Abstract

Scale formation and deposition in the subsurface and surface facilities have been recognized as a major cause of flow assurance issues in the oil and gas industry. Sulfate-based scales such as sulfates of calcium (anhydrite and gypsum) and barium (barite) are some of the commonly encountered scales during hydrocarbon production operations. Oilfield scales are a well-known flow assurance problem, which occurs mainly due to the mixing of incompatible brines. Researchers have largely focused on the rocks’ petrophysical property modifications (permeability and porosity damage) caused by scale precipitation and deposition. Little or no attention has been paid to their influence on the surface charge and wettability of calcite minerals. Thus, this study investigates the effect of anhydrite and barite scales’ presence on the calcite mineral surface charge and their propensity to alter the wetting state of calcite minerals. This was achieved vis-à-vis zeta-potential (ζ-potential) measurement. Furthermore, two modes of the scale control (slug and continuous injections) using ethylenediaminetetraacetic acid (EDTA) were examined to determine the optimal control strategy as well as the optimal inhibitor dosage. Results showed that the presence of anhydrite and barite scales in a calcite reservoir affects the colloidal stability of the system, thus posing a threat of precipitation, which would result in permeability and porosity damage. Also, the calcite mineral surface charge is affected by the presence of calcium and barium sulfate scales; however, the magnitude of change in the surface charge via ζ-potential measurement is insignificant to cause wettability alteration by the mineral scales. Slug and continuous injections of EDTA were implemented, with the optimal scale control strategy being the continuous injection of EDTA solutions. The optimal dosage of EDTA for anhydrite scale control is 5 and 1 wt % for the formation water and seawater environments, respectively. In the case of barite, in both environments, an EDTA dosage of 1 wt % suffices. Findings from this study not only further the understanding of the scale effects on calcite mineral systems but also provide critical insights into the potential of scale formation and their mechanisms of interactions for better injection planning and the development of a scale control strategy.
1. Introduction
Scale formation in subsurface and surface facilities has been recognized as a major concern in oil and gas production operations.1−3 These scales are sulfates such as calcium sulfate (anhydrite and gypsum), barium sulfate (barite), and calcium carbonate. Sulfate scale precipitation and deposition are serious oilfield operational problems that affect water (brine) injection systems.4,5 The most common cause of an oilfield scale is the mixing of brines that are incompatible. Brines are said to be incompatible if they interact chemically and precipitate minerals when mixed during water injection.3 For instance, when seawater (SW) with a high concentration of SO42+ and low concentrations of Ca2+ and Ba2+ is mixed with formation brine that contains high concentrations of Ca2+ and Ba2+ but a low concentration of SO42+, it causes the precipitation of CaSO4 and/or BaSO4.5−7 More so, when SW is mixed with the produced water for injection, scale precipitation is bound to occur.4
Commonly encountered sulfate scales in the oil and gas industry are barium sulfate (BaSO4) and calcium sulfate (CaSO4), which can be anhydrous (anhydrite)8 or hemihydrate (bassanite).9 Also unlike the CaSO4 scale, which has an inverse or retrograde solubility, BaSO4 solubility increases with temperature.8,10,11 A major concern of the retrograde solubility of CaSO4 (e.g., anhydrite) is scale formation at elevated temperatures.8 Although CaSO4 has a high solubility in water compared to that of BaSO4, under the same conditions, the scales of the two sulfates behave similarly except that CaSO4 is more soluble.12,13 Moreover, some scales are pH-dependent (calcium carbonate and sulfide scales), while some are not (e.g., calcium, barium, and strontium sulfate scales). Generally, pH-dependent scales are easier to treat than pH-independent scales.1,2 This is because pH-dependent scales can be easily treated by acid injection, while this is not suitable for pH-independent scales.14 Thus, it is better to inhibit their occurrence, which can be achieved using chemical inhibitors such as chelating agents,12,15,16 regulation of thermodynamic conditions of temperature and pressure, or by proper formulation of injected brine.1,17
The mechanisms of scale formation in reservoirs are (i) change in pressure and/or temperature, (ii) mixing of incompatible brines with different sulfate and cation (Ca2+ and Ba2+) concentrations, and (iii) brine evaporation and consequent increase in salt concentration.18 These scales impede flow and damage the reservoir and flow equipment,19 with the problem more intense at elevated temperatures.8,13
Sulfate-based scales limit and block oil and gas production by plugging the pore throats and causing wettability alterations.20 In water injection systems, sulfate scales could be deposited on pore walls or plug the pores, thus resulting in formation damage (porosity and permeability reduction), which leads to poor hydrocarbon recovery.18 Even though sulfate scales also get deposited on rocks and alter their surface properties, most researchers focus on scaling on steel surfaces21,22 and formation damage associated with scale deposition on rocks.18 BinMerdhah et al.18 investigated permeability reduction caused by BaSO4 scales in a sandstone formation. The authors by mixing SW and formation brine demonstrated that the permeability reductions reported are due to BaSO4 scale deposition on the pore surface. However, the effect of the deposited scale on the rock’s surface wettability was not reported.
Abbasi et al.5 investigated sulfate precipitation and scale formation in carbonate rocks by mixing different injection water with varying concentrations of sulfate and salinity [high and medium sulfate waters, SW, and low-salinity water (LSW)]. The authors showed that the mixing of brine with a high SO42– content increases the scale precipitation. The authors further employed the use of the zeta-potential (ζ-potential) for surface charge chemistry analysis to show that increasing the concentration of SO42– and, consequently, a higher magnitude of sulfate scales alters rock surface wettability into more water wet. However, they concluded that based on the results of the brine compatibility test, scanning electron microscopy (SEM), and ζ-potential, the optimal sulfate content should be defined to minimize the scale formation and obtain a high potential for wetting alteration simultaneously. Nikoo and Malayeri23 studied the impact of the rock surface type and topography during scale formation in scale/brine/rock interactions. They showed that different rock surfaces have different affinities for CaSO4 scales. Interfacial interactions between scales and the rock surface can be attributed to electrostatic interactions, an electrical double layer (EDL), Lifshitz–van der Waals forces, or Lewis acid–base interactions, and their magnitude is strongly salinity-dependent.24,25 However, Lewis’ acid–base interactions dominate for CaSO4 scale–rock interactions.
Studies have shown that sulfate scales can alter the surface properties and wetting behavior of rock surfaces.3,16,26 Although an extensive body of literature is available for bulk precipitation reactions,17,22 only a limited number of published articles5,27−29 focus on the effect of scales on rock surface chemistry which greatly influences its wetting behavior. Nikoo and Malayeri30 examined sulfate scale/brine/rock interfacial interactions and the rock wettability due to CaSO4 crystals. The authors used anhydrite, calcite, dolomite, and sandstone for their study and findings showed that the rocks become prone to scale formation and deposition as the fluid salinity and rock surface roughness are increased. Thus, researchers have recently faced the question as to what extent the surface chemistry, and thus the wetting behavior of rocks, is affected by sulfate scale deposition during the process of water injection. More so, scale introduction into the reservoir formation by a drilling fluid that contains a high concentration of barite has received less attention as to how it affects the near-wellbore wettability. Moreover, with this question yet unanswered, it has become important to study and understand the mechanisms of BaSO4 and CaSO4 precipitation and deposition on rocks. This is to help precisely predict scale occurrence and control the rock surface characteristics.
To the best of our knowledge, the available published works23,30 which have precisely investigated the affinity of the rock surface to scale formation are limited. Also, the wettability alteration potential of deposited scales on carbonate rock surfaces has not been investigated. The deposition of sulfate scales may affect the wetting characteristics of the rock surface, which, therefore, influence the ultimate recovery. Another point is the lack of experimental methods to verify the precise wetting potential of scales, their mechanism, and their effect on enhanced oil recovery. Thus, the current study reports systematic research (ζ-potential measurements) from the perspective of the surface charge by laboratory tests to assess the effect of sulfate-based scales on calcite mineral surface chemistry (charge). This stems from our earlier work20 which revealed that different oilfield operations, as well as scales, affect the net surface charge of calcite systems. Therefore, results from this study will enhance the understanding of the precise effect of scales on carbonate wettability and their implications in oilfield applications.
2. Wettability Alteration and the ζ-Potential
Due to the significant impact it has on the production capacity, reservoir wettability is a crucial concept in reservoir engineering and has been the focus of numerous studies over the years.31 Changes in the pore system (for instance, adsorption), which may be brought on by fluid–fluid interactions, rock–fluid interactions, rock mineralogy, and brine chemistry, have been substantially responsible for wettability alteration.32,33 These could cause the rock’s wettability to change completely or partially. Strong interfacial boundary conditions within the rock system that cause the fluid to be preferentially mobile in the presence of other fluids are what cause reservoir wettability.34 Therefore, it is mostly caused by a fluid that is either hydrophobic or hydrophilic and adheres to or coats the pore structure, making the carbonate rocks exhibit an oil-wetting state. Most largely confirmed in the literature, the wettability alteration of rocks is attributed to the adsorption of the most polar crude oil component (asphaltene).31 Asphaltene is said to be the heaviest and most polar crude oil component, soluble in aromatic fluids and insoluble in alkanes.35 Because of the electrochemical interactions, asphaltene coats the pore surface and alters its wettability. Furthermore, the rate of asphaltene adsorption is linearly correlated with the change in wettability.6,36−44 Regarding the relationship between the crude oil, asphaltene content, and adsorption, opposing views have been voiced. This could be because the different studies used varying experimental conditions and models which failed to account for the effect of brine and rock–fluid interactions.45−47
Since asphaltene is believed to be predominately negatively charged, mineral surfaces must be positively charged for asphaltene adsorption to occur.35 We have previously discussed the various minerals found in the reservoir, their surface charges, the composition of the brine, and the forms of salts that can create a mineral surface that can act as a precursor to asphaltene adsorption.20,32,33,48,49 According to the results of these investigations, the mineral–brine interface must be positively charged for asphaltene adsorption to occur because the electrostatic interaction is said to be the primary regulating mechanism.50 This consequently changes the wettability. Although various wettability alteration mechanisms have been identified in the literature studies,51−53 several factors, such as oilfield operations and ion-specific interactions, affect reservoir wettability.
Multivalent ionic exchange, an EDL expansion, electrostatic bond interactions, surface charge alteration, and calcite dissolution are the wettability modification mechanisms connected with carbonate rocks.32,54−58 This is highlighted in the report of Al-Bayati et al.59 who described the change in the carbonate rock samples and concluded that the observed recovery was caused by the EDL effect brought on by the injected fluid’s reduced ionic strength. Thus, understanding the electrostatic interaction at the rock surface caused by the salinity and composition of the brine is necessary to understand these mechanisms. Because of the mineral dissolution, adsorption of counterions, and formation of chemical complexes, colloidal particles in aqueous solutions acquire charges on their surfaces, as experimentally determined vis-à-vis ζ-potential measurements. When an electric field is applied to an ionic solution, particles having net charges move with a predetermined velocity, and thus, the determination of the particles’ ζ-potential uses this movement, known as electrophoretic mobility.60−62 The ζ-potential is the potential at a particle’s sliding plane in a medium, and its measurement can reveal details about a suspended particle’s surface charge, including its nature and size.63,64 Additionally, it provides information on a particle’s stability and flocculation potential. As a result, it has uses in the production of flocculants and dispersants as well as in water treatment.
ζ-potential values can indicate changes in the state of the surface, such as wettability alterations.32 It is a qualitative technique that depicts a change in surface chemistry by reduction or increase in magnitude and sign of the ζ-potential value. However, little or no correlation has been established with wettability other than a change in magnitude and sign of the ζ-potential, which could be due to adsorption of fluids on the particle surface or dissolution of ions from the surface. However, studies were conducted to see if the ζ-potential may be used as a special indicator of recovery for LSW.65 Streaming potential measurements and core flooding experiments were used by the authors to assess the crude oil/brine/rock system. Results showed that the recovery and ζ-potential of the water–oil and rock–water interfaces are correlated. Therefore, recovery was seen to be beneficial if both interfaces had the same surface charge; yet, inferences were made that the ζ-potential cannot be used as a sole tool for recovery predictions. Thus, at best, it can only be used as a qualitative check; thus, a comparison of wettability alteration using the ζ-potential measurement and the popular Amott–Harvey and USBM methods is not straightforward and can be misleading.8 Depending on the adsorbing fluid properties and the fluids’ propensity to interact with the surface, the ζ-potential can be used as a sign of wettability change. Additionally, the use of ζ-potential measurement in wettability alteration experiments gives researchers insight into wettability changes but does not allow in situ changes to be observed.66 Furthermore, a thorough understanding of the chemistry of the fluids and surfaces involved in the interactions is necessary to infer the wettability alterations from ζ-potential measurements.
To understand the interactions (intermolecular forces) acting on colloids or charged surfaces in an electrolyte solution, the Derjaguin–Landau–Verwey–Overbeek (DLVO) theory was developed. The DLVO theory is a classical explanation of the intermolecular forces (electrostatic repulsion and van der Waals attraction) responsible for the stability of colloids in a suspension. The effect of crude oil fractions, pH, and salinity on the film thickness on mineral surfaces using the DLVO theory is well documented in the literature studies.67,68 The principles of thin-film thickness measurement using atomic force microscopy (AFM) were developed in Basu and Sharma’s work.68 This was done to determine how much these factors affected wettability changes. According to the authors’ findings, a decrease in film thickness was seen when both salinity and pH values were reduced. A higher salinity and pH, however, led to a more stable film in the case of the nonpolar crude oil fractions. Because glass beads were used in the study and the pH range was only 5.5–8.0, these findings have some limitations. They thus give insights into the impact of salt interactions and pH circumstances on wettability alteration. To investigate the impact of altered water chemistry on oil recovery supported by the ζ-potential and contact angle measurement, Sanaei et al.69 used the DLVO theory and a surface complexation model. With the results showing the dominance of the surface charge in the observed recovery, the physics of improved recovery in LSW flooding could be understood. ζ-potential values and a measurement of the contact angle provided additional support for this; nevertheless, the impact of salt was not considered in their analysis. The presence of divalent cations and the sulfate ion is essential for determining surface wetness according to Tirjoo et al.70 who studied the impact of the brine ionic composition on wettability. More specifically, it clarified how to compute the film thickness using the DLVO theory.
3. Smart and Low Salinity Water
A causative operation that can result in a sulfate scale problem is the injection of smart water. Smart water is a brine whose chemical composition has been altered to achieve ion-specific interactions with the rock–fluid system.5,71−73 In most cases, the said smart water contains high and low dosages of SO42– and divalent cations, respectively.5 Smart water differs from LSW, which results from the dilution of formation brine [formation water (FW)] or SW.27 These two terms have been erroneously used in the literature;74 however, they mean two different brine systems. The effect of both brines has gained significant attention recently and continues to be an active area of research. Several studies are available in the literature on smart and LSW effects on reservoir wettability and oil recovery.27,59,75−78 For example, using AFM and contact angle and ζ-potential measurements, Al Maskari et al.79 described the impact of pH (caused by mineral dissolution in LSW) on carbonate surface wettability. Findings indicated that the contact angle decreased as pH increased, and this observation was corroborated by an increase in magnitude of the negative surface charge under the same circumstances. Additionally, the AFM’s adhesion test revealed that the adhesion force decreased as pH rose, indicating that the surface’s hydrophilicity had increased. Snosy et al.74 in a thorough investigation of the effect of LSW on carbonate reservoirs concluded that the recovery is controlled by the water’s composition rather than its salinity. More so, the concentrations of Na+, Mg2+, K+, and Cl– have the largest impact during the secondary recovery stage, whereas concentrations of K+, HCO3–, and SO42– and the mineralogy are the most important characteristics during the tertiary recovery stage. Similarly, Zekri et al.80 in an assessment of LSW injection in different wettability states using core flood experiments and effluent analysis concluded that the optimum LSW is a function of the wetting state of the rock. Thus, priority should be accorded to the brine composition rather than the salinity level.
In an analysis of the impact of LSW flooding on chalk core samples, Mokhtari et al.81 concluded that 10-fold diluted SW exhibits greater recovery than FW and SW. Furthermore, the scientists deduced from the ion effluent study that mineral dissolution cannot be the sole mechanism responsible for the observed recovery and that the effect of SO42– is not responsible for observed recovery in all situations. More recently, Tetteh et al.58 used the diffuse layer model (DLM) and triple-layer model (TLM) of surface complexation to model the carbonate rock wettability. The experimental ζ-potential values for the SW brine used in the investigation were more closely matched by the DLM than by the TLM according to the results. The computed interaction potential at the interfaces further demonstrated that low Mg2+ and high SO42– resulted in a more negative calcite–brine interface. Therefore, to achieve a water-wet state, a more negative interface surface charge is required.
As robust as the research on LSW and smart water is and their implications on oil recovery, it is not without a drawback, which includes sulfate scale precipitation and salt dispersion. The salt dispersion, which happens when LSW mixes with high-salinity water, is one of the downsides of LSW flooding; however, new research suggests that by adding around 200 ppm polymer, the salt dispersion can be decreased by roughly 70%, resulting in a lower volume of LSW usage.82 Also, with so many research articles emphasizing the benefits of increased SO42– concentration on oil recovery, no attention has been paid to the potential damage it can cause due to sulfate-based scale precipitation, which can result in wettability alterations and permeability damage. Thus, as stated in the preceding section, this research is aimed at understanding the effect of sulfate-based scales on the calcite mineral surface charge and the consequent wettability state.
4. Materials and Methods
4.1. Materials
The rock minerals used in this study include calcite, barite, and anhydrite samples with average particle sizes of 8.4, 19.01, and 8.26 μm for calcite, barite, and anhydrite, respectively. All chemicals used in this study were of the American Chemical Society (ACS) reagent grade. The synthetic brine solution compositions used (Table 1) mimic the Arabian Gulf SW and FW. The salts used in this study were purchased from Sigma Aldrich (Saint Louis, MO, USA).
Table 1. Ionic Composition of Arabian Gulf FW and SSW.83.
| ions | FW (ppm) | SW (ppm) |
|---|---|---|
| Na+ | 59,491 | 18,300 |
| Ca2+ | 19,040 | 650 |
| Mg2+ | 2439 | 2110 |
| SO42– | 350 | 4290 |
| Cl– | 132,060 | 32,200 |
| HCO3– | 354 | 120 |
| TDS | 213,734 | 57,670 |
4.2. Sample Preparation for ζ-Potential Measurement
Rock mineral particles were conditioned in deionized (DI) water, SW, and FW as well as in mixtures whose pH was modified to mimic that in oilfield operations. For all samples, the total dissolved mineral particles were 1 g, with the proportion of barite and anhydrite samples to achieve the required ratio added to the calcite mineral sample. After that, 10 mL of the fluid was then added to the sample mixture and allowed to stand undisturbed for 24 h. The pH adjustment of FW and SW was achieved using 0.1 mM NaOH and nitric acid to avoid surface etching.
4.3. ζ-Potential Measurement
An Anton Paar Litesizer 500 (Graz, Austria) machine was used for the sample mixture ζ-potential measurements. Before every measurement, the sample mixtures were centrifuged using a Hermle Z326K centrifuge (Gosheim, Germany). Samples were centrifuged at 23 °C for 2 min at 3000 rpm before the ζ-potential measurement, and the sample to be analyzed was taken from the clear supernatant. All reported measurements in this study are within ±2 mV standard deviation and an average of 20 runs for each sample.
4.4. pH Measurement
The pH value of the freshly prepared brines and all samples in this study was measured using the Thermo Scientific Orion Star A215 pH/conductivity benchtop multiparameter meter, with an accuracy of ±0.002.
5. Results and Discussion
The effect of sulfate-based scales on the calcite mineral surface charge is investigated using ζ-potential measurement. To provide a holistic picture of the effects, the influence of the varying proportions (wt %) of the scales (barite and anhydrite) on calcite surface chemistry is explored. As an initial step, the pH of the fluids (DI and synthetic brines) used in this study was recorded. The pH values were 7.64, 7.65, and 6.27 for DI water, SW, and FW, respectively. These pH values are the benchmark against which all reported pH values are compared in this study. This is done to infer governing mechanisms responsible for the observed surface charges.
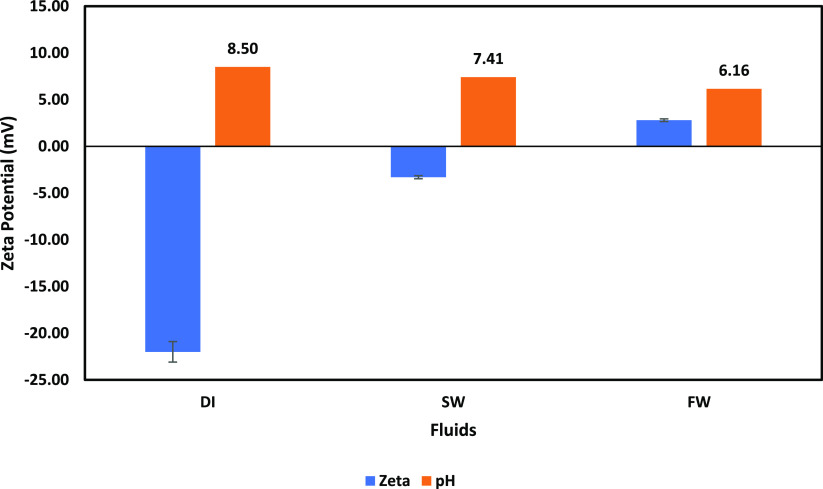
5.1. Calcite in DI Water, FW, and SW
The surface charge of the calcite mineral is first studied to serve as a benchmark against which the effect of scales would be compared. The ζ-potential of calcite minerals in DI water, SW, and FW is shown in Figure 1. Figure 1 shows that the calcite mineral in DI water and SW is negatively charged, while it shows a positive charge in FW. To understand the mechanism responsible for the observed surface charge, the initial and final pH values are critical. In the case of DI water, the initial pH was recorded as 7.64; however, after calcite mineral conditioning in DI water, the resulting pH of the calcite/DI mixture was 8.50. This points to the fact that H+ and OH– in the case of DI water are potential-determining ions (PDIs). Also, the increase in pH from 7.64 to 8.50 shows that the dominant interaction is due to the Hwater and Ocalcite interactions, resulting in more OHwater in the system and thus the shift in pH. In the case of SW and FW, no pH change is observed, which indicates that H+ and OH– are not PDIs. Furthermore, this implies that the surface charge development of the calcite mineral in SW and FW is due to their respective fluid compositions (ions).
Figure 1.
Calcite mineral surface charge in DI water, SW, and FW.
In the case of SW (Figure 1), the calcite mineral is negatively charged; however, the magnitude of the charge is small. The negative surface charge can be attributed to the presence of anions (Cl– and SO42–) in SW, especially SO42–. However, the low magnitude of the charge can be attributed to the competitive interaction of the cations (Na+, Ca2+, and Mg2+) with the calcite surface. Although due to the high SO42– concentration in SW, the surface remains negatively charged; however, this can be easily reversed by a change in the fluid composition. The reported calcite mineral surface charge in SW agrees with the report of Rodrigues et al.65 who studied the effect of controlled salinity flooding on the oil–water–carbonate system. Furthermore, our observations differ slightly from the report of Belhaj et al.84 who reported a higher magnitude of the surface charge value even though both cases agree on the nature (negative surface charge) of the calcite mineral surface charge in SW. This disparity can be attributed to the fact that the samples used in their study contain quartz and dolomite which resulted in an increased negative surface charge. However, in our study, pure calcite minerals were used. Similarly, our observation is corroborated by the report of Kasha et al.85 The authors used old and unaged limestone samples and ζ-potential measurements to describe the interactions of PDIs (Mg2+, Ca2+, and SO42–) on limestone. According to the authors’ findings, SO42– dominates in creating and maintaining a negative surface charge for both aged and unaged samples when additional PDIs are present.
The case of FW is similar to that of SW, in which no pH change was observed. However, the FW has a higher cation concentration than SW and has 10 times less SO42– than SW. Thus, the surface charge of calcite in FW is positive due to the adsorption of cations on the calcite surface. Figure 1 also shows that increased salinity affects the resulting surface charge of calcite minerals, and thus, salinity reduction may hold the key to the control of the mineral surface charge. This agrees with the report of Tetteh et al.,58 which demonstrated that an increase in cation concentration results in a reduced negative surface charge. More so, an increase in cation concentration results in the formation of a salt layer on the calcite mineral surface.86
5.2. Effect of the Anhydrite Scale on the Calcite Mineral Surface Charge
Anhydrite scales form due to acidic sulfate water and calcium present in the reservoir,87 and like calcite, their solubility decreases with increased temperature.11 The effect of anhydrite (1–20 wt %) on the surface charge of calcite minerals is shown in Figure 2. In the case of DI water (Figure 2A), the calcite mineral is observed to remain negatively charged, as shown in Figure 1, without the effect of anhydrite; however, the magnitude of the surface charge is reduced due to the presence of the anhydrite scale. The lowest magnitude of the surface charge is observed at the 1 wt % anhydrite concentration; however, above this concentration (3–20 wt %), the surface charge magnitude increased to approximately −10 mV. The surface charge at 1 wt % can be attributed to the dominance of Ca2+ at low anhydrite concentrations; however, above 1 wt %, SO42– dominated the interactions and thus the increased negative surface charge as the anhydrite scale concentration increases. Notably, no significant pH changes (Figure 2B) are observed with an increase in anhydrite scale concentration except at 15 wt %, which explains the reduction in the ζ-potential value. Furthermore, except at an anhydrite concentration of 1 wt %, the same surface charge is observed, which implies the complete coverage of the active sites on the calcite surface by the anhydrite scale. This also points to the fact that the anhydrite scale has the most impact on the calcite surface charge when present at low concentrations (<3 wt %).
Figure 2.
Effect of the anhydrite scale on the calcite mineral surface charge. (A) ζ-potential values of calcite/anhydrite mixtures in varying fluids. (B) pH values of the mixtures after 24 h conditioning. The X-axis represents fluids (DI water, SW, and FW) in which the mineral was conditioned.
The cases of SW and FW differ from those of DI water because they have dissolved ions, as shown in Table 1. Also, from the pH values (Figure 2B), no change is observed, and thus, the observed effect on the calcite mineral surface charge is due to the ionic composition of the brines. In the case of SW, at 1 wt % anhydrite, a slight reduction in the calcite mineral surface charge is observed from a value of −3.3 mV without anhydrite (Figure 1) to −2.2 mV in the presence of anhydrite (Figure 2A). Even though the SO42– concentration in SW was high, its effect only became pronounced at a 3 wt % anhydrite concentration. As the anhydrite scale concentration increased to 5 wt %, a charge reversal due to counter adsorption of the Ca2+ present in the system or collapse of the double layer with an increased SO42– density is observed. Furthermore, an increase in anhydrite scale concentration beyond 10 wt % (i.e., 15 and 20 wt %) does not increase the negative charge magnitude. This can be attributed to the saturation of the calcite surface with adsorbed SO42–. In the case of FW, a positive surface charge is observed at anhydrite concentrations of 1–5 wt % and 15 wt %, with a negative surface charge observed at 10 and 20 wt %. This is attributed to the adsorption of cations in FW on the surface and the counterbalance of SO42– from the anhydrite scale by the cations. Therefore, the effect of the anhydrite scale on the calcite mineral surface charge shows that except in the case of FW, the calcite mineral is negatively charged due to the sulfate ion from the scale. More so, the low values of the ζ-potential show that the presence of the anhydrite scale possesses no wettability alteration potential and does not in itself possess the capability to alter the wetting state of the calcite mineral.
5.3. Effect of the Barite Scale on the Calcite Mineral Surface Charge
The effect of barite on the calcite mineral surface charge is shown in Figure 3. Figure 3A shows the resulting ζ-potential values of the calcite mineral with varying concentrations of the barite scale in different fluids. On the other hand, the resulting pH of the mixture after 24 h conditioning is shown in Figure 3B. In the case of DI water (Figure 3A), an all-negative ζ-potential value is observed; however, the magnitude is less than that in the case of calcite without the barite scale (Figure 1). This reduction is attributed to the interaction of H+ with the calcite surface, causing an increase in pH (Figure 3B). Worthy of note is that the presence of the barite scale has no significant effect on the calcite mineral surface charge in DI water as the dominant mechanism at play is the PDIs (H+ and OH–).
Figure 3.
Effect of the barite scale on the calcite mineral surface charge. (A) ζ-potential values of the barite/calcite mixture. (B) pH of the resulting mixture after 24 h conditioning.
In the cases of SW and FW, a remarkable reduction of the calcite mineral ζ-potential is observed compared with the case of DI water. Recall that SW and FW possess ions otherwise not present in DI water, and thus, the effect of brine composition on the calcite mineral surface charge development is seen. As in the case of anhydrite (Figure 2), H+ and OH– are not PDIs in the cases of SW and FW, evident in the lack of pH change (Figure 3B). Therefore, the observed ζ-potential values are due to the brine composition. In both the cases of SW and FW, extremely low ζ-potential values are recorded. This infers the instability of the colloidal particles due to the scale presence. More so, even though an all-negative surface charge is recorded, it presents a threat of precipitation due to colloidal instability. In terms of the mechanism responsible for the observed surface charge, the presence of the scale has less effect on the calcite mineral surface charge in SW; however, its effect is pronounced in FW due to the charge reversal from positive (Figure 1) to negative (Figure 3). This implies that the presence of the scale in an FW environment reverses the surface charge of calcite to negative due to the adsorption of SO42– but has no significant effect in the SW environment as SW has a high sulfate concentration. This goes to say that the wettability alteration due to the barite scale only occurs in the FW environment. Furthermore, a closer look at the trends of the ζ-potential values shows double-layer compression due to an increased scale concentration or increased ionic strength in the system.
5.4. Effect of Oilfield Operations
Oilfield operations, which include drilling, stimulations, waterflooding, low salinity, polymer injections, and so forth, are implemented during the life of a field. These operations induce pH change around the wellbore, which impacts fluid interactions at the mineral–fluid interfaces.48 Thus, the effect of such pH changes along with the presence of scales is explored in this section.
5.4.1. Anhydrite
The effect of anhydrite (1–25 wt %) and oilfield-induced pH on the calcite mineral surface charge is shown in Figure 4. Also shown in this figure is the surface charge of calcite due to these two factors in different fluids (DI water, SW, and FW). In the case of DI water, regardless of the concentration of the anhydrite scale, the surface charge of calcite is observed to be negative; however, the magnitude varies with the scale concentration. Figure 4A shows the case of the 1 wt % anhydrite/calcite mixture in different fluids. In the case of DI water (Figure 4A), at pH values of 5 and 6, an average surface charge value of −4 mV is observed; however, with the decrease in pH toward the acidic pH region, a reduction in charge magnitude is recorded. This can be attributed to the interaction of H+ with the calcite mineral surface. Furthermore, the sudden increase in charge magnitude at pH 1 is caused by the compression of the double layer due to a high H+ density. On the other hand, the reduction in the surface charge at pH values of 9–11 is due to the compression of the double layer with an increase in OH– as the pH increases. The provided explanation is plausible because in an electrolyte solution, when two charged surfaces (mineral surface and ions) and the EDLs that accompany them approach one another, the EDLs initially start to overlap, resulting in the compression of the interfacial boundary before eventually collapsing under confinement.88 Altering the pH or the salt concentration offers other ways, as in our case to change the interfacial populations and chemical potentials and hence the relative stability of these structural components. Therefore, as earlier stated, the behavior of the calcite mineral in the DI water environment is controlled by the PDIs (H+ and OH–). In the case of SW, intermittent charge reversal is observed at pH values of 5–12. This can be attributed to the interplay between cation adsorption and sulfate ion adsorption as no observable pH change is recorded. However, in the acidic pH range (pH 1–4), the decrease in negative surface charge is due to the H+ ion interaction with the surface. In the case of FW, an all-positive surface condition is observed across all pH. This can be attributed to the high cation concentration in FW, most probably Na+, Mg2+, and Ca2+; however, the effect of pH becomes apparent in the alkaline pH range with the reduction in positive charge magnitude. This is corroborated by the work of Chen et al.77 who investigated the effect of pH and Ca2+ on the calcite–brine interface charge. The authors alluded that at any given pH, the surface species distribution varies, and thus, the effect of pH on the calcite–brine interface charge is due to the varying distribution of hydration sites (>CO3– and CaOH2+) on the calcite surface. Furthermore, calcite surface interactions with the ions are dependent on the adsorption reaction which is significantly reduced due to a high sulfate content.75
Figure 4.

Effect of the oilfield-induced pH and anhydrite scale on the calcite mineral surface charge. (A–F) 1, 5, 10, 15, 20, and 25 wt % anhydrite/calcite sample mixtures, respectively.
The case of 5 wt % anhydrite is shown in Figure 4B, and as can be observed, the surface charge is positive. In the case of SW, the highest magnitude of the positive charge is recorded at pH 5, with a reduction in surface charge magnitude observed as the pH value tends toward the acidic pH region. This reduction can be attributed to the compression of the double layer due to an increase in H+ density as the pH approaches a value of 1. In the alkaline pH region, with an increase in pH, the surface charge magnitude reduces due to the OH– interaction with the surface; however, at a pH value of 12, charge reversal is observed. In the case of FW (Figure 4B), a trend similar to that in SW is observed; however, the dominance of double-layer compression is more pronounced. Evident from Figure 4A–F is the fact that with an increase in anhydrite concentration, the surface charge across the pH values becomes positive, so a combined effect of induced pH and anhydrite concentration is what results in the surface charge reversal with the dominant mechanisms being ion adsorption and EDL compression.
5.4.2. Barite
The effect of the oilfield-induced pH and barite scale concentration on the calcite mineral surface charge is shown in Figure 5. This is of importance to the industry as barite, which serves as a weighting agent in drilling fluids, invades near-wellbore zones.89 To negate its effect on the fluid flow, acid treatment is implemented.90−92 This is to remove formation damage and mud cakes induced by the drilling operations. Other operations, such as SW injection and polymer or surfactant flooding, may follow during the life of the field. Thus, the question is if this operation-induced pH and the presence of barite have a significant effect on the calcite mineral’s surface charge? This question is what this section seeks to answer.
Figure 5.

Effect of the oilfield-induced pH and barite scale on the calcite mineral surface charge. (A–E) 1, 3, 5, 10, and 20 wt % barite/calcite sample mixtures, respectively.
Figure 5 shows the effect of different oilfield-induced pH and varying concentrations of the barite scale (1–20 wt %) on the calcite mineral surface charge. In the case of the 1 wt % barite/calcite mixture (Figure 5A), an all-positive surface charge is observed except at a pH value of 4 in the case of SW. In both the cases of SW and FW, the pH values (Figure S2) after 24 h conditioning show no observable change except in the extreme alkaline pH range. This implies that the recorded surface charge is not due to H+/OH–. In the case of SW (Figure 5A), the surface charge at a pH value of 6 is positive. This is due to the adsorption of BaBarite on the calcite mineral surface; however, with a decrease in pH (pH value 4.18), charge reversal is observed due to double-layer collapse. At an extremely acidic pH value (pH 2), the surface charge of the calcite mineral becomes positive, thus creating a surface susceptible to polar crude oil compound adsorption. On the other hand, an increase in pH (pH 8) first reduces the magnitude of the positive surface charge due to increased OH– in the system. However, further increase in the OH– density with pH increase results in double-layer compression which increases the positive charge magnitude at pH 10. Therefore, in the case of SW, the dominant mechanisms responsible for the observed surface charge are the ion adsorption and the EDL effect (compression and collapse). This is supported by the observed increase in pH toward the alkaline range (Figure S2A). With the surface being positively charged, an increase in OH– density should reduce the positive charge magnitude; however, the reverse is observed. This implies that the increased OH– density is causing a double-layer compression, resulting in more interactions of H+ with the surface.
In the case of FW (Figure 5A), at a pH value of 6, like in SW, the surface is positively charged; however, with a decrease in pH toward the acidic range, a reduction in the magnitude of the surface charge is observed. This can be attributed to the increased H+ density with a pH decrease. On the other hand, an increase in pH (8 and 10) results in a reduction of the positive charge magnitude due to the OH– density increase in pH. However, a sudden increase in positive charge magnitude was observed at pH 12. This sudden increase is attributed to the compression of the double layer due to the OH– density increase. Therefore, in the case of a 1 wt % barite/calcite mixture, the surface charge is positive and controlled by the pH via the EDL effect.
In the case of the 3 wt % barite/calcite mixture (Figure 5B), the pH trend (Figure S2B) shows that only in the extreme alkaline pH range does H+/OH– affect the surface charge development. In the case of SW, the surface is positively charged as in the case of 1 wt % (Figure 5A), however, with a lower magnitude. The lower magnitude is attributed to the increased sulfate ions in the system as the barite scale concentration (wt %) increased to 3 wt %. In the acid pH range, a decrease in pH from a pH value of 6 results in a slight increase in positive charge magnitude before charge reversal at pH 2. The increased magnitude and charge reversal are due to H+ ion adsorption as Ba2+ is released from the surface and double-layer collapse, respectively. On the other hand, at a pH value of 8, charge reversal to negative is recorded. However, with increased pH, an increase in positive charge magnitude is observed. The charge reversal can be attributed to the SO42– interaction at the pH; however, with increased OH– density, pH increases, and the EDL effect becomes pronounced.
The combined effect of oilfield-induced pH and the varying concentration of barite in the calcite system shown in Figure 5 shows a positive surface charge except in a few instances in the alkaline pH range (Figure 5D) where the surface was negatively charged. Other instances (Figure 5C,E) show positive surface charge conditions, with the dominant mechanisms of the surface charge development being the EDL effect and ion adsorption. Besides the positive surface charges that present flow assurance challenges, evident from the surface charge values (Figure 5) is the instability of the colloidal system. This is represented by the low and near-zero ζ-potential values. Thus, the barite/calcite system discussed above not only presents a surface condition that serves as a precursor for polar molecule adsorption but also presents threats of precipitation, which would impact formation permeability.
5.5. Scale Control
Formation damage due to scales is remedied by the injection of fluids to dissolve the deposited scale,90−93 stabilize the formation, and alter the wetting state of the rock.7,89,94 Another mechanism of scale control is the use of chelating agents which chelate specific ions from the rock surface and prevent their precipitation or deposition.95 Of the several chelating agents, the most used is ethylenediaminetetraacetic acid (EDTA). EDTA is reported to prevent anhydrite scale as well as alter the wetting state of the rock.93
Hassan and Al-Hashim96 reported the surface charge modification of the carbonate system using EDTA chelating agents (1, 3, 5, and 10 wt %). The authors analyzed the changes using ion concentration analysis via inductively coupled plasma-optical emission spectrometry (ICP-OES) and recovery evaluation using core flooding and spontaneous imbibition tests. Furthermore, nuclear magnetic resonance (NMR) was also used to evaluate the potential damage to the sample due to chelating agent treatment. Findings showed that the carbonate sample surface charge can be reversed to negative vis-à-vis the chelating agent. Also, an increase in chelating agent concentration results in an increased negative surface charge. The authors also alluded based on the ICP analysis that the surface charge modification is attributed to Ca2+ dissolution or chelating from the carbonate surface. This was further corroborated by the recovery measurements from the imbibition test and core flooding experiments.
Mady et al.97 reported the use of organophosphorus, nitrogen-free scale inhibitors for calcite and barite minerals. The authors conducted a compatibility test, efficiency evaluation, and density functional theory and solid-state computations as well as environmental friendliness examination of the proposed fluids. In comparison to the commercial inhibitors, the nitrogen-free scale inhibitors performed well, and the distance and number of PO(OH)2 and COOH on the inhibitor backbone were suggested to control the performance. Recently, a technique of converting anhydrite to calcite via a chelating agent was reported;94 however, in this study, a varying concentration of EDTA was employed to control the scale effect on the calcite mineral surface charge. This is to ascertain the optimal EDTA concentration required for anhydrite and barite scale inhibition. Furthermore, the mechanism responsible for the EDTA interaction was investigated using ion concentration analysis. Therefore, to evaluate whether the dominant mechanism is ion chelating or wettability alteration, the concentration of Ca2+ and Ba2+ was monitored in our study.
To understand the effect of EDTA solutions in chelating the cations from the mineral surface, 1 g of the minerals (calcite, anhydrite, and barite) was conditioned in EDTA of different concentrations (1, 5, and 10 wt %). After 24 h, the mixture was sonicated, and a sample was taken from the clear supernatant. The samples were then analyzed for the concentrations of Ca2+ and Ba2+ in the EDTA solution chelated from the mineral surfaces. This is to establish and affirm the earlier report by Mahmoud et al.94Figure 6 shows the results of the ion concentration analysis for the minerals. The effectiveness of the EDTA solution in chelating cations from the mineral surfaces is in the order anhydrite > calcite > barite. More so, the amount of cations chelated by the EDTA solutions increases with an increase in EDTA concentration in the cases of calcite and anhydrite. However, the reverse is observed in the case of barite as the highest amount of ions chelated by the EDTA solution is recorded at 1 wt % EDTA. This implies that unlike in the cases of calcite and anhydrite, a low concentration of EDTA is required to control barite scales in reservoir formation.
Figure 6.
Ion concentration analysis.
Two modes (Figure 7) of EDTA implementations [slug (Figure 7A) and continuous injections (Figure 7B)] and environments (primary and secondary production stages) were evaluated in this study. The primary and secondary production in this study means EDTA injection (slug or continuous) was implemented in the native reservoir state (FW) and after SW injection commenced, respectively. In the case of slug injection, EDTA contacts the mineral once, whereas in the case of continuous injection, the mineral is contacted multiple times.95
Figure 7.
Mode of chelating agent injection. (A) Slug injection. (B) Continuous injection.
5.5.1. Slug Injection
5.5.1.1. Anhydrite
Figure 8 shows the ζ-potential values of the effect of EDTA (1, 5, and 10 wt %) slug solution on the calcite/anhydrite and calcite/barite systems in FW and SW environments. In the case of a calcite/anhydrite system in the FW environment (Figure 8A), the highest magnitude of the negative charge is −15.8 mV. This is in the case of the 1 wt % EDTA slug being applied to a calcite/anhydrite system with 1 wt % anhydrite. For a calcite system with a 1 wt % anhydrite scale, the optimum EDTA concentration for slug treatment is 1 wt %. This is because increasing EDTA concentrations to 5 and 10 wt % results in a lower magnitude of the negative surface charge. This can be attributed to the compression of the double layer. Also, from the ζ-potential values at 5 and 10 wt % EDTA slug applications, it can be inferred that there exists a limit to the double-layer compression, beyond which the increased concentration of EDTA has no further effect. This is evident in the fact that the same value of the surface charge results regardless of the increased EDTA concentration from 5 to 10 wt %. For a calcite rock with a 5 wt % anhydrite scale system, a lower magnitude of the negative surface charge results compared to that in the case of a 1 wt % anhydrite scale. This is because, with a more anhydrite scale, more Ca2+, which is a PDI, is introduced into the system. More so, the highest magnitude of the negative surface charge was observed in the case of the 1 wt % EDTA slug, with a reduction in surface charge observed with an increase in EDTA concentration. This decrease in surface charge is due to the compression of the double layer, and in this case (5 wt % anhydrite), slug treatment with a 10 wt % EDTA solution is not sufficient to improve the colloidal stability of the system.
Figure 8.

Effect of slug injection on scale control. (A,B) ζ-potential values for the anhydrite/calcite case scenario in FW and SW, respectively. (C,D) ζ-potential values for the barite/calcite case scenario in FW and SW, respectively.
With an increase in anhydrite scale concentration to 10 wt %, a positively charged surface results with all tested EDTA concentrations (1–10 wt %), which is ineffective in reversing the calcite/anhydrite system surface charge. Thus, in the FW environment, the application of slug treatment is not effective due to the observed low negative and positive surface charges. This is attributed to the high concentration of cations in FW, which reduces the effect of the EDTA chelating agent on the calcite/anhydrite system. The effect of EDTA slug treatment on the calcite/anhydrite system in the SW environment, which has less cation concentration compared to that in FW, is shown in Figure 8B. In the case of a calcite system with a 1 wt % anhydrite scale, an increase in EDTA solution concentration is observed to improve the negative charge magnitude of the system. This agrees with the report of Hassan and Al-Hashim96 who reported carbonate system surface charge modification using an EDTA chelating agent. In the cases of 5 and 10 wt % anhydrite concentrations, the optimum EDTA concentration for slug treatment was determined to be 5 wt %. This is because a further increase in EDTA concentration above 5 wt % showed an insignificant difference. Thus, in the SW environment, calcite systems with 1 wt % and above 5 wt % anhydrite require 10 and 5 wt % EDTA solutions for slug treatment, respectively.
5.5.1.2. Barite
The effect of EDTA solution slug injection on the barite mineral scale in FW and SW is shown in Figure 8C,D, respectively. As seen from these figures, a negative surface charge results due to slug treatment. In the case of a barite/calcite system with 1 wt % barite in FW (Figure 8C), an all-negative surface charge results due to the slug injection of EDTA with the optimum EDTA concentration being 5 wt %. On the other hand (5 and 10 wt % barite), increased EDTA concentration results in a lower magnitude of the negative surface charge due to the compression of the double layer, and thus, the highest EDTA concentration required in both cases is 1 wt %. The plausible explanation for this is that at a low EDTA concentration, the combined effect of EDTA (chelating Ba2+) and sulfate (low concentration required for efficient adsorption) is responsible for the improved negative charge magnitude even with an increased barite concentration. However, with increased EDTA concentrations, this combined effect chelates more Ba2+ but compresses the double layer owing to the ionic strength of the solution. This effect is also prevalent with 10 wt % EDTA slug injection. Furthermore, this observation is supported by the earlier explanations shown in Figure 6. The case of the barite/calcite scale in the FW environment reveals that a low chelating agent concentration is required; however, even at this, the resulting surface charge is less than 10 mV, depicting threats of precipitation.
In the SW environment, at 1 wt % EDTA, the calcite mineral surface charge is positive, depicting the inefficiency of EDTA at this concentration. Also, at 1 wt %, an increased barite concentration results in a more positively charged surface. With an increased EDTA concentration to 5 wt %, the surface charge is reversed with the calcite system with 5 and 10 wt % barites having the highest and lowest magnitudes of the negative surface charge, respectively. Generally, the optimum EDTA concentration in the SW environment is 5 wt %, with a slightly higher magnitude of the negative surface charge compared to that in the FW environment.
The slug treatment of scales which involves single contact of the chelating agent with the mineral system is a convenient and more economical choice in field applications. However, in the cases of sulfate-based scales (anhydrite and barite), as shown in Figure 8, this is inefficient. Even though the slug injection of EDTA can reverse the surface charge in some instances, the propensity of mineral scale precipitation still exists. Thus, the examination of the continuous injection of EDTA solution for scale control is presented in the following section.
5.5.2. Continuous Injection
Continuous injection (see Figure 7B) of chelating agents into the formation is the alternative to slug injection. This often requires a low dosage of chemicals for economic concerns; thus, the most efficient chemical is that whose optimal concentration is exceptionally low and efficient. This is achieved by dosing the SW injected into the formation with the required chemical dosage. In this study, we investigate the use of 1–10 wt % EDTA for continuous injection in a calcite scale system of varying scale concentrations (1, 5, and 10 wt %).
5.5.2.1. Anhydrite
Figure 9 shows the effect of the continuous injection of 1, 5, and 10 wt % EDTA on the anhydrite/calcite system of 1, 5, and 10 wt % anhydrite. Also shown in this figure is the effect of the EDTA chelating agent in different environments [FW (Figure 9A–C) and SW (Figure 9D–F)]. In the FW environment (Figure 9A–C), the optimum EDTA concentration to achieve the highest magnitude of the negatively charged surface is 1, 1, and 5 wt % for 1, 5, and 10 wt % anhydrite/calcite systems, respectively. In an economic view, 1 wt % EDTA is optimal for low anhydrite scale conditions, whereas for higher scale percentages (10 ≥), 5 wt.% continuous injections will suffice. On the other hand, in the SW (Figure 9D–F) environment, 1 wt % EDTA continuous injection is the optimal injection dosage regardless of the anhydrite scale percentage. Thus, once SW injection has started, the dosage of the injected SW with a 1 wt % EDTA solution helps improve the colloidal stability against precipitation. This also ensures surface conditions that would not serve as precursors for polar crude oil compound adsorption and thus an operational strategy to minimize wettability alteration and formation damage.
Figure 9.

Effect of continuous EDTA chelating agent injection on the calcite/scale surface charge. (A–C) and (D–F) 1, 5, and 10 wt % anhydrite/calcite systems in FW and SW environments, respectively.
5.5.2.2. Barite
Figure 10 shows the effect of continuous injection of the EDTA solution on the barite/calcite system in the FW (Figure 10A–C) and SW (Figure 10D–F) environments. In the FW environment, regardless of the barite scale concentrations [1 wt % (Figure 10A), 5 wt % (Figure 10B), and 10 wt % (Figure 10C)], the optimal EDTA concentration required to achieve the highest magnitude of the negatively charged surface and improved colloidal stability is 1 wt %. Similarly, in the SW environment (Figure 10D–F), in all cases of barite scale concentrations (1, 5, and 10 wt %), injection of 1 wt % EDTA appears to be the optimal concentration. Although the case of 5 wt % EDTA results in a slightly higher magnitude of the negative surface charge, the question of the economics of the process gives 1 wt % an advantage. Thus, based on this criterion, the optimal EDTA concentration for both the FW and SW environments and at all barite scale concentrations is 1 wt %.
Figure 10.

Effect of continuous EDTA chelating agent injection on the calcite/scale surface charge. (A–C) and (D–F) 1, 5, and 10 wt % barite/calcite systems in FW and SW environments, respectively.
5.6. Wettability Alteration
Results thus far show that the presence of scales affects the surface charge of calcite minerals and thus affects the calcite mineral interactions. As earlier presented in the preceding section, for wettability alteration to occur due to the adsorption of crude oil polar compounds, the calcite surface charge must be opposite that of asphaltene which is said to be negatively charged. The results of the effect of the sulfate scale on the calcite mineral surface charge (Figures 2 and 3) showed that the presence of the scales makes the surface negatively charged except in the case of anhydrite in the FW environment. Thus, if the surface is negatively charged, wettability alteration due to the asphaltene molecule is mitigated. However, the change in the ζ-potential due to the presence of the scales possesses the threat of precipitation. The threat of scale precipitation is due to the instability of the colloidal system which is depicted by near-zero and low ζ-potential values (less than ±10) of the calcite/scale systems. This also leads to the inference that the presence of the scale does not affect the wettability of the calcite system but possesses a threat of permeability and porosity damage. Additionally, the injection of EDTA solution as a scale control method enhanced the magnitude of the system’s negative surface charge, thus improving the calcite surface’s ability to mitigate changes in wettability brought on by asphaltene adsorption.
Wettability alteration assessment using, for instance, the contact angle method was difficult to achieve in this study. The difficulties encountered that prevented the report of wettability alteration vis-à-vis contact angle measurements in this study involved making a core plug with anhydrite and barite scales at the proper percentage compositions. The samples must be heated and compressed under high pressure to create a core plug from the powder. After this, the core plugs would be cut into chips to quantify the contact angle. Additionally, cementing materials are employed to bind the powdered samples together to create the core plugs, and according to this study, a cementing material concentration greater than 30 weight percent is needed. This, in our opinion, would affect the recorded contact angle. To reduce the cementing material’s influence on surface contacts, a different study is devoted to the creation of representative core plugs.
6. Conclusions
The impact of sulfate-based scales (anhydrite and barite) on the calcite mineral surface charge is investigated in this study. Also, the effect of pH-inducing oilfield operations, as well as an evaluation of the optimal concentration of the chelating agent for scale control, is presented. Based on our findings, the following conclusions are reached.
-
1.
The presence of sulfate-based scales in calcite-rich formation results in precipitation-prone conditions even though the surface charge may be negative.
-
2.
Sulfate-based scales do not by themselves induce wettability alteration as they create a calcite mineral surface (negatively charged) that is inhibitive of asphaltene adsorption.
-
3.
The combined effect of sulfate scales and pH-inducing oilfield operations creates a positively charged calcite surface prone to polar crude oil compound adsorption and would consequently cause wettability alterations.
-
4.
Chelating agents are good candidates for sulfate-based scale control, with the best operational strategy being the continuous injection of chelating agents.
-
5.
The optimal concentrations of EDTA for control of the anhydrite scale in FW and SW environments are 5 and 1 wt % for the continuous injection strategy, respectively.
-
6.
The optimal EDTA concentration for the barite scale control in both the FW and SW environments is 1 wt % for the continuous injection mode.
Acknowledgments
The College of Petroleum and Geoscience at the King Fahd University of Petroleum & Minerals is acknowledged for the support and permission to publish this work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c03403.
pH values of the samples under study including the final pH values of the effect of oilfield operations as well as scale control strategies (PDF)
This work was funded by the KFUPM-KU Joint Research Program under the grant # KU-201004 and award # KU-KFUPM-2020-28.
The authors declare no competing financial interest.
Supplementary Material
References
- Olajire A. A. A Review of Oilfield Scale Management Technology for Oil and Gas Production. J. Pet. Sci. Eng. 2015, 135, 723–737. 10.1016/j.petrol.2015.09.011. [DOI] [Google Scholar]
- Verri G.; Sorbie K. S.; Silva D. A Rigorous General Workflow for Accurate Prediction of Carbonate and Sulphide Scaling Profiles in Oil and Gas Wells. J. Pet. Sci. Eng. 2017, 156, 673–681. 10.1016/j.petrol.2017.06.037. [DOI] [Google Scholar]
- Bader M. S. H. Sulfate Removal Technologies for Oil Fields Seawater Injection Operations. J. Pet. Sci. Eng. 2007, 55, 93–110. 10.1016/j.petrol.2006.04.010. [DOI] [Google Scholar]
- Stalker R.; Collins I. R.; Graham G. M.. The Impact of Chemical Incompatibilities in Commingled Fluids on the Efficiency of a Produced Water Reinjection System: A North Sea Example. International Symposium on Oilfield Chemistry; OnePetro, 2003; pp 441–453.
- Abbasi P.; Abbasi S.; Moghadasi J. Experimental Investigation of Mixed-Salt Precipitation during Smart Water Injection in the Carbonate Formation. J. Mol. Liq. 2020, 299, 112131. 10.1016/j.molliq.2019.112131. [DOI] [Google Scholar]
- Zhang P.; Tweheyo M. T.; Austad T. Wettability Alteration and Improved Oil Recovery in Chalk: The Effect of Calcium in the Presence of Sulfate. Energy Fuels 2006, 20, 2056–2062. 10.1021/ef0600816. [DOI] [Google Scholar]
- Mahmoud M. A. Evaluating the Damage Caused by Calcium Sulfate Scale Precipitation during Low- and High-Salinity-Water Injection. J. Can. Pet. Technol. 2014, 53, 141–150. 10.2118/164634-PA. [DOI] [Google Scholar]
- Isah A.; Arif M.; Hassan A.; Mahmoud M.; Iglauer S. A Systematic Review of Anhydrite-Bearing Reservoirs: EOR Perspective, CO2-Geo-Storage and Future Research. Fuel 2022, 320, 123942. 10.1016/j.fuel.2022.123942. [DOI] [Google Scholar]
- Liu T.; Artacho E.; Gázquez F.; Walters G.; Hodell D. Prediction of Equilibrium Isotopic Fractionation of the Gypsum/Bassanite/Water System Using First-Principles Calculations. Geochim. Cosmochim. Acta 2019, 244, 1–11. 10.1016/j.gca.2018.08.045. [DOI] [Google Scholar]
- Zarrouk S. J.; McLean K.. Operation and Management of Geothermal Wells. Geothermal Well Test Analysis; Academic Press, 2019. [Google Scholar]
- Zarrouk S. J.; McLean K.. Operation and Management of Geothermal Wells. Geothermal Well Test Analysis; Elsevier, 2019; pp 217–255. [Google Scholar]
- Abdelgawad K.; Mahmoud M.; Elkatatny S.; Patil S.. Effect of Calcium Carbonate on Barite Solubility Using a Chelating Agent and Converter. SPE International Conference on Oilfield Chemistry; OnePetro, 2019.
- AlAamri J.; AlDahlan M.; Al-Otaibi F. M.; Al-Ghamdi A.. Evaluation of a New Barium Sulfate Dissolver and the Effect of the Presence of Calcium Carbonate in the Dissolution Rate. Abu Dhabi International Petroleum Exhibition & Conference ADIP 2019; OnePetro, 2019.
- Kumar S.; Naiya T. K.; Kumar T. Developments in Oilfield Scale Handling towards Green Technology-A Review. J. Pet. Sci. Eng. 2018, 169, 428–444. 10.1016/j.petrol.2018.05.068. [DOI] [Google Scholar]
- Mahmoud M. A.; Abdelgawad K. Z. Chelating-Agent Enhanced Oil Recovery for Sandstone and Carbonate Reservoirs. SPE J. 2015, 20, 483–495. 10.2118/172183-PA. [DOI] [Google Scholar]
- Gamal H.; Al-Afnan S.; Elkatatny S.; Bahgat M. Barium Sulfate Scale Removal at Low-Temperature. Geofluids 2021, 2021, 5527818. 10.1155/2021/5527818. [DOI] [Google Scholar]
- Bukuaghangin O.; Sanni O.; Kapur N.; Huggan M.; Neville A.; Charpentier T. Kinetics Study of Barium Sulphate Surface Scaling and Inhibition with a Once-through Flow System. J. Pet. Sci. Eng. 2016, 147, 699–706. 10.1016/j.petrol.2016.09.035. [DOI] [Google Scholar]
- BinMerdhah A. B.; Yassin A. A. M.; Muherei M. A. Laboratory and Prediction of Barium Sulfate Scaling at High-Barium Formation Water. J. Pet. Sci. Eng. 2010, 70, 79–88. 10.1016/j.petrol.2009.10.001. [DOI] [Google Scholar]
- Mohammed I.; Al Shehri D.; Mahmoud M.; Kamal M. S.; Arif M.; Alade O. S.; Patil S. Investigation of Surface Charge at the Mineral/Brine Interface: Implications for Wettability Alteration. Front. Mater. 2022, 9, 891455. 10.3389/fmats.2022.891455. [DOI] [Google Scholar]
- Mohammed I.; Al Shehri D. A.; Mahmoud M.; Kamal M. S.; Alade O. Surface Charge Investigation of Reservoir Rock Minerals. Energy Fuels 2021, 35, 6003–6021. 10.1021/acs.energyfuels.1c00459. [DOI] [Google Scholar]
- Setta F. A.; Neville A. Efficiency Assessment of Inhibitors on CaCO3 Precipitation Kinetics in the Bulk and Deposition on a Stainless Steel Surface (316L). Desalination 2011, 281, 340–347. 10.1016/j.desal.2011.08.021. [DOI] [Google Scholar]
- Sanni O.; Charpentier T.; Kapur N.; Neville A.. Study of Surface Deposition and Bulk Scaling Kinetics in Oilfield Conditions Using an In-Situ Flow Rig. NACE Corrosion; OnePetro, 2015.
- Nikoo A. H.; Malayeri M. R. Interfacial Interactions between Scale-Brine and Various Reservoir Rocks. Colloids Surf., A 2021, 611, 125840. 10.1016/j.colsurfa.2020.125840. [DOI] [Google Scholar]
- Arsalan N.; Buiting J. J.; Nguyen Q. P. Surface Energy and Wetting Behavior of Reservoir Rocks. Colloids Surf., A 2015, 467, 107–112. 10.1016/j.colsurfa.2014.11.024. [DOI] [Google Scholar]
- Arsalan N.; Palayangoda S. S.; Burnett D. J.; Buiting J. J.; Nguyen Q. P. Surface Energy Characterization of Carbonate Rocks. Colloids Surf., A 2013, 436, 139–147. 10.1016/j.colsurfa.2013.06.004. [DOI] [Google Scholar]
- Elkatatny S.New Formulation for Iron Sulfide Scale Removal. SPE Middle East Oil Gas Show Conference; OnePetro. MEOS, Proc. 2017, 2017-March (March); pp 1809–1821.
- Taheriotaghsara M.; Eftekhari A. A.; Nick H. M. Adsorption- and Diffusion-Controlled Wettability Change in Modified Salinity Water Flooding. Energy Fuels 2020, 34, 13767–13781. 10.1021/acs.energyfuels.0c02493. [DOI] [Google Scholar]
- Nasr-El-Din H. A.; Al-Humaidan A. Y.. Iron Sulfide Scale: Formation, Removal and Prevention. In International Symposium on Oilfield Scale; Society of Petroleum Engineers, 2001.
- Chang F. F.; Civan F. Practical Model for Chemically Induced Formation Damage. J. Pet. Sci. Eng. 1997, 17, 123–137. 10.1016/S0920-4105(96)00061-7. [DOI] [Google Scholar]
- Nikoo A. H.; Malayeri M. R. On the Affinity of Carbonate and Sandstone Reservoir Rocks to Scale Formation – Impact of Rock Roughness. Colloids Surf., A 2021, 610, 125699. 10.1016/j.colsurfa.2020.125699. [DOI] [Google Scholar]
- Mohammed I.; Mahmoud M.; El-Husseiny A.; Al Shehri D.; Al-Garadi K.; Kamal M. S.; Alade O. S. Impact of Asphaltene Precipitation and Deposition on Wettability and Permeability. ACS Omega 2021, 6, 20091–20102. 10.1021/acsomega.1c03198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed I.; Al Shehri D.; Mahmoud M.; Kamal M. S.; Alade O. S. Impact of Iron Minerals in Promoting Wettability Alterations in Reservoir Formations. ACS Omega 2021, 6, 4022–4033. 10.1021/acsomega.0c05954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed I.; Al Shehri D.; Mahmoud M.; Kamal M. S.; Alade O. S. Feature Ranking and Modeling of Mineral Effects on Reservoir Rock Surface Chemistry Using Smart Algorithms. ACS Omega 2022, 7, 4194–4201. 10.1021/acsomega.1c05820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan K.; Gupta R.; Mohanty K. K. Wettability Altering Secondary Oil Recovery in Carbonate Rocks. Energy Fuels 2011, 25, 3966–3973. 10.1021/ef200449y. [DOI] [Google Scholar]
- Mohammed I.; Mahmoud M.; Al Shehri D.; El-Husseiny A.; Alade O. Asphaltene Precipitation and Deposition: A Critical Review. J. Pet. Sci. Eng. 2021, 197, 107956. 10.1016/j.petrol.2020.107956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwangi P.; Brady P. V.; Radonjic M.; Thyne G. The Effect of Organic Acids on Wettability of Sandstone and Carbonate Rocks. J. Pet. Sci. Eng. 2018, 165, 428–435. 10.1016/j.petrol.2018.01.033. [DOI] [Google Scholar]
- Clementz D. M.Alteration of Rock Properties by Adsorption of Petroleum Heavy Ends: Implications for Enhanced Oil Recovery. In SPE Enhanced Oil Recovery Symposium; Society of Petroleum Engineers: Tulsa, Oklahoma, 1982.
- Lin M.; Hua Z.; Li M. Surface Wettability Control of Reservoir Rocks by Brine. Pet. Explor. Dev. 2018, 45, 145–153. 10.1016/S1876-3804(18)30014-4. [DOI] [Google Scholar]
- Ghedan S.; Canbaz C. H.; Boyd D.; Mani G.; Haggag M.. Wettability Profile of a Thick Carbonate Reservoir by the New Rise in Core Wettability Characterization Method. Proceedings of the 14th Abu Dhabi International Petroleum Exhibition and Conference (ADIPEC ’10); OnePetro, 2010; pp 2203–2217.
- Xue H.; Wang C.; Jiang L.; Wang H.; Lv Z.; Huang J.; Xiao W. Asphaltene Precipitation Trend and Controlling Its Deposition Mechanism. Nat. Gas Ind. 2022, 9, 84–95. 10.1016/j.ngib.2021.12.001. [DOI] [Google Scholar]
- Ghosh A.; Chaudhuri P.; Kumar B.; Panja S. S. Review on Aggregation of Asphaltene Vis-a-Vis Spectroscopic Studies. Fuel 2016, 185, 541–554. 10.1016/j.fuel.2016.08.031. [DOI] [Google Scholar]
- Buenrostro-Gonzalez E.; Lira-Galeana C.; Gil-Villegas A.; Wu J. Asphaltene Precipitation in Crude Oils: Theory and Experiments. AIChE J. 2004, 50, 2552–2570. 10.1002/aic.10243. [DOI] [Google Scholar]
- Leontaritis K. J. Challenges and Solutions to Asphaltene and Wax Deposition. Offshore 1998, 58, 1–9. [Google Scholar]
- Gharbi K.; Benyounes K.; Khodja M. Removal and Prevention of Asphaltene Deposition during Oil Production: A Literature Review. J. Pet. Sci. Eng. 2017, 158, 351–360. 10.1016/j.petrol.2017.08.062. [DOI] [Google Scholar]
- Soulgani B. S.; Reisi F.; Norouzi F. Investigation into Mechanisms and Kinetics of Asphaltene Aggregation in Toluene/n-Hexane Mixtures. Pet. Sci. 2020, 17, 457–466. 10.1007/s12182-019-00383-3. [DOI] [Google Scholar]
- Ancheyta J.; Centeno G.; Trejo F.; Marroquín G.; García J. A.; Tenorio E.; Torres A. Extraction and Characterization of Asphaltenes from Different Crude Oils and Solvents. Energy Fuels 2002, 16, 1121–1127. 10.1021/ef010300h. [DOI] [Google Scholar]
- Riazi M. Influences of Asphaltene Deposition on Formation Damage and Gas Coning. Biomed. J. Sci. Technol. Res. 2018, 3, 3561–3565. 10.26717/BJSTR.2018.03.000960. [DOI] [Google Scholar]
- Mohammed I.; Al Shehri D.; Mahmoud M.; Kamal M. S.; Alade O.; Arif M.; Patil S. Effect of Native Reservoir State and Oilfield Operations on Clay Mineral Surface Chemistry. Molecules 2022, 27, 1739. 10.3390/molecules27051739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed I.; Al Shehri D.; Mahmoud M.; Kamal M. S.; Alade O. S. A Surface Charge Approach to Investigating the Influence of Oil Contacting Clay Minerals on Wettability Alteration. ACS Omega 2021, 6, 12841–12852. 10.1021/acsomega.1c01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C.; Li D.; Shi C.; Du Z.; Han W.; Zhu Y.; Dong H.; Fan X.; Wang C. Study on the Association Driving Force of Low Temperature Coal Tar Asphaltenes. J. Mol. Struct. 2022, 1254, 132361. 10.1016/j.molstruc.2022.132361. [DOI] [Google Scholar]
- Tetteh J. T.; Brady P. V.; Barati Ghahfarokhi R. Review of Low Salinity Waterflooding in Carbonate Rocks: Mechanisms, Investigation Techniques, and Future Directions. Adv. Colloid Interface Sci. 2020, 284, 102253. 10.1016/j.cis.2020.102253. [DOI] [PubMed] [Google Scholar]
- Hao J.; Mohammadkhani S.; Shahverdi H.; Esfahany M. N.; Shapiro A. Mechanisms of Smart Waterflooding in Carbonate Oil Reservoirs - A Review. J. Pet. Sci. Eng. 2019, 179, 276–291. 10.1016/j.petrol.2019.04.049. [DOI] [Google Scholar]
- Guo H.; Nazari N.; Esmaeilzadeh S.; Kovscek A. R. A Critical Review of the Role of Thin Liquid Films for Modified Salinity Brine Recovery Processes. Curr. Opin. Colloid Interface Sci. 2020, 50, 101393. 10.1016/j.cocis.2020.101393. [DOI] [Google Scholar]
- Crundwell F. K. On the Mechanism of the Dissolution of Quartz and Silica in Aqueous Solutions. ACS Omega 2017, 2, 1116–1127. 10.1021/acsomega.7b00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.; Zhang X.; Jiang B.; Li J.; Li T.; Shao X.; Cai W.; Wang H.; Zhang Y. Molecular Dynamics Simulation of Ion Adsorption and Ligand Exchange on an Orthoclase Surface. ACS Omega 2021, 6, 14952–14962. 10.1021/acsomega.1c00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas A. H.; Pourafshary P.; Wan Sulaiman W. R.; Jaafar M. Z.; Nyakuma B. B. Toward Reducing Surfactant Adsorption on Clay Minerals by Lignin for Enhanced Oil Recovery Application. ACS Omega 2021, 6, 18651. 10.1021/acsomega.1c01342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaeed S. M.; Zaki E. G.; Omar W. A. E.; Ashraf Soliman A.; Attia A. M. Guar Gum-Based Hydrogels as Potent Green Polymers for Enhanced Oil Recovery in High-Salinity Reservoirs. ACS Omega 2021, 6, 23421–23431. 10.1021/acsomega.1c03352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteh J. T.; Barimah R.; Korsah P. K. Ionic Interactions at the Crude Oil–Brine–Rock Interfaces Using Different Surface Complexation Models and DLVO Theory: Application to Carbonate Wettability. ACS Omega 2022, 7, 7199–7212. 10.1021/acsomega.1c06954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bayati A.; Karunarathne C. I.; Al Jehani A. S.; Al-Yaseri A. Z.; Keshavarz A.; Iglauer S. Wettability Alteration during Low-Salinity Water Flooding. Energy Fuels 2022, 36, 871–879. 10.1021/acs.energyfuels.1c03728. [DOI] [Google Scholar]
- Derkani M.; Fletcher A.; Abdallah W.; Sauerer B.; Anderson J.; Zhang Z. Low Salinity Waterflooding in Carbonate Reservoirs: Review of Interfacial Mechanisms. Colloids Interfaces 2018, 2, 20. 10.3390/colloids2020020. [DOI] [Google Scholar]
- Al-Araimi S.; Karimi M.; Al-Maamari R. S. Experimental Investigation of the Effect of Acid and Base on Wettability Alteration of a Calcite Surface. Energy Fuels 2021, 35, 11869–11883. 10.1021/acs.energyfuels.1c01167. [DOI] [Google Scholar]
- Awolayo A.; Sarma H.; Nghiem L. Brine-Dependent Recovery Processes in Carbonate and Sandstone Petroleum Reservoirs: Review of Laboratory-Field Studies, Interfacial Mechanisms and Modeling Attempts. Energies 2018, 11, 3020. 10.3390/en11113020. [DOI] [Google Scholar]
- Joekar-Niasar V.; Schreyer L.; Sedighi M.; Icardi M.; Huyghe J. Coupled Processes in Charged Porous Media: From Theory to Applications. Transp. Porous Media 2019, 130, 183. 10.1007/s11242-019-01257-3. [DOI] [Google Scholar]
- Alotaibi M. B.; Nasr-El-Din H. A. Electrokinetics of Limestone Particles and Crude-Oil Droplets in Saline Solutions. SPE Reservoir Eval. Eng. 2011, 14, 604–611. 10.2118/151577-PA. [DOI] [Google Scholar]
- Rodrigues R.; Levant M.; Klimenko A. Relevance of Zeta Potential as a Tool for Predicting the Response of Controlled Salinity Waterflooding in Oil–Water-Carbonate Systems. Fuel 2022, 324, 124629. 10.1016/j.fuel.2022.124629. [DOI] [Google Scholar]
- Isah A.; Arif M.; Hassan A.; Mahmoud M.; Iglauer S. Fluid–Rock Interactions and Its Implications on EOR: Critical Analysis, Experimental Techniques and Knowledge Gaps. Energy Rep. 2022, 8, 6355–6395. 10.1016/j.egyr.2022.04.071. [DOI] [Google Scholar]
- Basu S.; Sharma M. M. Measurement of Critical Disjoining Pressure for Dewetting of Solid Surfaces. J. Colloid Interface Sci. 1996, 181, 443–455. 10.1006/jcis.1996.0401. [DOI] [Google Scholar]
- Basu S.; Sharma M. M.. Investigating the Role of Crude-Oil Components on Wettability Alteration Using Atomic Force Microscopy. In International Symposium on Oilfield Chemistry; SPE: Houston, Texas, 1997.
- Sanaei A.; Tavassoli S.; Sepehrnoori K.. Investigation of Modified Water Chemistry for Improved Oil Recovery: Application of DLVO Theory and Surface Complexation Model. In SPE Western Regional Meeting; Society of Petroleum Engineers, 2018; Vol. 574, pp 131–145.
- Tirjoo A.; Bayati B.; Rezaei H.; Rahmati M. Molecular Dynamics Simulations of Asphaltene Aggregation under Different Conditions. J. Pet. Sci. Eng. 2019, 177, 392–402. 10.1016/j.petrol.2019.02.041. [DOI] [Google Scholar]
- Zallaghi M.; Khaz’ali A. R. Experimental and Modeling Study of Enhanced Oil Recovery from Carbonate Reservoirs with Smart Water and Surfactant Injection. Fuel 2021, 304, 121516. 10.1016/j.fuel.2021.121516. [DOI] [Google Scholar]
- Prabhakar S.; Melnik R. Wettability Alteration of Calcite Oil Wells: Influence of Smart Water Ions. Sci. Rep. 2017, 7, 17365. 10.1038/s41598-017-17547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi S. J.; Austad T.; Strand S. Water-Based Enhanced Oil Recovery (EOR) by “Smart Water”: Optimal Ionic Composition for EOR in Carbonates. Energy Fuels 2011, 25, 5173–5179. 10.1021/ef201019k. [DOI] [Google Scholar]
- Snosy M. F.; Abu El Ela M.; El-Banbi A.; Sayyouh H. Comprehensive Investigation of Low Salinity Waterflooding in Carbonate Reservoirs. J. Pet. Explor. Prod. Technol. 2021, 12, 701. 10.1007/s13202-021-01330-y. [DOI] [Google Scholar]
- Mosallanezhad A.; Kalantariasl A. Geochemical Simulation of Single-Phase Ion-Engineered Water Interactions with Carbonate Rocks: Implications for Enhanced Oil Recovery. J. Mol. Liq. 2021, 341, 117395. 10.1016/j.molliq.2021.117395. [DOI] [Google Scholar]
- El-Din Mahmoud M. A. Effect of Chlorite Clay-Mineral Dissolution on the Improved Oil Recovery From Sandstone Rocks During Diethylenetriaminepentaacetic Acid Chelating-Agent Flooding. SPE J. 2018, 23, 1880–1898. 10.2118/189988-PA. [DOI] [Google Scholar]
- Chen Y.; Ubaidah A.; Elakneswaran Y.; Niasar V. J.; Xie Q. Detecting PH and Ca2+ Increase during Low Salinity Waterflooding in Carbonate Reservoirs: Implications for Wettability Alteration Process. J. Mol. Liq. 2020, 317, 114003. 10.1016/j.molliq.2020.114003. [DOI] [Google Scholar]
- Yutkin M. P.; Mishra H.; Patzek T. W.; Lee J.; Radke C. J. Bulk and Surface Aqueous Speciation of Calcite: Implications for Low-Salinity Waterflooding of Carbonate Reservoirs. SPE J. 2018, 23, 84–101. 10.2118/182829-PA. [DOI] [Google Scholar]
- Al Maskari N. S.; Almobarak M.; Saeedi A.; Xie Q. Influence of PH on Acidic Oil-Brine-Carbonate Adhesion Using Atomic Force Microscopy. Energy Fuels 2020, 34, 13750–13758. 10.1021/acs.energyfuels.0c02494. [DOI] [Google Scholar]
- Zekri A.; Nantongo H.; Boukadi F. The Effect of Carbonate Rock Wettability on the Performance of Low Salinity Waterflooding: An Experimental Approach. J. Pet. Explor. Prod. Technol. 2021, 11, 4325–4338. 10.1007/s13202-021-01309-9. [DOI] [Google Scholar]
- Mokhtari R.; Anabaraonye B. U.; Afrough A.; Mohammadkhani S.; Feilberg K. L. Experimental Investigation of Low Salinity Water-Flooding in Tight Chalk Oil Reservoirs. J. Pet. Sci. Eng. 2022, 208, 109282. 10.1016/j.petrol.2021.109282. [DOI] [Google Scholar]
- Darvish Sarvestani A.; Rostami B.; Mahani H. Polymer-Enhanced Low-Salinity Brine to Control In Situ Mixing and Salt Dispersion in Low-Salinity Waterflooding. Energy Fuels 2021, 35, 10540–10550. 10.1021/acs.energyfuels.1c00871. [DOI] [Google Scholar]
- Abdel-Azeim S.; Sakthivel S.; Kandiel T. A.; Kanj M. Y. Specificity and Synergy at the Oil–Brine Interface: New Insights from Experiments and Molecular Dynamics Simulations. Energy Fuels 2021, 35, 14647–14657. 10.1021/acs.energyfuels.1c02133. [DOI] [Google Scholar]
- Belhaj A. F.; Singh N.; Sarma H. K.. Understanding the Interactions at Rock-Water and Oil-Water Interfaces during Controlled-Salinity Water Flooding. In Offshore Technology Conference Asia; OnePetro; Day 4 Fri, March 25, 2022; OTC, 2022.
- Kasha A.; Sauerer B.; Al-Hashim H.; Abdallah W. Interplay Between Ca 2+ , Mg 2+ , and SO 4 2– Ions and Their Influence on the Zeta-Potential of Limestone during Controlled-Salinity Waterflooding. Energy Fuels 2021, 35, 12982. 10.1021/acs.energyfuels.1c00940. [DOI] [Google Scholar]
- Koleini M. M.; Badizad M. H.; Mahani H.; Dastjerdi A. M.; Ayatollahi S.; Ghazanfari M. H. Atomistic Insight into Salinity Dependent Preferential Binding of Polar Aromatics to Calcite/Brine Interface: Implications to Low Salinity Waterflooding. Sci. Rep. 2021, 11, 11967. 10.1038/s41598-021-91402-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope D. M.; Bogie I.; Harvey C. C.; Menzies A. J.; Garcia S. E.. Calculation of Calcite and Anhydrite Scaling Potential in Geothermal Wells. In 7th Geothermal Workshop, 1985.
- Zachariah Z.; Espinosa-Marzal R. M.; Spencer N. D.; Heuberger M. P. Stepwise Collapse of Highly Overlapping Electrical Double Layers. Phys. Chem. Chem. Phys. 2016, 18, 24417–24427. 10.1039/C6CP04222H. [DOI] [PubMed] [Google Scholar]
- Nasr-El-Din H. A.; Al-Otaibi M. B.; Al-Qahtani A. A.; Samuel M. An Effective Fluid Formulation to Remove Drilling-Fluid MudCake in Horizontal and Multilateral Wells. SPE Drill. Completion 2007, 22, 26–32. 10.2118/87960-PA. [DOI] [Google Scholar]
- Siddig O.; Mahmoud A. A.; Elkatatny S. A Review of the Various Treatments of Oil-Based Drilling Fluids Filter Cakes. J. Pet. Explor. Prod. Technol. 2022, 12, 365–381. 10.1007/s13202-021-01427-4. [DOI] [Google Scholar]
- Mohamed A.; Elkatatny S.; Al-Majed A. Removal of Calcium Carbonate Water-Based Filter Cake Using a Green Biodegradable Acid. Sustainability 2020, 12, 994. 10.3390/su12030994. [DOI] [Google Scholar]
- Kremieniewski M.; Wiśniowski R.; Stryczek S.; Łopata P. Comparison of Efficient Ways of Mud Cake Removal from Casing Surface with Traditional and New Agents. Energies 2021, 14, 3653. 10.3390/en14123653. [DOI] [Google Scholar]
- Fredd C. N.; Fogler H. S. Alternative Stimulation Fluids and Their Impact on Carbonate Acidizing. SPE J. 1998, 3, 34–41. 10.2118/31074-pa. [DOI] [Google Scholar]
- Mahmoud M.; Kamal M. S.; Aljawad M. S.; Ali A.; Al-Nakhli A. Single-Stage Stimulation of Anhydrite-Rich Carbonate Rocks Using Chelating Agent: An Experimental and Modeling Investigation. SPE J. 2021, 26, 1144–1160. 10.2118/203840-PA. [DOI] [Google Scholar]
- Mohammed I.; Mahmoud M.; Al Shehri D.; Kamal M. S.; Alade O. S. Effects of the Reservoir Environment and Oilfield Operations on the Iron Mineral Surface Charge Development: An Insight into Their Role in Wettability Alteration. Energy Fuels 2022, 36, 1676–1687. 10.1021/acs.energyfuels.1c03909. [DOI] [Google Scholar]
- Hassan A. M.; Al-Hashim H. S. Surface Charge Study of EDTA Interaction with Carbonate Rock during Chelating Agent Flooding. J. Pet. Sci. Eng. 2020, 191, 107163. 10.1016/j.petrol.2020.107163. [DOI] [Google Scholar]
- Mady M. F.; Abdel-Azeim S.; Kelland M. A. Antiscaling Evaluation and Quantum Chemical Studies of Nitrogen-Free Organophosphorus Compounds for Oilfield Scale Management. Ind. Eng. Chem. Res. 2021, 60, 12175. 10.1021/acs.iecr.1c02441. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.