Abstract
Ruxolitinib is an important treatment for steroid refractory graft-versus-host disease (SR-GVHD). Therefore, we reported the updated results of a systematic review and meta-analysis of ruxolitinib as treatment for SR-GVHD. In addition, we wanted to compare the efficacy and safety between children and adults with SR-GVHD. Overall response rate (ORR) after ruxolitinib treatment was chosen as the primary end point. Complete response rate (CRR), infection, myelosuppression, and overall survival (OS) were chosen as secondary end points. A total of 37 studies were included in this meta-analysis, and 1,580 patients were enrolled. ORR at any time after ruxolitinib treatment was 0.77 [95% confidence interval (CI): 0.68–0.84] and 0.78 (95% CI: 0.74–0.81), respectively, for SR-aGVHD and SR-cGVHD. CRR at any time after ruxolitinib treatment was 0.49 (95% CI: 0.40–0.57) and 0.15 (95% CI: 0.10–0.23), respectively, for SR-aGVHD and SR-cGVHD. The ORRs at any time after treatment was highest in mouth SR-cGVHD, followed by skin, gut, joints and fascia, liver, eyes, esophagus, and lung SR-cGVHD. The incidence rate of infections after ruxolitinib treatment was 0.61 (95% CI: 0.45–0.76) and 0.47 (95% CI: 0.31–0.63), respectively, for SR-aGVHD and SR-cGVHD. The incidence rates of overall (grades I–IV) and severe (grades III–IV) cytopenia were 53.2% (95% CI: 16.0%–90.4%) and 31.0% (95% CI: 0.0–100.0%), respectively, for SR-aGVHD, and were 28.8% (95% CI:13.0%–44.6%) and 10.4% (95% CI: 0.0–27.9%), respectively, for SR-cGVHD. The probability rate of OS at 6 months after treatment was 63.9% (95% CI: 52.5%–75.2%) for SR-aGVHD. The probability rates of OS at 6 months, 1 year, and 2 years after treatment were 95% (95% CI: 79.5%–100.0%), 78.7% (95% CI: 67.2%–90.1%), and 75.3% (95% CI: 68.0%–82.7%), respectively, for SR-cGVHD. The ORR, CRR, infection events, and myelosuppression were all comparable between children and adults with SR-GVHD. In summary, this study suggests that ruxolitinib is an effective and safe treatment for SR-GVHD, and both children and adults with SR-GVHD could benefit from ruxolitinib treatment.
Keywords: acute graft-versus-host disease, chronic graft-versus-host disease, ruxolitinib, steroid-refractory, meta-analysis
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is one of the most important treatments for patients with hematological malignancies and non-malignant disease (1). However, graft-versus-host disease (GVHD) is still a common complication. It can severely influence the quality of life (2–5) and is an important cause of transplant-related mortality (6, 7). Corticosteroid is the first-line treatment for GVHD, but the response rate was approximate 50% (8), and a significant number of patients will experience steroid-refractory GVHD (SR-GVHD) (9). Thus far, there is no effective treatment for patients with SR-GVHD (10), and their survival rate is poor (11).
Ruxolitinib is a potent and selective oral inhibitor of Janus kinase (JAK) 1 and JAK2 and is an important treatment for myeloproliferative neoplasms (12). In addition, JAKs are well positioned to regulate GVHD. A variety of cytokines, which signal through the JAK/STAT pathways, play a critical role in regulation of the proliferation and activation on immune cell types that are important for GVHD pathogenesis (13). Recently, ruxolitinib is under investigation for the treatment of SR-GVHD, and it has been reported to be an important treatment for SR-GVHD (14–16). Ruxolitinib has been approved for the treatment of SR acute GVHD (aGVHD) and chronic GVHD (cGVHD) by the Unite States Food and Drug Administration in 2019 and 2021, respectively (17, 18).
Thus far, there are many clinical studies focused on ruxolitinib for SR-GVHD treatment, and Li et al. (19) had conducted a meta-analysis for ruxolitinib as the treatment for SR-GVHD in adults; however, only studies published before January 2019 were enrolled. Since 2019, many important research studies for ruxolitinib in SR-GVHD have been published (15, 16, 20–28). In addition, this meta-analysis did not include children, whereas several studies focused on ruxolitinib for treatment of SR-GVHD in children have been published since 2019 (29–32). No systematic review was designed to compare the efficacy of ruxolitinib for SR-GVHD treatment between children and adults.
Therefore, we reported the updated results of a systematic review and meta-analysis of ruxolitinib as treatment for SR-GVHD. In addition, we wanted to compare the efficacy and safety between children and adults with SR-GVHD.
Methods
Inclusion criteria
The inclusion criteria were as follows: (1) patients of any race, any sex, and all ages; (2) those diagnosed with SR-GVHD (i.e., aGVHD or cGVHD) after HSCT; and (3) those using ruxolitinib as the treatment for SR-GVHD. Reviews, duplicates, and conference abstracts were excluded. While assessing multiple reports from the same study, we selected the report containing more information or with a longer follow-up.
Search strategy
A literature search was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (33). The PubMed and Embase databases were searched, with the search strategy following the Population (patients with steroid refractory GVHD), Intervention (ruxolitinib), Outcomes [overall response rate (ORR), complete response rate (CRR), infection, myelosuppression, and overall survival (OS)], and Study framework (retrospective, prospective non-randomized, and randomized trials) (34): [(Glucocorticoid-Refractory) OR (steroid refractory) OR (steroid-refractory) OR (steroid resistant) OR (steroid-resistant) OR (corticosteroid refractory) OR (corticosteroid-refractory)] AND [(acute graft versus host disease) OR aGVHD OR cGVHD OR (chronic graft versus host disease) OR (graft versus host reaction)] AND [(Ruxolitinib) OR (Janus Kinase Inhibitors) OR (JAK Kinases Inhibitors) OR (jak kinases Inhibitors)].
Data extraction and outcomes
All data were independently extracted by two reviewers (Wen-Xuan Huo and Yang Yang) to ensure accuracy. Information on the following was extracted: study characteristics (e.g., study framework, first author, publish year, and follow-up period), patients (e.g., number, age, gender, and disease characteristics), transplantation (e.g., conditioning regimen, graft source, and type of transplant), GVHD (e.g., type, organ involved, and grade), ruxolitinib (e.g., dose, intervention time, and duration of treatment), and outcome parameters during the follow-up period.
The ORR after ruxolitinib treatment was chosen as the primary end point. The CRR, infection, myelosuppression, and OS were chosen as secondary end points. In addition, the response rates at 28 days and at 24 weeks after ruxolitinib were assessed in the analysis of SR-aGVHD and SR-cGVHD, respectively. Missing data were documented as “not available (NA)”. All data were extracted according to the Cochrane Handbook for Systematic Reviews of Interventions (35).
Statistical analysis
The “meta” package version 4.16-2 (36) was used to perform the meta-analysis. Statistical heterogeneity among studies was assessed using the I2 statistics and Cochran Q-test. The random-effects model was adopted, with the heterogeneity test showing I2 > 50% and P < 0.10. The subgroup comparison of adults and children was also conducted. The null hypothesis was set to no difference. A P-value < 0.05 was considered statistically significant to reject the null hypothesis. The results were analyzed by the boxplot using “ggplot2” package version 3.3.5 (37).
Results
Included studies
A total of 37 studies were included in this meta-analysis, and 1,580 patients were enrolled ( Tables 1 – 4 and Figure 1 ). Table 5 summarized the studies for every subgroup analysis.
Table 1.
Characteristics of 24 included studies (SR-aGVHD)*.
| Studies | Median age/year (range) | HLA matching (n) | SR-aGVHD grade (n) | Median time from SR-aGVHD diagnosis to the application of ruxolitinib/day (range) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MRD | mMRD | MUD | mMUD | I | II | III | IV | |||
| Zeiser, 2020 | 52.5 (12–73) | NA | NA | NA | NA | 2 | 50 | 68 | 30 | NA |
| Modemann, 2020 | 58.5 (21–73) | 3 | 0 | 9 | 6 | 0 | 0 | 9 | 9 | 87 (35–257) |
| Lancman, 2018 | 58 (33–61) | NA | NA | NA | NA | 1 | 3 | NA | NA | NA |
| Assouan, 2017 | 52 (26–65) | 5 | NA | 5 | NA | NA | NA | 6 | NA | 14 |
| Wei, 2021 | 30 (11–56) | 5 | NA | NA | NA | NA | 9 | 8 | 6 | 5 (1–79) |
| Leung, 2022 | 38 (19–63) | NA | NA | NA | NA | 0 | 13 | 4 | 5 | NA |
| Moiseev, 2020 | 17 (1–67) | 2 | NA | 19 | NA | 0 | 11 | 10 | 11 | 16 (5–113) |
| Jagasia, 2020 | 58 (18–73) | 18 | 11 | 27 | 10 | 0 | 23 | 34 | 14 | NA |
| Biliński, 2021 | 53.5 (22–66) | 2 | NA | 2 | NA | NA | NA | NA | 4 | NA |
| Liu, 2021 | 29 (13–63) | NA | NA | NA | NA | 0 | 7 | 22 | 11 | NA |
| Laisne, 2020 | 4.3 (0.4–14.5) | NA | NA | NA | NA | 0 | 7 | 13 | 9 | 91 (17–518) |
| González, 2018 | 11 (5–18) | NA | NA | NA | NA | 0 | 0 | 4 | 9 | 9 |
| Maldonado, 2017 | 51 (28–56) | 1 | NA | NA | NA | NA | NA | 3 | NA | NA |
| Maldonado, 2021 | 32 (26–48) | NA | NA | NA | NA | NA | NA | 2 | 7 | NA |
| Meng, 2019 | 23.5 (8–38) | 12 | NA | NA | NA | 0 | 6 | 5 | 1 | NA |
| Mozo, 2021 | 8.6 (0.8–18.1) | 3 | NA | 3 | NA | NA | NA | 2 | 6 | NA |
| Khandelwal, 2017 | 8.5 (1.6–16.5) | 1 | NA | 12 | NA | NA | 2 | 9 | 2 | 147 (55–538) |
| Zeiser, 2015 | 51 (21–75) | 13 | NA | 15 | NA | NA | NA | NA | NA | NA |
| Abedin, 2019 | 59 (46–70) | 6 | 2 | 10 | 1 | NA | 3 | 13 | 3 | 21 (3–162) |
| Dang, 2020 | 35 (19–55) | 8 | 2 | NA | NA | NA | NA | 9 | NA | NA |
| Uygun, 2020 | NA | 4 | NA | 17 | NA | NA | 2 | 4 | 7 | 28 (7–231) |
| Gómez, 2019 | 51 (0–73) | 33 | NA | 39 | NA | NA | 3 | 20 | NA | NA |
| Toama, 2020 | 55 (27–72) | 7 | NA | 24 | NA | NA | NA | NA | NA | 17 (2–280) |
| Zhao, 2020 | 29 (14–62) | 5 | 55 | 4 | NA | NA | NA | 22 | 42 | 8 (3–89) |
HLA, human leukocyte antigen; mMRD, mismatched related donor; MRD, matched related donor; mMUD, mismatched unrelated donor; MUD, matched unrelated donor; NA, not available; SR-aGVHD, steroid-refractory acute graft-versus-host disease.*Thirteen studies included both SR-aGVHD and SR-cGVHD analysis.
Table 4.
Characteristics of therapeutic response for 26 included studies (SR-cGVHD)*.
| Studies | Study design | N | Response/event (n) | Overall survival | Median follow-up (months) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ORR | ORR at 24 weeks | CRR | CRR at 24 weeks | 6 months | 12 months | 24 months | ||||
| Lancman, 2018 | Case report | 4 | NA | NA | 1 | NA | 1.00 | NA | NA | NA |
| Hurabielle, 2017 | Retrospective | 12 | NA | NA | 0 | NA | NA | NA | NA | NA |
| Maas-Bauer, 2020 | Retrospective | 23 | 17 | NA | 2 | NA | NA | NA | 0.75 | 30.00 |
| Zeiser, 2022 | RCT | 165 | NA | 82 | NA | 11 | NA | NA | NA | NA |
| Ferreira, 2021 | Retrospective | 35 | 31 | NA | 9 | NA | NA | NA | NA | 43.00 |
| Ferreira, 2018 | Case report | 20 | 15 | NA | 4 | NA | NA | NA | NA | 12.00 |
| Wei, 2021 | Retrospective | 32 | 25 | NA | 8 | NA | NA | NA | NA | 16.50 |
| Wang, 2021 | Retrospective | 70 | NA | 52 | NA | 34 | NA | 0.66 | NA | 13.37 |
| Leung, 2022 | Retrospective | 31 | NA | 26 | NA | 16 | NA | 0.94 | 0.81 | NA |
| Moiseev, 2020 | Prospective | 43 | 35 | NA | 9 | NA | NA | NA | NA | NA |
| González, 2018 | Prospective | 9 | NA | NA | 2 | NA | NA | NA | NA | NA |
| Kaurinovic, 2022 | Retrospective | 53 | 43 | NA | 28 | NA | NA | NA | NA | 20.00 |
| Maldonado, 2017 | Case report | 5 | 5 | NA | 1 | NA | NA | NA | NA | 12.00 |
| Maldonado, 2021 | Retrospective | 8 | 6 | NA | 4 | NA | NA | NA | NA | NA |
| Schoettler, 2019 | Case report | 5 | NA | NA | NA | NA | NA | NA | NA | 24.00 |
| Modi, 2018 | Retrospective | 46 | NA | 22 | NA | 5 | NA | 0.84 | NA | NA |
| Mozo, 2021 | Retrospective | 12 | 11 | NA | 1 | NA | NA | NA | 0.76 | NA |
| Zeiser, 2015 | Retrospective | 41 | 35 | NA | 3 | NA | 0.97 | NA | NA | 5.23 |
| Abedin, 2019 | Retrospective | 24 | 20 | NA | 3 | NA | NA | NA | NA | NA |
| Redondo, 2022 | Retrospective | 48 | 37 | NA | 7 | NA | NA | NA | 0.83 | 20.00 |
| Dang, 2020 | Retrospective | 28 | 22 | NA | NA | NA | NA | NA | NA | 5.00 |
| Uygun, 2020 | Retrospective | 15 | 13 | NA | 1 | NA | NA | NA | NA | NA |
| Gómez, 2019 | Retrospective | 56 | 32 | NA | 2 | NA | NA | 0.81 | NA | NA |
| Wu, 2021 | Retrospective | 41 | NA | 29 | NA | 15 | 0.88 | 0.66 | NA | 14.90 |
| Xue, 2021 | Retrospective | 36 | NA | 16 | NA | 4 | NA | 0.81 | 0.74 | NA |
| Zhao, 2021 | Retrospective | 30 | 20 | 17 | 3 | NA | NA | NA | 0.63 | 10.60 |
CRR, complete response rate; NA, not available; ORR, overall response rate; RCT, randomized controlled trials; SR-aGVHD, steroid-refractory acute graft-versus-host disease; SR-cGVHD, steroid-refractory chronic graft-versus-host disease.
*Thirteen studies included both SR-aGVHD and SR-cGVHD analysis.
Figure 1.
Selection scheme of studies. SR-GVHD, steroid-refractory graft-versus-host disease.
Table 5.
The number of studies included in the subgroup analysis.
| Subgroup | Studies included, No. (%) | |
|---|---|---|
| SR-aGVHD1 | SR-cGVHD1 | |
| ORR | ||
| At any time | n = 17 | n = 16 |
| Retrospective studies | 15 (88.2) | 15 (93.8) |
| Prospective unrandomized studies | 2 (11.8) | 1 (6.2) |
| RCT | NA | NA |
| At day 28 | n = 5 | NA |
| Retrospective studies | 2 (40.0) | NA |
| Prospective unrandomized studies | 2 (40.0) | NA |
| RCT | 1 (20.0) | NA |
| At week 24 | NA | n = 7 |
| Retrospective studies | NA | 6 (85.7) |
| Prospective unrandomized studies | NA | NA |
| RCT | NA | 1 (14.3) |
| CRR | ||
| At any time | n = 21 | n = 18 |
| Retrospective studies | 18 (85.7) | 16 (88.9) |
| Prospective unrandomized studies | 3 (14.3) | 2 (11.1) |
| RCT | NA | NA |
| At day 28 | n = 7 | NA |
| Retrospective studies | 4 (57.1) | NA |
| Prospective unrandomized studies | 2 (28.6) | NA |
| RCT | 1 (14.3) | NA |
| At week 24 | NA | n = 6 |
| Retrospective studies | NA | 5 (83.3) |
| Prospective unrandomized studies | NA | NA |
| RCT | NA | 1 (16.7) |
| Involved Organ Response | ||
| ORR 2 | n = 9 | n = 13 |
| Skin | 8 (88.9) | 13 (100.0) |
| Gut | 9 (100.0) | 11 (84.6) |
| Liver | 8 (88.9) | 9 (69.2) |
| Mouth | NA | 8 (61.5) |
| Eye | NA | 10 (76.9) |
| Lung | NA | 12 (92.3) |
| Joints and fascia | NA | 8 (61.5) |
| Esophagus | NA | 1 (7.7) |
| CRR 2 | n = 8 | n = 11 |
| Skin | 7 (87.5) | 11 (100.0) |
| Gut | 8 (100.0) | 9 (81.8) |
| Liver | 7 (87.5) | 7 (63.6) |
| Mouth | NA | 7 (63.6) |
| Eye | NA | 7 (63.6) |
| Lung | NA | 10 (90.9) |
| Joints and fascia | NA | 7 (63.6) |
| Esophagus | NA | 1 (9.1) |
| Hematologic Toxicities | ||
| Cytopenia 3 | n = 6 | n = 9 |
| Grades I–IV | 5 (83.3) | 9 (100.0) |
| Grades III–IV | 3 (50.0) | 5 (55.3) |
| Anemia 3 | n = 10 | n = 10 |
| Grades I–IV | 8 (80.0) | 8 (80.0) |
| Grades III–IV | 9 (90.0) | 10 (100.0) |
| Leukopenia 3 | n = 12 | n = 12 |
| Grades I–IV | 10 (83.3) | 9 (75.0) |
| Grades III–IV | 11 (91.7) | 10 (83.3) |
| Thrombocytopenia 3 | n = 14 | n = 12 |
| Grades I–IV | 12 (85.7) | 9 (75.0) |
| Grades III–IV | 11 (78.6) | 11 (91.7) |
| Infections4 | n = 14 | n = 18 |
| Total | 12 (85.7) | 16 (88.9) |
| Viral | 8 (57.1) | 9 (50.0) |
| Bacterial | 5 (35.7) | 8 (44.4) |
| Fungal | 4 (28.6) | 7 (38.9) |
| OS5 | n = 7 | n = 12 |
| 6 months | 7 (100.0) | 3 (25.0) |
| 1 year | – | 6 (50.0) |
| 2 years | – | 6 (50.0) |
CRR, complete response rate; NA, not available; ORR, overall response rate; OS, overall survival; RCT, randomized controlled trials; SR-aGVHD, steroid-refractory acute graft-versus-host disease; SR-cGVHD, steroid-refractory chronic graft-versus-host disease.
1 Twelve studies included both in the SR-aGVHD and SR-cGVHD analysis;
2 Twenty-one studies involved multiple organs in the analysis of ORRs;
Eighteen studies involved multiple organs in the analysis of CRRs.
3 Seven studies included both in the analysis of overall (grades I–IV) and severe (grades III–IV) cytopenia;
Fifteen studies included both in the analysis of overall (grades I–IV) and severe (grades III–IV) anemia;
Sixteen studies included both in the analysis of overall (grades I–IV) and severe (grades III–IV) leukopenia;
Seventeen studies included both in the analysis of overall (grades I–IV) and severe (grades III–IV) thrombocytopenia.
4 Fourteen studies involved in the analysis of multiple types of infection.
5 Three studies included both in the analysis of 6-month, 1-year, and 2-year OS in SR-cGVHD.
The bold values represent the total number of studies included in the analysis.
Table 2.
Characteristics of 26 included studies (SR-cGVHD)*.
| Studies | Median age/year (range) | HLA matching (n) | SR-cGVHD grade (n) | Median time from SR-cGVHD diagnosis to the application of ruxolitinib/day (range) | |||||
|---|---|---|---|---|---|---|---|---|---|
| MRD | mMRD | MUD | mMUD | Mild | Moderate | Severe | |||
| Lancman, 2018 | 52 (38–71) | NA | NA | NA | NA | NA | NA | NA | NA |
| Hurabielle, 2017 | 47 (21–67) | 4 | NA | 8 | NA | NA | NA | NA | NA |
| Maas-Bauer, 2020 | 49 (28–70) | NA | NA | NA | NA | NA | NA | 13 | NA |
| Zeiser, 2022 | 49 (13–73) | 91 | NA | 76 | NA | 1 | 67 | 97 | NA |
| Ferreira, 2021 | 54 (23–73) | 18 | NA | 8 | NA | 0 | 23 | 12 | 510 (30–2130) |
| Ferreira, 2018 | 46.5 (23–68) | 10 | NA | NA | NA | 0 | 11 | 9 | 480 |
| Wei, 2021 | 31 (11–54) | 19 | NA | 1 | NA | 2 | 10 | 20 | 17 (7–1239) |
| Wang, 2021 | 35 (13–63) | 27 | NA | 2 | 41 | 23 | 38 | 9 | NA |
| Leung, 2022 | 33 (21–64) | NA | NA | NA | NA | 7 | 15 | 7 | NA |
| Moiseev, 2020 | 21 (2–62) | 7 | NA | 30 | NA | 0 | 6 | 37 | 376 (28–3219) |
| González, 2018 | 11 (5–18) | NA | NA | NA | NA | 1 | 1 | 7 | 540 |
| Kaurinovic, 2022 | 60 (26–76) | NA | NA | NA | NA | 10 | 35 | 8 | NA |
| Maldonado, 2017 | 36 (26–52) | 1 | NA | 2 | NA | NA | 1 | 4 | 180 (90–540) |
| Maldonado, 2021 | 38.5 (22–59) | NA | NA | NA | NA | 2 | 1 | 5 | NA |
| Schoettler, 2019 | 10 (7–21) | NA | NA | 5 | NA | NA | 1 | 4 | 120 (75–720) |
| Modi, 2018 | 49 (21–77) | 15 | NA | 10 | 16 | 3 | 8 | 35 | NA |
| Mozo, 2021 | 12 (2.1–16) | 1 | NA | 8 | NA | NA | 8 | 4 | NA |
| Zeiser, 2015 | 55 (22–74) | 9 | NA | 17 | NA | NA | 6 | 35 | NA |
| Abedin, 2019 | 59 (45–70) | 11 | 1 | 12 | 0 | NA | 16 | 8 | NA |
| Redondo, 2022 | 49 (18–72) | 27 | NA | 19 | 2 | 1 | 29 | 18 | 150 (18–630) |
| Dang, 2020 | 30 (14–55) | 15 | 8 | 5 | NA | NA | 24 | NA | NA |
| Uygun, 2020 | NA | NA | NA | NA | NA | NA | 2 | 13 | 840 (210–1560) |
| Gómez, 2019 | NA | NA | NA | NA | NA | 0 | 28 | 28 | NA |
| Wu, 2021 | 31 (17–56) | 9 | NA | NA | NA | NA | 14 | 27 | 330 (18–2,157) |
| Xue, 2021 | 45 (19–71) | 10 | 14 | 12 | NA | NA | 9 | 27 | 654 (69–4,482) |
| Zhao,2021 | 27 (15–54) | 8 | 21 | 1 | NA | NA | 6 | 24 | 125 (27–1,598) |
HLA, human leukocyte antigen; mMRD, mismatched related donor; MRD, matched related donor; mMUD, mismatched unrelated donor; MUD, matched unrelated donor; NA, not available; SR-cGVHD, steroid-refractory chronic graft-versus-host disease. *Thirteen studies included both SR-aGVHD and SR-cGVHD analysis.
Table 3.
Characteristics of therapeutic response for 24 included studies (SR-aGVHD)*.
| Studies | Study design | N | Response/event (n) | cGVHD incidence (%) | 6-month overall survival | Median follow-up (months) | |||
|---|---|---|---|---|---|---|---|---|---|
| ORR | ORR at 28 days | CRR | CRR at 28 days | ||||||
| Zeiser, 2020 | RCT | 154 | NA | 96 | NA | 53 | NA | NA | 5.04 |
| Modemann, 2020 | Retrospective | 18 | 10 | NA | 8 | NA | 66.70 | NA | NA |
| Lancman, 2018 | Case report | 4 | NA | NA | 3 | 2 | NA | 0.75 | NA |
| Assouan, 2017 | Retrospective | 10 | NA | NA | 5 | NA | NA | 0.70 | 4.47 |
| Wei, 2021 | Retrospective | 23 | 20 | NA | 13 | NA | 21.74 | NA | 14.43 |
| Leung, 2022 | Retrospective | 26 | 19 | 19 | 15 | 8 | 11.54 | NA | NA |
| Moiseev, 2020 | Prospective | 32 | 24 | NA | 20 | NA | 37.50 | NA | NA |
| Jagasia, 2020 | Prospective | 71 | 52 | 39 | 40 | 19 | 5.63 | 0.51 | 10.77 |
| Biliński, 2021 | Case report | 4 | NA | NA | 2 | NA | NA | NA | NA |
| Liu, 2021 | Retrospective | 40 | 34 | NA | 25 | NA | 7.50 | 0.57 | 8.20 |
| Laisne, 2020 | Retrospective | 29 | NA | NA | 19 | 6 | NA | NA | 16.00 |
| González, 2018 | Prospective | 13 | NA | NA | 4 | NA | NA | NA | NA |
| Maldonado, 2017 | Case report | 3 | 3 | NA | 2 | NA | NA | NA | 24.00 |
| Maldonado, 2021 | Retrospective | 9 | 7 | NA | 4 | NA | NA | NA | NA |
| Meng, 2019 | Retrospective | 12 | 10 | NA | 7 | NA | NA | NA | NA |
| Mozo, 2021 | Retrospective | 8 | 7 | NA | 3 | NA | 12.50 | NA | NA |
| Khandelwal, 2017 | Retrospective | 13 | 5 | NA | 1 | NA | NA | NA | NA |
| Zeiser, 2015 | Retrospective | 54 | 44 | NA | 25 | NA | NA | 0.79 | 6.18 |
| Abedin, 2019 | Retrospective | 19 | 17 | 16 | 12 | 9 | NA | NA | NA |
| Dang, 2020 | Retrospective | 10 | 10 | NA | NA | NA | NA | NA | 2.50 |
| Uygun, 2020 | Retrospective | 13 | 11 | NA | 9 | NA | NA | NA | NA |
| Gómez, 2019 | Retrospective | 23 | 16 | NA | 5 | NA | NA | 0.47 | NA |
| Toama, 2020 | Retrospective | 36 | 14 | NA | 6 | NA | NA | NA | NA |
| Zhao, 2020 | Prospective | 64 | NA | 56 | NA | 47 | NA | 0.68 | 15.50 |
CRR, complete response rate; NA, not available; ORR, overall response rate; RCT, randomized controlled trials; SR-aGVHD, steroid-refractory acute graft-versus-host disease; SR-cGVHD, steroid-refractory chronic graft-versus-host disease.*Thirteen studies included both SR-aGVHD and SR-cGVHD analysis.
Response rate
SR-aGVHD
ORR after ruxolitinib treatment
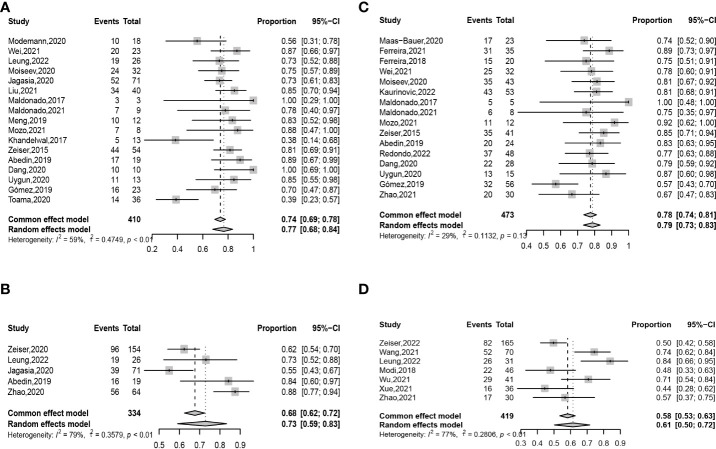
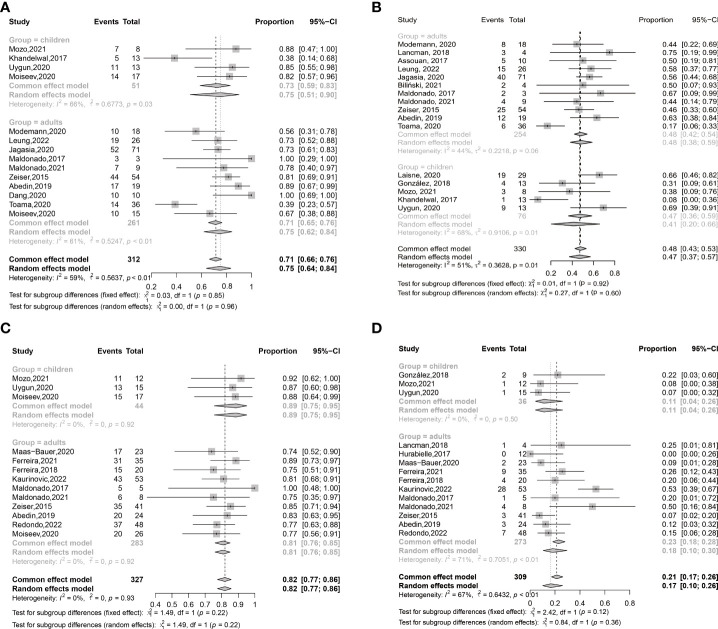
ORR at any time after ruxolitinib treatment was 0.77 (95% confidence interval (CI): 0.68–0.84) ( Figure 2A ). ORR at any time was 0.78 (95% CI: 0.67–0.86) in retrospective studies and was 0.74 (95% CI: 0.64–0.81) in prospective unrandomized studies ( Figures S1A, B ). In adults, ORR at any time was 0.75 (95% CI: 0.62–0.84), which was comparable with that in children (0.75 (95% CI: 0.51–0.90), P = 0.960) ( Table S1 , Table 6 , and Figure 3A ).
Figure 2.
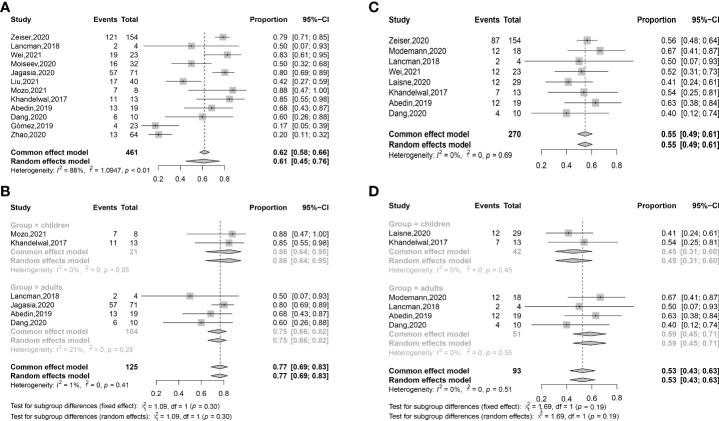
Forest plots of ORRs at any time (A) and day 28 (B) after ruxolitinib treatment in SR-aGVHD and the ORRs at any time (C) and week 24 (D) after ruxolitinib treatment in SR-cGVHD.
Table 6.
The summary table of comparisons between adults and children.
| Subgroup | Adults | Children | P-value | ||
|---|---|---|---|---|---|
| Cumulative incidence | 95% CI | Cumulative incidence | 95% CI | ||
| SR-aGVHD | |||||
| ORR | |||||
| At any time | 0.75 | 0.62–0.84 | 0.75 | 0.51–0.90 | 0.960 |
| CRR | |||||
| At any time | 0.48 | 0.42–0.54 | 0.41 | 0.20–0.66 | 0.601 |
| At day 28 | 0.32 | 0.24–0.41 | 0.21 | NA | NA |
| Infection | 0.75 | 0.66–0.82 | 0.86 | 0.64–0.95 | 0.296 |
| Viral infection | 0.59 | 0.59–0.71 | 0.45 | 0.31–0.60 | 0.193 |
| Cytopenia | |||||
| Grades I–IV | 37.3 | 0.0–82.1 | 53.8 | NA | NA |
| Anemia | |||||
| Grades I–IV | 24.7 | 0.0–55.3 | 25.0 | NA | NA |
| Grades III–IV | 26.0 | 7.0–45.0 | 25.0 | NA | NA |
| Leukopenia | |||||
| Grades I–IV | 34.3 | 0.6–68.1 | 26.0 | 0.0–100.0 | 0.645 |
| Grades III–IV | 28.0 | 14.8–41.2 | 26.0 | 0.0–100.0 | 0.904 |
| Thrombocytopenia | |||||
| Grades I–IV | 23.7 | 0.0–56.7 | 19.8 | 0.0–41.8 | 0.722 |
| Grades III–IV | 31.0 | 0.0–73.2 | 30.5 | 0.0–100.0 | 0.975 |
| SR-cGVHD | |||||
| ORR | |||||
| At any time | 0.81 | 0.76–0.85 | 0.89 | 0.75–0.95 | 0.222 |
| CRR | |||||
| At any time | 0.18 | 0.10-0.30 | 0.11 | 0.04 –0.26 | 0.359 |
| Infection | 0.37 | 0.18–0.61 | 0.42 | NA | NA |
| Anemia | |||||
| Grades I–IV | 20.7 | 0.0–57.9 | 8.3 | NA | NA |
| Grades III–IV | 5.0 | 0.0–15.6 | 0.0 | NA | NA |
| Leukopenia | |||||
| Grades I–IV | 11.5 | 2.6–20.4 | 8.3 | NA | NA |
| Grades III–IV | 9.8 | 2.3–17.3 | 0.0 | NA | NA |
| Thrombocytopenia | |||||
| Grades I–IV | 7.0 | 0.0–17.6 | 8.3 | NA | NA |
| Grades III–IV | 3.8 | 0.0–8.3 | 0.0 | NA | NA |
Figure 3.
The subgroup analysis of adults and children in ORRs (A) and CRRs (B) at any time after ruxolitinib treatment in SR-aGVHD; The subgroup analysis of adults and children in ORRs (C) and CRRs (D) at any time after ruxolitinib treatment in SR-cGVHD.
ORR at day 28 after ruxolitinib treatment was 0.73 (95% CI: 0.59–0.83) ( Figure 2B ). ORRs at day 28 were 0.78 (95% CI: 0.63–0.88) and 0.74 (95% CI: 0.45–0.91), respectively, in retrospective studies and prospective unrandomized studies ( Figures S1C, D ). In the randomized controlled trial (RCT), ORR at day 28 was 0.62.
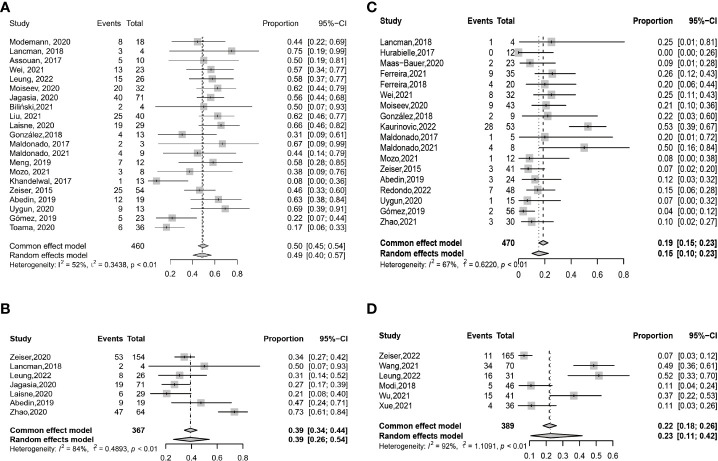
CRR after ruxolitinib treatment
CRR at any time after ruxolitinib treatment was 0.49 (95% CI: 0.40–0.57) ( Figure 4A ). CRRs at any time were 0.48 (95% CI: 0.38–0.58) and 0.55 (95% CI: 0.46–0.64), respectively, in retrospective studies and prospective unrandomized studies ( Figures S3A, B ). CRRs at any time were comparable between adults and children (0.48 (95% CI: 0.42–0.54) vs. 0.41 (95% CI: 0.20–0.66), P =0.601) ( Table S1 and Figure 3B ).
Figure 4.
Forest plots of CRRs at any time (A) and day 28 (B) after ruxolitinib treatment in SR-aGVHD and the CRRs at any time (C) and week 24 (D) after ruxolitinib treatment in SR-cGVHD.
CRR at day 28 after ruxolitinib treatment was 0.39 (95% CI: 0.26–0.54) ( Figure 4B ). CRRs at day 28 were 0.32 (95% CI: 0.23–0.43) and 0.50 (95% CI: 0.19–0.81), respectively, in retrospective studies and prospective unrandomized studies ( Figures S3C, D ). In the RCT, CRR at day 28 was 0.34. CRRs at day 28 were 0.32 (95% CI: 0.24–0.41) and 0.21, respectively, for adults and children.
SR-cGVHD
ORR after ruxolitinib treatment
ORR at any time after ruxolitinib treatment was 0.78 (95% CI: 0.74–0.81) ( Figure 2C ). ORRs at any time were 0.77 (95% CI: 0.73–0.81) and 0.81, respectively, in retrospective studies and prospective unrandomized studies ( Figure S2A ). In adults, ORR at any time was 0.81 (95% CI: 0.76–0.85), which was comparable with that in children (0.89 (95% CI: 0.75–0.95), P =0.222) ( Table S1 and Figure 3C ).
ORR at week 24 after ruxolitinib treatment was 0.61 (95% CI: 0.50–0.72) ( Figure 2D ). In retrospective studies, ORR at week 24 was 0.64 (95% CI: 0.51–0.75) ( Figure S2B ). In the RCT, ORR at week 24 was 0.50.
CRR after ruxolitinib treatment
CRR at any time after ruxolitinib treatment was 0.15 (95% CI: 0.10–0.23) ( Figure 4C ). CRR at any time was 0.15 (95% CI: 0.09–0.23) and 0.21 (95% CI: 0.12–0.34), respectively, in retrospective studies and prospective unrandomized studies, respectively ( Figures S4A, B ). In adults, CRR at any time was 0.18 (95% CI: 0.10-0.30), which was compared with that in children (0.11 (95% CI: 0.04 –0.26), P = 0.359) ( Figure 3D ).
CRR at week 24 after ruxolitinib treatment was 0.23 (95% CI: 0.11–0.42) ( Figure 4D ). In retrospective studies, CRR at week 24 was 0.29 (95% CI: 0.15–0.48) ( Figure S4C ). In the RCT, CRR at week 24 was 0.07.
Response according to the involved organs after ruxolitinib treatment
SR-aGVHD
The ORRs and CRRs at any time after treatment were showed in Table 7 . The CRRs at any time after treatment were highest in skin SR-aGVHD, followed by gut, and liver SR-aGVHD.
Table 7.
Response at any time according to the involved organs after ruxolitinib treatment.
| Subgroup | ORR | CRR | ||
|---|---|---|---|---|
| Cumulative incidence (%) | 95%CI | Cumulative incidence (%) | 95%CI | |
| SR-aGVHD | ||||
| Skin | 78.3 | 63.2–93.3 | 68.6 | 37.2–99.9 |
| Gut | 78.9 | 66.6–91.2 | 57.9 | 37.8–78.1 |
| Liver | 60.4 | 37.2–83.5 | 49.1 | 32.8–65.5 |
| SR-cGVHD | ||||
| Skin | 73.2 | 58.7–87.7 | 30.1 | 18.2–42.0 |
| Gut | 69.2 | 50.9–87.5 | 25.7 | 2.4–48.9 |
| Liver | 65.7 | 45.0–86.3 | 32.7 | 15.8–49.6 |
| Mouth | 76.5 | 61.5–91.5 | 34.0 | 24.7–43.3 |
| Eyes | 61.1 | 38.7–83.5 | 16.7 | 2.4–31.0 |
| Lung | 47.3 | 29.8–64.9 | 11.1 | 1.2–21.0 |
| Joints and fascia | 67.4 | 46.4–88.3 | 11.9 | 0.0–23.8 |
| Esophagus | 50.0 | NA | 0.0 | NA |
CI, confidence interval; CRR, complete response rate; NA, not available; ORR, overall response rate; SR-aGVHD, steroid-refractory acute graft-versus-host disease; SR-cGVHD, steroid-refractory chronic graft-versus-host disease.
SR-cGVHD
The ORRs at any time after treatment were highest in mouth SR-cGVHD, followed by skin and gut SR-cGVHD. The CRRs at any time after treatment was highest in mouth SR-cGVHD, followed by liver and skin SR-cGVHD ( Table 7 ).
Infections after ruxolitinib treatment
SR-aGVHD
The incidence rate of infections after ruxolitinib treatment was 0.61 (95% CI: 0.45–0.76). The frequency rates of infection after ruxolitinib treatment were comparable between children [0.86 (95% CI: 0.64–0.95)] and adults [0.75 (95% CI: 0.66–0.82), P = 0.296] ( Figures 5A, B ). The frequency rates of viral infections were 0.55 (95% CI: 0.49–0.61). The frequency rates of viral infection after ruxolitinib treatment were comparable between children [0.45 (95% CI: 0.31–0.60)] and adults [0.59 (95% CI: 0.59–0.71), P = 0.193] ( Figures 5C, D ).
Figure 5.
Forest plots of frequency rates of infections (A) and the subgroup analysis of adults and children (B) after ruxolitinib treatment in SR-aGVHD; Forest plots of frequency rates of viral infections (C) and the subgroup analysis of adults and children (D) after ruxolitinib treatment in SR-aGVHD.
SR-cGVHD
The incidence rate of infection after ruxolitinib treatment was 0.47 (95% CI: 0.31–0.63) ( Figure S5 ). The frequency rates of infection after ruxolitinib treatment were 0.37 (95% CI: 0.18–0.61) and 0.42, respectively, for adults and children. The frequency rates of viral infection were 0.29 (95% CI: 0.25–0.34) ( Figure S6 ).
Myelosuppression after ruxolitinib treatment
SR-aGVHD
The incidence rates of overall (grades I–IV) and severe (grades III–IV) cytopenia were 53.2% (95% CI: 16.0%–90.4%) and 31.0% (95% CI: 0.0%–100.0%), respectively. The incidence rate of overall cytopenia was 37.3% (95% CI: 0.0–82.1%) and 54.0%, respectively, for adults and children. The frequency rates of anemia, leukopenia, and thrombocytopenia were 45.6% (95% CI:19.8%–71.5%), 44.2% (95% CI: 24.4%–64.0%), and 40.6% (95% CI: 21.4%–59.8%), respectively ( Table 8 ).
Table 8.
Myelosuppression after ruxolitinib treatment.
| Subgroup | Total | Adults | Children | |||
|---|---|---|---|---|---|---|
| Cumulative incidence | 95% CI | Cumulative incidence | 95% CI | Cumulative incidence | 95% CI | |
| SR-aGVHD | ||||||
| Cytopenia | ||||||
| Grades I–IV | 53.2 | 16.0–90.4 | 37.3 | 0.0–82.1 | 53.8 | NA |
| Grades III–IV | 31.0 | 0.0–100.0 | 16.5 | 0.0–100.0 | NA | NA |
| Anemia | ||||||
| Grades I–IV | 45.6 | 19.8–71.5 | 24.7 | 0.0–55.3 | 25.0 | NA |
| Grades III–IV | 37.3 | 18.4–56.3 | 26.0 | 7.0-45.0 | 25.0 | NA |
| Leukopenia | ||||||
| Grades I–IV | 44.2 | 24.4–64.0 | 34.3 | 0.6–68.1 | 26.0 | 0.0–100.0 |
| Grades III–IV | 36.8 | 20.7–52.9 | 28.0 | 14.8–41.2 | 26.0 | 0.0–100.0 |
| Thrombocytopenia | ||||||
| Grades I–IV | 40.6 | 21.4–59.8 | 23.7 | 0.0–56.7 | 19.8 | 0.0–41.8 |
| Grades III–IV | 42.4 | 25.0–59.7 | 31.0 | 0.0–73.2 | 30.5 | 0.0–100.0 |
| SR-cGVHD | ||||||
| Cytopenia | ||||||
| Grades I–IV | 28.8 | 13.0–44.6 | 28.3 | 4.8–51.7 | NA | NA |
| Grades III–IV | 10.4 | 0.0–27.9 | 21.0 | 0.0–100.0 | NA | NA |
| Anemia | ||||||
| Grades I–IV | 35.1 | 13.2–57.0 | 20.7 | 0.0–57.9 | 8.3 | NA |
| Grades III–IV | 11.2 | 2.1–20.3 | 5.0 | 0.0–15.6 | 0.0 | NA |
| Leukopenia | ||||||
| Grades I–IV | 22.9 | 6.2–39.6 | 11.5 | 2.6–20.4 | 8.3 | NA |
| Grades III–IV | 8.9 | 4.7–13.1 | 9.8 | 2.3–17.3 | 0.0 | NA |
| Thrombocytopenia | ||||||
| Grades I–IV | 19.2 | 6.9–31.6 | 7.0 | 0.0–17.6 | 8.3 | NA |
| Grades III–IV | 10.2 | 3.6–16.8 | 3.8 | 0.0–8.3 | 0.0 | NA |
CI, confidence interval; NA, not available; SR-aGVHD, steroid-refractory acute graft-versus-host disease; SR-cGVHD, steroid-refractory chronic graft-versus-host disease.
SR-cGVHD
The incidence rates of overall and severe cytopenia were 28.8% (95% CI: 13.0%–44.6%) and 10.4% (95% CI: 0.0–27.9%), respectively. The frequency rates of anemia, leukopenia, and thrombocytopenia were 35.1% (95% CI: 13.2%–57.0%), 22.9% (95% CI: 6.2%–39.6%), and 19.2% (95% CI: 6.9%–31.6%), respectively ( Table 8 ).
OS
SR-aGVHD
The probability rate of OS at 6 months after treatment was 63.9% (95% CI: 52.5%–75.2%). The probability rates of OS at 6 months after treatment were 65.6% (95% CI: 49.1%–82.1%) and 59.5% (95% CI: 0.0%–100.0%), respectively, for retrospective studies and prospective unrandomized studies.
SR-cGVHD
The probability rates of OS at 6 months, 1 year, and 2 years after treatment were 95% (95% CI: 79.5%–100.0%), 78.7% (95% CI: 67.2%–90.1%), and 75.3% (95% CI: 68.0%–82.7%), respectively.
Discussion
Many studies have reported that ruxolitinib was effective treatment for patients with SR-GVHD, and we also observed that therapeutic response and survival seemed to be comparable between adults and children. To the best of our knowledge, this study is the most comprehensive systematic review to summarize the published studies and demonstrated the efficacy and safety of ruxolitinib treatment for SR-GVHD.
For SR-aGVHD, the ORRs of ruxolitinib at any time and at day 28 were 0.77 and 0.73, respectively. Many other therapeutic modalities were also applied to control SR-aGVHD. Prior data reported that the ORRs of antithymocyte globulin [ATG, (11, 38, 39)], extracorporeal photopheresis [ECP, (40–44)], mycophenolate mofetil [MMF, (45–50)], etanercept (51–55), daclizumab (56), inolimomab (56), and denileukin diftitox (56) were 0.30–0.31, 0.66–0.75, 0.31–0.67, 0.46–0.68, 0.71, 0.54, and 0.56, respectively. In addition, the ORR at day 28 after treatment was 0.54–0.56 for ATG (57, 58), and the ORRs at 1 month after treatment were 0.69, 0.55, and 0.56, respectively, for daclizumab (56), inolimomab (56), and denileukin diftitox (56). Thus, it seems that ruxolitinib has a higher ORR compared with most of the other second-line treatments in SR-aGVHD.
In this study, the CRRs of ruxolitinib at any time and at day 28 were 0.49 and 0.39, respectively, in patients with SR-aGVHD. The available data about the CRRs of ATG (11, 38, 39), ECP (40–44, 59–61), MMF (45, 46, 48, 50), etanercept (51, 52, 54, 55), mesenchymal stem cell (MSC) (62), daclizumab (56), inolimomab (56), and denileukin diftitox (56) were 0.08–0.14, 0.52–0.75, 0–0.31, 0–0.31, 0.09–0.65, 0.42, 0.30, and 0.37, respectively. In addition, the CRR at day 28 after treatment was 0.20–0.36 for ATG (57, 58), and the CRRs at 1 month after treatment were 0.37, 0.31, and 0.37, respectively, for daclizumab, inolimomab, and denileukin diftitox (56). Thus, the CRR of ruxolitinib did not seem to be more superior to other second-line treatments, which can be further improved.
For SR-cGVHD, the ORR of ruxolitinib at any time was 0.78. On the basis of data from previous systematic reviews, the ORRs of rituximab (63, 64), MMF (64), imatinib (64), MSC (64), methotrexate (64), ECP (43, 64), pentostatin (64), sirolimus (64), and thalidomide (64) were 0.66–0.68, 0.65, 0.58, 0.65, 0.70, 0.64–0.68, 0.54, 0.79, and 0.50, respectively. Ruxolitinib showed a higher ORR compared with most of the other second-line treatments in patients with SR-cGVHD.
Four studies reported that ruxolitinib could be used in the treatment for children with SR-GVHD (29–32); however, few studies compared the clinical outcomes of ruxolitinib between children and adults with SR-GVHD. In this study, we first observed that ORR, CRR, infection events, and myelosuppression were all comparable between adult and children, which suggested that ruxolitinib treatment was effective and safe for children with SR-GVHD.
In recently real-world studies, the ORR and CRR of basiliximab at day 28 were 0.66–0.79 and 0.52–0.61, respectively in patients with SR-aGVHD (65, 66). Compared with this study, the efficacy of basiliximab seemed to be compared with ruxolitinib. However, in the only successful RCT (REACH2 study) for SR-aGVHD, nine second-line treatments except interleukin-2 receptor antagonists were included as the best available treatments. Thus, comparing the safety and efficacy between ruxolitinib and basiliximab in SR-aGVHD seems to be an interesting clinical issue.
This study has several limitations. First, we observed that the ORR for ruxolitinib seemed to be superior to other drugs in SR-aGVHD and SR-cGVHD. However, differences existed in the patient selection or publication bias may influence the comparison between ruxolitinib and other drugs. Considering that most studies about SR-GVHD were single-arm–designed, we admitted that the comparison of ruxolitinib and other second-line therapies might be insufficient, and it is premature to conclude that ruxolitinib was superior to other drugs based on the results of our meta-analysis. REACH 2 and REACH 3 trials had observed that ruxolitinib was superior to most of the other second-line drugs in RCTs, and real-world analysis could help to further compare the efficacy and safety between ruxolitinib and other drugs in future. Second, the reducing accuracy of our result might due to heterogeneity of different studies in our analysis. Third, the sample of children was still relatively small, and the comparisons of efficacy and safety between adults and children were insufficient, which should be further identified in future.
Conclusion
In summary, this study suggests that ruxolitinib is an effective and safe treatment for SR-GVHD, and both children and adults with SR-GVHD could benefit from ruxolitinib treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
X-DM and M-ZS designed the study. SF, W-XH, and YY conducted data collection. M-ZS, SF, W-XH, and X-DM conducted data analysis and drafted manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (grant number 81621001), the Program of the National Natural Science Foundation of China (grant number 82170208), CAMS Innovation Fund for Medical Sciences (CIFMS) (grant number 2019-I2M-5-034), the Key Program of the National Natural Science Foundation of China (grant number 81930004), and the Fundamental Research Funds for the Central Universities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.954268/full#supplementary-material
References
- 1. Zhang XH, Chen J, Han MZ, Huang H, Jiang EL, Jiang M, et al. The consensus from the Chinese society of hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update. J Hematol Oncol (2021) 14(1):145. doi: 10.1186/s13045-021-01159-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yeshurun M, Weisdorf D, Rowe JM, Tallman MS, Zhang MJ, Wang HL, et al. The impact of the graft-versus-leukemia effect on survival in acute lymphoblastic leukemia. Blood Adv (2019) 3(4):670–80. doi: 10.1182/bloodadvances.2018027003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu J, Parasuraman S, Shah A, Weisdorf D. Mortality, length of stay and costs associated with acute graft-versus-host disease during hospitalization for allogeneic hematopoietic stem cell transplantation. Curr Med Res Opin (2019) 35(6):983–8. doi: 10.1080/03007995.2018.1551193 [DOI] [PubMed] [Google Scholar]
- 4. Modi A, Rybicki L, Majhail NS, Mossad SB. Severity of acute gastrointestinal graft-vs-host disease is associated with incidence of bloodstream infection after adult allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis (2020) 22(1):e13217. doi: 10.1111/tid.13217 [DOI] [PubMed] [Google Scholar]
- 5. Shen MZ, Hong SD, Lou R, Chen RZ, Zhang XH, Xu LP, et al. A comprehensive model to predict severe acute graft-versus-host disease in acute leukemia patients after haploidentical hematopoietic stem cell transplantation. Exp Hematol Oncol (2022) 11(1):25. doi: 10.1186/s40164-022-00278-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gratwohl A, Brand R, Frassoni F, Rocha V, Niederwieser D, Reusser P, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant (2005) 36(9):757–69. doi: 10.1038/sj.bmt.1705140 [DOI] [PubMed] [Google Scholar]
- 7. Barton-Burke M, Dwinell DM, Kafkas L, Lavalley C, Sands H, Proctor C, et al. Graft-versus-host disease: a complex long-term side effect of hematopoietic stem cell transplant. Oncol (Williston Park) (2008) 22(11 Suppl Nurse Ed):31–45. [PubMed] [Google Scholar]
- 8. Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American society of blood and marrow transplantation. Biol Blood Marrow Transplant (2012) 18(8):1150–63. doi: 10.1016/j.bbmt.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood (2010) 115(26):5412–7. doi: 10.1182/blood-2009-12-258442 [DOI] [PubMed] [Google Scholar]
- 10. Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European society for blood and marrow transplantation. Lancet Haematol (2020) 7(2):e157–67. doi: 10.1016/S2352-3026(19)30256-X [DOI] [PubMed] [Google Scholar]
- 11. Arai S, Margolis J, Zahurak M, Anders V, Vogelsang GB. Poor outcome in steroid-refractory graft-versus-host disease with antithymocyte globulin treatment. Biol Blood Marrow Transplant (2002) 8(3):155–60. doi: 10.1053/bbmt.2002.v8.pm11939605 [DOI] [PubMed] [Google Scholar]
- 12. Ajayi S, Becker H, Reinhardt H, Engelhardt M, Zeiser R, von Bubnoff N, et al. Ruxolitinib. Recent Results Cancer Res (2018) 212:119–32. doi: 10.1007/978-3-319-91439-8_6 [DOI] [PubMed] [Google Scholar]
- 13. Schroeder MA, Choi J, Staser K, DiPersio JF. The role of janus kinase signaling in graft-Versus-Host disease and graft versus leukemia. Biol Blood Marrow Transplant (2018) 24(6):1125–34. doi: 10.1016/j.bbmt.2017.12.797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jagasia M, Perales MA, Schroeder MA, Ali H, Shah NN, Chen YB, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood (2020) 135(20):1739–49. doi: 10.1182/blood.2020004823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-Host disease. N Engl J Med (2020) 382(19):1800–10. doi: 10.1056/NEJMoa1917635 [DOI] [PubMed] [Google Scholar]
- 16. Zeiser R, Polverelli N, Ram R, Hashmi SK, Chakraverty R, Middeke JM, et al. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-Host disease. N Engl J Med (2021) 385(3):228–38. doi: 10.1056/NEJMoa2033122 [DOI] [PubMed] [Google Scholar]
- 17. Przepiorka D, Luo L, Subramaniam S, Qiu J, Gudi R, Cunningham LC, et al. FDA Approval summary: Ruxolitinib for treatment of steroid-refractory acute graft-Versus-Host disease. Oncologist (2020) 25(2):e328–34. doi: 10.1634/theoncologist.2019-0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le RQ, Wang X, Zhang H, Li H, Przepiorka D, Vallejo J, et al. FDA Approval summary: Ruxolitinib for treatment of chronic graft-Versus-Host disease after failure of one or two lines of systemic therapy. Oncologist (2022) 27(6):493–500. doi: 10.1093/oncolo/oyac042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hui L, Qi L, Guoyu H, Xuliang S, Meiao T. Ruxolitinib for treatment of steroid-refractory graft-versus-host disease in adults: a systematic review and meta-analysis. Expert Rev Hematol (2020) 13(5):565–75. doi: 10.1080/17474086.2020.1738214 [DOI] [PubMed] [Google Scholar]
- 20. Wu H, Shi J, Luo Y, Tan Y, Zhang M, Lai X, et al. Evaluation of ruxolitinib for steroid-refractory chronic graft-vs-Host disease after allogeneic hematopoietic stem cell transplantation. JAMA Netw Open (2021) 4(1):e2034750. doi: 10.1001/jamanetworkopen.2020.34750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Risitano AM, Peffault de Latour R. Ruxolitinib for steroid-resistant acute GVHD. Blood (2020) 135(20):1721–2. doi: 10.1182/blood.2020005364 [DOI] [PubMed] [Google Scholar]
- 22. Killock D. REACH2: ruxolitinib for refractory aGvHD. Nat Rev Clin Oncol (2020) 17(8):451. doi: 10.1038/s41571-020-0385-z [DOI] [PubMed] [Google Scholar]
- 23. Alvarnas JC. Ruxolitinib for the treatment of steroid-refractory chronic graft-vs-Host disease-another hopeful step forward. JAMA Netw Open (2021) 4(1):e2035719. doi: 10.1001/jamanetworkopen.2020.35719 [DOI] [PubMed] [Google Scholar]
- 24. González Vicent M, Molina B, González de Pablo J, Castillo A, Díaz MÁ. Ruxolitinib treatment for steroid refractory acute and chronic graft vs host disease in children: Clinical and immunological results. Am J Hematol (2019) 94(3):319–26. doi: 10.1002/ajh.25376 [DOI] [PubMed] [Google Scholar]
- 25. Zhao Y, Wu H, Shi J, Luo Y, Li X, Lan J, et al. Ruxolitinib combined with etanercept induce a rapid response to corticosteroid-refractory severe acute graft vs host disease after allogeneic stem cell transplantation: Results of a multi-center prospective study. Am J Hematol (2020) 95(9):1075–84. doi: 10.1002/ajh.25898 [DOI] [PubMed] [Google Scholar]
- 26. Biliński J, Jasiński M, Tomaszewska A, Lis K, Kacprzyk P, Chmielewska L, et al. Fecal microbiota transplantation with ruxolitinib as a treatment modality for steroid-refractory/dependent acute, gastrointestinal graft-versus-host disease: A case series. Am J Hematol (2021) 96(12):E461–3. doi: 10.1002/ajh.26365 [DOI] [PubMed] [Google Scholar]
- 27. Shen M-Z, Liu X-X, Qiu Z-Y, Xu L-P, Zhang X-H, Wang Y, et al. Efficacy and safety of mesenchymal stem cells treatment for multidrug-resistant graft–host disease after haploidentical allogeneic hematopoietic stem cell transplantation. Ther Adv Hematol (2022) 13:20406207211072838. doi: 10.1177/20406207211072838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao JY, Liu SN, Xu LP, Zhang XH, Wang Y, Chen YH, et al. Ruxolitinib is an effective salvage treatment for multidrug-resistant graft-versus-host disease after haploidentical allogeneic hematopoietic stem cell transplantation without posttransplant cyclophosphamide. Ann Hematol (2021) 100(1):169–80. doi: 10.1007/s00277-020-04273-2 [DOI] [PubMed] [Google Scholar]
- 29. Laisne L, Neven B, Dalle JH, Galambrun C, Esvan M, Renard C, et al. Ruxolitinib in children with steroid-refractory acute graft-versus-host disease: A retrospective multicenter study of the pediatric group of SFGM-TC. Pediatr Blood Cancer (2020) 67(9):e28233. doi: 10.1002/pbc.28233 [DOI] [PubMed] [Google Scholar]
- 30. Moiseev IS, Morozova EV, Bykova TA, Paina OV, Smirnova AG, Dotsenko AA, et al. Long-term outcomes of ruxolitinib therapy in steroid-refractory graft-versus-host disease in children and adults. Bone Marrow Transplant (2020) 55(7):1379–87. doi: 10.1038/s41409-020-0834-4 [DOI] [PubMed] [Google Scholar]
- 31. Mozo Y, Bueno D, Sisinni L, Fernandez-Arroyo A, Rosich B, Martinez AP, et al. Ruxolitinib for steroid-refractory graft versus host disease in pediatric HSCT: high response rate and manageable toxicity. Pediatr Hematol Oncol (2021) 38(4):331–45. doi: 10.1080/08880018.2020.1868637 [DOI] [PubMed] [Google Scholar]
- 32. Uygun V, Karasu G, Daloglu H, Ozturkmen S, Kilic SC, Yalcin K, et al. Ruxolitinib salvage therapy is effective for steroid-refractory graft-versus-host disease in children: A single-center experience. Pediatr Blood Cancer (2020) 67(4):e28190. doi: 10.1002/pbc.28190 [DOI] [PubMed] [Google Scholar]
- 33. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med (2009) 151(4):264–9, W64. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 34. Miller SA, Forrest JL. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J Evidence Based Dental Pract (2001) 1(2):136–41. doi: 10.1016/S1532-3382(01)70024-3 [DOI] [Google Scholar]
- 35. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration (2011). Available from www.handbook.cochrane.org. [Google Scholar]
- 36. Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with r: a practical tutorial. Evid Based Ment Health (2019) 22(4):153–60. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wickham H. ggplot2: elegant graphics for data analysis. Switzerland:Springer; (2016). [Google Scholar]
- 38. Khoury H, Kashyap A, Adkins DR, Brown RA, Miller G, Vij R, et al. Treatment of steroid-resistant acute graft-versus-host disease with anti-thymocyte globulin. Bone Marrow Transplant (2001) 27(10):1059–64. doi: 10.1038/sj.bmt.1703032 [DOI] [PubMed] [Google Scholar]
- 39. Martin PJ, Schoch G, Fisher L, Byers V, Appelbaum FR, McDonald GB, et al. A retrospective analysis of therapy for acute graft-versus-host disease: secondary treatment. Blood (1991) 77(8):1821–8. doi: 10.1182/blood.V77.8.1821.1821 [DOI] [PubMed] [Google Scholar]
- 40. Jagasia M, Greinix H, Robin M, Das-Gupta E, Jacobs R, Savani BN, et al. Extracorporeal photopheresis versus anticytokine therapy as a second-line treatment for steroid-refractory acute GVHD: a multicenter comparative analysis. Biol Blood Marrow Transplant (2013) 19(7):1129–33. doi: 10.1016/j.bbmt.2013.04.018 [DOI] [PubMed] [Google Scholar]
- 41. Greinix HT, Volc-Platzer B, Kalhs P, Fischer G, Rosenmayr A, Keil F, et al. Extracorporeal photochemotherapy in the treatment of severe steroid-refractory acute graft-versus-host disease: a pilot study. Blood (2000) 96(7):2426–31. doi: 10.1182/blood.V96.7.2426 [DOI] [PubMed] [Google Scholar]
- 42. Messina C, Locatelli F, Lanino E, Uderzo C, Zacchello G, Cesaro S, et al. Extracorporeal photochemotherapy for paediatric patients with graft-versus-host disease after haematopoietic stem cell transplantation. Br J Haematol (2003) 122(1):118–27. doi: 10.1046/j.1365-2141.2003.04401.x [DOI] [PubMed] [Google Scholar]
- 43. Abu-Dalle I, Reljic T, Nishihori T, Antar A, Bazarbachi A, Djulbegovic B, et al. Extracorporeal photopheresis in steroid-refractory acute or chronic graft-versus-host disease: results of a systematic review of prospective studies. Biol Blood Marrow Transplant (2014) 20(11):1677–86. doi: 10.1016/j.bbmt.2014.05.017 [DOI] [PubMed] [Google Scholar]
- 44. Zhang H, Chen R, Cheng J, Jin N, Chen B. Systematic review and meta-analysis of prospective studies for ECP treatment in patients with steroid-refractory acute GVHD. Patient Prefer Adherence (2015) 9:105–11. doi: 10.2147/PPA.S76563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim JG, Sohn SK, Kim DH, Lee NY, Suh JS, Lee KS, et al. Different efficacy of mycophenolate mofetil as salvage treatment for acute and chronic GVHD after allogeneic stem cell transplant. Eur J Haematol (2004) 73(1):56–61. doi: 10.1111/j.1600-0609.2004.00247.x [DOI] [PubMed] [Google Scholar]
- 46. Krejci M, Doubek M, Buchler T, Brychtova Y, Vorlicek J, Mayer J. Mycophenolate mofetil for the treatment of acute and chronic steroid-refractory graft-versus-host disease. Ann Hematol (2005) 84(10):681–5. doi: 10.1007/s00277-005-1070-0 [DOI] [PubMed] [Google Scholar]
- 47. Baudard M, Vincent A, Moreau P, Kergueris MF, Harousseau JL, Milpied N. Mycophenolate mofetil for the treatment of acute and chronic GVHD is effective and well tolerated but induces a high risk of infectious complications: a series of 21 BM or PBSC transplant patients. Bone Marrow Transplant (2002) 30(5):287–95. doi: 10.1038/sj.bmt.1703633 [DOI] [PubMed] [Google Scholar]
- 48. Pidala J, Kim J, Perkins J, Field T, Fernandez H, Perez L, et al. Mycophenolate mofetil for the management of steroid-refractory acute graft vs host disease. Bone Marrow Transplant (2010) 45(5):919–24. doi: 10.1038/bmt.2009.252 [DOI] [PubMed] [Google Scholar]
- 49. Takami A, Mochizuki K, Okumura H, Ito S, Suga Y, Yamazaki H, et al. Mycophenolate mofetil is effective and well tolerated in the treatment of refractory acute and chronic graft-versus-host disease. Int J Hematol (2006) 83(1):80–5. doi: 10.1532/IJH97.05111 [DOI] [PubMed] [Google Scholar]
- 50. Furlong T, Martin P, Flowers ME, Carnevale-Schianca F, Yatscoff R, Chauncey T, et al. Therapy with mycophenolate mofetil for refractory acute and chronic GVHD. Bone Marrow Transplant (2009) 44(11):739–48. doi: 10.1038/bmt.2009.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. De Jong CN, Saes L, Klerk CPW, van der Klift M, Cornelissen JJ, Broers AEC. Etanercept for steroid-refractory acute graft-versus-host disease: A single center experience. PloS One (2017) 12(10):e0187184. doi: 10.1371/journal.pone.0187184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Busca A, Locatelli F, Marmont F, Ceretto C, Falda M. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol (2007) 82(1):45–52. doi: 10.1002/ajh.20752 [DOI] [PubMed] [Google Scholar]
- 53. Faraci M, Calevo MG, Giardino S, Leoni M, Ricci E, Castagnola E, et al. Etanercept as treatment of steroid-refractory acute graft-versus-Host disease in pediatric patients. Biol Blood Marrow Transplant (2019) 25(4):743–8. doi: 10.1016/j.bbmt.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 54. Ma CKK, Garcia-Cadenas I, Fox ML, Ai S, Nivison-Smith I, Milliken ST, et al. Poor prognosis in patients with steroid refractory acute graft versus host disease treated with etanercept: a multi-centre analysis. Bone Marrow Transplant (2018) 53(11):1478–82. doi: 10.1038/s41409-018-0215-4 [DOI] [PubMed] [Google Scholar]
- 55. Park JH, Lee HJ, Kim SR, Song GW, Lee SK, Park SY, et al. Etanercept for steroid-refractory acute graft versus host disease following allogeneic hematopoietic stem cell transplantation. Korean J Intern Med (2014) 29(5):630–6. doi: 10.3904/kjim.2014.29.5.630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shen MZ, Li JX, Zhang XH, Xu LP, Wang Y, Liu KY, et al. Meta-analysis of interleukin-2 receptor antagonists as the treatment for steroid-refractory acute graft-Versus-Host disease. Front Immunol (2021) 12:749266. doi: 10.3389/fimmu.2021.749266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Macmillan ML, Couriel D, Weisdorf DJ, Schwab G, Havrilla N, Fleming TR, et al. A phase 2/3 multicenter randomized clinical trial of ABX-CBL versus ATG as secondary therapy for steroid-resistant acute graft-versus-host disease. Blood (2007) 109(6):2657–62. doi: 10.1182/blood-2006-08-013995 [DOI] [PubMed] [Google Scholar]
- 58. Dugan MJ, DeFor TE, Steinbuch M, Filipovich AH, Weisdorf DJ. ATG plus corticosteroid therapy for acute graft-versus-host disease: predictors of response and survival. Ann Hematol (1997) 75(1-2):41–6. doi: 10.1007/s002770050310 [DOI] [PubMed] [Google Scholar]
- 59. Pidala J, Anasetti C. Glucocorticoid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant (2010) 16(11):1504–18. doi: 10.1016/j.bbmt.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 60. Shapiro RM, Antin JH. Therapeutic options for steroid-refractory acute and chronic GVHD: an evolving landscape. Expert Rev Hematol (2020) 13(5):519–32. doi: 10.1080/17474086.2020.1752175 [DOI] [PubMed] [Google Scholar]
- 61. Perfetti P, Carlier P, Strada P, Gualandi F, Occhini D, Van Lint MT, et al. Extracorporeal photopheresis for the treatment of steroid refractory acute GVHD. Bone Marrow Transplant (2008) 42(9):609–17. doi: 10.1038/bmt.2008.221 [DOI] [PubMed] [Google Scholar]
- 62. Elgaz S, Kuci Z, Kuci S, Bonig H, Bader P. Clinical use of mesenchymal stromal cells in the treatment of acute graft-versus-Host disease. Transfus Med Hemother (2019) 46(1):27–34. doi: 10.1159/000496809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kharfan-Dabaja MA, Mhaskar AR, Djulbegovic B, Cutler C, Mohty M, Kumar A. Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biol Blood Marrow Transplant (2009) 15(9):1005–13. doi: 10.1016/j.bbmt.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 64. Olivieri J, Manfredi L, Postacchini L, Tedesco S, Leoni P, Gabrielli A, et al. Consensus recommendations for improvement of unmet clinical needs–the example of chronic graft-versus-host disease: a systematic review and meta-analysis. Lancet Haematol (2015) 2(7):e297–305. doi: 10.1016/S2352-3026(15)00095-2 [DOI] [PubMed] [Google Scholar]
- 65. Liu SN, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Prognostic factors and long-term follow-up of basiliximab for steroid-refractory acute graft-versus-host disease: Updated experience from a large-scale study. Am J Hematol (2020) 95(8):927–36. doi: 10.1002/ajh.25839 [DOI] [PubMed] [Google Scholar]
- 66. Mo XD, Hong SD, Zhao YL, Jiang EL, Chen J, Xu Y, et al. Basiliximab for steroid-refractory acute graft-versus-host disease: A real-world analysis. Am J Hematol (2022) 97(4):458–69. doi: 10.1002/ajh.26475 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.