Abstract
A recent study indicated that Bacillus subtilis catabolizes acetoin by enzymes encoded by the acu gene cluster (F. J. Grundy, D. A. Waters, T. Y. Takova, and T. M. Henkin, Mol. Microbiol. 10:259–271, 1993) that are completely different from those in the multicomponent acetoin dehydrogenase enzyme system (AoDH ES) encoded by aco gene clusters found before in all other bacteria capable of utilizing acetoin as the sole carbon source for growth. By hybridization with a DNA probe covering acoA and acoB of the AoDH ES from Clostridium magnum, genomic fragments from B. subtilis harboring acoA, acoB, acoC, acoL, and acoR homologous genes were identified, and some of them were functionally expressed in E. coli. Furthermore, acoA was inactivated in B. subtilis by disruptive mutagenesis; these mutants were impaired to express PPi-dependent AoDH E1 activity to remove acetoin from the medium and to grow with acetoin as the carbon source. Therefore, acetoin is catabolized in B. subtilis by the same mechanism as all other bacteria investigated so far, leaving the function of the previously described acu genes obscure.
Acetoin is a product of fermentative metabolism in many prokaryotic and eukaryotic microorganisms and also in Bacillus spp. (20). For 70 years it has been known that bacteria are capable of using acetoin as their sole carbon source for growth (33). Detailed knowledge about the catabolism of acetoin was obtained from studies on a diversity of acetoin-utilizing bacteria, such as Pelobacter carbinolicus (21, 22), Clostridium magnum (16), Klebsiella pneumoniae (6), Alcaligenes eutrophus (23), and Pseudomonas putida (15). In those bacteria, acetoin breakdown is catalyzed by the acetoin dehydrogenase enzyme system (AoDH ES), which consists of thiamine PPi-dependent acetoin dehydrogenase (AoDH E1), dihydrolipoamide acetyltransferase (AoDH E2), and dihydrolipoamide dehydrogenase (AoDH E3) (21, 22). The structural genes of the AoDH ES acoA (encoding the α subunit of AoDH E1), acoB (encoding the β subunit of AoDH E1), and acoC (encoding AoDH E2) were found to be clustered in a colinear orientation in the genomes of the bacteria mentioned above (6, 15, 16, 22, 23). In P. carbinolicus, C. magnum, and K. pneumoniae acoL genes (encoding AoDH E3) are also part of the aco gene clusters.
The genes encoding the enzymes for acetoin formation form a single operon in Bacillus subtilis (24), and acetoin acts as an external carbon storage material that is synthesized and excreted during exponential growth. B. subtilis also utilizes acetoin by a yet-unknown pathway during the stationary growth phase, serving as a carbon and energy source during sporulation (19). In B. subtilis a gene cluster, acuABC, was recently identified. The disruption of acuA diminished the ability to utilize acetoin (10, 11). The acu genes are regulated by the adjacent ccpA, a trans-acting gene (10). The acuABC-encoded putative products do not exhibit any similarity to the aco-encoded components of the AoDH ESs mentioned above. Because of some structural similarity to the Escherichia coli ato operon, which encodes proteins involved in acetoacetate metabolism, the acuABC cluster of B. subtilis was instead supposed to encode proteins that catalyze the breakdown of acetoin or an unknown derivative by a similar mode as the ato-encoded system does, i.e., by coenzyme A transfer and subsequent cleavage (11). The unexpected designation of the recently described acu genes to acetoin catabolism in B. subtilis lacking homologies to the well-characterized aco genes prompted us to reinvestigate the catabolism of acetoin in B. subtilis. Understanding the catabolism of acetoin in B. subtilis will also be interesting from a historical perspective, since early studies on this subject resulted in the wrong hypothesis that acetoin is degraded via the 2,3-butanediol cycle which was also mentioned in some text books (e.g., reference 8).
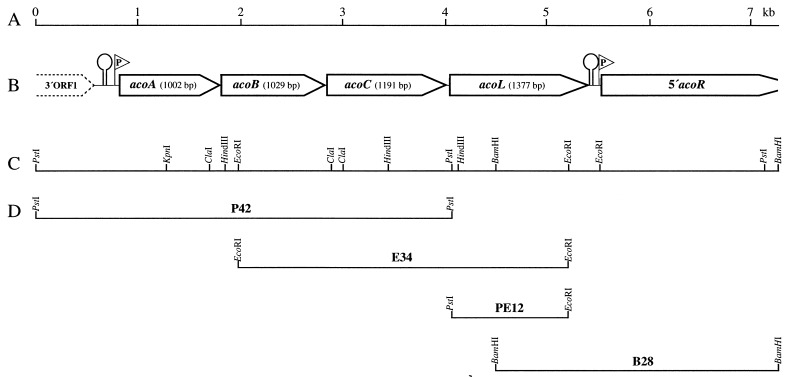
In order to investigate if B. subtilis 168 (DSMZ 402) possesses genes homologous to the known aco genes encoding the components involved in the AoDH ES, totally PstI-digested genomic DNA was screened by Southern hybridization (15) with a 1.6-kbp EcoRI fragment from C. magnum (16) containing the most conserved region of acoA and acoB and having a similar G+C content (22). This DNA probe gave a clear single signal, which corresponded to a 4.2-kbp PstI fragment. Restriction fragments of the corresponding size were separated electrophoretically and were isolated from the agarose gel by the filter technique (31). The fragments were subsequently ligated with PstI-restricted pBluescript SK(−) (Stratagene Cloning Systems, San Diego, Calif.) and transformed into E. coli DH5α (9) by the calcium chloride procedure (25). Colony hybridization (12) identified clones harboring a 4.2-kbp PstI fragment, which was referred to as P42 (Fig. 1). By employing subfragments of P42, a 3.4-kbp EcoRI fragment (referred to as E34) (Fig. 1) and a 2.8-kbp BamHI fragment (referred to as B28) (Fig. 1) were also identified. The nucleotide sequences of a region of 7,343 bp was obtained from both strands of P42, E34, and B28 (26); analysis of the nucleotide sequences, which was performed with the programs from the Heidelberg Unix Sequence Analysis Resources (release 4.0), revealed five major open reading frames (ORFs), which were designated acoA, acoB, acoC, acoL, and acoR (Fig. 1), capable of encoding polypeptides with molecular weights of 36,030, 36,821, 42,798, 48,822, and 64,938, respectively. The intergenic regions between acoA and acoB, acoB and acoC, acoC and acoL, and acoL and acoR comprised only 3, 13, 28, or 115 bp, respectively. In addition, ORF1 was identified 232 bp upstream from acoA. The obtained sequence was totally different from that of the acu gene cluster (10, 11). It was identical with the sequence recently deposited in the data bank by others (17, 34), except for a few minor deviations in the carboxy-terminal region of acoC and one deviation in acoR, which all occurred in not highly conserved regions. The sequence obtained in this study was submitted to the National Center for Biotechnology Information on 30 May 1997 under accession no. AF006075.
FIG. 1.
Molecular organization of the B. subtilis aco gene cluster. (A) Scale in kilobase pairs. (B) Structural genes of the aco gene cluster. The positions of putative promoters (P) and hairpin-like structures (loops) are indicated. (C) Relevant restriction sites of the sequenced region. (D) Relevant fragments of the analyzed region.
The deduced amino acid sequences of the identified ORFs were compared to data files in the SWISSPROT and EMBL data libraries in a homology search. Striking similarities between the acoABCL-encoded amino acid sequences and the corresponding enzyme components of the well-known AoDH ES from P. putida (15), P. carbinolicus (22), A. eutrophus (23), C. magnum (16), and K. pneumoniae (6) were found.
As in other thiamine PPi-dependent enzymes, a putative thiamine PPi-binding region (13) and other characteristic motifs (32) were identified in the central region of the deduced amino acid sequence of AcoA. The N-terminal region of B. subtilis AcoC shares most characteristics with the dihydrolipoamide acyltransferases of various origins—such as Lys43, which is presumably lipoylated, and Gly33 and Gly54, which flank this lysine residue—and with the corresponding C-terminal catalytic domains of various dihydrolipoamide acyltransferases, including the conserved putative active-site histidine-aspartate couple (3, 16, 22). AcoL exhibited the putative flavin adenine dinucleotide-binding domain (5) at the N-terminal region, an active disulfide bridge site at position Cys38 and Cys43, a putative NAD(H)-binding region in the central part, and the putative interface region at the C terminus and therefore matched the characteristic properties of dihydrolipoamide dehydrogenases (16, 22). Comparative sequence alignments of the putative acoR translational product revealed striking similarities to the conserved C domains of various −24/−12 promoter activating proteins, which are involved in the interaction with ς54-dependent RNA polymerases (29). ORF1 putatively encodes a 195-amino-residue polypeptide upstream from acoA (Fig. 1) that exhibits weak homologies to the DNA helicase-like protein or ribosomal protein L6.
A sequence that well matched the enterobacterial ς54 consensus sequence (30) and other specific upstream activator sequences of the aco gene clusters from A. eutrophus, C. magnum, and P. putida (15, 16, 23) was localized 39 bp upstream of acoA. In addition, 66 bp upstream of acoR a sequence exhibiting strong homology to the B. subtilis ς43 promoter consensus sequence (7) as well as to the E. coli ς70 (−35/−10) promoter sequence (14) was found. A second −24/−12-like promoter sequence was found 31 bp preceding the start codon of acoL. Inverted repeats were identified 6 bp downstream from acoL and 100 bp upstream from acoA, thus further indicating the separation of acoR and ORF1 from the genes of a putative acoABCL operon.
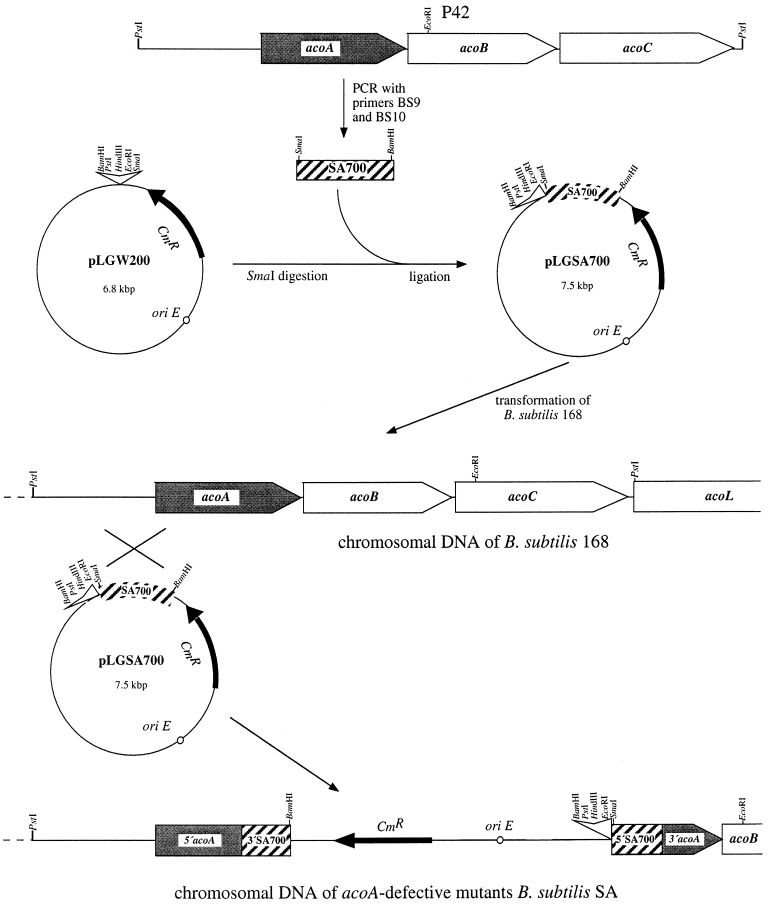
To investigate the physiological function of the aco genes for the acetoin catabolism in B. subtilis, the expression of intact AoDH ES was switched off by disruptive mutagenesis. For this purpose the integrational plasmid pLGSA700 was constructed (Fig. 2). This plasmid encoded part of the α subunit of AoDH E1, which is the key component of the AoDH ES (16, 22), and was used to disrupt the first gene of the aco cluster. After transforming B. subtilis 168, which was done as described previously (2), chloramphenicol-resistant strains were obtained; these were referred to as SA35, SA36, SA37, SA41, SA42, and SA43, respectively. The integration of pLGSA700 into B. subtilis 168 acoA through Campbell-type recombination (4) was confirmed by Southern hybridization (22, 25) of PstI- and EcoRI-digested genomic DNA with vector DNA (pLGW200) as a probe (Fig. 3). Whereas no signal was obtained in the DNA of the wild type, bands were detected in the DNAs of the acoA-defective mutants corresponding to PstI fragments of 8.4 kbp and to EcoRI fragments of 10.5 kbp, respectively. No signals corresponding to the size of pLGSA700 (7.5 kbp) were obtained. This result is in accordance with the integration of pLGSA700 by single crossover events into the SA700-homologous region of the acoA-defective mutants, which is located 1.1 kbp downstream of a PstI-site (Fig. 2) and 3.3 kbp downstream of an EcoRI-site (17).
FIG. 2.
Disruptive mutagenesis of acoA. By PCR a 700-bp DNA fragment of acoA, covering the inner region of acoA, was synthesized, and this fragment was inserted into pLGW200 (1). The ligation mixture was transformed into E. coli Top10 (Invitrogen, San Diego, Calif.) for amplification. The isolated recombinant plasmid pLGSA700 was transformed into cells of B. subtilis 168, which were selected for chloramphenicol resistance.
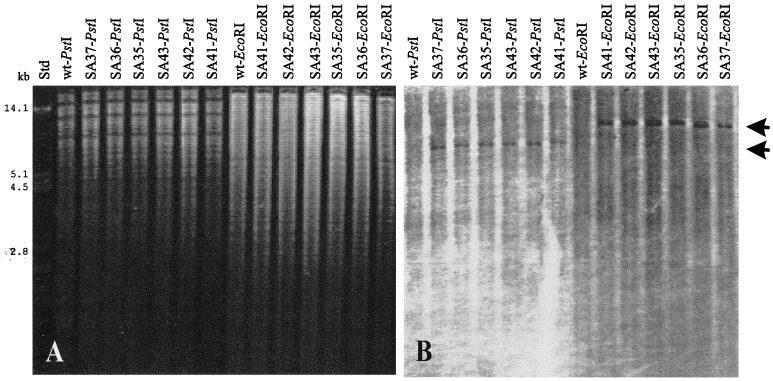
FIG. 3.
Localization of pLGSA700 integrations. PstI and EcoRI digests of genomic DNA from B. subtilis 168 (wt) and from pLGSA700-disrupted acoA mutants (SA35, SA36, SA37, SA41, SA42, and SA43) were separated in 0.8% (wt/vol) agarose gels, blotted onto a nylon membrane, and hybridized with biotin-labeled vector pLGW200 (22). The positions of PstI and EcoRI fragments which gave signals with the probe are indicated by arrows. The sizes of standard fragments (Std) are indicated at the left. (A) Agarose gel stained with ethidium bromide; (B) blot hybridized with probe pLGW200.
We investigated the growth of the strains in various solidified media. Cells were plated on solid TSS medium, which was supplemented with 0.01% (wt/vol) sodium glutamate (11). As main C sources, 0.2% (wt/vol) acetoin or 0.2% (wt/vol) glucose (positive control) was added. The following B. subtilis strains were tested: (i) 168 (wild type), (ii) an acoA mutant of 168 (SA35), and (iii) SMY (wild type); the third strain is the same one used by Grundy et al. (11). All three strains showed the same growth behavior: whereas good growth was obtained with glucose (positive control) after 1 day, only very weak growth was obtained both in the presence and absence of acetoin (negative control) even after 1 week of incubation. These results are not surprising, because acetoin (like 2,3-butanediol) is used by B. subtilis only during the stationary phase, when it serves as a C source for sporulation. Therefore, the degradation of acetoin is usually investigated with stationary cells (19).
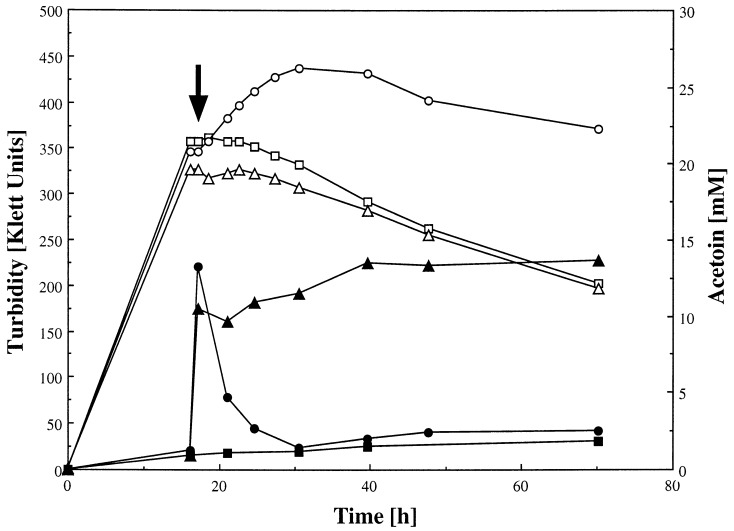
Growth was also investigated in liquid culture. Acetoin was added to stationary cells of B. subtilis 168 and acoA mutant SA35, which were grown in NSM medium (27), and the culture supernatant was monitored by gas chromatography as described before (28). As seen in Fig. 4, the mutant SA35 was unable to utilize acetoin as a carbon source, while the wild type started growing and kept doing so after the addition of acetoin. Regarding growth, the mutant behaved similar to the wild type without added acetoin. In accordance with this, AoDH E1 was present in extracts of stationary wild-type cells (0.016 U/mg of protein), while cell extracts of SA35 contained no detectable activity (<0.003 U/mg of protein) and gave no stained band in the protein pattern during activity staining. The mutation did not effect the cells’ ability to synthesize acetoin, which is obvious from the continuous, slight increase of the acetoin concentration in the medium, even after the addition of acetoin.
FIG. 4.
Acetoin utilization by B. subtilis 168 and the acoA-defective mutant SA35. Cells of B. subtilis 168 and of SA35 were cultivated in NSM at 37°C. At the time point indicated by an arrow, 10 mM acetoin was added to the culture of SA35 (▵, ▴) and to one culture of B. subtilis 168 (○, ●), whereas it was omitted from the other culture of B. subtilis 168 (□, ■). Growth (○, □, ▵) was monitored photometrically, and acetoin in the culture supernatants (●, ■, ▴) was measured gas chromatographically.
These experiments clearly demonstrated that an aco-encoded AoDH ES is present in B. subtilis and that it is the major enzyme system responsible for the catabolism of acetoin. From this and the striking similarities of the aco-encoded proteins of B. subtilis to the components of AoDH ESs in other bacteria, we conclude that B. subtilis possesses an enzyme system for the degradation of acetoin that is similar to the enzyme systems also responsible for the degradation of acetoin in all other phylogenetically and physiologically not related bacteria (6, 15, 16, 21–23). The function of the previously described acu genes (10, 11), the translational products of which have nothing in common with those in the well-investigated AoDH ES, remains unclear. It is very likely that these acu genes interfere with the acetoin catabolism in a nondirect way. To reveal the function of the acu genes, it will be necessary to assign an enzyme function to them. Recently, it was found that the acuC translational product exhibited significant sequence similarity to different eukaryotic histone deacetylases (18); it seems worthwhile to investigate whether AcuC has a regulatory function like these eucaryotic proteins.
Acknowledgments
The provision of plasmid pLGW200 by J. M. van Dijl and H. Tjalsma is gratefully acknowledged.
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (Ste 386/3-4) and by a fellowship of the Deutsche Forschungsanstalt für Luft- und Raumfahrt e. V. to Min Huang.
REFERENCES
- 1.Bolhuis A, Sorokin A, Azevedo V, Ehrlich S D, Braun P G, de Jong A, Venema G, Bron S, van Dijl J M. Bacillus subtilis can modulate its capacity and specificity for protein secretion through temporally controlled expression of the sipSgene for signal peptidase. Mol Microbiol. 1996;22:605–618. doi: 10.1046/j.1365-2958.1996.d01-4676.x. [DOI] [PubMed] [Google Scholar]
- 2.Bron S, Venema G. Ultraviolet inactivation and excision-repair in Bacillus subtilis. I. Construction and characterization of a transformable eightfold auxotrophic strain and two ultraviolet-sensitive derivatives. Mutat Res. 1972;15:1–10. doi: 10.1016/0027-5107(72)90086-3. [DOI] [PubMed] [Google Scholar]
- 3.Browner M F, Taroni F, Sztul E, Rosenberg L E. Sequence analysis, biogenesis, and mitochondrial import of the α-subunit of rat propionyl-coA carboxylase. J Biol Chem. 1989;264:12680–12685. [PubMed] [Google Scholar]
- 4.Campbell A. Episomes. Adv Genet. 1962;11:101–145. [Google Scholar]
- 5.Carothers D J, Pons G, Patel M S. Dihydrolipoamide dehydrogenase: functional similarities and divergent evolution of the pyridine nucleotide-disulfide oxidoreductases. Arch Biochem Biophys. 1989;268:409–425. doi: 10.1016/0003-9861(89)90309-3. [DOI] [PubMed] [Google Scholar]
- 6.Deng W-L, Chang H-Y, Peng H L. Acetoin catabolic system of Klebsiella pneumoniae CG43: sequence, expression, and organization of the acooperon. J Bacteriol. 1994;176:3527–3535. doi: 10.1128/jb.176.12.3527-3535.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doi R H, Wang L F. Multiple procaryotic ribonucleic acid polymerase sigma factors. Microbiol Rev. 1986;50:227–243. doi: 10.1128/mr.50.3.227-243.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottschalk G. Bacterial metabolism. 1st ed. New York, N.Y: Springer-Verlag; 1979. [Google Scholar]
- 9.Grant S G N, Jessee J, Bloom F R, Hanahan D. Differential plasmid rescue from transgenic mouse DNAs into Escherichia colimethylation-restriction mutants. Proc Natl Acad Sci USA. 1990;87:4645–4649. doi: 10.1073/pnas.87.12.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grundy F J, Turinsky A J, Henkin T M. Catabolic regulation of Bacillus subtilisacetate and acetoin utilization genes by CcpA. J Bacteriol. 1994;176:4527–4533. doi: 10.1128/jb.176.15.4527-4533.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grundy F J, Waters D A, Takova T Y, Henkin T M. Identification of genes involved in utilization of acetate and acetoin in Bacillus subtilis. Mol Microbiol. 1993;10:259–271. doi: 10.1111/j.1365-2958.1993.tb01952.x. [DOI] [PubMed] [Google Scholar]
- 12.Grunstein M, Hogness D. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci USA. 1975;72:3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkins C F, Borges A, Perham R N. A common structural motif in thiamin pyrophosphate-binding enzymes. FEBS Lett. 1989;255:77–82. doi: 10.1016/0014-5793(89)81064-6. [DOI] [PubMed] [Google Scholar]
- 14.Hawley D K, McClure W R. Compilation and analysis of Escherichia colipromoter DNA sequences. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang M, Oppermann F B, Steinbüchel A. Molecular characterization of the Pseudomonas putida2,3-butanediol catabolic pathway. FEMS Microbiol Lett. 1994;124:141–150. doi: 10.1111/j.1574-6968.1994.tb07276.x. [DOI] [PubMed] [Google Scholar]
- 16.Krüger N, Oppermann F B, Lorenzl H, Steinbüchel A. Biochemical and molecular characterization of the Clostridium magnumacetoin dehydrogenase enzyme system. J Bacteriol. 1994;176:3614–3630. doi: 10.1128/jb.176.12.3614-3630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Cummings N J, Daniel R A, Denizot F, Devine K M, Dusterhoft A, Ehrlich S D, Emmerson P T, Entian K D, Errington J, Fabret C, Ferrari E, Foulger D, Fritz C, Fujita M, Fujita Y, Fuma S, Galizzi A, Galleron N, Ghim S Y, Glaser P, Goffeau A, Golightly E J, Grandi G, Guiseppi G, Guy B J, Haga K, Haiech J, Harwood C R, Henaut A, Hilbert H, Holsappel S, Hosono S, Hullo M F, Itaya M, Jones L, Joris B, Karamata D, Kasahara Y, Klaerr-Blanchard M, Klein C, Kobayashi Y, Koetter P, Koningstein G, Krogh S, Kumano M, Kurita K, Lapidus A, Lardinois S, Lauber J, Lazarevic V, Lee S M, Levine A, Liu H, Masuda S, Mauel C, Medigue C, Medina N, Mellado R P, Mizuno M, Moestl D, Nakai S, Noback M, Noone D, O’Reilly M, Ogawa K, Ogiwara A, Oudega B, Park S H, Parro V, Pohl T M, Portetelle D, Porwollik S, Prescott A M, Presecan E, Pujic P, Purnelle B, Rapoport G, Rey M, Reynolds S, Rieger M, Rivolta C, Rocha E, Roche B, Rose M, Sadaie Y, Sato T, Scanlan E, Schleich S, Schroeter R, Scoffone F, Sekiguchi J, Sekowska A, Seror S J, Serror P, Shin B S, Soldo B, Sorokin A, Tacconi E, Takagi T, Takahashi H, Takemaru K, Takeuchi M, Tamakoshi A, Tanaka T, Terpstra P, Tognoni A, Tosato V, Uchiyama S, Vandenbol M, Vannier F, Vassarotti A, Viari A, Wambutt R, Wedler E, Wedler H, Weitzenegger T, Winters P, Wipat A, Yamamoto H, Yamane K, Yasumoto K, Yata K, Yoshida K, Yoshikawa H F, Zumstein E, Yoshikawa H, Danchin A. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 18.Leipe D D, Landsman D. Histone deacetylases, acetoin utilization proteins and acetylpolyamine amidohydrolases are members of an ancient protein superfamily. Nucleic Acids Res. 1997;25:3693–3697. doi: 10.1093/nar/25.18.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez J M, Thoms B. Beziehungen zwischen katabolischer Repression und Sporulation bei Bacillus subtilis. Arch Microbiol. 1976;109:181–186. doi: 10.1007/BF00425133. [DOI] [PubMed] [Google Scholar]
- 20.Nakano M M, Dailly Y P, Zuber P, Clark D P. Characterization of anaerobic fermentative growth of Bacillus subtilis: identification of fermentation end products and genes required for growth. J Bacteriol. 1997;179:6749–6755. doi: 10.1128/jb.179.21.6749-6755.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oppermann F B, Schmidt B, Steinbüchel A. Purification and characterization of acetoin:2,6-dichlorophenolindophenol oxidoreductase, dihydrolipoamide dehydrogenase, and dihydrolipoamide acetyltransferase of the Pelobacter carbinolicusacetoin dehydrogenase enzyme system. J Bacteriol. 1991;173:757–767. doi: 10.1128/jb.173.2.757-767.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oppermann F B, Steinbüchel A. Identification and molecular characterization of the aco genes encoding the Pelobacter carbinolicusacetoin dehydrogenase enzyme system. J Bacteriol. 1994;176:469–485. doi: 10.1128/jb.176.2.469-485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Priefert H, Hein S, Krüger N, Zeh K, Schmidt B, Steinbüchel A. Identification and molecular characterization of the Alcaligenes eutrophus H16 acooperon genes involved in acetoin catabolism. J Bacteriol. 1991;173:4056–4071. doi: 10.1128/jb.173.13.4056-4071.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renna M C, Najimudin N, Wink L R, Zahler S A. Regulation of the Bacillus subtilis alsS, alsD, and alsRgenes involved in post-exponential-phase production of acetoin. J Bacteriol. 1993;175:3863–3875. doi: 10.1128/jb.175.12.3863-3875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 26.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaeffer P, Millet J, Aubert J-P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA. 1965;54:704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinbüchel A, Schlegel H G. A multifunctional fermentative alcohol dehydrogenase from the strict aerobe Alcaligenes eutrophus: purification and properties. Eur J Biochem. 1984;141:555–564. doi: 10.1111/j.1432-1033.1984.tb08229.x. [DOI] [PubMed] [Google Scholar]
- 29.Thöny B, Hennecke H. The −24/−12 promoter comes of age. FEMS Microbiol Rev. 1989;63:341–358. doi: 10.1016/0168-6445(89)90028-4. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Gralla J D. Multiple in vivo roles for the −12-region elements of sigma 54 promoters. J Bacteriol. 1998;180:5626–5631. doi: 10.1128/jb.180.21.5626-5631.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weichenhan D. Fast recovery of DNA from agarose gels by centrifugation through blotting paper. Trends Genet. 1991;7:109. doi: 10.1016/0168-9525(91)90442-s. [DOI] [PubMed] [Google Scholar]
- 32.Wexler I D, Hemalatha S G, Patel M S. Sequence conservation in the α and β subunits of pyruvate dehydrogenase and its similarity to branched-chain α ketoacid dehydrogenase. FEBS Lett. 1991;282:209–213. doi: 10.1016/0014-5793(91)80479-m. [DOI] [PubMed] [Google Scholar]
- 33.Williams O B, Morrow M B. The bacterial destruction of acetyl-methyl-carbinol. J Bacteriol. 1928;16:43–48. doi: 10.1128/jb.16.1.43-48.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto H, Uchiyama S, Sekiguchi J. Cloning and sequencing of a 40.6 kb segment in the 73°–76° region of the Bacillus subtilischromosome containing genes for trehalose metabolism and acetoin utilization. Microbiology. 1996;142:3057–3065. doi: 10.1099/13500872-142-11-3057. [DOI] [PubMed] [Google Scholar]