Abstract
Polypharmacy, or the simultaneous use of multiple drugs to treat a single patient, is a common practice in psychiatry. Unfortunately, data on the health effects of commonly used combinations of medications are very limited. In this study, we therefore investigated the effects and interactions between two commonly prescribed psychotropic medications with sterol inhibiting side effects, trazodone (TRZ), an antidepressant, and aripiprazole (ARI), an antipsychotic. In vitro cell culture experiments revealed that both medications alone disrupted neuronal and astroglial sterol biosynthesis in dose-dependent manners. Furthermore, when ARI and TRZ were combined, exposure resulted in an additive 7-dehydrocholesterol (7-DHC) increase, as well as desmosterol (DES) and cholesterol decreases in both cell types. In adult mice, at baseline, we found that the three investigated sterols showed significant differences in distribution across the eight assessed brain regions. Furthermore, experimental mice treated with ARI or TRZ, or a combination of both medications for 8 days, showed strong sterol disruption across all brain regions. We show ARI or TRZ alone elevated 7-DHC and decreased DES levels in all brain regions, but with regional differences. However, the combined utilization of these two medications for 8 days did not lead to additive changes in sterol disturbances. Based on the complex roles of 7-DHC derived oxysterols, we conclude that individual and potentially simultaneous use of medications with sterol biosynthesis-inhibiting properties might have undesired side effects on the adult brain, with as yet unknown long-term consequences on mental or physical health.
Supplementary key words: trazodone, aripiprazole, polypharmacy, 7-dehydrocholesterol, cholesterol, desmosterol, side effects, brain regions, sterol homeostasis, mouse model
Abbreviations: 7-DHC, 7-dehydrocholesterol; ARI, aripiprazole; CHOL, cholesterol; DES, desmosterol; Dhcr7 or DHCR7, 7-dehydrocholesterol reductase; DHCR24, 24-dehydrocholesterol reductase; LAN, lanosterol; NBM, Neurobasal medium; SLOS, Smith-Lemli-Opitz syndrome; SRM, selective reaction monitoring; TRZ, trazodone; VEH, vehicle
Intact cholesterol biosynthesis is crucial for CNS homeostasis. The brain contains approximately 20%–25% of all cholesterol in the human body and relies on cholesterol biogenesis that is independent of the periphery (1). Genetic disruptions of the sterol synthesis pathway lead to several developmental disabilities, including, but not limited to, Smith-Lemli-Opitz syndrome (SLOS), lathosterolosis, and desmosterolosis (2, 3, 4). Impaired sterol biosynthesis has also been associated with many CNS disorders including major depression, schizophrenia, and Huntington’s and Alzheimer’s diseases (5, 6, 7, 8). Unfortunately, many commonly prescribed psychotropic medications also have sterol biosynthesis inhibiting effects (1, 9). Previous in vivo mouse experiments and patient biobank assessments revealed that aripiprazole, cariprazine, haloperidol, trazodone, and amiodarone are strong inhibitors of post-lanosterol biosynthesis (1, 10).
ARI is an atypical antipsychotic, with >6.6 million prescriptions in the United States in 2019 (https://clincalc.com/DrugStats/). ARI is primarily utilized for treatment of schizophrenia and bipolar disorder, and its beneficial effects are well documented (11, 12). TRZ is an antidepressant of the serotonin receptor antagonists and reuptake inhibitors family and it was prescribed approximately 24 million times in the United States in 2019 (https://clincalc.com/DrugStats/). The primary use of TRZ is the treatment of depression (13). However, TRZ has been also extensively utilized for off-label treatment of insomnia (14, 15), opioid withdrawal symptoms (16), alcohol withdrawal (17, 18, 19), dementia (15), fibromyalgia (20) and other conditions (21, 22).
Unfortunately, the side effects of ARI and TRZ have also been extensively documented (11, 23, 24, 25, 26, 27, 28, 29, 30), including their unwanted action on sterol biosynthesis: ARI and TRZ treatments lead to a strong elevation of 7-dehydrocholesterol (7-DHC) and decreased desmosterol (DES) levels in in vitro and in vivo animal models, as well as in human biomaterials (fibroblasts and plasma) (9, 31, 32, 33, 34). Importantly, 7-DHC is the most oxidizable lipid known to date, with the propagation rate constant 2,160 M−1s−1 (this is 200 times more than cholesterol and 10 times more than arachidonic acid) (35). 7-DHC spontaneously oxidizes and give rise to highly reactive 7-DHC-derived oxysterols (36). 7-DHC-derived oxysterols are toxic and affect cell viability, differentiation, and growth (37, 38, 39). One of the best characterized 7-DHC-derived oxysterols, 3β,5α-dihydroxycholest-7-en-6-one (DHCEO), has a profound effect on neuronal morphology, neurite outgrowth, and fasciculation, potentially through sonic hedgehog signaling (40). In addition to DHCEO, there are at least 20 other 7-DHC-derived oxysterols identified to date, and several of these have been found in mouse and human SLOS samples (36, 41). These 7-DHC-derived oxysterols are not only markers of oxidative stress (42) but are also biologically potent compounds capable of affecting sonic hedgehog signaling and interfering with immune response (43).
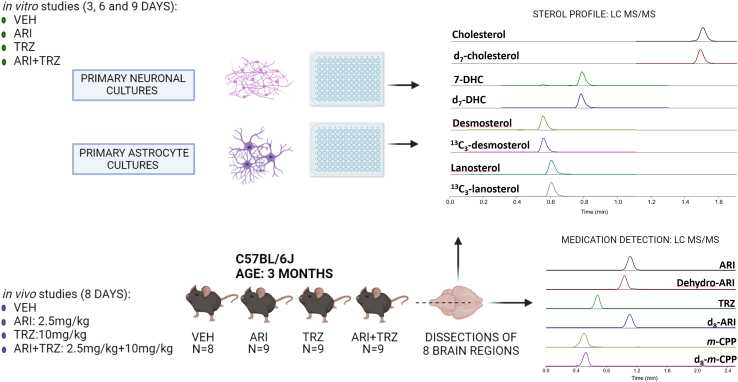
Polypharmacy is a nationwide and worldwide challenge (44, 45, 46, 47). Both ARI and TRZ are commonly prescribed, often utilized together as part of the treatment plan. Yet, the sterol biosynthesis inhibiting side effects of these two medications (alone or in combination) in adulthood have not been systematically explored to date. As a result, our study was designed to evaluate the effects of ARI, TRZ, and ARI+TRZ polypharmacy on adult brain sterol biosynthesis. Post-lanosterol lipid profiling was performed using LC/MS/MS. For our experiments we utilized in vitro primary neuronal and astroglial cultures, as well as in vivo studies on adult mice. The experimental design is presented in Fig. 1.
Fig. 1.
Experimental Design. In vitro experiments: Cortical neurons and astrocytes were cultured from C57BL/6J mice [embryonic day 18 (E18) for neurons and postnatal day 2 (P2) for astrocytes], and treated with five different concentrations of ARI, TRZ, or ARI+TRZ. CHOL, DES, 7-DHC, and LAN were analyzed by LC/MS/MS after 3, 6, and 9 days of treatment using the 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD) derivatization assay. In vivo experiments: Following daily exposure to ARI (2.5 mg/kg), TRZ (10 mg/kg), ARI+TRZ (2.5 mg/kg+10 mg/kg), or vehicle (VEH) for 8 days, eight brain regions of each adult mice were dissected (n=8–9/group). Sterols, ARI, TRZ, and their main metabolites were measured by LC/MS/MS.
Materials and methods
Chemicals
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich Co (St. Louis, MO). HPLC grade solvents were purchased from ThermoFisher Scientific Inc. (Waltham, MA). TRZ and ARI were obtained from Selleckchem (Radnor, PA) and dissolved in sterile DMSO solution for the experiments. All sterol standards, natural and isotopically labeled, used in this study are available from Kerafast, Inc. (Boston, MA).
Aripiprazole and trazodone injections in mice
Adult male C57Bl/6J stock # 000664 mice, 3 months old, were purchased from Jackson Laboratories. Mice were housed under a 12 h light-dark cycle at constant temperature (25°C) and humidity with ad libitum access to food (Teklad LM-485 Mouse/Rat Sterilizable Diet 7012) and water in Comparative Medicine at the University of Nebraska Medical Center (UNMC), Omaha, NE. In humans, TRZ (Desyrel) is given at a starting dose 150 mg/day and may be increased by 50 mg per day every 3–4 days to a maximum dose of 400 mg per day for outpatient use. For treatment of insomnia, TRZ is given at a starting dose of 50 mg/day. If we take a dose of 50 mg/60 kg human body weight, this translates to 0.83 mg/kg/day. Animal Equivalent dose (AED in mg/kg) is calculated as AED (mg/kg) = human dose (mg/kg) (50 mg per day) x Km ratio (12.3) = 10 mg/kg (48). As a result, we chose to use a low dose of 10 mg/kg in our mouse experiments, which translates back to about 50 mg/day in humans, depending on the weight of the patient. Based on these calculations and literature data, we used ARI (Abilify) at 2.5 mg/kg in our mouse experiments (which corresponds to ARI tablet 10–15 mg/day in humans). The range of doses in humans is 2 mg–30 mg/day (49). A total number of 35 adult male mice were used in our study with 9 animals assigned to each group, except for control group that had 8 mice. We applied intraperitoneal injections as a method for drug (or vehicle) delivery, every day at 8.00 AM. The treatment did not influence mouse body mass for the duration of the experiment (supplemental Fig. S1). All procedures were performed in accordance with the Guide for the Humane Use and Care of Laboratory Animals. The use of mice in this study was approved by the Institutional Animal Care and Use Committee of UNMC.
Tissue collection and preparation for sterol analysis
About 4 to 6 hours after the last injections, mice were euthanized with Isoflurane overdose (Forane® isofluranum, Abbott Laboratories Ltd; Lake Bluff, IL). Brains were dissected and brain regions were frozen in prechilled methylbutane and stored at −80°C. Frozen samples were sonicated in ice-cold PBS containing butylated hydroxytoluene and triphenylphosphine. The first set of aliquots (10 μl) of homogenized tissue were used for sterol extraction. The second set of aliquots (20 μl) of homogenized tissue were used for protein measurements. The protein was measured using BCA assay (Pierce™ BCA Protein Assay Kit, ThermoFisher Scientific, Waltham, MA). Sterol levels were normalized to protein measurements and expressed as nmol/mg protein. The third set of aliquots (100 μl) of homogenized tissue were used for drug measurements.
LC/MS/MS (selective reaction monitoring) analyses
Sterols were extracted and derivatized with 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD) as described previously (50) and placed in an Acquity UPLC system equipped with ANSI-compliant well plate holder coupled to a Thermo Scientific TSQ Quantis mass spectrometer equipped with an atmospheric pressure chemical ionization source. Then 10 μl was injected onto the column (Phenomenex Luna Omega C18, 1.6 μm, 100 Å, 2.1 mm × 100 mm) with 90% MeOH and 10% acetonitrile (0.1% v/v acetic acid) mobile phase for 1.7 min runtime at a flow rate of 500 μl/min. Natural sterols were analyzed by selective reaction monitoring (SRM) using the following transitions: Chol 369 → 369, 7-DHC 560 → 365, desmosterol 592 → 560, lanosterol 634 → 602, with retention times of 0.7, 0.4, 0.3, and 0.3 min, respectively. SRMs for the internal standards were set to d7-Chol 376 → 376, d7-7-DHC 567 → 372, 13C3-desmosterol 595 → 563, 13C3-lanosterol 637 → 605.
ARI and TRZ measurements
Medications were extracted from 100 μl aliquots for all brain regions using methyl tert-butyl ether and ammonium hydroxide as described previously (51). ARI levels were acquired in an Acquity UPLC system coupled to a Thermo Scientific TSQ Quantis mass spectrometer using an ESI source in the positive ion mode. Ten microliters of each sample was injected onto the column (Phenomenex Luna Omega C18, 1.6 μm, 100 Å, 2.1 × 50 mm2) using water (0.1% v/v acetic acid) (solvent A) and acetonitrile (0.1% v/v acetic acid) (solvent B) as the mobile phase. The gradient was 10%–40% B for 0.5 min; 40%–95% B for 0.4 min; 95% B for 1.5 min; 95%–10% B for 0.1 min; 10% B for 0.5 min. ARI, TRZ, and their metabolites were analyzed by SRM using the following transitions: ARI 448 → 285, dehydroaripiprazole 446 → 285, TRZ 372 → 176, m-CPP 197 → 153. The SRM for the internal standards (d8-ARI and d8-m-CPP) were set to 456 → 293 and 205 → 157, respectively. Final medication levels are reported as ng/mg of protein.
Primary neuronal cultures
Primary cortical neuronal cultures were prepared from E18 C57BL/6J mice as previously described (40, 52). The brain tissue was placed in prechilled HBSS solution (without Ca2+ or Mg2+), and two cortices were dissected, cut with scissors into small chunks of similar sizes, and transferred to Trypsin/EDTA (0.25%) for 25 min at 37°C. Trypsin was removed and residual trypsin was inactivated by adding Trypsin Inhibitor (Sigma, cat. no: T6522) and DNase for 5 min. The solution was removed and small tissue chunks were resuspended in Neurobasal medium (NBM) with B-27 supplement (Gibco, cat. no: 17504-044). Samples were triturated with a fire-polished Pasteur pipet. The cells were pelleted by centrifugation for 10 min at 80 g. The cell pellet was resuspended in NBM with B-27 supplement, and the cells were counted. The cells were plated on poly(d-lysine)-coated 96-well plates at 70,000 cells/well. The growth medium was NBM with B-27 supplement and Glutamax. Cells were incubated at 37°C in 5% CO2 for 3–10 days in presence and absence of different concentrations of ARI, TRZ, and ARI+TRZ.
Primary astrocyte cultures
Primary astrocyte cultures were prepared from postnatal day 2 (P2) C57BL/6J mice as previously described (53, 54). Brain tissue was placed in prechilled HBSS solution (without Ca2+ or Mg2+), and two cortices were dissected, cut with scissors into small chunks of similar sizes, and transferred to Trypsin/EDTA (0.5%) for 30 min at 37°C. Trypsin was removed and residual trypsin was inactivated by adding DMEM with 10% FBS. The cells were plated in 100 mm dishes and grown for 10–14 days until astrocytes fill the whole dish. At that time the astrocytes were trypsinized and plated in 96-well plates at 30,000 cells/well. On the following day, the medium was completely changed, and astrocytes were grown in NBM with B-27 supplement in the absence of cholesterol (same medium as for neuronal cells). Cells were incubated at 37°C in 5% CO2 for 3–10 days in the presence and absence of different concentrations of ARI, TRZ, and ARI+TRZ.
For the current study, we prepared three independent preparations of 3x96-well plates for primary cortical neurons and astrocytes. For experiments in both cell cultures, we treated the cells for 3, 6, or 9 days. All 3 time points showed a similar trend in sterol changes upon treatment.
At the end of the experiments in neuronal and astrocytes cultures, cell nuclei were stained with Hoechst dye and the total numbers of cells in each well were counted using ImageXpress Pico and cell counting algorithm in CellReporterXpress. After the medium was removed, wells were rinsed twice with 1× PBS and then stored at −80°C for lipid analysis. All samples were analyzed within 2 weeks after freezing. The results were concordant for cultures obtained from both embryonic stages and across three independent sets of experiments.
Statistical analyses
Statistical analyses were performed using GraphPad Prism 9 for Windows. Unpaired two-tailed t test was applied for individual comparisons between two groups. Discovery was determined using the two-stage linear stepup procedure of Benjamini, Krieger, and Yekutieli, with Q = 5%. Each analysis was performed individually, without assuming a consistent SD. Statistical test showed a normal distribution in all comparison groups. The P-values for statistically significant differences are highlighted in the figures and/or figure legends.
Results
ARI and TRZ alter sterol concentrations in both neurons and astrocytes
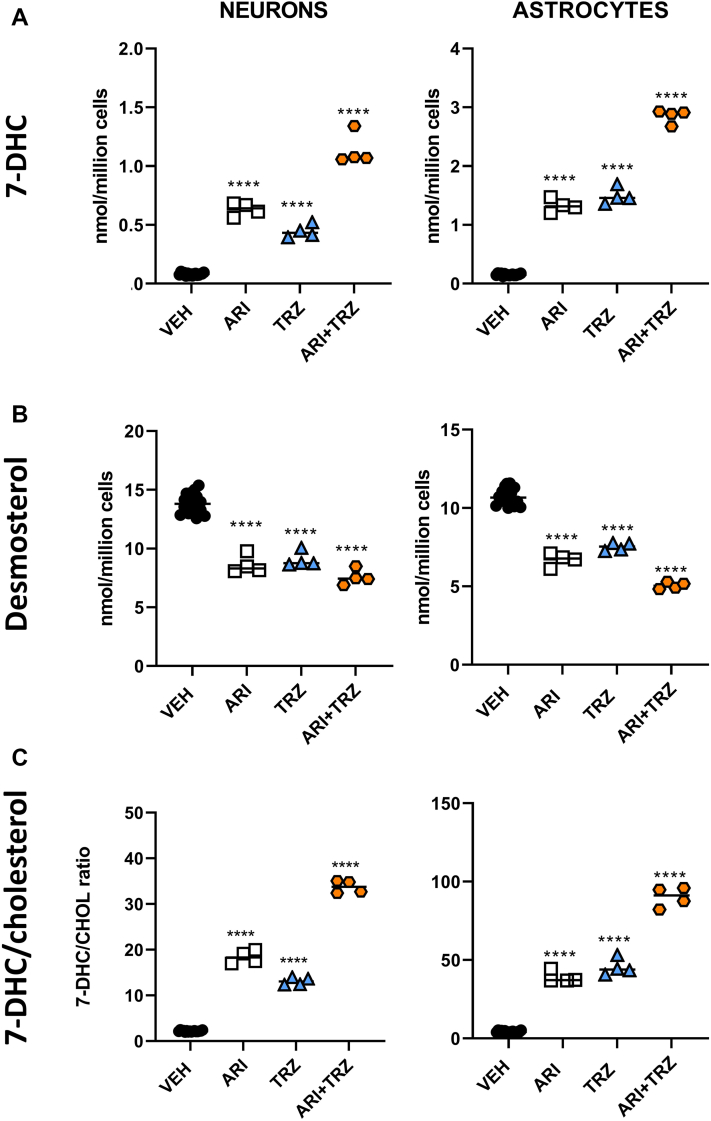
Primary cortical neuronal and astrocyte cultures were treated for 3, 6, and 9 days with five different concentrations of ARI, TRZ, or ARI+TRZ and sterols were analyzed with LC/MS/MS. While the cell viability was not affected over the range of concentrations used (0–100 nm for ARI, 0–500 nm for TRZ), the sterol profile was significantly altered by the treatment. The individual ARI and TRZ treatments significantly and robustly increased 7-DHC, decreased DES, and strongly altered the 7-DHC/CHOL ratio (Fig. 2). This was true for both neurons and astrocytes. Notably, the ARI+TRZ coadministration showed the strongest sterol biosynthesis effects on both cells, suggesting an additive effect between ARI and TRZ.
Fig. 2.
ARI and TRZ treatment alter sterol biosynthesis in primary neuronal and astrocyte cultures. A: 7-DHC levels, B: DES levels, and C: 7-DHC/CHOL. Cortical neurons and astrocytes were cultured and treated with five different concentrations of ARI, TRZ, or ARI+TRZ for 3–9 days. Data from 6 days of culturing are presented for vehicle treatment, 5 nmol ARI, 20 nmol TRZ, and ARI+TRZ (5 nmol+20 nmol). The response to all concentrations of ARI, TRZ, and ARI+TRZ drug treatments can be found in supplemental Figs. S2–S4. Treatments compared with vehicle exposure that reached significance are marked with black asterisks. Unpaired, two-tailed t test was used for statistical comparison of different treatments; ∗∗∗∗P<0.0001. Abbreviations: ARI, aripiprazole; TRZ, trazodone; ARI+TRZ, combined treatment with aripiprazole and trazodone; CNTR (vehicle), control group treated with vehicle. Note that treatment with ARI+TRZ showed an additive effect, increasing the 7-DHC/CHOL ratio by 15- to 20-fold over vehicle-treated cultures (P<0.001), compared with 8- to 9-fold and 6- to 10-fold baseline change for single drug ARI and TRZ treatment, respectively.
The full dose dependence profile for ARI, TRZ, and ARI+TRZ treatment is presented in supplemental Figs. S2–S4, with 7-DHC increases >100-fold in response to the highest concentrations of medications. In addition, at the highest concentrations of both medications reduced DES levels by up to 65%. These DES reductions were strongly correlated with CHOL reductions. Notably, while there were only minor differences in baseline sterol levels (e.g. sham-treated cells), both neuronal and astroglial cultures responded comparably to ARI, TRZ, and ARI+TRZ treatments with regard to 7-DHC, DES, and CHOL levels. However, we did not observe any significant differences in lanosterol (LAN) levels (data not shown).
Baseline sterol levels differ across adult brain regions
Brain regions of sham-treated adult mice exhibited quite variable baseline sterol levels (Fig. 3A). The comparison of CHOL and the two immediate precursors, 7-DHC and DES, showed a >2-fold difference across the CNS areas. The highest sterol levels were observed in medulla, pons, and midbrain, while the olfactory bulb had the lowest sterol levels. In general, the highest CHOL-containing regions had also the highest precursor levels. 7-DHC/CHOL and DESM/CHOL levels showed less variation across the investigated regions, except for olfactory bulb with higher values observed for both ratios (Fig. 3B). The data suggest that brain regions have somewhat different sterol requirements and homeostasis, but with relatively constant precursor/product ratio.
Fig. 3.
Sterol levels are different across the eight investigated brain regions of adult mice. A: The y-axis denotes sterol levels (CHOL, DES, and 7-DHC); x-axis denotes brain regions. Each symbol represents a single LC/MS/MS measured sample from a different mouse brain. B: DES/CHOL and 7-DHC/CHOL ratios across the investigated brain regions. Note that 1) baseline levels of CHOL are more that 500-fold higher than 7-DHC levels, 2) the ratio of 7-DHC to DES and CHOL is relatively constant across the different brain regions, and 3) that the medulla and pons have the highest levels of all three sterols.
ARI, TRZ, and their metabolites are detectable in the brains of treated mice
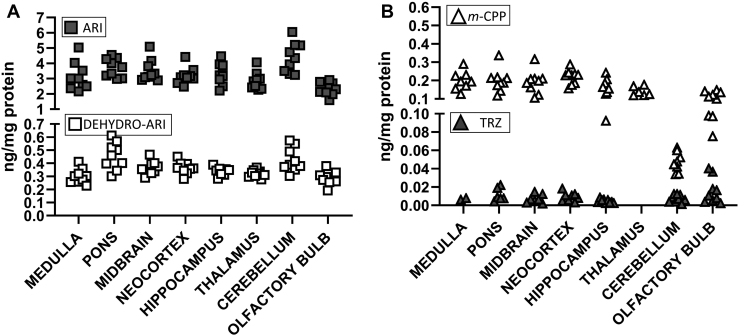
Adult mice were injected with either vehicle (VEH) or 2.5 mg/kg ARI or 10 mg/kg TRZ or both medications (ARI+TRZ) simultaneously. Mice were injected daily for 8 days. To confirm that the treatment reached the brain and to ascertain the uniformity of medication/metabolite distribution across the brain regions, ARI and TRZ concentrations were measured in eight different brain regions. Drug concentrations were only measured at the end of the experiment. Figure 4 presents medication levels in the ARI+TRZ treatment group.
Fig. 4.
ARI and TRZ are detected in the brain tissue. ARI (2.5 mg/kg) + TRZ (10 mg/kg) treatment data are reported. Each symbol denotes ARI, TRZ, or active metabolite concentration in a single ARI+TRZ-treated brain sample. ARI and its metabolite, dehydroaripiprazole (dehydro-ARI), and TRZ metabolite, meta-chlorophenylpiperazine (m-CPP), are readily detectable in all brain regions. TRZ was not detected in samples originating from thalamus and was only detected in two samples in medulla.
ARI and its metabolite dehydro-ARI were detected simultaneously in all investigated brain regions. Dehydroaripiprazole, a major and active ARI metabolite, was present in approximately 10-fold lower concentrations than ARI in all brain regions. TRZ alone was not detected in thalamus and was only detected in two samples in medulla while its active metabolite metachlorophenylpiperazine was detected at high concentration in all brain regions. Both medications and their metabolites showed similar levels across different brain regions. Similar results were obtained in mice treated with a single medication (TRZ or ARI) (data not shown). None of the compounds were detected in the VEH-injected mice.
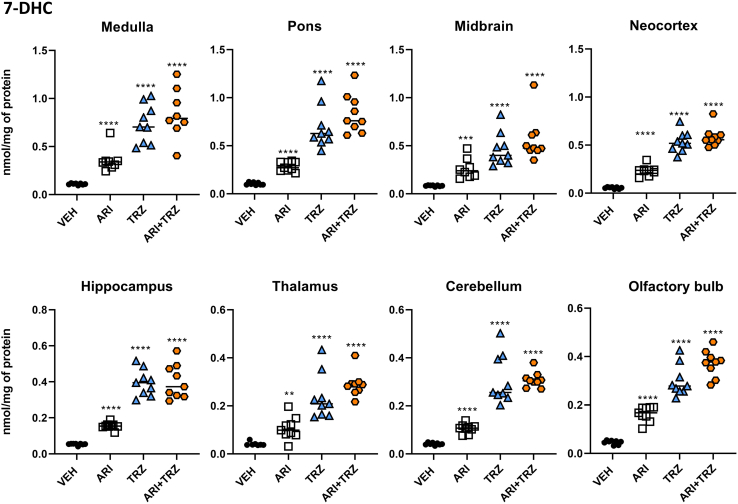
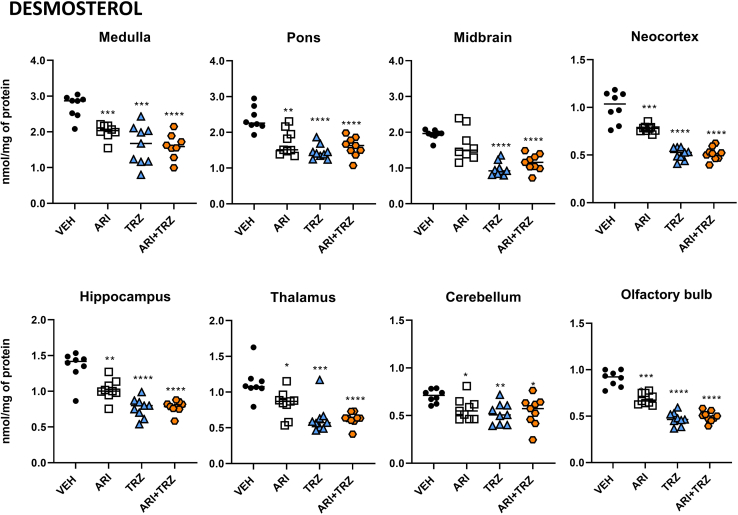
ARI and TRZ increase 7-DHC and decrease DES in the adult mouse brain
Next, we performed measurements of CHOL, 7-DHC, DES, and LAN levels in the drug-treated and sham-treated animals across the eight brain regions (n=8–9/group). Compared with vehicle-injected animals, treatment of mice with either individual drug (ARI or TRZ) or their combination (ARI+TRZ) robustly and significantly increased 7-DHC (Fig. 5) and decreased DES levels (Fig. 6) in all studied brain regions, altering 7-DHC/CHOL ratios (supplemental Fig. S5). While in the cell culture experiments ARI seemed to be a slightly more potent inhibitor of sterol biosynthesis than TRZ, in the adult mouse brain TRZ showed a much stronger inhibitory effect than ARI. ARI increased 7-DHC levels approximately 3-fold, while TRZ increased 7-DHC up to 10-fold in all brain regions. Importantly, it appeared that the ARI+TRZ group showed similar 7-DHC increases as the TRZ exposure alone. Changes in DES levels mirrored the 7-DHC changes across all three treatment groups. DES levels were decreased by 17%–30% by ARI treatment, 27%–50% by TRZ treatment, and 25%–50% by ARI+TRZ combination treatment.
Fig. 5.
ARI and TRZ treatment caused a strong and significant increase of 7-DHC levels in all brain regions of C57BL/6 mice. Each symbol represents a regional brain sample from a single mouse. The x-axis denotes animal groups; y- axis denotes 7-DHC concentration normalized to mg of protein. Black asterisk denotes comparison with baseline levels. Two-tailed groupwise t test was used for statistical comparisons, ∗∗∗∗P<0.0001. Abbreviations: ARI, aripiprazole; TRZ, trazodone; ARI+TRZ, combined treatment with aripiprazole and trazodone. 7-DHC fold changes over vehicle treatment for each brain region are presented in supplemental Fig. S6A.
Fig. 6.
ARI and TRZ treatment caused a strong and significant decrease in DES levels across all brain regions of C57BL/6 mice. Each symbol represents a regional brain sample from a single mouse. The x-axis denotes animal groups; y-axis denotes DES concentration normalized to mg of protein. Black asterisk denotes comparison with baseline levels. Two-tailed groupwise t test was used for statistical comparisons, ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001, ∗∗∗∗P<0.0001. Abbreviations: ARI, aripiprazole; TRZ, trazodone; ARI+TRZ, combined treatment with aripiprazole and trazodone. Note the strong DES decrease by all treatments. Percentage of desmosterol decrease for each brain region is presented in supplemental Fig. S6B.
Mean values for 7-DHC and DES in all groups for each brain region are shown in Table 1. Although all brain regions were strongly affected by ARI, TRZ, and ARI+TRZ treatment, the largest change was detected in the neocortex where ARI+TRZ treatment caused >10-fold increase of 7-DHC over control group. In contrast, the smallest, 4-fold 7-DHC increase was observed in thalamus (supplemental Fig. S6A). DES levels were most decreased by combined treatment in the neocortex (50%), thalamus, and olfactory bulb (45% decrease in both), while the lowest decrease was observed in the cerebellum (25% change) (supplemental Fig. S6B). Notably, with the TRZ or ARI exposure alone, or with the combined ARI+TRZ exposure, we did not observe a significantly changed CHOL or LAN levels (data not shown).
Table 1.
Sterol levels in different brain regions of mice treated with ARI, TRZ, or ARI+TRZ
| Region | Treatment | 7-DHC ± SEM | DES ± SEM |
|---|---|---|---|
| Medulla | VEH | 0.109 ± 0.003 | 2.71 ± 0.12 |
| ARI | 0.357 ± 0.043 | 2.01 ± 0.08 | |
| TRZ | 0.736 ± 0.067 | 1.62 ± 0.18 | |
| ARI+TRZ | 0.843 ± 0.092 | 1.59 ± 0.12 | |
| Pons | VEH | 0.104 ± 0.005 | 2.38 ± 0.12 |
| ARI | 0.284 ± 0.017 | 1.72 ± 0.12 | |
| TRZ | 0.695 ± 0.077 | 1.45 ± 0.07 | |
| ARI+TRZ | 0.834 ± 0.072 | 1.59 ± 0.09 | |
| Midbrain | VEH | 0.083 ± 0.002 | 1.94 ± 0.05 |
| ARI | 0.264 ± 0.04 | 1.66 ± 0.16 | |
| TRZ | 0.464 ± 0.058 | 0.98 ± 0.06 | |
| ARI+TRZ | 0.565 ± 0.081 | 1.15 ± 0.08 | |
| Cortex | VEH | 0.055 ± 0.003 | 1.01 ± 0.06 |
| ARI | 0.232 ± 0.017 | 0.78 ± 0.01 | |
| TRZ | 0.532 ± 0.036 | 0.51 ± 0.02 | |
| ARI+TRZ | 0.584 ± 0.036 | 0.51 ± 0.02 | |
| Hippocampus | VEH | 0.054 ± 0.002 | 1.35 ± 0.07 |
| ARI | 0.155 ± 0.006 | 1.02 ± 0.05 | |
| TRZ | 0.395 ± 0.025 | 0.77 ± 0.05 | |
| ARI+TRZ | 0.402 ± 0.032 | 0.78 ± 0.03 | |
| Thalamus | VEH | 0.041 ± 0.003 | 1.13 ± 0.08 |
| ARI | 0.107 ± 0.016 | 0.84 ± 0.06 | |
| TRZ | 0.237 ± 0.032 | 0.63 ± 0.07 | |
| ARI+TRZ | 0.289 ± 0.017 | 0.62 ± 0.03 | |
| Cerebellum | VEH | 0.043 ± 0.002 | 0.70 ± 0.02 |
| ARI | 0.106 ± 0.006 | 0.57 ± 0.04 | |
| TRZ | 0.309 ± 0.034 | 0.52 ± 0.04 | |
| ARI+TRZ | 0.309 ± 0.011 | 0.54 ± 0.05 | |
| Olfactory bulb | VEH | 0.046 ± 0.003 | 0.91 ± 0.03 |
| ARI | 0.163 ± 0.01 | 0.69 ± 0.02 | |
| TRZ | 0.299 ± 0.022 | 0.47 ± 0.02 | |
| ARI+TRZ | 0.372 ± 0.018 | 0.50 ± 0.02 |
All values are expressed as nmol/mg of protein ±SEM.
Abbreviations: 7-DHC, 7-dehydrocholesterol; ARI+TRZ, combined treatment of aripiprazole and trazodone; ARI, aripiprazole; VEH, vehicle-treated control group; DES, desmosterol; TRZ, trazodone.
Discussion
Polypharmacy is defined as the simultaneous use of multiple drugs by a single patient, for one or more conditions (55). It is increasingly common worldwide and contributes to the substantial burden of drug-related morbidity (56, 57). Quinn and Shah counted the incidence of drug combinations observed in four billion patient-months of outpatient prescription drug claims from 2007–2014 in the Truven Health MarketScan® Databases. They found that, among patients taking any prescription drug, half were exposed to two or more drugs, while 5% were exposed to eight or more. In Japan, the EGUIDE psychiatric project reported that 54% of schizophrenia patients received antipsychotic monotherapy, while 43% received combined therapy of two or more drugs. In patients with major depressive disorder, 59% received monotherapy and 25% received two or more antidepressants at the same time (44). Yet, we have very little information about the effects of this polypharmacy on brain homeostasis and overall health, especially in patients in whom treatment effects are routinely achieved by various combinations of drugs (e.g., neuropsychiatric diseases).
The studies of sterol biosynthesis inhibition by psychotropic medications were triggered by the initial observation of Hall et al., noting that patients utilizing ARI or TRZ, without any mutations in the 7-dehydrocholesterol reductase (DHCR7) gene, showed highly elevated 7-DHC levels in their plasma (31). Our research group recently replicated and expanded these findings on a group of psychiatric patients treated with either ARI or TRZ (32). In addition, in the most recent study, Cenik et al. have observed increased 7-DHC in human postmortem brain tissue due to TRZ usage, suggesting that DHCR7 inhibition is an integral part of TRZ action (22). In follow-up studies on sterol biosynthesis health, we found that multiple CNS-targeting medications are strong inhibitors of post-lanosterol biosynthesis, including (but not limited to) aripiprazole, cariprazine, haloperidol, trazodone, and amiodarone. This inhibition was consistently observed across all experimental systems utilized: Neuro2a cells, in vitro neuronal and astroglial cultures, human dermal fibroblasts, in vivo mouse studies, and human patient sera. We learned that the medication effects on sterol biosynthesis are complex and include inhibition of multiple enzymes in the pathway, including ARI and TRZ inhibition of DHCR7 and 24-dehydrocholesterol reductase (DHCR24) enzymes (1).
In our current study we found that the baseline levels of cholesterol and immediate cholesterol precursors (DES and 7-DHC) are not uniform across the eight studied brain regions. Brain regions rich in fiber tracts (medulla, pons, and midbrain) had the highest concentrations of all three sterols. However, in contrast to the regional distribution of cholesterol and its precursors in the brain, ARI and TRZ (and their metabolite) levels are mostly uniform across the various CNS regions. This could explain an interesting observation: while the pons had the highest sterol levels at baseline, the most robust sterol biosynthesis disruption by ARI and TRZ was observed in the neocortex. These findings raise an interesting question: will the potential behavioral changes due to disturbances in sterol biosynthesis first manifest themselves in behaviors that are primarily controlled by subcortical mechanisms (as they have the highest absolute sterol levels) or neocortex (which had the highest change in sterol precursors in response to medications). This can and should be tested in follow-up behavioral studies in a mouse model.
It is perhaps also noteworthy that the increase of 7-DHC levels and decrease of DES by TRZ and ARI might represent a double risk for the brain. Desmosterolosis, a condition arising from mutations in the DHCR24 gene, is lethal, underscoring the important role of DES in brain and body homeostasis (3, 58). Thus, the elevation of 7-DHC, combined with a strong decrease in DES levels, confirms impairment in both the Kandutsch-Russell and Bloch arms of the cholesterol biosynthesis pathway (59). Thus, impairment of both arms of the cholesterol biosynthesis pathways could ultimately result in changes in the fluidity of cell membranes, myelination, synaptic release, and many other cholesterol-dependent processes (1).
Admittedly, there are several limitations to our studies, which will have to be addressed by follow-up experimentation. The cross-species translatability of findings is always a concern. This concern is alleviated by the fact that cholesterol biosynthesis across the mammalian species is conserved (60) and that multiple publications show that ARI and TRZ utilization increases 7-DHC levels in the human plasma (31, 61) and brain tissue (22). In addition, we have shown previously that single drug treatments with ARI and TRZ are strong developmental inhibitors of sterol biosynthesis both in vitro and in vivo, with deleterious consequences on prenatal brain development (33, 34, 52, 61, 62). When these medications are given to pregnant transgenic Dhcr7+/- mice, offspring carrying the Dhcr7+/- genotype are particularly vulnerable: their 7-DHC levels rise to concentrations approaching those seen in the SLOS phenotype (33, 34, 62). However, without further experimentation, it is not clear how the ARI and TRZ-induced detrimental effects of the developing CNS relate to the 7-DHC increases that we observed in the adult animals. Simply, the exact neurobiological cascade of events that explain the effects of ARI and TRZ on sterol biosynthesis is not understood to date, and the clinical implications of these changes are unknown. In the current study, we observed significantly reduced cholesterol concentrations in our cell culture experiments, but this reduction was not observed in the brain regions of the adult mouse model. We believe that this can be explained by the stability and long turnover of cholesterol (63), especially as our treatment duration is much shorter than the typical ARI and TRZ treatment course in human patients. Similarly, while the ARI+TRZ effects were additive in cell cultures, we did not observe a similar additive effect in the brain of adult mice. This could suggest a different level of vulnerability of the developing versus adult brain: developing neurons and astrocytes robustly accumulate freshly synthesized cholesterol, while the cholesterol turnover in the adult brain is very slow (64). Thus, to observe reductions in cholesterol levels in the adult animals (and perhaps uncover an additive ARI+TRZ treatment effect on sterol biosynthesis), the treatment window with ARI and TRZ should be extended to much longer periods of time, mimicking the chronic treatment of human patients. Furthermore, based on our previous findings with cariprazine (62), we propose that the sterol biochemical profile is only disrupted while ARI or TRZ (and their active metabolites) is detectable in brain, and it remains unknown if specific microanatomy, gene expression, or neurochemical or connectivity changes persist long after the active compounds are washed out of the system. Notably, the focus of this study was post-lanosterol biosynthesis, as both ARI and TRZ interfere with DHCR7 function, which results in 7-DHC elevation and reduced DES levels. Thus, the primary effects of both medications are on DHCR7 and sterol biosynthesis and not on the clearance of the end product (cholesterol).
Based on these considerations, we propose that chronic treatments of psychiatric patients with ARI and/or TRZ will lead to long-term 7-DHC elevation and DES/CHOL reduction, potentially accounting to at least some of the commonly observed side effects of these treatments.
Conclusions
Sterol biosynthesis inhibition by ARI and TRZ is dose dependent, robust, and reproducible across in vivo and in vitro experimental systems and affects both neuronal and glial cells. Concurrent administration of TRZ and ARI has an additive effect on sterol biosynthesis disruption in developing neurons and astrocytes. In adult mice, the most robust 7-DHC elevation and DES decrease was observed in the neocortex. Further experimental evaluations are required to define the behavioral consequences of these post-lanosterol biosynthesis disruptions in the patient population who are receiving sterol-inhibiting medications or combinations of medications.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author within reasonable limits.
Supplemental data
This article contains supplemental data.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
Author contribution
Z. K., K. M., M. B. conceptualization; Z. K., K. M., M. B. methodology; Z. K., M. B., A. A., T. C. G.-M. formal analysis; Z. K., M. B., A. A., T. C. G.-M. investigation; Z. K., K. M. resources; Z. K., K. M., M. B. data curation; Z. K., K. M., M. B. writing – original draft; Z. K., K. M., M. B., A. A., T. C. G.-M. writing – review & editing; Z. K., K. M. supervision.
Funding and additional information
This work was supported by the National Institutes of Health Grants R01 MH110636 (K. M.) and R01 MH067234 (K. M., N. A. P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Zeljka Korade, Email: zeljka.korade@unmc.edu.
Karoly Mirnics, Email: karoly.mirnics@unmc.edu.
Supplemental data
References
- 1.Korade Z., Heffer M., Mirnics K. Medication effects on developmental sterol biosynthesis. Mol. Psychiatry. 2022;27:490–501. doi: 10.1038/s41380-021-01074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter F.D. Smith–Lemli–Opitz syndrome: pathogenesis, diagnosis and management. Eur. J. Hum. Genet. 2008;16:535–541. doi: 10.1038/ejhg.2008.10. [DOI] [PubMed] [Google Scholar]
- 3.Allen L.B., Genaro-Mattos T.C., Porter N.A., Mirnics K., Korade Z. Desmosterolosis and desmosterol homeostasis in the developing mouse brain. J. Inherit. Metab. Dis. 2019;42:934–943. doi: 10.1002/jimd.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie C., Turley S.D., Dietschy J.M. Cholesterol accumulation in tissues of the Niemann-pick type C mouse is determined by the rate of lipoprotein-cholesterol uptake through the coated-pit pathway in each organ. Proc. Natl. Acad. Sci. U. S. A. 1999;96:11992–11997. doi: 10.1073/pnas.96.21.11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müller C.P., Reichel M., Mühle C., Rhein C., Gulbins E., Kornhuber J. Brain membrane lipids in major depression and anxiety disorders. Biochim. Biophys. Acta. 2015;1851:1052–1065. doi: 10.1016/j.bbalip.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Vadakkan K. A structure-function mechanism for schizophrenia. Front. Psychiatry. 2012;3:108. doi: 10.3389/fpsyt.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valenza M., Leoni V., Tarditi A., Mariotti C., Bjorkhem I., Di Donato S., et al. Progressive dysfunction of the cholesterol biosynthesis pathway in the R6/2 mouse model of Huntington's disease. Neurobiol. Dis. 2007;28:133–142. doi: 10.1016/j.nbd.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Wolozin B. Cholesterol and the biology of Alzheimer's disease. Neuron. 2004;41:7–10. doi: 10.1016/s0896-6273(03)00840-7. [DOI] [PubMed] [Google Scholar]
- 9.Korade Z., Kim H.-Y.H., Tallman K.A., Liu W., Koczok K., Balogh I., et al. The effect of small molecules on sterol homeostasis: measuring 7-Dehydrocholesterol in Dhcr7-Deficient Neuro2a cells and human fibroblasts. J. Med. Chem. 2016;59:1102–1115. doi: 10.1021/acs.jmedchem.5b01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tallman K.A., Allen L.B., Klingelsmith K.B., Anderson A., Genaro-Mattos T.C., Mirnics K., et al. Prescription medications alter neuronal and glial cholesterol synthesis. ACS Chem. Neurosci. 2021;12:735–745. doi: 10.1021/acschemneuro.0c00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Citrome L. A review of aripiprazole in the treatment of patients with schizophrenia or bipolar I disorder. Neuropsychiatr. Dis. Treat. 2006;2:427–443. doi: 10.2147/nedt.2006.2.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muneer A. The treatment of adult bipolar disorder with aripiprazole: a systematic review. Cureus. 2016;8:e562. doi: 10.7759/cureus.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagiolini A., Comandini A., Catena Dell'Osso M., Kasper S. Rediscovering trazodone for the treatment of major depressive disorder. CNS Drugs. 2012;26:1033–1049. doi: 10.1007/s40263-012-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffer K.Y., Chang T., Vanle B., Dang J., Steiner A.J., Loera N., et al. Trazodone for Insomnia: a systematic review. Innov. Clin. Neurosci. 2017;14:24–34. [PMC free article] [PubMed] [Google Scholar]
- 15.La A.L., Walsh C.M., Neylan T.C., Vossel K.A., Yaffe K., Krystal A.D., et al. Long-term trazodone use and cognition: a potential therapeutic role for slow-wave sleep enhancers. J. Alzheimers Dis. 2019;67:911–921. doi: 10.3233/JAD-181145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurtz S.P., Buttram M.E., Margolin Z.R., Wogenstahl K. The diversion of nonscheduled psychoactive prescription medications in the United States, 2002 to 2017. Pharmacoepidemiol. Drug Saf. 2019;28:700–706. doi: 10.1002/pds.4771. [DOI] [PubMed] [Google Scholar]
- 17.Borras L., de Timary P., Constant E.L., Huguelet P., Eytan A. Successful treatment of alcohol withdrawal with trazodone. Pharmacopsychiatry. 2006;39:232. doi: 10.1055/s-2006-951385. [DOI] [PubMed] [Google Scholar]
- 18.Le Bon O., Murphy J.R., Staner L., Hoffmann G., Kormoss N., Kentos M., et al. Double-blind, placebo-controlled study of the efficacy of trazodone in alcohol post-withdrawal syndrome: polysomnographic and clinical evaluations. J. Clin. Psychopharmacol. 2003;23:377–383. doi: 10.1097/01.jcp.0000085411.08426.d3. [DOI] [PubMed] [Google Scholar]
- 19.Roccatagliata G., Albano C., Maffini M., Farelli S. Alcohol withdrawal syndrome: treatment with trazodone. Int. Pharmacopsychiatry. 1980;15:105–110. doi: 10.1159/000468420. [DOI] [PubMed] [Google Scholar]
- 20.Morillas-Arques P., Rodriguez-Lopez C.M., Molina-Barea R., Rico-Villademoros F., Calandre E.P. Trazodone for the treatment of fibromyalgia: an open-label, 12-week study. BMC Musculoskelet. Disord. 2010;11:204. doi: 10.1186/1471-2474-11-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fink H.A., MacDonald R., Rutks I.R., Wilt T.J. Trazodone for erectile dysfunction: a systematic review and meta-analysis. BJU Int. 2003;92:441–446. doi: 10.1046/j.1464-410x.2003.04358.x. [DOI] [PubMed] [Google Scholar]
- 22.Cenik B., Palka J.M., Thompson B.M., McDonald J.G., Tamminga C.A., Cenik C., et al. Desmosterol and 7-dehydrocholesterol concentrations in post mortem brains of depressed people: the role of trazodone. Transl. Psychiatry. 2022;12:139. doi: 10.1038/s41398-022-01903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodnick P.J., Jerry J.M. Aripiprazole: profile on efficacy and safety. Expert Opin. Pharmacother. 2002;3:1773–1781. doi: 10.1517/14656566.3.12.1773. [DOI] [PubMed] [Google Scholar]
- 24.Mendelson W.B. A review of the evidence for the efficacy and safety of trazodone in insomnia. J. Clin. Psychiatry. 2005;66:469–476. doi: 10.4088/jcp.v66n0409. [DOI] [PubMed] [Google Scholar]
- 25.Citrome L., Kalsekar I., Baker R.A., Hebden T. A review of real-world data on the effects of aripiprazole on weight and metabolic outcomes in adults. Curr. Med. Res. Opin. 2014;30:1629–1641. doi: 10.1185/03007995.2014.908280. [DOI] [PubMed] [Google Scholar]
- 26.Hall D.A., Agarwal P., Griffith A., Segro V., Seeberger L.C. Movement disorders associated with aripiprazole use: a case series. Int. J. Neurosci. 2009;119:2274–2279. doi: 10.3109/00207450903225553. [DOI] [PubMed] [Google Scholar]
- 27.Bernagie C., Danckaerts M., Wampers M., De Hert M. Aripiprazole and acute extrapyramidal symptoms in children and adolescents: a meta-analysis. CNS Drugs. 2016;30:807–818. doi: 10.1007/s40263-016-0367-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Etminan M., Procyshyn R.M., Samii A., Carleton B.C. Risk of extrapyramidal adverse events with aripiprazole. J. Clin. Psychopharmacol. 2016;36:472–474. doi: 10.1097/JCP.0000000000000543. [DOI] [PubMed] [Google Scholar]
- 29.Pena M.S., Yaltho T.C., Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord. 2011;26:147–152. doi: 10.1002/mds.23402. [DOI] [PubMed] [Google Scholar]
- 30.Rojo L.E., Gaspar P.A., Silva H., Risco L., Arena P., Cubillos-Robles K., et al. Metabolic syndrome and obesity among users of second generation antipsychotics: a global challenge for modern psychopharmacology. Pharmacol. Res. 2015;101:74–85. doi: 10.1016/j.phrs.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 31.Hall P., Michels V., Gavrilov D., Matern D., Oglesbee D., Raymond K., et al. Aripiprazole and trazodone cause elevations of 7-dehydrocholesterol in the absence of Smith-Lemli-Opitz Syndrome. Mol. Genet. Metab. 2013;110:176–178. doi: 10.1016/j.ymgme.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Korade Ž., Liu W., Warren E.B., Armstrong K., Porter N.A., Konradi C. Effect of psychotropic drug treatment on sterol metabolism. Schizophr Res. 2017;187:74–81. doi: 10.1016/j.schres.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korade Z., Allen L.B., Anderson A., Tallman K.A., Genaro-Mattos T.C., Porter N.A., et al. Trazodone effects on developing brain. Translational Psychiatry. 2021;11:85. doi: 10.1038/s41398-021-01217-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Genaro-Mattos T.C., Allen L.B., Anderson A., Tallman K.A., Porter N.A., Korade Z., et al. Maternal aripiprazole exposure interacts with 7-dehydrocholesterol reductase mutations and alters embryonic neurodevelopment. Mol. Psychiatry. 2019;24:491–500. doi: 10.1038/s41380-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamberson C.R., Muchalski H., McDuffee K.B., Tallman K.A., Xu L., Porter N.A. Propagation rate constants for the peroxidation of sterols on the biosynthetic pathway to cholesterol. Chem. Phys. Lipids. 2017;207:51–58. doi: 10.1016/j.chemphyslip.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu L., Korade Z., Rosado J.D.A., Liu W., Lamberson C.R., Porter N.A. An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2011;52:1222–1233. doi: 10.1194/jlr.M014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korade Z., Xu L., Shelton R., Porter N.A. Biological activities of 7-dehydrocholesterol-derived oxysterols: implications for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2010;51:3259–3269. doi: 10.1194/jlr.M009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfeffer B.A., Xu L., Porter N.A., Rao S.R., Fliesler S.J. Differential cytotoxic effects of 7-dehydrocholesterol-derived oxysterols on cultured retina-derived cells: dependence on sterol structure, cell type, and density. Exp. Eye Res. 2016;145:297–316. doi: 10.1016/j.exer.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu L., Korade Z., Porter N.A. Oxysterols from free radical chain oxidation of 7-Dehydrocholesterol: product and mechanistic studies. J. Am. Chem. Soc. 2010;132:2222–2232. doi: 10.1021/ja9080265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu L., Mirnics K., Bowman A.B., Liu W., Da J., Porter N.A., et al. DHCEO accumulation is a critical mediator of pathophysiology in a Smith-Lemli-Opitz syndrome model. Neurobiol. Dis. 2012;45:923–929. doi: 10.1016/j.nbd.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomita H., Hines K.M., Herron J.M., Li A., Baggett D.W., Xu L. 7-Dehydrocholesterol-derived oxysterols cause neurogenic defects in Smith-Lemli-Opitz syndrome. bioRxiv. 2021 doi: 10.1101/2021.01.30.428955. preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korade Z., Xu L., Mirnics K., Porter N.A. Lipid biomarkers of oxidative stress in a genetic mouse model of Smith-Lemli-Opitz syndrome. J. Inherit. Metab. Dis. 2013;36:113–122. doi: 10.1007/s10545-012-9504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corcoran R.B., Scott M.P. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quinn K.J., Shah N.H. A dataset quantifying polypharmacy in the United States. Sci. Data. 2017;4:170167. doi: 10.1038/sdata.2017.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siwek M., Woron J., Gorostowicz A., Wordliczek J. Adverse effects of interactions between antipsychotics and medications used in the treatment of cardiovascular disorders. Pharmacol. Rep. 2020;72:350–359. doi: 10.1007/s43440-020-00058-6. [DOI] [PubMed] [Google Scholar]
- 46.Miller C.H., Fleischhacker W.W. Managing antipsychotic-induced acute and chronic akathisia. Drug Saf. 2000;22:73–81. doi: 10.2165/00002018-200022010-00006. [DOI] [PubMed] [Google Scholar]
- 47.Cheine M., Ahonen J., Wahlbeck K. Beta-blocker supplementation of standard drug treatment for schizophrenia. Cochrane Database Syst Rev. 2000;3:CD000234. doi: 10.1002/14651858.CD000234. [DOI] [PubMed] [Google Scholar]
- 48.Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fleischhacker W.W. Aripiprazole. Expert Opin. Pharmacother. 2005;6:2091–2101. doi: 10.1517/14656566.6.12.2091. [DOI] [PubMed] [Google Scholar]
- 50.Genaro-Mattos T.C., Tallman K.A., Allen L.B., Anderson A., Mirnics K., Korade Z., et al. Dichlorophenyl piperazines, including a recently-approved atypical antipsychotic, are potent inhibitors of DHCR7, the last enzyme in cholesterol biosynthesis. Toxicol. Appl. Pharmacol. 2018;349:21–28. doi: 10.1016/j.taap.2018.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allen L.B., Genaro-Mattos T.C., Anderson A., Porter N.A., Mirnics K., Korade Z. Amiodarone Alters Cholesterol Biosynthesis through Tissue-Dependent Inhibition of Emopamil Binding Protein and Dehydrocholesterol Reductase 24. ACS Chem. Neurosci. 2020;11:1413–1423. doi: 10.1021/acschemneuro.0c00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allen L.B., Genaro-Mattos T.C., Anderson A., Porter N.A., Mirnics K., Korade Z. Amiodarone alters cholesterol biosynthesis through tissue-dependent inhibition of emopamil binding protein and dehydrocholesterol reductase 24. ACS Chem. Neurosci. 2020;11:1413–1423. doi: 10.1021/acschemneuro.0c00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tallman K.A., Allen L.B., Klingelsmith K.B., Anderson A., Genaro-Mattos T.C., Mirnics K., et al. Prescription medications alter neuronal and glial cholesterol synthesis. ACS Chem. Neurosci. 2021;12:735–745. doi: 10.1021/acschemneuro.0c00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Genaro-Mattos T.C., Anderson A., Allen L.B., Korade Z., Mirnics K. Cholesterol biosynthesis and uptake in developing neurons. ACS Chem. Neurosci. 2019;10:3671–3681. doi: 10.1021/acschemneuro.9b00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masnoon N., Shakib S., Kalisch-Ellett L., Caughey G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230. doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sergi G., Rui M.D., Sarti S., Manzato E. Polypharmacy in the elderly. Drugs & Aging. 2011;28:509–518. doi: 10.2165/11592010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 57.Rambhade S., Chakarborty A., Shrivastava A., Patil U.K., Rambhade A. A survey on polypharmacy and use of inappropriate medications. Toxicol. Int. 2012;19:68–73. doi: 10.4103/0971-6580.94506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waterham H.R., Koster J., Romeijn G.J., Hennekam R.C.M., Vreken P., Andersson H.C., et al. Mutations in the 3β-Hydroxysterol Δ24-Reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. Am. J. Hum. Genet. 2001;69:685–694. doi: 10.1086/323473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mazein A., Watterson S., Hsieh W.-Y., Griffiths W.J., Ghazal P. A comprehensive machine-readable view of the mammalian cholesterol biosynthesis pathway. Biochem. Pharmacol. 2013;86:56–66. doi: 10.1016/j.bcp.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang T., Yuan D., Xie J., Lei Y., Li J., Fang G., et al. Evolution of the cholesterol biosynthesis pathway in animals. Mol. Biol. Evol. 2019 doi: 10.1093/molbev/msz167. [DOI] [PubMed] [Google Scholar]
- 61.Genaro-Mattos T.C., Klingelsmith K.B., Allen L.B., Anderson A., Tallman K.A., Porter N.A., et al. Sterol biosynthesis inhibition in pregnant women taking prescription medications. ACS Pharmacol. Transl. Sci. 2021;4:848–857. doi: 10.1021/acsptsci.1c00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Genaro-Mattos T.C., Anderson A., Allen L.B., Tallman K.A., Porter N.A., Korade Z., et al. Maternal cariprazine exposure inhibits embryonic and postnatal brain cholesterol biosynthesis. Mol. Psychiatry. 2020;25:2685–2694. doi: 10.1038/s41380-020-0801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dietschy J.M. Central nervous system: cholesterol turnover, brain development and neurodegeneration. Biol. Chem. 2009;390:287–293. doi: 10.1515/BC.2009.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang J., Liu Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell. 2015;6:254–264. doi: 10.1007/s13238-014-0131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author within reasonable limits.