Abstract
Background
Although liver transplantation has been done successfully in elderly patients with hepatocellular carcinoma, these are likely well-selected patients. This study uses a large database of patients with hepatocellular carcinoma to explore treatment and potential candidacy for liver transplantation in the elderly.
Methods
Retrospective review of 1,533 hepatocellular carcinoma cases identified 2 groups: 475 patients 70 years or older (70 +) and 1,058 patients < 70 years. Demographics, risk factors, tumor characteristics, treatments, and survival were compared. Three- and 5-year survival rates were determined, and logistic regression was used to identify factors predictive of 3-year survival.
Results
Patients 70 + were more likely to have metabolic factors and less likely to have viral hepatitis, cirrhosis, hepatocellular carcinoma found with surveillance (21.7% vs 28.4%, P = .005), and hepatocellular carcinoma within Milan criteria (37.3% vs 43.8%, P = .019). Model for End-stage Liver Disease score was similar, but patients 70 + had higher mean creatinine and lower mean bilirubin. Patients 70 + were equally likely to undergo liver resection but less likely to undergo liver transplantation (0.4% vs 10.2%, P < .001). Three- and 5-year survival rates were significantly worse in 70 +, and predictors of 3-year survival included hepatocellular carcinoma found with surveillance, meeting Milan criteria, and normal alpha fetoprotein.
Discussion
Elderly patients with hepatocellular carcinoma were less likely to undergo liver transplantation potentially due to metabolic factors and advanced disease. Although there is no age cutoff for liver transplantation, elderly patients should be given realistic expectations of liver transplantation candidacy. Continued surveillance for hepatocellular carcinoma in elderly patients may allow for earlier diagnosis and improved liver transplantation candidacy.
Key Message
Hepatocellular carcinoma in patients who are 70 years or older can be managed with liver transplantation in select cases, but more patients will be managed with liver resection and nonoperative therapies.
Highlights
-
•
Most patients with hepatocellular carcinoma who are older than 70 years will not undergo liver transplant.
-
•
Older patients are more likely to have metabolic risk factors and comorbidities including diabetes, hypertension, hyperlipidemia, and nonalcoholic steatohepatitis.
-
•
Older patients have similar Model for End-stage Liver Disease score as younger patients; their scores are based on having more renal dysfunction and a lower bilirubin.
-
•
Older patients are less likely to have their hepatocellular carcinoma found with surveillance and are more likely to have cancer that is beyond Milan criteria which are generally used for transplant candidacy.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer in men and ninth most common cancer in women worldwide and was responsible for more than 830,000 deaths in 2020 [1]. Among the available therapeutic options for HCC, liver resection and transplantation offer the best long-term survival, with transplantation having superior disease-free survival [2]. However, transplantation is limited by the scarcity of available donor livers. In the United States, 8,906 liver transplantations (LTs) were performed in 2020, whereas more than 13,000 patients continue to wait for transplant. Approximately 17.1% of these patients have HCC. Of the waitlisted patients, 20.8% were 65 years or older in 2019 compared to 8.9% in 2009 [3]. The proportion of elderly patients with HCC listed for LT has also tripled from 2003 to 2017 [4].
With the limited number of available donor livers and LT being a high-risk procedure, transplant centers must carefully select recipients to maximize utilization of each liver. Although Organ Procurement and Transplantation Network data show that recipients older than 65 years have worse 5-year graft survival compared to all other age groups, several studies have shown comparable outcomes between elderly and younger LT recipients [3,[5], [6], [7]]. These studies utilized large databases from individual transplant centers or the Scientific Registry of Transplant Recipients, and they generally included patients who had been referred to transplant centers or undergone LT. These are thus patients who are already well-selected and likely the "best" elderly patients. There are likely many more patients who were not acceptable candidates for referral and whose outcomes have not been explored.
With an aging global population, HCC in the elderly is expected to rise. Aging itself can also be a risk factor in HCC development. In a national study of 504,646 patients from 2002 to 2013, each 5-year age increment was associated with a 1.24-fold increased risk in HCC development [8]. HCC in the elderly is associated with different clinical characteristics when compared to HCC in younger populations. The elderly are more likely to have medical comorbidities and metabolic diseases, such as diabetes, obesity, and nonalcoholic fatty disease/nonalcoholic steatohepatitis (NASH). Based on existing nonalcoholic fatty disease/NASH epidemiology data, modeling suggests that HCC related to NASH in the aging population will only continue to increase [9]. It is thus imperative that we develop an optimal management plan for the increasing elderly patients with HCC.
This study uses a detailed database of a large patient cohort with HCC from various referral sources to examine the proportion of elderly patients who would reasonably qualify for LT and to identify factors that may have affected their candidacy and outcome.
METHODS/MATERIALS
Patients
This was a retrospective study of 1,533 HCC cases referred over a 28-year period (1993–2021) to a group of physicians who were associated with the only LT and liver disease center in Hawaii. It was also the tertiary referral center for the American Territories of the Pacific Basin (including Samoa, Guam, Saipan, the Marshall Islands, and Federated States of Micronesia). These centers were initially affiliated with St Francis Medical Center before 2012 and then with the Queen's Medical Center after 2012. These comprised about 60%–70% of all the HCC cases in Hawaii. This study was approved by the Institutional Review Board of The University of Hawaii at Manoa. In compliance with ethical regulations, all data were deidentified prior to use and thus exempt from patient consent.
Most HCCs were diagnosed histologically by percutaneous biopsy or at surgery. In the first decade, consistent with the previous United Network for Organ Sharing policy regarding transplant for HCC, patients without histologic confirmation were included if they had a history of chronic liver disease, a mass of at least 2 cm seen on 2 imaging studies (ultrasound, computed tomographic [CT] scan, or magnetic resonance imaging [MRI]), and 1 of the following: (1) vascular blush evident on CT scan or MRI, (2) alpha fetoprotein (AFP) > 200 ng/mL, or (3) arteriogram confirming the tumor. More recently, the diagnosis of HCC was frequently made with imaging alone if a contrast-enhanced study (dynamic CT or MRI) showed typical arterial enhancement with "washout" in the venous phase, as described by the American Association for the Study of Liver Disease guidelines [10,11].
Data Collected
Information on demographics, medical history, laboratory data, tumor characteristics, treatment, and survival was collected from clinical records. Demographic data included age, sex, birthplace, and self-reported ethnicity. Ethnicity was then categorized as "White," "Asian," "Pacific Islander," "Hispanic," "Black," or "Other" (which included mixed ethnicity and Native Americans). Medical history included diabetes mellitus, hyperlipidemia, smoking, and risk factors for HCC: viral hepatitis, significant alcohol use (defined as 2 or more alcoholic beverages daily for at least 10 years), and other chronic liver diseases. We also recorded any known family history of HCC. Measured height and weight were used to determine body mass index (BMI). Obesity was defined as BMI ≥ 30.
Laboratory data were obtained within 2 weeks of the initial visit, which included bilirubin, albumin, prothrombin time with international normalized ratio (INR), creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), platelet count, and AFP. Hepatitis B and C serologies were obtained if unknown. We also noted patients with hepatitis B core antibody positivity in the absence of hepatitis B surface antigen positivity. AFP was categorized as "normal" if the value was less than 20 ng/mL.
We collected information on the presences of ascites, encephalopathy, and/or tumor rupture at presentation. Childs–Turcotte–Pugh score and Model for End-stage Liver Disease (MELD) score were calculated. AST/platelet ratio (APRI) was used as a surrogate marker for liver fibrosis.
Although our Liver Center recommended HCC surveillance in patients with chronic viral hepatitis and/or cirrhosis using biannual AFP and liver ultrasound, there was no uniform screening protocol used in the cohort as referring physicians used a combination of AFP and/or imaging (ultrasound, CT scan, or MRI) at variable intervals. HCC was deemed to be found with "surveillance" if the patient had a previous negative imaging study from 3 to 12 months before the positive study. Patients without surveillance had symptomatic HCC (pain, abdominal mass, weight loss, jaundice) or incidental HCC diagnosed with imaging for unrelated reasons. We also noted if patients had chronic viral hepatitis or known cirrhosis, for which surveillance would be warranted.
Tumor characteristics were determined with contrast-enhanced imaging. We recorded the largest tumor size, number of tumors, and whether the patients' tumor met Milan criteria (single tumor < 5 cm or 2–3 tumors all < 3 cm). Liver cirrhosis was determined by either liver biopsy or imaging.
Treatments
Treatments included liver resection, transplantation, locoregional therapies (radiofrequency ablation, cryosurgery, percutaneous ethanol injection, transarterial chemoembolization [TACE], or yttrium-90 radioembolization), and systemic therapies. Liver resection was considered in patients with good underlying liver function and no evidence of portal hypertension. LT was considered in patients with unresectable HCC within Milan criteria. All liver resections and transplantations were performed by a single surgical group practice.
Statistical Analysis
Patients were divided into 2 groups; patients less than 70 years of age (age < 70) and those who were 70 years or older (age 70 +) All analyses were performed using Excel (Microsoft) and SPSS statistical software (IBM, version 27). Categorical variables were analyzed using χ2 analysis, and post hoc testing was performed using the Bonferroni method as appropriate. Continuous variables were analyzed using Mann–Whitney U test. Odds ratios with 95% confidence intervals (CIs) were calculated using binary logistic regression. Overall survival was evaluated using Kaplan–Meier and Cox regression analyses.
Results
In the entire cohort of 1,533 patients, mean age was 63.7 years (SD 11.3 years). There were 1,145 men and 388 women. Ethnicity distribution was as follows: Asians, 869 (56.7%); White, 320 (20.9%); Pacific Islander, 241 (15.7%); Hispanic, 35 (2.3%); Black, 10 (0.7%); and Other, 58 (3.8%). Distribution of the Asian races was as follows: Japanese, 348; Filipino, 207; Chinese, 170; Korean, 70; Southeast, 65; and Mixed Asian, 9. In terms of birthplace, 61% were born in the United States, 28.4% were born in an Asian country, and 5.2% were born in a Pacific Island Nation/US Territory.
In terms of risk factors, 375 patients (24.5%) were hepatitis B surface antigen positive, and 166 (10.8%) were hepatitis B core antibody positive in the absence of hepatitis B surface antigen positivity. Hepatitis C positivity was noted in 615 patients (40.1%), and 212 patients had NASH (13.8%). Significant alcohol use was noted in 660 patients (43.1%), and smoking history was noted in 947 patients (61.8%). However, only 205 (13.4%) were currently smoking. A family relative with HCC was noted in 102 patients (6.6%).
Table 1 compares demographics and risk factors between the 2 groups. Both groups had a male predominance. However, this predominance was less evident in the older patients. Ethnicity distribution was also different between the groups, notably with 71.7% of the age 70 + group being Asian. Hepatitis B surface antigen and hepatitis C were more likely to be positive in the age < 70 group, whereas there was a higher proportion of the age 70 + group who had hepatitis B core antibody positivity only. Older patients were more likely to have NASH, diabetes, hypertension, and hyperlipidemia, whereas younger patients were more likely to have obesity and higher mean BMI. Older patients were less likely to have a screenable disease (53.8% vs 83.8%, P < .001) and were less likely to have their HCC found with surveillance (21.6% vs 28.4%, P < .005).
Table 1.
Patient demographics and risk factors
| Age < 70 (n = 1056) | Age 70 + (n = 477) | P value | |
|---|---|---|---|
| Male, no. (%) | 827 (78.3) | 318 (66.7) | P < .001 |
| Ethnicity | P < .001 | ||
| Asian, no. (%) | 527 (49.9) | 342 (71.7) | |
| White, no. (%) | 250 (23.7) | 70 (14.7) | |
| Pacific Islander, no. (%) | 194 (18.4) | 47 (9.9) | |
| Hispanic, no. (%) | 26 (2.5) | 9 (1.9) | |
| Black, no. (%) | 10 (0.9) | 0 (0.0) | |
| Other, no. (%) | 49 (4.6) | 9 (1.9) | |
| Finished high school, no. (%) | 637 (83.1) | 271 (78.6) | P = .07 |
| Hepatitis B surf Ag positive, no. (%) | 299 (28.4) | 76 (16.1) | P < .001 |
| Hepatitis B core Ab positive, no. (%) | 100 (9.5) | 66 (14) | P = .01 |
| Hepatitis C positive, no. (%) | 509 (48.4) | 106 (22.3) | P < .001 |
| Alcohol, no. (%) | 522 (49.6) | 138 (29.1) | P < .001 |
| NASH, no. (%) | 90 (8.5) | 122 (25.8) | P < .001 |
| HCC found with surveillance, no. (%) | 300 (28.4) | 103 (21.6) | P = .005 |
| Screenable disease, no. (%) | 885 (83.8) | 256 (53.8) | P < .001 |
| Previous other cancer, no. (%) | 109 (10.3) | 93 (19.5) | P < .001 |
| Smoking, no. (%) | 688 (66.2) | 259 (54.8) | P < .001 |
| Diabetes mellitus, no. (%) | 324 (30.7) | 229 (48.1) | P < .001 |
| Hyperlipidemia, no. (%) | 215 (20.8) | 212 (45.2) | P < .001 |
| Hypertension, no. (%) | 463 (51.4) | 319 (77.8) | P < .001 |
| Obesity (BMI > 30), no. (%) | 257 (26.7) | 74 (17.7) | P < .001 |
| BMI, mean (SD), kg/m2 | 27.6 (8.4) | 26.1 (4.9) | P < .001 |
Age 70 + patients had a larger mean tumor size and were more likely to have single tumors but less likely to have HCC that met Milan criteria. Older patients had a lower APRI score and were less likely to have cirrhosis (Table 2). Although MELD scores were similar in the groups, younger patients had higher bilirubin and prothrombin time and a lower creatinine.
Table 2.
Tumor characteristics and laboratory values
| Age < 70 (n = 1056) | Age 70 + (n = 477) | P value | |
|---|---|---|---|
| Tumor size, mean (SD), cm | 5.6 (4.4) | 6.2 (4.3) | P < .001 |
| Bilateral, no. (%) | 134 (14.7) | 57 (13.3) | P = .51 |
| Single tumor, no. (%) | 690 (67.2) | 350 (73.8) | P = .009 |
| Met Milan criteria, no. (%) | 462 (43.8) | 178 (37.4) | P = .02 |
| Normal AFP, no. (%) | 414 (39.4) | 212 (45.0) | P = .04 |
| AFP, mean (SD), ng/mL | 11,980 (54576) | 9743 (60365) | P = .006 |
| Cirrhosis, no. (%) | 774 (74.4) | 303 (64.6) | P < .001 |
| Rupture, no. (%) | 40 (3.8) | 18 (3.8) | P = .99 |
| APRI, mean (SD) | 0.79 (0.84) | 0.45 (0.64) | P < .001 |
| MELD, mean (SD) | 10.5 (4.5) | 10.2 (4.09) | P = .56 |
| Childs–Turcotte–Pugh Score, mean (SD) | 6.5 (2.0) | 5.9 (1.4) | P < .001 |
| Bilirubin, mean (SD), mg/dL | 1.7 (2.7) | 1.2 (1.7) | P < .001 |
| Albumin, mean (SD), g/dL | 3.52 (0.74) | 3.71 (0.63) | P < .001 |
| Prothrombin time, mean (SD), s | 14.6 (2.4) | 14.1 (2.0) | P < .001 |
| INR, mean (SD) | 1.18 (0.27) | 1.13 (0.23) | P < .001 |
| Platelets, mean (SD), 103/μL | 161.3 (95.8) | 183.0 (92.5) | P < .001 |
| Creatinine, mean (SD), mg/dL | 0.98 (0.71) | 1.18 (0.93) | P < .001 |
| AST, mean (SD), IU/L | 91.5 (84.8) | 61.8 (54.2) | P < .001 |
| ALT, mean (SD), IU/L | 67.0 (60.5) | 50.6 (45.2) | P < .001 |
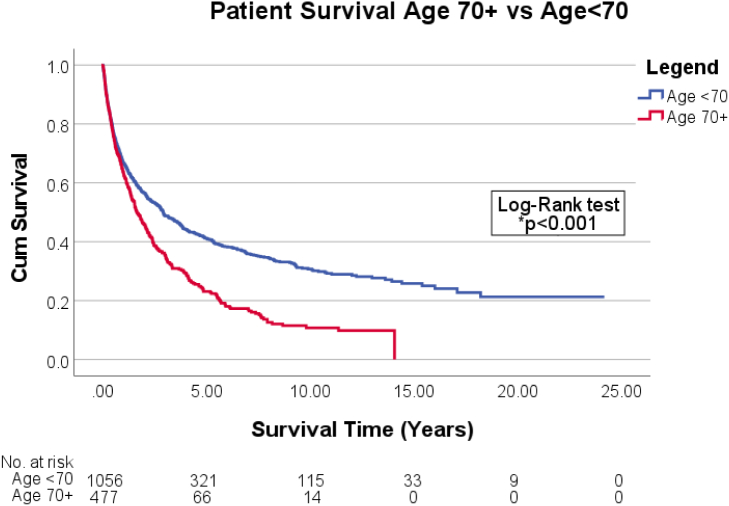
Age 70 + patients were just as likely to undergo liver resection as those who were age < 70. Significantly more patients in the age < 70 group underwent LT compared to those age 70 + (10.2% vs 0.4%, P < .001). One-year survival was similar for both groups but significantly worse for older patients at 3 and 5 years (Table 3). On Kaplan–Meier analysis, age 70 + has worse mean median survival time (1.65 years; 95% CI: 1.34–1.96) than age < 70 group (2.91 years; 95% CI: 2.39–3.43) (Fig 1). The significant positive predictors for 3- and 5-year survival were HCC within Milan criteria and HCC found on surveillance. Age 70 + was the strongest negative predictor of 3- and 5-year survival (Table 4).
Table 3.
Patient treatment and outcome
| Age < 70 (n = 1056) | Age 70 + (n = 477) | P value | |
|---|---|---|---|
| Transplantation, no. (%) | 108 (10.2) | 2 (0.4) | P < .001 |
| Liver resection, no. (%) | 211 (20.0) | 84 (17.6) | P = .28 |
| Nonsurgical therapy only, no. (%) | 499 (47.3) | 266 (55.8) | P = .002 |
| No therapy, no. (%) | 241 (22.8) | 125 (26.5) | P = .15 |
| 1-y survival, no. (%) | 667 (66.0) | 282 (62.3) | P = .16 |
| 3-y survival, no. (%) | 432 (45.8) | 132 (31.5) | P < .001 |
| 5-y survival, no. (%) | 321 (35.8) | 66 (16.8) | P < .001 |
Fig 1.
Overall patient survival by age group.
Table 4.
Predictors of 3- and 5-year survival by binary logistic regression
| Factors |
3-y survival |
5-y survival |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age 70 + | 0.49 (0.35, 0.69) | P < .001 | 0.32 (0.22, 0.47) | P < .001 |
| Male | 1.01 (0.71, 1.42) | P = .975 | 1.18 (0.81, 1.72) | P = .39 |
| Hepatitis B S Ag positive | 1.24 (0.82, 1.88) | P = .315 | 1.09 (0.69, 1.71) | P = .714 |
| Hepatitis C | 1.03 (0.69, 1.53) | P = .889 | 0.87 (0.57, 1.35) | P = .54 |
| Alcohol | 0.83 (0.60, 1.14) | P = .248 | 0.62 (0.44, 0.88) | P = .008 |
| NASH | 0.71 (0.43, 1.17) | P = .174 | 0.59 (0.33, 1.06) | P = .08 |
| Screened for HCC | 1.51 (1.08, 2.10) | P = .015 | 1.45 (1.02, 2.06) | P = .04 |
| Other cancer | 0.94 (0.63, 1.39) | P = .739 | 1.02 (0.67, 1.57) | P = .91 |
| Smoking | 0.77 (0.57, 1.05) | P = .101 | 0.64 (0.46, 0.89) | P = .008 |
| Diabetes | 0.68 (0.49, 0.94) | P = .019 | 0.58 (0.41, 0.83) | P = .003 |
| Hyperlipidemia | 1.28 (0.90, 1.83) | P = .169 | 1.04 (0.70, 1.54) | P = .86 |
| Hypertension | 0.83 (0.61, 1.13) | P = .233 | 0.78 (0.56, 1.08) | P = .13 |
| Normal AFP | 1.88 (1.41, 2.51) | P < .001 | 1.56 (1.14, 2.13) | P = .005 |
| Milan criteria | 3.34 (2.47, 4.53) | P < .001 | 3.00 (2.15, 4.18) | P < .001 |
| Cirrhosis | 0.82 (0.58, 1.16) | P = .254 | 1.00 (0.68, 1.46) | P = .99 |
| APRIa | 0.74 (0.59, 0.92) | P = .008 | 0.82 (0.65, 1.03) | P = .09 |
| MELDb | 0.88 (0.84, 0.91) | P < .001 | 0.88 (0.84, 0.92) | P < .001 |
APRI: AST to platelet ratio index.
MELD: Model for end stage liver disease.
DISCUSSION
Many studies have shown that elderly patients can tolerate liver resection, locoregional therapy, and systemic therapies for management of HCC. In a large Italian multicenter retrospective cohort study, 1,834 HCC cases from 1987 to 2004 were separated into the elderly (age ≥ 70, n = 614) and younger (< 70, n = 1104) and were followed for a median of 15 months [12]. With propensity matching, the median survival was equivalent between the older and younger patients for each therapy: hepatic resection, percutaneous ablation, TACE, or other/palliation for treatment. They concluded that age over 70 did not affect life expectancy and the overall applicability of each therapy for HCC. Similar conclusions were made in separate single center studies performed in Taiwan and Korea, in which elderly patients had comparable survival outcomes to younger patients undergoing the same therapy, including surgical resection, ablation, or TACE [13,14].
Several studies have compared LT in older and younger patients. One of the earliest retrospective studies reported 1,446 LT recipients over a 13-year period. Overall, elderly patients (age ≥ 60, n = 241) had worse survival than younger patients (age < 60, n = 1205), with age over 60 being an independent risk factor for poor survival. Worse survival was noted in older patients with low albumin, marked prolonged INR, Childs–Turcotte–Pugh score > 10 points, severely malnourished, and hospitalized patients. However, older patients with bilirubin < 10 mg/dL had similar survival outcomes as younger patients [15]. Lipshutz et al compared LT outcomes in 62 patients aged 70 or older to 864 patients age 50–59 years. Although there was no difference in survival between the groups, death after the first year was generally due to chronic conditions, including cardiovascular disease, for which advanced age was a strong risk factor [16]. Sonny et al in a retrospective study of 223 older (age ≥ 60 years) LT recipients and 515 younger recipients found no difference in 1-year mortality or composite outcome. However, age > 65 years was an independent predictor for 1-year mortality. Coronary artery disease and arrhythmias were independent predictors for 1-year composite outcome. They suggested that carefully selected older recipients had comparable outcomes to younger recipients, but the elderly needed better pretransplant evaluation and cardiovascular disease optimization [17]. Finally, in a retrospective study of 1,529 HCC patients from 2002 to 2019, long-term outcomes were analyzed for 538 LT patients and 162 patients who had liver resection, stratified by age. Patients older than 65 were less likely to be considered or listed for LT (27% vs 73%, P < .01). However, for patients who underwent LT, the overall survival, disease-specific survival, and disease-free survival were not significantly different between the groups. LT patients older than 65 years were more likely to be disease-free and alive at the end of the study period. They suggested that older candidates were less likely to be considered for transplant, but judicious selection can lead to comparable survival to younger patients [5].
In these studies on LT, the study population consisted of patients who were referred to transplant centers or had undergone LT. These are inherently highly selected patients that medical professionals had assessed and deemed fit enough to tolerate the stress of LT. Thus, it is not a great surprise that the elderly had comparable posttransplant outcomes to the younger patients. However, this is with the caveat that these were carefully selected patients. Our study offers the unique perspective of HCC treatment in a heterogeneous population using a database with a broad referral pattern of all-comers. Consistent with many studies, we also showed that elderly patients were more likely to have NASH and associated metabolic risk factors. Our elderly patients were also less likely to have underlying viral hepatitis or cirrhosis. The lower mean APRI and higher mean platelet count also agree with lower incidence of cirrhosis and portal hypertension previously described in the elderly with HCC. Although MELD scores were similar, elderly patients were more likely to have a higher creatinine and lower bilirubin, suggesting that the elevation in MELD score in the elderly is mainly from a renal, rather than hepatic, etiology.
Despite less severe underlying liver disease, our elderly patients had more advanced HCC, as indicated by larger tumor size or being beyond Milan criteria. This is within the context of the elderly patients less likely to have HCC detected with surveillance. Within our study population, less than 1% of the elderly underwent LT compared to more than 10% of the younger patients. Elderly patients were more likely to have nonoperative, locoregional therapy. Associated metabolic disease and more advanced HCC outside of Milan criteria likely contributed to decreased transplant candidacy. With logistic regression analysis, the only factors that contributed to survival included having HCC found with surveillance, normal AFP, and tumor within Milan criteria. Taken together, this suggested that surveillance and early detection of HCC in the elderly patients may have allowed for more treatment options, including LT, and better outcome.
Multiple retrospective studies have demonstrated the potential benefit of surveillance in enhancing early detection and ultimately improving survival [[18], [19], [20]]. The value of surveillance can be especially seen in countries like Japan, where LT is not as readily available. The Japan Society of Hepatology published clinical guidelines with recommendation of ultrasonographic and biomarker screening as frequently as every 3–4 months in high-risk patients [21]. With superior surveillance, Japanese centers have been able to find 63.5% of 19,536 HCCs in a 2-year period with a solitary nodule, with 56.6% being less than 3 cm [22]. In a study of 1,797 patients diagnosed with surveillance from two US and two Japanese centers, the Japanese patients were diagnosed with smaller tumor size and less advanced stage and were more likely to undergo curative treatment [23]. Median survival time was also longer in patients from Japan. After propensity matching analysis, there was better survival in Japanese patients who underwent resection. This study underlies the importance of a robust HCC surveillance program in improving overall patient outcome.
This study was limited as it was retrospectively designed and from a single institution with a high proportion of Asian patients. We likely had a higher influence of hepatitis B, as many patients emigrated from Asian or Pacific Island nations where hepatitis B was endemic. Furthermore, the specific reasons for a patient not undergoing LT were unclear. Insurance, financial, or psychosocial reasons may have contributed to noncandidacy. The exact cause of death was unknown, so it is unclear if death was specifically due to HCC.
Although this study may not completely reflect the population of all US centers, our study had granular data on risk factors and laboratory studies that are not typically collected in administrative databases. This study also provided information that would not be available from transplant centers that primarily evaluate early-stage HCC patients. LT is possible in well-selected elderly patients, but most elderly patients will not undergo LT. Although transplant centers may consider use of extended-criteria donors to increase the transplant rate in this population, it is unclear if this is the optimal solution. This study suggests that elderly patients are more likely to have metabolic comorbidities, NASH, and larger tumors. The elderly are less likely to have HCC that is found with surveillance or meets Milan criteria; however, it is possible that elderly patients have less advanced underlying liver disease that would warrant surveillance. Physicians should continue to refer appropriate elderly patients for LT, but patients should be given realistic expectations that many will not be candidates. Future studies will be needed to determine if better efforts at HCC surveillance in elderly patients will allow more elderly patients to undergo LT.
Author Contribution
The following are author contributions listed by author:
Wong: research design, performance of the research, writing of the paper.
Lee: data analysis, writing of the paper.
Karasaki: writing of the paper, research design.
Ogihara: writing of the paper, critical analysis.
Tran: writing of the paper, critical analysis.
Conflict of Interest
Dr Wong is on the speakers bureau for Eisai and Helsinn. All other authors have no conflicts to declare.
Funding Source
This study was partially supported by National Institute of Health 1U01CA230690-01.
Ethics Approval
This study was approved by the Institutional Review Board of The University of Hawaii at Manoa. In compliance with ethical regulations, all data were deidentified prior to use and thus exempt from patient consent.
Footnotes
Financial Support:
This study was partially supported by National Institute of Health grant number 1U01CA230690-01.
Dr Linda Wong is on the speakers bureau for Eisai and Helsinn. All other authors have no financial disclosures
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021 doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Sotiropoulos G.C., Malago M., Machairas N., Fouzas I., Paul A. AGMA score: a novel prognostic score for patients undergoing liver transplant for hepatocellular carcinoma. Transplant Proc. 2019;51(6):1923–1925. doi: 10.1016/j.transproceed.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Kwong A.J., Kim W.R., Lake J.R., et al. OPTN/SRTR 2019 annual data report: liver. Am J Transplant. 2021;21(S2):208–315. doi: 10.1111/ajt.16978. [DOI] [PubMed] [Google Scholar]
- 4.Cullaro G., Rubin J.B., Mehta N., Lai J.C. The differential impact of age among liver transplant candidates with and without HCC. Liver Transpl. 2020;26(3):349–358. doi: 10.1002/lt.25662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed O., Vachharajani N., Chang S.H., et al. Access to liver transplantation for hepatocellular carcinoma: does candidate age matter? J Am Coll Surg. 2021;233(1):140–151. doi: 10.1016/j.jamcollsurg.2021.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Gómez Gavara C., Esposito F., Gurusamy K., et al. Liver transplantation in elderly patients: a systematic review and first meta-analysis. HPB (Oxford) 2019;21(1):14–25. doi: 10.1016/j.hpb.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 7.Wilson G.C., Quillin R.C., 3rd, Wima K., et al. Is liver transplantation safe and effective in elderly (≥ 70 years) recipients? A case-controlled analysis. HPB (Oxford) 2014;16(12):1088–1094. doi: 10.1111/hpb.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi S.W., Choi J.S., Yi J.J., Lee Y.H., Han K.J. Risk factors for hepatocellular carcinoma by age, sex, and liver disorder status: a prospective cohort study in Korea. Cancer. 2018;124(13):2748–2757. doi: 10.1002/cncr.31406. [DOI] [PubMed] [Google Scholar]
- 9.Estes C., Razavi H., Loomba R., Younossi Z., Sanyal A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heimbach J.K., Kulik L.M., Finn R.S., et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 11.Roberts L.R., Sirlin C.B., Zaiem F., et al. Imaging for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatology. 2018;67:401–421. doi: 10.1002/hep.29487. [DOI] [PubMed] [Google Scholar]
- 12.Mirici-Cappa F., Gramenzi A., Santi V., et al. Treatments for hepatocellular carcinoma in elderly patients are as effective as in younger patients: a 20-year multicentre experience. Gut. 2010;59(3):387–396. doi: 10.1002/hep.29466. [DOI] [PubMed] [Google Scholar]
- 13.Liu P.H., Hsu C.Y., Lee Y.H., et al. Uncompromised treatment efficacy in elderly patients with hepatocellular carcinoma: a propensity score analysis. Medicine. 2014;93(28) doi: 10.1097/MD.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y.J., Kang B.K., Kim E.S., et al. Hepatocellular carcinoma in the elderly: clinical characteristics, treatment, survival analysis in Korean patients older than 70 years. J Korean Med Sci. 2012;27(10):1147–1154. doi: 10.3346/jkms.2012.27.10.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy M.F., Somasundar P.S., Jennings L.W., et al. The elderly liver transplant recipient: a call for caution. Ann Surg. 2001;233(1):107–113. doi: 10.1097/00000658-200101000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipshutz G.S., Hiatt J., Ghobrial R.M., et al. Outcome of liver transplantation in septuagenarians: a single-center experience. Arch Surg. 2007;142(8):775–784. doi: 10.1001/archsurg.142.8.775. [DOI] [PubMed] [Google Scholar]
- 17.Sonny A., Kelly D., Hammel J.P., Albeldawi M., Zein N., Cywinski J.B. Predictors of poor outcome among older liver transplant recipients. Clin Transplant. 2015;29:197–203. doi: 10.1111/ctr12500. [DOI] [PubMed] [Google Scholar]
- 18.Choi D.T., Kum H.C., Park S., et al. Hepatocelluular carcinoma screening is associated with increased survival of patients with cirrhosis. Clin Gastroenterol Hepatol. 2019;17(5):976–987. doi: 10.1016/j.cgh.2018.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramai D., Singh J., Candan S., et al. Utilization of hepatocellular carcinoma surveillance programs in patients with cirrhosis: a systematic review and meta-analysis. Clin Gastroenterol. 2022 doi: 10.1097/MCG.0000000000001668. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Wolf E., Rich N.E., Marrero J.A., Parikh N.D., Singal A.G. Use of hepatocellular carcinoma surveillance in patients with cirrhosis: a systematic review and meta-analysis. Hepatology. 2021;73(2):713–725. doi: 10.1002/hep.31309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokudo N., Takemura N., Hasegawa K., et al. Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th HSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49(10):1109–1113. doi: 10.1111/hepr.13411. [DOI] [PubMed] [Google Scholar]
- 22.Kudo M. Surveillance, diagnosis and treatment outcomes of hepatocellular carcinoma in Japan, 2021 update. Liver Cancer. 2021;10:167–180. doi: 10.1159/000516491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toyodo H., Hiraoka A., Olivares J., et al. Outcome of hepatocellular carcinoma detected during surveillance: comparing USA and Japan. Clin Gastroenterol Hepatol. 2021;19:2379–2388. doi: 10.1016/j.cgh.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]