Abstract
Persons at clinical high-risk for psychosis (CHR) are characterised by specific neurocognitive deficits. However, the course of neurocognitive performance during the prodromal period and over the onset of psychosis remains unclear. The aim of this meta-analysis was to synthesise results from follow-up studies of CHR individuals to examine longitudinal changes in neurocognitive performance. Three electronic databases were systematically searched to identify articles published up to 31 December 2021. Thirteen studies met inclusion criteria. Study effect sizes (Hedges' g) were calculated and pooled for each neurocognitive task using random-effects meta-analyses. We examined whether changes in performance between baseline and follow-up assessments differed between: (1) CHR and healthy control (HC) individuals, and (2) CHR who did (CHR-T) and did not transition to psychosis (CHR-NT). Meta-analyses found that HC individuals had greater improvements in performance over time compared to CHR for letter fluency (g = −0.32, p = 0.029) and digit span (g = −0.30, p = 0.011) tasks. Second, there were differences in longitudinal performance of CHR-T and CHR-NT in trail making test A (TMT-A) (g = 0.24, p = 0.014) and symbol coding (g = −0.51, p = 0.011). Whilst CHR-NT improved in performance on both tasks, CHR-T improved to a lesser extent in TMT-A and had worsened performance in symbol coding over time. Together, neurocognitive performance generally improved in all groups at follow-up. Yet, evidence suggested that improvements were less pronounced for an overall CHR group, and specifically for CHR-T, in processing speed tasks which may be a relevant domain for interventions aimed to enhance neurocognition in CHR populations.
Key words: CHR, cognition, processing speed, psychosis, UHR
Introduction
Robust deficits in neurocognition are evident in the early stages of psychosis development among people at clinical high-risk for psychosis (CHR) (Catalan et al., 2021; Hedges et al., 2022; Seidman et al., 2016). As these deficits are less pronounced than individuals with first-episode psychosis (FEP) compared to healthy control (HC) individuals (Sheffield, Karcher, & Barch, 2018), reviews indirectly comparing cross-sectional studies of FEP and CHR samples have suggested a potential neurocognitive decline prior to or over the transition to psychosis (Giuliano et al., 2012; Mesholam-Gately, Giuliano, Goff, Faraone, & Seidman, 2009). However, follow-up studies of CHR cohorts have shown inconsistent evidence for a decline (Bora & Murray, 2014): some have reported a decline in visual memory, processing speed (Wood et al., 2007) and verbal fluency (Lee et al., 2014), whereas others have observed stable cognitive deficits over time (Allott et al., 2019; Metzler et al., 2015). An improved understanding of longer-term cognitive changes in CHR populations, and particularly over illness onset for those who transition to psychosis (CHR-T), may provide insights for clinical research and inform early interventions targeting cognitive decline (Catalan et al., 2021).
To date, only one systematic review and meta-analysis has examined longitudinal changes in neurocognitive function of CHR individuals (Bora & Murray, 2014). Results indicated a general improvement in performance over time (i.e. stability of deficits), which did not significantly differ between CHR individuals and HCs with the exception of the verbal fluency domain. Here, the magnitude of improvement was significantly more pronounced in HC than in the CHR group. The main limitation of the meta-analysis was the small number of included studies, which meant that individual-task analysis was not always feasible. Instead, task performance was combined and analysed as global or domain-level cognition scores (Bora & Murray, 2014). Since this meta-analysis, several large cohort studies have published results on longitudinal neurocognition in CHR samples and over longer follow-up times, including the North American Prodrome Longitudinal Study (NAPLS-2) (Addington et al., 2019; Velikonja et al., 2021) and the Personal Assessment and Crisis Evaluation (PACE) Clinic (Allott et al., 2019). Given the increase in published studies examining longitudinal neurocognitive performance in CHR samples, an updated review is required.
The aim of the present study was to meta-analytically examine changes in neurocognitive functioning in specific tasks over two assessments among (1) CHR compared to HCs, as well as (2) CHR-T compared to CHR-NT individuals. In the current paper, we sought to address some of the limitations in the design of the earlier meta-analysis. First, we aimed to conduct analyses of performance in individual tasks, which may be a more effective approach for identifying longitudinal changes in specific cognitive processes, some of which may be differentially impaired (Brewer et al., 2006; Szöke et al., 2008). Second, we extended the analysis to examine whether changes in specific neurocognition were associated with transition to psychosis among CHR, which may help to characterise CHR-T individuals (Fusar-Poli et al., 2020). Third, we have applied a robust method for calculating effect sizes from data collected at multiple time points recommended by Morris (2008), who has comprehensively assessed the precision and stability of effect sizes from repeated measures designs.
Methods
Selection procedure
The systematic review protocol was registered on PROSPERO (CRD42020207568) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009). Three independent authors (E.H., S.S., C.S.) carried out systematic literature searches in Medline, Embase and PsycINFO databases until 31 December 2021. Identified articles were screened first by title and abstract for possible inclusion. Full text of relevant papers was then reviewed for eligibility. A manual search of the reference lists of included articles was also conducted.
Search strategy and eligibility criteria
Literature searches were implemented using the following key terms: (‘at risk mental state’ OR ‘ultra high risk’ OR ‘UHR’ OR ‘clinical high risk’ OR ‘psychosis risk’ OR ‘prodrome’ OR ‘psychosis’ OR ‘basic symptoms’) AND (‘neurocognit*’ OR ‘cognit*’ OR ‘neuropsych*’) AND (‘retest’ OR ‘longitudinal’ OR ‘chang*’ OR ‘follow-up’ OR ‘course’).
Studies were included if they (1) were original research articles published in English; (2) included individuals who met CHR criteria, as defined by any validated scale including the Comprehensive Assessment of At-Risk Mental States (CAARMS) (Yung et al., 2005) and Structured Interview for Psychosis-risk Syndromes (SIPS) (McGlashan, Walsh, & Woods, 2010); (3) included a comparison group of HCs or provided data separately for CHR-T and CHR-NT groups; (4) reported raw neurocognitive test scores from two assessments and (5) administered the same cognitive test at both assessments. Studies were excluded if they: (1) were unpublished studies, reviews, conference abstracts or case reports; (2) had overlapping samples on the same cognitive measure; (3) only examined cognitive performance in FEP, schizophrenia or bipolar disorder samples (no CHR sample); (4) included intervention therapies to improve cognition between assessments and (5) reported only composite cognition or standardised z-scores in the original article and could not provide the raw data upon request. For example, when studies only reported composite or standardised scores, corresponding authors were contacted by email to obtain the raw group data on individual task performance. For overlapping samples, the study with the largest sample size was chosen.
Data extraction and risk of bias
Three researchers independently extracted data from included studies using a structured coding form (E.H., C.S., S.S.). Sample characteristics (e.g. number of subjects at first and second assessment, age at baseline, follow-up months) and details of neurocognitive measures [e.g. task used, domain, means and standard deviations (s.d.s) of results at both assessments] were extracted for CHR, HC, CHR-T and CHR-NT groups. The means and s.d.s of subgroups (i.e. CHR-T and CHR-NT) were pooled together using Equations 23.2 and 23.3 (Borenstein, Hedges, Higgins, & Rothstein, 2009) to calculate performance for an overall group (i.e. CHR), if it was not reported. Data extraction forms were compared to verify accuracy. Any inconsistencies were resolved under supervision of senior researchers (M.K., H.D.). Study risk of bias was assessed using a modified version of the Newcastle–Ottawa Scale (NOS) for cohort studies, which rates study quality from 0 to 8 stars across three categories: selection, comparability and exposure/outcome (Wells et al., 2011) (online Supplementary Table S1). Although there is no threshold for determining ‘good’ quality studies, accumulating stars index reduced risk of bias. This tool has been validated for longitudinal observational studies and has been used in previous meta-analyses of CHR samples (Catalan et al., 2021; Fusar-Poli et al., 2012; Salazar de Pablo, Catalan, & Fusar-Poli, 2020).
Outcome measures
Across studies, neurocognitive data were grouped by task and group comparisons (CHR v. HC; CHR-T v. CHR-NT). Each task was separately meta-analysed. To ensure analyses were sufficiently powered, tasks with less than three independent studies were excluded from the meta-analyses. Individual tasks that were analysed included Trail Making Test A (TMT-A) and B (TMT-B), Brief Assessment of Cognition Scale (BACS) symbol coding, semantic fluency, letter fluency, Continuous Performance Task – Identical Pairs (CPT-IP), Rey Auditory Verbal Learning Test (RAVLT) immediate recall, California Verbal Learning Test (CVLT) immediate recall, Wechsler Adult Intelligence Scale (WAIS) block design and digit span. For consistency of interpretations, task outcome measures were categorised into neurocognitive domains based on the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) criteria (Kern et al., 2008; Nuechterlein et al., 2008) and in line with two published meta-analyses examining baseline cognition in CHR populations (Catalan et al., 2021; Fusar-Poli et al., 2012). These included: (1) processing speed, (2) attention/vigilance, (3) verbal learning and memory, (4) visuospatial ability, (5) executive functioning and (5) working memory (see online Supplementary Methods 1 for individual tasks involved).
Statistical analyses
Methods used by researchers to calculate effect sizes from repeated measures designs have been examined in terms of precision, robustness and bias. Morris (2008) proposed an optimal methodology which calculates the effect size using the pre- and post-condition means, s.d.s and sample sizes of two independent groups. Therefore, for each study, we calculated the Hedges' g effect size (which is the Cohen's effect size corrected for small sample bias; Lakens, 2013) and its variance from Equations 8 and 25 provided by Morris (2008). If participants were lost to follow-up, sample sizes at the second assessment were used in the meta-analyses. The effect size variance requires an estimation of the correlation coefficient, rho, between first and second neurocognitive measures. Although rho is not usually reported in publications, it can be calculated from study data if the mean (and s.d.) pre, post and change values are reported. These data were provided by Allott et al. (2019) and the mean weighted rho across neurocognitive tasks was determined at 0.64 [95% confidence interval (CI) 0.58–0.70]. Therefore, rho was set to 0.65 for each meta-analysis but was adjusted from 0.65 to 0.58 and 0.70 in sensitivity analyses, consistent with the CIs from Allott et al. (2019), to examine the strength of results. To enable direct comparisons of effect sizes, we used the same methodological approach across all the neurocognitive tasks. However, this methodology for calculating effect size estimates assumes homogeneity of variance between the comparison groups (Morris, 2008). We used Bartlett's (1937) test to assess the assumption of equal variances. For studies where this assumption of homogeneity did not hold, we conducted a second sensitivity analysis to verify our findings. In the sensitivity analysis, Hedges' g study effect sizes were recalculated from equations provided by Morris and DeShon (2002) which do not rely on the assumption of equal variances (Equation 6 and the corresponding sampling variance in Table 2).
For each neurocognitive task, study effect sizes were combined using a random-effects inverse-weighted variance model (DerSimonian & Laird, 1986) for (1) CHR participants v. HCs and (2) CHR-NT v. CHR-T participants. Meta-analyses were conducted in Microsoft Excel using standard meta-analytical equations taken from the Major Depressive Disorder Neuroimaging Database (Kempton et al., 2011), which are identical to the METAN command (Llamas-Velasco, Contador, Villarejo-Galende, Lora-Pablos, & Bermejo-Pareja, 2015) in STATA (StataCorp, 2017). In terms of validation, previous meta-analyses have used this method in parallel with STATA and produced the same results (Bromis, Calem, Reinders, Williams, & Kempton, 2018; Kempton et al., 2011). In the meta-analyses, where changes in neurocognition from baseline to follow-up significantly differed between groups, estimated mean scores were plotted to visualise these changes in performance. To note, as our analyses examine change over time, we are not able to comment on statistically significant group differences at individual time points. Between-study heterogeneity was estimated using the Cochran Q test (χ2 and p value) and the degree of heterogeneity was measured by the I2 statistic. I2 values above 75% indicate high heterogeneity (Higgins, Thompson, Deeks, & Altman, 2003). Potential effect size moderators can be explored using meta-regression when at least 10 studies are available (Sharp, 1998). Publication bias was assessed using the Egger's test (Egger, Smith, Schneider, & Minder, 1997) when at least six studies were included to ensure the test was adequately powered (Sutton, Duval, Tweedie, Abrams, & Jones, 2000). Tests were two-sided and statistical significance was set at p < 0.05.
Results
Study characteristics
Of 9804 unique articles that were identified in the literature search, 76 full-text articles were assessed for possible inclusion (see online Supplementary Fig. S1 for the study selection procedure). Seven authors were successfully contacted to provide additional neurocognitive data required for the meta-analysis (Addington et al., 2019; Allott et al., 2019; Barbato et al., 2013; Fujioka et al., 2020; Lam et al., 2018; Lee et al., 2014; Liu et al., 2015). Thirteen studies met inclusion criteria for the meta-analyses (Addington et al., 2019; Allott et al., 2019; Barbato et al., 2013; Becker et al., 2010; Fujioka et al., 2020; Jahshan et al., 2010; Lam et al., 2018; Lee et al., 2014; Liu et al., 2015; Metzler et al., 2015; Shin et al., 2016; Wood et al., 2007; Woodberry et al., 2013) (see online Supplementary Table S2 for characteristics of the study database). Although there were overlapping samples for certain tasks reported by (1) Allott et al. (2019) and Wood et al. (2007), and (2) Lee et al. (2014) and Shin et al. (2016), only the latter of each pair included a HC group. Therefore, these two studies (Shin et al., 2016; Wood et al., 2007) were included in CHR v. HC meta-analyses. Follow-up time of studies ranged from 6 months to 13.1 years (online Supplementary Table S2).
Longitudinal neurocognitive functioning in CHR compared to HC individuals
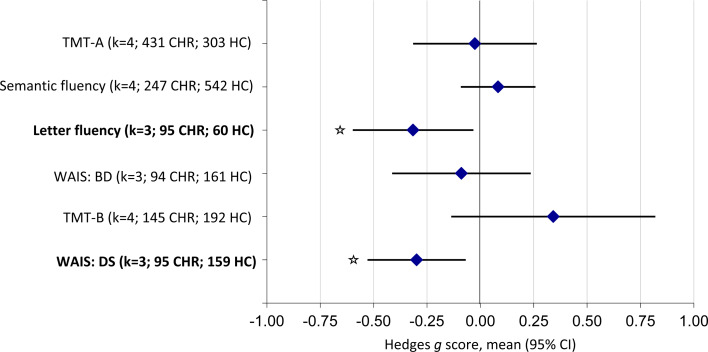
Eight studies were included in the CHR v. HC meta-analyses, comprising a total of 794 CHR and 787 HC individuals. Changes in neurocognitive performance significantly differed between CHR and HC individuals on letter fluency tests (g = −0.32; 95% CI −0.60 to −0.03; p = 0.029) and WAIS digit span (g = −0.30; 95% CI −0.53 to −0.07; p = 0.011) (online Supplementary Figs S2 and S4). For letter fluency, HCs improved significantly more than the CHR group (online Supplementary Fig. S3). For WAIS digit span, results indicated that there were little differences in performance at baseline, but HCs improved over time and CHR individuals did not (online Supplementary Fig. S5). There were no differences in TMT-A, semantic fluency, WAIS block design or TMT-B tasks (Fig. 1; Table 1), indicating that there was no significantly different improvement between CHR and HC groups.
Fig. 1.
Neurocognitive task-level functioning in CHR individuals compared to HC individuals. A negative effect size demonstrated an improvement in the HC compared to the CHR group. However, this is reversed for TMTs as higher scores indicate poorer performance on these tasks. Tasks highlighted in bold indicate significant results (p < 0.05).
Table 1.
Neurocognitive task-level functioning of individuals at CHR compared with HC individuals
| Task | Studies | Number of participants | Mean baseline scorea | Mean change score at follow-upa | Effect size | Heterogeneity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHR | HC | CHR | HC | CHR | HC | Hedges' g | 95% CI | z | p value | Q | I2 | p value | ||
| TMT-A | 4 | 431 | 303 | 30.18 | 26.45 | −3.55 | −3.36 | −0.03 | −0.32 to 0.26 | 0.17 | 0.862 | 8.53 | 64.82 | 0.036* |
| Semantic fluency | 4 | 247 | 542 | 33.65 | 39.45 | 0.99 | 0.57 | 0.08 | −0.09 to 0.26 | 0.93 | 0.352 | 4.06 | 26.04 | 0.255 |
| Letter fluency | 3 | 95 | 60 | 29.19 | 30.23 | 0.48 | 3.40 | −0.32 | −0.60 to −0.03 | 2.19 | 0.029* | 0.37 | 0.00 | 0.834 |
| WAIS-R/III block design | 3 | 94 | 161 | 21.97 | 24.37 | 2.04 | 1.70 | −0.09 | −0.41 to 0.24 | 0.54 | 0.592 | 3.39 | 40.99 | 0.184 |
| TMT-B | 4 | 145 | 192 | 71.10 | 60.98 | −0.52 | −8.53 | 0.34 | −0.14 to 0.82 | 1.40 | 0.162 | 14.46 | 79.26 | 0.002* |
| WAIS-R/III digit span | 3 | 95 | 159 | 11.18 | 11.13 | −0.07 | 1.00 | −0.30 | −0.53 to −0.07 | 2.54 | 0.011* | 1.44 | 0.00 | 0.487 |
Tasks highlighted in bold indicate significant results (p < 0.05).
Estimated as the non-weighted mean performance from included studies.
*p < 0.05.
Longitudinal neurocognitive functioning in CHR-T compared to CHR-NT individuals
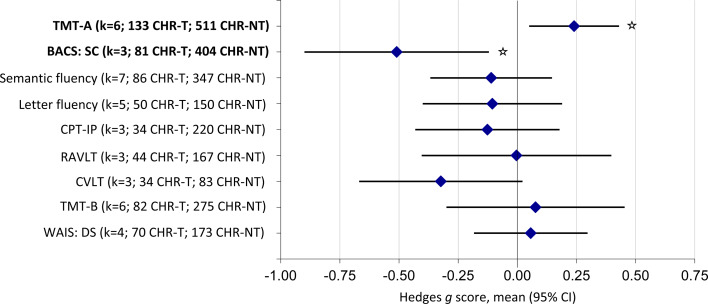
Eleven studies were included in the CHR-T v. CHR-NT meta-analyses, consisting of 227 CHR-T and 806 CHR-NT individuals. Changes in neurocognitive performance differed between CHR-T and CHR-NT individuals in TMT-A task (g = 0.24; 95% CI 0.05–0.43; p = 0.014) and BACS symbol coding subtest (g = −0.51; 95% CI −0.89 to −0.12; p = 0.011) (online Supplementary Figs S6 and S8). For TMT-A, CHR-NT improved significantly more than the CHR-T group (online Supplementary Fig. S7). For BACS symbol coding, CHR-T had higher scores than CHR-NT at baseline. However, CHR-T performance had worsened at follow-up, where CHR-NT had improved over time (online Supplementary Fig. S9). There were no significant differences in semantic or letter fluency, CPT-IP, RAVLT, CVLT, TMT-B or WAIS digit span tests (Fig. 2; Table 2). Results indicated that CHR-T and CHR-NT group performance on these tasks were both unchanged at follow-up, or had improved at a similar rate over time.
Fig. 2.
Neurocognitive task-level functioning in CHR individuals who developed psychosis (CHR-T) compared to those who did not develop psychosis (CHR-NT). A negative effect size demonstrated an improvement in the CHR-NT compared to the CHR-T group. However, this is reversed for TMTs as higher scores indicate poorer performance on these tasks. Tasks highlighted in bold indicate significant results (p < 0.05).
Table 2.
Neurocognitive task-level functioning of individuals at clinical high-risk for psychosis who did (CHR-T) and did not (CHR-NT) transition to psychosis
| Task | Studies | Number of participants | Mean baseline scorea | Mean change score at follow-upa | Effect size | Heterogeneity | Publication bias | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHR-T | CHR-NT | CHR-T | CHR-NT | CHR-T | CHR-NT | Hedges' g | 95% CI | z | p value | Q | I2 | p value | p value | ||
| TMT-A | 6 | 133 | 511 | 32.07 | 29.36 | −0.45 | −3.99 | 0.24 | 0.05 to 0.43 | 2.46 | 0.014* | 5.82 | 14.11 | 0.324 | 0.11 |
| BACS symbol coding | 3 | 81 | 404 | 65.47 | 61.52 | −4.33 | 4.45 | −0.51 | −0.89 to −0.12 | 2.56 | 0.011* | 4.16 | 51.90 | 0.125 | – |
| Semantic fluency | 7 | 86 | 347 | 29.02 | 32.43 | −1.08 | 0.70 | −0.16 | −0.44 to 0.11 | 1.15 | 0.249 | 10.22 | 41.31 | 0.116 | 0.77 |
| Letter fluency | 5 | 50 | 150 | 23.63 | 25.45 | −0.96 | 1.74 | −0.14 | −0.42 to 0.15 | 0.94 | 0.347 | 0.97 | 0.00 | 0.914 | – |
| CPT-IP | 3 | 34 | 220 | 1.40 | 3.30 | 0.34 | −0.10 | −0.13 | −0.43 to 0.18 | 0.81 | 0.416 | 0.71 | 0.00 | 0.702 | – |
| RAVLT | 3 | 44 | 167 | 41.45 | 46.08 | 1.58 | 0.86 | 0.00 | −0.40 to 0.40 | 0.02 | 0.986 | 3.65 | 45.18 | 0.161 | – |
| CVLT/-II | 3 | 34 | 83 | 36.50 | 39.37 | 1.04 | 2.64 | −0.32 | −0.67 to 0.02 | 1.84 | 0.066 | 0.82 | 0.00 | 0.662 | – |
| TMT-B | 6 | 82 | 275 | 75.15 | 68.10 | −3.55 | −5.58 | 0.08 | −0.30 to 0.45 | 0.40 | 0.689 | 14.81 | 66.24 | 0.011* | 0.40 |
| WAIS/-R/II digit span | 4 | 70 | 173 | 11.83 | 13.67 | 0.30 | 0.15 | 0.06 | −0.18 to 0.30 | 0.46 | 0.643 | 1.68 | 0.00 | 0.641 | – |
Tasks highlighted in bold indicate significant results (p < 0.05).
Estimated as the non-weighted mean performance from included studies.
*p < 0.05.
Heterogeneity, study quality and publication bias
Heterogeneity across the studies was small to high (Tables 1 and 2). Potential effect size moderators could not be explored due to insufficient power to perform meta-regressions. Where publication bias could be assessed, we reported no significant evidence of bias (all p > 0.05) (Table 2). In terms of study risk of bias, NOS scores ranged from five to seven (mean = 5.88; median = 6.00) in the CHR v. HC meta-analysis and from two to seven (mean = 5.45; median = 6.00) in the CHR-T v. CHR-NT meta-analysis (online Supplementary Table S2).
Sensitivity analysis
By increasing rho (the correlation between baseline and follow-up neurocognitive measures) to 0.70, no change in significant results was observed. We did, however, detect an additional significant difference in longitudinal performance of CVLT among CHR-T and CHR-NT groups (g = −0.32; 95% CI −0.64 to −0.004; p = 0.047), where the CHR-NT had improved more than CHR-T. When rho was modified to 0.58, there was no change in significant results compared to rho at 0.65.
We conducted a second sensitivity analysis recalculating study effect sizes where we could not assume homogeneity of variance of comparison groups. In the sensitivity analysis, significant differences between CHR and HC in longitudinal performance of letter fluency (g = −0.33; 95% CI −0.62 to −0.04; p = 0.046) and WAIS digit span (g = −0.30; 95% CI −0.53 to −0.07; p = 0.011) remained. In keeping with our earlier findings, we also reported differences in longitudinal performance of TMT-A (g = 0.30; 95% CI 0.06 to 0.55; p = 0.016) and BACS symbol coding (g = −0.51; 95% CI −0.90 to −0.11; p = 0.012) among CHR-T and CHR-NT individuals. Therefore, the results of the second sensitivity analysis supported those of our main analysis.
Discussion
In this systematic review and meta-analysis, we first observed that longitudinal improvements in verbal fluency and digit span task performance were significantly more pronounced in HC compared to CHR individuals. Our second main finding was that performance over time in TMT-A and symbol coding tasks significantly differed between CHR-T and CHR-NT individuals. Whilst CHR-NT improved in performance on both tasks, CHR-T improved to a lesser degree in TMT-A and had worsened performance in symbol coding at follow-up. To our knowledge, this is the first comprehensive meta-analysis of longitudinal neurocognitive task performance in CHR-T and CHR-NT samples.
Our meta-analysis of longitudinal neurocognition in 697 CHR and 761 HC individuals demonstrated that performance in both groups generally improved between baseline and follow-up assessments. This may reflect the magnitude of practice effects, particularly for meta-analyses that included studies with shorter follow-up intervals (Calamia, Markon, & Tranel, 2012). We did, however, detect small effect size differences in longitudinal performance of digit span and letter fluency tasks, where improvements at follow-up were significantly more pronounced in HCs. An earlier meta-analysis of longitudinal cognition reported the same findings for letter fluency but did not have enough studies to analyse digit span performance in CHR (Bora & Murray, 2014). However, deficits in digit span are well-established in FEP (Mesholam-Gately et al., 2009) and schizophrenia patients (Fatouros-Bergman, Cervenka, Flyckt, Edman, & Farde, 2014). Furthermore, Bora and Murray (2014) did report that improvements over follow-up in the working memory domain, which comprises digit span performance, were significantly greater in HC than FEP. Findings are also in line with birth cohort studies that report developmental lags in cognitive performance from childhood at age 8 years among adults with psychotic disorder compared to HC individuals (Mollon, David, Zammit, Lewis, & Reichenberg, 2018). Developmental lags in cognitive functioning have also been identified between ages 9 and 16 years among children at-risk who present with a triad of antecedent markers of schizophrenia compared to typically developing children (Dickson et al., 2018). Our results showed reduced cognitive improvement of CHR individuals between assessments, which may reflect underlying structural and functional brain abnormalities in the prefrontal and anterior cingulate cortex of CHR and FEP individuals; key regions of working memory and verbal fluency function (Fusar-Poli et al., 2007, 2011). Still, our results should be interpreted cautiously as we are limited by heterogeneity attributable to both the CHR phenotype and to primary studies, such as short follow-up times (up to 2 years).
Prior research has suggested that any potential decline in neurocognition may be specific to individuals who transition to psychosis (Bora & Murray, 2014). As stated earlier, only one meta-analysis has examined the course of neurocognition in CHR across two assessments, but there was insufficient data to conduct task analysis for CHR-T and CHR-NT groups (Bora & Murray, 2014). Of nine tasks analysed in the present review, we observed small to moderate effect sizes differences in longitudinal processing speed, indexed by performance on both TMT-A (g = 0.24) and symbol coding tasks (g = −0.51). Improvements in TMT-A were significantly more pronounced among CHR-NT than CHR-T individuals. For the symbol coding task, performance was in fact higher in the CHR-T group at baseline but there was evidence of worsening performance at the follow-up assessment, whereas the CHR-NT group had improved. Interestingly, processing speed, and specifically symbol coding, has been recognised as the largest deficit in schizophrenia (Dickinson, Ramsey, & Gold, 2007), as well as in CHR samples (Seidman et al., 2010), relative to other common neurocognitive measures. Our results may indicate that some decline or lag in processing speed performance may occur later during the prodromal phase in those who develop psychosis (Seidman et al., 2010). This is of importance given the known relationship between poorer performance on trail making and symbol coding tasks and poorer social and role functioning among CHR individuals (Carrión et al., 2011) and highlights the need to develop interventions to address these impairments prior to the onset of psychosis. Although few randomised controlled trials (RCTs) have examined the effectiveness of cognitive remediation therapies on neurocognition and functioning in CHR groups, some do provide evidence that cognitive remediation may improve performance in select cognitive domains, such as processing speed and verbal memory (Choi et al., 2017; Loewy et al., 2016), and social functioning (Friedman-Yakoobian, Parrish, Eack, & Keshavan, 2022; Piskulic, Barbato, Liu, & Addington, 2015). Of interest, in a double-blind RCT directly targeting processing speed deficits, CHR participants who underwent processing speed training had significantly improved scores on WAIS-III symbol coding task as well as enhanced social adjustment at follow-up compared to an active control group (Choi et al., 2017).
To our knowledge, this is the largest comprehensive meta-analysis characterising longitudinal neurocognitive functioning in CHR individuals to date. We have extended previous research by Bora and Murray (2014) to compare changes in specific task performance of CHR-T and CHR-NT individuals. An additional strength of our review is that we applied a robust analytic approach to calculate effect sizes from repeated measures designs (Morris, 2008) and our results did not change during sensitivity analyses. However, limitations of the current paper must also be noted. There were several tasks that could not be meta-analytically examined due to an insufficient number of included studies and our approach to analyse the data at the task level. Though heterogeneity was typically low, considerable heterogeneity was observed for TMT-B in the meta-analyses. However, due to limited studies, we could not perform meta-regression analyses to investigate heterogeneity, exploring potential moderator variables, such as changes in symptoms, medication use or length of follow-up (which may reflect practice effects). Lastly, although we were able to examine changes in neurocognition of CHR, our meta-analyses consisted of data collected from two assessments. As a result, the interpretation of our findings is limited. Of 13 articles included in the meta-analyses, two studies examined neurocognition at more than two assessments but had small sample sizes for the transition group at follow-up (Lam et al., 2018; Lee et al., 2014). Therefore, we have limited insight into the nonlinear trajectories of neurocognition in CHR and over psychosis onset in CHR-T. Future research collecting repeated data at multiple time points in larger CHR cohorts is warranted.
To conclude, the current meta-analysis suggests that, despite general improvements in neurocognition among CHR, there are some differences in task performance over 2 years in CHR compared to HC as well as CHR-T relative to CHR-NT. These longitudinal differences were observed in processing speed and working memory domains. Taken together, these results suggest that tasks related to processing speed and working memory may be key targets for interventions aimed at improving neurocognitive deficits in clinical high-risk populations.
Acknowledgements
The authors thank S. Morris for his advice on the statistical methods and J. Addington, L. Liu, K. Allott, M. Fujioka, J. Lee, K. Lim, T. Lee and C. Liu for providing the additional data necessary to complete the meta-analysis. C. See was supported by the UK Medical Research Council (MR/N013700/1). S. Si was supported by the China Scholarship Council. H. Dickson is affiliated with the National Institute for Health Research (NIHR) Specialist Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, Psychology and Neuroscience, King's College London, United Kingdom. M. Kempton was supported by a Medical Research Council Fellowship (grant MR/J008915/1).
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291722001830.
click here to view supplementary material
Conflict of interest
The authors declare no conflict of interest.
References
- Addington, J., Stowkowy, J., Liu, L., Cadenhead, K. S., Cannon, T. D., Cornblatt, B. A., … Woods, S. W. (2019). Clinical and functional characteristics of youth at clinical high-risk for psychosis who do not transition to psychosis. Psychological Medicine, 49(10), 1670–1677. doi: 10.1017/S0033291718002258 [DOI] [PubMed] [Google Scholar]
- Allott, K., Wood, S. J., Yuen, H. P., Yung, A. R., Nelson, B., Brewer, W. J., … Lin, A. (2019). Longitudinal cognitive performance in individuals at ultrahigh risk for psychosis: A 10-year follow-up. Schizophrenia Bulletin, 45(5), 1101–1111. doi: 10.1093/schbul/sby143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbato, M., Colijn, M. A., Keefe, R. S., Perkins, D. O., Woods, S. W., Hawkins, K. A., … Addington, J. (2013). The course of cognitive functioning over six months in individuals at clinical high risk for psychosis. Psychiatry Research, 206(2–3), 195–199. doi: 10.1016/j.psychres.2012.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett, M. S. (1937). Properties of sufficiency and statistical tests. Proceedings of the Royal Society of London, Series A: Mathematical and Physical Sciences, 160(901), 268–282. [Google Scholar]
- Becker, H., Nieman, D., Wiltink, S., Dingemans, P., Van de Fliert, J., Velthorst, E., … Linszen, D. (2010). Neurocognitive functioning before and after the first psychotic episode: Does psychosis result in cognitive deterioration? Psychological Medicine, 40(10), 1599–1606. doi: 10.1017/S0033291710000048 [DOI] [PubMed] [Google Scholar]
- Bora, E., & Murray, R. M. (2014). Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: Do the cognitive deficits progress over, or after, the onset of psychosis? Schizophrenia Bulletin, 40(4), 744–755. doi: 10.1093/schbul/sbt085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein, M., Hedges, L. V., Higgins, J. P. T., & Rothstein, H. R. (2009). Complex data structures. In: Introduction to meta-analysis (Chapter 23). West Sussex, UK: John Wiley & Sons, Ltd. [Google Scholar]
- Brewer, W. J., Wood, S. J., Phillips, L. J., Francey, S. M., Pantelis, C., Yung, A. R., … McGorry, P. D. (2006). Generalized and specific cognitive performance in clinical high-risk cohorts: A review highlighting potential vulnerability markers for psychosis. Schizophrenia Bulletin, 32(3), 538–555. doi: 10.1093/schbul/sbj077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromis, K., Calem, M., Reinders, A. A., Williams, S. C., & Kempton, M. J. (2018). Meta-analysis of 89 structural MRI studies in posttraumatic stress disorder and comparison with major depressive disorder. American Journal of Psychiatry, 175(10), 989–998. doi: 10.1176/appi.ajp.2018.17111199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamia, M., Markon, K., & Tranel, D. (2012). Scoring higher the second time around: Meta-analyses of practice effects in neuropsychological assessment. The Clinical Neuropsychologist, 26(4), 543–570. doi: 10.1080/13854046.2012.680913 [DOI] [PubMed] [Google Scholar]
- Carrión, R. E., Goldberg, T. E., McLaughlin, D., Auther, A. M., Correll, C. U., & Cornblatt, B. A. (2011). Impact of neurocognition on social and role functioning in individuals at clinical high risk for psychosis. American Journal of Psychiatry, 168(8), 806–813. doi: 10.1176/appi.ajp.2011.10081209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan, A., Salazar de Pablo, G., Aymerich, C., Damiani, S., Sordi, V., Radua, J., … Fusar-Poli, P. (2021). Neurocognitive functioning in individuals at clinical high risk for psychosis: A systematic review and meta-analysis. JAMA Psychiatry, 78(8), 859–867. doi: 10.1001/jamapsychiatry.2021.1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J., Corcoran, C. M., Fiszdon, J. M., Stevens, M., Javitt, D. C., Deasy, M., … Pearlson, G. D. (2017). Pupillometer-based neurofeedback cognitive training to improve processing speed and social functioning in individuals at clinical high risk for psychosis. Psychiatric Rehabilitation Journal, 40(1), 33. doi: 10.1037/prj0000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian, R., & Laird, N. (1986). Meta-analysis in clinical trials. Controlled Clinical Trials, 7(3), 177–188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- Dickinson, D., Ramsey, M. E., & Gold, J. M. (2007). Overlooking the obvious: A meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Archives of General Psychiatry, 64(5), 532–542. doi: 10.1001/archpsyc.64.5.532 [DOI] [PubMed] [Google Scholar]
- Dickson, H., Cullen, A. E., Jones, R., Reichenberg, A., Roberts, R. E., Hodgins, S., … Laurens, K. R. (2018). Trajectories of cognitive development during adolescence among youth at-risk for schizophrenia. Journal of Child Psychology and Psychiatry, 59(11), 1215–1224. doi: 10.1111/jcpp.12912 [DOI] [PubMed] [Google Scholar]
- Egger, M., Smith, G. D., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315(7109), 629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatouros-Bergman, H., Cervenka, S., Flyckt, L., Edman, G., & Farde, L. (2014). Meta-analysis of cognitive performance in drug-naïve patients with schizophrenia. Schizophrenia Research, 158(1–3), 156–162. doi: 10.1016/j.schres.2014.06.034 [DOI] [PubMed] [Google Scholar]
- Friedman-Yakoobian, M. S., Parrish, E. M., Eack, S. M., & Keshavan, M. S. (2022). Neurocognitive and social cognitive training for youth at clinical high risk (CHR) for psychosis: A randomized controlled feasibility trial. Schizophrenia Research, 243, 302–306. doi: 10.1016/j.schres.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka, M., Kirihara, K., Koshiyama, D., Tada, M., Nagai, T., Usui, K., … Kasai, K. (2020). Mismatch negativity predicts remission and neurocognitive function in individuals at ultra-high risk for psychosis. Frontiers in Psychiatry, 11, 770. doi: 10.3389/fpsyt.2020.00770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli, P., Borgwardt, S., Crescini, A., Deste, G., Kempton, M. J., Lawrie, S., … Sacchetti, E. (2011). Neuroanatomy of vulnerability to psychosis: A voxel-based meta-analysis. Neuroscience & Biobehavioral Reviews, 35(5), 1175–1185. doi: 10.1016/j.neubiorev.2010.12.005 [DOI] [PubMed] [Google Scholar]
- Fusar-Poli, P., de Pablo, G. S., Correll, C. U., Meyer-Lindenberg, A., Millan, M. J., Borgwardt, S., … Arango, C. (2020). Prevention of psychosis: Advances in detection, prognosis, and intervention. JAMA Psychiatry, 77(7), 755–765. doi: 10.1001/jamapsychiatry.2019.4779 [DOI] [PubMed] [Google Scholar]
- Fusar-Poli, P., Deste, G., Smieskova, R., Barlati, S., Yung, A. R., Howes, O., … Borgwardt, S. (2012). Cognitive functioning in prodromal psychosis: A meta-analysis. Archives of General Psychiatry, 69(6), 562–571. doi: 10.1001/archgenpsychiatry.2011.1592 [DOI] [PubMed] [Google Scholar]
- Fusar-Poli, P., Perez, J., Broome, M., Borgwardt, S., Placentino, A., Caverzasi, E., … McGuire, P. (2007). Neurofunctional correlates of vulnerability to psychosis: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 31(4), 465–484. doi: 10.1016/j.neubiorev.2006.11.006 [DOI] [PubMed] [Google Scholar]
- Giuliano, A. J., Li, H., Mesholam-Gately, R. I., Sorenson, S. M., Woodberry, K. A., & Seidman, L. J. (2012). Neurocognition in the psychosis risk syndrome: A quantitative and qualitative review. Current Pharmaceutical Design, 18(4), 399–415. doi: 10.2174/138161212799316019 [DOI] [PubMed] [Google Scholar]
- Hedges, E. P., Dickson, H., Tognin, S., Modinos, G., Antoniades, M., van der Gaag, M., … Kempton, M. J. (2022). Verbal memory performance predicts remission and functional outcome in people at clinical high-risk for psychosis. Schizophrenia Research: Cognition, 28, 100222. doi: 10.1016/j.scog.2021.100222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P., Thompson, S. G., Deeks, J. J., & Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahshan, C., Heaton, R. K., Golshan, S., & Cadenhead, K. S. (2010). Course of neurocognitive deficits in the prodrome and first episode of schizophrenia. Neuropsychology, 24(1), 109–120. doi: 10.1037/a0016791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton, M. J., Salvador, Z., Munafò, M. R., Geddes, J. R., Simmons, A., Frangou, S., & Williams, S. C. (2011). Structural neuroimaging studies in major depressive disorder: Meta-analysis and comparison with bipolar disorder. Archives of General Psychiatry, 68(7), 675–690. doi: 10.1001/archgenpsychiatry.2011.60 [DOI] [PubMed] [Google Scholar]
- Kern, R. S., Nuechterlein, K. H., Green, M. F., Baade, L. E., Fenton, W. S., Gold, J. M., … Marder, S. R. (2008). The MATRICS consensus cognitive battery, part 2: Co-norming and standardization. American Journal of Psychiatry, 165(2), 214–220. doi: 10.1176/appi.ajp.2007.07010043 [DOI] [PubMed] [Google Scholar]
- Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t tests and ANOVAs. Frontiers in Psychology, 4, 863. doi: 10.3389/fpsyg.2013.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, M., Lee, J., Rapisarda, A., See, Y. M., Yang, Z., Lee, S.-A., … Keefe, R. S. E. (2018). Longitudinal cognitive changes in young individuals at ultrahigh risk for psychosis. JAMA Psychiatry, 75(9), 929–939. doi: 10.1001/jamapsychiatry.2018.1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, T. Y., Shin, Y. S., Shin, N. Y., Kim, S. N., Jang, J. H., Kang, D.-H., & Kwon, J. S. (2014). Neurocognitive function as a possible marker for remission from clinical high risk for psychosis. Schizophrenia Research, 153(1–3), 48–53. doi: 10.1016/j.schres.2014.01.018 [DOI] [PubMed] [Google Scholar]
- Liu, C.-C., Hua, M.-S., Hwang, T.-J., Chiu, C.-Y., Liu, C.-M., Hsieh, M. H., … Hwu, H.-G. (2015). Neurocognitive functioning of subjects with putative pre-psychotic states and early psychosis. Schizophrenia Research, 164(1–3), 40–46. doi: 10.1016/j.schres.2015.03.006 [DOI] [PubMed] [Google Scholar]
- Llamas-Velasco, S., Contador, I., Villarejo-Galende, A., Lora-Pablos, D., & Bermejo-Pareja, F. (2015). Physical activity as protective factor against dementia: A prospective population-based study (NEDICES). Journal of the International Neuropsychological Society, 21(10), 861–867. doi: 10.1017/S135561771500 [DOI] [PubMed] [Google Scholar]
- Loewy, R., Fisher, M., Schlosser, D. A., Biagianti, B., Stuart, B., Mathalon, D. H., & Vinogradov, S. (2016). Intensive auditory cognitive training improves verbal memory in adolescents and young adults at clinical high risk for psychosis. Schizophrenia Bulletin, 42(Suppl_1), S118–S126. doi: 10.1093/schbul/sbw009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan, T., Walsh, B., & Woods, S. (2010). The psychosis-risk syndrome: Handbook for diagnosis and follow-up. New York, NY: Oxford University Press. [Google Scholar]
- Mesholam-Gately, R. I., Giuliano, A. J., Goff, K. P., Faraone, S. V., & Seidman, L. J. (2009). Neurocognition in first-episode schizophrenia: A meta-analytic review. Neuropsychology, 23(3), 315. https://psycnet.apa.org/doi/10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Metzler, S., Dvorsky, D., Wyss, C., Müller, M., Gerstenberg, M., Traber-Walker, N., … Heekeren, K. (2015). Changes in neurocognitive functioning during transition to manifest disease: Comparison of individuals at risk for schizophrenic and bipolar affective psychoses. Psychological Medicine, 45(10), 2123–2134. doi: 10.1017/S0033291715000057 [DOI] [PubMed] [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollon, J., David, A. S., Zammit, S., Lewis, G., & Reichenberg, A. (2018). Course of cognitive development from infancy to early adulthood in the psychosis spectrum. JAMA Psychiatry, 75(3), 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, S. B. (2008). Estimating effect sizes from pretest-posttest-control group designs. Organizational Research Methods, 11(2), 364–386. [Google Scholar]
- Morris, S. B., & DeShon, R. P. (2002). Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological Methods, 7(1), 105. [DOI] [PubMed] [Google Scholar]
- Nuechterlein, K. H., Green, M. F., Kern, R. S., Baade, L. E., Barch, D. M., Cohen, J. D., … Marder, S. R. (2008). The MATRICS consensus cognitive battery, part 1: Test selection, reliability, and validity. American Journal of Psychiatry, 165(2), 203–213. doi: 10.1176/appi.ajp.2007.07010042 [DOI] [PubMed] [Google Scholar]
- Piskulic, D., Barbato, M., Liu, L., & Addington, J. (2015). Pilot study of cognitive remediation therapy on cognition in young people at clinical high risk of psychosis. Psychiatry Research, 225, 93–98. 10.1016/j.psychres.2014.10.021. [DOI] [PubMed] [Google Scholar]
- Salazar de Pablo, G., Catalan, A., & Fusar-Poli, P. (2020). Clinical validity of DSM-5 attenuated psychosis syndrome: Advances in diagnosis, prognosis, and treatment. JAMA Psychiatry, 77(3), 311–320. doi: 10.1001/jamapsychiatry.2019.3561 [DOI] [PubMed] [Google Scholar]
- Seidman, L. J., Giuliano, A. J., Meyer, E. C., Addington, J., Cadenhead, K. S., Cannon, T. D., … Cornblatt, B. A. (2010). Neuropsychology of the prodrome to psychosis in the NAPLS consortium: Relationship to family history and conversion to psychosis. Archives of General Psychiatry, 67(6), 578–588. doi: 10.1001/archgenpsychiatry.2010.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman, L. J., Shapiro, D. I., Stone, W. S., Woodberry, K. A., Ronzio, A., Cornblatt, B. A., … Woods, S. W. (2016). Association of neurocognition with transition to psychosis: Baseline functioning in the second phase of the North American Prodrome Longitudinal Study. JAMA Psychiatry, 73(12), 1239–1248. doi: 10.1001/jamapsychiatry.2016.2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp, S. (1998). Meta-analysis regression. Stata Technical Bulletin, 7(42), 16–22. [Google Scholar]
- Sheffield, J. M., Karcher, N. R., & Barch, D. M. (2018). Cognitive deficits in psychotic disorders: A lifespan perspective. Neuropsychology Review, 28(4), 509–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, Y. S., Kim, S.-Y., Lee, T. Y., Hur, J.-W., Shin, N. Y., Kim, S. N., … Kwon, J. S. (2016). Longitudinal change in neurocognition and its relation to symptomatic and functional changes over 2 years in individuals at clinical high-risk for psychosis. Schizophrenia Research, 174(1–3), 50–57. doi: 10.1016/j.schres.2016.03.024 [DOI] [PubMed] [Google Scholar]
- StataCorp. (2017). Stata Statistical Software: Release 15. StataCorp LLC.
- Sutton, A. J., Duval, S. J., Tweedie, R., Abrams, K. R., & Jones, D. R. (2000). Empirical assessment of effect of publication bias on meta-analyses. BMJ, 320(7249), 1574–1577. doi: 10.1136/bmj.320.7249.1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szöke, A., Trandafir, A., Dupont, M.-E., Meary, A., Schürhoff, F., & Leboyer, M. (2008). Longitudinal studies of cognition in schizophrenia: Meta-analysis. The British Journal of Psychiatry, 192(4), 248–257. doi: 10.1192/bjp.bp.106.029009 [DOI] [PubMed] [Google Scholar]
- Velikonja, T., Velthorst, E., Zinberg, J., Cannon, T., Cornblatt, B., Perkins, D., … McGlashan, T. (2021). Childhood trauma and cognitive functioning in individuals at clinical high risk (CHR) for psychosis. Development and Psychopathology, 33(1), 53–64. doi: 10.1017/S095457941900155X [DOI] [PubMed] [Google Scholar]
- Wells, G. A., Shea, B., O'Connell, D., Petersen, J., Welch, V., Losos, M., & Tugwell, P. (2011). The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Retrieved 21 September 2021 from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Wood, S. J., Brewer, W. J., Koutsouradis, P., Phillips, L. J., Francey, S. M., Proffitt, T. M., … Pantelis, C. (2007). Cognitive decline following psychosis onset: Data from the PACE clinic. The British Journal of Psychiatry, 191(Suppl. 51), s52–s57. doi: 10.1192/bjp.191.51.s52 [DOI] [PubMed] [Google Scholar]
- Woodberry, K. A., McFarlane, W. R., Giuliano, A. J., Verdi, M. B., Cook, W. L., Faraone, S. V., & Seidman, L. J. (2013). Change in neuropsychological functioning over one year in youth at clinical high risk for psychosis. Schizophrenia Research, 146(1–3), 87–94. doi: 10.1016/j.schres.2013.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung, A. R., Yung, A. R., Pan Yuen, H., Mcgorry, P. D., Phillips, L. J., Kelly, D., … Buckby, J. (2005). Mapping the onset of psychosis: The comprehensive assessment of at-risk mental states. Australian and New Zealand Journal of Psychiatry, 39(11–12), 964–971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291722001830.
click here to view supplementary material