Abstract
Background
Functional capacity assessment is a critical step in the preoperative evaluation to identify patients at increased risk of cardiac complications and disability after major noncardiac surgery. Smartphones offer the potential to objectively measure functional capacity but are limited by inaccuracy in patients with poor functional capacity. Open-source methods exist to analyze accelerometer data to estimate gait cadence (steps/min), which is directly associated with activity intensity. Here, we used an updated Step Test smartphone application with an open-source method to analyze accelerometer data to estimate gait cadence and functional capacity in older adults.
Methods
We performed a prospective observational cohort study within the Frailty, Activity, Body Composition and Energy Expenditure in Aging study at the University of Chicago. Participants completed the Duke Activity Status Index (DASI) and performed an in-clinic 6-min walk test (6MWT) while using the Step Test application on a study smartphone. Gait cadence was measured from the raw accelerometer data using an adaptive empirical pattern transformation method, which has been previously validated. A 6MWT distance of 370 m was used as an objective threshold to identify patients at high risk. We performed multivariable logistic regression to predict walking distance using a priori explanatory variables.
Results
Sixty patients were enrolled in the study. Thirty-seven patients completed the protocol and were included in the final data analysis. The median (IQR) age of the overall cohort was 71 (69–74) years, with a body mass index of 31 (27–32). There were no differences in any clinical characteristics or functional measures between participants that were able to walk 370 m during the 6MWT and those that could not walk that distance. Median (IQR) gait cadence for the entire cohort was 110 (102–114) steps/min during the 6MWT. Median (IQR) gait cadence was higher in participants that walked more than 370 m during the 6MWT 112 (108–118) versus 106 (96–114) steps/min; p = 0.0157). The final multivariable model to identify participants that could not walk 370 m included only median gait cadence. The Youden's index cut-point was 107 steps/min with a sensitivity of 0.81 (95% CI: 0.77, 0.85) and a specificity of 0.57 (95% CI: 0.55, 0.59) and an AUCROC of 0.69 (95% CI: 0.51, 0.87).
Conclusions
Our pilot study demonstrates the feasibility of using gait cadence as a measure to estimate functional capacity. Our study was limited by a smaller than expected sample size due to COVID-19, and thus, a prospective study with preoperative patients that measures outcomes is necessary to validate our findings.
Keywords: Triaxial accelerometer, Gait, Mobile technology, Raw data, Wearable physical activity monitoring
Introduction
Functional assessment is a critical component of the preoperative evaluation as it guides preoperative cardiac testing and informs the prediction of morbidity, disability-free survival, and discharge to post-acute care [1, 2, 3, 4, 5]. The 6-min walk test (6MWT) and Duke Activity Status Index (DASI) are commonly studied and validated preoperative functional assessments [4, 6]. We previously combined these assessments together in a smartphone application (Step Test) to facilitate their clinical administration as each takes time to administer in busy preoperative clinics [7]. Step Test administers the DASI questionnaire and then instructs patients to perform a 6MWT. The smartphone measures the number of steps walked using the accelerometer and gyroscope during the 6MWT and estimates the total number of steps walked. However, our application demonstrated decreased accuracy in total steps counted as compared to a research grade pedometer and estimated distance walked in patients with poor functional status [8, 9].
To overcome the accuracy limitations, we applied a previously validated and open-source method, adaptive empirical pattern transformation (ADEPT), to analyze the accelerometer output and estimate steps walked from the smartphone device during the 6MWT [10]. ADEPT allows for adaptability to different walking stride patterns to quantify cadence and overcomes the limitation of relying on commercial software to analyze acceleration data. Additionally, rather than estimated distance walked, we used gait cadence, defined as the number of steps walked per minute, to serve as a measure of functional capacity. Gait cadence is strongly associated with activity intensity as a sustained gait cadence of 110 steps/min is associated with 4 metabolic equivalents, a critical threshold for the preoperative evaluation [11, 12]. Further, gait cadence calculated using this software has demonstrated strong associations with mobility, physical performance, and fatigability measures in older adults [13]. Smartphones have two distinct advantages as compared to physical activity trackers to perform preoperative functional assessments. They have the ability to implement timed walk tests to measure patients' capability to increase activity intensity and identify patients unable to reach 110 steps/min and they facilitate the use of ADEPT to analyze accelerometer output rather than the proprietary software of the device. This is especially important as physical activity trackers have demonstrated decreased accuracy in ambulatory older adults [14].
In this work, we build upon our previous application and describe our updated pilot study of Step Test. We hypothesize that gait cadence will be able to accurately identify older adults with poor functional capacity as measured by the 6MWT. To test our hypothesis, we administered both measures using Step Test in a cohort of older adults oversampled for frailty. We chose to implement this study in a sample of older adults with poor functional capacity because they previously demonstrated decreased step count accuracy in our prior study.
Materials and Methods
Study Design and Population
This study was conducted as part of a larger prospective cohort study performed at the University of Chicago called the Frailty, Activity, Body Composition and Energy Expenditure in Aging (FACE Aging) study. Older adults, 65 years of age or older, were recruited from the community surrounding the University of Chicago's primary geriatrics practice site. Exclusion criteria included hospitalization, surgery, or procedure within 2 months of enrolling in the study; a change in thyroid (e.g., levothyroxine) or diuretic (e.g., furosemide, hydrochlorothiazide, or spironolactone) medication dose within 2 months; use of oral steroids; use of β-blocker (e.g., metoprolol, atenolol, or carvedilol); persistent hyperglycemia >250; life expectancy less than 1 year; and history of moderate or advanced dementia or Montreal Cognitive Assessment ≤18 of 30 points. Data collection for the FACE Aging study occurred over multiple evaluations: (1) baseline survey and physical exam, and (2) a 1-year follow-up survey and physical exam. For the current study, we recruited participants at the 1-year follow-up evaluation in the FACE Aging study. Participants were provided a USD 10 gift card for participation in the study procedures. This study was approved by the University of Chicago Institutional Review Board and informed written consent was obtained from all participants prior to enrollment. This article adheres to the applicable TREND guidelines for this nonrandomized study.
All adult patients presenting to the FACE Aging 1-year follow-up were approached for enrollment unless they were nonambulatory or had an absolute contraindication to performing a 6MWT such as unstable angina, myocardial infarction within 30 days, resting heart rate >120 bpm, a systolic blood pressure >180 mm Hg, or a diastolic blood pressure >100 mm Hg [15]. The use of assistive walking devices such as canes, walkers, or other devices and the use of supplemental oxygen were not contraindications for enrollment in the study. Participants were approached for enrollment in the study between August of 2019 and February of 2021. The enrollment period was prolonged due to the COVID-19 pandemic.
Clinical Characteristics
A medical history, demographics, height, and weight were obtained from the baseline FACE aging study visit. The Charlson Comorbidity Index was calculated and used to estimate the comorbidity burden [16]. The 1-year FACE Aging assessment includes the Fried frailty phenotype and Short Physical Performance Battery and the American Geriatrics Society fall risk questionnaire consisting of two questions: (1) Have you had more than 2 falls in the last 12 months? (2) Do you have difficulty with walking or balance? [17, 18].
Step Test Smartphone Application
Our previous Step Test application consisted of two primary components: (1) DASI questionnaire and (2) 6MWT. Those components remained the same for this study as described previously, but the accelerometer output has been modified [7]. Previously the CMPedometer class from Apple's Core Motion framework was used to estimate a step count based on data retrieved from the device's motion coprocessor. The coprocessor records data from the device's sensors, including the raw accelerometer and gyroscope data. However, those methods are proprietary, and their algorithms are unknown to researchers. Therefore, we were unable to comprehensively evaluate the performance and characteristics of how the application generated the step count.
In this work, ADEPT was used to analyze the raw accelerometer output from the smartphone [10]. ADEPT is a statistical pattern recognition method optimized for precise (at sub-second level) identification of start time and duration of individual walking strides in raw accelerometry data. The method uses a predefined template and detects its repetitions in the data set by maximizing the local correlation between a collection of scale-transformed templates and the data. The ADEPT algorithm has been previously validated and is freely available for download as an adept R package [19]. Following the ADEPT paper recommendations, in our application, a one-dimensional vector magnitude summary of three-dimensional raw acceleration data was used for strides identification. All ADEPT segmented strides were validated for correctness by visual inspection by the study team (D.R., S.R.). The first 20 s of data from each 6MWT were excluded from analysis to allow each patient to reach a steady state of walking [20]. Thus, we only estimated gait cadence for 5 of the minutes of the 6MWT, excluding the final 40 s. ADEPT-derived duration of each segmented stride, expressed in seconds, was converted to cadence (steps/min). Finally, a median of cadence values was computed separately for each participant [10].
Six-Minute Walk Test
The 6MWT was administered by the same research assistant according to the American Thoracic Society guidelines which included the script detailing the instructions on how to perform a 6MWT and voice prompts at each minute during the test [15]. An indoor 30-meter track in a hallway was used to administer the test. Encouragement was not provided during the test. The same iPhone 8 (Apple, Cupertino, CA, USA) was used to standardize the device for all the 6MWT. The smartphone was then placed in either the patient's front pants pocket or attached to a waist belt using a belt clip (Stalion Secure Belt Clip Holster; Stalion®), depending on patient preference. Participants were allowed to choose the wear location as prior studies have not identified any differences between the hip and pocket during timed walk tests [8]. Upon completion of the test, the research personnel would directly measure the total distance walked and confirm that the results of the Step Test application had been successfully sent securely to a University of Chicago server.
Outcome
The primary outcome was defined as whether a patient walked a distance farther than 370 m during the in-clinic 6MWT. This distance was used as this threshold identifies patients at risk of perioperative cardiac events and disability-free survival after major noncardiac surgery [4].
Sample Size and Power Analysis
The primary outcome was a 6MWT distance walked greater than 370 m. While the mean (standard deviation) walking distance for preoperative patients was 473 (98) m, we estimated that the mean for our population would be lower since it is older and has a higher comorbidity burden [4]. Given our patient population, we estimated that the probability of walking 370 m would be 50% and that the median gait cadence for older adults would be 115 steps/min [21]. Thus, older adults with an slower cadence (≈100 steps/min) would have a probability of 30% to achieve the same distance. Thus, we estimated that a sample size of 60 participants would yield a power of 80% at significance level alpha = 0.05, using powerlog in STATA. Due to enrollment difficulties secondary to the COVID-19 pandemic, we were only able to enroll 45 participants with 37 participants included in the final analysis, and thus only have estimated power of 60% to detect a 20% difference.
Statistical Analysis
First, sample summaries of data set variables were computed. Categorical variables are reported as a number and percentage; continuous variables are reported as a mean and standard deviation or median and interquartile range (IQR) if the variable values were not normally distributed. The Shapiro-Wilk test and histogram plots were used to assess for normality of the distributions. Differences between groups of participants based on 6MWT distance were evaluated using χ2 tests or Wilcox rank-sum method.
A multivariable logistic regression was used to predict whether a patient was able to walk 370 m during the 6MWT. Three different versions of the model were considered which varied in the set of explanatory variables used: (1) median gait cadence only; (2) median gait cadence, height; (3) DASI score only. The median gait cadence, height, and DASI score variables were a priori determined to impact 6MWT distance. A receiver operating characteristic (ROC) curve was used to assess the predictive performance of the models. The Youden's index was used to identify the cutoff on logistic regression probabilities that maximized sensitivity and specificity. A two-sided test assuming statistical significance level alpha = 0.05 was used to determine statistical significance of model coefficients. R software was used for accelerometer output analysis and STATA 15 was used for all the remaining part of data analysis.
Role of Funding Source
The Carol and George Abramson fund for aging and longevity funded the updated build and study phones that were used for this pilot study. The funding agency had no role in study design, data collection, data analysis, data interpretation, writing, or submission of the report.
Results
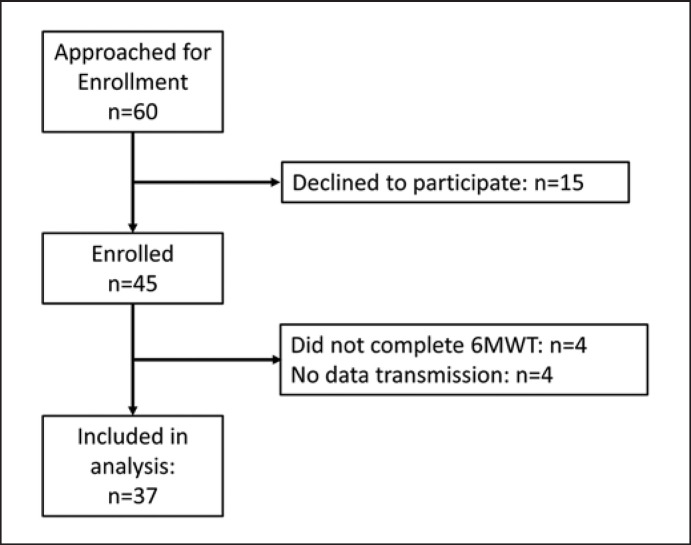
A total of 45 participants were enrolled in the study. Enrollment began on August 28th, 2019, and was paused on March 16th, 2021, after enrolling 29 participants due to the COVID-19 pandemic. We began enrollment again on August 1st, 2020, and continued until February of 2021 where we enrolled the final 16 participants. Figure 1 details the enrollment procedures. Of the 45 participants that were enrolled 4 were unable to complete the walk and thus have incomplete data. One participant fell during the walk. The participant did not disclose to our study nurse that he required a walking device (nor did the participant bring the device) during walking and did not use a device during the 6MWT despite asking about assistive devices as part of our study protocol. The patient was seen by a healthcare provider and no injury was sustained. The event was promptly reported to the IRB and we were allowed to continue enrollment without modifying any additional study procedures. Furthermore, there were 3 errors in data transmission and ADEPT was not able to identify walking strides successfully for 1 patient's data. The final cohort used in the statistical analysis included 37 participants.
Fig. 1.
CONSORT diagram that illustrates patient enrollment throughout the study. 6MWT, 6-min walk test.
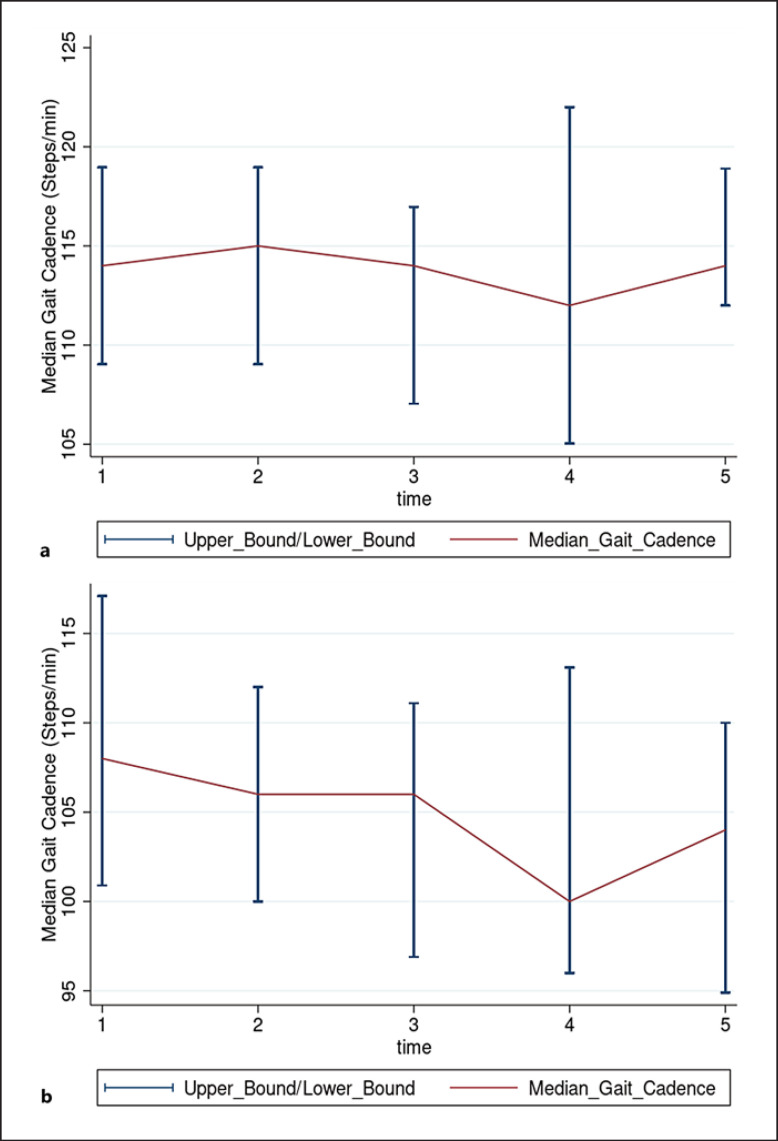
Patient characteristics are shown in Table 1. The median (IQR) age of the overall cohort was 71 (69–74) years, with a body mass index of 31 (27–32). African American/Black women made up most of our cohort (85%) as the local community around the University of Chicago is predominantly African American. There were no differences in any clinical characteristics or functional measures between participants that were able to walk 370 m during the 6MWT and those that could not walk that distance. Median (IQR) gait cadence for the entire cohort was 110 (102–114) steps/min during the 6MWT. The minute-level aggregates of cadence estimates for the two groups can be seen in Figure 2a, b. Median (IQR) gait cadence was higher in participants that walked more than 370 m during the 6MWT 112 (108–118) versus 106 (96–114) steps/min; p = 0.016.
Table 1.
Demographics and patient characteristics
| Characteristics | Entire cohort N = 37 | Short 6MWT distance N = 21 | Long 6MWT distance N = 16 | p value |
|---|---|---|---|---|
| Age, median (IQR), years | 71 (69–74) | 70 (69–73) | 72 (69–76) | 0.229 |
| Sex, n (%) | ||||
| Male | 5 (13) | 2 (10) | 3 (19) | 0.416 |
| Female | 32 (87) | 19 (90) | 13 (81) | |
| BMI, median (IQR) | 30.5 (26.6–32.3) | 31.6 (30.0–35.9) | 28.1 (26.3–30.6) | 0.067 |
| Height, m | 1.63 (1.89–1.69) | 1.62 (1.58–1.67) | 1.67 (1.61–1.70) | 0.160 |
| Weight, kg | 83 (72–89) | 84 (73–93) | 82 (72–85) | 0.348 |
| Race | ||||
| White | 1 (3) | 0 | 1 (6) | 0.245 |
| African American | 36 (97) | 21 (100) | 15 (94) | |
| Walking assist device (cane, walker) | 4 | 3 | 1 | 0.435 |
| Functional measures | ||||
| Short physical performance battery | 11 (10–12) | 11 (10–12) | 11 (10–12) | 0.181 |
| Physical frailty | ||||
| Not frail | 17 (46) | 9 (43) | 8 (50) | 0.224 |
| Pre-frail | 19 (51) | 11 (42) | 8 (50) | |
| Frail | 1 (3) | 1 (5) | 0 | |
| Charlson comorbidity score (IQR) | 1 (0–1) | 1 (0–2) | 0 (0–1) | 0.033 |
| Fall screen | 8 (22%) | 6 (29%) | 2 (13%) | 0.239 |
| Median gait cadence (steps/min) | 110 (102–114) | 106 (96–114) | 112 (108–118) | 0.009 |
BMI, body mass index.
Fig. 2.
a, b Median gait cadence stratified by walking distance (370 m). Patients who walked more than 370 meters had a higher gait cadence than those that walked less. a is the group of patients that walked >370 m during the 6MWT and is the group of patients that walked <370 m (b).
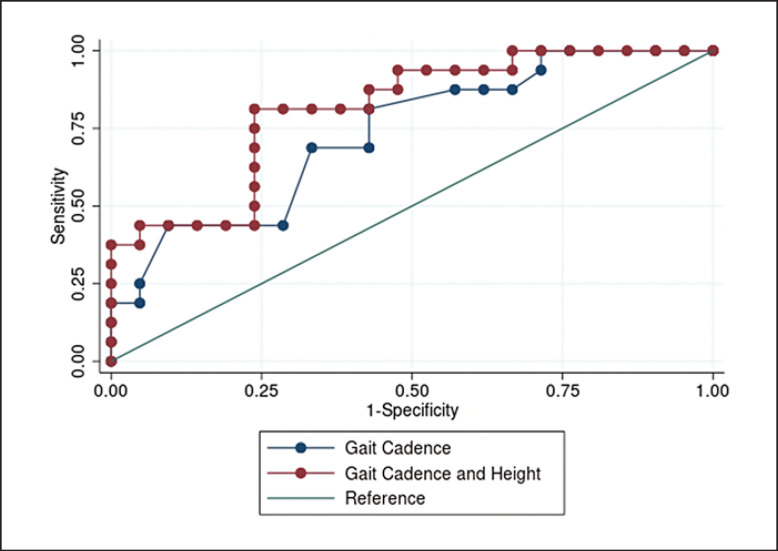
We performed logistic regressions to identify independent variables that were predictive of 6MWT distance. Table 2 shows the summary of the three logistic regression models that used various combinations of median gait cadence, height, and DASI scores as explanatory variables and used 6MWT distance-based-group assignments as the outcome. The model that included only the DASI score was not significant (β-coefficient, 95% CI) (0.049, −0.001, 0.107; p = 0.103), whereas the models that included median gait cadence and height were significant. Figure 3 illustrates the ROC curves and the model that included median gait cadence (Model 1) alone was not inferior to the model that included median gait cadence and height (Model 2) (p = 0.291). The Youden's index for Model 1 identified a cut-point of 107 steps/min as the optimal cut-point with a sensitivity of 0.81 (95% CI: 0.77, 0.85), a specificity of 0.57 (95% CI: 0.55, 0.59), and an AUCROC of 0.69 (95% CI: 0.51, 0.87) to identify participants that cannot walk at least 370 m during a 6MWT.
Table 2.
Model and AUC for the receiver operating characteristic for the 3 models that were tested to predict 6MWT distance threshold
| Variable | Model 1 β-coefficient | 95% CI | p value | AUC (95% CI) | Model 2 β-coefficient | 95% CI | p value | AUC (95% CI) | Model 3 β-coefficient | 95% CI | p value | AUC (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median gait cadence (steps/min) | 0.198 | 0.028, 0.369 | 0.023 | 0.734 (0.571,0.900) | 0.323 | 0.066, 0.579 | 0.014 | 0.810 (0.669,0.950) | − | − | − | 0.640 (0.457, 0.822) |
| Height (m) | − | − | − | − | 11.67 | 1.06, 22.28 | 0.031 | − | − | − | − | |
| DASI score | − | − | − | − | − | − | − | − | 0.049 | −0.001, 0.107 | 0.103 |
There was no difference between the AUCs of all the models in the ability to predict patients that would walk >370 m during 6MWT (p = 0.215). CI, confidence interval; 6MWT, 6-min walk test; DASI, Duke Activity Status Index; AUC, area under the curve.
Fig. 3.
ROC curves for models 1 and 2. Model 1 includes median gait cadence alone, and model 2 includes median gait cadence and height in meters. No difference was identified between the two ROC curves.
Discussion
In this prospective cohort of older adults, median gait cadence captured using a smartphone application during the 6MWT and calculated using an open-source processing method was significantly lower among older adults unable to walk at least 370 m. A gait cadence of 107 steps/min during a 6MWT distinguished older adults with an inability to walk a minimum functional threshold of 370 m with reasonably high sensitivity (80%) and modest specificity (57%). We used Youden's Index to determine the optimal gait cadence that provided the best balance of sensitivity and specificity. As our smartphone application is a screening test to identify high-risk surgical patients. As a screening, prioritizing sensitivity is favored; however, often at the expense of specificity as in our study. Therefore, all patients screening “positive” using this app would need further confirmation of their functional risk by doing in-person objective testing like the 6MWT. Future studies may be required to recalibrate the sensitivity and specificity to determine the optimal gait cadence to identify at-risk patients. Our results are limited by a small sample size that was secondary to the ongoing COVID-19 pandemic as we could not reach our target number of participants. A larger prospective cohort with perioperative patients is still necessary to confirm our findings and confirm the optimal gait cadence threshold to identify patients at risk of moderate-to-severe major complications after noncardiac surgery.
Gait cadence measured from a smartphone during a timed walk test provides a novel objective functional assessment. Walking is the most frequent physical activity performed by older adults and walking intensity provides a measure of functional capacity. Step-based metrics generated from wearable devices, such as smartphones and smartwatches, have focused on daily step count or distance walked as measures of functional assessment [8, 22, 23, 24]. However, step counts and distance walked represent the volume of an activity (walking) but do not necessarily reflect the intensity of that activity, which is at the core of functional assessments before surgery. Gait cadence is a functional measure that is positively correlated with absolute activity intensity such that an increase in cadence is associated with an increase in absolute activity intensity [12]. In a recent prospective cohort of older adults, gait cadence was strongly predictive of absolute activity intensity independent of sex, age, or leg length [12]. Further, a gait cadence of 110 steps/min identified absolutely defined moderate-intensity activity (>4 metabolic equivalents) mirroring our 107 steps/min as the optimal cut-point to identify decreased walking capacity [12]. The use of gait cadence as a functional assessment tool can improve upon the 6MWT distance as there is no need to adjust distance estimates for older, shorter or female patients that have been shown to have shorter stride lengths [25].
The use of smartphones also enables data collection to occur outside of the clinical setting. Developing approaches to improve performance of functional assessments before surgery is critical as currently less than 10% of older adults receive functional assessments prior to surgery [26]. Our approach uses existing patient-owned technology and while our original application used iOS it can be adapted to Android devices. To improve performance of objective functional assessments, our next steps include deploying our smartphone application to patients before their in-clinic appointment. Patients will perform a timed walk test in a long hallway and gait cadence will be measured during the walk using the application. The results can be used during the in-clinic visit to guide future testing and consideration of prehabilitation before surgery. Gait cadence is also an easy metric for patients to understand and could help communicate prehabilitation intensity targets to them [27]. Gait cadence during a timed walk may be an ideal metric to increase performance of objective preoperative functional assessments.
Our approach of using an open-source software to perform accelerometer analysis advances the field of wearables as it does not rely on proprietary algorithms from the device makers [28]. Specifically employing the ADEPT method overcomes two distinct challenges with wearable activity monitors (1) ability to generalize across studies that use different devices and (2) the ability to adapt the analysis to patients with poor functional status. Prior studies that have used wearables in the perioperative period have used data analysis for the accelerometer output provided by the wearable device manufacturer or using proprietary software algorithms [24, 29]. Apple has developed traditional functional capacity metrics, such as peak oxygen consumption and 6MWT distance, that use passive data collection from the Apple Watch to provide estimates of functional capacity. These metrics do not require the user to perform a 6MWT, rather the device will estimate the expected distance based on user activity using proprietary algorithms produced by Apple. The major limitation of these approaches is that the analysis from the device is not known to researchers or clinicians and can be subject to change over time depending on the company. Further, this limits the generalizability of any finding across devices as differences in analysis may lead to different results. This is critical as certain devices (e.g., Apple Watch) may be out of financial reach for many patients to adopt and so creating a generalizable platform to perform these objective functional assessments is essential to adoption. Our work used ADEPT, a previously validated software platform, to analyze raw accelerometer output sampled at a frequency of 30 Hz, which is a sampling frequency easily obtainable by many wearable accelerometers. The use of a data analysis method that can be applied to all the different wearable devices and is not dependent on device software greatly improves generalizability.
The other advantage of our approach is the ability to adapt stride pattern recognition among patients with poor functional capacity. For this study, ADEPT automatically identified individual stride patterns in raw accelerometry data of older adults, followed by manual visual inspection-based validation of all segmented strides done by study personnel (D.R., S.R.) to ensure that the stride patterns were valid for each walk. This approach was critical as the proprietary software from smartphones underperformed when tested in participants with slower walking speeds, as we demonstrated in our previous application and has been demonstrated in other studies [7, 9]. In our cohort, 1 patient was not included in the final analysis as ADEPT was unable to appropriately segment walking strides. The participants gait consisted of a highly variable shuffling pattern such that individual strides were not able to be segmented. We believe the inability for ADEPT to segment this patient's gait pattern confirms our approach as this would be a sign of poor functional status as the patient's 6MWT distance was only 106 m.
We have identified the following limitations in our study. We did not evaluate our application in a preoperative population of older adults and thus cannot generalize our findings to that population. We chose the FACE Aging cohort as it includes older adults with poor functional capacity and the ideal population to test whether our approach is feasible and accurate in that patient population. A larger study that involves patients presenting for major noncardiac surgery is warranted to confirm our findings that gait cadence can serve as a measure of functional status to identify high-risk patients. Further, it remains unclear whether our threshold of 107 steps/min will identify patients at risk of complications after surgery. While this threshold is associated with moderate-intensity activity, it remains unclear what the optimal threshold is to identify high-risk perioperative patients. Additionally, while a short 6MWT test distance is associated with 30-day recovery, 12-month disability-free survival, and cardiovascular outcomes from the METS trial, we cannot generalize that gait cadence will also be associated with those outcomes. Finally, the within-person measurement reliability of gait cadence between different 6MWT in the same patient cannot be inferred from our study. We measured gait cadence during a single in-clinic 6MWT, and it remains unclear how consistent median gait cadence is across different timed walks in the same patient. Future studies are needed to determine the consistency of gait cadence.
Conclusion
Gait cadence during a 6MWT performed moderately well to identify participants that cannot walk farther than 370 m. Additional studies are still needed to clarify the optimal cut-points of gait cadence to identify high-risk patients and to determine whether walk tests can be performed outside of the clinic environment and generate similar results. Smartphone-based gait cadence is an objective functional capacity assessment that may inform management during preoperative clinic visits and potentially identify high-risk patients in the perioperative period.
Statement of Ethics
This study protocol was reviewed and approved by the University of Chicago Institutional Review Board (IRB), approval number IRB 13-0443. Written informed consent was obtained from participants to participate in the study.
Conflict of Interest Statement
Dr. Daniel Rubin is the president of DRDR Mobile Health, a company that creates mobile applications for healthcare, including functional capacity assessment applications. He has engaged in consulting for mobile applications as well. He has not taken any salary or money from the company. He has also engaged in expert witness testimony.
Funding Sources
Carol and George Abramson Fund for Aging and Longevity, Chicago, IL provided funds for the development and study of the mobile application.
Author Contributions
Daniel S. Rubin, MD, MS: This author helped with the conception, acquisition of data, analysis, drafting and revising for content, and final approval and is held accountable for all aspects of the work. Sylvia L. Ranjeva, MD, PhD: This author helped with the conception, analysis, and drafting and revising for final content and is held accountable. Jacek K. Urbanek, PhD; Marta Karas, PhD; and Maria Lucia L. Madariaga, MD: These authors helped with the analysis and drafting and revising for final content and are held accountable for all aspects of the work. Megan Huisingh-Scheetz, MD: This author helped with the conception, analysis, and drafting and revising for final content and is held accountable for all aspects of the work.
Data Availability Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants but are available from the corresponding author (D.S.R.) upon reasonable request.
Acknowledgments
The authors are grateful to Ms. Cornelia Bailey, MS, Strategic Innovation Consultant IT Services, and Fritz Anderson, JD, Senior iOS Developer IT Services, for their assistance in developing and programming the Step Test application and Corliss Taylor for enrollment and recruitment of patients even during the pandemic.
References
- 1.Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014 Dec 9;64((22)):e77–137. doi: 10.1016/j.jacc.2014.07.944. [DOI] [PubMed] [Google Scholar]
- 2.Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, Hert SD, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA) Eur Heart J. 2014 Sep 14;35((35)):2383–431. doi: 10.1093/eurheartj/ehu282. [DOI] [PubMed] [Google Scholar]
- 3.Balentine CJ, Naik AD, Berger DH, Chen H, Anaya DA, Kennedy GD. Postacute care after major abdominal surgery in elderly patients: intersection of age, functional status, and postoperative complications. JAMA Surg. 2016 Aug 1;151((8)):759–66. doi: 10.1001/jamasurg.2016.0717. [DOI] [PubMed] [Google Scholar]
- 4.Shulman MA, Cuthbertson BH, Wijeysundera DN, Pearse RM, Thompson B, Torres E, et al. Using the 6-minute walk test to predict disability-free survival after major surgery. Br J Anaesth. 2019 Jan;122((1)):111–9. doi: 10.1016/j.bja.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Martin D, Romain B, Pache B, Vuagniaux A, Guarnero V, Hahnloser D, et al. Physical activity and outcomes in colorectal surgery: a pilot prospective cohort study. Eur Surg Res. 2020;61((1)):23–33. doi: 10.1159/000507578. [DOI] [PubMed] [Google Scholar]
- 6.Wijeysundera DN, Pearse RM, Shulman MA, Abbott TEF, Torres E, Ambosta A, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet. 2018 Jun 30;391((10140)):2631–40. doi: 10.1016/S0140-6736(18)31131-0. [DOI] [PubMed] [Google Scholar]
- 7.Rubin DS, Dalton A, Tank A, Berkowitz M, Arnolds DE, Liao C, et al. Development and pilot study of an iOS smartphone application for perioperative functional capacity assessment. Anesth Analg. 2020 Sep;131((3)):830–9. doi: 10.1213/ANE.0000000000004440. [DOI] [PubMed] [Google Scholar]
- 8.Brooks GC, Vittinghoff E, Iyer S, Tandon D, Kuhar P, Madsen KA, et al. Accuracy and usability of a self-administered 6-minute walk test smartphone application. Circ Heart Fail. 2015 Sep;8((5)):905–13. doi: 10.1161/CIRCHEARTFAILURE.115.002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Major MJ, Alford M. Validity of the iPhone M7 motion co-processor as a pedometer for able-bodied ambulation. J Sports Sci. 2016 Dec;34((23)):2160–4. doi: 10.1080/02640414.2016.1189086. [DOI] [PubMed] [Google Scholar]
- 10.Karas M, Stra Czkiewicz M, Fadel W, Harezlak J, Crainiceanu CM, Urbanek JK. Adaptive empirical pattern transformation (ADEPT) with application to walking stride segmentation. Biostatistics. 2021;22((2)):331–47. doi: 10.1093/biostatistics/kxz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tudor-Locke C, Han H, Aguiar EJ, Barreira TV, Schuna JM, Jr, Kang M, et al. How fast is fast enough? Walking cadence (steps/min) as a practical estimate of intensity in adults: a narrative review. Br J Sports Med. 2018 Jun;52((12)):776–88. doi: 10.1136/bjsports-2017-097628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tudor-Locke C, Mora-Gonzalez J, Ducharme SW, Aguiar EJ, Schuna JM, Jr, Barreira TV, et al. Walking cadence (steps/min) and intensity in 61-85-year-old adults: the CADENCE-adults study. Int J Behav Nutr Phys Act. 2021 Sep 23;18((1)):129. doi: 10.1186/s12966-021-01199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urbanek JK, Zipunnikov V, Harris T, Crainiceanu C, Harezlak J, Glynn NW. Validation of gait characteristics extracted from raw accelerometry during walking against measures of physical function, mobility, fatigability, and fitness. J Gerontol A Biol Sci Med Sci. 2018 Apr 17;73((5)):676–81. doi: 10.1093/gerona/glx174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tedesco S, Sica M, Ancillao A, Timmons S, Barton J, O'Flynn B. Accuracy of consumer-level and research-grade activity trackers in ambulatory settings in older adults. PLoS One. 2019;14((5)):e0216891. doi: 10.1371/journal.pone.0216891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002 Jul 1;166((1)):111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40((5)):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995 Mar 2;332((9)):556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56((3)):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 19.Karas M, Urbanek JK, Illiano VP, Bogaarts G, Crainiceanu CM, Dorn JF. Estimation of free-living walking cadence from wrist-worn sensor accelerometry data and its association with SF-36 quality of life scores. Physiol Meas. 2021 Jun 29;42((6)) doi: 10.1088/1361-6579/ac067b. [DOI] [PubMed] [Google Scholar]
- 20.Urbanek JK, Zipunnikov V, Harris T, Fadel W, Glynn N, Koster A, et al. Prediction of sustained harmonic walking in the free-living environment using raw accelerometry data. Physiol Meas. 2018 Feb 28;39((2)):02nt02. doi: 10.1088/1361-6579/aaa74d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagasaki H, Itoh H, Hashizume K, Furuna T, Maruyama H, Kinugasa T. Walking patterns and finger rhythm of older adults. Percept Mot Skills. 1996 Apr;82((2)):435–47. doi: 10.2466/pms.1996.82.2.435. [DOI] [PubMed] [Google Scholar]
- 22.McConnell MV, Shcherbina A, Pavlovic A, Homburger JR, Goldfeder RL, Waggot D, et al. Feasibility of obtaining measures of lifestyle from a smartphone app: the myheart counts cardiovascular health study. JAMA Cardiol. 2017 Jan 1;2((1)):67–76. doi: 10.1001/jamacardio.2016.4395. [DOI] [PubMed] [Google Scholar]
- 23.McDermott MM, Spring B, Berger JS, Treat-Jacobson D, Conte MS, Creager MA, et al. Effect of a home-based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease: the HONOR randomized clinical trial. JAMA. 2018 Apr 24;319((16)):1665–76. doi: 10.1001/jama.2018.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmichael H, Overbey DM, Hosokawa P, Goode CM, Jones TS, Barnett CC, Jr, et al. Wearable technology-a pilot study to define “Normal” postoperative recovery trajectories. J Surg Res. 2019 Dec;244:368–73. doi: 10.1016/j.jss.2019.06.057. [DOI] [PubMed] [Google Scholar]
- 25.Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003 Feb;123((2)):387–98. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- 26.Deiner S, Fleisher LA, Leung JM, Peden C, Miller T, Neuman MD. Adherence to recommended practices for perioperative anesthesia care for older adults among US anesthesiologists: results from the ASA committee on geriatric anesthesia-perioperative brain health initiative ASA member survey. Perioper Med. 2020;9:6. doi: 10.1186/s13741-020-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozemek C, Strath SJ, Riggin K, Harber MP, Imboden MT, Kaminsky LA. Pedometer feedback interventions increase daily physical activity in phase III cardiac rehabilitation participants. J Cardiopulm Rehabil Prev. 2020;40((3)):183–8. doi: 10.1097/HCR.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 28.Karas M, Bai J, Strączkiewicz M, Harezlak J, Glynn NW, Harris T, et al. Accelerometry data in health research: challenges and opportunities. Stat Biosci. 2019 Jul;11((2)):210–37. doi: 10.1007/s12561-018-9227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daskivich TJ, Houman J, Lopez M, Luu M, Fleshner P, Zaghiyan K, et al. Association of wearable activity monitors with assessment of daily ambulation and length of stay among patients undergoing major surgery. JAMA Netw Open. 2019;2((2)):e187673. doi: 10.1001/jamanetworkopen.2018.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants but are available from the corresponding author (D.S.R.) upon reasonable request.