Abstract
Molecularly imprinted biodegradable polymers are receiving considerable attention in drug delivery due to their ability of targeted recognition and biocompatibility. This study reports the synthesis of a novel fluorescence-active magnetic molecularly imprinted drug carrier (MIDC) using a glucose-based biodegradable cross-linking agent for the delivery of anticancer drug docetaxel. The magnetic molecularly imprinted polymer (MMIP) was characterized through scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction spectroscopy, and vibrating sample magnetometry (VSM). The MMIP presented a magnetization value of 0.0059 emu g–1 and binding capacity of 72 mg g–1 with docetaxel. In vitro and in vivo studies were performed to observe the effectiveness of the MIDC for drug delivery. The cell viability assay suggested that the MMIP did not present toxic effects on healthy cells. The magnetic property of the MMIP allowed quick identification of the drug carrier at the target site by applying the external magnetic field to mice (after 20 min of loading) and taking X-ray images. The novel MMIP-based drug carrier could thus deliver the drug at the target site without affecting the healthy cells.
1. Introduction
Docetaxel (DTX) is an extract from a rare Pacific yew tree, Taxus briefolia, and hence is classified as a plant alkaloid.1 DTX possesses a side chain with tertbutyl carbamate ester and a carbon 10 hydroxy functional group. By binding and stabilizing tubulins, DTX prevents disintegration of the cell’s physiological microtubules, resulting in G2/M cell cycle arrest and cell death. DTX also suppresses the anti-apoptotic expression of the Bcl2 gene and supports the expression of the p27 inhibition cell cycle.2−4 Its mechanism of action is effective against a wide variety of cancer tumors,4 including breast, ovarian, gastric, and prostate cancer.5−8
Several drug carriers such as polymer drug conjugates,9,10 polymer protein conjugates,11,12 polymer micelles,13,14 polymeric nanoparticles,15 and gold nanoparticles16,17 have been used as carriers for a variety of drugs previously. Few drug nanocarriers such as galactosamine-d-α-tocopherol polyethene glycol 1000 succinate-poly(lactide) (Gal-pD-TPGS-PLA/NPs) loaded with DTX have recently been reported with 55% release time in 14 days.18 Previous studies used chitosan-coated solid lipid nanoparticles (CS-SLNs) containing docetaxel for development and optimization. The high-pressure hot homogenization approach was used to create solid lipid nanoparticles (SLNs) with and without chitosan coats, but the targeted selectivity and release of DTX were feeble.19 Monoolein cubic nanoparticles containing docetaxel were another carrier of DTX prepared by a top-down procedure through the homogenization technique with different amphiphile concentrations with a release rate of 24 h.20 Therefore, further research on drug carriers focused on overcoming the side effects and fast release of DTX and improving its cancer effects is immensely required. Among various techniques, molecular imprinting polymerization is being deeply explored to synthesize biodegradable drug carriers. They possess a dominating characteristic of molecular recognition depending on the form, size, and interactions of the cavity produced by the template.21,22 Monomers usually form these polymers in the presence of a target molecule as the template and an excess of cross-linker.23 MIPs are extremely efficient artificially manufactured receptors capable of rapid mass transfer and have high binding capacity.24,25 They display considerable extraction capabilities and can be modified to incorporate various properties like fluorescence activity, magnetic activity, etc. Magnetic MIPs have received prominent attention basically due to their outstanding properties such as magnetic susceptibility, small size, and high coercivity. They have also been considered suitable candidates in fields like bioengineering, catalysis, and bioseparation.26 In molecularly imprinted polymer systems, recent magnetic separation techniques have received a lot of attention to facilitate separation by introducing an outside magnetic field.27,28 Such systems are capable of delivering loaded drugs at specific sites.29 Various polymeric as well as sol–gel modifications can be performed to design magnetic imprinted drug carriers. One such example is Fe3O4@SiO2 nanoparticle drug delivery systems.30 The superparamagnetic core shell of iron silica is ideal for biomedical applications. They are protected and made acceptable for in vivo use by the surrounding polymeric layer. Iron nanoparticles are preferred over other forms of nanocarrier cancer therapy because their advantageous magnetic characteristics, higher chemical stability, minimal toxicity, and simplicity of surface modification are considered preferable to those of other nanocarriers for tumor-specific delivery of anticancer drugs.31,32 Additionally, iron nanoparticles of smaller sizes have several additional benefits, such as improved pharmacokinetics, better in vivo stability, and site-specific delivery of loaded cargo as well as increased internalization through the tumor vasculature (enhanced permeability and retention effect) and successful escape from the reticuloendothelial system (RES).33 Furthermore, these synthetic or natural biodegradable materials in drug delivery systems enable them to be implanted in living organisms without further treatment.34,35 Other qualities such as stability in the 3D network, customizable drug release, elasticity, and flexibility with good mechanical characteristics are also beneficial in drug systems.36 It has been shown that cross-linking polymers are usually stable and can protect their dimensions for longer periods. They offer a uniform biodegradability that may directly be changed by cross-linking density.37 It has been observed that biologically degradable cross-linking polymers are among the most promising materials in targeted and regulated drug delivery.38 Few papers are encountered in the literature regarding biodegradable molecularly imprinted polymers possessing magnetic characteristics and their applications in targeted drug delivery.39−41
Hence, to further explore the unique traits of imprinted materials, we synthesized a novel fluorescent active magnetic molecularly imprinted polymer (MMIP) using glucose as the cross-linker. The synthesized MMIP was studied for its ability to deliver targeted drug docetaxel, an anticancer drug. Furthermore, the selection of glucose as the cross-linker describes this material as green, quick, and easy for rapid release of docetaxel at the target site.
2. Materials and Methods
2.1. Materials and Reagents
Ferric chloride (FeCl3) and ferrous sulfate heptahydrate (FeSO4·7H2O), ammonia solution (33%), trisodium citrate (Na3C6H5O7, 99.0%), tetraethyl orthosilicate (TEOS, 99.9%), fluorescein-5-isothiocyanate (FITC), 3-aminopropyl trimethoxysilane vol (APTS), 3-trimethoxysilyl propyl methacrylate (MPS) 99.9–101%, toluene, triethylamine, methacryloyl chloride (99%), decane, 2,2′-azobis-2-methylpropionitrile (AIBN, 99.7%), sodium dodecyl sulfate (SDS), absolute ethanol (99.8%), acetone, and acetonitrile (ACN) were purchased from Sigma Aldrich. Double distilled water was used for washing.
2.2. Synthesis of Magnetic Nanoparticles
The magnetic nanoparticles of Fe3O4 were synthesized by the co-precipitation method.42 20 mmol of ferric chloride and 10 mmol of ferrous sulfate were mixed in 140 mL of water. The obtained solution was added into a two-neck round-bottom flask and purged under a N2 atmosphere for 30 min. In this flask, 33 mL of 2 M ammonia solution was added dropwise under constant stirring till the precipitation of magnetic nanoparticles. After centrifugation, the brownish-black magnetic nanoparticles were washed with deionized water. An external magnetic field was applied to separate the nanoparticles. The isolated nanoparticles were then re-dispersed in 200 mL of water with 0.3 M trisodium citrate and heated at 70 °C for 2 h. After that, the nanoparticles were washed with acetone three times to remove the excess citrate and dried in a desiccator.
2.2.1. Modification of Magnetic Nanoparticles with Silica
The citrate-modified magnetic nanoparticles were dispersed in 50 mL of water in an ultrasonic bath for 10 min. Next, 2 mL of dispersed nanoparticles was taken and diluted to 40 mL with distilled water. An ammonia solution (33%, 5 mL) and 140 mL of ethanol were added and mechanically stirred for 18 h. In the next step, tetraethyl orthosilicate (1 mL) was added dropwise to the mixture of dispersed nanoparticles and the reaction was allowed to proceed at room temperature for 16 h. The obtained material was then washed with distilled water and ethanol to remove any unreacted reagent. The silica-coated magnetic nanoparticles were dispersed in ethanol with the help of an ultrasonic tub.
2.2.2. Synthesis of FITC Magnetic Nanoparticles
The silica-coated fluorescent nanoparticles were synthesized according to the method by Huang et al.43 A solution of fluorescence isothiocyanate FITC (0.04 g) in 10 mL of ethanol with APTS (0.22 mL) was mixed together and stirred for 18 h in the dark (solution A). Solution A was added to the flask containing silica-coated magnetic nanoparticles and mechanically agitated for 16 h. Fabricated FITC nanoparticles were obtained after drying at room temperature.
2.2.3. Modification with Methacryloxypropyl Trimethoxysilane
Finally, Fe3O4@SiO2@FITC nanoparticles were modified with methacryloxypropyl trimethoxysilane (MPS). For this purpose, 50 mL of toluene and 3 mL of MPS were mixed with Fe3O4@SiO2@FITC magnetic nanoparticles, and the reaction was performed at 70 °C with magnetic stirring for 15 h. After magnetic separation, these nanoparticles (Fe3O4@SiO2@FITC@CH2=CH2) were modified with a double bond, washed with distilled water and ethanol, and stored in a desiccator at 40 °C.
2.2.4. Synthesis of the Cross-Linker
Glucose (1 mmol) and dry ACN were mixed in a Schlenk flask (10 mL). A few drops of N (C2H5)3 (3 mmol) triethylamine and a solution of methacryloyl chloride (3 mmol) prepared in ACN were added into the Schlenk flask and stirred at 0 °C for 3 h. Finally, the unstable organics and extra methacryloyl chloride were rotary-evaporated, and the resultant solid was dried in a vacuum oven at 40 °C.
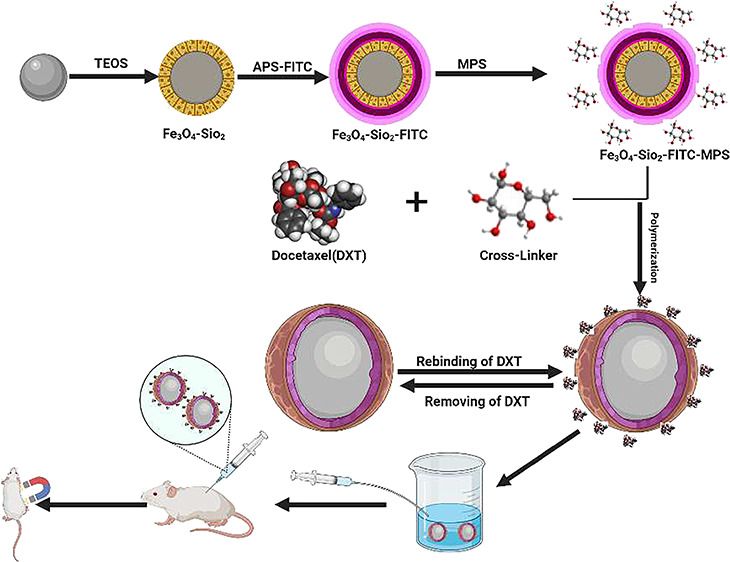
2.2.5. Fabrication of Molecularly Imprinted Polymer Nanoparticles
The MIP-coated Fe3O4 magnetic nanoparticles were synthesized using a microemulsion polymerization process. In the first step, to form a template monomer complex, cross-linker glucose (monomer) and docetaxel were dissolved in ACN/DMSO solution in a ratio of 5:1 (v/v). The solution was shaken for 5 h at room temperature. In the next step, 120 mg of modified magnetic nanoparticles and 60 mg of AIBN were added into ACN/DMSO solution and sonicated for 10 min. The reaction was continued under a N2 atmosphere at 60 °C, and after 20 h, the fabricated imprinted polymer was collected using a strong external magnetic field, which was then washed with water and acetone.
Similarly, the non-imprinted polymer (NIP) was also synthesized using the same procedure in the absence of docetaxel. In the next step, both the drug-loaded MIP and NIP were administered orally to the mice with the help of a gavage. The mice’s blood plasma was taken for drug detection and analyzed through HPLC.
2.3. Characterization of Nanomaterials
FTIR was used to study the functional groups present on the newly synthesized drug carrier. FTIR was performed using a Bruker Alpha II (USA) within the range 500–4000 cm–1. The carbon, hydrogen, and oxygen contents were determined with a CHN analyzer (CHNO/S 2400 from PerkinElmer, USA). The phase angle of the materials was determined with X-ray Power diffraction (XRD-Powder D8 [Advance, Bruker Germany] with Cu Kα radiation = 0.154 nm). The operational condition of XRD was a current of 30 mA and voltage of 40 kV for 2θ (20–80°). The particle size was determined using a scanning electron microscope (SEM) with an EDS and the E-Beam lithograph FEI Nova 450 NanoSEM. The magnetometry of magnetic nanoparticles, silica-coated magnetic nanoparticles, and MMIP nanoparticles was determined with VSM (Model 7407, Lakeshore, USA). The elemental composition was determined using EDS. In vitro drug location was performed with an X-ray analysis technique. The degradation of the MMIPs was performed at various pH levels. The dosage response of cell viability was studied with an MTT assay.
2.4. X-ray Analysis
For X-ray analysis, materials were made into tablets and placed on a gavage syringe. Two mice were taken and labeled with two dots at the tail for control and three dots at the tail for tablet containing medicine. An external magnetic field was used to attract materials. 30 min after administering the dose, X-ray images were taken. A magnetic field applied outside the body attracts the material, as evidenced by white spots on X-rays. Chloroform was used to make the mice comatose.
2.5. Blood Sample Analysis
The blood plasmas from blood samples of the mice were first separated through centrifugation. Then, the obtained plasmas were added to a mixture of acetic acid and methanol (8:2 v/v) in order to extract the drug. The extracted drug sample was analyzed through HPLC.
HPLC was performed using a Shimadzu LC-10 AT HPLC system (Japan) with a 266 nm UV detector and a reversed-phase column (ODS, C18 4.6 mm × 250 mm, 5 μ waters USA). The mobile phase consisted of methanol:phosphate buffer (90:10 v/v), and the flow rate was adjusted to 0.5 mL min–1.
3. Results and Discussion
The synthetic scheme involves the MIP modified with fluorescence-active functional group FITC. Moreover, the magnetic properties were also introduced to the MIP through the incorporation of Fe3O4 nanoparticles. The main advantage of FITC is to locate or detect the drug inside the body (in vivo study) through fluorescence, and the purpose of glucose was to serve as a biodegradable cross-linker replacing harmful cross-linkers such as tannic acid44 commonly used in conventional synthesis.
3.1. Characterization
3.1.1. Scanning Electron Microscopy
Figure 1 shows the morphology of Fe3O4 NPs, Fe3O4-SiO2 NPs, and the MMIP nanoparticles. The Fe3O4 NPs were in minor accretion, according to published data of scanning electron microscopy,45 with an average size of about 70 nm. Fe3O4 NPs were modified with TEOS (tetraethyl orthosilicate) to produce core–shell Fe3O4-SiO2 NPs with an average size range of 105 nm. The silica layer in the case of Fe3O4-SiO2 NPs was approximately 30 to 36 nm-thick. The shape of the MIP nanoparticles produced after the polymerization process remained spherical, and the diameter increased to 250 nm, indicating that this synthetic technique for MIP synthesis was successful. For measurement, two different nanoscales of 5 and 10 μm were used as given in Figure 1. The size of particles was confirmed by using software (Image J1.52v/ java 1.8.0_112 (64 bit).
Figure 1.
Scanning electron microscope images of Fe3O4 (a), silica-coated nanoparticles (b), and MMIPs (c).
3.1.2. Elemental Detection Analysis
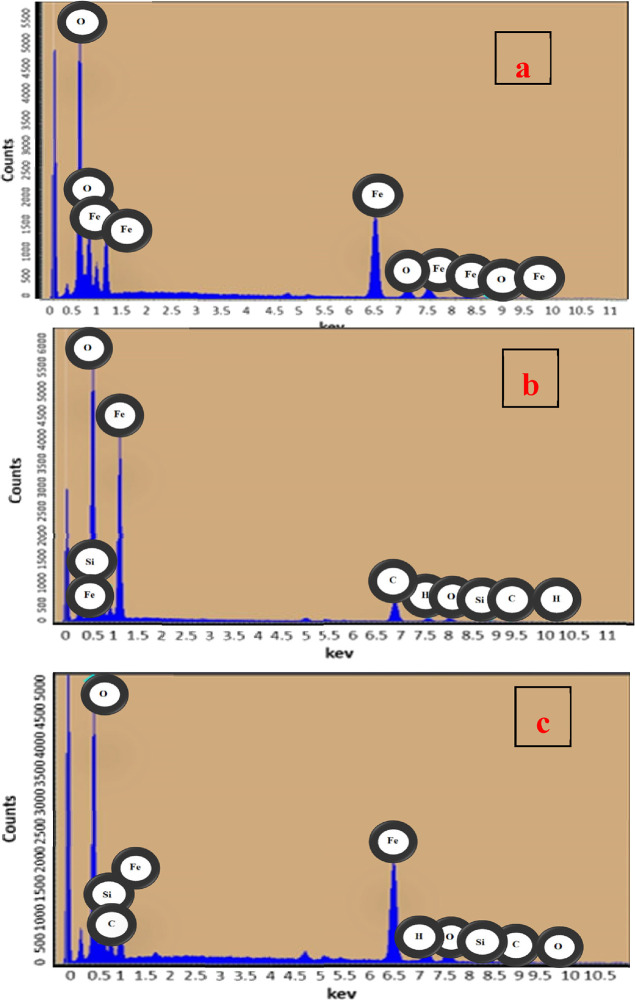
The elemental properties of materials were assessed through EDS analysis. The Fe, O, H, and Na peaks in Figure 2a indicate that iron nanoparticles were loaded with sodium citrate. The Fe, O, H, Na, S, and Si peaks in Figure 2b confirm the coating of silica. The peak at around 23 keV corresponds to S in the fluorescent layer, and the relatively higher amount of C in the MMIP pattern supports the production of a multicore–shell structure nanoparticle, as shown in Figure 2c.
Figure 2.
EDX diagrams of (a) Fe3O4, (b) silica-coated nanoparticles, and (c) the molecularly imprinted polymer.
3.1.3. Vibrating Sample Magnetometer
In Figure 3, magnetic nanoparticles (Fe3O4), silica-coated magnetic nanoparticles (Fe3O4-SiO2), and the magnetic molecularly imprinted polymer (MMIP) loaded with docetaxel were tested using a vibrating sample magnetometer (VSM). Because of the pure magnetic material with no coatings or alterations, Fe3O4 nanoparticles have the highest capacity magnetization value. The magnetization of the second material is then reduced due to amalgamation with SiO2. After coating Fe3O4-SiO2 with cross-linkers and producing a polymer, the saturation magnetization decreased significantly. The magnetic characteristics of samples were thus altered to some extent after incorporating various non-magnetic layers on Fe3O4 acting as the core, but they were still magnetic enough to respond to an external force (Table 1). A similar behavior was observed in previous studies. According to the trend of magnetization given in the literature for the synthesis of magnetic MIPs, the magnetization value reduced with the formation of the core shell and then decreased further after polymer formation.46,47
Figure 3.
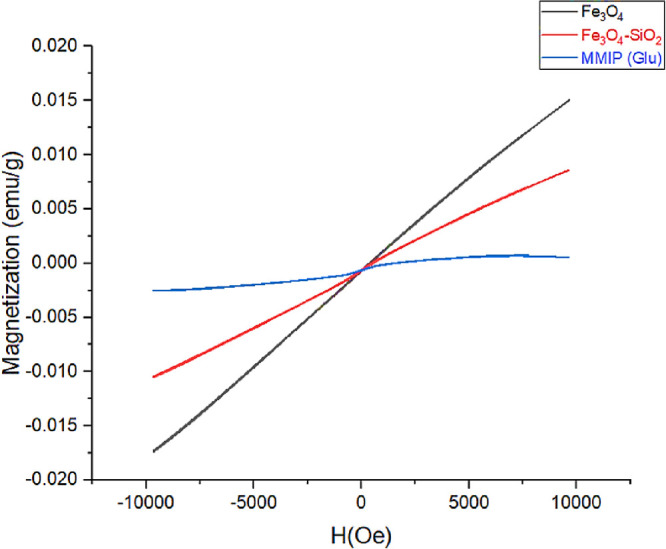
Vibrating sample magnetometer hysteresis loop of Fe3O4, Fe3O4-SiO2, and MMIPs.
Table 1. Magnetic Character of Fe3O4, Fe3O4-SiO2, and MMIP.
| material | magnetization (emu/g) |
|---|---|
| Fe3O4 | 0.01509 |
| Fe3O4-SiO2 | 0.00861 |
| MMIP | 0.000587 |
3.1.4. Fourier Transform Infrared Spectroscopy
Fourier transform infrared spectroscopy (FTIR) is a technique for detecting various functional groups in organic and inorganic molecules. Figure 4a displays an Fe3O4 spectrograph with only one conspicuous signal at 580 cm–1, indicating that only Fe3O4 is present. In Figure 4b, a new strong peak appeared at 1085 cm–1 correlated to the Si–O–Si stretching vibration, and another peak appeared at around 800 cm–1, which was referred to the Si–O stretching vibration and showed the successful encapsulation of SiO2 onto the surface of Fe3O4. In Figure 4c, the peaks at 3600, 2990, 2850, 1730, 1200, 1090, and 580 cm–1 represent many functional groups such as OH, C–H, C=O, C–C, and some bending frequencies of this spectrograph.
Figure 4.
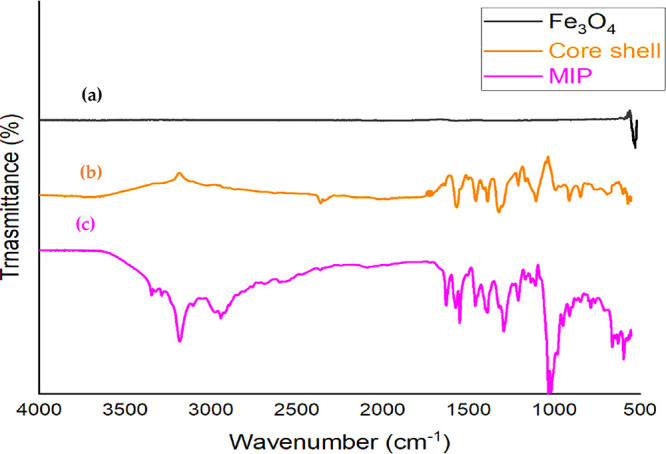
IR spectra of iron nanoparticles Fe3O4 (a), core–shell (b), and the magnetic molecularly imprinted polymer (c).
3.1.5. X-ray Diffraction Analysis
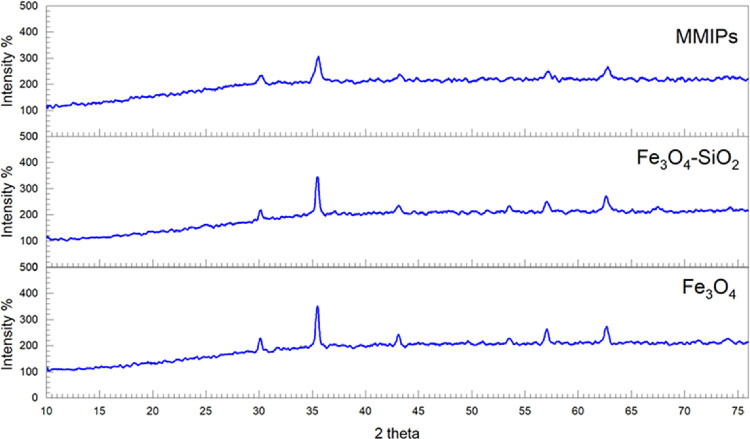
The crystalline structure of the drug carrier was determined by X-ray diffraction analysis. XRD was used to examine the structural characteristics of generated Fe3O4, core shells, and MMIPs. Diffraction peaks in the 2θ region of 10° to 75° (30°, 35°, 43.5°, 53.6°, 56.0°, and 63°) with crystalline planes of (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1), and (4 4 0), respectively (JCPDS NO. 19-0629), were in agreement with the typical spectrum of Fe3O4 NPs and identical to its core–shell, as shown in Figure 5. Broad peaks in the region of 2θ = 12° to 26° were observed in the XRD patterns of synthesized MMIPs, indicating the formation of an amorphous polymeric layer on the surface of Fe3O4 NPs. Furthermore, prominent reflection peaks in the range of 2θ = 30° to 75° were in agreement with the Fe3O4 pattern. The similarity of the patterns of Fe3O4, core–shell, and MMIPs revealed that the crystallography of magnetic NPs was maintained during the creation of MMIPs on the surface of Fe3O4. The intensity of signals or peaks decreases when we move from Fe3O4 to core–shell, and the intensity was further lowered from core–shell to the final product, which indicated the coating of silica and polymerization on Fe3O4.
Figure 5.
XRD graph of Fe3O4, silica-coated nanoparticles, and the magnetic molecularly imprinted polymer.
3.2. X-ray Analysis, MTT Assay, and Binding Capacity
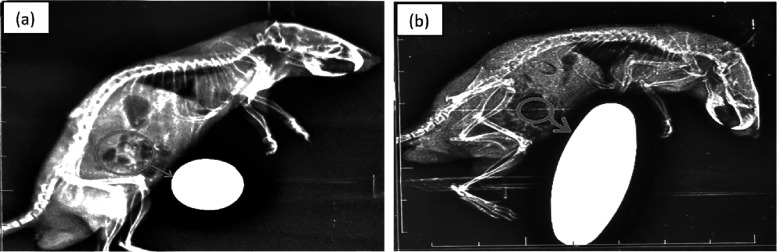
The designed drug carrier possesses the benefit of quick identification through direct external application of a magnetic field. This was made possible by incorporating iron oxide nanoparticles in the drug carrier. Figure 6 confirms that the drug loaded with the carrier reached near the magnet after the mice were administered with the dose.
Figure 6.
X-ray analysis image of mice loaded with (without drug) a tablet dose (a) and a MIP tablet dose (b).
The dose–response data was used to measure the toxicity of the polymer toward healthy cells. Hence, the MTT (3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide) assay was performed describing the percentage of living cells. The cell viability of the polymer was measured against non-cancerous cell lines (HepG2) and is given in Figure 7. This data showed that by increasing the concentration of polymer (MIDC), the percentage of living cells increased, proving that the polymer is non-toxic to healthy cells. When the concentration was changed from 0.3 to 100 μg, the percentage of living cells increased from 60% to 83% and these values were calculated after 72 h.
Figure 7.
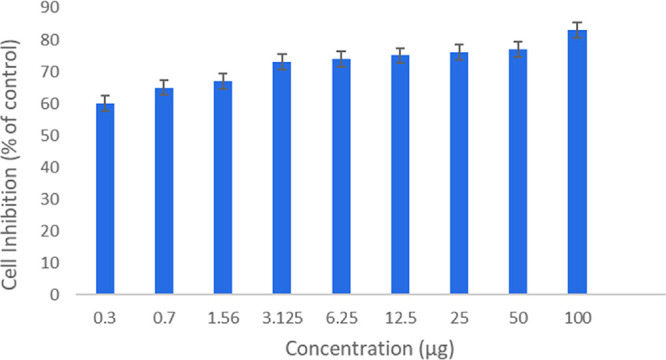
Effect of concentration of MIDC on cell viability.
The amount of docetaxel bound to the MMIP was calculated using eq 1.
| 1 |
where q (mg g–1), C0 (mg mL–1), Cf (mg L–1), V (L), and m (g) are the adsorption capacity, initial and final concentrations of DTX, sample volume, and mass of MMIP, respectively. The binding capacity was thus found to be 72 mg g–1.
3.3. Blood Sample Analysis
The drug released into the blood stream was investigated by analyzing the blood plasmas collected from the mice that were orally administered with the MMIP and MNIP. In Figure 8a, the primary standard drug was used as a reference and its peak appeared after 4 min of sample loading. In the case of the MNIP, the peak of the docetaxel did not appear in the HPLC chromatogram (Figure 8b), while the relevant peak of the drug was observed at a retention time of 4 min in the chromatogram obtained by using the MMIP (Figure 8c). This confirms the drug release from the MIP into the blood at the targeted site. Figure 8d shows the overlap of all the three chromatograms. Three types of chromatograms of the sample, standard, and MMIPs without the drug were taken for such analysis. From these values, the percentage recovery was calculated by applying the following equation
Cfound and Creal were calculated from their respective peak areas, and Cadded is the spiked amount. The percentage recovery of 96% (n = 9) was thus obtained.
Figure 8.
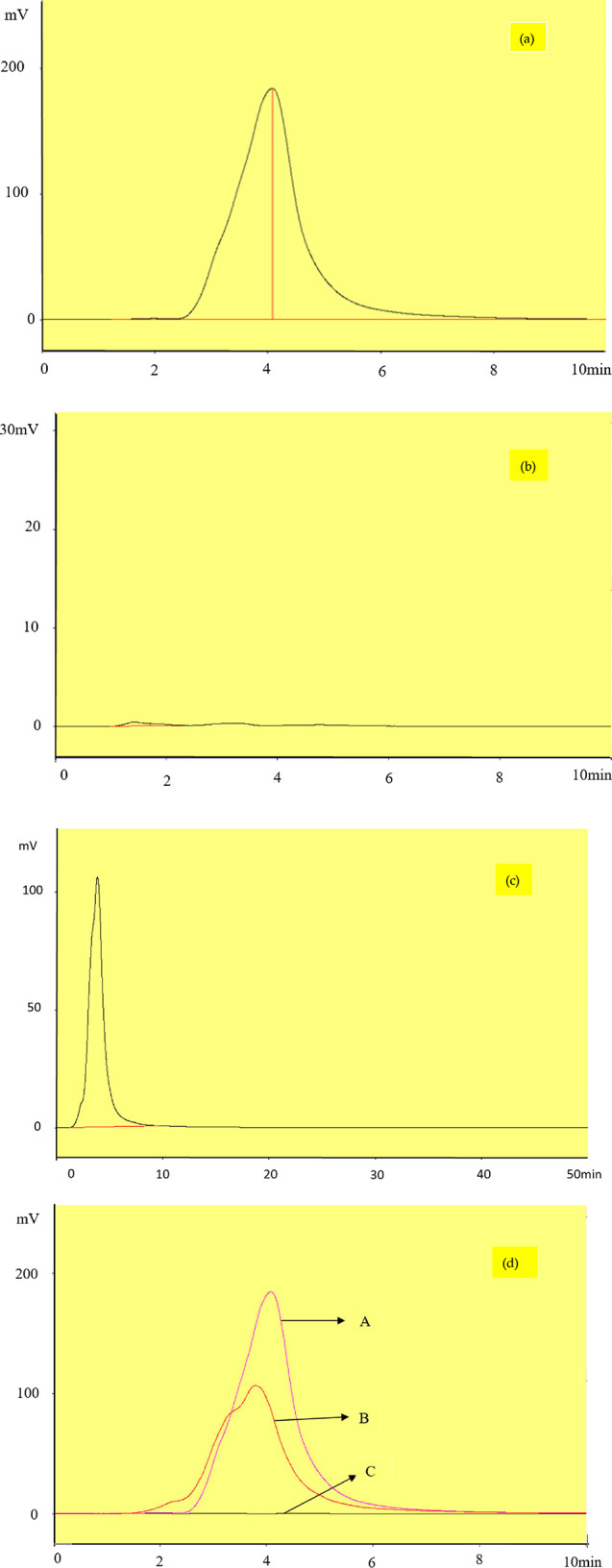
Chromatogram of 100 mg L–1 mobile phase (90–10 methanol/phosphate buffer (v/v %), flow rate of 0.5 mL min–1: (a) standard docetaxel, (b) blood plasma loaded with the MNIP, (c) and blood plasma loaded with MMIPs; (d) overlay of three chromatograms (a–c).
The expected mechanism of drug loading and release involves the formation of hydrogen bonds between the drug and cross-linker. Docetaxel contains various functional groups such as amino, hydroxyl, and carbonyl that can interact with glucose (cross-linker). These interactions are possibly reversible in aqueous medium; therefore, it was anticipated that the drug would probably release in the stomach due to its favorable environment.
3.4. Degradation Test
Studying the material’s self-degradation ability provides the most vital information for its selection in biomedical uses because its presence in the body for an extended period may be detrimental. At pH = 3, which is close to the stomach environment, and pH = 7.35, which is close to the physical state, the degradation of the MMIP was investigated (Table 2). The deterioration rate at pH = 3 (stomach environment) was faster than at pH 7.35, according to the data (Figure 9). Furthermore, the data show that material breakdown was faster in acidic and basic environments than in the body’s natural pH of 7.4. The discovered results are connected to the glucose-based cross-linker in the molecularly imprinted polymer structure.
Table 2. Values of Percentage Remaining Weight against Time at Different pH Levels.
| sr. no. | soaking time (day) | weight remaining (%) at pH 3 | weight remaining (%) at pH 7.35 |
|---|---|---|---|
| 1 | 1 | 100 | 100 |
| 2 | 2 | 93 | 96 |
| 3 | 3 | 91 | 93 |
| 4 | 4 | 88 | 92 |
| 5 | 5 | 87 | 91 |
| 6 | 6 | 86 | 90 |
| 7 | 7 | 84 | 88 |
| 8 | 8 | 83 | 88 |
| 9 | 9 | 82 | 87 |
| 10 | 10 | 82 | 86 |
Figure 9.
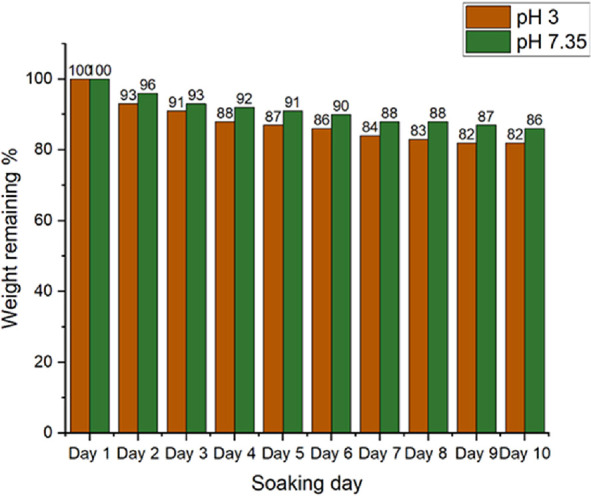
Degradation of the magnetic molecularly imprinted polymer at neutral and acidic pH.
3.5. Comparative Study
Though docetaxel is among one of the commonly employed anticancer drugs, its targeted drug delivery through MMIP has not been studied previously. The MMIP synthesized in this work for this purpose presented a binding capacity of 72 mg g–1. This value was compared with other drugs that have also been studied for the targeted delivery using MIPs as carriers. It is eminent to mention here that these MIPs were obtained through different synthetic schemes. Table 3 clearly depicts that the MMIP reported in this study presented a qe value comparable to others and hence can successfully be selected as a potential drug carrier for the targeted delivery.
Table 3. Comparison of the Current Study with the Previously Reported Work.
4. Conclusions
This study was based on the synthesis of fluorescence-active MMIP using a glucose cross-linker to create docetaxel-loaded unique multicore–shell structure nanocarriers. The final product was in the nano range, which can facilitate its in vivo movement. The MMIP exhibited an acceptable magnetic property for its detection through an external magnetic field. Moreover, due to the need for non-toxic materials in medical applications, several studies were developed to prove the biocompatibility of such materials. The MTT viability assay showed the MMIP’s non-toxic nature. In vitro drug release in simulated stomach fluid showed a fast release profile. A qe value of 72 mg g–1 describes its ability to load a considerable amount of drug. Overall, the findings show that the described MMIP can be exploited as a substantial device in drug delivery applications for malignant tissue treatment due to its exceptional features and diversified performances.
Acknowledgments
This research has been funded by the Research Deanship of University of Ha’il, Saudi Arabia, through the Project RG-20 122. The authors are grateful to higher Education Commission (HEC), and the Department of Pharmacy Bahauddin Zakariya University Multan, Pakistan for providing the lab facility to conduct this research work.
The authors declare no competing financial interest.
Notes
All the data in this study is provided in the manuscript.
References
- Clarke S. J.; Rivory L. P. Clinical pharmacokinetics of docetaxel. Clin. Pharmacokinet. 1999, 36, 99–114. 10.2165/00003088-199936020-00002. [DOI] [PubMed] [Google Scholar]
- Snyder J. P.; Nettles J. H.; Cornett B.; Downing K. H.; Nogales E. The binding conformation of Taxol in β-tubulin: a model based on electron crystallographic density. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 5312–5316. 10.1073/pnas.051309398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvon A. M. C.; Wadsworth P.; Jordan M. A. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol. Biol. Cell 1999, 10, 947–959. 10.1091/mbc.10.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Poppel H. Recent docetaxel studies establish a new standard of care in hormone refractory prostate cancer. Can. Urol. 2005, 12, 81–85. [PubMed] [Google Scholar]
- Chevallier B.; Fumoleau P.; Kerbrat P.; Dieras V.; Roche H.; Krakowski I.; Azli N.; Bayssas M.; Lentz M. A.; Van Glabbeke M. Docetaxel is a major cytotoxic drug for the treatment of advanced breast cancer: a phase II trial of the Clinical Screening Cooperative Group of the European Organization for Research and Treatment of Cancer. J. Clin. Oncol. 1995, 13, 314–322. 10.1200/JCO.1995.13.2.314. [DOI] [PubMed] [Google Scholar]
- Fossella F. V.; Lee J. S.; Shin D. M.; Calayag M.; Huber M.; Perez-Soler R.; Murphy W. K.; Lippman S.; Benner S.; Glisson B. Phase II study of docetaxel for advanced or metastatic platinum-refractory non-small-cell lung cancer. J. Clin. Oncol. 1995, 13, 645–651. 10.1200/JCO.1995.13.3.645. [DOI] [PubMed] [Google Scholar]
- Kaye S. B.; Piccart M.; Aapro M.; Francis P.; Kavanagh J. Phase II trials of docetaxel (Taxotere®) in advanced ovarian cancer—an updated overview. Eur. J. Cancer 1997, 33, 2167–2170. 10.1016/S0959-8049(97)00363-8. [DOI] [PubMed] [Google Scholar]
- Mavroudis D.; Kourousis C.; Androulakis N.; Kalbakis K.; Agelaki S.; Kakolyris S.; Souglakos J.; Sarra E.; Vardakis N.; Hatzidaki D.; Sarmonis G.; Georgoulias V. Frontline treatment of advanced gastric cancer with docetaxel and granulocyte colony-stimulating factor (G-CSF): a phase II trial. Am. J. Clin. Oncol. 2000, 23, 341–344. 10.1097/00000421-200008000-00005. [DOI] [PubMed] [Google Scholar]
- Ekladious I.; Colson Y. L.; Grinstaff M. W. Polymer–drug conjugate therapeutics: advances, insights and prospects. Nat. Rev. Drug Discovery 2019, 18, 273–294. 10.1038/s41573-018-0005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariatinia Z. Big family of nano- and microscale drug delivery systems ranging from inorganic materials to polymeric and stimuli-responsive carriers as well as drug-conjugates. J. Drug Delivery Sci. Technol. 2021, 66, 102790. 10.1016/j.jddst.2021.102790. [DOI] [Google Scholar]
- Trzebicka B.; Szweda R.; Kosowski D.; Szweda D.; Otulakowski Ł.; Haladjova E.; Dworak A. Thermoresponsive polymer-peptide/protein conjugates. Prog. Polym. Sci. 2017, 68, 35–76. 10.1016/j.progpolymsci.2016.12.004. [DOI] [Google Scholar]
- Stevens C. A.; Kaur K.; Klok H. A. Self-assembly of protein-polymer conjugates for drug delivery. Adv. Drug Delivery Rev. 2021, 174, 447–460. 10.1016/j.addr.2021.05.002. [DOI] [PubMed] [Google Scholar]
- Yokoyama M. Polymeric micelles as drug carriers: their lights and shadows. J. Drug Targeting 2014, 22, 576–583. 10.3109/1061186X.2014.934688. [DOI] [PubMed] [Google Scholar]
- Shi L.; Lu S.; Sun T.; Xi G.; Chen Z.; Xu K.; Zhao X.; Shen M.; Jia T.; Zhao X. Robust fluorescent amphiphilic polymer micelle for drug carrier application. New J. Chem. 2021, 45, 9409–9415. 10.1039/D1NJ01473K. [DOI] [Google Scholar]
- Zhang W. J.; Hong C. Y.; Pan C. Y. Polymerization-induced self-assembly of functionalized block copolymer nanoparticles and their application in drug delivery. Macromol. Rapid Commun. 2019, 40, 1800279. 10.1002/marc.201800279. [DOI] [PubMed] [Google Scholar]
- Siddique S.; Chow J. C. L. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. 10.3390/app10113824. [DOI] [Google Scholar]
- He J. S.; Liu S. J.; Zhang Y. R.; Chu X. D.; Lin Z. B.; Zhao Z.; Qiu S. H.; Guo Y. G.; Ding H.; Pan Y. L.; Pan J. H. The application of and strategy for gold nanoparticles in cancer immunotherapy. Front. Pharmacol. 2021, 12, 687399. 10.3389/fphar.2021.687399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D.; Tao W.; Zhang H.; Liu G.; Wang T.; Zhang L.; Zeng X.; Mei L. Docetaxel (DTX)-loaded polydopamine-modified TPGS-PLA nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Acta Biomater. 2016, 30, 144–154. 10.1016/j.actbio.2015.11.031. [DOI] [PubMed] [Google Scholar]
- Dawoud M. Chitosan coated solid lipid nanoparticles as promising carriers for docetaxel. J. Drug Delivery Sci. Technol. 2021, 62, 102409. 10.1016/j.jddst.2021.102409. [DOI] [Google Scholar]
- Dawoud M.; Abourehab M. A. S.; Abdou R. Monoolein cubic nanoparticles as novel carriers for docetaxel. J. Drug Delivery Sci. Technol. 2020, 56, 101501. 10.1016/j.jddst.2020.101501. [DOI] [Google Scholar]
- Ndunda E. N. Molecularly imprinted polymers—a closer look at the control polymer used in determining the imprinting effect: a mini review. J. Mol. Recognit. 2020, 33, e2855 10.1002/jmr.2855. [DOI] [PubMed] [Google Scholar]
- Vasapollo G.; Sole R. D.; Mergola L.; Lazzoi M. R.; Scardino A.; Scorrano S.; Mele G. Molecularly imprinted polymers: present and future prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. 10.3390/ijms12095908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köse K.; Kehribar D. Y.; Uzun L. Molecularly imprinted polymers in toxicology: A literature survey for the last 5 years. Environ. Sci. Pollut. Res. 2021, 28, 35437–35471. 10.1007/s11356-021-14510-4. [DOI] [PubMed] [Google Scholar]
- Ostovan A.; Ghaedi M.; Arabi M.; Asfaram A. Hollow porous molecularly imprinted polymer for highly selective clean-up followed by influential preconcentration of ultra-trace glibenclamide from bio-fluid. J. Chromatogr. A. 2017, 1520, 65–74. 10.1016/j.chroma.2017.09.026. [DOI] [PubMed] [Google Scholar]
- Dong C.; Shi H.; Han Y.; Yang Y.; Wang R.; Men J. Molecularly imprinted polymers by the surface imprinting technique. Eur. Polym. J. 2021, 145, 110231. 10.1016/j.eurpolymj.2020.110231. [DOI] [Google Scholar]
- Gopi S.; Amalraj A.; Sukumaran N. P.; Haponiuk J. T.; Thomas S. Biopolymers and their composites for drug delivery: a brief review. Macromol. Symp. 2018, 380, 1800114. 10.1002/masy.201800114. [DOI] [Google Scholar]
- Ansari S. Application of magnetic molecularly imprinted polymer as a versatile and highly selective tool in food and environmental analysis: Recent developments and trends. Trends Anal. Chem. 2017, 90, 89–106. 10.1016/j.trac.2017.03.001. [DOI] [Google Scholar]
- Ansari S.; Karimi M. Recent configurations and progressive uses of magnetic molecularly imprinted polymers for drug analysis. Talanta 2017, 167, 470–485. 10.1016/j.talanta.2017.02.049. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Feng Z.; Li S.; Lu Y.; Hu Y.; Fan H.; Zhai X. Preparation of Magnetic Molecularly Imprinted Polymers for Selective Recognition and Determination of Clenbuterol in Pork Samples. J. Chem. 2020, 8820262. 10.1155/2020/8820262. [DOI] [Google Scholar]
- Yang C.; Li Y.; Yang Y.; Chen Z. Overview of strategies to improve therapy against tumors using natural killer cell. J. Immunol. Res. 2020, 8459496. 10.1155/2020/8459496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeilpour M.; Zahmatkesh S. Chemistry, Palladium nanoparticles immobilized on EDTA-modified Fe3O4@ SiO2: a highly stable and efficient magnetically recoverable catalyst for the Heck–Mizoroki coupling reactions. Inorg. Nano-Met. Chem. 2019, 49, 267–276. 10.1080/24701556.2019.1661445. [DOI] [Google Scholar]
- Hashemi-Moghaddam H.; Kazemi-Bagsangani S.; Jamili M.; Zavareh S. Evaluation of magnetic nanoparticles coated by 5-fluorouracil imprinted polymer for controlled drug delivery in mouse breast cancer model. Int. J. Pharm. (Amsterdam, Neth.) 2016, 497, 228–238. 10.1016/j.ijpharm.2015.11.040. [DOI] [PubMed] [Google Scholar]
- Wahajuddin; Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445. 10.2147/IJN.S30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Lorenzo C.; Concheiro A. Smart drug delivery systems: from fundamentals to the clinic. Chem. Commun. 2014, 50, 7743–7765. 10.1039/C4CC01429D. [DOI] [PubMed] [Google Scholar]
- Sung Y. K.; Kim S. W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 1–12. 10.1186/s40824-020-00190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogay V.; Mun E. A.; Kudaibergen G.; Baidarbekov M.; Kassymbek K.; Zharkinbekov Z.; Saparov A. Progress and prospects of polymer-based drug delivery systems for bone tissue regeneration. Polymer 2020, 12, 2881. 10.3390/polym12122881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M.; Xiao J.; Li H.; Hai L.; Yang K.; Li J.; Wang Z.; Deng L.; He D. Magnetically retained and glucose-fueled hydroxyl radical nanogenerators for H2O2-self-supplying chemodynamic therapy of wound infections. Mater. Sci. Eng., C 2021, 131, 112522. 10.1016/j.msec.2021.112522. [DOI] [PubMed] [Google Scholar]
- Qin Y. T.; Feng Y. S.; Ma Y. J.; He X. W.; Li W. Y.; Zhang Y. K. Tumor-sensitive biodegradable nanoparticles of molecularly imprinted polymer-stabilized fluorescent zeolitic imidazolate framework-8 for targeted imaging and drug delivery. ACS Appl. Mater. Interfaces 2020, 12, 24585–24598. 10.1021/acsami.0c05154. [DOI] [PubMed] [Google Scholar]
- He S.; Zhang L.; Bai S.; Yang H.; Cui Z.; Zhang X.; Li Y. Advances of molecularly imprinted polymers (MIP) and the application in drug delivery. Eur. Polym. J. 2021, 143, 110179. 10.1016/j.eurpolymj.2020.110179. [DOI] [Google Scholar]
- Shevchenko K. G.; Garkushina I. S.; Canfarotta F.; Piletsky S. A.; Barlev N. A. Nano-molecularly imprinted polymers (nanoMIPs) as a novel approach to targeted drug delivery in nanomedicine. RSC Adv. 2022, 12, 3957–3968. 10.1039/D1RA08385F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.; Poma A. Advances in molecularly imprinted polymers as drug delivery systems. Molecules 2021, 26, 3589. 10.3390/molecules26123589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.; Hu J.; Fu S. Controlled synthesis of magnetite– silica nanocomposites via a seeded sol– gel approach. J. Phys. Chem. C 2009, 113, 7646–7651. 10.1021/jp900868d. [DOI] [Google Scholar]
- Huang J.; Liu H.; Men H.; Zhai Y.; Xi Q.; Zhang Z.; Zhang J.; Yin Z.; Li L. Molecularly imprinted polymer coating with fluorescence on magnetic particle. Macromol. Res. 2013, 21, 1021–1028. 10.1007/s13233-013-1134-2. [DOI] [Google Scholar]
- Chung K. T.; Wong T. Y.; Wei C. I.; Huang Y. W.; Lin Y. Tannins and human health: a review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. 10.1080/10408699891274273. [DOI] [PubMed] [Google Scholar]
- Dong H.; Zhao F.; He Q.; Xie Y.; Zeng Y.; Zhang L.; Tang L.; Zeng G. Physicochemical transformation of carboxymethyl cellulose-coated zero-valent iron nanoparticles (nZVI) in simulated groundwater under anaerobic conditions. Sep. Purif. Technol. 2017, 175, 376–383. 10.1016/j.seppur.2016.11.053. [DOI] [Google Scholar]
- Cheng Y.; Nie J.; Li J.; Liu H.; Yan Z.; Kuang L. Synthesis and characterization of core–shell magnetic molecularly imprinted polymers for selective recognition and determination of quercetin in apple samples. Food Chem. 2019, 287, 100–106. 10.1016/j.foodchem.2019.02.069. [DOI] [PubMed] [Google Scholar]
- Asadi E.; Abdouss M.; Leblanc R. M.; Ezzati N.; Wilson J. N.; Azodi-Deilami S. In vitro/in vivo study of novel anti-cancer, biodegradable cross-linked tannic acid for fabrication of 5-fluorouracil-targeting drug delivery nano-device based on a molecular imprinted polymer. RSC Adv. 2016, 6, 37308–37318. 10.1039/C6RA03704F. [DOI] [Google Scholar]
- Zhang Z.; Chen X.; Rao W.; Chen H.; Cai R. Synthesis and properties of magnetic molecularly imprinted polymers based on multiwalled carbon nanotubes for magnetic extraction of bisphenol A from water. J. Chromatogr., B 2014, 965, 190–196. 10.1016/j.jchromb.2014.06.031. [DOI] [PubMed] [Google Scholar]
- Hande P. E.; Samui A. B.; Kulkarni P. S. A molecularly imprinted polymer with flash column chromatography for the selective and continuous extraction of diphenyl amine. RSC Adv. 2015, 5, 73434–73443. 10.1039/C5RA11965K. [DOI] [Google Scholar]
- Sedghi R.; Yassari M.; Heidari B. Thermo-responsive molecularly imprinted polymer containing magnetic nanoparticles: Synthesis, characterization and adsorption properties for curcumin. Colloids Surf., B 2018, 162, 154–162. 10.1016/j.colsurfb.2017.11.053. [DOI] [PubMed] [Google Scholar]