Abstract
Background
Intracavernosal injection therapy (ICI) is an effective intervention used to treat erectile dysfunction (ED). It has been proposed that caution should be exercised when prescribing ICI to patients currently taking anticoagulants (AC) due to the theoretical increased risk of bleeding, however, there is limited literature describing complication rates of actively anticoagulated patients utilizing ICI.
Aim
We sought to determine whether there was a difference in bleeding and other complications in a cohort of patients using ICI therapy with or without concurrent AC use.
Methods
We reviewed our institutional electronic health record and identified 168 patients who were seen in our clinic from January to August 2020 who had either currently or previously utilized ICI therapy for ED treatment. These patients were surveyed regarding their ICI therapy as well as given the erectile dysfunction inventory for treatment satisfaction questionnaire. Data from 85 patients was obtained; 43 concurrently using AC during ICI therapy and 42 with no AC use. Fisher's exact test for categorical variables and a 2-tailed t-test were used with P < .05 considered to be significant.
Outcome
Documented bleeding events (eg, bruising, hematoma), complications, and mean erectile dysfunction inventory for treatment satisfaction scores were compared between the 2 groups.
Results
There were more absolute bleeding complications in the AC group vs the no AC group, with 3 of 43 AC patients (7%, 95% confidence interval: 2.4–18.6) and 0/42 no AC patients (0%, 95% confidence interval: 0–8.4) experiencing some type of bleeding complication on ICI. However, there was no statistically significant difference found in overall or stratified documented bleeding events and complications between the 2 groups.
Clinical Implications
Patients with concurrent AC usage on ICI therapy reported a higher rate of absolute bleeding complications than our non-AC group.
Strengths and Limitations
The strength of this study is addressing question of safety of ICI therapy in patients with concurrent AC usage. Limitations include single-center retrospective study design and underpowered sample size limiting confidence with which conclusions from data should guide future patient counseling regarding ICI risks.
Conclusion
Findings from a single-center cohort of patients suggest that ICI therapy may be a safe and effective treatment modality for ED in patients with concurrent anticoagulant usage, however, given the higher rate of absolute bleeding events in our AC cohort, future assessment in a higher-powered study is warranted in determining a more accurate estimation of risk or propensity for bleeding complications in patients on AC using ICI therapy.
Blum KA, Mehr JP, Green T, et al. Complication Rates in Patients Using Intracavernosal Injection Therapy for Erectile Dysfunction With or Without Concurrent Anticoagulant Use—A Single-Center, Retrospective Pilot Study. Sex Med 2022;10:100535.
Key Words: Erectile Dysfunction, Intracavernosal Injection, Anticoagulation, Bleeding, Erectile Dysfunction Inventory for Treatment Satisfaction (EDITS) Questionnaire
INTRODUCTION
Intracavernosal injection therapy (ICI) is an effective form of medication used to treat erectile dysfunction (ED), commonly serving as an alternative to oral ED medications, such as PDE5i therapy.1, 2, 3 Current American Urological Association guidelines indicate that men with ED be informed regarding the option of ICI therapy for management and treatment.4 Composition of these injections can vary, ranging from monotherapies, such as papaverine (non-selective PDE5i) and alprostadil (PGE1 stimulating cAMP release), to combination medications, such as Trimix (alprostadil, phentolamine (a non-selective alpha adrenergic antagonist), and papaverine) and Bimix (phentolamine, papaverine).1,5, 6, 7, 8, 9 More recently, Aviptadil, a synthetic Vasoactive Intestinal Polypeptide, has been developed and is primarily used in combination with phentolamine.10 Usage of ICI therapy involves injecting these medications into the corpus cavernosum which then produces an erection.
While not an absolute contraindication, it has been recommended that patients exercise caution in using ICI therapy while on anticoagulants (AC) due to increased propensity for injection site bleeding following injection.11,12 To date, there is limited literature comparing complication rates among patients undergoing ICI usage with or without concurrent AC use. A study by Limoge et al13 did follow a group of 26 patients with ED using Vacuum Erection Devices and ICI therapy for 6 months while anticoagulated on warfarin and found no difference in adverse events when compared to the general urological population. There was no anticoagulated group followed within the study. Various studies evaluating complication and side effect profiles in ED patients undergoing ICI have shown that common issues, as well as reasons for therapy discontinuation, include penile pain, prolonged erection, penile fibrosis, and hematoma.11,14, 15, 16, 17, 18, 19
The aim of this study was to determine: is there a difference in complication rates in patients using ICI therapy with or without concurrent AC use, with special attention to bleeding events, such as hematoma or hemorrhage? Our goal was to determine whether there is a clinically significant difference in these complications rates between the 2 groups and determine patient's treatment satisfaction through the Erectile Dysfunction Inventory for Treatment Satisfaction (EDITS) validated questionnaire.20 ICI is one of the more effective and viable ED treatment options available and dissuading patients on AC from utilizing ICI due to a theoretical increased risk of bleeding may be unnecessarily removing an effective form of treatment from their available options.
METHODS
Following Institutional Review Board Approval, a retrospective review was conducted to identify patients seen in clinic from January to August 2020 who either currently or have previously utilized ICI therapy for ED treatment. For all patients identified, a survey was administered regarding their experience with ICI therapy, including confirmation of ICI usage, length and timeframe of usage, complications experienced, AC usage during ICI usage, and clinical indication for AC if the patient was taking the medication (Figure 1). During this time, patients were also administered the 14-item validated EDITS questionnaire.20 Patients’ outcomes were reviewed until October 2021 to allow for adequate follow-up time to evaluate for the presence of any complications related to ICI usage as well as concurrent anticoagulant medication usage. Complications were quantified per patient as a single complication noted per type irrespective of frequency of occurrence. If a patient reported multiple different complications, such as penile pain and curvature, both were marked as an individual complication. Primary etiology of patient's ED was determined through institutional electronic medical record review and were grouped into 3 cohorts; prostate cancer-related treatment, other cancer treatment, or other causes.
Figure 1.
Patient call checklist used during patient interview.
The primary outcome measured in this study was documented bleeding events, such as bruising, hematoma, or otherwise unspecified bleeding events. Secondary outcomes measured were the presence of nonvasculogenic complications and patient treatment satisfaction using mean EDITS scores between groups.
Statistical analysis was performed using R 3.6.2 (R Foundation For Statistical Computing, Vienna, Austria) where fisher's exact test for categorical variables was used to compare documented bleeding events and complication rates between the AC and non-AC groups. Chi-square and fisher's exact test for categorical variables were used to compare differences in number of patients of each race in the 2 respective groups. Kruskall-Wallis test was used to compare median age and median follow-up time between the 2 groups. A 2-tailed t-test was used to compare mean EDITS scores between the 2 groups. P < .05 was considered to be significant.
RESULTS
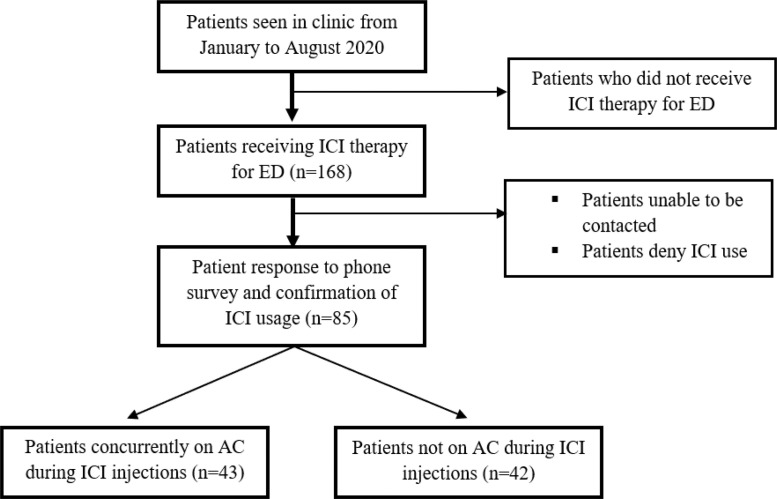
A total of 168 patients met criteria for assessment. Of these, 85 patients had questionnaire data available for review (Figure 2). There were 43 (51%) patients concurrently using AC during ICI therapy and 42 (49%) with no AC use. Median age of the entire cohort was 65 years (interquartile range [IQR] 61–69) with a median follow up time of 20.6 months (IQR 14.0–43.7). Fully abstracted patient demographics between cohorts are available in Table 1.
Figure 2.
Diagram of patient selection for both groups.
Table 1.
Patient demographics
| Variables | Anticoagulation | No anticoagulation | P-value |
|---|---|---|---|
| N | 43 | 42 | - |
| Age, median (IQR) | 65 (61–69) | 63.5 (60–68) | .22 |
| Race, n (%) | - | - | - |
| White | 37 (86) | 29 (69) | .06 |
| Black | 5 (12) | 7 (17) | .51 |
| Hispanic | 1 (2.3) | 4 (9.5) | .16 |
| Asian | 0 (0.0) | 2 (4.8) | .24 |
| Median follow-up time, mo. (IQR) | 20.5 (15.0–52.5) | 20.7 (14.6–39.5) | .32 |
IQR = interquartile range.
The most common ED etiology was prostate cancer related treatment for both AC and non-AC groups at 81% and 91%, respectively. Other cancer related treatment was the etiology for 16% of the AC group and 9.5% for the non-AC group. These treatments included chemotherapeutic or surgical interventions for bladder cancer, rectal cancer, melanoma, and acute myeloid leukemia, and others.
Table 2 itemizes the types of medications used in the AC group as well as documented bleeding events and complications in both groups. The primary AC medication used was ASA-81 with 91% (n = 39) of the group concurrently using this during their ICI injections. There was no statistically significant difference found in overall rate of documented bleeding events between the 2 groups (P= .24). Stratification of bleeding events including bruising, hematoma, and otherwise unspecified bleeding events similarly did not showing a statistically significant difference between groups (P= 1.0). Although there were more absolute bleeding complications in the AC group (n = 3) vs the non-AC group (n = 0), there was no statistically significant difference in the complication rate (P= .24).
Table 2.
Anticoagulation medications and complications
| Variables | Anticoagulation (n = 43) | No anticoagulation (n = 42) | P-value |
|---|---|---|---|
| Anti-coagulation, n (%) | - | - | - |
| ASA-81 | 39 (91) | - | - |
| ASA-325 | 2 (4.7) | - | - |
| Apixaban | 1 (2.3) | - | - |
| Clopidogrel | 1 (2.3) | - | - |
| Documented bleeding, n (%, 95% CI) | - | - | - |
| Penile bruising | 1 (2.3, 0.4–12.1) | 0 (0.0, 0.0–8.4) | 1.0 |
| Penile hematoma | 1 (2.3, 0.4–12.1) | 0 (0.0, 0.0–8.4) | 1.0 |
| Unspecified penile bleeding | 1 (2.3, 0.4–12.1) | 0 (0.0, 0.0–8.4) | 1.0 |
| Complications, n (%, 95% CI) | - | - | - |
| Priapism | 3 (7.0, 2.4–18.6) | 3 (7.1, 2.5–19.0) | 1.0 |
| Penile bleeding | 3 (7.0, 2.4–18.6) | 0 (0.0, 0.0–8.4) | .24 |
| Penile pain | 3 (7.0, 2.4–18.6) | 1 (2.4, 0.4–12.3) | .62 |
| Penile burning | 1 (2.3, 0.4–12.1) | 2 (4.8, 1.3–15.8) | .62 |
| Fever | 0 (0.0, 0.0–8.2) | 1 (2.4, 0.4–12.3) | .50 |
| Penile curvature | 1 (2.3, 0.4–12.1) | 0 (0.0, 0.0–8.4) | 1.0 |
CI = confidence interval.
The most common nonvasculogenic complications in the AC group were priapism (n = 3), penile bleeding (n = 3), and penile pain (n = 3), while the most common complication in the non-AC group was priapism (n = 3). Other complications queried included penile burning, fever, and penile curvature. There was no significant difference found in these complication rates between the 2 groups, specifically penile pain (P= .6), penile curvature (P= 1.0), penile burning (P= .6), priapism (P= 1.0), and fever (P= .5).
Treatment satisfaction that was captured using the EDITS20 validated questionnaire, was completed by 95% (n = 41) of the AC group and 91% (n = 38) of the non-AC group. No statistically significant difference in mean EDITS score was observed among the 2 groups, with a score of 62.47 in the AC cohort and 69.74 in the non-AC cohort, respectively (P= .21).
Additionally, the cohort was compared to those excluded due to being unable to be contacted to determine if significant differences exist between the 2 groups- both overall and stratified by AC and no AC usage. There were 85 patients in our overall cohort, 43 with AC and 42 with no AC usage and in the excluded patient cohort, there were 83 patients, 43 with AC usage and 40 with no AC usage. There was no statistically significant difference in median age in years between the 2 overall groups, 67 (IQR: 61–71) for the included cohort vs 65 (IQR: 61–69) for excluded cohort (P= .29). When stratified by AC (P= .22) and no AC (P= .78) there was no statistically significant difference found in either cohort. There was no statistically significant difference in distribution of race between the 2 groups, both overall (P= .14), and when stratified by AC (P= .09) and no AC (P= .63). Additionally, there was also no statistically significant difference in etiology of ED between the 2 groups, both overall (P= .55), and when stratified by AC (P= .82) and no AC (P= .94).
DISCUSSION
Although our data suggests that there is no statistically significant difference in bleeding events or complication rates in patients utilizing ICI therapy with or without concurrent anticoagulant use, it is extremely important to note that the absolute rate of bleeding events was in fact higher in the AC group vs the no AC group. Specifically, there was no statistically significant difference in bruising, hematoma, or otherwise unspecified bleeding events between the 2 groups and there was no statistically significant difference in nonvasculogenic complications including priapism, penile pain, penile burning, penile curvature, and fever. However, in the AC group, 7% of patients reported some type of bleeding complication vs 0% of patients in the no AC group experiencing any type of bleeding on ICI therapy.
This study is relevant as there is limited literature available on complication rates in patients on anticoagulants utilizing ICI therapy. With little data available to drive clinical decision making on this topic, anecdotal experience and the theoretical risk of bleeding have been primarily guiding recommendations against ICI in anticoagulated ED patients. One such study reported in 1996 attempted to address this clinical question and included data from 26 men utilizing a Vacuum Erection Device and ICI therapy while anticoagulated on warfarin over 6 months.13 The authors observed 3 cases of ecchymosis and 1 case of priapism from the injections. This rate of ecchymosis, 12% (n = 3), is higher than the rate in our AC group, 7.0% (n = 3), however we did observe a higher proportion of priapism, 7.0% (n = 3) vs 3.8% (n = 1) in their study.13 Furthermore, this study was limited as the authors compared the difference in complication rates to rates in the general urological population in previously published literature rather than in a non-AC comparison group within the study itself.
When observing rates of complications in our study, it is important to note the 95% confidence interval (CI), which is quite wide in several metrics measured in our data. For instance, the 95% CI in our documentation of bleeding events ranges from 0.4% to 12.1%. When looking at overall rates of bleeding in the AC cohort, that interval increases to 2.4–18.6%. While it is likely, or possibly assumed, that this range should narrow with a higher-powered study, it remains a limitation in drawing definitive conclusions from our data especially when comparing our complications to a non-AC group. Even if the upper bounds of such 95% CI do not reflect observed reality in a clinical setting, it must be considered in the absence of more robust data.
ICI has been and will continue to be an effective treatment in the management of ED, however, the increased rate of absolute contraindications in our AC cohort indicates the importance that patients are counseled on potential risks that injection therapy for ED treatment possesses. The absence of a statistically significant difference in bleeding and complication rates between our AC and no AC groups is likely due to the limited sample size and power of our study and should not serve as the clinical takeaway of findings presented here. Our results should instead serve as a reminder that patients who are on AC be counseled and educated appropriately on potential side effects and complications that may occur while on ICI therapy. The exact likelihood of these complications in clinical practice is less clear, however, with a 95% CI of bleeding complications reaching up to 19% in our AC group compared to 8% in our no AC group, this is still likely a significant enough rate such that patients continue to be advised on potential risks of bleeding.
Patients possessing certain contraindications to ICI therapy need to be identified accurately and counseled appropriately. Absolute contraindications include known hypersensitivity to the medications involved, diseases predisposing to priapism, such as sickle hemoglobinopathies, severe coagulopathies, history of bleeding diathesis, and penile implants.5,11,12,21 However, it is equally important that patients are not incorrectly excluded from accessing various modalities of ED treatment, ICI included, in the absence of supporting data. While not expressly an absolute contraindication, it has been advised that patients taking AC medications exercise caution when using injection therapy due to the increased propensity for bleeding. This concern has made physicians hesitant to prescribe this potent and effective ED medication. Indeed, the FDA approval for Alprostadil (marketed as CAVERJECT by Pfizer Inc., New York, NY, USA, and under the brand name EDEX by Endo Pharmaceuticals, Malvern, PA, USA) notes concurrent usage with anticoagulants as one of their warnings and precautions for consideration prior to usage of the medication.11 This recommendation, while intended to be in the best interest of the patient, is not data driven, evidenced by the paucity of literature on the topic. The solution for prospective ICI patients currently on AC is likely at the intersection of appropriate caution and risk-stratification while continuing to confidently utilize the medication following patient-provider decision making and not opting for other ED treatments solely due to AC status. Neither completely avoiding ICI in AC patients nor absence of any mention of bleeding risk are prudent or safe given the currently available literature.
The lack of available data was a motivating force behind this current study to determine whether this standing recommendation of avoiding ICI therapy solely due to a patient's anticoagulant usage is unnecessary and potentially harmful to ED patient treatment. The data of our study suggests that while complication rates, and specifically bleeding events, did not statistically significantly differ between AC and non-AC patients using ICI therapy, given the difference in absolute bleeding rates, patients should continue to be educated on the bleeding risks of ICI therapy on concurrent AC. However, while our study is one of the largest contemporary datasets to date analyzing complication rates in concurrently anticoagulated patients on ICI, our results are insufficient to provide conclusive evidence to confidently to dissuade the concomitant use of ICI and anticoagulants in ED patients. This data will need to be validated through higher powered studies with a larger cohort.
Further follow-up should be geared towards determining whether the difference in complication rates between the AC and non-AC groups that we observed, including no statistically significant difference but a difference in absolute rate, is within the acceptable range for AC men on ICI. More specifically, even if differences in absolute complication rates are found in future higher-powered studies, does this rate still fall below the threshold of statistical significance such that it not be deemed a risk or deterrent to treatment? Until then, and perhaps even in the presence of such data, patients should continue to be appropriately counseled on the risk of such complications.
This study has limitations, including those that are inherent in a single-center retrospective study design as well as mentioned above. Additional limitations include the study's sample size of 85 patients, which was in part due to our 49% response rate of patients in our original inclusion criteria. Therefore, it is possible that those patients unable to be reached for a survey could have affected this study's outcomes compared to our current analyses. To understand if the patients not included in our study differed in any substantial way from those included, we compared median age, race, and etiology of ED between both overall cohorts, as well as between the AC groups and the non-AC groups. Of the 83 patients not included in our study, 43 utilized AC concurrently on ICI while 40 did not use AC. There was no significant difference in median age, race or etiology of ED between the 2 total cohorts as well as when stratified by AC usage or no AC usage.
Patients were also not stratified by the type of AC medication and doing so would have allowed for more granular data analyses to be performed to determine if certain medications exhibit significantly higher rates of complications or bleeding events. Nevertheless, despite these limitations the benefit of our current study is that it reports on one of the largest contemporary datasets available.
Future investigation on this topic would benefit from a detailed focus on comparison of bleeding complications, side-effects, and outcomes of ICI therapy patients with concurrent anticoagulation medication usage to those not on AC in a larger and more highly powered cohort.
CONCLUSION
Findings from a single-center cohort of patients suggest that ICI therapy may be a safe and effective treatment modality for ED in patients with concurrent anticoagulant usage. However, given our observed difference in absolute complication rates even in the absence of a statistically significant difference between AC and no AC groups, further investigation should be conducted prior to confidently determining that anticoagulation medications not serve as a deterrent for utilizing ICI therapy in the treatment of ED. Future management of patients with ED on AC considering ICI should involve appropriate education regarding bleeding and complication risk, while also not unnecessarily avoiding what has shown to be an effective ED treatment option. Future assessment in a higher-powered study is warranted in determining a more accurate estimation of risk for bleeding complications in patients on AC using ICI therapy.
STATEMENT OF AUTHORSHIP
Justin P Mehr: methodology, software, validation, formal analysis, investigation, data curation, writing–review and editing, visualization, and project administration. Kyle A Blum: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing-original draft, writing–review and editing, visualization, and administration. Lauren Conroy: writing–review and editing. Run Wang: conceptualization, methodology, resources, writing-original draft, writing–review and editing, supervision, and project administration. Sravan Panuganti: conceptualization. Travis Green: conceptualization, methodology, validation, formal analysis, investigation, resources, writing-original draft, writing–review and editing, visualization, supervision, and project administration. Vanessa Marino, Daniel Kim, Kailash Panchapakesan, and Liam Murphy: investigation and data curation.
Footnotes
Conflict of Interest: Run Wang, MD, FACS is a consultant for Boston Scientific, Coloplast, and Teleflex.
Funding: No outside funding was provided for this project.
REFERENCES
- 1.Chung E. A review of current and emerging therapeutic options for erectile dysfunction. Med Sci (Basel) 2019;7 doi: 10.3390/medsci7090091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bearelly P, Phillips EA, Pan S, et al. Long-term intracavernosal injection therapy: Treatment efficacy and patient satisfaction. Int J Impot Res. 2020;32:345–351. doi: 10.1038/s41443-019-0186-z. [DOI] [PubMed] [Google Scholar]
- 3.El-Sakka AI. What is the current role of intracavernosal injection in management of erectile dysfunction? Int J Impot Res. 2016;28:88–95. doi: 10.1038/ijir.2016.14. [DOI] [PubMed] [Google Scholar]
- 4.Burnett AL, Nehra A, Breau RH, et al. Erectile dysfunction: AUA guideline. J Urol. 2018;200:633–641. doi: 10.1016/j.juro.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Belew D, Klaassen Z, Lewis RW. Intracavernosal injection for the diagnosis, evaluation, and treatment of erectile dysfunction: A review. Sex Med Rev. 2015;3:11–23. doi: 10.1002/smrj.35. [DOI] [PubMed] [Google Scholar]
- 6.Ledda A. Erectile dysfunction: Intracavernous treatment. Curr Med Res Opin. 2000;16(Suppl. 1):s59–s62. doi: 10.1185/0300799009117041. [DOI] [PubMed] [Google Scholar]
- 7.Zentgraf M, Baccouche M, Jünemann KP. Diagnosis and therapy of erectile dysfunction using papaverine and phentolamine. Urol Int. 1988;43:65–75. doi: 10.1159/000281308. [DOI] [PubMed] [Google Scholar]
- 8.Moncada I, Martinez-Salamanca J, Ruiz-Castañe E, et al. Combination therapy for erectile dysfunction involving a PDE5 inhibitor and alprostadil. Int J Impot Res. 2018;30:203–208. doi: 10.1038/s41443-018-0046-2. [DOI] [PubMed] [Google Scholar]
- 9.Seyam R, Mohamed K, Akhras AA, et al. A prospective randomized study to optimize the dosage of trimix ingredients and compare its efficacy and safety with prostaglandin E1. Int J Impot Res. 2005;17:346–353. doi: 10.1038/sj.ijir.3901313. [DOI] [PubMed] [Google Scholar]
- 10.Duncan C, Omran GJ, Teh J, et al. Erectile dysfunction: A global review of intracavernosal injectables. World J Urol. 2019;37:1007–1014. doi: 10.1007/s00345-019-02727-5. [DOI] [PubMed] [Google Scholar]
- 11.Pfizer Inc. Caverject (Alprostadil Injection) [package insert].U.S. Food and Drug Administration, 2017, Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020379s032lbl.pdf. Accessed December 30, 2021.
- 12.Sharlip ID. Evaluation and nonsurgical management of erectile dysfunction. Urol Clin North Am. 1998;25:647–659. doi: 10.1016/s0094-0143(05)70054-9. [DOI] [PubMed] [Google Scholar]
- 13.Limoge JP, Olins E, Henderson D, et al. Minimally invasive therapies in the treatment of erectile dysfunction in anticoagulated cases: a study of satisfaction and safety. J Urol. 1996;155:1276–1279. doi: 10.1016/s0022-5347(01)66241-4. [DOI] [PubMed] [Google Scholar]
- 14.Casabé A, Bechara A, Cheliz G, et al. Drop-out reasons and complications in self-injection therapy with a triple vasoactive drug mixture in sexual erectile dysfunction. Int J Impot Res. 1998;10:5–9. doi: 10.1038/sj.ijir.3900307. [DOI] [PubMed] [Google Scholar]
- 15.Kunelius P, Lukkarinen O. Intracavernous self-injection of prostaglandin E1 in the treatment of erectile dysfunction. Int J Impot Res. 1999;11:21–24. doi: 10.1038/sj.ijir.3900377. [DOI] [PubMed] [Google Scholar]
- 16.Tsai YS, Lin JS, Lin YM. Safety and efficacy of alprostadil sterile powder (S. Po., CAVERJECT) in diabetic patients with erectile dysfunction. Eur Urol. 2000;38:177–183. doi: 10.1159/000020277. [DOI] [PubMed] [Google Scholar]
- 17.Chen RN, Lakin MM, Montague DK, et al. Penile scarring with intracavernous injection therapy using prostaglandin E1: A risk factor analysis. J Urol. 1996;155:138–140. [PubMed] [Google Scholar]
- 18.Chew KK, Stuckey BG. Clinical course of penile fibrosis in intracavernosal prostaglandin E1 injection therapy: A follow-up of 44 patients. Int J Impot Res. 2003;15:94–98. doi: 10.1038/sj.ijir.3900951. [DOI] [PubMed] [Google Scholar]
- 19.He L, Wen J, Jiang X, et al. Long-term efficacy and safety of self-intracavernous injection of prostaglandin E1 for treatment of erectile dysfunction in China. Andrologia. 2011;43:208–212. doi: 10.1111/j.1439-0272.2010.01091.x. [DOI] [PubMed] [Google Scholar]
- 20.Althof SE, Corty EW, Levine SB, et al. EDITS: development of questionnaires for evaluating satisfaction with treatments for erectile dysfunction. Urology. 1999;53:793–799. doi: 10.1016/s0090-4295(98)00582-2. [DOI] [PubMed] [Google Scholar]
- 21.Jain A, OA Iqbal, Alprostadil, in StatPearls. 2022, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.: Treasure Island (FL).