Abstract
Intracellular hydrogen peroxide is regulated in Escherichia coli by OxyR in response to the metabolic production of H2O2. Here, we show that the untranslated oxyS RNA controlled by OxyR has a role in this regulation. The oxyS transcript appears to affect the metabolic output of H2O2 rather than the removal of H2O2 by catalases-hydroperoxidases.
The intracellular steady-state concentration of hydrogen peroxide (H2O2) depends on its rates of generation and dismutation. The major source of superoxide (O2−) and H2O2 in Escherichia coli cells is the respiratory chain, which accounts for as much as 90% of the total production of H2O2 (10, 12). Even though the metabolic generation of H2O2 varies >10-fold during growth, E. coli cells maintain the intracellular concentration of hydrogen peroxide within a narrow range (0.20 ± 0.05 μM). This regulation is accomplished by a continuous variation in the degree of activation of the OxyR protein, which in turn governs transcription of katG, the gene encoding catalase-hydroperoxidase I (catalase–HP-I) (8).
Although catalase–HP-I constitutes the major detoxification system for endogenous H2O2 in exponential-phase E. coli cultures (8, 14), other regulated activities are involved in controlling H2O2 levels: ΔoxyR strains have a higher concentration of H2O2 and are more susceptible to exogenous oxidative stress than strains mutated only in katG (8).
In addition to catalase–HP-I, OxyR controls the expression of seven to eight other proteins in Salmonella typhimurium and E. coli (4, 11, 19). One of these, the ahpFC-encoded alkyl hydroperoxide reductase, was reported to react with H2O2 in vitro (20), but the activity did not contribute measurably to the regulation of the H2O2 concentration in vivo (8). Another member of the OxyR regulon, oxyS, was recently reported to have posttranscriptional regulatory functions (1). Strains overexpressing oxyS had a reduced rate of spontaneous and H2O2-induced mutagenesis, and cells carrying a deletion had near-wild-type resistance to challenge with exogenous H2O2 (1). These results prompted us to evaluate the possible role of oxyS in H2O2 homeostasis.
Bacterial strains and experimental procedures.
The strains of E. coli used in this study are listed in Table 1. Strains BGF416 and BGF420 were constructed by transduction (18) of the ΔoxyS2::Cm allele from strain GS035 into strains AB1157 and BGF611, respectively. Plasmid poxyS has the oxyS gene under the control of the tac promoter in a multicopy vector; even without induction by isopropyl-β-d-thiogalactoside, the level of the oxyS transcript expressed from poxyS is similar to the level seen in H2O2-treated wild-type E. coli (1). Plasmid psyxO has the gene in the reverse orientation. Cells were inoculated into Luria-Bertani (LB) broth (18) containing the appropriate antibiotic and incubated overnight at 37°C with gentle shaking (100 rpm). For experimental measurements, the saturated cultures were diluted 100-fold into fresh LB broth and incubated at 37°C for 3 h (optical density at 600 nm, ∼1). Antibiotics were used at the following concentrations (in micrograms per milliliter): tetracycline, 12.5; streptomycin, 50; chloramphenicol, 25; and ampicillin, 100. The intracellular concentration of H2O2 was assessed with peroxidase-mediated scopoletin oxidation as previously described (9). Total catalase activity was assayed by monitoring the disappearance of H2O2 at 240 nm in cell homogenates as described previously (8, 23) and normalized to the protein concentration determined with bovine serum albumin as the standard (15). The rate of H2O2 production was calculated from the experimental values for H2O2 and catalase concentrations as previously described (3, 8). The rate of O2− production was measured in membrane preparations by monitoring the superoxide dismutase (SOD)-sensitive rate of cytochrome c reduction at 550 nm (ɛreduced − ɛoxidized = 21 mM−1 cm−1) (2, 12). The reaction mixtures consisted of 50 mM potassium phosphate buffer (pH 7.4), 20 μM cytochrome c, 100 μM NADH, and membrane protein (∼0.2 mg/ml), with or without 50 U of bovine CuZn SOD. The rate of respiration by cell cultures was measured with a Clark-type electrode (5). Three-hour cultures were placed in the chamber of an oxygraph, and O2 concentration was monitored for 5 to 10 min. Values are expressed in nanoatoms of O2 per minute per 106 cells. The temperature was 37°C. The cellular sensitivity to H2O2 was assessed on plates. Three-hour cultures were plated on LB plates, and a filter disk (10-mm diameter, Whatman no. 1) was soaked with 150 mmol of H2O2 in water and placed in the center of the plate. After 12 to 16 h, the diameter of growth inhibition was measured (11). The frequency of spontaneous mutations to rifampin resistance was measured by plating on LB plates containing 125 μg of rifampin per ml; Rifr colonies were scored after 24 h of incubation at 37°C (11) and normalized to the number of cells plated (8).
TABLE 1.
Bacterial strains and plasmids of E. coli used in this study
| Strain or plasmid | Genotype or relevant characteristic | Source or reference |
|---|---|---|
| Strain | ||
| AB1157 | F−thr-1 leuB6 proA2 his-4 thi-1 argE2 lacY1 galK2 rpsL supE44 ara-14 xyl-15 mtl-1 tsx-33 | Laboratory stock |
| BGF611 | As AB1157, but katG17::Tn10 | 8 |
| BGF416 | As AB1157, but ΔoxyS2::Cm | This study |
| BGF420 | As BGF611, but ΔoxyS2::Cm | This study |
| GS035 | K-12 ΔoxyS2::Cm | 1 |
| RK4936 | araD139 (argF-lac)205 glbB5301 non-(gyrA219) relA1 rpsL150 metE70 btuB::Tn10 | Laboratory stock |
| TA4112 | As RK4936, but Δ(oxyRS-btuB)3 | 4 |
| Plasmid | ||
| pKK177-3 | Ampr vector | Pharmacia |
| poxyS | pKK177-3 derivative carrying oxyS | 1 |
| psyxO | pKK177-3 derivative carrying oxyS in the reverse orientation | 1 |
Regulation of intracellular concentrations of hydrogen peroxide.
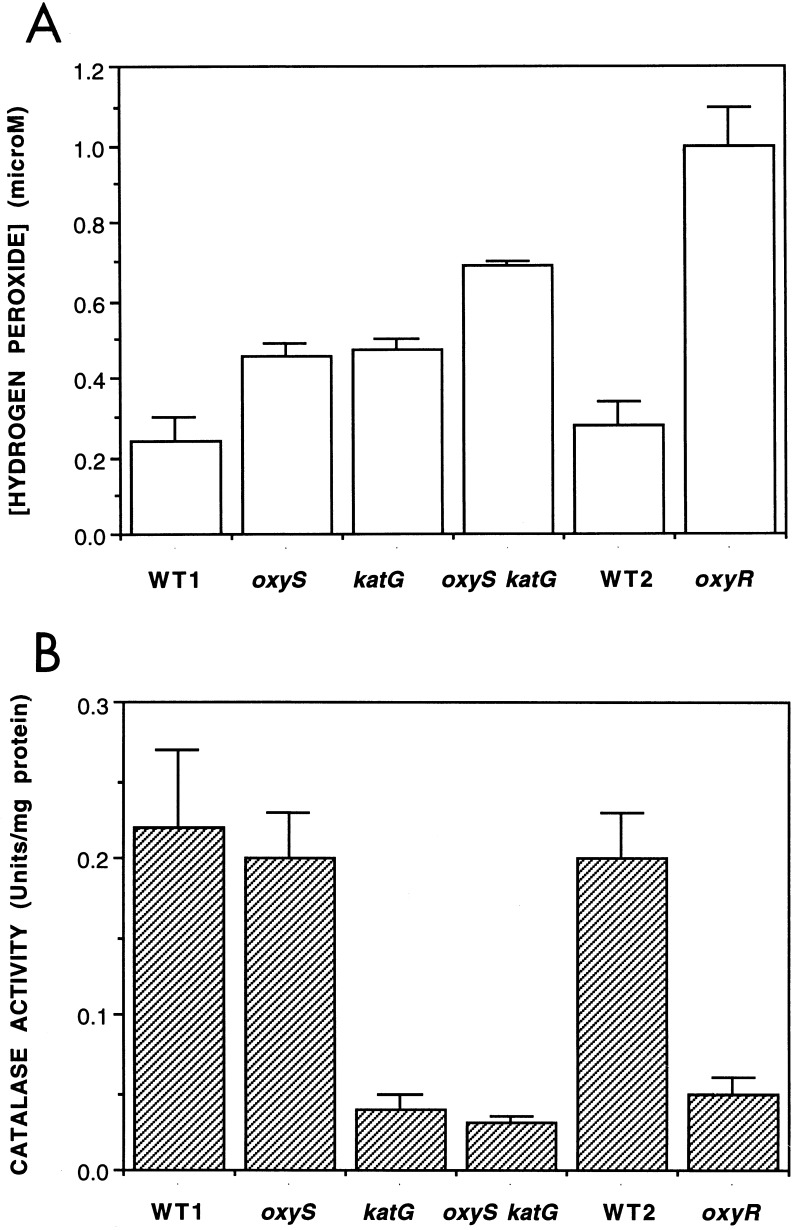
The level of H2O2 in the oxyS-deficient strain BGF416 (in exponential phase) was ∼2-fold higher than the level measured for the parental oxyS+ strain (Fig. 1A), consistent with regulation mediated by oxyS. This increase was similar to that observed for a katG strain and less than that for a ΔoxyRS strain (Fig. 1A). We therefore tested whether mutations in both oxyS and katG would act synergistically to elevate the intracellular H2O2 concentration. A ΔoxyS katG double mutant (BGF420) had an ∼3-fold increase in H2O2 concentration (Fig. 1A), which shows that katG and oxyS play independent roles in the OxyR-dependent regulation of H2O2.
FIG. 1.
Effect of genetic deficiency in oxyS or katG on the intracellular concentration of hydrogen peroxide or catalase activity. (A) Steady-state H2O2 concentrations in intact cells. (B) Total catalase activity in cell extracts. Values are the means of four to six independent experiments ± SEMs. Strain abbreviations: WT1, AB1157 (oxyRS+ katG+); oxyS, BGF416 (oxyR+ oxyS2::Cm); katG, BGF611 (oxyRS+ katG17::Tn10); oxyS katG, BGF420 (oxyR+ oxyS2::Cm katG17::Tn10); WT2, RK4936 (oxyRS+ katG+); oxyR, TA4112 [Δ(oxyRS-btuB)3].
Increased steady-state concentrations of H2O2 can result from decreases in the rate of its decomposition or increases in the rate of H2O2 production. The catalase–HP-I is the major H2O2-decomposing activity in exponentially growing E. coli (8, 14), and it is possible that oxyS regulates katG by a posttranscriptional mechanism (1) distinct from the transcriptional activation of katG mediated by OxyR. However, the deletion of oxyS did not alter the total catalase activity in either the wild-type or the katG background (Fig. 1B). As previously reported, the katG mutant strain had an ∼70% lower catalase activity due to the lack of a functional catalase–HP-I (the remainder is the katE-encoded enzyme [8, 14]).
Since oxyS did not seem to affect expression of the primary H2O2 scavenging activity, we hypothesized that oxyS might influence the cellular generation of H2O2, most of which arises from the dismutation of O2− by SOD (10). We therefore tested the ΔoxyS, katG, and ΔoxyRS strains for changes in the rate of O2− production. The rates of superoxide anion production in membrane preparations (12) increased 6-fold in the ΔoxyS strain and 2.5-fold in the ΔoxyRS strain compared to their wild-type counterparts (Table 2). Expression of oxyS from the multicopy plasmid poxyS (1) complemented the ΔoxyS phenotype by preventing the increased superoxide production (Table 2). In the wild-type strain, the rate of O2− production was not changed significantly by poxyS, the vector plasmid, or a plasmid with oxyS in the reverse orientation (psyxO [1]) (Table 2 and data not shown).
TABLE 2.
Effect of oxyS mutation on the rates of H2O2 production in intact cells and O2− production in isolated membranesa
| Strain (relevant genotype) | d[H2O2]/dt (μM/s) |
d[O2−]/dt
(nmol/min/mg of protein)
|
|
|---|---|---|---|
| Without poxyS | With poxyS | ||
| AB1157 (oxyRS+) | 2.1 ± 0.7 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| BGF416 (oxyR+ ΔoxyS) | 3.6 ± 0.7 | 1.7 ± 0.5 | 0.3 ± 0.1 |
| BGF611 (oxyRS+ katG) | 0.9 ± 0.7 | 0.15 ± 0.1 | 0.08 ± 0.02 |
| RK4936 (oxyRS+) | 2.2 ± 0.5 | 0.20 ± 0.03 | 0.3 ± 0.1 |
| TA4112 (ΔoxyRS) | 1.9 ± 0.5 | 0.5 ± 0.1 | 0.16 ± 0.04 |
The rates of H2O2 production were calculated with the experimental values for H2O2 and catalase concentrations (Fig. 1). The rates of O2− production were measured in membranes isolated from 3-h cultures of the strains listed. Values are means of four to six independent experiments ± SEMs.
Unexpectedly, there seemed to be a small decrease in O2− production in the katG strain BGF611, but this was not statistically significant (Table 2). However, this strain is oxyR proficient, and it may be that there is diminished O2− production due to OxyR-dependent induction of oxyS as a result of the increased H2O2 concentration in this strain (Fig. 1A). Indeed, forcing the increased expression of oxyS by itself with poxyS was sufficient to decrease O2− production in BGF611 as well as in the ΔoxyRS strain TA4112 (Table 2).
The rates of H2O2 production were calculated from the experimental values for the steady-state H2O2 concentration and the total catalase concentration (3, 8). Deletion of oxyS in strain BGF416 increased H2O2 production 1.7-fold, compared to a 6-fold increase in O2− generation in this strain (Table 2). We have not determined whether H2O2 production is decreased by poxyS in the ΔoxyS strain. In principle, the 2:1 O2−-H2O2 stoichiometry of superoxide dismutation (3) would predict a maximal threefold increase in H2O2 generation in the ΔoxyS strain. However, reactions other than SOD can consume O2− (7), and such reactions may contribute to this difference. The lack of a significant increase in H2O2 production in the ΔoxyRS strain (TA4112) (Table 2) could also be related to non-SOD pathways consuming superoxide.
The metabolic production of O2− and H2O2 in E. coli depends on the number of active respiratory chain units per cell and on the energetic state of the chains, which can be altered by directing the electron flux through components with higher or lower energetic efficiency (coupled versus uncoupled components) (10, 22). Therefore, changes in growth conditions or in the proportions of coupled and uncoupled components would determine the rate of free radical production at the respiratory chain. The effect of oxyS on the metabolic production of superoxide and hydrogen peroxide reported here could be due to this type of regulation. Three of the eight oxyS-regulated genes reported so far, fhlA, gadB, and uhpT, are involved in energy metabolism, although they have not been related directly to electron transport processes. The fhlA gene encodes a transcriptional activator of the hydrogenase pleiotropic operon (hypABCDE) (16, 17). The gadB gene encodes a glutamic acid decarboxylase (21), and uhpT encodes the sugar phosphate transporter (6, 13). Perhaps oxyS represses some energy pathways that leak more O2− and are deleterious to cells under oxidative stress.
To test the hypothesis that oxyS deletion affects the energy metabolism of the cells, we measured the rates of respiration in intact wild-type and ΔoxyS cells. Oxygen uptake by exponentially growing cells was significantly increased in the ΔoxyS strain (mean rate of O2 consumption ± standard error of the mean [SEM] for three independent experiments, 0.77 ± 0.02 nanoatoms of O2/min/106 cells; wild type, 0.51 ± 0.06 nanoatoms of O2/min/106 cells), and this effect was largely suppressed by introduction of the multicopy plasmid poxyS (0.63 ± 0.07 nanoatoms of O2/min/106 cells). As seen for the rate of O2− production (Table 2), expression of additional oxyS from poxyS in the wild-type strain may slightly decrease the rate of respiration (from 0.51 ± 0.06 to 0.44 ± 0.04 nanoatoms of O2/min/106 cells). We therefore conclude that oxyS does affect cellular respiration. More-detailed studies will be required to delineate the mechanism underlying this regulation.
We previously reported that twofold increases in the rate of production of H2O2 are enough to trigger a substantial OxyR-dependent transcription of katG (10) and that twofold increases in the steady-state concentration of H2O2 significantly increased the frequency of spontaneous mutation (8). We therefore tested whether the lack of oxyS or oxyS and katG resulted in phenotypic changes. We measured the frequency of spontaneous mutation (a sensitive marker of oxidative DNA damage [8]) and the cellular sensitivity to exogenous H2O2 in the various strains. Compared to the wild-type strain (AB1157), both parameters were unchanged in the ΔoxyS strain (BGF416), but there were significant increases in spontaneous mutation (2.8-fold) and H2O2 sensitivity (1.5-fold) in the ΔoxyS katG strain (BGF420) (Table 3). Multicopy oxyS had an antimutagenic effect in the ΔoxyS and katG strains and possibly in the oxyRS+ strain (Table 3). Multicopy oxyS in the double mutant strain BGF420 (ΔoxyS katG), decreased the frequency of spontaneous mutation to almost the same value as obtained the katG strain without poxyS (Table 3). Interestingly, multicopy oxyS did not complement the mutator phenotype of the ΔoxyRS strain (Table 3). This result suggests that, in addition to oxyS, either katG or some other OxyR-dependent activity is critical for limiting mutagenesis by endogenous H2O2. Alternatively, oxyS in poxyS is not regulated in response to oxidative stress (1), so the level of the RNA does not adjust to changing H2O2 production.
TABLE 3.
Oxidative damage and sensitivity to H2O2 in exponentially growing E. colia
| Strain (relevant genotype) | Mutation frequency (Rifr
colonies/109 cells)
|
H2O2 sensitivity (killing zone, mm) | |
|---|---|---|---|
| Without poxyS | With poxyS | ||
| AB1157 (oxyRS+) | 10 ± 2 | 6 ± 5 | 8 ± 1 |
| BGF416 (oxyR+ ΔoxyS) | 11 ± 2 | 5 ± 1 | 9 ± 1 |
| BGF611 (oxyRS+ katG) | 16 ± 1 | 8 ± 1 | 10 ± 1 |
| BGF420 (oxyS katG) | 28 ± 2 | 20 ± 2 | 12 ± 1 |
| RK4936 (oxyRS+) | 7 ± 1 | 5 ± 1 | 7 ± 2 |
| TA4112 (ΔoxyRS) | 80 ± 8 | 100 ± 10 | 17 ± 1 |
Three-hour cultures were plated on LB plates with rifampin or LB plates with a filter paper soaked in H2O2, as described in the text. Values are means of four to six independent experiments ± SEMs.
Multicopy oxyS did not significantly alter the sensitivity to hydrogen peroxide in any of the strains listed (data not shown), in agreement with the lack of regulation of katG by oxyS (Fig. 1B).
Our results show an oxyS-dependent regulation of the intracellular production of oxygen free radicals. In this way, the oxyS pathway and the OxyR-dependent induction of catalase–HP-I would provide two independent and complementary mechanisms to limit the levels of H2O2 during aerobic growth and possibly under oxidative stress. Elimination of either pathway is still compatible with almost normal aerobic growth, but elimination of both oxyS and katG produces a ΔoxyRS-like phenotype with significant increases in the frequency of spontaneous mutations and sensitivity to H2O2. Defining the mechanism(s) by which oxyS limits O2− production in respiring E. coli may reveal general pathways to avoid oxidative damage.
Acknowledgments
We are grateful to members of the laboratory for discussions. We thank G. Storz for providing us with strain GS035 and plasmids poxyS and psyxO.
This work was supported by grants from the National Institutes of Health (CA37831 to B.D. and P30 ES00002 to J. Brain). B.G.-F. acknowledges the generous support of a fellowship from the Francis Families Foundation.
REFERENCES
- 1.Altuvia S, Weinstein-Fischer D, Zhang A, Postow L, Storz G. A small, stable RNA induced by oxidative stress: role as a pleiotropic regulator and antimutator. Cell. 1997;90:43–53. doi: 10.1016/s0092-8674(00)80312-8. [DOI] [PubMed] [Google Scholar]
- 2.Boveris A. Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods Enzymol. 1984;105:429–435. doi: 10.1016/s0076-6879(84)05060-6. [DOI] [PubMed] [Google Scholar]
- 3.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 4.Christman M F, Morgan R W, Jacobson F S, Ames B N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985;41:753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- 5.Estabrook R W. Mitochondrial respiratory control and the polarographic measurement of ADP:O ratios. Methods Enzymol. 1967;10:41–47. [Google Scholar]
- 6.Friedrich M J, Kadner R J. Nucleotide sequence of the uhp region of Escherichia coli. J Bacteriol. 1987;169:3556–3563. doi: 10.1128/jb.169.8.3556-3563.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner P R, Fridovich I. Inactivation-reactivation of aconitase in Escherichia coli. A sensitive measure of superoxide radical. J Biol Chem. 1992;267:8757–8763. [PubMed] [Google Scholar]
- 8.González-Flecha B, Demple B. Homeostatic regulation of intracellular hydrogen peroxide concentration in aerobically growing Escherichia coli. J Bacteriol. 1997;179:382–388. doi: 10.1128/jb.179.2.382-388.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzálex-Flecha B, Demple B. Intracellular generation of superoxide as a by-product of Vibrio harveyi luciferase expressed in Escherichia coli. J Bacteriol. 1994;176:2293–2299. doi: 10.1128/jb.176.8.2293-2299.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Flecha B, Demple B. Metabolic sources of hydrogen peroxide in aerobically growing Escherichia coli. J Biol Chem. 1995;270:13681–13687. doi: 10.1074/jbc.270.23.13681. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg J T, Demple B. Overproduction of peroxide-scavenging enzymes in Escherichia coli suppresses spontaneous mutagenesis and sensitivity to redox-cycling agents in oxyR-mutants. EMBO J. 1988;7:2611–2617. doi: 10.1002/j.1460-2075.1988.tb03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imlay J A, Fridovich I. Assay of metabolic superoxide production in Escherichia coli. J Biol Chem. 1991;266:6957–6965. [PubMed] [Google Scholar]
- 13.Island M D, Wei B-Y, Kadner R J. Structure and function of the uhp genes for the sugar phosphate transport system in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1992;174:2754–2762. doi: 10.1128/jb.174.9.2754-2762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loewen P C, Switala J, Triggs-Raine B L. Catalases HPI and HPII in Escherichia coliare induced independently. Arch Biochem Biophys. 1985;243:144–149. doi: 10.1016/0003-9861(85)90782-9. [DOI] [PubMed] [Google Scholar]
- 15.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 16.Lutz S, Jacobi A, Schlensog V, Bohm R, Sawers G, Bock A. Molecular characterization of an operon (hyp) necessary for the activity of the three hydrogenase isoenzymes in Escherichia coli. Mol Microbiol. 1991;5:123–135. doi: 10.1111/j.1365-2958.1991.tb01833.x. [DOI] [PubMed] [Google Scholar]
- 17.Maier T, Jacobi A, Sauter M, Böck A. The product of the hypBgene, which is required for nickel incorporation into hydrogenases, is a novel guanine nucleotide-binding protein. J Bacteriol. 1993;175:630–635. doi: 10.1128/jb.175.3.630-635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 19.Morgan R W, Christman M F, Jacobson F S, Storz G, Ames B N. Hydrogen peroxide-inducible proteins in Salmonella typhimuriumoverlap with heat shock and other stress proteins. Proc Natl Acad Sci USA. 1986;83:8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niimura Y, Poole L B, Massey V. Amphibacillus xylanus NADH oxidase and Salmonella typhimurium alkyl-hydroperoxide reductase flavoprotein components show extremely high scavenging activity for both alkyl hydroperoxide and hydrogen peroxide in the presence of S. typhimuriumalkyl-hydroperoxide reductase 22-kDa protein component. J Biol Chem. 1995;270:25645–25650. doi: 10.1074/jbc.270.43.25645. [DOI] [PubMed] [Google Scholar]
- 21.Smith D K, Kassam T, Singh B, Elliott J F. Escherichia colihas two homologous glutamate decarboxylase genes that map to distinct loci. J Bacteriol. 1992;174:5820–5826. doi: 10.1128/jb.174.18.5820-5826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trumpower B L, Gennis R B. Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: the enzymology of coupling electron transfer reactions to transmembrane proton translocation. Annu Rev Biochem. 1994;63:675–716. doi: 10.1146/annurev.bi.63.070194.003331. [DOI] [PubMed] [Google Scholar]
- 23.Visick J E, Clarke S. RpoS- and OxyR-independent induction of HPI catalase at stationary phase in Escherichia coli and identification of rpoSmutations in common laboratory strains. J Bacteriol. 1997;179:4158–4163. doi: 10.1128/jb.179.13.4158-4163.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]