has received a lot of attention in the last two decades, suggesting that this is a hot issue for research. In addition, as shown in Figure 6, the research hotspots in this area have evolved in the last decade. 10 years ago, there was concern about the relevance of vitamin D to specific diseases (such as HIV infections, tuberculosis) and associated mechanisms (such as “sustained virological response,” “nf kappa b,” and “d receptor polymorphism”). The maturation of research in this area will lead to increasing concerns of researchers regarding the use of vitamin D for diseases' prevention or treatment. Therefore, it is not surprising that “randomized controlled trials” and “placebo controlled trials” have become a hot topic of study (Figure 6), indicating that researchers were beginning to investigate the efficacy of vitamin D applied to clinical diseases.
The most cited article (Table 2) published in Science by Liu and colleagues discussed the role of vitamin D in the treatment of tuberculosis (TB). It was demonstrated that Toll-like receptor (TLR) activation of human macrophages was followed by the upregulation of VDR and vitamin D-1 hydroxylase gene expression, resulting in cathelicidin induction and intracellular killing of Mycobacterium tuberculosis (35). The research further suggested that, for the first time, the increased susceptibility to tuberculosis in African Americans was associated with low serum levels of 25(OH)D, which was insufficient to sustain the induction of antimicrobial peptide cathelicidin messenger RNA. Other in vitro findings revealed that calcitriol mediated the response of the host to Mycobacterium tuberculosis infection by inducing reactive oxygen intermediate (36), and the antimicrobial peptide cathelicidin (37) that triggers autophagy (38).
Based on the results of many such studies, researchers have maintained a lively interest in the relationship between vitamin D and tuberculosis for the past two decades. Interestingly, these clinical trials yielded very different conclusions. A recent study in Indonesia showed that compared to the placebo group, fever and cough of TB subsided faster in the vitamin D supplementation group (39). Meanwhile, a meta-analysis that summarized the effect of vitamin D supplementation on the prognosis of patients with pulmonary TB considered it a combination therapy (40). However, another recent randomized controlled trial (RCT) conducted for 3 years showed that vitamin D supplementation did not reduce the risk of TB infection or TB disease compared to placebo among Mongolian schoolchildren who were vitamin D-deficient (41). Not only that, but also the polymorphism of VDR correlated with study results. Calcitriol regulates the immune response via binding to the VDR which is expressed aboard antigen-presenting cells and active lymphocytes, thereby modulating the transcription of vitamin D-responsive genes (8). Of note, human VDR carrying the t allele of the TaqI VDR polymorphism or the f allele of the FokI VDR polymorphism associates with different or even opposite performance in Mycobacterium infection (42). One of the multi-center RCTs conducted by Martineau and colleagues showed that adjunctive high-dose vitamin D3 (2.5mg per dose, four times in total) reduced the time to sputum culture conversion in adult TB patients with TaqI VDR polymorphism (43). Other RCTs in Mongolian adults showed that in patients carrying one or more minor variations in the gene encoding VDR, adjuvant vitamin D (one-time oral supplementation of 14,000 IU per week) speeded up the conversion of sputum cultures (44). However, in the entire study population, the supplementation of vitamin D presented no effect on the time to sputum culture conversion (44). Research on this topic continues and is controversial, which may partly explain why the keywords “d receptor” and “d receptor polymorphism” have drawn extensive attention in the last few decades (Table 3 and Figure 6).
At present, TB still ranks as the 13th cause of death and the second leading infectious disease contributor, only second to COVID-19 globally (45). In 2020, a population of 1.5 million people died from TB (45). Countries with a high TB burden accounted for the vast majority of new TB cases, with India leading the way, followed by other developing countries such as China, Indonesia, Pakistan, and South Africa. It is not surprising, therefore, that there has been a boost in the research in these countries recently (Figure 3), and we should be pleased about this. If the impact could be demonstrated in larger-scale studies, the public health implications would be clarified, as improved vitamin D status could improve innate immunity and contribute to the prevention and treatment of TB infection.
Besides TB, the relationship between vitamin D and acute respiratory infections (ARIs) has also been extensively discussed (Figures 4, 5). Several studies of RCTs reveal that vitamin D supplementation has a protective effect against influenza (46, 47). The sixth-ranked highly cited article (presented in Table 2) showed that serum 25(OH)D levels were inversely associated with acute upper respiratory tract infection (48). The association may be more significant in patients with respiratory diseases such as asthma and chronic obstructive pulmonary disease (48). Furthermore, it was suggested that children with low vitamin D status were related to a significantly higher risk of admission to the intensive care unit (ICU) and invasive mechanical ventilation (49). The underlying mechanisms of vitamin D against respiratory viral infections involve antiviral and anti-inflammatory effects, such as increased viral killing, reduced pro-inflammatory cytokine production, and protection of the integrity of tight junctions, thus keeping immune cells from invading lungs (50, 51). The second most cited literature (Table 2) also demonstrated that vitamin D deficiency was associated with an elevated risk of occurring ARIs (20). The systematic review and meta-analysis indicated that vitamin D supplementation was safe and could prevent acute respiratory infections on the whole. Amazingly, a daily or weekly regimen was more efficient than a one-time injection (20). Moreover, patients with extreme vitamin D deficiency and those who did not receive high doses of vitamin D benefited the most (20). More importantly, in 2021, researchers updated the meta-analysis of aggregated data from 48,488 participants with an age range from 0 to 95 years. The data again reported a small but significant beneficial effect of vitamin D supplementation on the association with the risk of one or more ARIs compared to placebo (52). Meanwhile, the protective effect of vitamin D was relevant to a daily dose of 400–1,000 IU for about 12 months in the 1–15.9 year age groups while was independent of different baseline 25(OH)D concentrations (52), which was in contrast to the previous findings.
Similar to the controversial relationship between TB and vitamin D, there are many different voices in the debate about the association of ARIs with vitamin D. A recent RCT showed that in young healthy Canadian children with a high 25(OH)D baseline, the high-dose vitamin D oral supplementation group (2,000 IU/day) lacked an effect on the incidence of upper respiratory tract infections compared with the regular-dose group (400 IU/day) (53). A large, double-blind, placebo-controlled D-Health Trial conducted for 5 years in Australia suggested that oral vitamin D3 (60,000 IU per month) failed to influence the incidence of upper respiratory infections (54). However, there was some benefit of taking vitamin D that patients receiving vitamin D had fewer days (0.5 days) of symptoms than those in the control group (54). Certainly, the researchers also concluded that the difference, while statistically significant, was of unclear clinical meaning. The protective effect of vitamin D remains controversial even in groups with severe vitamin D deficiency (41, 55). Since ARIs are prevalent in children younger than 5 years old (56) and lower respiratory tract infections are one of the leading causes of death in these children (57), more research is required and worth continuing in future.
Research at the frontier and in future
As discussed in the above section, the implications of vitamin D supplementation for respiratory infections have been widely addressed, with both proponents and opponents holding their views. The emerging COVID-19 outbreak further escalates the debate. Figure 6 also confirms the trend that since 2020, research related to COVID-19 is undoubtedly the hottest topic.
Since its outbreak, COVID-19 has been of great concern worldwide. The disease is a severe lower respiratory tract viral infection which is caused by a highly infectious, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It is demonstrated that vitamin D possesses anti-inflammatory and antioxidant characteristics against COVID-19 infection (58). In vitro findings revealed that calcitriol exhibited antiviral activity toward SARS-CoV-2. Another important study showed that vitamin D attenuated lipopolysaccharide-induced acute lung injury through the renin–angiotensin system (RAS) by regulating the expression of angiotensin-converting enzyme 2 (ACE2) in rats (59). Lower 25(OH)D and 1,25(OH)2D levels were independently associated with upregulation of RAS activity and angiotensin 2 concentrations (60). The excessive level of RAS activation is related to a poorer prognosis of COVID-19 (61). As displayed in Table 5, the tenth-ranked article summarizes the role of vitamin D in the pathogenesis of SARS-CoV-2 and specifically highlights its modulation of the immune dysfunctional response following cytokine storm in critically ill patients (62). The multiple mechanisms by which vitamin D modulates the immune system include inhibition of SARS-CoV-2 access and replication, reduction in pro-inflammatory cytokine concentrations, increased anti-inflammatory cytokine levels, enhanced natural antimicrobial peptide production, and activation of defense cells capable of destroying SARS-CoV-2, such as macrophages (62). Since there has been a lot of research on this subject, it is understandable that keywords related to it are popular in last 2 years (Figure 6).
Interestingly, in contrast to other respiratory viral infections that tend to be prevalent in children, severe COVID-19 cases are less likely to be seen in infants and young children (63, 64). In the top-cited article of 2021 (Table 5), Zimmermann and colleagues reviewed this issue, suggesting that vitamin D was one of the important influencing factors (65). In most countries, vitamin D supplementation is routinely taken by children or infants, while vitamin D deficiency is more common in the elderly (66). According to the investigation from 2011 to 2014, the prevalence of deficient and inadequate risk of vitamin D in the United States was lowest in kids aged 1–5 years (23).
Researchers were also interested in the correlation between low vitamin D levels and the severity, incidence, and mortality of COVID-19. Of the highly cited articles in 2021 (Table 5), five out of 10 are on this issue (ranked third (67), fourth (68), sixth (69), seventh (70), and 10 (71), respectively) but there are certain differences in their conclusions. In general, the association between lower vitamin D status and COVID-19 is affirmed and it is further acknowledged that vitamin D deficiency may increase the risk of COVID-19 incidence (67–71). However, the correlation between vitamin D and the severity of COVID-19 is conflicting. The meta-analysis conducted by Kazemiand and colleagues suggested that although the results on the relationship between vitamin D deficiency and ICU admissions, pulmonary comorbidities, and hospitalizations were not consistent among studies, most of them showed a positive correlation between 25(OH)D and COVID-19 severity and mortality (71). Cheng and colleagues also indicated that vitamin D deficiency affected COVID-19 hospitalization and severity among the Chinese population (70). However, other two studies (67, 69) took the opposite view that the severity of COVID-19 and vitamin D deficiency was not relevant. It was also suggested that vitamin D supplementation may be protective against COVID-19-related ICU admissions (69), especially in frail older adults (72–74), although more solid evidence was needed as also suggested by previous bibliometric analysis (75).
In fact, the debate between these articles is also a microcosm of the research field's controversy. At present, the correlation between vitamin D deficiency and COVID-19 is still disputed, with some studies suggesting that these two are irrelevant (76–78). A systematic review and meta-analysis conducted by Ghasemian and colleagues showed that there was no significant association between vitamin D status and higher mortality rates of COVID-19 (79). In the multi-center RCT performed by Murai and colleagues, a single high oral dose of 200,000 IU cholecalciferol did not lead to a significant reduction in the hospital stay of patients with moderate-to-severe COVID-19 compared to placebo (80). Notably, participants in this study received different concomitant medications and took vitamin D for a longer period of time (mean 10.3 days) after the onset of symptoms. Therefore, it is uncertain whether the null result is related to this delayed treatment.
Actually, many variables seem to contribute to the inconsistency and discrepancy of the complicated role of vitamin D in infections. Factors, such as variations in in vitro and in vivo studies, different sample sizes and ages, different clinical trial designs, and different supplementation dose regimens, may account for the controversial results of vitamin D in the prevention and treatment of infectious diseases. It should be aware that there are fundamental differences between vitamin D RCT designs versus drug RCT designs (81, 82). One important point is that any conclusions about the health benefits of a certain dose of vitamin D supplementation must be informed by the baseline 25(OH)D concentrations in the study population and the vitamin D status achieved after treatment (81). In addition, since there are so many factors that affect vitamin D, the body concentration of vitamin D might vary, for example, seasonally. Moreover, the definition, threshold, and indicators of vitamin D deficiency also vary between countries and organizations (83). Another underestimated factor is the impact of DBP. The circulating DBP level is variable, and changes in DBP levels may affect the assessment of vitamin D status. A recent study demonstrated that while total 25(OH)D levels were significantly lower in critically ill patients, the calculated free 25(OH)D concentrations were not decreased compared to controls. Therefore, measuring only the total 25(OH)D concentration may lead to an underestimation of vitamin D status and an overestimation of the number of patients with vitamin D deficiency (84). Meanwhile, DBP polymorphisms may be associated with COVID-19 prevalence and mortality (85). Median plasma concentrations of 25(OH)D also depend on DBP polymorphisms (86). Further studies are needed to investigate the association between DBP and vitamin D status. In general, despite some developments, our knowledge in this area still lags far behind. The mechanism of vitamin D involvement in the immune system is not fully elucidated. Therefore, the deciphering of the causality of vitamin D in the development of infections remains challenging. Although we are glad to see that new data on the health benefits of vitamin D continue to emerge in this field, more hypothesis-driven studies are required in future.
Developments in this field have also led to the updating of vitamin D guidelines. In June 2020, the Scientific Advisory Committee on Nutrition (SACN) released a rapid review on vitamin D and acute respiratory tract infections and concluded that the evidence was insufficient to support vitamin D supplementation to specifically prevent ARIs in the general UK population. Interestingly, in December 2020, SACN updated the rapid review and concluded that “there may be some benefit from daily, low-dose vitamin D supplementation” in reducing risk of ARIs (87). These recommendations are also consistent with the UK government guidelines launched on 22 December 2020, granting people at high risk from COVID-19 the option to receive 4 months of daily 10 microgram (400 IU) vitamin D supplements for free (88).
It should be aware that vitamin D deficiency is relatively common in individuals of all age groups worldwide (89). Dietary patterns, mandatory supplementation strategies, age, latitude, urbanization, air pollution, sunscreen usage, lifestyle, skin pigmentation, and genetic factors are all associated with vitamin D status (13, 22, 89, 90). Interestingly, studies showed that vitamin D did not impact the immune effect of flu vaccines (91, 92). Thus, vitamin D supplements may be more convenient and acceptable as a way to prevent the flu than medications and flu vaccines because of their safety and many other benefits for healthy skeletons. Moreover, despite the tremendous efforts of frontline health workers, morbidity and mortality of COVID-19 continue to rise globally. While in an ideal world all health decisions should be grounded on overwhelming proof, times of crisis may require a slightly different set of rules as well as more prompt judgment, and certainly, solid evidence remains a necessity.
Considering the wide range of populations affected by vitamin D deficiency, the multiple diseases associated with it, and the relatively simple means of supplementation, it is still worthwhile to continue research in this area. Even a small experimental benefit will be of significant public health importance in a large-scale population. Special attention should also be paid to those who are prone to vitamin D deficiency, many of whom are associated with poorer clinical outcomes. Authorities should be aware of the issues and take initiatives to improve the health status of the population and consequently reduce the burden on health care resources and society.
Strengths and limitations
CiteSpace is not a complete replacement for systematic retrieval and still has certain limitations to be addressed. First, we obtained the literature data through the WoSCC database, but with the constant updating of the database, there is some discrepancy between the results of this study and the actual number of literature available now. Second, this study included only articles and reviews, and the quality of the literature collected was mixed. Third, only English papers were selected for this study. The above reasons may render our analysis not so comprehensive. Besides some limitations, the literature-based visual analysis still certainly provides a basis for scholars to understand the research objects, hotspots, and trends in the field of vitamin D and infection rapidly.
Conclusion
Although the role of vitamin D on bone health has long been widely recognized, its ability to modulate immune responses and attenuate acute infectious processes has been emphasized in the last 20 years. Our article presents the developments in the field over the past two decades, from the impact of vitamin D on pathogenesis to the effects on therapeutic outcomes in various infectious diseases. Our results indicate the significant contribution of developed Western countries in this field, as well as the increasing number of countries/regions engaged in the subject of research. Despite numerous encouraging and promising findings, there is not yet a consensus on the role of vitamin D in the prevention and control of infectious diseases. As the COVID-19 epidemic keeps escalating, the overall context of the immunomodulatory effects of vitamin D in infections deserves further investigation.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XL had the idea for the article and collected data. XL and YD performed the search and analysis. WH prepared the draft of the manuscript. XL critically revised the work. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by the Scientific Research Project of Hunan Provincial Health Commission (202117010086) and Natural Science Foundation of Hunan Province, China (Grant No. 2021JJ40832).
conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Holick MF. Cancer, sunlight and vitamin D. J Clin Transl Endocrinol. (2014) 1:179–86. 10.1016/j.jcte.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. (2004) 80(Suppl. 6):1689S−96. 10.1093/ajcn/80.6.1689S [DOI] [PubMed] [Google Scholar]
- 3.Seamans KM, Cashman KD. Existing and potentially novel functional markers of vitamin D status: a systematic review. Am J Clin Nutr. (2009) 89:1997S−2008. 10.3945/ajcn.2009.27230D [DOI] [PubMed] [Google Scholar]
- 4.Bikle DD, Schwartz J. Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front Endocrinol. (2019) 10:317. 10.3389/fendo.2019.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yousefzadeh P, Shapses SA, Wang X. Vitamin D binding protein impact on 25-hydroxyvitamin D levels under different physiologic and pathologic conditions. Int J Endocrinol. (2014) 2014:981581. 10.1155/2014/981581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, Haddad JG, et al. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Investig. (1999) 103:239–51. 10.1172/JCI5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Speeckaert MM, Speeckaert R., Geel Nv, Delanghe. Vitamin D binding protein: a multifunctional protein of clinical importance Adv Clin Chem. (2014) 63:1–57. 10.1016/B978-0-12-800094-6.00001-7 [DOI] [PubMed] [Google Scholar]
- 8.Szymczak I, Pawliczak R. The active metabolite of vitamin D3 as a potential immunomodulator. Scand J Immunol. (2016) 83:83–91. 10.1111/sji.12403 [DOI] [PubMed] [Google Scholar]
- 9.Hossein-nezhad A, Spira A, Holick MF. Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: a randomized double-blind clinical trial. PLoS ONE. (2013) 8:58725. 10.1371/journal.pone.0058725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirvani A, Kalajian TA, Song A, Holick MF. Disassociation of vitamin D's calcemic activity and non-calcemic genomic activity and individual responsiveness: a randomized controlled double-blind clinical trial. Sci Rep. (2019) 9:17685. 10.1038/s41598-019-53864-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ. Vitamin D: metabolism. Endocrinol Metab Clin North Am. (2010) 39:243–53. 10.1016/j.ecl.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev. (2019) 40:1109–51. 10.1210/er.2018-00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JA, Mahdi AA, Sharma D, Karunanand B, et al. Blood lead levels in occupationally exposed workers involved in battery factories of Delhi-NCR region: effect on vitamin D and calcium metabolism Indian. J Clin Biochem. (2020) 35:80–7. 10.1007/s12291-018-0797-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Fang F, Tang J, Jia L, Feng Y, Xu P, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. (2019) 366:4673. 10.1136/bmj.l4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang TJ. Vitamin D and cardiovascular disease. Annu Rev Med. (2016) 67:261–72. 10.1146/annurev-med-051214-025146 [DOI] [PubMed] [Google Scholar]
- 16.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. (2004) 80(Suppl. 6):1678S−88. 10.1093/ajcn/80.6.1678S [DOI] [PubMed] [Google Scholar]
- 17.Emerging Risk Factors Collaboration/EPIC-CVD/vitamin D studies collaboration . Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: observational and Mendelian randomisation analyses. Lancet Diabetes Endocrinol. (2021) 9:837–46. 10.1016//S2213-8587(21)00263-1 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ. (2014) 349:6330. 10.1136/bmj.g6330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kempker JA, Tangpricha V, Ziegler TR, Martin GS. Vitamin D in sepsis: from basic science to clinical impact. Critical Care. (2012) 16:316. 10.1186/cc11252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. (2017) 356:6583. 10.1136/bmj.i6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vassiliou AG, Jahaj E, Orfanos SE, Dimopoulou I, Kotanidou A. Vitamin D in infectious complications in critically ill patients with or without COVID-19. Metabol Open. (2021) 11:100106. 10.1016/j.metop.2021.100106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cashman KD, Dowling KG, Skrabakova Z, Gonzalez-Gross M, Valtuena J, Henauw SD, et al. Vitamin D deficiency in Europe: pandemic?. Am J Clin Nutr. (2016) 103:1033–44. 10.3945/ajcn.115.120873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrick KA, Storandt RJ, Afful J, Pfeiffer CM, Schleicher RL, Gahche JJ, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. (2019) 110:150–7. 10.1093/ajcn/nqz037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roth DE, Abrams SA, Aloia J, Bergeron G, Bourassa MW, Brown KH, et al. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low- and middle-income countries. Ann N Y Acad Sci. (2018) 1430:44–79. 10.1111/nyas.13968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lips P. Jongh RTd, Schoor NMv. Trends in vitamin D status around the world. JBMR Plus. (2021) 5:10585. 10.1002/jbm4.10585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C, Song M. Visualizing a field of research: a methodology of systematic scientometric reviews. PLoS ONE. (2019) 14:0223994. 10.1371/journal.pone.0223994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang A, Lv Q, Chen F, Wang D, Liu Y, Shi W. Identification of recent trends in research on vitamin D: A quantitative and co-word analysis. Med Sci Monit. (2019) 25:643–55. 10.12659/MSM.913026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanat BB, Yavuzer H. Top 100 articles on vitamin D: Bibliometric versus altmetric analysis. Bratisl Lek Listy. (2022) 123:160–71. 10.4149/BLL_2022_027 [DOI] [PubMed] [Google Scholar]
- 29.Deluca HF. History of the discovery of vitamin D and its active metabolites. Bonekey Rep. (2014) 3:479. 10.1038/bonekey.2013.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallah EM, Hamad MF, Elmanaseer MA, Qinna NA, Idkaidek NM, Arafat TA, et al. Plasma concentrations of 25-hydroxyvitamin D among Jordanians: effect of biological and habitual factors on vitamin D status. BMC Clin Pathol. (2011) 11:8. 10.1186/1472-6890-11-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaikh AMA, Abaalkhail B, Soliman A, Kaddam I, Aseri K, Saleh YA, et al. Prevalence of vitamin D deficiency and calcium homeostasis in Saudi children. J Clin Res Pediatr Endocrinol. (2016) 8:461–7. 10.4274/jcrpe.3301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golbahar J, Al-Saffar N, Diab DA, Al-Othman S, Darwish A, Al-Kafaji G. Predictors of vitamin D deficiency and insufficiency in adult Bahrainis: a cross-sectional study. Public Health Nutr. (2014) 17:732–8. 10.1017/S136898001300030X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ardawi MM, Sibiany AM, Bakhsh TM, Qari MH, Maimani AA. High prevalence of vitamin D deficiency among healthy Saudi Arabian men: relationship to bone mineral density, parathyroid hormone, bone turnover markers, and lifestyle factors. Osteoporos Int. (2012) 23:675–86. 10.1007/s00198-011-1606-1 [DOI] [PubMed] [Google Scholar]
- 34.Hwalla N, Dhaheri ASA, Radwan H, Alfawaz HA, Fouda MA, Al-Daghri NM, et al. The prevalence of micronutrient deficiencies and inadequacies in the Middle East and approaches to interventions. Nutrients. (2017) 9:229. 10.3390/nu9030229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. (2006) 311:1770–3. 10.1126/science.1123933 [DOI] [PubMed] [Google Scholar]
- 36.Sly LM, Lopez M, Nauseef WM, Reiner NE. 1alpha,25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Bio Chem. (2001) 276:35482–93. 10.1074/jbc.M102876200 [DOI] [PubMed] [Google Scholar]
- 37.Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. (2007) 178:7190–8. 10.4049/jimmunol.178.11.7190 [DOI] [PubMed] [Google Scholar]
- 38.Yuk J, Shin D, Lee H, Yang C, Jin HS, Kim K, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. (2009) 6:231–43. 10.1016/j.chom.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 39.Tamara L, Kartasasmita CB, Alam A, Gurnida DA. Effects of vitamin D supplementation on resolution of fever and cough in children with pulmonary tuberculosis: a randomized double-blind controlled trial in Indonesia. J Glob Health. (2022) 12:04015. 10.7189/jogh.12.04015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu H, Xiong X, Zhu M, Wei J, Zhuo K, Cheng D. Effects of vitamin D supplementation on the outcomes of patients with pulmonary tuberculosis: a systematic review and meta-analysis. BMC Pulm Med. (2018) 18:108. 10.1186/s12890-018-0677-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganmaa D, Uyanga B, Zhou X, Gantsetseg G, Delgerekh B, Enkhmaa D, et al. Vitamin D supplements for prevention of tuberculosis infection and disease. N Engl J Med. (2020) 383:359–68. 10.1056/NEJMoa1915176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roth DE, Soto G, Arenas F, Bautista CT, Ortiz J, Rodriguez R, et al. Association between vitamin D receptor gene polymorphisms and response to treatment of pulmonary tuberculosis. J Infect Dis. (2004) 190:7–920. 10.1086/423212 [DOI] [PubMed] [Google Scholar]
- 43.Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. (2011) 377:242–50. 10.1016/S0140-6736(10)61889-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ganmaa D, Munkhzul B, Fawzi W, Spiegelman D, Willett WC, Bayasgalan P, et al. High-dose vitamin D3 during tuberculosis treatment in Mongolia. A randomized controlled trial. Am J Respir Crit Care Med. (2017) 196:628–37. 10.1164/rccm.201705-0936OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization . Tuberculosis. https://www.who.int/en/news-room/fact-sheets/detail/tuberculosis (2022). (accessed April 28, 2022).
- 46.Zhu Z, Zhu X, Gu L, Zhan Y, Chen L, Li X. Association between vitamin D and influenza: meta-analysis and systematic review of randomized controlled trials. Front Nutr. (2022) 8:799709. 10.3389/fnut.2021.799709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou J, Du J, Huang L, Wang Y, Shi Y, Lin H. Preventive effects of vitamin D on seasonal influenza A in Infants: a multicenter, randomized, open, controlled clinical trial. J Pediatr Infect Dis. (2018) 37:749–54. 10.1097/INF.0000000000001890 [DOI] [PubMed] [Google Scholar]
- 48.Ginde AA, Mansbach JM, Camargo CAJ. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the third national health and nutrition examination survey. Arch Intern Med. (2009) 169:384–90. 10.1001/archinternmed.2008.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hurwitz JL, Jones BG, Penkert RR, Gansebom S, Sun Y, Tang L, et al. Low retinol-binding protein and vitamin D levels are associated with severe outcomes in children hospitalized with lower respiratory tract infection and respiratory syncytial virus or human metapneumovirus detection. J Pediatr. (2017) 187:323–7. 10.1016/j.jpeds.2017.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. (2015) 7:4240–70. 10.3390/nu7064240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khare D, Godbole NM, Pawar SD, Mohan V, Pandey G, Gupta S, et al. Calcitriol [1, 25[OH]2 D3] pre- and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cells. Eur J Nutr. (2013) 52:1405–15. 10.1007/s00394-012-0449-7 [DOI] [PubMed] [Google Scholar]
- 52.Jolliffe DA, Camargo CAJ, Sluyter JD, Aglipay M, Aloia JF, Ganmaa D, et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. (2021) 9:276–92. 10.1136/thorax-2020-BTSabstracts.105 [DOI] [PubMed] [Google Scholar]
- 53.Aglipay M, Birken CS, Parkin PC, Loeb MB, Thorpe K, Chen Y, et al. Effect of high-dose vs standard-dose wintertime vitamin D supplementation on viral upper respiratory tract infections in young healthy children. JAMA. (2017) 318:245–54. 10.1001/jama.2017.8708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pham H, Waterhouse M, Baxter C, Romero BD, McLeod DSA, Armstrong BK, et al. The effect of vitamin D supplementation on acute respiratory tract infection in older Australian adults: an analysis of data from the D-Health Trial. Lancet Diabetes Endocrinol. (2021) 9:69–81. 10.1016/S2213-8587(20)30380-6 [DOI] [PubMed] [Google Scholar]
- 55.Camargo CA, Sluyter J, Stewart AW, Khaw K, Lawes CMM, Toop L, et al. Effect of monthly high-dose vitamin D supplementation on acute respiratory infections in older adults: a randomized controlled trial. Clin Infect Dis. (2020) 71:311–7. 10.1093/cid/ciz801 [DOI] [PubMed] [Google Scholar]
- 56.Simoes EAF, Cherian T, Chow J, et al. “Acute respiratory infections in children,” In: Jamison DT, Breman JG, Measham AR, et al, editors. Disease Control Priorities in Developing Countries 2nd edition Washington, DC: The International Bank for Reconstruction and Development/The World Bank. (2006). [PubMed] [Google Scholar]
- 57.GBD 2017 Causes of death collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1736–88. 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gimenez VMM, Inserra F, Tajer CD, Mariani J, Ferder L, Reiter RJ, et al. Lungs as target of COVID-19 infection: Protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment. Life Sci. (2020) 254:117808. 10.1016/j.lfs.2020.117808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H. Vitamin D alleviates lipopolysaccharideinduced acute lung injury via regulation of the reninangiotensin system. Mol Med Rep. (2017) 16:7432–8. 10.3892/mmr.2017.7546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, et al. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chim Acta. (2010) 411:1354–60. 10.1016/j.cca.2010.05.037 [DOI] [PubMed] [Google Scholar]
- 61.Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. (2020) 24:422. 10.1186/s13054-020-03120-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumar R, Rathi H, Haq A, Wimalawansa SJ, Sharma A. Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID-19. Virus Res. (2021) 292:198235. 10.1016/j.virusres.2020.198235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. (2020) 174:882–9. 10.1001/jamapediatrics.2020.1467 [DOI] [PubMed] [Google Scholar]
- 64.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. (2020) 109:1088–95. 10.1111/apa.15270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. (2020). 10.1136/archdischild-2020-320338 [DOI] [PubMed] [Google Scholar]
- 66.Mosekilde L. Vitamin D and the elderly. Clin Endocrinol. (2005) 62:265–81. 10.1111/j.1365-2265.2005.02226.x [DOI] [PubMed] [Google Scholar]
- 67.Hernandez JL, Nan D, Fernandez-Ayala M, Garcia-Unzueta M, Hernandez-Hernandez MA, Lopez-Hoyos M, et al. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metabol. (2021) 106:1343–53. 10.1210/clinem/dgaa733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu N, Sun J, Wang X, Zhang T, Zhao M, Li H. Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis. Int J Infect Dis. (2021) 104:58–64. 10.1016/j.ijid.2020.12.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bassatne A, Basbous M, Chakhtoura M, Zein OE, Rahme M, Fuleihan GE. The link between COVID-19 and VItamin D (VIVID): A systematic review and meta-analysis. Metab Clin Exp. (2021) 119:154753. 10.1016/j.metabol.2021.154753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo X, Liao Q, Shen Y, Li H, Cheng L. Vitamin D deficiency Is associated with COVID-19 incidence and disease severity in chinese people [corrected]. J Nutr. (2021) 151:103–98. 10.1093/jn/nxaa332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kazemi A, Mohammadi V, Aghababaee SK, Golzarand M, Clark CCT, Babajafari S. Association of vitamin D status with SARS-CoV-2 infection or COVID-19 severity: a systematic review and meta-analysis. Adv Nutr. (2021) 12:1636–58. 10.1093/advances/nmab012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giannini S, Passeri G, Tripepi G, Sella S, Fusaro M, Arcidiacono G, et al. Effectiveness of in-hospital cholecalciferol use on clinical outcomes in comorbid COVID-19 patients: a hypothesis-generating study. Nutrients. (2021) 13:219. 10.3390/nu13010219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castillo ME, Costa LME. Barrios JMV, Diaz JFA, Miranda JL, Bouillon R, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. (2020) 203:105751. 10.1016/j.jsbmb.2020.105751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gonen MS, Alaylioglu M, Durcan E, Ozdemir Y, Sahin S, Konukoglu D, et al. Rapid and effective vitamin D supplementation may present better clinical outcomes in COVID-19 (SARS-CoV-2) patients by altering serum INOS1, IL1B, IFNg, Cathelicidin-LL37, and ICAM1. Nutrients. (2021) 13:4047. 10.3390/nu13114047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dayal D, Gupta B, Surulinathi M, Nanda P. Covid-19 and vitamin D deficiency: a scientometric assessment of global publications during 2020-21. J Young Pharm. (2021) 13:89–S94. 10.5530/jyp.2021.13s.77 [DOI] [Google Scholar]
- 76.Brandao CMA, Chiamolera MI, Biscolla RPM, Lima JVJ, Ferrer CMDF, Prieto WH, et al. No association between vitamin D status and COVID-19 infection in São Paulo, Brazil. Arch Endocrinol Metab. (2021) 65:381–5. 10.20945/2359-3997000000343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jevalikar G, Mithal A, Singh A, Sharma R, Farooqui KJ, Mahendru S, et al. Lack of association of baseline 25-hydroxyvitamin D levels with disease severity and mortality in Indian patients hospitalized for COVID-19. Sci Rep. (2021) 11:6258. 10.1038/s41598-021-85809-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Osman W, Fahdi FA, Salmi IA, Khalili HA, Gokhale A, Khamis F. Serum calcium and vitamin D levels: correlation with severity of COVID-19 in hospitalized patients in royal hospital, Oman. Int Journal Infect Dis. (2021) 107:153–63. 10.1016/j.ijid.2021.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghasemian R, Shamshirian A, Heydari K, Malekan M, Alizadeh-Navaei R, Ebrahimzadeh MA, et al. The role of vitamin D in the age of COVID-19: a systematic review and meta-analysis. Int J Clin Pract. (2021) 75:e14675. 10.1111/ijcp.14675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CSC, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. (2021) 325:1053–60. 10.1001/jama.2020.26848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heaney RP. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev. (2014) 72:48–54. 10.1111/nure.12090 [DOI] [PubMed] [Google Scholar]
- 82.Pilz S, Trummer C, Theiler-Schwetz V, Grubler MR, Verheyen ND, Odler B, et al. Critical appraisal of large vitamin D randomized controlled trials. Nutrients. (2022) 14:303. 10.3390/nu14020303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinology. (2017) 13:466–79. 10.1038/nrendo.2017.31 [DOI] [PubMed] [Google Scholar]
- 84.Palmer D, Soule S, Gaddam RR, Elder P, Chambers S, Doogue M. Unbound vitamin D concentrations are not decreased in critically ill patients. Intern Med J. (2022) 52:89–94. 10.1111/imj.15096 [DOI] [PubMed] [Google Scholar]
- 85.Batur LK, Hekim N. The role of DBP gene polymorphisms in the prevalence of new coronavirus disease 2019 infection and mortality rate. J Med Virol. (2021) 93:1409–13. 10.1002/jmv.26409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lordan R. Notable developments for vitamin D Amid the COVID-19 pandemic, but caution warranted overall: a narrative review. Nutrients. (2021) 13:740. 10.3390/nu13030740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scientific Advisory Committee on Nutrition (SACN) . SACN Rapid Review: Vitamin D and Acute Respiratory Tract Infections. https://www.gov.uk/government/publications/sacn-rapid-review-vitamin-d-and-acute-respiratory-tract-infections (2020). (accessed June 16, 2022)
- 88.Department of Health &Social Care. Vitamin D and Clinically Extremely Vulnerable (CEV) Guidance. (2021). https://www.gov.uk/government/publications/vitamin-d-for-vulnerable-groups/vitamin-d-and-clinically-extremely-vulnerable-cev-guidance. (accessed April 28, 2022)
- 89.Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. (2014) 144 Pt A:138–45. 10.1016/j.jsbmb.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goswami R, Gupta N, Goswami D, Marwaha RK, Tandon N, Kochupillai N. Prevalence and significance of low 25-hydroxyvitamin D concentrations in healthy subjects in Delhi. Am J Clin Nutr. (2000) 72:472–5. 10.1093/ajcn/72.2.472 [DOI] [PubMed] [Google Scholar]
- 91.Goncalves-Mendes N, Talvas J, Duale C, Guttmann A, Corbin V, Marceau G, et al. Impact of vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: a randomized placebo-controlled trial. Front Immunol. (2019) 10:65. 10.3389/fimmu.2019.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee M, Lin C, Lei W, Chang H, Lee H, Yeung C, et al. Does vitamin D deficiency affect the immunogenic responses to influenza vaccination? A systematic review and meta-analysisNutrients. (2018) 10:409. 10.3390/nu10040409 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.