Abstract
Background
Chronic kidney disease (CKD) has become a global public health problem nowadays. As cardiovascular diseases (CVDs) are the primary cause of death in advanced CKD patients, much attention has been paid to resolving their cardiovascular complications. However, managing CKD and cardiovascular complications is still a big challenge for nephrologists, as satisfactory treatments are still lacking. Platelets, the second most abundant cells in the blood, are the major participants of hemostasis, thrombosis, and wound healing. In recent years, platelets have been reported in various physiological and pathological processes, including CKD and CKD-related CVDs.
Keywords: Platelets, Chronic kidney disease, Cardiovascular complications
Introduction
Chronic kidney disease (CKD) is a kind of disease in which kidney damage or decreased glomerular filtration rate (<60 mL/min per 1.73 m2) exists for more than 3 months. It has become a global health problem, as the morbidity achieved approximately 10–13% [1]. A large proportion of CKD patients will gradually progress to end-stage kidney disease and eventually need replacement therapy with dialysis for life and even kidney transplantation [2]. Although extensive studies indicate that CKD progression is characterized by tubular atrophy, glomerular sclerosis, interstitial fibrosis, and peritubular capillary rarefaction, the pathological mechanisms of CKD have not been fully elucidated.
Dramatically, in CKD patients, the most common complications and major causes of death are cardiovascular diseases (CVDs) [3]. Thus, the type 4 cardio-renal syndrome was defined as the CVDs induced by CKD. However, it remains largely unknown how CKD facilitates the development of CVDs. Therefore, exploring the mechanisms of CKD and its cardiovascular complications is either beneficial for finding preventive and therapeutic targets or reducing the morbidity and mortality of CKD patients.
During the past decades, platelets have been found with extensive and versatile functions. As we all know, platelets are essential participants in acute coronary syndromes and are involved in atherosclerosis and thrombosis [4]. Besides, accumulating evidence suggests that platelets are involved in the pathogenesis of CKD, as they are hyperactivated and can participate in the processes of chronic inflammation, oxidative stress, immunoreaction, and fibrosis associated with CKD progression [5]. Therefore, understanding the functions and mechanisms of platelets in CKD and identifying effective intervention targets of platelets provide broad prospects for CKD patients, with an ultimate aim to reduce cardiovascular morbidity and mortality. In this review, we mainly focus on the roles and mechanisms of activated platelets in CKD and CKD-related CVDs.
Platelet and Its Vesicles
Platelets are anucleate blood cells with a 2–4 μm diameter. Platelets originate from proplatelets derived from megakaryocytes predominantly situated at bone marrow sinusoids [5] and a recent study also provided evidence for megakaryocytes and platelet production in the lung [6]. The traditional functions of platelets are hemostasis, thrombosis, and wound healing [7].
Platelets contain a great variety of cell surface receptors and adhesion molecules, which make them respond quickly to stimuli such as injury or infection [5]. As they are highly sensitive to environmental changes and are present in high numbers in the circulation, they are the first cells to arrive at sites of acute injury, where they interact with endothelial cells and leukocytes. In response to different stimuli, resting platelets become active and differentiate into different subtypes according to the activation of different surface receptors and signaling integrin molecules. Once activated, platelets begin to change shape, degranulate, and release microvesicles to recruit additional platelets and other immune cells [5].
Platelets carry three different types of microvesicles: α-granules, dense granules, and lysosomes. These microvesicles contain various biomolecules, including over 300 kinds of different proteins and other bioactive mediators, such as P-selectin, thrombospondin, platelet-derived growth factor (PDGF), thromboxane, and platelet-activating factor. In addition, platelet microvesicles contain mRNAs and miRNAs that can be transferred to other cells, modulating their gene transcription and protein synthesis. Through the release of microvesicles, platelets can rapidly modulate molecular processes that regulate coagulation, inflammation, fibrosis, and redox, all of which are associated with CKD and CKD-related CVDs [8].
Hyperactivated Platelets Contribute to the Progress of CKD
Platelets are hyperactivated in CKD patients. This can be detected by the increased expression of P-selectin, thromboxane A2, CC-chemokine ligand 5(CCL5), CD154, platelet factor 4(PF4), etc. [9, 10]. Clinically, it was also detected that mean platelet volume was obviously increased with the progression of CKD. Many substances lead to platelet activation in CKD patients. On the other hand, hyperactivated platelets contribute to the progress of CKD in turn.
Platelets Release Proinflammatory Factors and Interact with Inflammatory Cells to Accelerate Renal Inflammation and Fibrosis in CKD
Chronic, systemic, and low-grade inflammation is a long and generalized process, which can be usually observed in CKD patients [11]. Renal inflammation plays a central role in the initiation and progression of renal fibrosis and CKD-induced complications. Multiple pieces of evidence show there are bidirectional relationships between platelets and inflammation.
On the one hand, inflammation can induce platelet activation, evidenced by the increase in platelet aggregation and the interaction of platelets with monocyte [12]. On the other hand, platelets can release multiple proinflammatory factors such as PF4, stromal cell-derived factor-1, epithelial neutrophil-activating protein 78, IL-1β, CD40 Ligand (CD40L), and CCL5, all of which further accelerate the development of CKD [13, 14]. Platelet-derived proinflammatory factors can trigger a switch of endothelial cells to a more inflammatory phenotype [15], which will subsequently release inflammatory cytokines such as IL-8, CCL2, etc. Therefore, activated platelets in CKD contribute to leukocyte recruitment.
In CKD, the interactions between platelets, monocytes, and endothelial cells are enhanced, facilitated by the increases in inflammatory cytokine levels and cell adhesion molecules in these cell types [16]. Platelet-derived extracellular vesicles (EVs) activate endothelial cells and leukocytes by surface molecules CD41 and CD62P. Additionally, platelet EVs can induce the migration and proliferation of vascular smooth muscle cells (VSMCs), as prolonged incubation of VSMCs with platelet EVs results in increased adhesiveness for THP1 monocytic cells and an increase in IL-6 production [17]. These findings indicate that platelet EVs have proinflammatory effects and promote endothelial dysfunction. Besides, incubating VSMCs with platelet-derived EVs led to a phenotypic transition towards a synthetic phenotype, as evidenced by the morphological changes and the reduced expression of contractile marker calponin [17]. Therefore, platelets can amplify the inflammation by releasing EVs under CKD conditions.
Particularly, activated platelets can express CD40 and CD154, the latter of which is also termed CD40L. CD40 is a transmembrane glycoprotein receptor expressed in platelets and endothelial cells. However, CD40L in platelets is only expressed upon activation and always in a soluble form [18]. In CKD patients, the level of CD40L from platelet microparticles was obviously increased, especially in CKD 4–5 stages [19]. According to the binding of CD40 and CD40L, CD40L on platelets induces endothelial cells to secrete chemokines and express adhesion molecules, thereby generating signals for the recruitment and extravasation of leukocytes at the site of injury [20]. This study revealed that platelets could directly initiate an inflammatory response of the vessel wall. Under this condition, endothelial cells upregulate the surface adhesion molecules E-selectin, VCAM1, and ICAM1, release CCL2, and further boost the recruitment of leukocytes, such as macrophages and neutrophils [21]. All these actions above promote the creation of a constantly inflammatory environment in CKD patients.
Some inflammatory factors are reported to be associated with renal fibrosis. After activation, platelets can increase the release of inflammatory factors in CKD [13]. The profibrotic inflammatory factors such as CCL5, TNF-a, TGF-β, and PDGF are reported to be up-regulated, while the antifibrotic factors, such as AMPK and IL-10, are down-regulated in the kidneys of CKD patients. Among them, TGF-β drives renal fibrosis by activating lots of signaling molecules and plays a central role in renal fibrosis [11]. It is reasonable to infer platelet can contribute to systemic inflammation and promote renal fibrosis in CKD patients, which is a characteristic process connecting inflammatory factors, recruitment of leukocytes, and activated fibrotic signal pathways.
Moreover, platelets are also involved in the phenotypic change of macrophages to promote fibrosis of CKD. Macrophages belong to the mononuclear phagocytic system [22]. Based on their functions and anatomical location, macrophages are divided into different subpopulations [23]. In the kidneys, macrophages can be broadly classified into two different subtypes: classically activated (M1) macrophages (which can release inflammatory factors) and alternatively activated (M2) macrophages (which can release TGF-β-promoting fibrosis) [24, 25]. With the development of CKD, M1 macrophages can switch to fibrotic-M2 macrophages [24]. A study illustrated that by incubating platelet-derived EVs with monocytes, platelet EVs can preferably bind to monocytes, and platelet EVs can be absorbed by phagocytic over time [26]. Thus, monocytes can harbor the platelet markers. Prolonged incubation of monocytes with platelet EVs results in a remarkable change of surface marker expression, indicating a polarization of the monocytes to M2-type macrophages [26]. Therefore, it is a novel thrombo-inflammatory pathway that platelets may mediate the monocytes to M2-type macrophages and probably contribute to the progress of CKD by M2 macrophages.
MiRNAs Released from Platelets Play a Vital Role in Fibrosis of CKD
Although devoid of a nucleus and lacking genomic DNA, circulating human platelets retain as much as 45% of the Refseq genes in the form of mRNAs [27]. Platelets contain an abundant and diverse array of mRNAs and miRNAs. MiRNAs are noncoding RNAs with a length of 20–25 nucleotides. The binding of miRNAs to their respective target mRNAs promotes degradation of the mRNAs [28]. Clinical research investigated the circulating platelets of 10 CKD patients and five age- and sex-matched healthy subjects. They found that platelet mRNA and miRNA transcriptome was altered in CKD patients and could be restored partially upon dialysis [29]. Platelet-derived miRNAs can be internalized by recipient cells including endothelial cells, macrophages, and VSMCs, where the altered miRNAs may take part in the molecular processes of oxidative stress, inflammation, and fibrosis of CKD [30]. Therefore, some circulating miRNAs have been suggested as promising noninvasive biomarkers in CKD patients. Microarray screening revealed miRNAs from activated platelets or platelet microparticles mainly include miRNA-223, miRNA-126, miRNA-21, miRNA-191, miRNA-150, miRNA-24, and miRNA-197 [30, 31, 32].
The miRNA-21 is an evolutionarily conservative miRNA and almost exists in all types of cells, among which platelets are the major sources [33, 34]. MiRNA-21 is very stable in the blood and performs vital regulatory roles in health and disease [33]. A study in 2015 found a strong up-regulation of miRNA-21 in the kidneys of mice with unilateral ureteral obstruction and also in the kidneys of patients with severe kidney fibrosis. In addition, their data also indicated that circulating miR-21 levels were associated with renal fibrosis [35]. Another study reported miRNA-21 could contribute to fibrogenesis and epithelial injury by suppressing the expression of peroxisome proliferator-activated receptor (PPAR)-α, which is a major regulator of the mitochondrial β-oxidation pathways [36]. Genetic deletion of miRNA-21 in mice dramatically reduced interstitial fibrosis, glomerulosclerosis, tubular injury, and inflammation and prevented CKD progression. Inhibition of miRNA-21 was protective against TGF-β-induced fibrogenesis and inflammation in glomerular and interstitial cells, likely as the result of enhanced PPARα/RXR activity and improved mitochondrial function in CKD mice [37]. MiRNA-21 also upregulates extracellular signal-regulated kinase (ERK) signaling in the kidney. Both ERK1/2 and TGF-β/Smad signaling pathways seem to be emphasized in the development of kidney fibrosis in diabetic models [38]. Moreover, miRNA-21 and miRNA-124 also activate the profibrotic genes in human podocytes and tubular cells in a model of IgA nephropathy [39]. All these studies indicate that miRNA-21 plays an essential role in the fibrosis of CKD, and it can be a candidate target for antifibrotic therapies.
MiRNA-223 is the most abundant miRNA from platelet microvesicles. It is considered to be associated with several inflammatory disorders including diabetes-type 2, sepsis, and rheumatoid arthritis. MiRNA-223 can be delivered into vascular endothelial cells, where it participates in the process of inflammation in CKD [40]. Studies have revealed the close relationship between miRNA-223 and the NLRP3 gene in several disease models including IgA nephropathy, atherosclerosis, and diabetic cardiomyopathy [41, 42]. Thus, it is reasonable to predict that miRNA-223 may promote fibrosis of the kidneys by activating inflammation.
Although miRNAs have great potential and more and more research is exploring the functions of miRNAs, the studies regarding the detailed molecular mechanisms of platelet-derived miRNAs in CKD are quite limited. From existing data, we can infer that platelet-derived miRNAs can have extraordinary functions in regulating fibrosis in CKD, and targeting specific platelet-derived miRNAs could be a novel therapeutic approach to treating renal fibrosis. Therefore, further studies are needed to elucidate the molecular mechanism of miRNAs.
Platelets Accelerate Glomerulosclerosis in CKD Progression
Glomerulosclerosis is an important progressive pathological process that appears in almost all kinds of CKDs as well as the natural aging process. Glomerulosclerosis is defined as the obstruction of glomerular capillaries and loss of podocytes by extracellular matrix (ECM) deposition [43]. The glomerulus contains four different cell types including parietal epithelial cells, endothelial cells, podocytes, and mesangial cells [44], among which podocytes are the most important in maintaining the structure of the glomerular filtration. In recent years, platelets have been reported to influence the normal functions of glomeruli and participate in the process of glomerulosclerosis.
First of all, activated platelets may affect the functions of podocytes and the remodeling of GBM. As mentioned before, platelets are a primary blood reservoir for CD154. In the glomerulus, CD40 is synthesized by podocytes and can be detectable in kidney tissue sections. Activated platelet supernatants induced matrix metalloproteinases 9 (MMP-9) mRNA synthesis in podocytes, an effect reduced by anti-CD40 antibody [45]. This study uncovered the potential role of platelets through the CD40/CD154 signaling pathway in the control of GBM synthesis and degradation. In addition, there are still some studies that reported CD154 may contribute to the regulation of matrix remodeling proteins, particularly through the induction of MMP-9 in other disease models [46, 47]. In a word, the platelet-derived CD154 activates the CD40/CD154 signaling pathway to modulate matrix remodeling through the synthesis of MMPs in podocytes, further contributing to CKD progression.
Second, platelet secretory factors influence mesangial cell proliferation in glomerulosclerosis. Fibronectin, PF4, 12-hydroxyeicosatetraenoic acid, TGF-β, and PDGF, all can be released by platelets and almost all of them are related to mesangial cell proliferation [48, 49, 50, 51]. A study reported that fibronectin could promote mesangial cell migration and proliferation in vitro and contribute to extracellular matrix formation and tissue remodeling during glomerular disease [48]. Moreover, this research also proposed a hypothesis that fibronectin derived from platelets and macrophages served as a provisional matrix involved with mesangial cell migration into glomerular lesions [48]. Platelets are also crucial in mesangial cell injury to renal matrix expansion in an acute glomerular wound repair to chronic kidney injury animal model. Platelets inhibition significantly reduced TGF-β overexpression, fibrinogen deposition, and glomerular matrix expansion in this acute glomerular wound repair model [52].
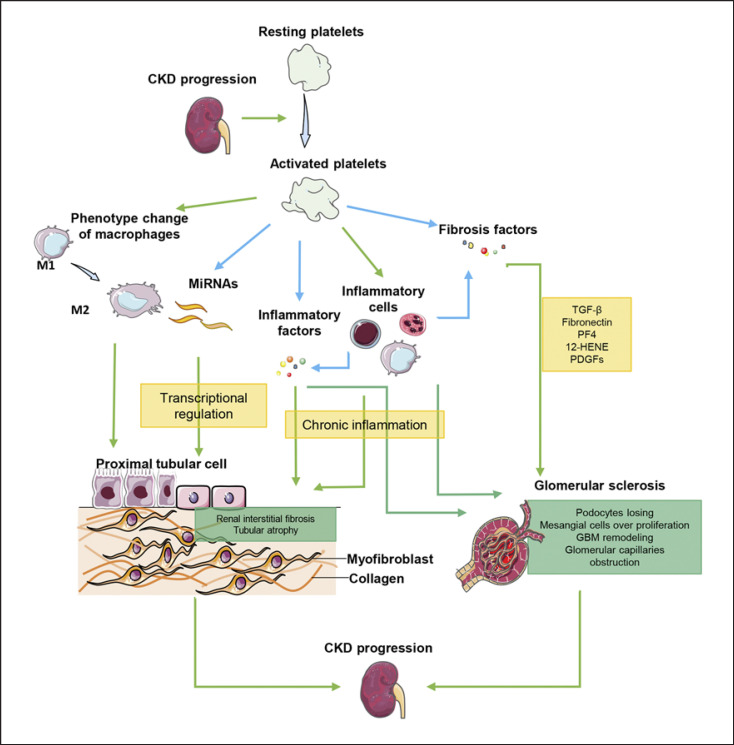
In conclusion, platelets may involve in ECM remodeling, cell migration, and proliferation to stimulate glomerular remodeling. The mechanisms of platelets in CKD progression are shown in Figure 1.
Fig. 1.
The mechanisms of platelets in CKD progression. Platelets can not only release miRNAs, inflammatory factors, and fibrosis factors to directly promote renal fibrosis, but also interact with inflammatory cells to promote CKD progression indirectly.
Involvement of Activated Platelets in Cardiovascular Complications of CKD
Cardiovascular complications of CKD mainly include cardiomyopathy, atherosclerosis, calcification, and subsequent result in heart failure, cerebrovascular, cardiovascular death, and so on [53]. CVD accounts for 40–50% of deaths among patients with end-stage kidney disease [54], which is much higher than that in age- and sex-matched people [55].
A clinical study stated that for patients with CKD stage 5 or receiving dialysis treatment, higher platelet counts tend to be associated with a greater risk of CVD events [56]. It was also discovered that patients with CVDs had higher mean platelet volumes than those without CVDs in CKD [57]. Our group also reported that platelet counts, plateletcrit, and platelet distribution width were associated with CVD events in CKD patients without dialysis [58]. The changes in platelet indices in CKD patients indicate the vital role of platelets in CKD-related CVDs. To reduce the morbidity of cardiovascular complications in CKD, it has become a common therapy to use acetylsalicylic acid, clopidogrel, etc. as antiplatelet agents. A significant reduction in cardiovascular mortality was observed in CKD patients who received aspirin alone or in combination with a β-blocker compared to those who did not receive either medication [59]. What's more, although there is still a lack of evidence using acetylsalicylic acid as cardiovascular primary prevention in CKD patients, research has clearly shown aspirin administration resulted in an absolute risk reduction of major CVD events [60]. The information on antiplatelet agents in CKD and CVDs is listed in Table 1.
Table 1.
Platelet inhibitors used in CKD and CKD related CVDs
| Medication | Dose | Comments |
|---|---|---|
| Acetylsalicylic acid | 100 mg/day | Reduced effect in CKD stages 4 and 5 |
|
| ||
| Clopidogrel | 75 mg/day | Reduced effect in CKD stages 4 and 5 |
|
| ||
| Prasugrel | 10 mg/day | More safety in bleeding events |
|
| ||
| Ticagrelor | 180 mg/day | A higher antiplatelets efficiency but a higher incidence of bleeding especially in CKD stages 4 and 5 |
Under CKD conditions, many sera pathophysiological factors can lead to the activation of platelets. These factors, like accumulated uremic toxins and inflammatory cytokines, induce the overproduction of platelet microvesicles. They can be directly absorbed by vascular cells and even mediate platelet-monocyte aggregation, further leading to vascular calcification (VC), atherosclerosis, and heart fibrosis in CKD patients. In a word, platelets play a vital role in the cardiovascular complication progression of CKD patients. The functions of platelets in cardiovascular complications of CKD patients have been explored, which will be discussed below.
Platelets Expedite VC of CKD
Vascular calcification (VC) is defined as mineral deposition in the vasculature in a form of calcium-phosphate complexes [61]. VC often occurs with aging but is prevalent in patients with hypertension, CKD, or diabetes [61]. Different from other pathological types, CKD-induced VC often occurs in the medial layer. Even in the early stage of CKD, the rate of VC increases obviously. Many factors influence the progression of VC in CKD, such as oxidative stress, endothelial dysfunction, and the increased levels of proinflammatory cytokines.
As mentioned before, platelets can directly release many inflammatory factors that can contribute to the persistent inflammatory state in CKD and recruit more inflammatory cells, further enhancing the interactions between VSMCs and inflammatory cells [62]. These interactions result in phenotypic switching of VSMCs. The phenotype of osteochondrogenic VSMCs can enhance cell migration and proliferation and eventually facilitate VC [63]. This might be a general process that happened in the development of VC in CKD patients.
Furthermore, platelets can also express and release osteocalcin (OC). OC is one of the most abundant noncollagenous proteins in bone and is primarily generated by osteoblasts during bone formation. Recent data indicated OC was closely related to VC [64, 65]. OC exists in δ-granules of human platelets and is released upon platelet activation. In CKD patients, the total plasma OC concentration was higher when compared with that in the control group [66]. In the calcium-deposit area, there is an evident co-localization between OC and platelets, thus platelets probably secrete OC to promote the early stage of VC [64].
In conclusion, platelets can affect the inflammatory state either by releasing proinflammatory factors or releasing OC to promote VC in CKD. Further studies are needed to explore the detailed molecular mechanisms underlying the particular functions of OC and inflammatory factors from platelets in CKD-related VC.
Platelets Facilitate Vascular Fibrosis and Atherosclerosis in CKD
Both vascular fibrosis and atherosclerosis are momentous pathological processes in cardiovascular complications. Damage to vascular endothelial cells is the origin of vascular fibrosis and atherosclerosis and usually happens in CKD with a high frequency. The process of vascular endothelial cell damage often refers to premature senescence of endothelial cells, cell transition from an endothelial to a mesenchymal phenotype, endothelial cell dysfunction, and vascular fibrosis [67].
As we all know, platelets act essentially in vascular wound healing. At sites of vascular injury where endothelium is damaged or removed, clot formation and vessel contraction immediately occur, which is mediated largely by substances such as thromboxane and PDGF from activated platelets [5]. Then VSMCs are dedifferentiated and proliferated to repair the vascular injuries, with their phenotype shifting from a quiescent contractile phenotype to a highly synthetic and proliferating cell type. However, excessive repair of injuries by VSMCs can lead to intimal hyperplasia and fibrosis, which is engaged in the pathogenesis of atherosclerosis [7]. Current studies clearly show that platelets play an essential part in atherosclerotic lesions. Even more, activated platelets are present in the circulating blood of atherosclerotic individuals throughout the atherosclerotic process [5]. CKD, even at early stages, can increase the risk of atheromatous plaques [68].
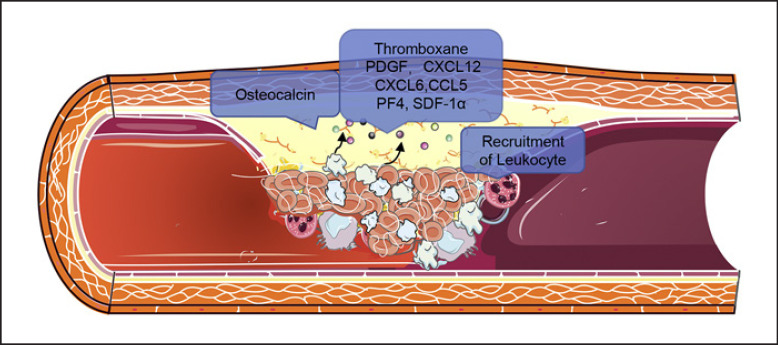
From a general position, activated platelets can release a plethora of chemokines, including CXCL4 or PF4, CCL5, CXCL12 or stromal cell-derived factor-1α (SDF-1α), and CXCL16, all of which initiate or promote local inflammatory processes at sites of vascular injury and atherosclerosis. Moreover, genome-wide miRNA sequencing of VSMCs cocultured with activated platelets identified significant increases in platelet-derived miRNA-223. MiRNA-223 appears to directly target PDGFRβ (in VSMCs), reversing the injury-induced dedifferentiation and intimal hyperplasia [69]. Thus, platelets may have bidirectional functions in vascular fibrosis, including initiating an immediate repair process and excessive repair in a delayed manner. In CKD, the aggregation of the circulating activated platelets and platelet-leukocytes is enhanced, promoting the development of atherosclerosis. The chemokines released by platelets in VC, vascular fibrosis and atherosclerosis progression are shown in Figure 2.
Fig. 2.
Activated platelets can secret lots of chemokines to promote VC and atherosclerosis in CKD.
There still exist some other functions of platelets in vascular fibrosis and atherosclerosis in CKD. Platelet-derived miRNAs are also proved to be implicated in the initiation and progression of atherosclerosis through the regulation of lipid metabolism, inflammatory response, oxidative stress, endothelial function, angiogenesis, and plaque formation [30]. MiRNA-126, mainly released by platelets, is thought to become a biomarker of CVDs because it was considerably elevated in vascular damage and endothelial dysfunction according to the detection in myocardial infarction patients. A positive association between circulating miRNA-126 and fatal myocardial infarction has been reported recently [70]. MiRNA-126 can control vascular inflammation by affecting the adhesion of white blood cells to the endothelium and has a positive function in CVDs. In addition, miRNA197, another miRNA shed mainly by platelets, is up-regulated in CKD patients. MiRNA-197 might facilitate dyslipidemia in metabolic syndrome, hence leading to the progression of CVDs [71]. Clinical research confirmed that an elevated level of miRNA-197 could be a predictor of cardiovascular death in a large patient cohort with coronary artery disease [72].
In a word, platelets are classic cells that participate in fibrosis and atherosclerosis. However, the particularity of CKD revealed some different roles of platelets. Thus, for one thing, platelets can secrete plenty of chemokines to enhance interactions between endothelial cells and leukocytes to enhance vascular fibrosis and atherosclerosis indirectly; for another thing, platelets can produce miRNAs to influence the transcription of key genes in the process of vascular fibrosis and atherosclerosis in CKD.
Platelets Deteriorate Cardiac Remodeling of CKD Both Directly and Indirectly
Aberrant cardiac remodeling with hypertrophy and fibrosis is one of the major pathological changes of CKD-associated CVDs. In CKD patients, uremic cardiomyopathy is a specialized cardiac pathology characterized by aberrant cardiac remodeling [73].
Previous studies have illustrated that activated platelets can release some pathophysiological factors including serotonin, thromboxane A2, platelet-activating factor, PDGF, etc. to participate in cardiac remodeling by regulating endothelial and VSMCs [74]. For example, a study showed that platelets and platelet-released serotonin (5-HT) were directly involved in the functional regulation of neonatal rat cardiac fibroblasts by enhancing the secretion of TGF-β1 and promoting their migration and differentiation to promote cardiac remodeling [75]. A recent study also provided evidence that platelet-specific p38α contributed to cardiac remodeling via the MAPK/P38 signal pathway, and platelet-specific p38α-deficient mice had improved cardiac function, reduced infarct size, decreased inflammatory response, and microthrombus in a myocardial infarction model [76]. Furthermore, the P2y12 receptor, one of the predominant activating receptors for platelets, promoted pressure overload–induced cardiac remodeling via platelet-driven inflammation in mice [77]. These studies clearly indicated that platelets and their products participate in cardiac remodeling directly. In addition, platelets can interact with those inflammatory cells and lead to cardiac remodeling indirectly, which is similar to the inflammatory functions of platelets in other cardiac pathological processes of CKD complications such as atherosclerosis and VC.
Platelet-derived miRNAs are also deeply engaged in cardiac remodeling. A clinical study suggested miRNA-21 as a novel biomarker for elderly patients with type 2 cardiorenal syndrome, as obviously the elevated level of serum miRNA-21 was found in these patients [78]. Similarly, miRNA-21-5p is a mediator of left ventricular remodeling through its regulation of PPARα [79]. In addition, miRNA-21 can control cardiac hypertrophy and affect the overall structure and functions of the heart by regulating the signaling pathway of ERK-MAP kinase [80]. What's more, overexpression of miRNA-24 in cultured rat cardiomyocytes resulted in hypertrophic growth. This indicates that miRNA-24 may regulate the development of cardiac remodeling [81]. Last but not least, miRNA-223 can regulate the gene expression of NLRP3 and conduce to fibrosis and inflammation of myocardial tissues in diabetic cardiomyopathy [42, 82]. All these studies clearly showed that platelet-derived miRNAs were closely related to cardiac remodeling. The functions of platelet-derived miRNAs in CKD and CKD-related CVDs are listed in Table 2.
Table 2.
Platelets-derived miRNAs in CKD and CKD related CVDs
| MiRNAs | Pathology | Effect | Mechanisms | References |
|---|---|---|---|---|
| MiRNAs in CKD | ||||
| miRNA-223 | IgA nephropathy, atherosclerosis, diabetic cardiomyopathy | Inflammation, apoptosis of vascular endothelial cell | Insulin-like growth factor 1 receptor, NLRP3 inflammasome | [40, 41, 42] |
| miRNA-21 | CKD, cardiorenal syndrome type 4 | Glomerulosclerosis, interstitial fibrosis, tubular injury, and inflammation | ERK1/2, TGF-β/Smad signaling, left ventricular remodeling | [36, 37, 38, 39] |
| MiRNAs in CVDs | ||||
| miRNA-223 | Atherosclerosis | VSMCs dedifferentiation and intimal hyperplasia | PDGFRβ | [69] |
| miRNA-126 | Myocardial infarction | Vascular damage and endothelial dysfunction, vascular inflammation | − | [70] |
| miRNA-197 | Metabolic syndrome | Dyslipidemia | − | [71] |
| miRNA-21 | Type 2 cardiorenal syndrome | Left ventricular remodeling, cardiac hypertrophy | ERK-MAP, left ventricular remodeling | [78, 79, 80] |
| miRNA-24 | Heart failure | Cardiac remodeling | − | [81] |
In CKD patients, the high frequency of cardiac remodeling is strongly linked to platelet activation, which causes a cascade of downstream reactions. In a recent study, Yang et al. [83] found that platelets were significantly activated in 5/6 nephrectomy-operated mice, while cardiac remodeling was significantly ameliorated when platelets were effectively depleted. They further found that activated platelets released PF4 and induced macrophages to polarize toward a specific phenotype intermediate between the previously characterized M1 and M4 phenotypes. Then activated macrophages secreted MMP7, which could cleave a wide range of ECM proteins including collagen I, thereby leading to the process of cardiac remodeling during uremia. This study provided a potential mechanism of cardiac remodeling in uremic mice [83].
In conclusion, inappropriate platelet activation can affect the cardiac remodeling of CKD. They can facilitate macrophage dysfunction in cardiac remodeling in uremic mice. What's more, platelets can also recruit proinflammatory cells or shed miRNAs to precipitate cardiac remodeling.
Conclusion
Here, we review the pathogenic roles of platelets in CKD and its cardiovascular complications. Besides thrombosis, the hyperactivated platelets also release large amounts of cytokines and chemokines that directly or indirectly contribute to CKD progression and the development of cardiovascular complications. Meanwhile, the hyperactivated platelets can also be swallowed by other cells, wherein they shed miRNA to affect their activities. Given these pleiotropic roles of platelets, we anticipate that more pathogenic mechanisms of platelet hyperactivation in CKD need to be defined. What's more, the prolonged and persistent state of inflammation in CKD patients is closely linked to coagulation disorder. Inflammation results in activation of coagulation, and coagulation also affects inflammatory activity. Proinflammatory cytokines and other mediators are capable of activating the coagulation system and leading to thrombin generation. As a result of the central role of platelets in the process of thrombosis, there exists an upward tendency in thrombotic risk in CKD patients. So oral anticoagulants are commonly used drugs in patients with CKD. Of note, both thrombosis and hemorrhage are prevalent in CKD; it remains unclear how CKD affects hemostasis via regulating platelet activity. Further studies focusing on the distinctive regulation of platelet activity by different stages of CKD or the special treatment during CKD may reconcile this contradiction. We believe that deeply resolving these questions will help explain the intrinsic mechanism of the high morbidity and mortality of CVD in CKD as well as provide new therapeutic targets for CKD-associated CVD.
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
Funding Sources
This study was supported by research grants from the Key Program of the Natural Science Foundation of China (No. 82030023), the Natural Science Foundation of China (No. 32171104, 81873605), the Chongqing Science and Technology Talent Program (cstc2021ycjh-bgzxm0145), and the Personal Training Program for Clinical Medicine Research of Army Medical University (No. 2018XLC1007).
Author Contributions
Shuiqin Gong: writing the draft of the review; Chenyu Wang: graphic drawing of the figures; Jiachuan Xiong, Ke Yang, and Jinghong Zhao: revising the review; Ke Yang and Jinghong Zhao: designing and approving this work.
References
- 1.Ammirati AL. Chronic kidney disease. Rev Assoc Med Bras. 2020 Jan 13;66((Suppl 1)):s03–9. doi: 10.1590/1806-9282.66.S1.3. [DOI] [PubMed] [Google Scholar]
- 2.Himmelfarb J, Ikizler TA. Hemodialysis. N Engl J Med. 2010 Nov 4;363((19)):1833–45. doi: 10.1056/NEJMra0902710. [DOI] [PubMed] [Google Scholar]
- 3.House AA. Cardio-renal syndrome type 4: epidemiology, pathophysiology and treatment. Semin Nephrol. 2012 Jan;32((1)):40–8. doi: 10.1016/j.semnephrol.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Yeung J, Li W, Holinstat M. Platelet signaling and disease: targeted therapy for thrombosis and other related diseases. Pharmacol Rev. 2018 Jul;70((3)):526–48. doi: 10.1124/pr.117.014530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Meijden PEJ, Heemskerk JWM. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019 Mar;16((3)):166–79. doi: 10.1038/s41569-018-0110-0. [DOI] [PubMed] [Google Scholar]
- 6.Lefrancais E, Looney MR. Platelet biogenesis in the lung circulation. Physiology. 2019 Nov 1;34((6)):392–401. doi: 10.1152/physiol.00017.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holinstat M. Normal platelet function. Cancer Metastasis Rev. 2017 Jun;36((2)):195–8. doi: 10.1007/s10555-017-9677-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golebiewska EM, Poole AW. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015 May;29((3)):153–62. doi: 10.1016/j.blre.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen MPB, Florquin S, Roelofs JJTH. The role of platelets in acute kidney injury. Nat Rev Nephrol. 2018 Jul;14((7)):457–71. doi: 10.1038/s41581-018-0015-5. [DOI] [PubMed] [Google Scholar]
- 10.Stepniewska J, Dolegowska B, Chrusciana M, Golembiewska E, Malinowska-Jedraszczyk A, Marchelek-Mysliwiec M, et al. Platelet: derived CD154 antigen in patients with chronic kidney disease. Clin Biochem. 2016 Feb;49((3)):243–7. doi: 10.1016/j.clinbiochem.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Lv W, Booz GW, Wang Y, Fan F, Roman RJ. Inflammation and renal fibrosis: recent developments on key signaling molecules as potential therapeutic targets. Eur J Pharmacol. 2018 Feb 5;820:65–76. doi: 10.1016/j.ejphar.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuchtriegel G, Uhl B, Puhr-Westerheide D, Pornbacher M, Lauber K, Krombach F, et al. Platelets guide leukocytes to their sites of extravasation. PLoS Biol. 2016 May;14((5)):e1002459. doi: 10.1371/journal.pbio.1002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gros A, Ollivier V, Ho-Tin-Noé B. Platelets in inflammation: regulation of leukocyte activities and vascular repair. Front Immunol. 2014;5:678. doi: 10.3389/fimmu.2014.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cloutier N, Pare A, Farndale RW, Schumacher HR, Nigrovic PA, Lacroix S, et al. Platelets can enhance vascular permeability. Blood. 2012 Aug 9;120((6)):1334–43. doi: 10.1182/blood-2012-02-413047. [DOI] [PubMed] [Google Scholar]
- 15.Gawaz M, Brand K, Dickfeld T, Pogatsa-Murray G, Page S, Bogner C, et al. Platelets induce alterations of chemotactic and adhesive properties of endothelial cells mediated through an interleukin-1-dependent mechanism. Implications for atherogenesis. Atherosclerosis. 2000 Jan;148((1)):75–85. doi: 10.1016/s0021-9150(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 16.van der Poll T, Parker RI. Platelet activation and endothelial cell dysfunction. Crit Care Clin. 2020 Apr;36((2)):233–53. doi: 10.1016/j.ccc.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Vajen T, Benedikter BJ, Heinzmann ACA, Vasina EM, Henskens Y, Parsons M, et al. Platelet extracellular vesicles induce a pro-inflammatory smooth muscle cell phenotype. J Extracell Vesicles. 2017;6((1)):1322454. doi: 10.1080/20013078.2017.1322454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inwald DP, McDowall A, Peters MJ, Callard RE, Klein NJ. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res. 2003 May 16;92((9)):1041–8. doi: 10.1161/01.RES.0000070111.98158.6C. [DOI] [PubMed] [Google Scholar]
- 19.Mortberg J, Lundwall K, Mobarrez F, Wallen H, Jacobson SH, Spaak J. Increased concentrations of platelet- and endothelial-derived microparticles in patients with myocardial infarction and reduced renal function- a descriptive study. BMC Nephrol. 2019 Mar 1;20((1)):71. doi: 10.1186/s12882-019-1261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998 Feb 5;391((6667)):591–4. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 21.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011 Apr;11((4)):264–74. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 22.Nelson PJ, Rees AJ, Griffin MD, Hughes J, Kurts C, Duffield J. The renal mononuclear phagocytic system. J Am Soc Nephrol. 2012 Feb;23((2)):194–203. doi: 10.1681/ASN.2011070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009 Apr;9((4)):259–70. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guiteras R, Flaquer M, Cruzado JM. Macrophage in chronic kidney disease. Clin Kidney J. 2016 Dec;9((6)):765–71. doi: 10.1093/ckj/sfw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Harris DC. Macrophages in renal disease. J Am Soc Nephrol. 2011 Jan;22((1)):21–7. doi: 10.1681/ASN.2010030269. [DOI] [PubMed] [Google Scholar]
- 26.Chimen M, Evryviadou A, Box CL, Harrison MJ, Hazeldine J, Dib LH, et al. Appropriation of GPIbalpha from platelet-derived extracellular vesicles supports monocyte recruitment in systemic inflammation. Haematologica. 2020 May;105((5)):1248–61. doi: 10.3324/haematol.2018.215145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, et al. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood. 2011 Oct 6;118((14)):e101–11. doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vettori S, Gay S, Distler O. Role of microRNAs in fibrosis. Open Rheumatol J. 2012;6:130–9. doi: 10.2174/1874312901206010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plé H, Maltais M, Corduan A, Rousseau G, Madore F, Provost P. Alteration of the platelet transcriptome in chronic kidney disease. Thromb Haemost. 2012 Oct;108((4)):605–15. doi: 10.1160/TH12-03-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuentes E, Palomo I, Alarcón M. Platelet miRNAs and cardiovascular diseases. Life Sci. 2015 Jul 15;133:29–44. doi: 10.1016/j.lfs.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 31.Willeit P, Zampetaki A, Dudek K, Kaudewitz D, King A, Kirkby NS, et al. Circulating microRNAs as novel biomarkers for platelet activation. Circ Res. 2013 Feb 15;112((4)):595–600. doi: 10.1161/CIRCRESAHA.111.300539. [DOI] [PubMed] [Google Scholar]
- 32.Pordzik J, Pisarz K, De Rosa S, Jones AD, Eyileten C, Indolfi C, et al. The potential role of platelet-related microRNAs in the development of cardiovascular events in high-risk populations, including diabetic patients: a review. Front Endocrinol. 2018;9:74. doi: 10.3389/fendo.2018.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krzywinska O, Bracha M, Jeanniere C, Recchia E, Kedziora Kornatowska K, Kozakiewicz M. Meta-analysis of the potential role of miRNA-21 in cardiovascular system function monitoring. Biomed Res Int. 2020;2020:4525410. doi: 10.1155/2020/4525410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenike AE, Halushka MK. miR-21: a non-specific biomarker of all maladies. Biomark Res. 2021 Mar 12;9((1)):18. doi: 10.1186/s40364-021-00272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glowacki F, Savary G, Gnemmi V, Buob D, Van der Hauwaert C, Lo-Guidice JM, et al. Increased circulating miR-21 levels are associated with kidney fibrosis. PLoS One. 2013;8((2)):e58014. doi: 10.1371/journal.pone.0058014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Science Transl Med. 2012 Feb 15;4((121)):121ra18. doi: 10.1126/scitranslmed.3003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S, et al. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J Clin Invest. 2015 Jan;125((1)):141–56. doi: 10.1172/JCI75852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng X, Gao W, Dang Y, Liu X, Li Y, Peng X, et al. Both ERK/MAPK and TGF-Beta/Smad signaling pathways play a role in the kidney fibrosis of diabetic mice accelerated by blood glucose fluctuation. J Diabetes Res. 2013;2013:463740. doi: 10.1155/2013/463740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milas O, Gadalean F, Vlad A, Dumitrascu V, Gluhovschi C, Gluhovschi G, et al. Deregulated profiles of urinary microRNAs may explain podocyte injury and proximal tubule dysfunction in normoalbuminuric patients with type 2 diabetes mellitus. J Investig Med. 2018 Apr;66((4)):747–54. doi: 10.1136/jim-2017-000556. [DOI] [PubMed] [Google Scholar]
- 40.Pan Y, Liang H, Liu H, Li D, Chen X, Li L, et al. Platelet-secreted microRNA-223 promotes endothelial cell apoptosis induced by advanced glycation end products via targeting the insulin-like growth factor 1 receptor. J Immunol. 2014 Jan 1;192((1)):437–46. doi: 10.4049/jimmunol.1301790. [DOI] [PubMed] [Google Scholar]
- 41.Li L, Gong Z, Xue P, Wang D, Xu M, Sui S, et al. Expression of miRNA-223 and NLRP3 gene in IgA patients and intervention of traditional Chinese medicine. Saudi J Biol Sci. 2020 Jun;27((6)):1521–6. doi: 10.1016/j.sjbs.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu D, Zhang X, Chen X, Yang S, Chen H. Inhibition of miR-223 attenuates the NLRP3 inflammasome activation, fibrosis, and apoptosis in diabetic cardiomyopathy. Life Sci. 2020 Sep 1;256:117980. doi: 10.1016/j.lfs.2020.117980. [DOI] [PubMed] [Google Scholar]
- 43.Meng XM, Nikolic-Paterson DJ, Lan HY. Inflammatory processes in renal fibrosis. Nat Rev Nephrol. 2014 Sep;10((9)):493–503. doi: 10.1038/nrneph.2014.114. [DOI] [PubMed] [Google Scholar]
- 44.Shabaka A, Tato Ribera A, Fernández-Juárez G. Focal segmental glomerulosclerosis: state-of-the-art and clinical perspective. Nephron. 2020;144((9)):413–27. doi: 10.1159/000508099. [DOI] [PubMed] [Google Scholar]
- 45.Rigothier C, Daculsi R, Lepreux S, Auguste P, Villeneuve J, Dewitte A, et al. CD154 induces matrix metalloproteinase-9 secretion in human podocytes. J Cell Biochem. 2016 Dec;117((12)):2737–47. doi: 10.1002/jcb.25571. [DOI] [PubMed] [Google Scholar]
- 46.Schonbeck U, Mach F, Sukhova GK, Murphy C, Bonnefoy JY, Fabunmi RP, et al. Regulation of matrix metalloproteinase expression in human vascular smooth muscle cells by T lymphocytes: a role for CD40 signaling in plaque rupture? Circ Res. 1997 Sep;81((3)):448–54. doi: 10.1161/01.res.81.3.448. [DOI] [PubMed] [Google Scholar]
- 47.Mach F, Schonbeck U, Fabunmi RP, Murphy C, Atkinson E, Bonnefoy JY, et al. T lymphocytes induce endothelial cell matrix metalloproteinase expression by a CD40L-dependent mechanism: implications for tubule formation. Am J Pathol. 1999 Jan;154((1)):229–38. doi: 10.1016/S0002-9440(10)65269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barnes JL, Hastings RR, De la Garza MA. Sequential expression of cellular fibronectin by platelets, macrophages, and mesangial cells in proliferative glomerulonephritis. Am J Pathol. 1994 Sep;145((3)):585–97. [PMC free article] [PubMed] [Google Scholar]
- 49.Couser WG. Pathogenesis of glomerular damage in glomerulonephritis. Nephrol Dial Transplant. 1998;13((Suppl 1)):10–5. doi: 10.1093/ndt/13.suppl_1.10. [DOI] [PubMed] [Google Scholar]
- 50.Lianos EA, Bresnahan BA, Pan C. Mesangial cell immune injury. Synthesis, origin, and role of eicosanoids. J Clin Invest. 1991 Aug;88((2)):623–31. doi: 10.1172/JCI115347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abboud HE. Growth factors and the mesangium. J Am Soc Nephrol. 1992 Apr;2((10 Suppl)):S185–9. doi: 10.1681/ASN.V210s185. [DOI] [PubMed] [Google Scholar]
- 52.Peters H, Eisenberg R, Daig U, Liefeldt L, Westenfeld R, Gaedeke J, et al. Platelet inhibition limits TGF-beta overexpression and matrix expansion after induction of anti-thy1 glomerulonephritis. Kidney Int. 2004 Jun;65((6)):2238–48. doi: 10.1111/j.1523-1755.2004.00630.x. [DOI] [PubMed] [Google Scholar]
- 53.Sadeghi-Alavijeh O, Tadayyon M, Caplin B. Chronic kidney disease-associated cardiovascular disease: scope and limitations of animal models. Cardiovasc Endocrinol. 2017 Dec;6((4)):120–7. doi: 10.1097/XCE.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsieh YP, Chang CC, Kor CT, Yang Y, Wen YK, Chiu PF. Mean corpuscular volume and mortality in patients with CKD. Clin J Am Soc Nephrol. 2017 Feb 7;12((2)):237–44. doi: 10.2215/CJN.00970116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Modi ZJ, Lu Y, Ji N, Kapke A, Selewski DT, Dietrich X, et al. Risk of cardiovascular disease and mortality in young adults with end-stage renal disease: an analysis of the US Renal Data System. JAMA Cardiol. 2019 Apr 1;4((4)):353–62. doi: 10.1001/jamacardio.2019.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun J, Axelsson J, Machowska A, Heimburger O, Barany P, Lindholm B, et al. Biomarkers of cardiovascular disease and mortality risk in patients with advanced CKD. Clin J Am Soc Nephrol. 2016 Jul 7;11((7)):1163–72. doi: 10.2215/CJN.10441015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ju HY, Kim JK, Hur SM, Woo SA, Park KA, Park MY, et al. Could mean platelet volume be a promising biomarker of progression of chronic kidney disease? Platelets. 2015;26((2)):143–7. doi: 10.3109/09537104.2014.890179. [DOI] [PubMed] [Google Scholar]
- 58.Yu Z, Xiong J, Yang K, Huang Y, He T, Yu Y, et al. The association between platelet indices and cardiovascular events in chronic kidney disease patients without dialysis. Int Urol Nephrol. 2021 May;53((5)):961–71. doi: 10.1007/s11255-020-02696-4. [DOI] [PubMed] [Google Scholar]
- 59.McCullough PA, Sandberg KR, Borzak S, Hudson MP, Garg M, Manley HJ. Benefits of aspirin and beta-blockade after myocardial infarction in patients with chronic kidney disease. Am Heart J. 2002 Aug;144((2)):226–32. doi: 10.1067/mhj.2002.125513. [DOI] [PubMed] [Google Scholar]
- 60.Jardine MJ, Ninomiya T, Perkovic V, Cass A, Turnbull F, Gallagher MP, et al. Aspirin is beneficial in hypertensive patients with chronic kidney disease: a post-hoc subgroup analysis of a randomized controlled trial. J Am Coll Cardiol. 2010 Sep 14;56((12)):956–65. doi: 10.1016/j.jacc.2010.02.068. [DOI] [PubMed] [Google Scholar]
- 61.Lee SJ, Lee IK, Jeon JH. Vascular calcification-new insights into its mechanism. Int J Mol Sci. 2020 Apr 13;21((8)):21. doi: 10.3390/ijms21082685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glorieux G, Cohen G, Jankowski J, Vanholder R. Platelet/leukocyte activation, inflammation, and uremia. Semin Dial. 2009 Jul–Aug;22((4)):423–7. doi: 10.1111/j.1525-139X.2009.00593.x. [DOI] [PubMed] [Google Scholar]
- 63.Schurgers LJ, Akbulut AC, Kaczor DM, Halder M, Koenen RR, Kramann R. Initiation and propagation of vascular calcification is regulated by a concert of platelet- and smooth muscle cell-derived extracellular vesicles. Front Cardiovasc Med. 2018;5:36. doi: 10.3389/fcvm.2018.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foresta C, Strapazzon G, De Toni L, Fabris F, Grego F, Gerosa G, et al. Platelets express and release osteocalcin and co-localize in human calcified atherosclerotic plaques. J Thromb Haemost. 2013 Feb;11((2)):357–65. doi: 10.1111/jth.12088. [DOI] [PubMed] [Google Scholar]
- 65.Mizokami A, Kawakubo-Yasukochi T, Hirata M. Osteocalcin and its endocrine functions. Biochem Pharmacol. 2017 May 15;132:1–8. doi: 10.1016/j.bcp.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 66.Kratz M, Zelnick LR, Trenchevska O, Jeffs JW, Borges CR, Tseng HH, et al. Relationship between chronic kidney disease, glucose homeostasis, and plasma osteocalcin carboxylation and fragmentation. J Ren Nutr. 2021 May;31((3)):248–56. doi: 10.1053/j.jrn.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goligorsky MS. Pathogenesis of endothelial cell dysfunction in chronic kidney disease: a retrospective and what the future may hold. Kidney Res Clin Pract. 2015 Jun;34((2)):76–82. doi: 10.1016/j.krcp.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gracia M, Betriu A, Martinez-Alonso M, Arroyo D, Abajo M, Fernandez E, et al. Predictors of subclinical atheromatosis progression over 2 years in patients with different stages of CKD. Clin J Am Soc Nephro. 2016 Feb 5;11((2)):287–96. doi: 10.2215/CJN.01240215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeng Z, Xia L, Fan X, Ostriker AC, Yarovinsky T, Su M, et al. Platelet-derived miR-223 promotes a phenotypic switch in arterial injury repair. J Clin Invest. 2019 Mar 1;129((3)):1372–86. doi: 10.1172/JCI124508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zampetaki A, Willeit P, Tilling L, Drozdov I, Prokopi M, Renard JM, et al. Prospective study on circulating MicroRNAs and risk of myocardial infarction. J Am Coll Cardiol. 2012 Jul 24;60((4)):290–9. doi: 10.1016/j.jacc.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 71.Karolina DS, Tavintharan S, Armugam A, Sepramaniam S, Pek SL, Wong MT, et al. Circulating miRNA profiles in patients with metabolic syndrome. J Clin Endocrinol Metab. 2012 Dec;97((12)):E2271–6. doi: 10.1210/jc.2012-1996. [DOI] [PubMed] [Google Scholar]
- 72.Schulte C, Molz S, Appelbaum S, Karakas M, Ojeda F, Lau DM, et al. miRNA-197 and miRNA-223 predict cardiovascular death in a cohort of patients with symptomatic coronary artery disease. PLoS One. 2015;10((12)):e0145930. doi: 10.1371/journal.pone.0145930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Di Lullo L, House A, Gorini A, Santoboni A, Russo D, Ronco C. Chronic kidney disease and cardiovascular complications. Heart Fail Rev. 2015 May;20((3)):259–72. doi: 10.1007/s10741-014-9460-9. [DOI] [PubMed] [Google Scholar]
- 74.Cirillo P, Golino P, Ragni M, Battaglia C, Pacifico F, Formisano S, et al. Activated platelets and leucocytes cooperatively stimulate smooth muscle cell proliferation and proto-oncogene expression via release of soluble growth factors. Cardiovasc Res. 1999 Jul;43((1)):210–8. doi: 10.1016/s0008-6363(99)00006-1. [DOI] [PubMed] [Google Scholar]
- 75.Yabanoglu S, Akkiki M, Seguelas MH, Mialet-Perez J, Parini A, Pizzinat N. Platelet derived serotonin drives the activation of rat cardiac fibroblasts by 5-HT2A receptors. J Mol Cell Cardiol. 2009 Apr;46((4)):518–25. doi: 10.1016/j.yjmcc.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 76.Shi P, Zhang L, Zhang M, Yang W, Wang K, Zhang J, et al. Platelet-specific p38alpha deficiency improved cardiac function after myocardial infarction in mice. Arterioscler Thromb Vasc Biol. 2017 Dec;37((12)):e185–96. doi: 10.1161/ATVBAHA.117.309856. [DOI] [PubMed] [Google Scholar]
- 77.Wu L, Zhao F, Dai M, Li H, Chen C, Nie J, et al. P2y12 receptor promotes pressure overload-induced cardiac remodeling via platelet-driven inflammation in mice. Hypertension. 2017 Oct;70((4)):759–69. doi: 10.1161/HYPERTENSIONAHA.117.09262. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y, Liang Y, Zhao W, Fu G, Li Q, Min X, et al. Circulating miRNA-21 as a diagnostic biomarker in elderly patients with type 2 cardiorenal syndrome. Sci Rep. 2020 Mar 17;10((1)):4894. doi: 10.1038/s41598-020-61836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chuppa S, Liang M, Liu P, Liu Y, Casati MC, Cowley AW, et al. MicroRNA-21 regulates peroxisome proliferator-activated receptor alpha, a molecular mechanism of cardiac pathology in cardiorenal syndrome type 4. Kidney Int. 2018 Feb;93((2)):375–89. doi: 10.1016/j.kint.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008 Dec 18;456((7224)):980–4. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 81.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006 Nov 28;103((48)):18255–60. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haneklaus M, O'Neill LA, Coll RC. Modulatory mechanisms controlling the NLRP3 inflammasome in inflammation: recent developments. Curr Opin Immunol. 2013 Feb;25((1)):40–5. doi: 10.1016/j.coi.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 83.Yang Y, Ma L, Wang C, Song M, Li C, Chen M, et al. Matrix metalloproteinase-7 in platelet-activated macrophages accounts for cardiac remodeling in uremic mice. Basic Res Cardiol. 2020 Apr 9;115((3)):30. doi: 10.1007/s00395-020-0789-z. [DOI] [PubMed] [Google Scholar]