Abstract
To investigate the relationships between communicative and critical health literacy (CCHL) and anxiety and depressive symptoms (ADs) in pregnant women during the coronavirus disease 2019 (COVID-19) pandemic. A cross-sectional study was conducted and 5466 pregnant women responded in Japan in September 2020. A Kessler 6 scale (K6) score ≥ 10, an Edinburgh Postnatal Depression Scale (EPDS) score ≥ 13, and four CCHL groups were analyzed using a logistic regression model and trend test. The proportions of pregnant women with a K6 score ≥ 10 and EPDS score ≥ 13 were 13.5 and 15.4%, respectively. In comparisons with the low CCHL group, the adjusted odds ratio (95% CI) for anxiety symptoms was 0.770 (0.604–0.982) in the high CCHL group, while those for depressive symptoms were 0.777 (0.639–0.946), 0.665 (0.537–0.824), and 0.666 (0.529–0.838) in the lower, higher, and high CCHL groups (all p < 0.05), respectively, after adjustments for potential confounding factors, such as age, weeks of gestation, complications, history, number of children, marital status, education, employment, and income. Higher CCHL was associated with significantly lower adjusted odds ratios for anxiety (p for trend = 0.019) and depressive symptoms (p for trend < 0.001). These results suggest a relationship between CCHL and ADs in pregnant women during the COVID-19 pandemic.
Subject terms: Anxiety, Depression, Preventive medicine
Introduction
In 2020, a worldwide pandemic of novel coronavirus disease 2019 (COVID-19) emerged from Wuhan. Due to mandatory changes in lifestyles under the declaration of a lockdown or emergency state, greater mental health difficulties have been reported in general populations1–3.
After the COVID-19 pandemic, the prevalence of major depressive symptoms with an Edinburgh postnatal depression scale (EPDS) score ≥ 13 was 15% for pregnant women in Europe countries, while that of a generalized anxiety symptom score ≥ 10 was 11%4. The prevalence of depression with an EPDS score ≥ 10 was 16.1% in Wuhan, China, and that of anxiety with a self-rating anxiety scale score ≥ 50 was 13.9%5. In a national survey conducted in Mexico, 17.5% of participants had an EPDS score of more than 14 points6. In Japan, the prevalence of depression with an EPDS score ≥ 10 was reportedly 35.5% in pregnant women, while that of anxiety with a Kessler 6 scale (K6) score ≥ 5 was 39.9%7. A meta-analysis revealed that the pooled prevalence was 18.7% for anxiety and 25.1% for depression8. Although these studies were conducted in various countries and used different cut-off points for EDPS as well as different anxiety scales, anxiety and depressive symptoms were commonly reported in pregnant women after the COVID-19 pandemic.
Since the COVID-19 pandemic, some studies have suggested the urgent need to screen and treat mental health conditions9,10 and provide obstetric counseling and psychological support7,10 for pregnant women with high anxiety and depression. Health literacy is a personal ability to find, assess, and use health information. Previous studies reported the importance of ensuring a correct understanding of health information and relevant behavior at the onset of novel infectious diseases11–14. Although a study reported health literacy, anxiety, and depression among pregnant women15, the impact of health literacy for COVID-19 on anxiety and depressive symptoms in pregnant women remains unclear. We hypothesized that health literacy may be related to anxiety and depressive symptoms and that an approach to health literacy that reduces these symptoms needs to be proposed. Therefore, the aim of the present study was to the clarify relationships between anxiety and depressive symptoms and communicative and critical health literacy (CCHL) in pregnant women during the COVID-19 pandemic.
Results
A total of 5739 pregnant women answered the online survey, and 5466 (95.2%) were analyzed after the exclusion of those with missing values (age = 7, expected delivery date = 5, CCHL = 81, K6 = 167, and EPDS = 13).
The characteristics and backgrounds of participants are shown in Table 1. The proportions of pregnant women in their 30 s, after 28 weeks of gestation, and pregnant for the first time were 63.9, 56.6, and 64.7%, respectively. Pregnant women with complications were those with threatened premature delivery (7.8%), gestational diabetes mellitus (4.2%), placental malposition (1.7%), and multiple pregnancies (1.4%), respectively. The proportion of participants with fetal disorder or fetal growth restriction, gestational hypertension, and other complications were less than 1%. Pregnant women with a medical history of 1% or more included miscarriage (19.4%), mental disease (4.3%), premature birth (1.9%), and fatality (1.5%). A total of 94.5% of pregnant women were married and living together, 57.6% were educated beyond university level, 19.5% were unemployed, and 46.0% had an annual household income of less than 7 million (Yen). Mean (SD) CCHL, K6, and EPDS were 17.5 (3.6), 4.6 (4.4), and 7.0 (5.5), respectively.
Table 1.
Characteristics of pregnant woman (n = 5466).
| n | % | |
|---|---|---|
| Age group | ||
| ≤ 19 yr | 14 | 0.3 |
| 20–29 yr | 1608 | 29.4 |
| 30–39 yr | 3493 | 63.9 |
| 40–49 yr | 351 | 6.4 |
| Weeks of gestation | ||
| Early pregnancy (≤ 15 wk) | 851 | 15.6 |
| Mid-pregnancy (16–27 wk) | 1520 | 27.8 |
| Late pregnancy (≥ 28 wk) | 3095 | 56.6 |
| Number of children born | ||
| 0 | 3535 | 64.7 |
| 1 | 1388 | 25.4 |
| 2 | 427 | 7.8 |
| ≥ 3 | 101 | 1.8 |
| Unknown | 15 | 0.3 |
| Complications during pregnancya, yes | ||
| Threatened premature delivery | 424 | 7.8 |
| Fetal disorder or fetal growth restriction | 51 | 0.9 |
| Placental malposition | 93 | 1.7 |
| Multiple pregnancy | 76 | 1.4 |
| Gestational hypertension | 29 | 0.5 |
| Gestational diabetes mellitus | 231 | 4.2 |
| Other | 970 | 17.7 |
| Medical historya | ||
| Miscarriage | 1061 | 19.4 |
| Fatal death | 84 | 1.5 |
| Premature birth | 106 | 1.9 |
| Hypertension | 38 | 0.7 |
| Diabetes mellitus | 25 | 0.5 |
| Mental disease | 235 | 4.3 |
| Other | 1098 | 20.1 |
| Marital status | ||
| Married and live together | 5165 | 94.5 |
| Married and separated | 159 | 2.9 |
| Unmarried with a partner | 63 | 1.2 |
| Unmarried without a partner | 57 | 1.0 |
| Other | 6 | 0.1 |
| Unknown | 16 | 0.3 |
| Education | ||
| Junior high school | 96 | 1.8 |
| High school | 803 | 14.7 |
| College | 1389 | 25.4 |
| University | 2748 | 50.3 |
| Graduate school | 397 | 7.3 |
| Unknown | 33 | 0.6 |
| Current employment status | ||
| Full-time | 2330 | 42.6 |
| Part-time | 482 | 8.8 |
| Housewife or student | 1536 | 28.1 |
| On leave | 990 | 18.1 |
| Unemployed | 75 | 1.4 |
| Unknown | 53 | 1 |
| Household income, yen | ||
| < 1 million | 34 | 0.6 |
| 1–3.99 million | 626 | 11.5 |
| 4–6.99 million | 1854 | 33.9 |
| 7–9.99 million | 1361 | 24.9 |
| ≥ 10 million | 964 | 17.6 |
| Unknown | 627 | 11.5 |
| K6, mean, SD, score | 4.6 | 4.4 |
| EPDS, mean, SD, score | 7.0 | 5.5 |
| CCHL scale, mean, SD, score | 17.5 | 3.6 |
CCHL scale communicative and critical health literacy scale, K6 Kessler 6 scale, EPDS Edinburgh postnatal depression scale.
aMultiple answers.
Among pregnant women, 744 (13.6%) showed anxiety symptoms and 844 (15.4%) had depressive symptoms (Table 2). Anxiety and depressive symptoms (ADs) were related to age, the number of children born, two complications during pregnancy (threatened premature delivery and fetal disorder or fetal growth restriction), a history of mental disease, marital status, education level, employment status, household income, and CCHL, while anxiety symptoms were related to a history of hypertension (Table 2).
Table 2.
Comparison of anxiety and depressive symptoms for each variable in pregnant woman.
| K6 scores | p valuea | EPDS scores | p valuea | |||
|---|---|---|---|---|---|---|
| < 10 | ≥ 10 | < 13 | ≥ 13 | |||
| n (%) | n (%) | n (%) | n (%) | |||
| All | 4722 (86.4) | 744 (13.6) | 4622 (84.6) | 844 (15.4) | ||
| Age group | ||||||
| ≤ 19 yr | 9 (0.2) | 5 (0.7) | < 0.001 | 8 (0.2) | 6 (0.7) | < 0.001 |
| 20–29 yr | 1344 (28.5) | 264 (35.5) | 1288 (27.9) | 320 (37.9) | ||
| 30–39 yr | 3054 (64.7) | 439 (59.0) | 3012 (65.2) | 481 (57.0) | ||
| 40–49 yr | 315 (6.7) | 36 (4.8) | 314 (6.8) | 37 (4.4) | ||
| Weeks of gestation | ||||||
| Early pregnancy (≤ 15 wk) | 719 (15.2) | 132 (17.7) | 0.123 | 705 (15.3) | 146 (17.3) | 0.202 |
| Mid-pregnancy (16–27 wk) | 1307 (27.7) | 213 (28.6) | 1279 (27.7) | 241 (28.6) | ||
| Late pregnancy (≥ 28 wk) | 2696 (57.1) | 399 (53.6) | 2638 (57.1) | 457 (54.1) | ||
| Number of children born | ||||||
| 0 | 3029 (64.1) | 506 (68.0) | < 0.001 | 2946 (63.7) | 589 (69.8) | < 0.001 |
| 1 | 1218 (25.8) | 170 (22.8) | 1218 (26.4) | 170 (20.1) | ||
| 2 | 386 (8.2) | 41 (5.5) | 377 (8.2) | 50 (5.9) | ||
| ≥ 3 | 79 (1.7) | 22 (3.0) | 73 (1.6) | 28 (3.3) | ||
| Unknown | 10 (0.2) | 5 (0.7) | 8 (0.2) | 7 (0.8) | ||
| Complications during pregnancy, yes | ||||||
| Threatened premature delivery | 352 (7.5) | 72 (9.7) | 0.035 | 323 (7.0) | 101 (12.0) | < 0.001 |
| Fetal disorder or fetal growth restriction | 38 (0.8) | 13 (1.7) | 0.013 | 35 (0.8) | 16 (1.9) | 0.002 |
| Placental malposition | 85 (1.8) | 8 (1.1) | 0.155 | 80 (1.7) | 13 (1.5) | 0.694 |
| Multiple pregnancy | 68 (1.4) | 8 (1.1) | 0.430 | 59 (1.3) | 17 (2.0) | 0.092 |
| Gestational hypertension | 25 (0.5) | 4 (0.5) | 0.570b | 23 (0.5) | 6 (0.7) | 0.285b |
| Gestational diabetes mellitus | 209 (4.4) | 22 (3.0) | 0.064 | 196 (4.2) | 35 (4.1) | 0.901 |
| Other | 832 (17.6) | 138 (18.5) | 0.538 | 819 (17.7) | 151 (17.9) | 0.905 |
| Medical history, yes | ||||||
| Miscarriage | 927 (19.6) | 134 (18.0) | 0.299 | 913 (19.8) | 148 (17.5) | 0.134 |
| Fatal death | 73 (1.5) | 11 (1.5) | 0.899 | 70 (1.5) | 14 (1.7) | 0.754 |
| Premature birth | 93 (2.0) | 13 (1.7) | 0.683 | 92 (2.0) | 14 (1.7) | 0.520 |
| Hypertension | 34 (0.7) | 4 (0.5) | 0.578 | 26 (0.6) | 12 (1.4) | 0.006 |
| Diabetes mellitus | 22 (0.5) | 3 (0.4) | 1.000a | 19 (0.4) | 6 (0.7) | 0.261b |
| Mental disease | 137 (2.9) | 98 (13.2) | < 0.001 | 144 (3.1) | 91 (10.8) | < 0.001 |
| Other | 943 (20.0) | 155 (20.8) | 0.586 | 932 (20.2) | 166 (19.7) | 0.741 |
| Marital status | ||||||
| Married and live together | 4486 (95.0) | 679 (91.3) | < 0.001 | 4403 (95.3) | 762 (90.3) | < 0.001 |
| Married and separated | 133 (2.8) | 26 (3.5) | 120 (2.6) | 39 (4.6) | ||
| Unmarried with a partner | 47 (1.0) | 16 (2.2) | 41 (0.9) | 22 (2.6) | ||
| Unmarried without a partner | 39 (0.8) | 18 (2.4) | 43 (0.9) | 14 (1.7) | ||
| Other | 5 (0.1) | 1 (0.1) | 5 (0.1) | 1 (0.1) | ||
| Unknown | 12 (0.3) | 4 (0.5) | 10 (0.2) | 6 (0.7) | ||
| Education | ||||||
| Junior high school | 69 (1.5) | 27 (3.6) | < 0.001 | 62 (1.3) | 34 (4.0) | < 0.001 |
| High school | 670 (14.2) | 133 (17.9) | 630 (13.6) | 173 (20.5) | ||
| College | 1199 (25.4) | 190 (25.5) | 1158 (25.1) | 231 (27.4) | ||
| University | 2401 (50.8) | 347 (46.6) | 2383 (51.5) | 366 (43.3) | ||
| Graduate school | 355 (7.5) | 42 (5.6) | 361 (7.8) | 36 (4.3) | ||
| Unknown | 28 (0.6) | 5 (0.7) | 29 (0.6) | 4 (0.5) | ||
| Current employment status | ||||||
| Full-time | 2073 (43.9) | 257 (34.5) | < 0.001 | 2021 (43.7) | 309 (36.6) | < 0.001 |
| Part-time | 419 (8.9) | 63 (8.5) | 414 (9.0) | 68 (8.1) | ||
| Housewife or student | 1291 (27.3) | 245 (32.9) | 1287 (27.8) | 249 (29.5) | ||
| On leave | 842 (17.8) | 148 (19.9) | 814 (17.6) | 176 (20.9) | ||
| Unemployed | 54 (1.1) | 21 (2.8) | 46 (1.0) | 29 (3.4) | ||
| Unknown | 43 (0.9) | 10 (11.3) | 40 (0.9) | 13 (1.5) | ||
| Household income, yen | ||||||
| < 1 million | 27 (0.6) | 7 (0.9) | < 0.001 | 23 (0.5) | 11 (1.3) | < 0.001 |
| 1–3.99 million | 503 (10.7) | 123 (16.5) | 474 (10.3) | 152 (18.0) | ||
| 4–6.99 million | 1570 (33.2) | 284 (38.2) | 1543 (33.4) | 311 (36.8) | ||
| 7–9.99 million | 1220 (25.8) | 141 (19.0) | 1196 (25.9) | 165 (19.5) | ||
| ≥ 10 million | 860 (18.2) | 104 (14.0) | 862 (18.6) | 102 (12.1) | ||
| Unknown | 542 (11.5) | 85 (11.4) | 524 (11.3) | 103 (12.2) | ||
| CCHL scale | ||||||
| Low (first quartile) | 1245 (26.4) | 225 (30.2) | 0.048 | 1184 (25.6) | 286 (26.9) | < 0.001 |
| Lower (second quartile) | 1395 (29.5) | 228 (30.6) | 1376 (29.8) | 247 (29.7) | ||
| Higher (third quartile) | 1147 (24.3) | 166 (22.3) | 1141 (24.7) | 172 (24.0) | ||
| High (fourth quartile) | 935 (19.8) | 125 (16.8) | 621 (19.9) | 139 (19.4) | ||
CCHL scale communicative and critical health literacy scale, K6 Kessler 6 scale, EPDS Edinburgh postnatal depression scale.
aUsing a Chi-squared test.
bUsing Fisher’s exact test.
Table 3 shows the crude odd ratios (95% confidence intervals, CI) for ADs for each variable in pregnant woman using a univariate logistic regression model analysis. Pregnant women of an older age, higher education level, and higher CCHL had less ADs. Women in late pregnancy had less anxiety symptoms than those in early pregnancy. In comparisons with first-time pregnancies, pregnant women with one or two children had fewer ADs, while those with three children had more ADs. Pregnant women with complications (threatened premature delivery and fetal disorder or fetal growth restriction), a history of mental disease, separated and unmarried, housewives or students, those on leave, and those who were unemployed had more ADs. A history of hypertension was associated with more severe depressive syndrome.
Table 3.
Relationships between anxiety and depressive symptoms for each variable in pregnant woman (n = 5466).
| Univariable logistic regression model analysis | ||||||
|---|---|---|---|---|---|---|
| K6 score ≥ 10 | p value | EPDS score ≥ 13 | p value | |||
| CORs | 95% CI | CORs | 95% CI | |||
| Age group | ||||||
| ≤ 19 yr | 1.000 | 1.000 | ||||
| 20–29 yr | 0.354 | 0.118–1.063 | 0.064 | 0.331 | 0.114–0.961 | 0.042 |
| 30–39 yr | 0.259 | 0.086–0.776 | 0.016 | 0.213 | 0.074–0.616 | 0.004 |
| 40–49 yr | 0.206 | 0.065–0.647 | 0.007 | 0.157 | 0.052–0.478 | 0.001 |
| Weeks of gestation | ||||||
| Early pregnancy (≤ 15 wk) | 1.000 | 1.000 | ||||
| Mid-pregnancy (16–27 wk) | 0.888 | 0.701–1.123 | 0.573 | 0.910 | 0.726–1.140 | 0.411 |
| Late pregnancy (≥ 28 wk) | 0.806 | 0.651–0.998 | 0.048 | 0.837 | 0.682–1.026 | 0.086 |
| Number of children born | ||||||
| 0 | 1.000 | 1.000 | ||||
| 1 | 0.836 | 0.694–1.006 | 0.058 | 0.698 | 0.581–0.838 | < 0.001 |
| 2 | 0.636 | 0.455–0.889 | 0.008 | 0.663 | 0.488–0.903 | 0.009 |
| ≥ 3 | 1.667 | 1.030–2.699 | 0.038 | 1.918 | 1.230–2.992 | 0.004 |
| Unknown | 2.993 | 1.019–8.793 | 0.046 | 4.376 | 1.581–12.115 | 0.004 |
| Complications during pregnancy, yes vs no | ||||||
| Threatened premature delivery | 1.330 | 1.019–1.736 | 0.036 | 1.809 | 1.428–2.292 | < 0.001 |
| Fetal disorder or fetal growth restriction | 2.192 | 1.162–4.135 | 0.015 | 2.533 | 1.395–4.597 | 0.002 |
| Placental malposition | 0.593 | 0.286–1.229 | 0.160 | 0.888 | 0.492–1.603 | 0.694 |
| Multiple pregnancy | 0.744 | 0.356–1.554 | 0.431 | 1.590 | 0.922–2.741 | 0.095 |
| Gestational hypertension | 1.016 | 0.352–2.926 | 0.977 | 1.432 | 0.581–3.527 | 0.435 |
| Gestational diabetes mellitus | 0.658 | 0.421–1.028 | 0.066 | 0.977 | 0.677–1.411 | 0.901 |
| Other | 1.065 | 0.872–1.300 | 0.538 | 1.012 | 0.835–1.225 | 0.905 |
| Medical history, yes vs no | ||||||
| Miscarriage | 0.899 | 0.736–1.099 | 0.299 | 0.864 | 0.713–1.046 | 0.134 |
| Fatal death | 0.956 | 0.505–1.810 | 0.889 | 1.097 | 0.615–1.956 | 0.754 |
| Premature birth | 0.885 | 0.493–1.590 | 0.683 | 0.831 | 0.471–1.464 | 0.521 |
| Hypertension | 0.745 | 0.264–2.106 | 0.579 | 2.550 | 1.281–5.073 | 0.008 |
| Diabetes mellitus | 0.865 | 0.258–2.897 | 0.814 | 1.735 | 0.691–4.356 | 0.241 |
| Mental disease | 5.077 | 3.868–6.655 | < 0.001 | 3.758 | 2.859–4.941 | < 0.001 |
| Other | 1.055 | 0.871–1.276 | 0.585 | 0.969 | 0.806–1.166 | 0.841 |
| Marital status | ||||||
| Married and live together | 1.000 | 1.000 | ||||
| Married and separated | 1.292 | 0.842–1.981 | 0.241 | 1.878 | 1.298–2.717 | < 0.001 |
| Unmarried with a partner | 2.249 | 1.268–3.989 | 0.006 | 3.101 | 1.837–5.234 | < 0.001 |
| Unmarried without a partner | 3.049 | 1.734–5.361 | < 0.001 | 1.881 | 1.024–3.455 | 0.042 |
| Other | 1.321 | 0.153–11.327 | 0.799 | 1.156 | 0.135–9.905 | 0.895 |
| Unknown | 2.202 | 0.708–6.848 | 0.173 | 3.467 | 1.256–9.567 | 0.016 |
| Education | ||||||
| Junior high school | 1.000 | 1.000 | ||||
| High school | 0.507 | 0.313–0.822 | 0.006 | 0.501 | 0.319–0.786 | 0.003 |
| College | 0.405 | 0.253–0.648 | < 0.001 | 0.364 | 0.234–0.566 | < 0.001 |
| University | 0.369 | 0.233–0.584 | < 0.001 | 0.280 | 0.182–0.432 | < 0.001 |
| Graduate school | 0.302 | 0.175–0.523 | < 0.001 | 0.182 | 0.106–0.312 | < 0.001 |
| Unknown | 0.456 | 0.160–1.305 | 0.143 | 0.252 | 0.082–0.775 | 0.016 |
| Current employment status | ||||||
| Full-time | 1.000 | 1.000 | ||||
| Part-time | 1.213 | 0.903–1.629 | 0.200 | 1.074 | 0.809–1.426 | 0.620 |
| Housewife or student | 1.531 | 1.268–1.848 | < 0.001 | 1.265 | 1.056–1.516 | 0.011 |
| On leave | 1.418 | 1.141–1.762 | 0.002 | 1.414 | 1.155–1.731 | < 0.001 |
| Unemployed | 3.137 | 1.864–5.279 | < 0.001 | 4.123 | 2.552–6.663 | < 0.001 |
| Unknown | 1.876 | 0.931–3.778 | 0.078 | 2.126 | 1.124–4.019 | 0.020 |
| Household income, yen | ||||||
| < 1 million | 1.000 | 1.000 | ||||
| 1–3.99 million | 0.943 | 0.401–2.216 | 0.893 | 0.671 | 0.319–1.407 | 0.291 |
| 4–6.99 million | 0.698 | 0.301–1.618 | 0.401 | 0.421 | 0.203–0.873 | 0.020 |
| 7–9.99 million | 0.446 | 0.191–1.042 | 0.062 | 0.288 | 0.138–0.603 | < 0.001 |
| ≥ 10 million | 0.466 | 0.198–1.098 | 0.081 | 0.247 | 0.117–0.522 | < 0.001 |
| Unknown | 0.605 | 0.255–1.433 | 0.253 | 0.411 | 0.194–0.869 | 0.020 |
| CCHL scale | ||||||
| Low (first quartile) | 1.000 | 1.000 | ||||
| Lower (second quartile) | 0.904 | 0.741–1.104 | 0.323 | 0.743 | 0.616–0.896 | 0.002 |
| Higher (third quartile) | 0.801 | 0.645–0.994 | 0.044 | 0.624 | 0.508–0.767 | < 0.001 |
| High (fourth quartile) | 0.740 | 0.585–0.935 | 0.012 | 0.625 | 0.501–0.779 | < 0.001 |
CCHL scale communicative and critical health literacy scale, K6 Kessler 6 scale, EPDS Edinburgh postnatal depression scale, CORs crude odds ratios, 95% CI 95% Confidence interval.
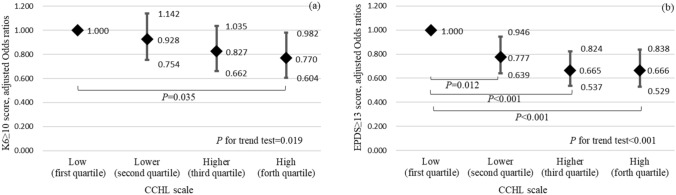
Spearman’s correlation coefficients for all potential confounding factor pairs were less than 0.597 (see Supplementary Table S1), and independent variables (22 items) multiplied 30 were less than 744 for a K6 score ≥ 10 and 844 for an EPDS score ≥ 13 in the multivariable logistic regression models. After adjustments for potential confounding factors, such as age, weeks of gestation, complications, medical history, the number of children, marital status, education level, employment status, and household income, the adjusted odds ratio (95% CI) for anxiety symptoms was significantly lower in the high CCHL group than in the low CCHL group [0.779 (0.604–0.982)]. The adjusted odds ratio (95% CI) for depressive symptoms was significantly lower in the lower CCHL group [0.777 (0.639–0.946)], higher CCHL group [0.665 (0.537–0.824)], and high CCHL group [0.666 (0.529–0.838)] than in the low CCHL group. A trend test showed significant trends in the adjusted odds ratio for anxiety symptoms (p for trend = 0.019) and depressive symptoms (p for trend < 0.001) (Fig. 1a,b). Supplementary Table S2 shows adjusted odds ratios (95% CI) for ADs to other variables.
Figure 1.
Anxiety (a) and depressive (b) symptoms in pregnant women in four CCHL groups. CCHL communicative and critical healthy literacy scale. Using a multivariable logistic regression model analysis with odds ratios (rhombus), 95% confidence intervals (bar), and p values, and the p for trend tests after adjustments for age, weeks of pregnancy, number of children born, complications during pregnancy, medical history, marital status, education, current employment status, and household income.
Discussion
In the present study, anxiety symptoms with a K6 score ≥ 10 in the high CCHL group and depressive symptoms with an EPDS score ≥ 13 in the lower, higher, and high CCHL groups showed significantly lower adjusted odds ratios than those in the lower CCHL group. The proportion of participants with ADs was lower in the low to high CCHL groups. To the best of our knowledge, this is the first study to report relationships between ADs and CCHL in pregnant women during the COVID-19 pandemic.
During the COVID-19 pandemic, 13.5% of pregnant women had anxiety symptoms with a K6 score ≥ 10 while 15.4% had depressive symptoms with an EPDS score ≥ 13 in the present study. These results were consistent with previous findings showing higher anxiety and depression in pregnant women after COVID-19 infection4,7,8, but were lower than those reported in other previous studies, with 7.5% of pregnant women having a K6 score (≥ 10)16 and 9.5% with a high EPDS score (≥ 13)17 before COVID-19 infection. This survey was conducted in September 2020. Although the cumulative incidence of patients with and deaths from COVID-19 was not high at that time in Japan, it was steadily increasing every day18,19. The high levels of ADs among pregnant women due to the COVID-19 pandemic were further validated in the present study.
On the other hand, approximately 60% or more of pregnant women in the present study were first-time mothers older than 30 years and in the last trimester of pregnancy at the time of the survey. Anxiety about pregnancy and childbirth was superimposed on COVID-19 infection, which was associated with more ADs. Further studies are warranted to develop strategies that improve ADs in pregnant women.
According to a basic 5-item CCHL showing that internal consistency was adequately high (Cronbach’s α = 0.86)20, for COVID-19, we used the CCHL and obtained Cronbach’s α = 0.78. Moreover, in consideration of some potential confounding factors associated with ADs, such as sociodemographic and socioeconomic factors21, we used a multivariable logistic model to analyze relationships with CCHL, and found that CCHL was still a dependent factor affecting ADs in pregnant women during the COVID-19 pandemic. As one upstream factor, health literacy is an individual’s ability to influence downstream health conditions22 or mortality rates23. In the present study, we measured CCHL for COVID-19. An insufficient knowledge of COVID-19 infection was suggested to be associated with ADs in pregnant women. Therefore, the present results are significant and reliable.
Vaccination and effective medication against COVID-19 are the only strategies for ADs caused by COVID-19. With the spread of vaccines worldwide, the Japan Society of Obstetrics and Gynecology has also recommended vaccines for pregnant women24. The widespread vaccination of pregnant women is expected to reduce ADs caused by COVID-19. However, the complete control of COVID-19 has not yet been achieved. Even if COVID-19 is eventually controlled, humans will undoubtedly encounter novel infections again in the future. The ability to collect, analyze, and use health information is a skill that is beneficial for everyone. In the present study, we focused on pregnant women and examined relationships between ADs and CCHL. As suggested by Paakkari and Okan25, CCHL may provide a solution that reduces ADs due to the impact of a novel infectious pandemic.
While not limited to pregnant women, many studies reported that the level of health literacy on COVID-19 was not only a cause of increased mental disorders, but also increased future anxiety26,27. Therefore, health literacy-based policy decisions and the provision of information as well as accurate knowledge and appropriate actions against novel infectious diseases are important28,29. However, the situation for pregnant women is more complex. In the present study, in addition to CCHL, weeks of gestation, the number of children, complications, a history of mental disease, marital status, education level, and employment status were related to ADs (Supplement Table S1). In consideration of these factors, detailed support appears to be needed while enhancing health literacy. For example, it is important to provide preconception and health education in routine prenatal classes30.
The limitations of the present study need to be addressed. (1) Since the present study had a cross-sectional design, causal relationships were not identified. (2) ADs were assessed using a self-reported questionnaire and not by a medical doctor; therefore, information bias was not avoided. (3) This was an online survey study; therefore, selection bias existed and the reproducibility of the results obtained cannot be evaluated. However, this was a large-scale survey with few missing values, respondents were from all prefectures in Japan, and the representative sample size was obtained. (4) Since there are no data on psychiatric comorbidities in pregnant women, a medical history of mental disease was adjusted for. (5) The careful interpretation of the present results is necessary for generalization to other populations due to the above limitations.
In conclusion, the present results suggest that CCHL had an impact on anxiety and depressive symptoms in pregnant women during the COVID-19 pandemic. Since COVID-19 has not yet been eradicated, psychological stress in pregnant women is likely to increase. Therefore, mental care and health literacy are considered to be equally important for pregnant women.
Methods
Study design and subjects
A cross-sectional study was conducted using an online survey for pregnant women in Japan between September 1st and 30th, 2020, through leaflets delivered to medical facilities and placed on social networking sites, such as Facebook, Twitter, and Line. The Japan Society of Obstetrics and Gynecology (https://www.jsog.or.jp/), Yokohama City University School of Medicine (https://www.yokohama-cu.ac.jp/academics/med/index.html), Pregnant Women Health Initiative (https://pw-hi.jp/), and Registration for COVID-19 complicated pregnancy in Japan (https://www.med.kobe-u.ac.jp/cmv/covid/) were used. Participants older than 20 years or married minors 16–19 years old, who are considered to be adults under Japanese Civil Law at the time of the survey (https://elaws.e-gov.go.jp/document?lawid=129AC0000000089) and have sufficient judgment under Ethical Guidelines Guidance in Japan (https://www.mhlw.go.jp/content/000946358.pdf), were recruited in the present study. Exclusion criteria were unmarried women between the ages of 16 and 19 years old or women younger than 16 years old, women whose gestational weeks and expected date of delivery did not match, or one of the CCHL, K6, and EPDS items was defective and inadequate. Informed consent to participate in this study was obtained from potential participants prior to answering the questionnaire and one of the most secure online questionnaire sites, “SurveyMonkey™” was used. At the beginning of the survey, written informed consent on WEB site stated that the survey was voluntary and that even if potential participants began to respond, it may be stopped prematurely. In addition, the agreement that pressing the submit button before and after answering the questions was considered the final agreement was a requirement to proceed to the actual research website. Information sent on the internet was encrypted and converted into data through a secure server without individual information. Online survey questions included the characteristics and socioeconomic status of pregnant women, such as age, weeks of gestation, number of children, complications during pregnancy, medical history, marital status, education level, employment status, and household income.
Assessments of anxiety and depressive symptoms as well as health literacy
K631 and EDPS32 with a 5-point Likert scale were used to assess ADs in pregnant women. In the present study, Cronbach’s coefficient alpha of K6 and EPDS were 0.860 and 0.875, respectively. A cut-off value of a K6 score of 10 indicated anxiety symptoms in pregnant women, while an EPDS score of 13 indicated depressive symptoms.
The CCHL levels of pregnant women were assessed using a basic 5-item, and a 5-point Likert scale of CCHL20,33 for COVID-19. The 5 items consist of the following: (1) the ability to gather information on COVID-19 from various sources; (2) the ability to select information necessary for oneself (on childbirth and postpartum) from a large amount of information on COVID-19; (3) an understanding of information on COVID-19 and the ability to convey it to others; (4) the ability to judge the reliability of information on COVID-19; and (5) the ability to decide on plans and actions to prevent infection based on information on COVID-19. In the present study, Cronbach’s coefficient alpha of CCHL was 0.783.
Statistical analysis
Sociodemographic factors, pregnancy-related factors, medical history, anxiety symptoms (K6), depressive symptoms (EPDS), and CCHL were assessed using descriptive statistics. To analyze the trend relationship with ADs, CCHL scores were divided into four groups: low (first quartile), lower (second quartile), higher (third quartile), and high (fourth quartile) levels, based on the quartile of the distribution of CCHL.
The proportions of anxiety symptoms (K6 score ≥ 10) and depressive symptoms (EPDS score ≥ 13) for each variable were analyzed using the Chi-squared test or Fisher’s exact test when the expected number of zero cells was 20% or more. A univariable logistic regression model was used to examine the relationship between each variable and ADs. To analyze the relationship between the four CCHL groups of pregnant women and ADs, multivariable logistic regression models and trend tests were conducted after adjustments for all potential confounding factors, such as age, weeks of pregnancy, number of children born, complications during pregnancy, medical history, marital status, education, current employment status, and household income. The multicollinearity of the input variables was confirmed with Spearman’s correlation coefficient less than 0.9. The multivariate overfitting was checked by multiplying the number of input variables by 30 and below the number of K6 scores ≥ 10 or EPDS scores ≥ 13.
All statistical analyses were performed using a two-tailed test and an assumed type I error rate of 0.05. Statistical analyses were performed using IBM SPSS Statistics 27 for Windows (IBM Japan, Tokyo, Japan).
Ethical approval and informed consent
The present study was approved by the Institutional Ethics Committee of Yokohama City University (B 200800046), and all methods were performed in accordance with the Declaration of Helsinki, relevant guidelines, and regulations. Consent to participate in the present study was obtained by confirmation from participants at the start of questionnaire responses.
Supplementary Information
Acknowledgements
We thank Prof. Shigeru Saito, Prof. Satoshi Hayakawa, Prof. Kei Kawana, Assoc. prof. Satoru Ikenoue, Assoc. Prof. Masashi Deguchi, Prof. Masayo Takada, and Prof. Ichiro Morioka for their helpful advice and cooperation with the recruitment of study participants. This work was supported by the 2020 Grant for the Health and Labor Sciences Research Grant “Studies toward the construction of appropriate support delivery system for pregnant women during the COVID-19 pandemic”.
Author contributions
Y.H. contributed to making the questionnaire and the statistical analysis and wrote the initial draft of the manuscript. E.M. designed the study protocol, obtained the grant, and contributed to finalizing the manuscript. G.K., S.O., T.U., and Y.S. contributed to the questionnaire. A.Y., A.H., and K.K. contributed to the review of the questionnaire. T.I., T.K., and H.Y. contributed to supervising the protocol, and reviewed and approved the manuscript. All authors contributed to the interpretation of results.
Data availability
The datasets used and/or analyzed during the current study available on the following web site of the Department of Obstetrics and Gynecology, Yokohama City University Graduate School of Medicine, Japan. The datasets on CCHC in pregnant women is available at https://pw-hi.jp/.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-18405-3.
References
- 1.Gloster AT, et al. Impact of COVID-19 pandemic on mental health: An international study. PLoS One. 2020;15:e0244809. doi: 10.1371/journal.pone.0244809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.González-Blanco L, et al. COVID-19 lockdown in people with severe mental disorders in Spain: Do they have a specific psychological reaction compared with other mental disorders and healthy controls? Schizophr. Res. 2020;223:192–198. doi: 10.1016/j.schres.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skoda EM, et al. Severely increased generalized anxiety, but not COVID-19-related fear in individuals with mental illnesses: A population based cross-sectional study in Germany. Int. J. Soc. Psychiatry. 2021;67:550–558. doi: 10.1177/0020764020960773. [DOI] [PubMed] [Google Scholar]
- 4.Ceulemans M, et al. Mental health status of pregnant and breastfeeding women during the COVID-19 pandemic—A multinational cross-sectional study. Acta Obstet. Gynecol. Scand. 2021;100:1219–1229. doi: 10.1111/aogs.14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu K, et al. Mental health among pregnant women under public health interventions during COVID-19 outbreak in Wuhan, China. Psychiatry Res. 2021;301:113977. doi: 10.1016/j.psychres.2021.113977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medina-Jimenez V, et al. The impact of the COVID-19 pandemic on depression and stress levels in pregnant women: A national survey during the COVID-19 pandemic in Mexico. J. Matern. Fetal Neonatal Med. 2020;26:1–3. doi: 10.1080/14767058.2020.1851675. [DOI] [PubMed] [Google Scholar]
- 7.Obata S, et al. Psychological stress among pregnant and puerperal women in Japan during the coronavirus disease 2019 pandemic. J. Obstet. Gynaecol. Res. 2021;47:2990–3000. doi: 10.1111/jog.14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghazanfarpour M, et al. Prevalence of anxiety and depression among pregnant women during the COVID-19 pandemic: A meta-analysis. J. Psychosom. Obstet. Gynaecol. 2021;24:1–12. doi: 10.1080/0167482X.2021.1929162. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, et al. Mental health among pregnant women with COVID-19-related stressors and worries in the United States. Birth. 2021;48:470–479. doi: 10.1111/birt.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koyucu RG, Karaca PP. The Covid 19 outbreak: Maternal mental health and associated factors. Midwifery. 2021;99:103013. doi: 10.1016/j.midw.2021.103013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abel T, McQueen D. Critical health literacy and the COVID-19 crisis. Health Promot. Int. 2021;35:1612–1613. doi: 10.1093/heapro/daaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matterne U, et al. Health literacy in the general population in the context of epidemic or pandemic coronavirus outbreak situations: Rapid scoping review. Patient Educ. Couns. 2021;104:223–234. doi: 10.1016/j.pec.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melki J, et al. Mitigating infodemics: The relationship between news exposure and trust and belief in COVID-19 fake news and social media spreading. PLoS One. 2021;16:e0252830. doi: 10.1371/journal.pone.0252830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wijaya MC, Kloping YP. Validity and reliability testing of the Indonesian version of the eHealth Literacy Scale during the COVID-19 pandemic. Health Inform. J. 2021;27:1460458220975466. doi: 10.1177/1460458220975466. [DOI] [PubMed] [Google Scholar]
- 15.Luong TC, et al. Fear, anxiety and depression among pregnant women during COVID-19 pandemic: Impacts of healthy eating behaviour and health literacy. Ann. Med. 2021;53:2120–2131. doi: 10.1080/07853890.2021.2001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzumori N, et al. Study of relationship between mode of conception and non-specific psychological distress in women undergoing noninvasive prenatal testing. J. Reprod. Infertil. 2020;21:189–193. [PMC free article] [PubMed] [Google Scholar]
- 17.Usuda K, Nishi D, Okazaki E, Makino M, Sano Y. Optimal cut-off score of the Edinburgh Postnatal Depression Scale for major depressive episode during pregnancy in Japan. Psychiatry Clin. Neurosci. 2017;71:836–842. doi: 10.1111/pcn.12562. [DOI] [PubMed] [Google Scholar]
- 18.Ministry of Health, Labour and Welfare of Japan. https://www.mhlw.go.jp/stf/covid-19/kokunainohasseijoukyou.html (2020).
- 19.World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int (2020).
- 20.Ishikawa H, Nomura K, Sato M, Yano E. Developing a measure of communicative and critical health literacy: A pilot study of Japanese office workers. Health Promot. Int. 2008;23:269–274. doi: 10.1093/heapro/dan017. [DOI] [PubMed] [Google Scholar]
- 21.Cena L, et al. Prevalence of maternal antenatal and postnatal depression and their association with sociodemographic and socioeconomic factors: A multicentre study in Italy. J. Affect. Disord. 2021;15:217–221. doi: 10.1016/j.jad.2020.09.136. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell SE, Sadikova E, Jack BW, Paasche-Orlow MK. Health literacy and 30-day postdischarge hospital utilization. J. Health Commun. 2012;17:325–338. doi: 10.1080/10810730.2012.715233. [DOI] [PubMed] [Google Scholar]
- 23.Baker DW, et al. Health literacy and mortality among elderly persons. Arch. Intern. Med. 2007;167:1503–1509. doi: 10.1001/archinte.167.14.1503. [DOI] [PubMed] [Google Scholar]
- 24.Hayakawa S, Komine-Aizawa S, Takada K, Kimura T, Yamada H. Anti-SARS-CoV-2 vaccination strategy for pregnant women in Japan. Obstet. Gynaecol. Res. 2021;47:1958–1964. doi: 10.1111/jog.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paakkari L, Okan O. COVID-19: Health literacy is an underestimated problem. Lancet Public Health. 2020;5:e249–e250. doi: 10.1016/S2468-2667(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duplaga M, Grysztar M. The Association between Future Anxiety, Health Literacy and the Perception of the COVID-19 Pandemic: A Cross-Sectional Study. Healthcare (Basel) 2021;9:43. doi: 10.3390/healthcare9010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermans L, Van den Broucke S, Gisle L, Demarest S, Charafeddine R. Mental health, compliance with measures and health prospects during the COVID-19 epidemic: The role of health literacy. BMC Public Health. 2021;21:1365. doi: 10.1186/s12889-021-11437-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durmuş V. Differences in health literacy level of patients from public and private hospitals: A cross-sectional study in Turkey. Public Health. 2021;200:77–83. doi: 10.1016/j.puhe.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abel T, McQueen D. Critical health literacy in pandemics: The special case of COVID-19. Health Promot. Int. 2021;36:1473–1481. doi: 10.1093/heapro/daaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker R, Drakeley S, Boyle J. Preconception women's views of promoting preconception women's health in Australia. Health Promot. J. Aust. 2021;2:22–28. doi: 10.1002/hpja.402. [DOI] [PubMed] [Google Scholar]
- 31.Furukawa TA, et al. The performance of the Japanese version of the K6 and K10 in the World Mental Health Survey Japan. Int. J. Methods Psychiatr. Res. 2008;17:152–158. doi: 10.1002/mpr.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozinszky Z, Dudas RB. Validation studies of the Edinburgh Postnatal Depression Scale for the antenatal period. J. Affect. Disord. 2015;176:95–105. doi: 10.1016/j.jad.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 33.Ishikawa H, Takeuchi T, Yano E. Measuring functional, communicative, and critical health literacy among diabetic patients. Diabetes Care. 2008;31:874–879. doi: 10.2337/dc07-1932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available on the following web site of the Department of Obstetrics and Gynecology, Yokohama City University Graduate School of Medicine, Japan. The datasets on CCHC in pregnant women is available at https://pw-hi.jp/.